Abstract
Background:
Organizational readiness for change, defined as the collective preparedness of organization members to enact changes, remains understudied in implementing sepsis survivor transition-in-care protocols. Effective implementation relies on collaboration between hospital and post-acute care informants, including those who are leaders and staff. Therefore, our cross-sectional study compared organizational readiness for change among hospital and post-acute care informants.
Methods:
We invited informants from 16 hospitals and five affiliated HHC agencies involved in implementing a sepsis survivor transition-in-care protocol to complete a pre-implementation survey, where organizational readiness for change was measured via the Organizational Readiness to Implement Change (ORIC) scale (range 12–60). We also collected their demographic and job area information. Mann-Whitney U-tests and linear regressions, adjusting for leadership status, were used to compare organizational readiness of change between hospital and post-acute care informants.
Results:
Eighty-four informants, 51 from hospitals and 33 from post-acute care, completed the survey. Hospital and post-acute care informants had a median ORIC score of 52 and 57 respectively. Post-acute care informants had a mean 4.39-unit higher ORIC score compared to hospital informants (p = 0.03).
Conclusions:
Post-acute care informants had higher organizational readiness of change than hospital informants, potentially attributed to differences in health policies, expertise, organizational structure, and priorities. These findings and potential inferences may inform sepsis survivor transition-in-care protocol implementation. Future research should confirm, expand, and examine underlying factors related to these findings with a larger and more diverse sample. Additional studies may assess the predictive validity of ORIC towards implementation success.
1 Background
Older adults are vulnerable to poor health outcomes while transitioning from hospital to post-acute care, including home health care (HHC), outpatient appointments, rehabilitation care, and skilled or long-term care (1–4). This may be due to incomplete information transfer, medication reconciliation errors, and poor communication between and within transferring and receiving healthcare institutions (1–4). Transition-in-care protocols, defined as evidence-based guidelines and interventions intended to facilitate smooth care continuity as patients move between different healthcare institutions or stages of care (1–4), are critical to achieving positive patient outcomes, including fewer rehospitalizations, lower mortality, and better quality of life (4–8). Sepsis survivors, an at-risk population for high long-term morbidity and mortality, may especially benefit from transition-in-care protocols as they are nearly twice as likely to be rehospitalized within 30 days compared to the general patient population (18%–26% vs. 13.9%) within the United States (U.S.) (9, 10). Approximately 40% of sepsis survivor rehospitalizations in the U.S. are due to ambulatory care sensitive conditions (11). This suggests that they could have been prevented with early and intensive post-discharge follow-up, which may be facilitated by transition-in-care protocols (11).
Examples of transition-in-care protocols include the Transitional Care Model (1, 12–16), Care Transitions Intervention (1, 17, 18), and Better Outcomes for Older Adults Through Safe Transitions (1, 19). These protocols have been used for patients with advanced age, heart failure, depression, cognitive impairment, and multimorbidity (13–16, 18, 19). Key components of these protocols include discharge planning, nurse or physician post-discharge follow-up, and patient education (1, 4, 12–19). One transition-in-care protocol specific to sepsis survivors transitioning from hospital to home is I-TRANSFER (Improving TRansitions ANd outcomeS oF sEpsis suRvivors) and consists of an initial HHC nursing visit within two days, a second HHC nursing visit within seven days, and an outpatient appointment within seven days after hospital discharge (20). A previous comparative effectiveness national study using Medicare claims data from 170,571 sepsis survivors discharged from hospitals to HHC informed this protocol (21). This study found that sepsis survivors who received the I-TRANSFER protocol had a 41% relative reduction in 30-day rehospitalizations compared to those who did not (21). However, only 28% of sepsis survivors in the study received this type of care (21).
Transition-in-care protocols are often implemented by informants, or staff and leaders, within transferring (hospital) and receiving (post-acute care) healthcare institutions (6). Staff are those who execute tasks, follow protocols, or provide direct patient care. They mainly consist of clinicians and administrators. Clinicians include physicians, nurses, advance practice providers, physical and occupational therapists, social workers, and case managers. Examples of administrators include quality improvement professionals and information technology specialists. Leaders are those who are designated, based on their role, to instill motivation, build morale, create shared visions, and manage changes throughout the healthcare institution (22). They may include chief officers, directors, and upper-level management.
Successful protocol implementation is influenced by various individual, organizational, and external factors (4, 23). Individual factors include the role, motivation, expertise, and skills of the staff and leaders involved in the implementation (4, 23). Organizational factors may include staffing ratios, resource allocation, organizational priorities, and established internal workflows (4, 23). These workflows are systematic internal steps and processes followed by the transferring and receiving healthcare institutions when discharging or accepting patients. External factors may include health policies and quality initiatives from professional health organizations or government agencies (4, 23). An example of a government agency is the U.S. Centers for Medicare and Medicaid Services (CMS), which provides oversight on healthcare quality and safety provided by U.S. healthcare institutions (24).
Another important but unexplored factor within the context of implementing transition-in-care protocols is the organizational readiness for change among informants across the transferring (hospital) and receiving (post-acute care) healthcare institutions. Organizational readiness for change, defined by Weiner's theory on readiness for change, is the collective capacity of organizational members to psychologically and behaviorally embrace and implement organizational changes (25). High organizational readiness for change suggests greater collective capability and efficacy among organizational members towards implementing and adapting to changes, leading to more persistence and cooperation in adopting new initiatives (26). Meanwhile, low readiness for change contributes to resisting and viewing change as undesirable, leading to unsuccessful implementation efforts (26). To our knowledge, previous studies on organizational readiness for change focused on a single healthcare institution type, such as hospitals (23, 27, 28), but not across different types.
