
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hematol., 30 May 2023
Sec. Blood Cancer
Volume 2 - 2023 | https://doi.org/10.3389/frhem.2023.1160049
This article is part of the Research TopicHematopoietic Stress in Stem Cell Homeostasis and Disease PathogenesisView all 5 articles
Large granular lymphocytic leukemia (LGLL) is a clonal lymphoproliferative disease with a slow course and considerable difficulties in correct diagnosis and therapy. T-LGLL is the most prevalent subtype of LGLL, accounting for approximately 85%. T-LGLL co-existence with solid malignancies is relatively rare. CD20-positive T-cell neoplasm is a rare disease in clinics. In this study, we report a case of CD20-positive T-LGLL with renal cell carcinoma (RCC) that was eventually diagnosed by splenectomy and nephrectomy. The accumulation of cases will contribute to diagnosing and treating CD20-positive T-LGLL complicated with solid tumors.
LGLL is a rare group of chronic lymphoproliferative diseases that is incompletely understood, and approximately 85% of the reported cases are T-LGLL subtype (1, 2). T-LGLL is typically identified in larger cells than normal lymphocytes, which contain abundant cytoplasm and distinct azurophilic granules. Most T-LGLL cases express characteristic immunophenotypes, CD3+, CD4-, CD8+, CD16+, CD57+, TCRαβ+, CD5dim, and/or CD7dim, and the clonal rearrangement of TCRαβ, indicating a diagnosis of T-cell type LGLL (3–6). However, not all LGL leukemias are created equal (7). Neither morphological characteristics nor immunophenotypes are totally specific. These features may vary, and occasionally clonally expanded lymphocytes with characteristic immunophenotypes may lack LGL morphology on a peripheral blood smear. In particular, STAT3 and STAT5B mutations may be present in leukemic cells, and their presence has been linked to diagnosing LGL diseases. STAT3 mutations are detected in 30%–40% of T-LGL leukemia (8–10). Hence, STAT3 and STAT5B mutations were proposed in the latest WHO classification of LGLL.
Rare variants include TCR γδ+ variants and CD4+ TCR αβ+ cases. Compared to CD8+ T-LGL leukemia, CD4+ T-LGLL demonstrated unique clinical features, including a low incidence of cytopenia, autoimmune phenomena, and increased association with neoplasia (11). Rivero A et al. reported that CD4+ T-LGLL had a higher association with concurrent solid tumors (27%) compared with CD8+ T-LGLL (10%) (12). It has been reported that Tγδ LGLL, compared with Tαβ variant, displays distinctive features with a less indolent form of the disease and shorter overall survival (OS). Moreover, Vδ2 negative patients displayed a higher frequency of symptomatic disease (13, 14).
CD20 is a transmembrane protein expressed uniformly from pre-B cells to mature B cells. Hence, it has long been considered a pan-B cell antigen. Hultin et al. (15) reported low expression of CD20 antigen in human T lymphocyte subsets. However, abnormal CD20 expression in T-cell lymphomas has rarely been reported (16–21). To date, CD20-positive T-LGLL has been reported sporadically (22, 23).
Renal cell carcinoma (RCC) is one of the most lethal malignant tumors worldwide, accounting for approximately 3% of all adult malignancies and over 90% of renal neoplasms. Clear cells are the most commonly diagnosed subtype (70%–75% of all cases) (24).
Concurrent presentation and implications of solid tumors with T-LGLL are rare, and the number of reported cases remains sparse. Here, we present a rare case of CD20-positive T-LGLL with renal clear cell carcinoma that was initially inconclusive but was successfully diagnosed and treated by splenectomy and nephrectomy and a literature review.
A 60-year-old male had thrombocytopenia, leukopenia for more than five years, and splenomegaly for three years. As he was asymptomatic, the patient neglected to seek medical attention for further diagnosis and treatment. He was admitted to our department in November 2021 for a strong recommendation by a neurologist. He was admitted to the Department of Neurology one month previously because of a cerebral infarction. He had thrombopenia and a giant spleen during hospitalization, and the neurologist repeatedly emphasized and suggested further diagnosis and treatment in the Department of Hematology. The patient had neither superficially swollen lymph nodes nor hepatomegaly. No skin rash was observed. His medical history included type 2 diabetes for one year and cerebral infarction for seven months. Physical examination revealed splenomegaly with line I: +16 cm, line II: +16 cm, and line III: -1 cm. Laboratory test results were as follows: hemoglobin (HB), 134 g/L; platelets (PLT), 64×109/L; white blood cells (WBC) 1.1×109/L, neutrophils 0.71×109/L, lymphocytes 0.22×109/L. No viral or autoimmune disorders were observed. The patient underwent an enhanced abdominal computed tomography (CT) scan because of the giant spleen. Contrast-enhanced CT and magnetic resonance imaging (MRI) revealed a heterogeneously enhanced mass highly suspected to be a malignant disease in the upper pole of the left kidney (Supplementary figure 1). A peripheral blood smear revealed a few lymphocytes, no large granular lymphocytes, or teardrop erythrocytes. Peripheral blood flow cytometry (FCM) revealed a high proportion of NK cells, accounting for 37.79% of the lymphocytes. The proportion of lymphocytes in the bone marrow increased to 45.6%, primarily mature lymphocytes with occasional hair and tails. FCM analysis revealed 20.6% CD2+/cCD3+/CD3+/CD8+/CD4-/CD5dim/CD7part/CD20+/CD56-/CD57dim/TCRαβ+/TCRγδ-/CD19-/CD79a-/CD38-populations (Figure 1 and Supplementary figure 2). T cells predominated in the bone marrow biopsy with Immunochemistry showing CD3+/CD4-/CD8+/CD57-/GranzymeB-/CD20+/CD19-/PAX-5-/CD79a- (Figure 2A). T cell receptor (TCR)Vβ repertoire analysis by flow cytometry displayed 47.13% of cells with Vβ9 clonality (Supplementary table 1). PCR analysis detected clonal TCR β and TCR γ gene rearrangements (Figure 3A, 4A).
