
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Genet. , 17 February 2025
Sec. Genetics of Common and Rare Diseases
Volume 16 - 2025 | https://doi.org/10.3389/fgene.2025.1536000
Background: Congenital eyelid coloboma (CEC) is a rare genetic disease, manifesting as a congenital partial or total defect of the eyelid. In this study, we report a pedigree with CEC caused by a novel pathogenic variant in JMJD6.
Case report: The proband was a 3-year-old girl who presented with a congenital coloboma of the left upper eyelid, accompanied by hypoplasia of the ipsilateral eyebrow. Karyotype analysis was normal. Whole-exome sequencing (WES) identified a novel pathogenic variant in JMJD6 (c.941+75G > T), which was classified as a likely pathogenic (LP) and de novo variant. To date, this variant has not been reported.
Conclusion: Our study found a novel pathogenic variant in JMJD6 (c.941+75G > T), which broadens the CEC phenotype spectrum and JMJD6 gene variant spectrum, providing a basis for clinical diagnosis, genetic counseling, and treatment.
Congenital eyelid coloboma (CEC) is a rare congenital malformation first reported by the French physician Jacques Guillemeau in 1585. The term “coloboma” is derived from the Greek word Κολόβωμα, meaning a defect or hole in the tissue (Donaldson, 2012). CEC manifests as a congenital partial or total defect of the eyelid, leading to corneal exposure and potentially associated with eyebrow defects. Symptoms may include ocular irritation, impaired visual acuity, and even affect psychological wellbeing (Smith et al., 2015; Ng et al., 2024). It occurs more frequently in the upper eyelid than in the lower eyelid and can be either unilateral or bilateral (Smith et al., 2015). CEC is common in certain craniofacial syndromes, such as Goldenhar syndrome (Rooijers et al., 2020; Singh et al., 2020), Treacher Collins syndrome (Ng et al., 2024), and CHARGE syndrome (Lodhi et al., 2010). The etiology of CEC is unclear, involving both genetic and environmental factors, such as exposure to certain harmful substances early in pregnancy. Previous studies have suggested that congenital eyelid defects may be associated with variants in genes such as ABCB6 (Wang et al., 2012), FZD5 (Liu et al., 2016), PAX6 (Azuma et al., 2003), and SALL2 (Kelberman et al., 2014).
In this study, we report the case of a patient with congenital eyelid coloboma caused by a JMJD6 pathogenic variant in one individual from a monozygotic twin pregnancy. Our findings expand the variant spectrum of JMJD6 and provide novel insights into the molecular and clinical analysis of complex inherited eye disease phenotypes.
The proband (Figure 1, II-1) was a 3-year-old girl who presented to the Department of Plastic and Reconstructive Surgery at Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, with the chief complaint of a congenital coloboma of the left upper eyelid, which had been present since birth. According to the patient’s father, she was born with a full-thickness partial coloboma of the left upper eyelid measuring approximately 6 mm in length, located on the medial third of the eyelid, and was accompanied by hypoplasia of the ipsilateral eyebrow on the lateral third. Closure of the patient’s left eye revealed exposure of the left cornea (Figure 1). Slit-lamp examination revealed no obvious abnormalities in the cornea, pupil, conjunctiva, or other ocular structures, and the pupil demonstrated a sensitive response to light reflex. The presence of Bell’s phenomenon in the patient could explain the absence of corneal pathology despite significant corneal exposure. The patient did not present with any other evident congenital anomalies, including the skull, external ear, spine, and other areas. However, it is interesting that she has a monochorionic monoamniotic twin sister (II-2) from the same oosperm, who should have exactly the same genetic information as the patient, but does not exhibit any eyelid abnormalities (Figure 2). However, the patient showed no significant differences in growth, development, intelligence, or social skills compared to her monozygotic twin sister. The mother had no history of exposure to radiation or hazardous chemicals during pregnancy, and the pregnancy was uneventful without any complications. Both parents are of Han ethnicity with no history of a consanguineous marriage. There is no relevant family history of similar conditions or other congenital anomalies among the patient’s parents and other relatives.