Effective implementation of transition-in-care protocols requires interdisciplinary collaborations between informants from hospital and post-acute care, both of which may have different organizational structures, care expertise, priorities, and relevant health policies. These factors may contribute to differences in organizational readiness for change. Understanding these differences may inform tailored strategies and health policies for implementing transition-in-care protocols, leading to better interdisciplinary collaborations and better patient outcomes.
Given the understudied nature of organizational readiness for change in transition-in-care protocol implementation and the potential benefits these protocols offer sepsis survivors, our study (1) described and compared informant characteristics based on leadership status and healthcare institution, and (2) determined whether healthcare institution is a predictor of organizational readiness for change. In this study we focused on the post-acute care institutions of outpatient clinics and HHC agencies.
2 Materials and methods
2.1 Overview and participants
This cross-sectional study was part of a larger type 1 hybrid effectiveness implementation science study that aims to measure the effectiveness and implementation of the I-TRANSFER protocol (20). Five healthcare systems (consisting of hospitals and outpatient clinics) and their five affiliated HHC agencies were purposively selected for implementing this protocol (20). They are diverse in size and geographic region (18). Three HHC agencies were owned by their affiliated healthcare system while the other two were free-standing (20). Some healthcare systems consisted of multiple hospitals. Specifically, one healthcare system had one hospital, another had two, another had three, another had four, and another had six, making a total of 16 hospitals involved in implementing the I-TRANSFER protocol. The larger study was approved by the Institutional Review Board of the University of Pennsylvania and VNS Health. Other healthcare systems and HHC agencies reviewed the protocol and granted permission after determining there was “no research engagement” of their patients or by their employees.
Informants eligible for our smaller cross-sectional study include staff and leaders from the five healthcare systems and HHC agencies. These informants were directly involved in implementing the I-TRANSFER protocol. They were recruited from our larger implementation science study. Findings from our smaller cross-sectional study are reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supplementary File 1) (29).
2.2 Data collection
Those eligible were invited and completed a pre-implementation organizational readiness for change survey via REDCap® (30, 31). This survey included questions on demographics (specifically gender, race, and ethnicity), job area (via 27 options), and healthcare institution (healthcare system or HHC). Example of job areas include outpatient staff, acute care director, and HHC case manager. They were allowed to select multiple job area options or type one in if no options applied. The survey was available from March 22, 2021 to October 15, 2021.
Organizational readiness for change was measured using the Organizational Readiness for Implementing Change (ORIC) scale (Supplementary File 2), which was developed by Shea et al. (26) and based on Weiner's theory on readiness for change. Although originally in English (26), the scale has been translated and psychometrically validated in multiple languages, such as German, Danish, French, and Brazilian Portuguese (32–35). ORIC contains 12 items, each rated on a five-point scale ranging from one (disagree) to five (agree) and are summed to generate an ORIC score ranging from 12 to 60 (26). This scale also includes two subscales: change commitment (five items) and change efficacy (seven items) (26). Change commitment refers to organizational members’ capability to implement change, while change efficacy represents their confidence and motivation to do so (26, 36). Subscale scores are calculated similarly to the ORIC score, with change commitment scores ranging from five to 25 and change efficacy scores from seven to 35 (26). Higher ORIC and subscale scores indicate higher organizational readiness for change, change commitment, and change efficacy (26).
To prepare for data analysis, job area was delineated by leadership status and was categorized into leader or staff. Healthcare institution was also operationalized as a dichotomous variable, indicating whether participants worked in either hospitals or post-acute care. Informants employed in healthcare systems and had job areas focused on coordinating outpatient appointments were categorized under post-acute care.
2.3 Data analysis
Cronbach's alpha was measured to assess the internal consistency of ORIC, change commitment, and change efficacy among informants. To describe the informant sample, frequencies and percentages were calculated for categorical measures (i.e., demographics, healthcare institution, and leadership status). Mean, standard deviation (SD), median, and interquartile range (IQR) were calculated for ORIC scores.
Box plots were used to illustrate the distribution of ORIC, change commitment, and change efficacy scores among informants in hospitals and post-acute care. Frequency bar graphs were also made to show informant responses to the 12 individual ORIC items. Responses were then separated based on healthcare institution (hospital vs. post-acute care). Stratified summary tables were produced to compare informants’ characteristics between healthcare institution as well as between leadership status.
Pearson's Chi-Square and Fisher's exact tests were used to test associations between categorical measures. The non-parametric Mann-Whitney U-test was used to test bivariate associations between ORIC score and the dichotomous measures of healthcare institution and leadership status. Finally, linear regression was used to assess the extent to which healthcare institution is predictive of ORIC score. We also adjusted for leadership status for there were significantly more informants who were leaders in post-acute care than in hospitals.
An alpha level of 0.05 was used to determine statistical significance. Data analysis was done using R version 4.2.2 (37). The package “ggplot2” was used create the box plot and bar graphs (38).
3 Results
Out of the 122 eligible informants who received the survey, 84 (68.85%) completed it and were included in the analysis. This included 51 (60.71%) from hospitals and 33 (39.29%) from post-acute care. Most informants were female (59/84, 70.24%), white (69/84, 82.14%), and non-Hispanic/non-Latino (78/84, 92.86%). Among those from post-acute care, four (12.12%) were from outpatient clinics and 29 (87.88%) were from HHC agencies. The sample was almost evenly split between informants in leadership (44/84, 52.38%) and staff roles (40/84, 47.62%). The mean score was 52.44 (SD 8.05) for ORIC, 22.15 (SD 3.45) for change commitment, and 30.28 (SD 4.93) for change efficacy. Supplementary File 3 describes the characteristics of the 84 informants.
