- 1Department of Medical Laboratories, College of Applied Medical Sciences, Majmaah University, Al-Majmaah, Saudi Arabia
- 2Department of Business Administration, Business School, Al al-Bayt University, Mafraq, Jordan
- 3Department of Pharmaceutics and Pharmaceutical Technology, Taif University, Taif, Saudi Arabia
- 4Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Majmaah University, Majmaah, Saudi Arabia
- 5Pharmaceutical Chemistry Department, College of Pharmacy, Al-Ayen University, Thi-Qar, Iraq
- 6Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 7College of Medical Technology, Imam Ja’afar Al-Sadiq University, Al-Muthanna, Iraq
- 8Medical Laboratory Technology Department, College of Medical Technology, The Islamic University, Najaf, Iraq
- 9Genetics Research Center, Department of Genetics and Breeding, The University of Guilan, Rasht, Iran
Background: An increasing number of studies have suggested the relationship between single-nucleotide polymorphisms (SNPs) in toll-like receptor (TLR) genes and gastric cancer (GC) susceptibility; however, the available evidence is contradictory. This meta-analysis aimed to comprehensively evaluate whether the SNPs within the TLR family are related to GC development.
Methods: PubMed, Scopus, and China National Knowledge Infrastructure (CNKI) were systematically searched up to May 2023 to obtain the pertinent publications. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were applied to examine the associations using the random-effects model.
Results: A total of 45 studies with 25,831 participants (cases: 11,308; controls: 14,523) examining the relation of 18 different SNPs in the TLR family to GC were analyzed. Variations in TLR-4 rs4986790, TLR-4 rs4986791, TLR-5 rs5744174, and TLR-9 rs187084 were significantly associated with increased risk of GC in different genetic models. No significant association was detected for TLR-2-196 to -174de (Delta22), TLR-2 rs3804100, TLR-4 rs11536889, TLR-4 rs11536878, TLR-4 rs2770150, TLR-4 rs10116253, TLR-4 rs1927911, TLR-4 rs10983755, TLR-4 rs10759932, TLR-4 rs1927914, and TLR-10 rs10004195.
Conclusion: These findings indicate that variations in TLR-4, TLR-5, and TLR-9 genes were found to be potential risk factors for GC.
Introduction
Gastric cancer (GC) is the fifth most prevalent kind of malignancy and the third main cause of cancer-related death globally (Castano-Rodriguez et al., 2014), accounting for 8.8% of total cancer-associated mortalities, with 5-year survival rates less than 30% (Castaño-Rodríguez et al., 2014). GC is a complex disease with a multifactorial etiology that includes the combined impacts of lifestyle, bacteria, and host and environmental factors (Gao et al., 2020). However, Helicobacter pylori infection is an established risk factor for GC, but only 0.1%–4% of infected people develop GC, highlighting the etiological involvement of environmental predisposing factors and host genetics in GC development (Companioni et al., 2014).
Changes in the host immune elements, such as toll-like receptors (TLRs), may affect the development of GC via their crucial involvement in triggering adaptive and innate immune responses (Sultan et al., 2022). Ten TLRs have been identified on the surfaces of immune cells as well as gastric epithelial cells in humans (Eed et al., 2020). TLRs deliver the first line of host defense against damaging pathogens by recognizing pathogen-associated molecular patterns (PAMPs) expressed by the majority of microorganisms, such as unmethylated CpG motifs (TLR-9), flagella (TLR-5), lipopolysaccharides (LPSs) and lipoproteins (TLR-1,-2,-4,-5, and -6), peptidoglycan (TLR-2), and microbial nucleic acids (TLR-3,-7,-8, and -9) (Castano-Rodriguez et al., 2014; Li et al., 2021). Moreover, TLR-10 expressed by the gastric epithelial cells plays a key role in the identification of multiple patterns of H. pylori LPSs (Li et al., 2021). The contribution of TLRs in gastric tumorigenesis has been suggested by previous studies (de Oliveira and Silva, 2012; Castaño-Rodríguez et al., 2014). It has been detected that TLR-3 and TLR-4 are highly expressed in cancer cells (Li et al., 2021). Evidence has also shown that the overexpression of TLR-5 is associated with increased GC cell proliferation and may act as a biomarker for GC (Song et al., 2011; Kasurinen et al., 2019). Furthermore, TLR-7 expression has been negatively linked to the viability of GC cells (Jiang et al., 2016), and the TLR-7 agonist has been examined as a possible vaccine formulation in GC immunotherapy (Wang X-D. et al., 2015). TLR-9 is also expressed aberrantly in GC, and H. pylori DNA could trigger TLR-9-mediated GC cell invasion (Gao et al., 2020). Single-nucleotide polymorphisms (SNPs) within TLR genes may lead to a change in their expression along with dysregulation of their signaling pathways, resulting in disturbed secretion of inflammatory mediators and enabling H. pylori to cause persistent infection and affecting gastric immunopathology and GC susceptibility (Eed et al., 2020; Sultan et al., 2022).
In recent studies, polymorphisms in TLR genes have been suggested to be associated with the risk of GC (de Oliveira and Silva, 2012; Companioni et al., 2014; Dargiene et al., 2018). However, the results have been inconsistent among various ethnic populations, and the small sample sizes of individual studies limit their statistical power to detect associations. This systematic review and meta-analysis was performed to comprehensively and quantitatively evaluate the relationship between TLR gene polymorphisms and the risk of GC.
Materials and methods
This meta-analysis was performed according to the PRISMA protocol for systematic review and meta-analysis (Moher et al., 2015).
Search strategy
Without language restriction, we systematically searched PubMed, Scopus, and China National Knowledge Infrastructure (CNKI) up to 30 May 2023 to find studies examining the relation of polymorphisms in the TLR family to the risk of GC using the following terms: (((((Toll-like receptors[Title/Abstract]) OR (Toll-like receptor[Title/Abstract])) OR (TLR[Title/Abstract])) AND (((((variant*[Title/Abstract]) OR (Polymorphism*[Title/Abstract])) OR (mutation[Title/Abstract])) OR (allele[Title/Abstract])) OR (genotype[Title/Abstract]))) AND ((stomach[Title/Abstract]) OR (gastric[Title/Abstract]))) AND ((((((tumor[Title/Abstract]) OR (cancer[Title/Abstract])) OR (neoplasm[Title/Abstract])) OR (neoplasia[Title/Abstract])) OR (carcinoma[Title/Abstract])) OR (adenocarcinoma[Title/Abstract])). The lists of references within the reviews and pertinent studies were manually searched for possible additional publications. Initially, all studies obtained through PubMed and Scopus were entered into a citation manager software (EndNote) to screen the identified studies according to the inclusion/exclusion criteria. Then, a complementary systematic search was conducted in CNKI for additional studies. Some authors were experts in multiple languages, including Chinese. Nevertheless, except for one non-English study (Chinese) (Zeng et al., 2011a), all other articles published in non-English languages were excluded from the meta-analysis based on their English titles/abstracts because they had unrelated exposures or outcomes.