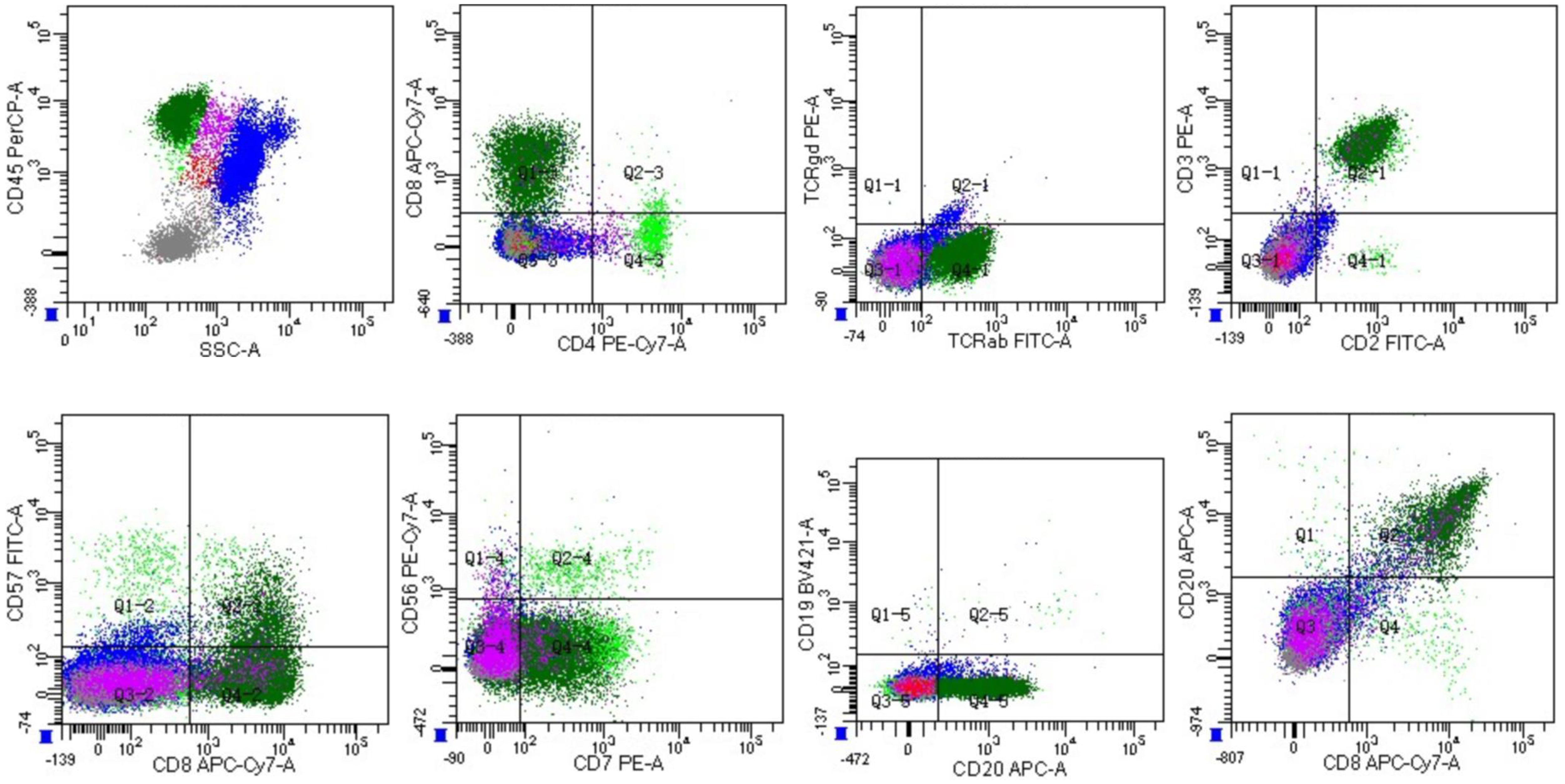
Figure 1 Flow cytometric analysis of bone marrow cells with strong CD45 gating. The analysis subsequently revealed a population with a CD2+CD3+CD4-CD7+CD8+CD19-CD20+CD56- phenotype comprising 20.6% of marrow nucleated cells (dark green).