Although hospitals and post-acute care both had 22 informants who were leaders, the proportion of leaders was higher in post-acute care (22/33, 66.67%) compared to hospitals (22/51, 35.48%) (p = 0.04). Neither healthcare institution nor leadership status were significantly associated with gender, race, or ethnicity (p > 0.05). However, leadership status was significantly associated with ORIC, change efficacy, and change commitment scores (p < 0.04). Table 1 stratifies these characteristics by healthcare institution and leadership status. Supplementary File 4 contains the frequency bar graphs showing informant responses to the 12 individual ORIC items, and Supplementary File 5 separated these responses by healthcare institution (hospitals and post-acute care).
Table 1
| Characteristics | Hospital | Post-acute care | P-value | Leader | Staff | P-value |
|---|---|---|---|---|---|---|
| (n = 51) n (%) | (n = 33) n (%) | (n = 44) n (%) | (n = 40) n (%) | |||
| Gender | 0.7 | 0.3 | ||||
| Male | 16 (31.37%) | 9 (27.27%) | 11 (25.00%) | 14 (35.00%) | ||
| Female | 35 (68.63%) | 24 (72.73%) | 33 (75.00%) | 26 (65.00%) | ||
| Race | 0.8 | 0.2 | ||||
| White | 42 (89.36%) | 27 (84.38%) | 39 (92.86%) | 30 (78.95%) | ||
| Asian | 4 (8.51%) | 5 (15.63%) | 3 (7.14%) | 6 (15.79%) | ||
| Black | 1 (2.13%) | 0 (0.00%) | 0 (0.00%) | 1 (2.63%) | ||
| Pacific Islander | 1 (2.13%) | 0 (0.00%) | 0 (0.00%) | 1 (2.63%) | ||
| Ethnicity | 0.5 | >0.9 | ||||
| Hispanic/Latino | 2 (4.17%) | 0 (0.00%) | 1 (2.50%) | 1 (2.50%) | ||
| Non-Hispanic/Non-Latino | 46 (95.83%) | 32 (100%) | 39 (97.50%) | 39 (97.50%) | ||
| Leadership Status | 0.04* | N/A | N/A | N/A | ||
| Leader | 22 (43.14%) | 22 (66.67%) | ||||
| Staff | 29 (56.86%) | 11 (35.48%) | ||||
| Healthcare Institution | N/A | N/A | N/A | 0.04* | ||
| Hospital | 22 (50.00%) | 29 (72.50%) | ||||
| Post-Acute Care | 22 (50.00%) | 11 (27.50%) |
| Organizational Readiness for Change | Median (IQR) | Median (IQR) | P-value | Median (IQR) | Median (IQR) | P-value |
|---|---|---|---|---|---|---|
| ORIC | 52.50 | 57.00 | 0.03* | 56.00 | 54.00 | 0.4 |
| (45.50, 59.00) | (53.50, 60.00) | (51.25, 59.75) | (46.25, 59.75) | |||
| Change Commitment | 23.00 | 24.00 | 0.02* | 24.00 | 23.00 | 0.4 |
| (18.75, 25.00) | (22.75, 25.00) | (20.50, 25.00) | (20.00, 25.00) | |||
| Change Efficacy | 31.00 | 33.00 | 0.04* | 32.41 | 30.50 | 0.4 |
| (24.75, 35.00) | (29.75, 35.00) | (29.25, 35.00) | (26.25, 35.00) |
Characteristics based on healthcare institution and leadership Status of informants.
Indicates statistical significance.
The median ORIC score was 52.50 (IQR 45.50, 59.00) among hospital informants compared to 57.00 (IQR 53.50, 60.00) among post-acute informants (p = 0.03) (26). For change commitment, the median score was 23.00 (IQR 18.75, 25.00) among hospital informants and 24.00 (IQR 22.75, 25.00) among post-acute informants (p = 0.02). Meanwhile, the median change efficacy score was 31.00 (IQR 24.75, 35.00) among hospital informants and 33.00 (IQR 29.75, 35.00) among post-acute informants (p = 0.04). There were no significant score differences based on leadership status. Figure 1 contains the box plots showing the ORIC, change commitment, and change efficacy score distributions, separated by healthcare institution. Cronbach's alpha was 0.96 for ORIC, 0.93 for change commitment, and 0.92 for change self-efficacy.