Eligibility criteria
Studies with the following criteria were eligible to be included in the present analysis: 1) investigated the association between gene polymorphisms in TLRs (TLR-1, TLR-2, TLR-3, TLR-4, TLR-5, TLR-6, TLR-7, TLR-8, TLR-9, or TLR-10) as the exposure and GC as the outcome; 2) studies with cohort or case-control design; and 3) provided genotype frequency in both cases and controls. We excluded studies that did not have a control group, studies with irrelevant exposure/outcomes, gene expression papers, letters, conference papers, case reports, book chapters, publications with unextractable data, and studies on precancerous gastric lesions. Moreover, studies were excluded from the meta-analysis if the genotypic frequency of the control group deviated from the Hardy–Weinberg equilibrium (HWE). However, for the studies that investigated several polymorphisms, polymorphisms that were not in HWE were excluded from the analysis, and only polymorphisms that were in HWE were included in the meta-analysis. Two reviewers screened titles/abstracts and full texts of the potentially related studies for eligibility assessment based on the inclusion/exclusion criteria. The disagreements regarding the eligibility of studies were resolved by a group discussion among all authors.
Data extraction and quality assessment
Two investigators independently extracted the data using a standardized data extraction sheet, and disagreements were resolved by discussion between the two investigators, and if necessary, a third reviewer was consulted. The following data were obtained for each publication: the first author’s name, country, sample size of cases and controls, ethnicity, minor allele frequency (MAF) for the control group, HWE, year of publication, and genotype frequencies in controls and cases. The quality of the included publications was assessed using the Q-Genie tool. This tool includes 11 questions to be marked on a 7-point Likert scale for assessing several aspects of the genetic association studies. Overall scores ≤35 indicate poor-quality studies, those >35 and ≤45 indicate moderate-quality studies, and those >45 indicate good-quality studies (Sohani et al., 2015).
Statistical analysis
The HWE in control groups was tested using the chi-squared test, and p < 0.05 indicated significant disequilibrium (Alizadeh et al., 2016). The strength of the relation between the TLR polymorphisms and the odds of GC risk was examined using the pooled odds ratio (OR) with a 95% confidence interval (CI) using the random-effects model (DerSimonian–Laird approach) (DerSimonian and Laird, 1986) under five genetic models, namely, the allelic, recessive, dominant, homozygote contrast, and heterozygote contrast models. The heterogeneity among studies was evaluated by the Q-test and I2 statistics; p-value <0.1 was considered a remarkable evidence of heterogeneity (Abyadeh et al., 2019; Nazari et al., 2022). Subgroup analyses based on ethnicity, age, and source of controls (community-based or hospital-based) were conducted to assess the possible sources of heterogeneity. Publication bias was assessed by funnel plots and Egger’s test, and p-values less than 0.05 were considered statistically significant (Alizadeh et al., 2017). All analyses were conducted using Stata software (version 13.0; Stata Corporation, College Station, TX).
Results
Description of studies
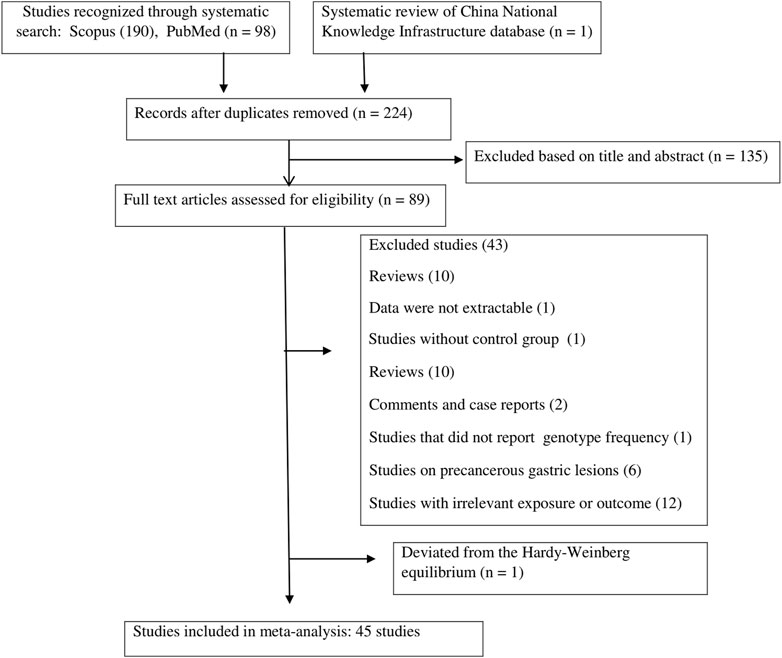
A total of 289 publications were obtained through the systematic search of databases. After removing duplicate papers (n = 65) and excluding irrelevant studies based on titles/abstracts (n = 135), a total of 89 studies underwent full-text screening. Of these, 43 publications were excluded according to the inclusion/exclusion criteria. Figure 1 shows the process of study selection and the excluded publications with the specific reasons. For some studies that investigated several SNPs in TLR genes, the genotype frequencies for controls followed the HWE in some SNPs but deviated from the HWE for other SNPs (Schmidt et al., 2011; Ravishankar Ram et al., 2015; Simawaranon et al., 2017; De Re et al., 2019; Susi et al., 2019; Tongtawee et al., 2019). For these studies, only the SNPs that followed the HWE were included in the meta-analysis. Finally, a total of 45 publications (Zeng et al., 2011a; de Oliveira and Silva, 2012; Castano-Rodriguez et al., 2014; Castaño-Rodríguez et al., 2014; Companioni et al., 2014; Dargiene et al., 2018; Eed et al., 2020; Gao et al., 2020; Li et al., 2021; Sultan et al., 2022; Garza-Gonzalez et al., 2007; Hold et al., 2007; Tahara et al., 2007; Santini et al., 2008; Trejo-de la et al., 2008; Hishida et al., 2009; Hold et al., 2009; Hishida et al., 2010; Huang et al., 2010; Rigoli et al., 2010; Zeng et al., 2011b; Kupcinskas et al., 2011; Schmidt et al., 2011; De Oliveira et al., 2013; He et al., 2013; Kim et al., 2013; Wang et al., 2013; Huang et al., 2014; Kutikhin et al., 2014; Li et al., 2014; Qadri et al., 2014; He et al., 2015; Liu et al., 2015; Ravishankar Ram et al., 2015; Stubljar et al., 2015; Wei et al., 2015; Mukherjee et al., 2016; Simawaranon et al., 2017; Xu et al., 2017; He et al., 2018a; De Re et al., 2019; Huang et al., 2019; Susi et al., 2019; Tongtawee et al., 2019; de Matos Lourenço et al., 2020; Gonzalez-Hormazabal et al., 2021), with a total sample size of 25,831 participants (cases: 11,308; controls: 14,523), examining the relation of 18 different SNPs in the TLR family to GC, were eligible to be included in the present meta-analysis. The analyzed studies were published from 2007 to 2022. Moreover, we systematically reviewed the results of the studies on less common SNPs of TLRs and GC that were not eligible for meta-analysis (with only one available effect size) (Zeng et al., 2011b; Kim et al., 2013; Yang et al., 2013; Castano-Rodriguez et al., 2014; Companioni et al., 2014; Liu et al., 2015; De Re et al., 2019; Wang et al., 2019; Gao et al., 2020; Gonzalez-Hormazabal et al., 2021; Li et al., 2021) to have a comprehensive evaluation. Of the included studies in the meta-analysis, 12 studies were on Caucasian populations (Hold et al., 2007; Santini et al., 2008; Hold et al., 2009; Rigoli et al., 2010; Kupcinskas et al., 2011; Companioni et al., 2014; Kutikhin et al., 2014; Stubljar et al., 2015; Dargiene et al., 2018; De Re et al., 2019; Eed et al., 2020; Sultan et al., 2022), seven studies were on Latino populations (Garza-Gonzalez et al., 2007; Trejo-de la et al., 2008; de Oliveira and Silva, 2012; De Oliveira et al., 2013; Susi et al., 2019; de Matos Lourenço et al., 2020; Gonzalez-Hormazabal et al., 2021), and 26 studies were on Asian populations. The mean age of participants ranged from 44.6 ± 15.9 to 66.26 ± 16.32 years. Quality assessment scores of the studies based on the Q-Genie tool are reported in Supplementary Table S1. The overall quality scores of the studies ranged from 23 to 55. Among the studies, 21 studies were rated to be of poor quality, 10 studies were rated to be of moderate quality, and four studies were rated to be of high quality. The genotype frequencies and other characteristics of studies included in the meta-analysis are given in Table 1.