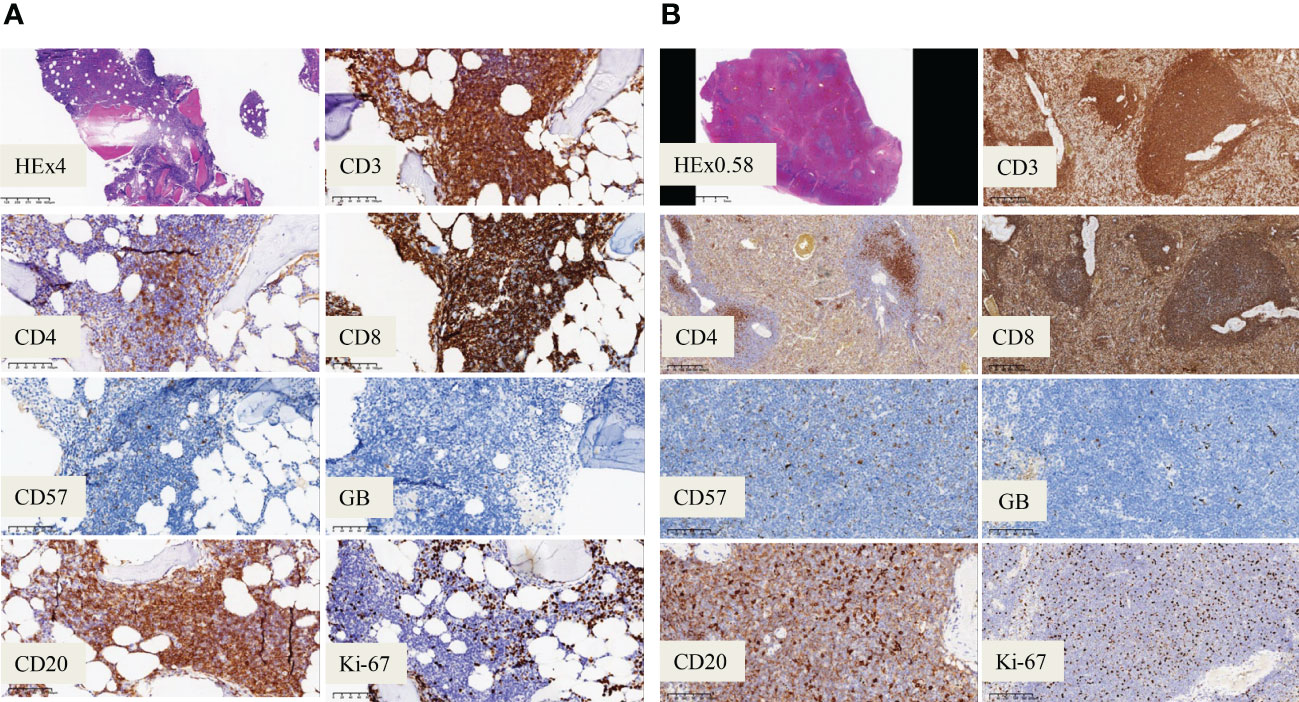
Figure 2 Immunohistochemistry analysis. (A) Immunochemistry analysis showing CD20+ /CD3+ /CD8+ /CD57- /GranzymeB- phenotype of the bone marrow cells (magnification, ×200). (B) Immunochemistry analysis on spleen showing CD20 weak+ /CD3+ /CD8+ /CD57- /GranzymeB- cells (magnification, ×200).