Figure 1
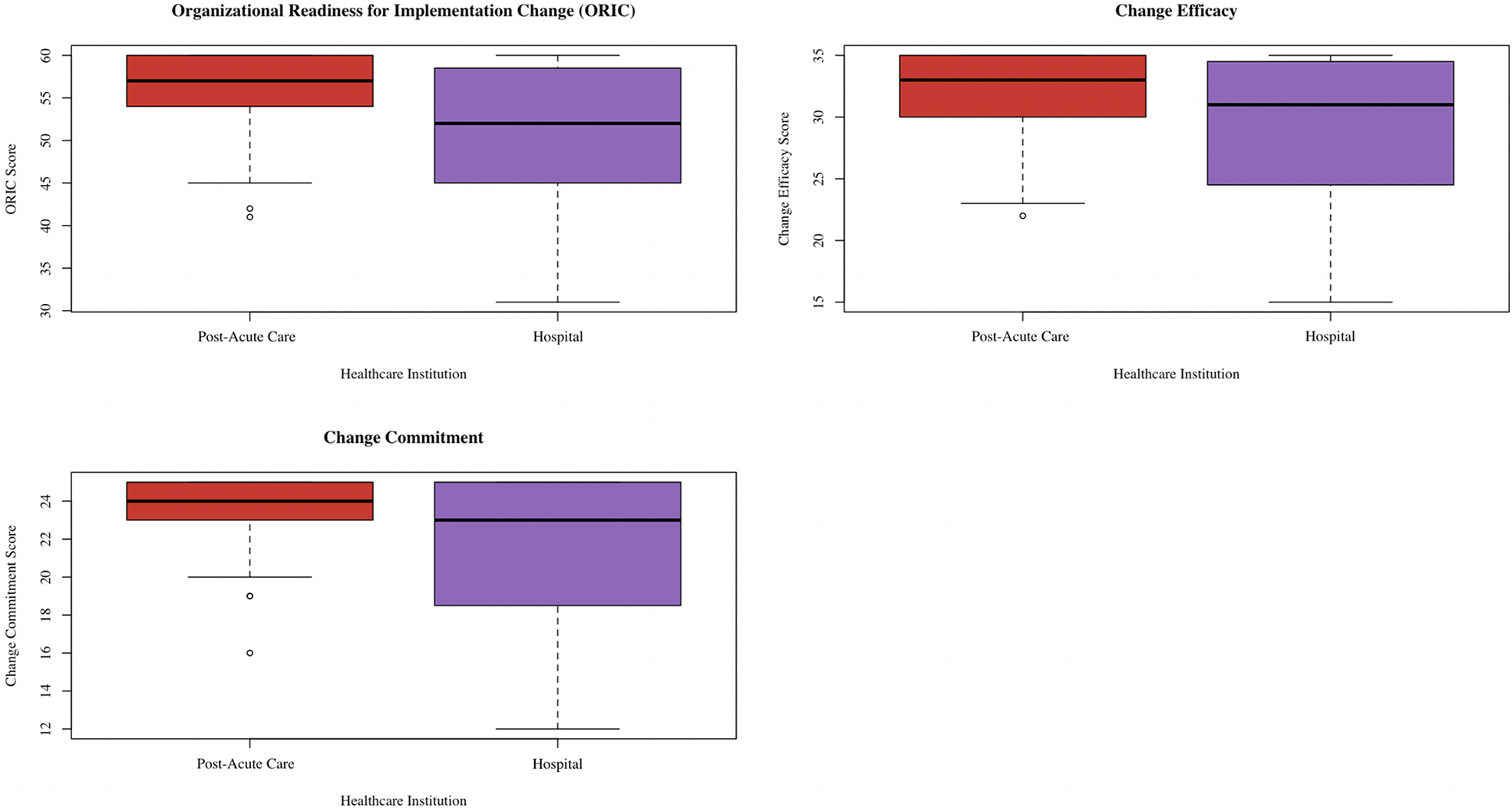
Box plot showing distribution of ORIC, change commitment, and change efficacy scores separated by healthcare institution. This box plot was created via the ggplot2 package (38) in R (37).
A simple linear regression model showed that post-acute informants had higher estimated mean ORIC (4.57 ± 1.74 units, p = 0.01), change commitment (1.94 ± 0.75 units, p = 0.01), and change efficacy (2.63 ± 1.07, p = 0.02) scores compared to hospital informants (Supplementary File 6). After adjusting for leadership status, post-acute care remained a significant predictor of ORIC (B = 4.39 ± 1.80 units, p = 0.02), change commitment (B = 1.93 ± 0.78 units, p = 0.01), and change efficacy (B = 2.46 ± 1.10 units, p = .03) (Table 2). Leadership status was not significantly associated with ORIC, change commitment, or change efficacy.
Table 2
| Variables | Regression coefficient | Standard error | 95% confidence interval | t value | P-value |
|---|---|---|---|---|---|
| ORIC | |||||
| Intercept | 51.07 | 1.48 | 48.12, 54.02 | 34.45 | <0.01* |
| Healthcare institution | |||||
| Hospitals | Reference | ||||
| Post-acute care | 4.39 | 1.80 | 0.81, 7.96 | 2.44 | 0.02* |
| Leadership status | |||||
| Leader | Reference | ||||
| Staff | −0.76 | 1.76 | −4.25, 2.74 | −0.43 | 0.67 |
| Change commitment | |||||
| Intercept | 21.42 | 0.64 | 20.16, 22.69 | 33.67 | <0.01* |
| Healthcare institution | |||||
| Hospitals | Reference | ||||
| Post-acute care | 1.93 | 0.78 | 0.39, 3.46 | 2.50 | 0.01* |
| Leadership status | |||||
| Leader | Reference | ||||
| Staff | −0.05 | 0.75 | −1.55, 1.45 | −0.07 | 0.95 |
| Change efficacy | |||||
| Intercept | 29.65 | 0.91 | 27.84, 31.46 | 32.59 | <0.01* |
| Healthcare institution | |||||
| Hospitals | Reference | ||||
| Post-acute care | 2.46 | 1.10 | 0.27, 4.66 | 2.23 | 0.03* |
| Leadership status | |||||
| Leader | Reference | ||||
| Staff | −0.70 | 1.08 | −2.85, 1.44 | −0.65 | 0.52 |
Multiple linear regression of ORIC after adjusting for leadership Status.
Indicates statistical significance.
4 Discussion
Informants from our study exhibited higher organizational readiness for change compared to those from other pre-implementation and implementation science studies. These studies focused on a midwifery model of care (average ORIC score = 41.5) (27), an electronic lung cancer patient outcome reporting system (average ORIC score = 47.24) (28), and a doula–hospital partnership program (average ORIC score = 49.96) (39), all within hospital settings. To our knowledge, our study is the first to examine organizational readiness for change towards implementing a sepsis survivor transition-in-care protocol from hospital to home.