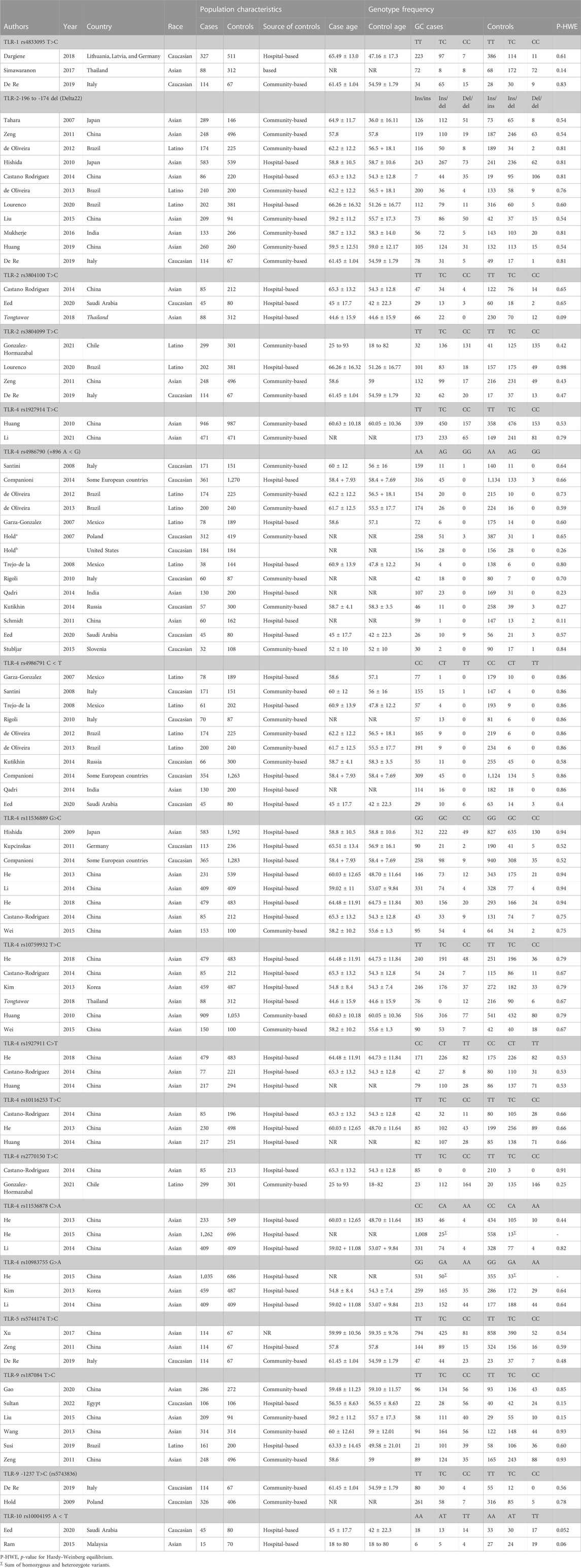
TABLE 1. Characteristics of eligible studies considered for the association between toll-like receptor family gene polymorphism and GC risk in the meta-analysis.
Systematic review of rare polymorphisms in TLR genes and GC
The characteristics of studies on less common SNPs of TLRs and GC that were not included in the meta-analysis but were included in the systematic review are presented in Supplementary File S1 (1, 3, 4, 7, 25, 39, 42, 49, and 57–59). Castano-Rodriguez et al. (2014) investigated the relation between TLR-4 SNPs (rs10759931, rs11536891, rs11536898, rs2149356, and rs5030728) and GC risk in a Chinese population and found that rs10759931 in TLR-4 could provide protection against GC in the dominant model and heterozygote contrast model, while no association for other SNPs was detected. Companioni et al. (2014) examined the effect of TLR-4 SNPs (rs1329061, rs1329060, rs1329057, and rs10491851) in Caucasian populations and reported that the carriers of the variant allele of the rs10491851 in TLR-4 were significantly at lower risk of GC. In the study by Gonzalez-Hormazabal et al. (2021) in Chile on TLR-4-2 rs7656411, TLR-4 rs1554973, TLR-4 rs7037117, TLR-4 rs913930, and TLR-5 rs75977922, a significantly increased odds of GC was observed for carriers of the variant allele/genotype of TLR-5 rs75977922. No significant association was identified between the TLR-1 rs5743681 in German (Yang et al., 2013), TLR-2 rs1898830 in Korean (Kim et al., 2013), TLR-8 rs3764880 in Italian (De Re et al., 2019), and TLR-9 rs164640 in Chinese (Gao et al., 2020) populations and the risk of GC. In contrast, variations in TLR-3 1377C T (rs3775290) (Liu et al., 2015) and TLR-4 rs1057317 (Trejo-de la et al., 2015; Wang et al., 2019) in Chinese populations were significantly linked to the increased risk of GC among the risk-allele carriers. Li et al. (2021), in 2020, revealed a significant negative relationship between the TLR-4 rs7873784 (dominant model: OR = 0.17 (95% CI: 0.09–0.33) and TLR-4 rs7869402 [dominant model: OR = 0.61 (95% CI: 0.40–0.92)] and GC risk, while no association was reported for TLR-3 rs5743303, TLR-4 rs1927914, TLR-5 rs1640816, and TLR-7 rs3853839 and GC risk. Moreover, in the Chinese population, compared to carriers of the C allele, carriers of the risk allele (T) of TLR-3 1377C < T (rs3775290) showed a 2.05-fold elevated odds of GC, OR = 2.02 (95% CI: 1.41–2.98) (Liu et al., 2015). Zeng et al. (2011b) also investigated the association of two common SNPs in TLR-2 and TLR-5 genes (TLR-5 rs2072493, TLR-5-889T>C, and TLR-2 -688G>T) with GC and reported that these variants are not significant predictors of GC susceptibility. Overall, these findings evidentiate that polymorphisms within the TLR genes may be potential predictors of GC; however, the available data for these SNPs are rare, and further studies are required to assess these relationships.