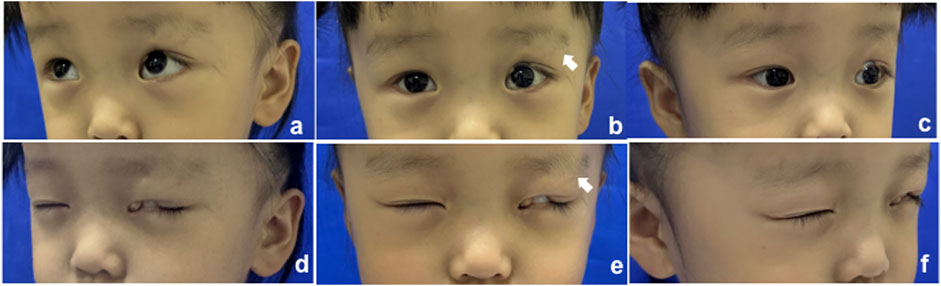
Figure 1. Clinical manifestation of the proband; the white arrow indicates the defect of the eyebrow. (A–C) Open eyes; (D–F) closed eyes.
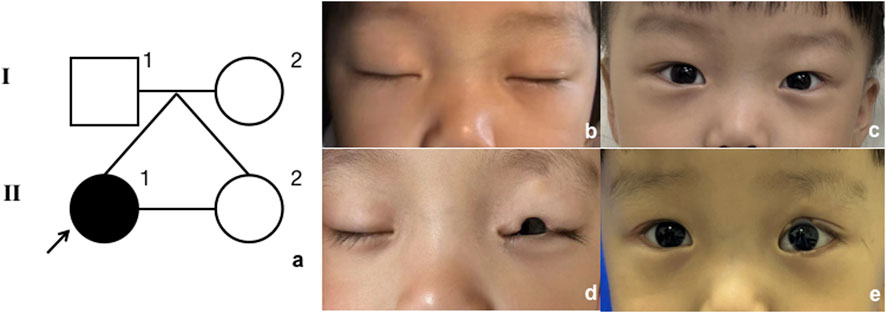
Figure 2. Model of inheritance. (A) Pedigree diagram. Black arrow indicates the proband. (B, C) Proband’s twin sister, closed and open eyes; (D, E) proband, closed and open eyes.
Karyotype analysis confirmed that the patient’s karyotype is 46, XX, indicating a normal female chromosomal composition. The analysis was conducted using G-banding after cell culture. No abnormalities were observed in chromosome number (46 chromosomes) or structure (Supplementary Figure S1).
In addition to the clinical examinations and karyotype analysis, analysis of variants via whole-exome sequencing (WES) (Zhu et al., 2015) using peripheral blood was performed for II-1, II-2, I-1, and I-2, which was conducted by Beijing Genomics Institution Co., Ltd. (BGI, China) (Supplementary Table S1). The reference genome GRCh38 was used for sequence alignment; on average, 86,142,132 clean reads were obtained per sample, and Burrows–Wheeler Aligner (BWA) (Rajan-Babu et al., 2021) was used to align the clean reads of each sample to the human reference genome sequence. The average sequencing depth was × 104.0. An average of 122,142 single-nucleotide polymorphisms (SNPs) and 23,173 insertions and deletions (InDels) were found in all samples. First, we focused on genes with variants present in the patient but absent in the parents and the twin sibling. We then excluded genes classified as benign or with low pathogenic risk according to the American College of Medical Genetics and Genomics (ACMG) guidelines (Ghosh et al., 2017). Subsequently, genes inconsistent with the patient’s clinical manifestations were filtered out using databases such as gnomAD, ExAC, dbSNP, GeneCards, and the 1000 Genomes Project (Coppieters et al., 2016). We found the most major variant associated with the clinical features located at the heterozygous variant of JMJD6 (NM_015167.3: c.941+75G > T), which was a de novo variant. The JMJD6 variant was not recorded in the aforementioned databases. According to the ACMG guidelines (Coppieters et al., 2016), the variant is classified as “likely pathogenic” based on the presence of strong evidence (PS2: de novo occurrence), moderate evidence (PM2: rarity in population databases), and supporting evidence (PP1: co-segregation with the disease in the family). However, the variant was located in the intron. According to the prediction results from the Rare Disease Data Center (RDDC) RNA splicer algorithms (https://rddc.tsinghua-gd.org/) (Zhang et al., 2023), this alteration in the sequence may lead to the formation of a pseudo-exon. This results in a 485-bp insertion, causing a frameshift variant and premature termination (Figure 3). Consequently, it induces alternative splicing, affects protein coding, and leads to a splicing-related disease (Tao et al., 2024).