The higher organizational readiness for change observed within our study may be due to (1) the already established internal workflows within the five healthcare systems and HHC agencies involved in implementing the I-TRANSFER protocol and (2) the quality and financial priorities set by Centers for Medicare and Medicaid Services (CMS) to prevent rehospitalizations (24, 40–42). Established internal workflows facilitate patient referral from hospital to post-acute care and transition-in-care protocol integration, reflecting the compatibility construct within the Consolidated Framework for Implementation Research (CFIR) (43, 44). This construct is defined as the alignment between existing workflows and the innovation to be implemented (43, 44). CMS launched initiatives, such as the Home Health Quality Reporting Program (HHQRP) and the Hospital Readiness Readmissions Reduction Program (HRRP), financially incentivizes quality care within hospitals and HHC (24, 40). This corresponds with the CFIR external pressure construct, which includes external initiatives and policies influencing implementation (43, 44). Compatibility of a transition-in-care protocol with existing workflows and external pressures may potentially increase collective capability and motivation towards implementation, leading to higher organizational readiness of change.
We compared organizational readiness of change between informants from hospitals and post-acute care (HHC and outpatient clinics) to inform sepsis survivor transition-in-care protocol implementation. Those from post-acute care had a 4.39-unit higher mean organizational readiness for change score compared to those from hospitals. Although studies have yet to compare hospital and post-acute care, potential explanations for our findings may be related to external health policies, quality measures, priorities, organizational structures, and expertise.
Health policies and quality measures set by the CMS HHQRP may influence organizational readiness for change among HHC agencies (24, 40). According to HHQRP health policies, HHC agencies must initiate a start-of-care visit within two days post-discharge. In addition, the HHQRP financially incentivizes HHC agencies to reduce 30-day rehospitalization rates (24, 40). As sepsis survivors are at high risk for 30-day rehospitalizations (9, 10), HHC informants have a strong incentive to implement sepsis survivor transition-in-care protocols aligning with these requirements, such as the I-TRANSFER protocol (20). This increases informant resolve, contributing to higher organizational readiness for change (26). Furthermore, a systematic review showed that the HHQRP contributes to HHC agency engagement in quality improvement initiatives focused on staffing, quality monitoring, and care coordination redesign, suggesting that health policies and quality measures enable HHC informants towards implementing transition-in-care protocols (40). Thus, maintaining these health policies and quality measures may be essential towards increasing organizational readiness for change.
Hospital informants may prioritize reducing 30-day rehospitalizations, but other health conditions may have a higher priority over sepsis. According to the CMS Hospital Readiness Readmissions Reduction Program (HRRP), hospitals having excess 30-day rehospitalizations for heart failure, myocardial infarction, chronic obstructive pulmonary disease, coronary artery bypass graft surgery, total knee or hip arthroplasty, and pneumonia will face reduced payments from CMS (42). As such, hospital informants may be less inclined to prioritize implementing sepsis survivor transition-in-care protocols as sepsis is not part of HRRP, resulting in lower organizational readiness for change. Given the poor outcomes among sepsis survivors (11, 45), we recommend sepsis be added to HRRP to incentivize hospitals towards implementing transition-in-care protocols tailored to sepsis survivors.
Organizational structure differences may also explain differences in organizational readiness. Although studies have yet to confirm, hospitals may have more complex organizational structures with multiple layers of committees than HHC agencies and outpatient clinics. Receiving approval from these hierarchical committees is required prior to implementing a transition-in-care protocol but may delay the implementation timeline. This delay due to bureaucratic processes may reduce implementation buy-in, motivation, and organizational readiness for change. However, the less hierarchical structure within HHC and outpatient clinics may lead to a more streamlined approach towards implementing protocols. One strategy is to organize a hospital-wide committee dedicated to improving sepsis outcomes, leading to centralized efforts towards expediting transition-in-care protocol implementation and mitigating bureaucratic barriers.
HHC agency and outpatient informants may have more expertise than hospital informants in implementing transition-in-care protocols, potentially due to differences in practice scope. Hospitals provide care to patients with diverse, acute, and complex health conditions requiring specialized services and inpatient monitoring, while HHC agencies and outpatient clinics provide mainly post-acute care, rehabilitation, and primary care to patients who transitioned from hospital care. Thus, HHC agency and outpatient informants may have expertise in monitoring and managing health conditions to prevent rehospitalizations. This expertise makes them more positioned than hospital informants towards caring out transition-in-care protocols, increasing their readiness for implementing change. Another strategy is to create interdisciplinary teams consisting of post-acute care and hospital informants to plan and execute transition-in-care protocols.
Our findings and inferences should be interpreted with caution. The study limited post-acute care to HHC and outpatient appointments. Additional studies may include additional post-acute care institutions, such as skilled nursing and rehabilitation care. While our findings are statistically significant, research is needed to establish their clinical significance. We described potential explanations for our findings, but more studies are needed to substantiate them.
Distributing surveys for data collection may have introduced selection bias, and those who completed the survey may have higher organizational readiness of change than those who did not. Due to the size of the healthcare institutions, we recruited more hospital than post-acute informants, and only four within post-acute were from outpatient clinics and the rest were from HHC. Thus, our findings are skewed towards HHC and hospitals as we did not have enough data to elucidate organizational readiness for change among outpatient informants.
Our informant sample had a larger proportion of leaders from post-acute care than from hospitals, which may have influenced responses to ORIC. Additional factors, such as clinician fatigue, time constraints, and work environments may have also influenced organizational readiness for change but were not captured within our study. Most of our informants identified as White and Non-Hispanic/Latino, limiting our findings’ generalizability to only those with similar sociodemographic characteristics or from similar HHC agencies, hospitals, and outpatient clinics.
