- 1Clinical Pathology Unit, Polyclinic of Bari, Bari, Italy
- 2Hematology and Bone Marrow Transplantation Unit, Azienda Ospedaliera Unicersitaria Consorziale (AOUC) Policlinico, Bari, Italy
Background: IgD Multiple Myeloma is a rare form of plasma cell dyscrasia and accounts for approximately 1-2% of all cases of Multiple Myeloma. It mainly affects young, male subjects; it is characterized by an aggressive course, a high production of Bence Jones protein, acute renal failure and an often unfortunate outcome compared to the other isotypes of MM. A distinctive feature is the lack of a monoclonal peak on serum protein electrophoresis (SPE).
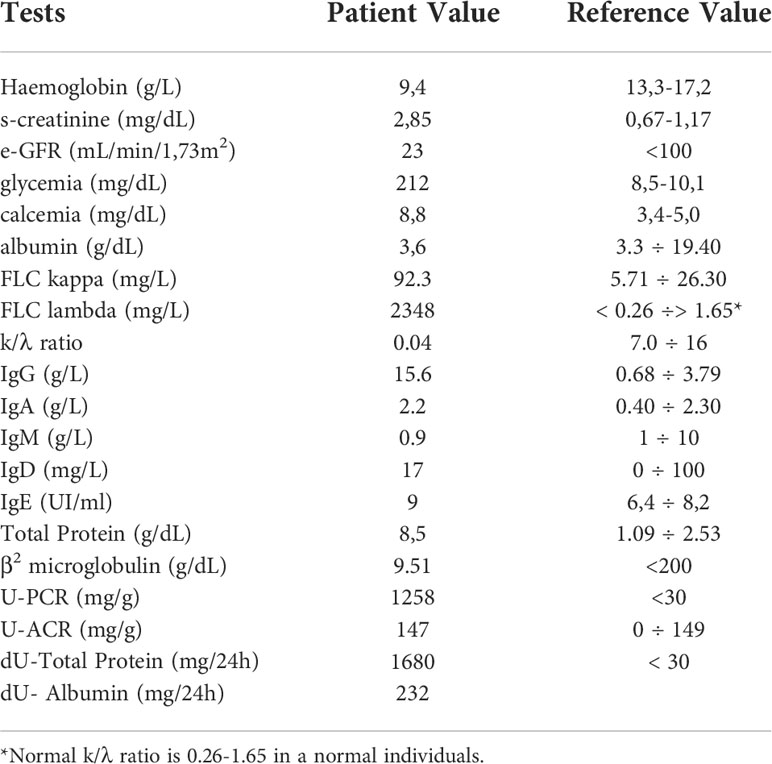
Case report: a 57-year-old man with pain in his left lower limb and weight loss goes to the Emergency Department (Emergency Department). Laboratory tests performed showed normocytic normochromic anemia (Hemoglobin 9.4 g/dL), acute renal failure (s-creatinine 2.85 mg/dL, e-GFR 23 mL/min/1.73 m². serum protein electrophoresis (SPE) detected only mild polyclonal in the gamma zone with no evidence of any monoclonal peak.
Results: serum immunofixation (s-IFE) showed a monoclonal IgD λ band and a monoclonal λ band. The Free Light Chains (s-FLC) measurement showed a ratio of 0.04. The bone marrow biopsy confirmed an infiltration of> 20% of clonal plasma cells; renal biopsy diagnosed “cast nephropathy”.
Conclusion: IgD λ/λ Multiple Myeloma is a rare form of this disease with a poor prognosis; an early and correct laboratory diagnosis is crucial for appropriate treatment and effective monitoring in order to improve patient outcome.
Introduction
Multiple Myeloma, represents about 10% of hematological neoplasms, is characterized by the clonal proliferation of plasma cells that produces a monoclonal protein almost always visible on the electrophoretic trace (SPE). IgD MM is a rare form of this disease (1-2%) and is characterized by, compared to the other isotypes, greater frequency in male subjects (M/F: 1.6/1);, absence of monoclonal protein on serum protein electrophoresis, low survival, higher frequency of the elevated isotype IgD λ compared to IgD k, hypercalcemia, incidence of extramedullary infiltration, bone lesions and often associated with amyloidosis to read (AL) (1–3).