Due to the patient’s full-thickness eyelid defect, with the defect involving one-third to one-half of the eyelid’s horizontal length and surrounding skin in good condition, the “Tenzel” myocutaneous advancement flap (Kim et al., 2008) was selected to restore the anatomical continuity and functionality of the eyelid while maximizing the preservation of the natural appearance of the periocular area. An inverse semicircular incision was made at the lateral canthus, dissecting in a submuscular plane up to the orbital rim. Skin and orbicularis oculi flaps were created, and the superior branch of the lateral canthal ligament was identified and severed. The orbital septum was released, and the conjunctiva of the fornix was dissected and advanced anteriorly to serve as a lining for the rotational flap. Successive sutures were placed on the lower edge of the flap, which was then rotated medially. The eyelid was pulled medially to align the defect edges, and the tarsal margins were reattached with a 7-0 suture. The orbicularis oculi muscle was closed with a 5-0 suture, and the skin was sutured with 7-0 sutures. This resulted in a small dog ear inferiorly, which was excised. The flap was sutured to the periosteum at the overlap of the orbital rim with 4-0 polypropylene sutures and secured to the inferior branch of the lateral canthal ligament. The canthotomy incision was then closed with a 7-0 suture. The postoperative recovery at 1 week and 2 months is shown in Figure 4, and the patient’s guardian expressed satisfaction with the outcome, and no adverse events occurred during the treatment.
The gene JMJD6, whose full name is Jumonji domain-containing protein 6, arginine demethylase and lysine hydroxylase, is also known as the phosphatidylserine receptor (PTDSR). It is located at 17q25.1 and encodes a nuclear protein with a JmjC domain, which is predicted to play an important role in gene regulation through its demethylation or hydroxylation activity on histone and transcription factors (Chang et al., 2007). Bose et al. revealed that the ablation of JMJD6 function in mice leads to delayed terminal differentiation in multiple organs, including the eyes. This disruption in eye development can result in severe abnormalities, ranging from retinal defects to unilateral or bilateral anophthalmia (Böse et al., 2004). Another study also found that JMJD6 plays an important role in the eye development of Xenopus through GSK3β RNA splicing and Wnt/β-catenin signaling (Shin et al., 2019).
In our study, we identified a novel intronic variant in JMJD6 (c.941+75G > T) through WES and associated it with CEC. This finding highlights a potential link between JMJD6 and eyelid development. Although previous research has emphasized its role in broader ocular development, our report adds a new possible dimension by associating it with CEC, expanding the phenotypic spectrum of this gene. We acknowledge that the association between the JMJD6 variant and eyelid coloboma is currently limited by the lack of functional validation. The identified splicing variant (c.941+75G > T) requires further experimental analysis, including RNA-level evaluation to assess its stability, processing, and translation impacts, as well as protein-level studies to confirm its pathogenicity. Functional tests are essential to elucidate the variant’s mechanistic role in eyelid development.
Interestingly, the proband’s monozygotic twin sister did not exhibit eyelid coloboma despite theoretically having identical genetic material. This suggests that the younger sister’s embryo might have acquired de novo variants or that environmental factors during embryogenesis influenced gene expression. The proband presented with isolated eyelid coloboma without systemic developmental or intellectual impairments, further emphasizing the significance of this specific variant.