Moving forward, future research may focus on the ORIC measure and assessing organizational readiness for change longitudinally throughout implementation. Our study's ORIC Cronbach's alpha values indicated high internal reliability, like those reported by other studies (23, 27, 46). This suggests consistent internal scale consistency across different populations. However, ORIC cut-off points for poor, fair, good, and excellent organizational readiness for change have yet to be established, and additional studies may determine the predictive validity of ORIC towards implementation success (27, 47). As implementation proceeds, informants may encounter barriers and facilitators, such as those related to time constraints, staff training, and care coordination (4). As such, researchers may consider measuring organizational readiness for change throughout implementation.
To conclude, our study lays a foundation for understanding organizational readiness for change in implementing transition-in-care protocols for sepsis survivors, but its limitations require a cautious interpretation. Present results should be considered exploratory and additional research is needed to confirm these findings, measure clinical significance, and elucidate underlying factors among larger and more diverse samples.
5 Conclusion
Our cross-sectional study found that informants from post-acute care had higher organizational readiness for change compared to those from hospitals for implementing sepsis survivor transition-in-care protocols. Findings may inform tailored strategies and health policies for implementing transition-in-care protocols for sepsis survivors, an at-risk population for high long-term morbidity and mortality. Strategies include creating a hospital-wide committee to improve sepsis survivor outcomes and launching interdisciplinary teams, consisting of post-acute care and hospital informants, to coordinate care for sepsis survivors transitioning from hospital to home. The CMS HHQRP should continue incentivizing HHC agencies to maintain timely HHC start-of-care visits and reduce 30-day rehospitalizations. Health policy makes may consider adding sepsis to the CMS HRRP to encourage hospitals to prioritize improving sepsis survivor outcomes.
Although our study is exploratory and its findings require a cautious interpretation, they inform effect sizes and lay the foundation for future studies on organizational readiness for change towards implementing sepsis survivor transition-in-care protocols. Future work should confirm and expand upon our findings by recruiting larger and more diverse samples, studying additional factors potentially associated with organizational readiness for change, and by investigating the predictive relationship between baseline organizational readiness for change and implementation success.
Statements
Data availability statement
The raw de-identified data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Ethics statement
The study involving human participants was reviewed and approved by Institutional Review Boards of the University of Pennsylvania and VNS Health and was conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review boards waived the requirement of written informed consent for participation from the participants because this study was granted exempt status. Other healthcare systems and HHC agencies within the parent I-TRANSFER study reviewed the protocol and granted permission after determining there was “no research engagement” of their patients or by their employees. Nevertheless, verbal informed consent was sought and obtained from all human participants prior to their participation in the interviews.
Author contributions
ES: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. RQ: Methodology, Writing – review & editing. MAS: Conceptualization, Writing – review & editing. JS: Methodology, Writing – review & editing. KBH: Conceptualization, Writing – review & editing. SY: Writing – review & editing. KSP: Writing – review & editing. NH: Conceptualization, Writing – review & editing. PG: Data curation, Writing – review & editing. MOC: Writing – review & editing. SO: Writing – review & editing. KHB: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is supported by the National Institute of Nursing Research (NINR) grant number 5R01NR016014. ES, JS, KSP, and SO were supported by NINR T32NR009356. JS is also supported by National Heart, Lung, and Blood Institute (NHLBI) K99HL169940. MOC was supported by a Betty Irene Moore Fellowship for Nurse Leaders and Innovators. The content is solely the responsibility of the authors and does not represent the perspectives of the NINR, NHLBI, and the Betty Irene Moore Fellowship for Nurse Leaders and Innovators.
Acknowledgments
Momin M. Malik from the University of Pennsylvania School of Social Policy & Practice offered constructive guidance on the statistical methodology associated with this manuscript. The manuscript was solely written by the authors. ChatGPT version 4.0 was used for proofreading and to improve readability. Afterwords, the authors reviewed and reworded the manuscript as needed and take full responsibility for its content.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhs.2024.1436375/full#supplementary-material
References
1.
Enderlin CA McLeskey N Rooker JL Steinhauser C D’Avolio D Gusewelle R et al Review of current conceptual models and frameworks to guide transitions of care in older adults. Geriatr Nurs (Minneap). (2013) 34(1):47–52. 10.1016/j.gerinurse.2012.08.003
2.
Tan M Lang D . Effectiveness of nurse leader rounding and post-discharge telephone calls in patient satisfaction: a systematic review. JBI Database System Rev Implement Rep. (2015) 13(7):154–76. 10.11124/01938924-201513070-00015
3.
Naylor MD Aiken LH Kurtzman ET Olds DM Hirschman KB . The importance of transitional care in achieving health reform. Health Aff. (2011) 30(4):746–54. 10.1377/hlthaff.2011.0041
4.
Fakha A Groenvynck L de Boer B van Achterberg T Hamers J Verbeek H . A myriad of factors influencing the implementation of transitional care innovations: a scoping review. Implement Sci. (2021) 16(1):21. 10.1186/s13012-021-01087-2
5.
Mohamedsharif A Elfeaki M Bushra R Gemperli A . Effectiveness of hospital-to-home transitional care interventions and consultation for implementation in Sudan: a scoping review of systematic reviews. Front Health Serv. (2023) 3:1288575. 10.3389/frhs.2023.1288575
6.
Braet A Weltens C Sermeus W . Effectiveness of discharge interventions from hospital to home on hospital readmissions: a systematic review. JBI Database System Rev Implement Rep. (2016) 14(2):106–73. 10.11124/jbisrir-2016-2381
7.