Meta-analysis
The overall pooled analyses and subgroup analyses by ethnicity are reported in Table 2. In the overall analyses, TLR-4 rs4986790 was significantly associated with an increased risk of GC (dominant model: OR = 1.51, 95% CI: 1.12–2.03; recessive model: OR = 2.58, 95% CI: 1.06–6.30; allelic model: OR = 1.50, 95% CI: 1.12–2.01; homozygote model: OR = 2.58, 95% CI: 1.05–6.34; and heterozygote model (AG vs. AA): OR = 1.46, 95% CI: 1.09–1.97) (Figure 2). An increased odds of GC was also found for variations in TLR-4 rs4986791 (dominant model: OR = 1.53, 95% CI: 1.15–2.04; allelic model: OR = 1.56, 95% CI: 1.15–2.11; heterozygote model (CT vs. CC): OR = 1.45, 95% CI: 1.12–1.87) (Figure 3). Additionally, the analysis revealed a significant association between TLR-9 rs187084 and GC risk (dominant model: OR = 1.40, 95% CI: 1.01–1.95; recessive model: OR = 1.51, 95% CI: 1.003–2.27; allelic model: OR = 1.33, 95% CI: 1.03–1.73; homozygote model (CC vs. TT): OR = 1.77, 95% CI: 1.07–2.91) (Figure 4). No significant association with GC risk was observed for TLR-2 -196 to -174de (Delta22), TLR-2 rs3804100, TLR-4 rs11536889, TLR-4 rs11536878, TLR-4 rs2770150, TLR-4 rs10116253, TLR-4 rs1927911, TLR-4 rs10983755, TLR-4 rs10759932, TLR-4 rs1927914, TLR-9 rs352140, and TLR-10 rs10004195 (Table 2).
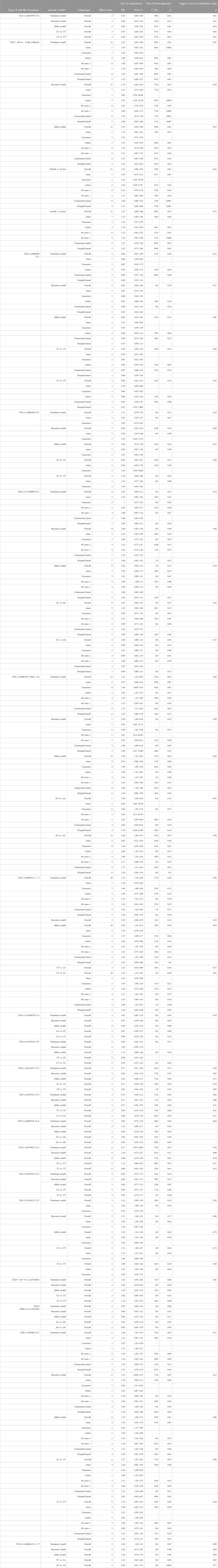
TABLE 2. Main results and subgroup analysis by ethnicity for the meta-analysis of various types of toll-like receptors and gastric cancer susceptibility.
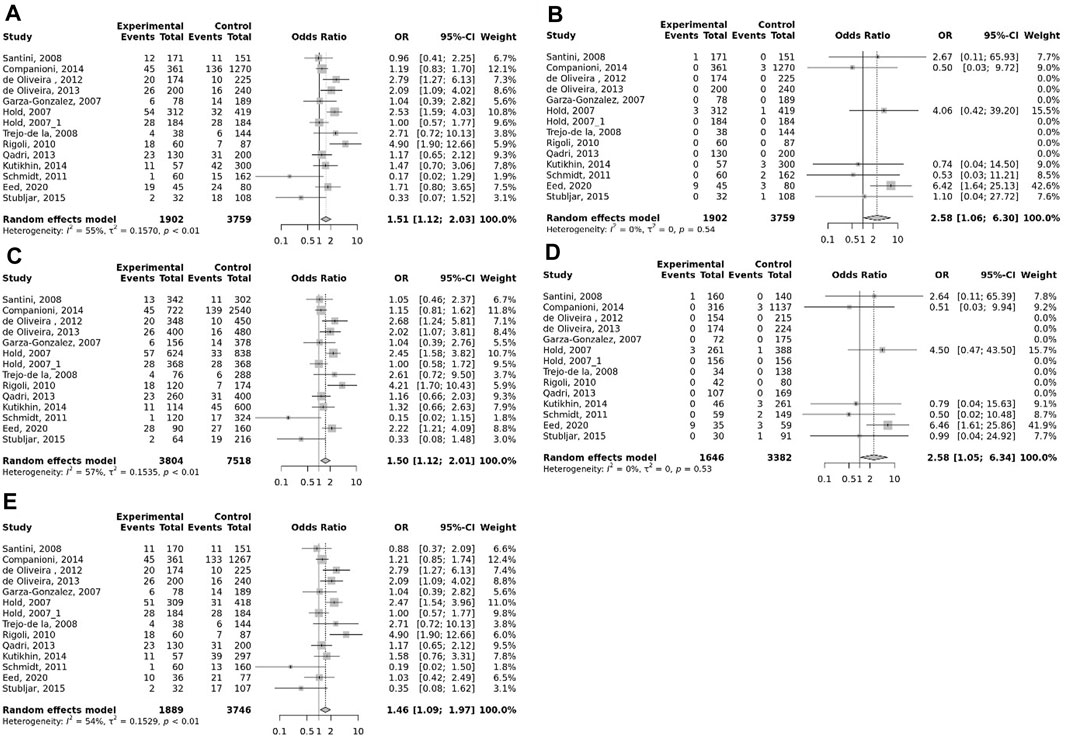
FIGURE 2. Meta-analysis of the association between TLR-4 rs4986790 (+896 A<G) polymorphism with gastric cancer in dominant (A), recessive (B), allelic (C), homozygote (GG vs. AA) (D), and heterozygote (AG vs. AA) (E) models.