This study reports the first case of a JMJD6 intronic pathogenic variant (c.941+75G > T) linked to congenital eyelid coloboma, expanding the known phenotypic spectrum of JMJD6 variants and their role in eyelid morphogenesis. Despite limitations, our findings highlight the gene’s importance in ocular development and provide insights for diagnosis and prognosis. Future research should validate the variant’s function and support diagnostic guidelines, furthering the understanding of JMJD6-related congenital eyelid abnormalities.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by the Human Investigation Committee (IRB) of Shanghai Ninth People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
XL: conceptualization, data curation, software, visualization, and writing–original draft. YZ: conceptualization, methodology, and writing–original draft. GC: data curation, funding acquisition, project administration, and writing–review and editing. WS: conceptualization, formal analysis, funding acquisition, investigation, supervision, and writing–review and editing. YZ: conceptualization, methodology, project administration, resources, validation, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Interdisciplinary Program of Shanghai Jiao Tong University (YG2022QN048), the National Natural Science Foundation of China (No. 82372548), and Shanghai Municipal Key Clinical Specialty (2023ZZ02023-ZWYJZX02).
The authors are grateful to the patients who were research volunteers and to technical assistance from Beijing Genomics Institution Co., Ltd. (BGI, Shenzhen, China).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1536000/full#supplementary-material
Azuma, N., Yamaguchi, Y., Handa, H., Tadokoro, K., Asaka, A., Kawase, E., et al. (2003). Mutations of the PAX6 gene detected in patients with a variety of optic-nerve malformations. Am. J. Hum. Genet. 72, 1565–1570. doi:10.1086/375555
Böse, J., Gruber, A. D., Helming, L., Schiebe, S., Wegener, I., Hafner, M., et al. (2004). The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J. Biol. 3, 15. doi:10.1186/jbiol10
Chang, B., Chen, Y., Zhao, Y., and Bruick, R. K. (2007). JMJD6 is a histone arginine demethylase. Science 318, 444–447. doi:10.1126/science.1145801
Coppieters, F., Ascari, G., Dannhausen, K., Nikopoulos, K., Peelman, F., Karlstetter, M., et al. (2016). Isolated and syndromic retinal dystrophy caused by biallelic mutations in RCBTB1, a gene implicated in ubiquitination. Am. J. Hum. Genet. 99, 470–480. doi:10.1016/j.ajhg.2016.06.017
Donaldson, I. M. L. (2012). How blood was let in the sixteenth century: Jacques Guillemeau, La Chirurgie Françoise. 1594. J. R. Coll. Physicians Edinb 42, 375–377. doi:10.4997/JRCPE.2012.418
Ghosh, R., Oak, N., and Plon, S. E. (2017). Evaluation of in silico algorithms for use with ACMG/AMP clinical variant interpretation guidelines. Genome Biol. 18, 225. doi:10.1186/s13059-017-1353-5
Kelberman, D., Islam, L., Lakowski, J., Bacchelli, C., Chanudet, E., Lescai, F., et al. (2014). Mutation of SALL2 causes recessive ocular coloboma in humans and mice. Hum. Mol. Genet. 23, 2511–2526. doi:10.1093/hmg/ddt643
Kim, H. S., Kim, J. W., and Yu, D. S. (2008). Semicircular (Tenzel) flap for malignant melanoma involving the palpebral conjunctiva and skin of an eyelid. J. Eur. Acad. Dermatol Venereol. 22, 102–103. doi:10.1111/j.1468-3083.2007.02166.x