Naylor MD Hirschman KB McCauley K Shaid EC Hanlon AL Whitehouse CR et al MIRROR-TCM: multisite replication of a randomized controlled trial - transitional care model. Contemp Clin Trials. (2022) 112:106620. 10.1016/j.cct.2021.106620
8.
Hirschman KB Shaid E Bixby MB Badolato DJ Barg R Byrnes MB et al Transitional care in the patient-centered medical home: lessons in adaptation. J Healthc Qual. (2017) 39(2):67–77. 10.1097/01.JHQ.0000462685.78253.e8
9.
Jiang H Hensche M . Characteristics of 30-Day All-Cause Hospital Readmissions, 2016-2020. HCUP Statistical Brief #304. (2023). Available online at:https://hcup-us.ahrq.gov/reports/statbriefs/sb248-Hospital-Readmissions-2010-2016.jsp(cited 2024 April 5).
10.
Goodwin AJ Ford DW . Readmissions among sepsis survivors: risk factors and prevention. Clin Pulm Med. (2018) 25(3):79–83. 10.1097/CPM.0000000000000254
11.
Prescott H Langa K Iwashyna T . Forty percent of hospitalizations after severe sepsis are potentially preventable. Crit Care. (2014) 18(S2):35. 10.1186/cc14038
12.
Naylor MD Van Cleave J . Transitional care model. In: Meleis AI, editor.Transitions Theory: Middle-Range and Situation-Specific Theories in Nursing Research and Practice. New York: Springer Publishing (2010). p. 459–65.
13.
Morkisch N Upegui-Arango LD Cardona MI van den Heuvel D Rimmele M Sieber CC et al Components of the transitional care model (TCM) to reduce readmission in geriatric patients: a systematic review. BMC Geriatr. (2020) 20(1):345. 10.1186/s12877-020-01747-w
14.
Berthelsen C Møller N Bunkenborg G . Transitional care model for older adults with multiple chronic conditions: an evaluation of benefits utilising an umbrella review. J Clin Nurs. (2024) 33(2):481–96. 10.1111/jocn.16913
15.
Naylor MD Brooten DA Campbell RL Maislin G McCauley KM Schwartz JS . Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. (2004) 52(5):675–84. 10.1111/j.1532-5415.2004.52202.x
16.
Pauly M V Hirschman KB Hanlon AL Huang L Bowles KH Bradway C et al Cost impact of the transitional care model for hospitalized cognitively impaired older adults. J Comp Eff Res. (2018) 7(9):913–22. 10.2217/cer-2018-0040
17.
Parry C Coleman EA Smith JD Frank J Kramer AM . The care transitions intervention: a patient-centered approach to ensuring effective transfers between sites of geriatric care. Home Health Care Serv Q. (2003) 22(3):1–17. 10.1300/J027v22n03_01
18.
Aronow H Fila S Martinez B Sosna T . Depression and coleman care transitions intervention. Soc Work Health Care. (2018) 57(9):750–61. 10.1080/00981389.2018.1496514
19.
Robertson DA . Evaluation of a modified BOOST tool in the acute care setting. J Nurs Care Qual. (2017) 32(1):62–70. 10.1097/NCQ.0000000000000200
20.
O’Connor M Kennedy EE Hirschman KB Mikkelsen ME Deb P Ryvicker M et al Improving transitions and outcomes of sepsis survivors (I-TRANSFER): a type 1 hybrid protocol. BMC Palliat Care. (2022) 21(1):98. 10.1186/s12904-022-00973-w
21.
Deb P Murtaugh CM Bowles KH Mikkelsen ME Khajavi HN Moore S et al Does early follow-up improve the outcomes of sepsis survivors discharged to home health care? Med Care. (2019) 57(8):633–40. 10.1097/MLR.0000000000001152
22.
Sfantou D Laliotis A Patelarou A Sifaki- Pistolla D Matalliotakis M Patelarou E . Importance of leadership style towards quality of care measures in healthcare settings: a systematic review. Healthcare. (2017) 5(4):73. 10.3390/healthcare5040073
23.
Sharma N Herrnschmidt J Claes V Bachnick S De Geest S Simon M . Organizational readiness for implementing change in acute care hospitals: an analysis of a cross-sectional, multicentre study. J Adv Nurs. (2018) 74(12):2798–808. 10.1111/jan.13801
24.
Centers for Medicare & Medicaid Services. State operations manual appendix B: guidance to surveyors home health agencies. (2020). Available online at:https://qsep.cms.gov/data/2505/SOM_APPENDIX_B_FOR_HHA.pdf(cited 2024 April 20).
25.
Weiner BJ Lewis MA Linnan LA . Using organization theory to understand the determinants of effective implementation of worksite health promotion programs. Health Educ Res. (2008) 24(2):292–305. 10.1093/her/cyn019
26.
Shea CM Jacobs SR Esserman DA Bruce K Weiner BJ . Organizational readiness for implementing change: a psychometric assessment of a new measure. Implement Sci. (2014) 9(1):7. 10.1186/1748-5908-9-7
27.
Adelson P Yates R Fleet JA McKellar L . Measuring organizational readiness for implementing change (ORIC) in a new midwifery model of care in Rural South Australia. BMC Health Serv Res. (2021) 21(1):368. 10.1186/s12913-021-06373-9
28.
Girgis A Bamgboje-Ayodele A Rincones O Vinod SK Avery S Descallar J et al Stepping into the real world: a mixed-methods evaluation of the implementation of electronic patient reported outcomes in routine lung cancer care. J Patient Rep Outcomes. (2022) 6(1):70. 10.1186/s41687-022-00475-6
29.