At the time of diagnosis, 20-40% of patients present with acute renal failure with “cast nephropathy” caused by the intratubular precipitation of the free light chains of immunoglobulin (4, 5).
We report a challenging case in the laboratory diagnosis of a biclonal IgD λ/λ Multiple Myeloma in a patient with impaired renal function, anemia, without hypercalcemia and with an electrophoretic trace of monoclonal peak serum proteins.
Case report
A 57-year-old male patient with a history of hypertension and type 2 diabetes mellitus being treated with oral antidiabetics, he arrived he was admitted to at the Emergency Department (Emergency Department) of the University Hospital Polyclinic of Bari for pain in the left lower limb, asthenia and weight loss. On physical examination he presented obesity (body weight 112kg; height 173cm; BMI of 37.12), referred with an inexplicable weight loss of about 10 kg in two months. The first level laboratory investigations are performed showed in Table 1 a normocytic normochromic anemia (Hemoglobin 9.4 g/dL) (13.3-17.2 g/dL); acute renal failure (AKI) ((s-creatinine 2.85 mg/dL) (0.67-1.17 mg/dL) and eGFR 23 mL/min/1.73m2), blood glucose 212 mg/dL (<100 mg/dL), total protein 8.5 g/dL (6.4 - 8.2 g/dL), albumin 3.6 g/dL (3.4-5.0 g/dL), calcium 8.8 mg/dL (8.5-10.1 g/dL), U-PCR (U-Protein/U-Creatinine Ratio) 1258 mg/g (<200mg/g) and U-ACR (U-Albumin/U- Creatinine Ratio) 146 mg/g (<30 mg/g). For a better understanding of the renal damage, further laboratory investigations were performed and renal biopsy planned in a diabetic and hypertensive patient.
Serum protein electrophoresis (CZE capillary method SEBIA-France instrumentation) showed a mild hypergammaglobulinemia without any monoclonal peak; the s-Free Light Chains λ (sFLCL) and the s-Free Light Chains k (sFLCK) (Freelite immunoturbidimetric method Optilite instrumentation The Binding Site, Birmingham, UK) were respectively 2348 mg/L (5.71-26.30 mg/L) and 92.3 mg/L (3.3-19.40 mg/L) with a k/λ ratio of 0.04. Total Protein 24h Urine (UTP) was 1680 mg/24h (0–149) and Albumin 24h Urine was 232mg/24h (<30). Total IgG, IgM, IgA and IgE were performed with immunonephelometric method on Dimension Vista 1500 analyzer (Siemens Healthineers). The total IgD was determined with the immunonephelometric method on the BN ProSpec System analyzer (Siemens Healthineers) (Table 1).
The laboratory diagnostic work-up was completed with serum (s-IFE) and urinary (u-IFE) immunofixation performed on agarose gel (Hydrasis Sebia France) for the search for monoclonal immunoglobulins and the Bence Jones protein. The laboratory diagnostic work-up was completed with serum (s-IFE) and urinary (u-IFE) immunofixation performed on agarose gel (Hydrasis Sebia France) to detect the monoclonal immunoglobulins and the Bence Jones protein. The first s-IFE was performed using standard anti-IgG, IgA, IgM, Kappa and lambda antisera which showed the presence of a monoclonal band of lambda type (λ) not binding any heavy chain (Figure 1A). The second s-IFE was performed using anti IgD and IgE kappa and lambda (Sebia Instrumentation, Francia) antisera which showed the presence of a monoclonal, homogeneous and compact band, type IgD band and a monoclonal lambda band (λ) (Figure 1B, 2). The visualization of the agarose gel showed that the monoclonal band of the IgD heavy chain had different electrophoretic motility of the lambda light chain and were not aligned with each other. The correct characterization of the two monoclonal immunoglobulins required an additional s-IFE using an immunosubtraction technique. Patient serum (10µL) was incubated at 4° C for 48h with 100µL of anti-kappa antiserum and 100µL of anti-lambda antiserum respectively. After centrifugation of the samples, an s-IFE was performed on both supernatants: the disappearance on the agarose gel tracks of the IgD λ and λ monoclonal bands reacted with anti IgD and anti-λ antisera respectively confirmed the presence of the complete IgD λ and λ free (Figure 1C). Urinary immunofixation (u-IFE) on agarose gel (Hydrasis Sebia France) showed a marked presence of λ-type Bence Jones protein of 925 mg/24h.