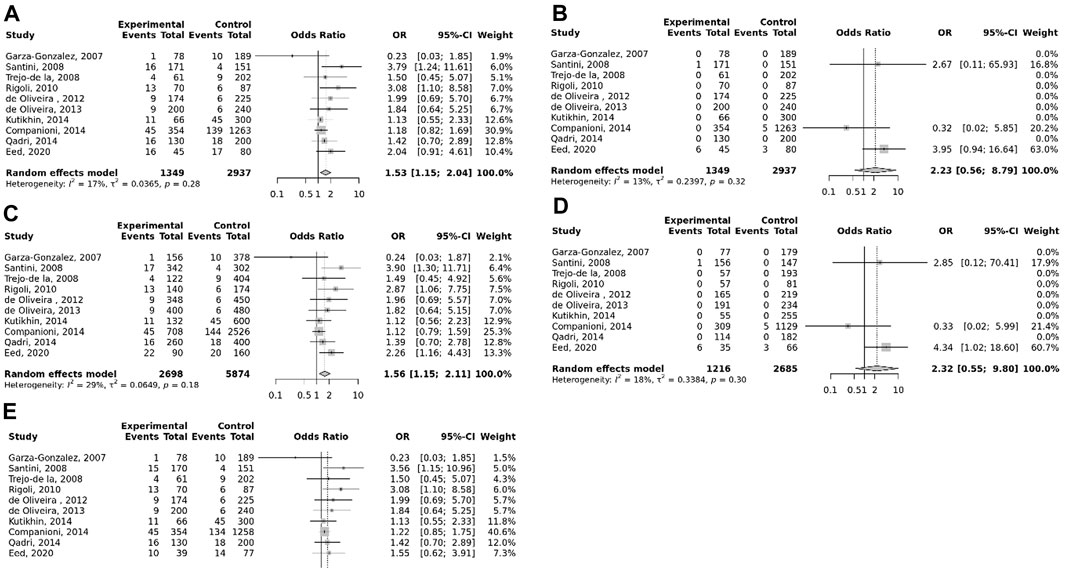
FIGURE 3. Meta-analysis of the association between TLR-4 rs4986791 C<T polymorphism with gastric cancer in dominant (A), recessive (B), allelic (C), homozygote (TT vs. CC) (D), and heterozygote (CT vs. CC) (E) models.
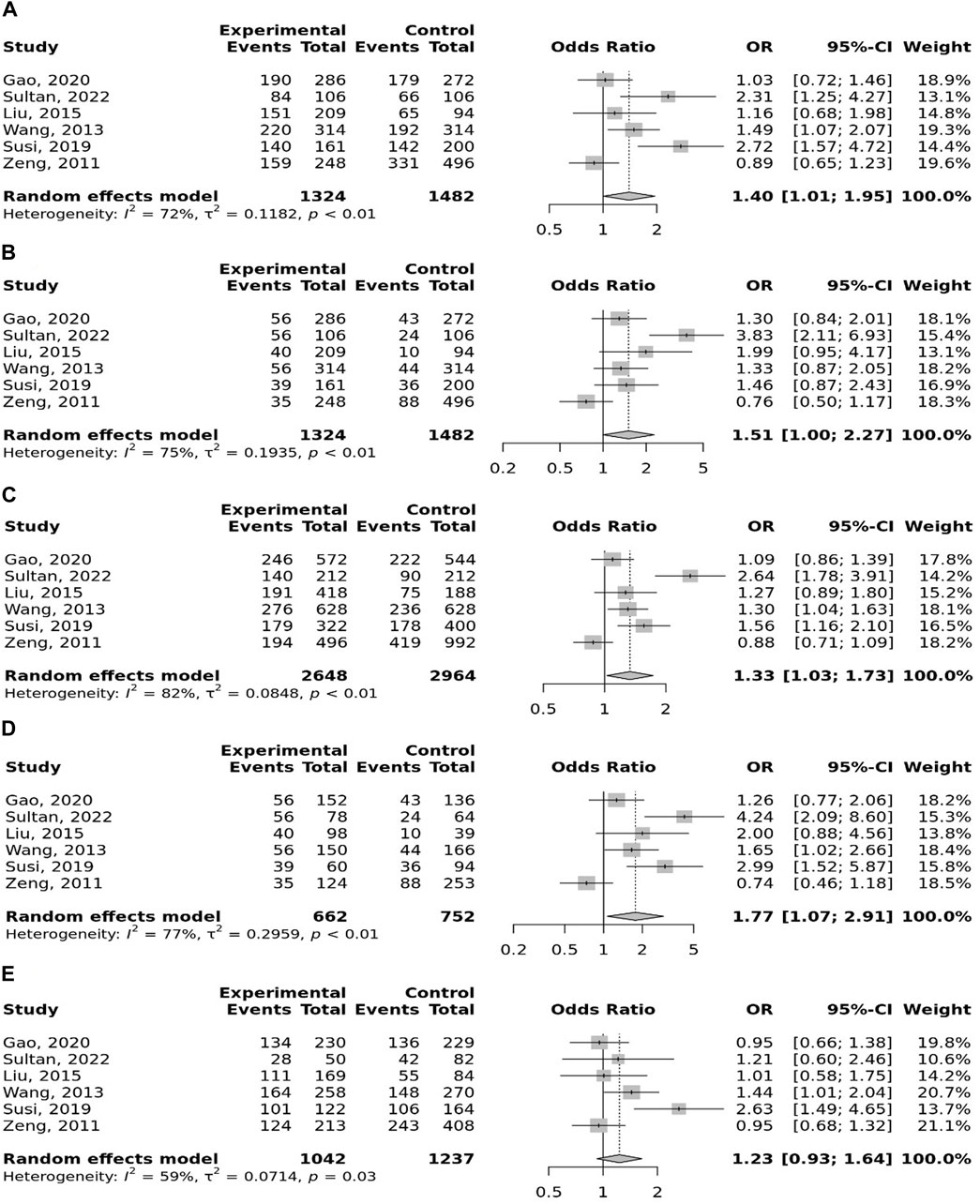
FIGURE 4. Meta-analysis of the association between TLR-9 rs187084 T>C polymorphism with gastric cancer in dominant (A), recessive (B), allelic (C), homozygote (CC vs. TT) (D), and heterozygote (TC vs. TT) (E) models.
Subgroup analyses
In the stratified analysis, the association of TLR-4 rs4986790 with GC was supported by the subgroups of Caucasian, Latino, people of age ≤60 years, and studies with the community-based control group (Table 2). Moreover, the association of TLR-4 rs4986791 with GC was supported by the subgroups of Caucasian, people of age ≤60 years, and studies with the community-based control group (Table 2). In different genetic models, there was a positive relationship between TLR-5 rs5744174 and GC susceptibility (recessive model: OR = 1.71, 95% CI: 1.26–2.31; allelic model: OR = 1.25, 95% CI: 1.11–1.40; homozygote model (CC vs. TT): OR = 1.74, 95% CI: 1.28–2.37) (Figure 5), which was also observed in the Asian population (Table 2). In the stratified analysis, the association of TLR-9 rs187084 with GC was supported by the subgroups of Caucasian, Latino, people of age ≤60 years, and studies with the community-based control group (Table 2).