Vandenbroucke JP von Elm E Altman DG Gøtzsche PC Mulrow CD Pocock SJ et al Strengthening the reporting of observational studies in epidemiology (STROBE). Epidemiology. (2007) 18(6):805–35. 10.1097/EDE.0b013e3181577511
30.
Harris PA Taylor R Thielke R Payne J Gonzalez N Conde JG . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42(2):377–81. 10.1016/j.jbi.2008.08.010
31.
Harris PA Taylor R Minor BL Elliott V Fernandez M O’Neal L et al The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. 10.1016/j.jbi.2019.103208
32.
Lindig A Hahlweg P Christalle E Scholl I . Translation and psychometric evaluation of the German version of the organisational readiness for implementing change measure (ORIC): a cross-sectional study. BMJ Open. (2020) 10(6):e034380. 10.1136/bmjopen-2019-034380
33.
Storkholm MH Mazzocato P Tessma MK Savage C . Assessing the reliability and validity of the danish version of organizational readiness for implementing change (ORIC). Implement Sci. (2018) 13(1):78. 10.1186/s13012-018-0769-y
34.
Ruest M Léonard G Thomas A Desrosiers J Guay M . French cross-cultural adaptation of the organizational readiness for implementing change (ORIC). BMC Health Serv Res. (2019) 19(1):535. 10.1186/s12913-019-4361-1
35.
Bomfim RA Braff EC Frazão P . Adaptação transcultural e propriedades psicométricas da versão em português (brasil) do questionário prontidão organizacional para implementação de mudança para implementação de mudança em serviços de saúde. Revista Brasileira de Epidemiologia. (2020) 23:e200100. 10.1590/1980-549720200100
36.
Weiner BJ . A theory of organizational readiness for change. Implement Sci. (2009) 4(1):67. 10.1186/1748-5908-4-67
37.
R Core Team. A Language and Environment for Statistical Computing (Version 4.2.2). Vienna, Austria: R Foundation for Statistical Computing (2022).
38.
Wickham H . Ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag (2016). Available online at:https://ggplot2.tidyverse.org
39.
DaCosta MC Mogaka J Gebhardt L Goff SL Qasba N Attanasio L . Readiness to implement a doula–hospital partnership program. J Obstet Gynecol Neonatal Nurs. (2024) 53(2):197–206. 10.1016/j.jogn.2023.12.001
40.
Shetty KD Robbins MW Saliba D Campbell KN Castora-Binkley M Damberg CL . Home health agency adoption of quality improvement activities and association with performance. J Am Geriatr Soc. (2021) 69(11):3273–84. 10.1111/jgs.17368
41.
Pozniak A Lammers E Mukhopadhyay P Cogan C Ding Z Goyat R et al Association of the home health value-based purchasing model with quality, utilization, and medicare payments after the first 5 years. JAMA Health Forum. (2022) 3(9):e222723. 10.1001/jamahealthforum.2022.2723
42.
Zuckerman RB Joynt Maddox KE Sheingold SH Chen LM Epstein AM . Effect of a hospital-wide measure on the readmissions reduction program. N Engl J Med. (2017) 377(16):1551–8. 10.1056/NEJMsa1701791
43.
Damschroder LJ Reardon CM Opra Widerquist MA Lowery J . Conceptualizing outcomes for use with the consolidated framework for implementation research (CFIR): the CFIR outcomes addendum. Implement Sci. (2022) 17(1):7. 10.1186/s13012-021-01181-5
44.
Damschroder LJ Reardon CM Widerquist MAO Lowery J . The updated consolidated framework for implementation research based on user feedback. Implement Sci. (2022) 17(1):75. 10.1186/s13012-022-01245-0
45.
Prescott HC Costa DK . Improving long-term outcomes after sepsis. Crit Care Clin. (2018) 34(1):175–88. 10.1016/j.ccc.2017.08.013
46.
Sanders KA Wolcott MD McLaughlin JE D’Ostroph A Shea CM Pinelli NR . Organizational readiness for change: preceptor perceptions regarding early immersion of student pharmacists in health-system practice. Res Social Adm Pharm. (2017) 13(5):1028–35. 10.1016/j.sapharm.2017.03.004
47.
Weiner BJ Clary AS Klaman SL Turner K Alishahi-Tabriz A . Organizational readiness for change: what we know, what we think we know, and what we need to know. In: AlbersBShlonskyAMildonR, editors. Implementation Science 30. Cham, Switzerland: Springer (2020). p. 101–44.
Summary
Keywords
sepsis survivors, transitions in care, organizational readiness for change, implementation science, healthcare system, home health care (HHC), transition-in-care protocols, hospital to home
Citation
Sang E, Quinn R, Stawnychy MA, Song J, Hirschman KB, You SB, Pitcher KS, Hodgson NA, Garren P, O'Connor M, Oh S and Bowles KH (2024) Organizational readiness for change towards implementing a sepsis survivor hospital to home transition-in-care protocol. Front. Health Serv. 4:1436375. doi: 10.3389/frhs.2024.1436375
Received
22 May 2024
Accepted
14 August 2024
Published
06 September 2024
Volume
4 - 2024
Edited by
Lauren Clack, University of Zurich, Switzerland
Reviewed by
Janet C. Long, Macquarie University, Australia
Aline Wolfensberger, University Hospital Zürich, Switzerland
Updates
Copyright
© 2024 Sang, Quinn, Stawnychy, Song, Hirschman, You, Pitcher, Hodgson, Garren, O'Connor, Oh and Bowles.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Elaine Sang esang@upenn.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.