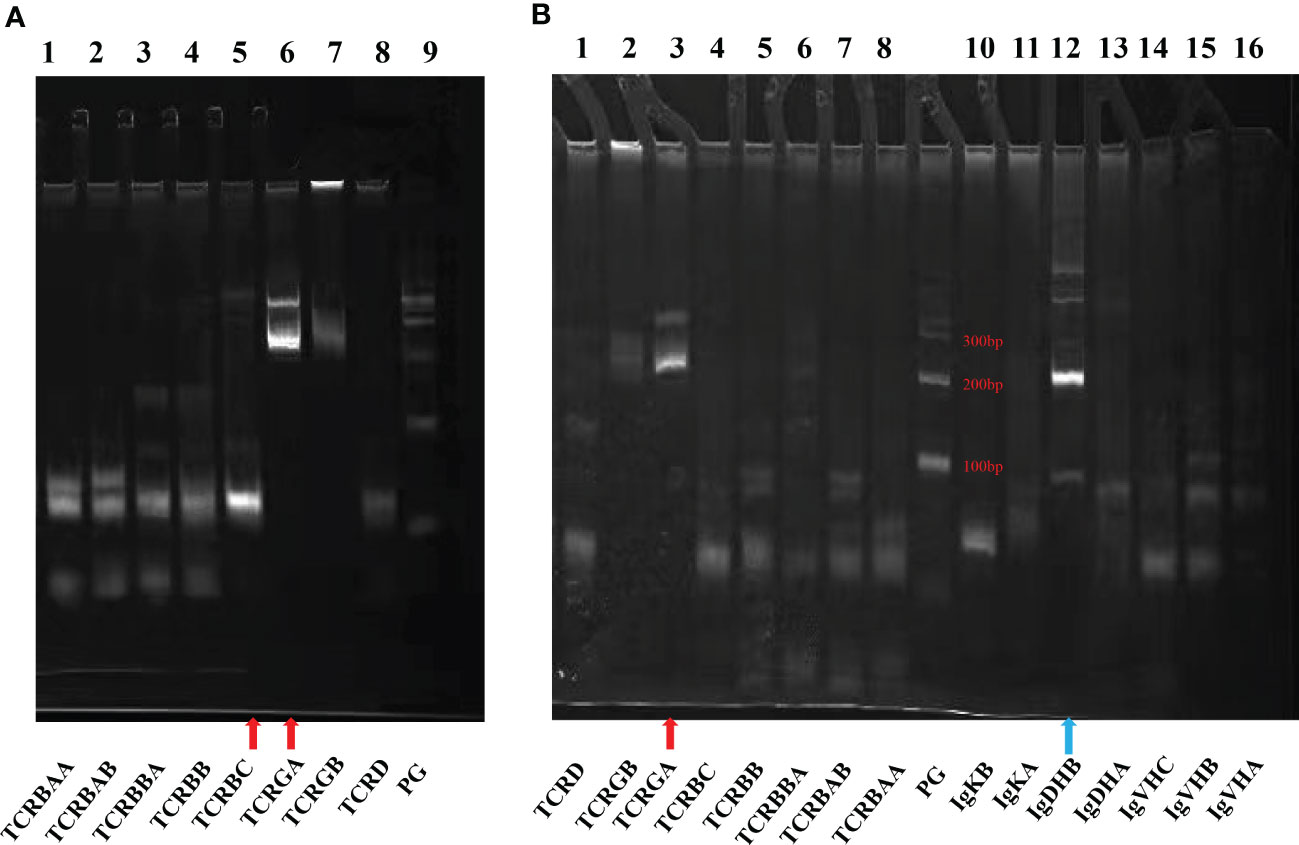
Figure 3 Agarose gel electrophoresis demonstrating results of PCR for antigen receptor rearrangements. The kit adopts Euroclonality/BIOMED-2 test system. (A) Bone marrow: TCR (Lanes 1-8). (B) Spleen: TCR (Lanes 1-8) and Ig genes (Lanes 10-16). Red arrow: TCRGA (Lane A6 and Lane B3) and TCRBC (Lane A5) were detected rearranged bands. Blue arrow: IgDHB (Lane B12) was a polyclonal PCR product and a nonspecific band of Ig.
Cytogenetics revealed a normal karyotype. Negative results were obtained for JAK2 V617F, JAK2 exon12, MPL W515L/K, and CALR. Second-generation sequencing (NGS) did not reveal any mutations in STAT3, STAT5b, or TP53 in this patient. A repeat bone marrow evaluation revealed a similar abnormal immunophenotype and clonality after a review by experienced hematopathologists. The patient underwent positron emission tomography with computed tomography (CT) to further clarify the condition. Splenectomy and radical nephrectomy were performed under general anesthesia on Jan 20, 2022. Postoperative pathology and immunohistochemistry demonstrated as follows: 1. The upper pole of the left kidney tissue was a clear cell RCC (WHO/ISUP grade I. 2. Spleen tissue: CD2+/CD3+/CD4-/CD5+/CD7+/CD8+/CD10-/CD56-/CD57-/TIA-1-/GranzyeB-/EBER-/CD20Weak+/CD19-/CD79a-/PAX-5- Ki-67 5% (Figure 2B). Spleen flow cytometric analysis revealed a T-cell population (73.5% of the total lymphocyte count) with an immunophenotype CD3+CD8+ and a reversed CD4/CD8 cell ratio (Supplementary figure 3). Based on the standardized multiplex PCRs as developed by the European BIOMED-2 collaborative study (25), TCRβ and TCRγ gene rearrangements were detected, whereas the immunoglobulin heavy chain (IgH) gene rearrangement was negative (Figure 3B).
After splenectomy, subsequent hematological follow-up revealed persistent lymphocytosis. During a follow-up visit in March 2022, a peripheral blood smear revealed that lymphocytes with granules accounted for 40% of nucleated cells in peripheral blood. These lymphocytes were rich in light-blue cytoplasm and contained several coarse or fine azurophilic granules (Figure 4B).