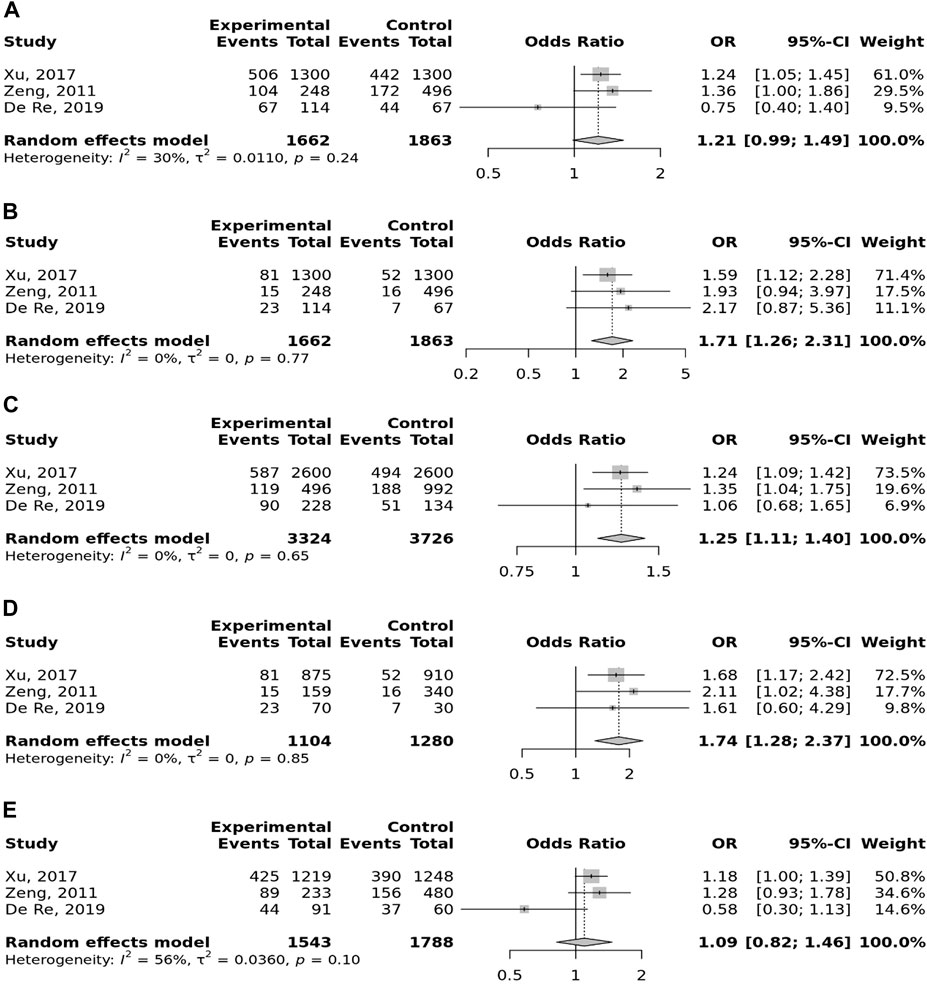
FIGURE 5. Meta-analysis of the association between TLR-5 rs5744174 T>C polymorphism with gastric cancer in dominant (A), recessive (B), allelic (C), homozygote (CC vs. TT) (D), and heterozygote (TC vs. TT) (E) models.
Heterogeneity and publication bias
For the SNPs of TLR-2 rs3804099, TLR-2 rs3804100, TLR-4 rs11536889, TLR-4 rs4986791, TLR-4 rs11536878, TLR-4 rs2770150, TLR-5 rs5744174, and TLR-9 rs352140, no significant heterogeneity was detected across the studies, but the test of heterogeneity was significant for other investigated SNPs (Table 2). Egger’s test of publication bias revealed no evidence of publication bias for most SNPs (Table 2). Funnel plots for publication bias for SNPs with ≥10 studies under the dominant model are given in Supplementary File S2.
Discussion
This meta-analysis examined the association of 18 common SNPs in the TLR family to GC risk. The results indicated that variations in TLR-4 rs4986790, TLR-4 rs4986791, TLR-5 rs5744174, and TLR-9 rs187084 were significantly associated with increased risk of GC in different genetic models.
Given the close relationship between inflammation and carcinogenesis, several studies have attempted to disclose the role of TLRs in GC progression. However, the available data were contradictory. The protein function of TLRs might be damaged by SNPs in genes coding TLRs, resulting in altered vulnerability to malignancies (Wang et al., 2023). Previous meta-analyses on the relation of TLR SNPs to GC have only focused on four common SNPs in the TLR family, namely, TLR-2-196 to -174de (Delta22), TLR-4 rs11536889, TLR-4 rs4986790, and TLR-4 rs498679, with inconclusive results. While the study by Castano-Rodriguez indicated a significant association of TLR-2-196 to -174de (Delta22) with GC (Castaño-Rodríguez et al., 2013), the meta-analysis by Chen et al. (2013) failed to find an association. In line with our findings, a previous meta-analysis by Zhao et al. identified a significant direct association between TLR-4 rs4986790 and GC, while, in contrast to the present meta-analysis, no association was found for TLR-4 rs4986791 (Zhao et al., 2013). Confirming our results, the significant relation of TLR-4 rs4986790 and TLR-4 rs4986791 variations to GC has been proposed by another meta-analysis (Zhou et al., 2014). Moreover, similar to our study, two previous meta-analyses by Wang X. et al. (2015) and Chen et al. (2013) did not find a significant relationship between TLR-4 rs11536889 and GC. The present study is the first meta-analysis showing a significant relationship between TLR-5 rs5744174 and TLR-9 rs187084 and genetic susceptibility to GC. The differences in the results of the previous meta-analyses may be due to the small number of the analyzed studies and, thus lack of sufficient statistical power to detect the true associations.