Liu, C., Widen, S. A., Williamson, K. A., Ratnapriya, R., Gerth-Kahlert, C., Rainger, J., et al. (2016). A secreted WNT-ligand-binding domain of FZD5 generated by a frameshift mutation causes autosomal dominant coloboma. Hum. Mol. Genet. 25, 1382–1391. doi:10.1093/hmg/ddw020
Lodhi, A. A., Junejo, S. A., Khanzada, M. A., Sahaf, I. A., and Siddique, Z. K. (2010). Surgical outcome of 21 patients with congenital upper eyelid coloboma. Int. J. Ophthalmol. 3, 69–72. doi:10.3980/j.issn.2222-3959.2010.01.16
Ng, J. J., Zhong, A., Massenburg, B. B., Binenbaum, G., Wu, M., Romeo, D. J., et al. (2024). Periorbital outcomes and vision risk stratification in treacher collins syndrome. Plast. Reconstr. Surg. doi:10.1097/PRS.0000000000011563
Rajan-Babu, I.-S., Peng, J. J., Chiu, R., IMAGINE StudyCAUSES StudyLi, C., et al. (2021). Genome-wide sequencing as a first-tier screening test for short tandem repeat expansions. Genome Med. 13, 126. doi:10.1186/s13073-021-00932-9
Rooijers, W., Caron, C. J. J. M., Loudon, S. E., Padwa, B. L., Dunaway, D. J., Forrest, C. R., et al. (2020). Ocular and adnexal anomalies in craniofacial microsomia: a systematic review. Int. J. Oral Maxillofac. Surg. 49, 1107–1114. doi:10.1016/j.ijom.2020.03.003
Shin, J. Y., Son, J., Kim, W. S., Gwak, J., and Ju, B.-G. (2019). Jmjd6a regulates GSK3β RNA splicing in Xenopus laevis eye development. PLoS One 14, e0219800. doi:10.1371/journal.pone.0219800
Singh, M., Kaur, M., Grewal, A. M., Yangzes, S., Yadav, D., Zadeng, Z., et al. (2020). Ophthalmic features and management outcomes of 30 children having Goldenhar syndrome. Int. Ophthalmol. 40, 667–675. doi:10.1007/s10792-019-01227-0
Smith, H. B., Verity, D. H., and Collin, J. R. O. (2015). The incidence, embryology, and oculofacial abnormalities associated with eyelid colobomas. Eye (Lond). 29, 492–498. doi:10.1038/eye.2014.335
Tao, Y., Zhang, Q., Wang, H., Yang, X., and Mu, H. (2024). Alternative splicing and related RNA binding proteins in human health and disease. Signal Transduct. Target Ther. 9, 26. doi:10.1038/s41392-024-01734-2
Wang, L., He, F., Bu, J., Zhen, Y., Liu, X., et al. (2012). ABCB6 mutations cause ocular coloboma. Am. J. Hum. Genet. 90, 40–48. doi:10.1016/j.ajhg.2011.11.026
Zhang, Y., Bi, S., Dai, L., Zhao, Y., Liu, Y., and Shi, Z. (2023). Clinical report and genetic analysis of a Chinese neonate with craniofacial microsomia caused by a splicing variant of the splicing factor 3b subunit 2 gene. Mol. Genet. Genomic Med. 11, e2268. doi:10.1002/mgg3.2268
Keywords: congenital eyelid coloboma, karyotype analysis, whole-exome sequencing, JMJD6 gene, “Tenzel” flap, case report
Citation: Li X, Zhang Y, Chai G, Su W and Zhang Y (2025) Case report: A novel intronic JMJD6 likely pathogenic variant (c.941+75G > T) associated with congenital eyelid coloboma in one of the identical twin sisters. Front. Genet. 16:1536000. doi: 10.3389/fgene.2025.1536000
Received: 28 November 2024; Accepted: 30 January 2025;
Published: 17 February 2025.
Edited by:
Fan Jin, Zhejiang University, ChinaReviewed by:
Htoo Aung Wai, University of Southampton, United KingdomCopyright © 2025 Li, Zhang, Chai, Su and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijie Su, rosiewudi@163.com; Yan Zhang, zhangy1330@sh9hospital.org.cn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.