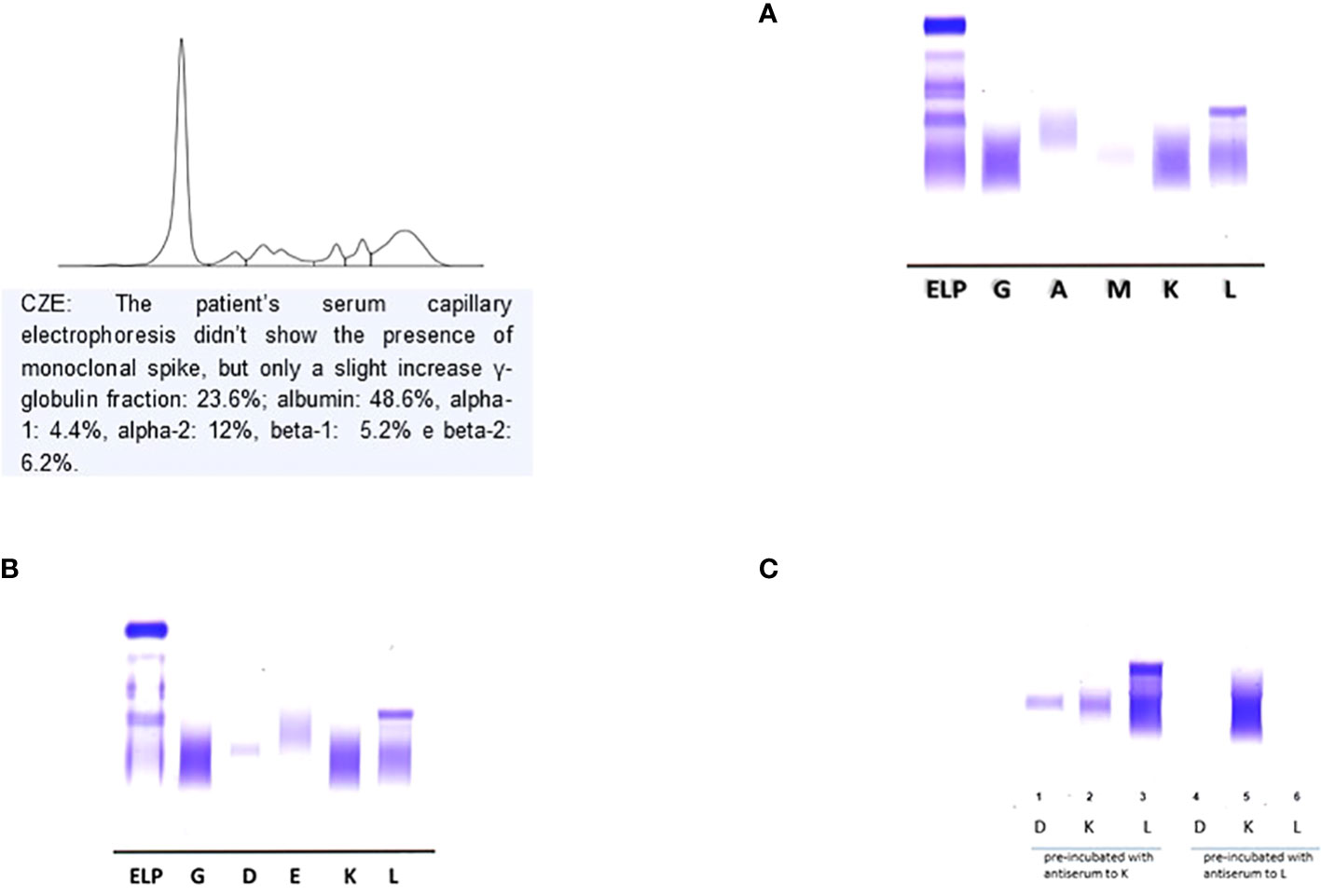
Figure 1 Serum Immunofixation with antisera standard anti- IgG, IgA, IgM kappa e lambda. It is highlighted a monoclonal lambda light chains. (A) Serum Immunofixation with antisera standard anti-IgG anti-IgA, anti- IgM, anti- kappa e anti-lambda. (B) Serum Immunofixation with antisera for IgD and IgE. It is highlighted a monoclonal band IgD heavy chain no corresponding monoclonal lambda light chains. (C) : Immunosubtraction technique: on anes 1-2-3- was applied the sample precipitated with antiserum anti-kappa; on lanes 4-5-6- the sample precipitated with antiserum anti-lambda; the disappearance in the latter of the band confirmed the presence of monoclonal IgD λ and free λ chains.