Mechanistically, genetic variations in TLRs may result in impaired TLR signaling and alter their affinity to the ligands, leading to variations in immune responses of the gastric mucosa against carcinogens and dysregulated inflammation, leading to modulation of H. pylori infection and, thus, GC risk (Zhou et al., 2014; Tongtawee et al., 2019). TLR signaling plays a crucial role in shaping the tumor microenvironment (Wang et al., 2023). Polymorphisms in TLR genes could influence the recruitment and activation of immune cells, tumor-associated inflammation, and immune evasion mechanisms employed by cancer cells, which all affect cancer susceptibility (Paulos et al., 2007). TLR-2 activation results in the activation of nuclear factor-κB (NF-κB) and functions as an innate immune response (de Matos Lourenço et al., 2020). It has been suggested that NF-κB is a main mediator of inflammation-induced tumor progression (Wang et al., 2023). The rs3804099 variants may reduce TLR-2-related chronic inflammation and induce apoptosis (Kim and Karin, 2011; Semlali et al., 2017; Mohamed et al., 2020), resulting in decreased risk of GC. Furthermore, the TLR-5 rs5744174 may affect the recognition and binding affinity of TLR-5 toward bacterial flagellin, leading to compromised antibacterial responses and prolonged exposure to pathogens, thus promoting gastric oncogenesis (Castaño-Rodríguez et al., 2014). TLR-9 impacts tumor development and progression through various molecular mechanisms, such as activation of immune response, induction of apoptosis, modulation of angiogenesis, and regulation of tumor cell proliferation and survival. TLR-9 activation in GC cells leads to the production of pro-inflammatory cytokines and chemokines, such as TNF-alpha, IL-6, and IL-8 (Karapetyan et al., 2020). These molecules attract immune cells, such as macrophages and dendritic cells, to the tumor microenvironment. This immune response helps in tumor recognition, elimination, and control (Karapetyan et al., 2020). TLR-9 activation can induce apoptosis in GC cells. This is mediated by the activation of caspases, which are enzymes involved in the execution of apoptosis (Sindhava et al., 2017). TLR-9 can influence the angiogenesis that is required for tumor growth and metastasis. Studies have shown that TLR-9 activation can inhibit angiogenesis by suppressing the production of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) (He et al., 2018b). Activation of TLR-9 impacts the proliferation and survival of GC cells (Tang et al., 2022). It can activate signaling pathways, such as the nuclear factor kappa-light-chain-enhancer of activated B-cell (NF-κB) pathway, which is involved in promoting cell proliferation and survival (Tsujimura et al., 2004). Regarding TLR-5, induction of TLR-5 leads to the production of pro-inflammatory cytokines and chemokines, such as interleukin 1 beta (IL-1β), IL-6, and IL-8. These molecules attract immune cells, including macrophages and natural killer cells, to the tumor microenvironment. This immune response aids in tumor recognition and elimination (Tallant et al., 2004). TLR-5 plays a role in the integrity and barrier function of the epithelial layer in the stomach (Feng et al., 2023). This is achieved through the activation of various signaling pathways, such as the NF-κB pathway (Tallant et al., 2004), which regulates the expression of genes involved in tight junction formation and epithelial cell adhesion (Al-Sadi et al., 2016). A well-functioning epithelial barrier helps prevent the infiltration of potentially harmful agents and pathogens into the underlying tissues (Mitamura et al., 2021). TLR-5 activation has been shown to affect the invasive potential and metastatic spread of GC cells (Castaño-Rodríguez et al., 2014). This effect is mediated by the suppression of various molecular pathways involved in invasive behavior, such as the matrix metalloproteinase (MMP) family enzymes that degrade the extracellular matrix (Jiang et al., 2017), a critical step in tumor invasion and metastasis (Zhuyan et al., 2020). Studies have also demonstrated that activation of TLR-5 promotes apoptosis in cancer cells through the activation of caspases and the upregulation of pro-apoptotic factors (Zeng et al., 2006). This helps in reducing tumor burden and inhibiting tumor progression. Upon binding to molecular patterns, TLR-4 triggers the activation of inflammatory pathways, leading to the release of pro-inflammatory cytokines, chemokines, and various immune cells (Escoubet-Lozach et al., 2011). Chronic inflammation can promote tumor growth, angiogenesis, and metastasis in GC (Landskron et al., 2014). TLR-4 signaling can activate the NF-κB pathway (Verstrepen et al., 2008). Activation of NF-κB promotes the expression of genes involved in cell survival, proliferation, and angiogenesis, which can contribute to the development and progression of GC (Rastogi et al., 2023). TLR-4 activation has been shown to induce epithelial–mesenchymal transition (EMT), a process in which epithelial cells acquire a mesenchymal phenotype (Shi et al., 2016). EMT is associated with increased invasive potential and metastasis in cancer cells (Huang et al., 2015). TLR-4-induced EMT in GC is mediated through signaling pathways such as Snail, Twist, and ZEB1, which are known regulators of EMT markers (Jing et al., 2012). TLR-4 activation can contribute to the activation of cancer-associated fibroblasts (CAFs) and the development of fibrosis in the GC microenvironment (Janovec et al., 2021). CAFs can promote GC progression through the secretion of growth factors, extracellular matrix remodeling, and immune modulation (Kobayashi et al., 2019). TLR-4 signaling can directly impact tumor cell behavior. Activation of TLR-4 promotes cell survival and proliferation through signaling pathways such as phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), and the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) (JAK/STAT) (Vivarelli et al., 2004; Korneev et al., 2017). These pathways regulate various aspects of cell growth, survival, and apoptosis resistance, ultimately influencing GC cell behavior (Hu et al., 2014; Sun et al., 2015). It is important to note that the molecular mechanisms of TLRs in GC are still an area of active research, and further studies are needed to fully understand the complexities of the TLR-mediated effects on GC development and progression. Accordingly, the SNPs of TLRs might change the aforementioned pathways, resulting in cancer proneness. These findings can contribute to the development of targeted preventive strategies and personalized therapeutic interventions for individuals at high risk of GC.
Our systematic review and meta-analysis provided a comprehensive evaluation of the current evidence for the association of TLR SNPs to GC risk. To the best of our knowledge, this meta-analysis was the largest review to date exploring the association between TLR-2-196 to -174de (Delta22), TLR-4 rs11536889, TLR-4 rs4986790, and TLR-4 rs4986791 polymorphisms and GC risk. This study was also the first meta-analysis on the relation of TLR-1 rs4833095, TLR-2 rs3804100, TLR-2 rs3804099, TLR-4 rs1927914, TLR-4 rs11536878, TLR-4 rs2770150, TLR-4 rs10116253, TLR-4 rs1927911, TLR-4 rs10983755, TLR-4 rs10759932, TLR-5 rs5744174, TLR-9 rs5743836, TLR-9 rs187084, and TLR-10 rs10004195 SNPs to GC susceptibility.
As a strength, no evidence of publication bias was detected, and studies that deviated from the HWE were excluded from the analyses. Nevertheless, some limitations of the present meta-analysis should be considered. First, there was significant heterogeneity across the analyzed publications. To improve the reliability of the results, the random-effects model was used for analyses. Moreover, stratified analysis revealed that ethnicity, the source of controls, and the age of participants were the sources of the observed heterogeneity. The association of the TLR polymorphisms with GC was modified by ethnicity, the source of controls, and the age of participants, indicating that these factors have contributed to the differences in the results of the previous studies. Therefore, future studies should consider these factors when investigating the association between TLR polymorphisms and gastric cancer. Second, the interactions between gene–gene and gene–environmental factors, including diet, alcohol intake, smoking, and physical activity, potentially affect GC carcinogenesis; due to the unavailability of data, we could not consider interaction analyses in this study. Third, for some SNPs, a small number of studies were included, and the findings of some subgroups may be at risk of bias because of relatively low statistical power to detect associations. Therefore, findings from the stratified analyses should be interpreted cautiously and need additional validation. We also could not perform subgroup analysis by the sex of subjects because of the lack of sex-specific data in original studies. Moreover, data for the effects of the haplotype analyses and gene–environmental factor interactions on GC were not reported sufficiently in the included original studies; thus, we could not report haplotype analyses and gene–environment interactions. Lastly, no sufficient information was available for African populations, and our result, therefore, may not be expandable to these populations. Thus, further research is required to assess the impact of these SNPs on GC in various ethnicities, particularly those of African ethnicities.