Accordingly, T-LGLL was diagnosed according to the 2016 revision of WHO classification of lymphoid neoplasms (6). The patient was ultimately diagnosed with CD20-positive T-LGLL with renal clear cell carcinoma based on the pathological results of renal surgery and positive CD20 expression. His postoperative hematological follow-up revealed persistent lymphocytosis. The latest follow-up CBC test (Mar 31, 2023) revealed a WBC of 8.0×109/L, an ALC of 2.73×109/L, HB 163 g/L, and PLT 225×109/L with LGL comprising 51.2% of lymphocytes. Additionally, it still presented a TCRVb9 clone. However, peripheral blood FCM revealed a high proportion of NK cells, accounting for 58.4% of lymphocytes. The recent immunophenotype of bone marrow (Mar 31, 2023) revealed same phenotype of CD3+/CD4-/CD8+/CD19-/CD20+/CD56-/CD57few/CD79a-/CD94-/TCRαβ+/TCRγδ-. PCR analysis of BM detected clonal TCR β and TCR γ gene rearrangements without immunoglobulin gene rearrangement (Supplementary figure 4). Thus far, the patient has been in good condition without any special discomfort or symptoms of B.
The course of T-LGLLs is generally indolent. Approximately one-third of patients display no symptoms at the time of diagnosis, and splenomegaly is observed in 20%–50% of patients (26). In this case, the main presentation was splenomegaly with neutropenia and thrombocytopenia for a long time, and the patient presented with a low lymphocyte count. Consequently, we did not detect LGL morphology in peripheral blood smears before splenectomy. Furthermore, no mutations were found in STAT3 or STAT5b, as per NGS studies. Initially, the diagnosis was ambiguous, and the patient faced a dilemma regarding whether or not to perform a total splenectomy. Additionally, imaging of the patient’s left kidney suggested a renal tumor. However, the pathological nature of this disease is unclear. We performed a total splenectomy and partial left nephrectomy under general anesthesia on Jan 20, 2022. Follow-up revealed persistent lymphocytosis in the patient’s peripheral blood. During one of his follow-up visits in March 2022, a peripheral blood smear revealed that lymphocytes containing granules comprised 40% of nucleated cells in the peripheral blood. These lymphocytes were cytoplasm-rich, light blue, and contained several coarse or fine azurophilic granules.
Our patient also discovered interesting immunophenotypes, with T lymphocytes co-expressing the CD20 antigen. There have been a few reports of CD20+ T-cell malignancies (16–21). Most cases exhibited monoclonal rearrangements of TCR β without IgH genes. However, CD20-positive T-LGLLs have been sporadically reported. A literature review revealed that Sonoko Nakano-Akamatsu et al. and Koji Miyazaki et al. reported cases of CD20-positive T-LGLL (22, 23). Interestingly, both leukemia cell lines demonstrated monoclonal rearrangement of TCR β without IgH gene rearrangement, whereas another case reported monoclonal rearrangement of TCR β and IgH genes in CD20+ LGL leukemia cells (19). However, the mechanism and role of aberrant CD20 expression on malignant T-cells remain unclear. There are several possible hypotheses that may explain CD20 expression in T-LGLL. The first hypothesis is that normal circulating CD20-positive T-cells may undergo neoplastic transformation (27). Second, CD20 may be considered an activation marker (28–30). The third is a clonal proliferation of the small population of bone marrow T-cells expressing CD20 (21). Notably, the possibility of cross-reacting antigens should also be considered.
We ultimately diagnosed T-LGLL with RCC based on cell morphology, immunophenotype, pathological immunohistochemistry, and 8ji9000TCR rearrangement Coincidentally, we discovered many coexisting malignancies in our LGLL patients. Several studies initially reported less than 10% of non-hematologic malignancies in LGLL (31, 32). In 2010, Bareau et al. described solid tumors in 4.4% of LGLL patients (10/229) (31). However, later studies have depicted a significantly higher incidence of solid tumors in LGLL. In 2015, Viny et al. reported the presence of solid cancers in 17.3% LGLL patients (27/156) (33). More recently, in 2021, Dong et al. reported a proportion of 66/319 (20.7%) solid neoplasms in their LGLL cohort and confirmed similar results (34). Overall, the most common primary tumor localizations from the above three mentioned largest LGLL cohorts were: prostate (22 cases), breast (20 cases), lung (10 cases), and skin (10 cases) cancers. Notably, six cases of kidney malignancies co-existence with LGLL were demonstrated in these LGLL cohorts (31, 33, 34). We observed only one case of T-LGL with renal clear-cell carcinoma in the literature. Qiu et al. reported a case of T-LGLL combined with renal cancer and discovered that the co-existence of the two might not be coincidental. Their co-pathogenesis may be associated with immune dysregulation (35). Despite undoubtedly increasing evidence, there are insufficient data on the pathogenic link between LGLL and solid neoplasms. There are various explanations for the association between LGLL and solid neoplasms. One hypothesis is that tumors may be an antigenic trigger and that LGL expansion is a strong antitumor response. Another hypothesis is that the immunosuppressive state induced by LGLL may contribute to the pathogenesis of other malignancies. It is unclear whether solid tumors are more common in patients with LGLL or whether the higher incidence reflects an increased incidence of these cancers in older patients (36–38). Given the fact that LGL is currently involved in the control of neoplastic growth, in our case, we assume that LGL proliferation begins as a result of a reactive process, and this concept is also consistent with the increase of NK cells (58.4%) detected at the latest test (Mar 31, 2023).