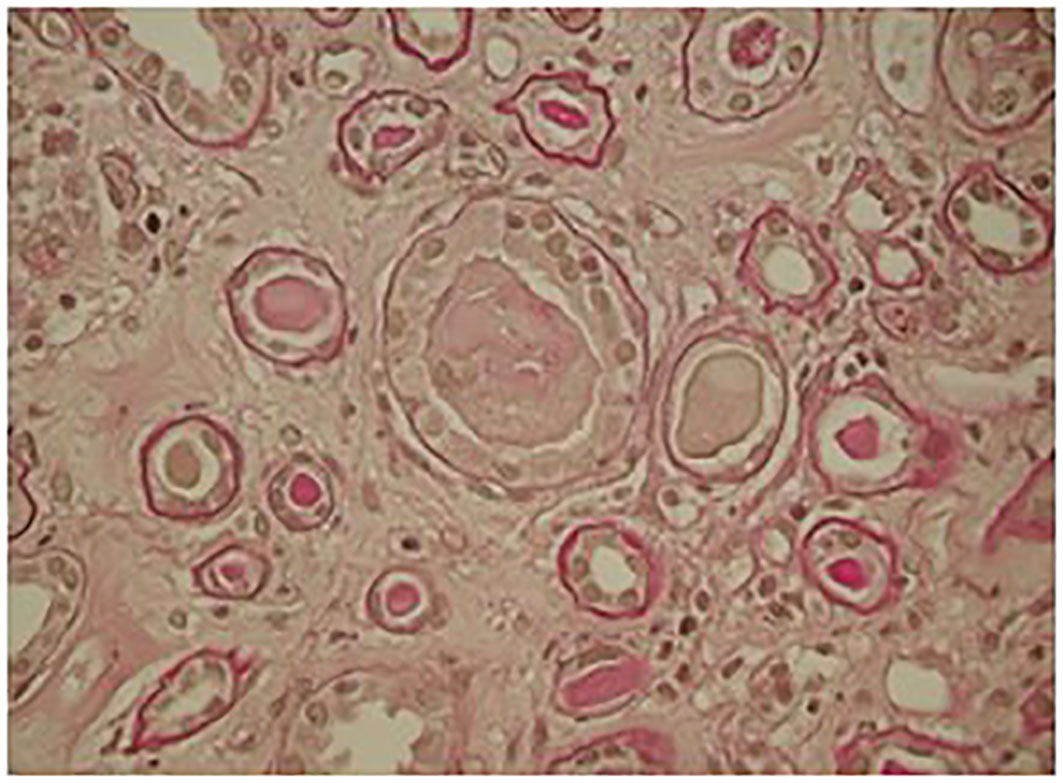
Figure 2 Kidney biopsy: light microscopy showed ischemic glomeruli; intratubular casts, fracturated appearance giant cell cast reaction.
These laboratory data led clinicians to perform an urgent hematology work-up for the diagnosis of Multiple Myeloma. Immunophenotypic analysis by flow cytometry, performed on a bone marrow aspirate sample, showed revealed a proportion 7% of CD38 and CD138 positive plasma cells polyclonal, which had cytoplasmic lambda light chain restriction and expressed CD117, but were CD19, CD45 and CD56 negative. Immunohistochemistry on osteo-medullary biopsy sections revealed focal infiltration> 20% of plasma cells positive for CD138, immunoglobulin D heavy chain and lambda light chain. The bone marrow biopsy detected plasma cell infiltrate> 20%, positive for CD138, immunoglobulin D heavy chain and lambda light chain.
Conventional G-banding analysis revealed a normal 46 XY male karyotype, while fluorescence in situ hybridization (FISH) with Vysis DNA probes was negative for the most common high-risk markers.