Currently, there is no standard protocol for treating T-LGLL due to a lack of randomized prospective trials. Therefore, the choice of initial and subsequent treatment for LGLL is primarily based on experience or retrospective analysis of a limited number of cases. Currently, therapeutic approaches for T-LGLL in clinical practice largely rely on immunosuppressive agents, including methotrexate, cyclophosphamide, cyclosporine A, purine nucleoside analogs, and splenectomy. Considering the indolent course of the disease in most T-LGLL patients, it is recommended that asymptomatic patients have the option of watchful waiting, with laboratory and clinical physical examinations every 3–6 months. Splenectomy may be considered for T-LGLL with symptomatic splenomegaly or significant hemocytopenia caused by splenomegaly. Splenectomy results in LGLL patients are rarely reported and vary in limited reports.
A literature review revealed that the overall response rate of T-LGLL treated with splenectomy was 56% (31/55); however, sustained responses were infrequent (31, 32, 39–43). Five of 13 T-LGLL cases who underwent splenectomy exhibited clinical improvement in the French registry study, while 11 of 19 patients responded at the Cleveland Clinic (43, 44). However, the main complications of splenectomy are thrombosis, infection, and symptom aggravation. Additionally, postoperative analysis revealed that splenectomy did not clear LGL clone, and LGL continued to expand. Consequently, splenectomy must weigh the pros and cons. In our case, splenectomy was chosen as the diagnostic and treatment option for significant splenomegaly. After surgery, the patient was in good condition without any clinical symptoms. Currently, no treatment is available, and the patient is undergoing close follow-up in the outpatient department.
This is the first case report with a final diagnosis of CD20-expressing T-LGLL and renal cancer after splenectomy and nephrectomy. In our clinical routine, variable presentations and phenotypes of T-LGLL are underappreciated. In our case, the morphology was not initially identified, and the immunophenotype suggested T-LGLL; however, it was not entirely specific. Subsequently, the peripheral blood lymphocyte count increased, and splenectomy and nephrectomy revealed typical large granular lymphocytes. The final diagnosis was T-LGLL and renal cancer with CD20 expression, demonstrating the importance of immunophenotyping in assisting with T-LGLL diagnosis. In addition, it demonstrated that resection of the spleen could be used as a diagnostic and treatment method without removing large granular lymphocytes. This case also reminds us that T-LGLL is difficult to diagnose and easily overlooked in clinical practice.
This case demonstrated that T-LGLL presents a diagnostic challenge. When the cell morphology of these diseases is initially inadequate for diagnosis, and the immunophenotype and immunohistochemistry are atypical, the diagnosis and treatment of splenic marginal zone lymphoma can be referred to, and splenectomy may be a more appropriate option.
Here, we describe a rare case of CD20-positive T-LGL leukemia accompanied by renal neoplasia. Our experience with splenectomy as a diagnostic method and treatment strategy provides a reference for choice in this patient group. However, further data are required to elucidate the implications of CD20 expression on the concurrent presentation of these disorders and clarify whether these cases constitute a unique biological and clinical disease entity.
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YJ and FR reviewed relevant literature and wrote the manuscript. SW and JX provided relevant pathological findings and original images. YW and JT verified the relevant content and revised the manuscript. JX created, reviewed, and revised the manuscript. All authors read and agreed to submit the present manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by HawMei Research Foundation of Ningbo No.2 Hospital (Grant No. 2022HMKY21) and Clinical Medicine Special Fund Project of Zhejiang Medical Association (Grant No. 2022ZYC-A162).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2023.1160049/full#supplementary-material
1. Cheon HJ, Dziewulska KH, Moosic KB, Olson KC, Gru AA, Feith DJ, et al. Advances in the diagnosis and treatment of Large granular lymphocytic leukemia. Curr Hematol Malig Rep (2020) 15:103–12. doi: 10.1007/s11899-020-00565-6
2. Lamy T, Loughran TP Jr. How I treat LGL leukemia. Blood (2011) 117:2764–74. doi: 10.1182/blood-2010-07-296962
3. Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood (2017) 129:1082–94. doi: 10.1182/blood-2016-08-692590
4. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
5. Lundell R, Hartung L, Hill S, Perkins SL, Bahler DW. T-Cell large granular lymphocyte leukemias have multiple phenotypic abnormalities involving pan-t-cell antigens and receptors for MHC molecules. Am J Clin Pathol (2005) 124:937–46. doi: 10.1309/ph7x-78hf-4fw4-prkw
6. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
7. Semenzato G, Calabretto G, Barilà G, Gasparini VR, Teramo A, Zambello R. Not all LGL leukemias are created equal. Blood Rev (2023) 20:101058. doi: 10.1016/j.blre.2023.101058
8. Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmäki H, Andersson EI, et al. Somatic STAT3 mutations in Large granular lymphocytic leukemia. N Engl J Med (2012) 366:1905–13. doi: 10.1056/NEJMoa1114885
9. Barilà G, Teramo A, Calabretto G, Vicenzetto C, Gasparini VR, Pavan L, et al. Stat3 mutations impact on overall survival in large granular lymphocyte leukemia: a single-center experience of 205 patients. Leukemia (2020) 34:1116–24. doi: 10.1038/s41375-019-0644-0
10. Andersson EI, Tanahashi T, Sekiguchi N, Gasparini VR, Bortoluzzi S, Kawakami T, et al. High incidence of activating STAT5B mutations in CD4-positive T-cell large granular lymphocyte leukemia. Blood (2016) 128:2465–68. doi: 10.1182/blood-2016-06-724856
11. Lima M, Almeida J, Dos Anjos Teixeira M, Alguero Md Mdel C, Santos AH, Balanzategui A, et al. TCRalphabeta+/CD4+ large granular lymphocytosis: a new clonal T-cell lymphoproliferative disorder. Am J Pathol (2003) 163:763–71. doi: 10.1016/s0002-9440(10)63703-0
12. Rivero A, Mozas P, Jiménez L, López-Guerra M, Colomer D, Bataller A, et al. Clinicobiological characteristics and outcomes of patients with T-cell Large granular lymphocytic leukemia and chronic lymphoproliferative disorder of natural killer cells from a single institution. Cancers (Basel) (2021) 13:3900. doi: 10.3390/cancers13153900
13. Teramo A, Binatti A, Ciabatti E, Schiavoni G, Tarrini G, Barila G, et al. Combined immunophenotypic and molecular evaluation contributes to better define TCRγδ lymphoproliferative disorders. Nat Commun (2022) 13:3298–308. doi: 10.1038/s41467-022-31015-x
14. Barilà G, Grassi A, Cheon H, Teramo A, Calabretto G, Chahal J, et al. Tγδ LGLL identifies a subset with more symptomatic disease: analysis of an international cohort of 137 patients. Blood (2023) 141:1036–46. doi: 10.1182/blood.2021013489
15. Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-b cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry (1993) 14:196–204. doi: 10.1002/cyto.990140212
16. Kakinoki Y, Hashiguchi J, Ishio T, Chiba K, Niino D, Ohshima K. CD20-positive primary gastric T-cell lymphoma poorly responding to initial treatment with rituximab plus CHOP, and a literature review. Int J Hematol (2015) 102:702–8. doi: 10.1007/s12185-015-1841-x
17. Kawano R, Niino D, Ohshima K. Six cases of CD20-positive adult T-cell leukemia. J Clin Exp Hematop (2016) 56:119–25. doi: 10.3960/jslrt.56.119
18. Li YL, Wang HP, Zhang C, Zhai ZM. CD20-positive primary nasal peripheral T-cell lymphoma: an analysis of one case and review of the literature. Cytometry B Clin Cytom (2020) 98:348–54. doi: 10.1002/cyto.b.21852
19. Akashi K, Shibuya T, Nakamura M, Oogami A, Harada M, Niho Y. Large Granular lymphocytic leukaemia with mixed T-cell/B-cell phenotype. Br J Haematol (1998) 100:291–4. doi: 10.1046/j.1365-2141.1998.00552.x
20. Tamayose K, Sato N, Ando J, Sugimoto K, Oshimi K. CD3-negative, CD20-positive T-cell prolymphocytic leukemia: case report and review of the literature. Am J Hematol (2002) 71:331–5. doi: 10.1002/ajh.10224
21. Algino KM, Thomason RW, King DE, Montiel MM, Craig FE. CD20 (pan-b cell antigen) expression on bone marrow-derived T cells. Am J Clin Pathol (1996) 106:78–81. doi: 10.1093/ajcp/106.1.78
22. Nakano-Akamatsu S, Takahashi R, Sekioka Y, Hosokawa Y, Inaba T. CD20- and CD56-positive T-cell Large granular lymphocyte leukemia in a human T-cell leukemia virus type 1 carrier. Int J Hematol (2007) 86:348–51. doi: 10.1532/IJH97.07076
23. Miyazaki K, Ohsaka M, Suzuki Y, Danbara M, Horie R, Higashihara M. CD20-positive T-cell large granular lymphocyte leukemia: case report and review of the literature. Intern Med (2009) 48:1443–7. doi: 10.2169/internalmedicine.48.2141
24. Chow W-H, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol (2010) 7:245–57. doi: 10.1038/nrurol.2010.46
25. van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia (2003) 17:2257–317. doi: 10.1038/sj.leu.2403202