The skeletal x-ray and total body CT were negative for osteolytic lesions; PET-CT showed widespread hypermetabolic activity throughout the bone marrow. Optical microscopy renal biopsy revealed glomeruli with signs of ischemia and thickening of the capsule and retraction of the floc; widespread signs of tubular distress with flattening of the epithelium and detachment of epithelial cells from the basement membrane and several protein cylinders in the tubular lumen, some of them showing clear margins of fracture associated with rupture of the tubular wall; Tubular atrophy associated with interstitial fibrosis was present in 50% of the cortical tissue. The microscopic picture was compatible with acute tubular necrosis with aspects of cast nephropathy (Figure 2).
The patient’s final diagnosis was λ/λ IgD bicyclonal multiple myeloma and stage IV Chronic Kidney Disease (CKD stage IV).
Therapy with Bortezomib, Doxorubicin and Dexamethasone was started immediately and the patient was scheduled for autologous stem cell transplantation.
Discussion
Multiple Myeloma represents about 10% of all hematological neoplasms and IgD MM is a rare subtype of this disease (1-2%); the electrophoretic pattern is different from that of the IgA and IgG MM isotypes but more similar to the Light Chain MM isotype, in fact most of the IgD MM have a normal electrophoretic pattern or hypogammaglobulinemia. This electrophoretic specificity can be explained on the basis of the intrinsic characteristics of IgD which have an average life of 2.8 days and are present in plasma in low concentrations (0-10 mg/dL) (6, 7). To avoid that an IgD MM is erroneously diagnosed as an LCMM, laboratory investigations must include the use of anti IgD and anti IgE antisera whenever the s-IFE with standard anti-IgG, IgA, IgM, kappa and lambda antisera only shows one band monoclonal type k or λ (8). Furthermore, the interpretation of the electrophoretic pattern can be further complicated by the presence in the patient’s serum of free light chains with almost identical mobility to complete immunoglobulin (Ig). The characterization of monoclonal Ig in the serum of the patient with monoclonal gammopathies is usually simple and a single determination is sufficient to identify the specific isotype (9). In our case, to confirm that the free light chains highlighted in the s-IFE were the product of a proliferative clone secreting free light chains and not the excess production of the complete IgD (free quota), it was necessary to carry out a further s-IFE after immunosubtraction as a further diagnostic test. The evidence on agarose gel of the disappearance of both IgD and lambda light chains has supported the hypothesis of a double neoplastic clone and the diagnosis of MM IgD λ/λ.
The analytical difficulties encountered could be related to the low IgD concentration, to the partial overlap of the second clone and, as already highlighted by other authors, to the poor avidity and inaccessibility of the anti light chain antisera in the intact Ig (10, 11). Knowledge of MM IgDλ/λ and its laboratory analytical specificities are essential to avoid a misdiagnosis of LCMM. Bence Jones proteinuria is present in 71 to 96% of patients and cast nephropathy is the most common pathophysiological mechanism of kidney damage. The light chains precipitated in the distal tubule form obstructing protein cylinders with consequent atrophy of the same and interstitial fibrosis. Acute renal failure (AKI) with no apparent cause with high 24-hour proteinuria, associated with bone pain, must lead to suspicion of IgD Multiple Myeloma (12, 13).
Clinical laboratories performing diagnostic work-up for MM should always perform s-IFE with anti-IgD and anti-IgE antisera, in the presence of a single monoclonal k- or λ-type band, and an agarose gel after immunosubtraction as a follow-up diagnostic test when there is no correspondence in the deposition zone between the monoclonal free light chain and the heavy chain. The technique used for immunosubtraction is simple, inexpensive and allows for the integration of u-IFE and s-FLC information, directing the diagnosis towards a bi-cyclonal MM. This laboratory information will be useful above all in the response to therapy and will improve the patient’s outcome.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written Informed Consent was obtained for the publication of any potentially identifiable images or data included in this article.