26. Loughran TP. Clonal diseases of large granular lymphocytes. Blood (1993) 82:1–14. doi: 10.1182/blood.V82.1.1.bloodjournal8211
27. Quintanilla-Martinez L, Preffer F, Rubin D, Ferry JA, Harris NL. CD20+ T-cell lymphoma. neoplastic transformation of a normal T-cell subset. Am J Clin Pathol (1994) 102:483–9. doi: 10.1093/ajcp/102.4.483
28. Murayama Y, Mukai R, Sata T, Matsunaga S, Noguchi A, Yoshikawa Y. Transient expression of CD20 antigen (pan b cell marker) in activated lymph node T cells. Microbiol Immunol (1996) 40:467–41. doi: 10.1111/j.1348-0421.1996.tb01096.x
29. Sun T, Akalin A, Rodacker M, Braun T. CD20 positive T cell lymphoma: is it a real entitiy? J Clin Pathol (2004) 57:442–4. doi: 10.1136/jcp.2003.011734
30. Lee KY, Jeon SY, Hong JW, Kim YH, Song KH, Kim KH. CD20 positive T cell lymphoma involvement of skin. Ann Dermatol (2011) 23:529–35. doi: 10.5021/ad.2011.23.4.529
31. Bareau B, Rey J, Hamidou M, Donadieu J, Morcet J, Reman O, et al. Analysis of a French cohort of patients with large granular lymphocyte leukemia: a report on 229 cases. Haematologica (2010) 95:1534–41. doi: 10.3324/haematol.2009.018481
32. Dhodapkar MV, Li CY, Lust JA, Tefferi A, Phyliky RL. Clinical spectrum of clonal proliferations of T-large granular lymphocytes: a T-cell clonopathy of undetermined significance? Blood (1994) 84:1620–7. doi: 10.1182/blood.V84.5.1620.1620
33. Viny AD, Maciejewski JP. High rate of both hematopoietic and solid tumors associated with large granular lymphocyte leukemia. Leuk Lymphoma (2015) 56:503–4. doi: 10.3109/10428194.2014.927459
34. Dong N, Castillo Tokumori F, Isenalumhe L, Zhang Y, Tandon A, Knepper TC, et al. Large Granular lymphocytic leukemia - a retrospective study of 319 cases. Am J Hematol (2021) 96:772–80. doi: 10.1002/ajh.26183
35. Qiu ZY, Wang Y, Zhang J, Zhang Z, Fan L, Wang R, et al. T-Cell large granular lymphocytic leukemia associated with renal cell carcinoma: a case report. Med (Baltimore) (2018) 97:e13064. doi: 10.1097/MD.0000000000013064
36. O’Keefe CL, Plasilova M, Wlodarski M, Risitano AM, Rodriguez AR, Howe E, et al. Molecular analysis of TCR clonotypes in LGL: a clonal model for polyclonal responses. J Immunol (2004) 172:1960–9. doi: 10.4049/jimmunol.172.3.1960
37. Wlodarski MW, O’Keefe C, Howe EC, Risitano AM, Rodriguez A, Warshawsky I, et al. Pathologic clonal cytotoxic T-cell responses: nonrandom nature of the T-cell-receptor restriction in large granular lymphocyte leukemia. Blood (2005) 106:2769–80. doi: 10.1182/blood-2004-10-4045
38. Clemente MJ, Wlodarski MW, Makishima H, Viny AD, Bretschneider I, Shaik M, et al. Clonal drift demonstrates unexpected dynamics of the T-cell repertoire in T-large granular lymphocyte leukemia. Blood (2011) 118:4384–93. doi: 10.1182/blood-2011-02-338517
39. Loughran TP Jr, Starkebaum G, Clark E, Wallace P, Kadin ME. Evaluation of splenectomy in large granular lymphocyte leukaemia. Br J Haematol (1987) 67:135–40. doi: 10.1111/j.1365-2141.1987.tb02316.x
40. Gentile TC, Hadlock KG, Uner AH, Delal B, Squiers E, Crowley S, et al. Large Granular lymphocyte leukaemia occurring after renal transplantation. Br J Haematol (1998) 101:507–12. doi: 10.1046/j.1365-2141.1998.00712.x
41. Brinkman K, van Dongen JJ, van Lom K, Groeneveld K, Misere JF, van der Heul C. Induction of clinical remission in T-large granular lymphocyte leukemia with cyclosporin a, monitored by use of immunophenotyping with vbeta antibodies. Leukemia (1998) 12:150–4. doi: 10.1038/sj.leu.2400907
42. Subbiah V, Viny AD, Rosenblatt S, Pohlman B, Lichtin A, Maciejewski JP. Outcomes of splenectomy in T-cell large granular lymphocyte leukemia with splenomegaly and cytopenia. Exp Hematol (2008) 36:1078–83. doi: 10.1016/j.exphem.2008.04.005
43. Mohan SR, Maciejewski JP. Diagnosis and therapy of neutropenia in large granular lymphocyte leukemia. Curr Opin Hematol (2009) 16:27–34. doi: 10.1097/MOH.0b013e32831c8407
Keywords: CD20, T-cell large granular lymphocyte leukemia, renal cell carcinoma, splenectomy, nephrectomy
Citation: Jin Y, Ren F, Wang S, Xu J, Wu Y, Tang J and Xu J (2023) Case Report: A rare case of CD20-positive T-cell large granular lymphocyte leukemia with renal cell carcinoma: a challenging diagnosis. Front. Hematol. 2:1160049. doi: 10.3389/frhem.2023.1160049
Received: 24 February 2023; Accepted: 11 May 2023;
Published: 30 May 2023.
Edited by:
Francesco Di Raimondo, University of Catania, ItalyCopyright © 2023 Jin, Ren, Wang, Xu, Wu, Tang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfen Xu, WHVqaWFuZmVuMjAxM0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.