Author contributions
Author Contributions: Conceptualization, TT and VB; methodology, LM and AM; Software, VB and RL; validation, FDS and TT; formal analysis, VB; investigation, LM and AM; resources, RR; data curation, RL; writing—original draft preparation, TT; writing—review and editing, TT and VB; visualization, LM and AM; supervision, TT; project administration, FDS; funding acquisition, RR. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pisani F, Petrucci MT, Giannelli D, Bongarzoni V, Montanaro M, De Stefano V, et al. IgD multiple myeloma a descriptive report of 17 cases: Survival and response to therapy. J Exp Clin Cancer Res (2012) 31(1):17. doi: 10.1186/1756-9966-31-17
2. Pandey S, Kyle RA. Unusual myelomas: A review of IgD and IgE variants. Oncology (Williston Park) (2013) 27(8):798–803.
3. Liu J, Hu X, Jia Y, Lu J, Lee JH, Kim K, et al. Clinical features and survival outcomes in IgD myeloma: A study by Asia myeloma network (AMN). Leukemia (2021) 35(6):1797–802. doi: 10.1038/s41375-020-01060-w
4. Modi J, Kamal J, Eter A, El-Sayegh S, El-Charabaty E. Immunoglobulin d multiple myeloma with rapidly progressing renal failure. J Clin Med Res (2015) 7(8):653–5. doi: 10.14740/jocmr2210w
5. Hutchison CA, Baturman V, Behrens J, Bridoux F, Sirac C, Dispenzieri A, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol (2011) 8(1):43–51. doi: 10.1038/nrneph.2011.168
6. El Maataoui A, Fares S, Taoufik A. Immunoglobulin d-lambda multiple myeloma and a review of the licterature. Clin Med Rev Case Rep (2021) 8:341. doi: 10.23937/2378-3656/1410341
7. Selene II, Jose JA, Khalil MJ, Faisal MS, Malik MN. Presentation petterns, diagnostic markers, management strategies, and outcomes of IgD multiple mieloma: A systemic review of licterature. Cureus (2019) 11(2):e4011. doi: 10.7759/cureus.4011
8. Willrich MAV, Murray DL, Kylr R. Laboratory testing for monoclonal gammopathies: Focus on monoclonal gammopathy of undeterminated significance and smoldering multiple myeloma. Clin Biochem (2018) 51:38–47. doi: 10.1016/j.clinbiochem.2017.05.001
9. Katzmann JA, Hyle RA, Benson J, Larson DR, Snyder MR, Lust JA, et al. Screening panels for detection of monoclonal gammopathies. Clin Chem (2009) 55(8):1517–22. doi: 10.1373/clinchem.2009.126664
10. Cejka J, Kithier K. IgD myeloma protein with “Unreactive” light determinants. Clin Chem (1979) 25/8:1495–8. doi: 10.1093/clinchem/25.8.1495
11. Rowe DS, Fahey JL. A new class of human immnoglobulin. J Exp Med (1965) 121:185–99. doi: 10.1084/jem.121.1.185
12. Blade’ J, Lust JA, Kyle RA. Immunoglobulin d multiple myeloma: Presenting feature, response to therapy, and survival in a series of 53 cases. J Clin Oncol (1994) 12(11):2398–404. doi: 10.1200/JCO.1994.12.11.2398
Keywords: Multiple Myeloma, IgD lambda/lambda, monoclonal spike, serum electrophoresis, acute renal failure
Citation: Troiano T, Brescia V, De Marinis L, Marinaccio A, Lovero R, Rizzi R and Di Serio F (2022) Case Report: A case of IgD lambda/lambda Multiple Myeloma in patient with acute renal failure and without monoclonal spike in serum electrophoresis. Front. Hematol. 1:974392. doi: 10.3389/frhem.2022.974392
Received: 21 June 2022; Accepted: 29 July 2022;
Published: 16 September 2022.
Edited by:
Francesc Bosch, Vall d’Hebron University Hospital, SpainReviewed by:
John P. Campbell, University of Bath, United KingdomFrancesca Fazio, Department of Translational and Precision Medicine Hematology Sapienza University of Rome, Italy
Copyright © 2022 Troiano, Brescia, De Marinis, Marinaccio, Lovero, Rizzi and Di Serio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Lovero, cm9iZXJ0b2xvdmVybzY5QGdtYWlsLmNvbQ==
 Teresa Troiano1
Teresa Troiano1 Vincenzo Brescia
Vincenzo Brescia Anna Marinaccio
Anna Marinaccio Roberto Lovero
Roberto Lovero