Conclusion
In conclusion, this meta-analysis revealed that variations in TLR-4, TLR-5, and TLR-9 genes were potential risk factors of GC. Additional well-designed, large-scale studies, with more detailed information concerning gene–gene and gene–environmental interactions, should be conducted on different races, especially for less investigated SNPs, to yield a better understanding of the relationship between TLR SNPs and GC risk.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
AAA: formal analysis, project administration, data curation, and writing–original draft. SA-H: data curation, formal analysis, writing–original draft, investigation, and methodology. HOA: writing–original draft, conceptualization, project administration, software, and validation. SA: project administration, validation, writing–original draft, investigation, methodology, resources, and visualization. MN: investigation, methodology, validation, writing–original draft, data curation, and formal analysis. AH: formal analysis, investigation, methodology, validation, conceptualization, and writing–review and editing. AA: conceptualization, data curation, project administration, and writing–original draft. AFA: data curation, writing–original draft, formal analysis, investigation, and methodology. HA: formal analysis, project administration, software, supervision, validation, and writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1280051/full#supplementary-material
References
Abyadeh, M., Djafarian, K., Heydarinejad, F., Alizadeh, S., and Shab-Bidar, S. (2019). Association between apolipoprotein E gene polymorphism and Alzheimer's disease in an Iranian population: a meta-analysis. J. Mol. Neurosci. 69, 557–562. doi:10.1007/s12031-019-01381-1
Alizadeh, S., Djafarian, K., Alizadeh, H., Mohseni, R., and Shab-Bidar, S. (2017). Common variants of vitamin D receptor gene polymorphisms and susceptibility to coronary artery disease: a systematic review and meta-analysis. Lifestyle Genomics 10 (1-2), 9–18. doi:10.1159/000455914
Alizadeh, S., Djafarian, K., Moradi, S., and Shab-Bidar, S. (2016). C667T and A1298C polymorphisms of methylenetetrahydrofolate reductase gene and susceptibility to myocardial infarction: a systematic review and meta-analysis. Int. J. Cardiol. 217, 99–108. doi:10.1016/j.ijcard.2016.04.181
Al-Sadi, R., Guo, S., Ye, D., Rawat, M., and Ma, T. Y. (2016). TNF-α modulation of intestinal tight junction permeability is mediated by NIK/IKK-α axis activation of the canonical NF-κB pathway. Am. J. pathology 186 (5), 1151–1165. doi:10.1016/j.ajpath.2015.12.016
Castaño-Rodríguez, N., Kaakoush, N. O., Goh, K.-L., Fock, K. M., and Mitchell, H. M. (2013). The role of TLR2, TLR4 and CD14 genetic polymorphisms in gastric carcinogenesis: a case-control study and meta-analysis. PloS one 8 (4), e60327. doi:10.1371/journal.pone.0060327
Castaño-Rodríguez, N., Kaakoush, N. O., and Mitchell, H. M. (2014). Pattern-recognition receptors and gastric cancer. Front. Immunol. 5, 336. doi:10.3389/fimmu.2014.00336
Castano-Rodriguez, N., Kaakoush, N. O., Pardo, A. L., Goh, K.-L., Fock, K. M., and Mitchell, H. M. (2014). Genetic polymorphisms in the Toll-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer. Hum. Immunol. 75 (8), 808–815. doi:10.1016/j.humimm.2014.06.001
Chen, J., Hu, S., Liang, S., Chen, Q., Yang, Q., Zheng, W., et al. (2013). Associations between the four toll-like receptor polymorphisms and the risk of gastric cancer: a meta-analysis. Cancer Biotherapy Radiopharm. 28 (9), 674–681. doi:10.1089/cbr.2012.1395
Companioni, O., Bonet, C., Munoz, X., Weiderpass, E., Panico, S., Tumino, R., et al. (2014). Polymorphisms of Helicobacter pylori signaling pathway genes and gastric cancer risk in the European prospective investigation into cancer-eurgast cohort. Int. J. cancer 134 (1), 92–101. doi:10.1002/ijc.28357
Dargiene, G., Streleckiene, G., Skieceviciene, J., Leja, M., Link, A., Wex, T., et al. (2018). TLR1 and PRKAA1 gene polymorphisms in the development of atrophic gastritis and gastric cancer. J. Gastrointest. Liver Dis. 27 (4), 363–369. doi:10.15403/jgld.2014.1121.274.tlr
de Matos Lourenço, C., Susi, M. D., do Nascimento, M. C. A., Junior, V. S., Vila, A. P. S., Rodrigues-Flemming, G. H., et al. (2020). Characterization and strong risk association of TLR2 del-196 to-174 polymorphism and Helicobacter pylori and their influence on mRNA expression in gastric cancer. World J. Gastrointest. Oncol. 12 (5), 535–548. doi:10.4251/wjgo.v12.i5.535
De Oliveira, J. G., Rossi, A. F. T., Nizato, D. M., Miyasaki, K., and Silva, A. E. (2013). Profiles of gene polymorphisms in cytokines and Toll-like receptors with higher risk for gastric cancer. Dig. Dis. Sci. 58, 978–988. doi:10.1007/s10620-012-2460-5
de Oliveira, J. G., and Silva, A. E. (2012). Polymorphisms of the TLR2 and TLR4 genes are associated with risk of gastric cancer in a Brazilian population. World J. gastroenterology WJG 18 (11), 1235–1242. doi:10.3748/wjg.v18.i11.1235
De Re, V., Repetto, O., De Zorzi, M., Casarotto, M., Tedeschi, M., Giuffrida, P., et al. (2019). Polymorphism in toll-like receptors and Helicobacter pylori motility in autoimmune atrophic gastritis and gastric cancer. Cancers 11 (5), 648. doi:10.3390/cancers11050648
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. trials 7 (3), 177–188. doi:10.1016/0197-2456(86)90046-2
Eed, E. M., Hawash, Y. A., Khalifa, A. S., Alsharif, K. F., Alghamdi, S. A., Almalki, A. A., et al. (2020). Association of toll-like receptors 2, 4, 9 and 10 genes polymorphisms and Helicobacter pylori-related gastric diseases in Saudi patients. Indian J. Med. Microbiol. 38 (1), 94–100. doi:10.4103/ijmm.IJMM_20_164
Escoubet-Lozach, L., Benner, C., Kaikkonen, M. U., Lozach, J., Heinz, S., Spann, N. J., et al. (2011). Mechanisms establishing TLR4-responsive activation states of inflammatory response genes. PLoS Genet. 7 (12), e1002401. doi:10.1371/journal.pgen.1002401
Feng, S., Zhang, C., Chen, S., He, R., Chao, G., and Zhang, S. (2023). TLR5 signaling in the regulation of intestinal mucosal immunity. J. Inflamm. Res. 16, 2491–2501. doi:10.2147/JIR.S407521
Gao, F., Qin, J., Wei, X., Tian, X., Dong, W., Dang, T., et al. (2020). Polymorphisms of TLR9 gene are associated with a decreased risk of H. pylori infection in a Chinese population. Transl. cancer Res. 9 (2), 683–689. doi:10.21037/tcr.2019.11.45
Garza-Gonzalez, E., Bosques-Padilla, F. J., Mendoza-Ibarra, S. I., Flores-Gutierrez, J. P., Maldonado-Garza, H. J., and Perez-Perez, G. I. (2007). Assessment of the toll-like receptor 4 Asp299Gly, Thr399Ile and interleukin-8-251 polymorphisms in the risk for the development of distal gastric cancer. BMC cancer 7 (1), 70–75. doi:10.1186/1471-2407-7-70
Gonzalez-Hormazabal, P., Pelaez, D., Musleh, M., Bustamante, M., Stambuk, J., Pisano, R., et al. (2021). NOD1 rs2075820 (p. E266K) polymorphism is associated with gastric cancer among individuals infected with cag PAI-positive H. pylori. Biol. Res. 54, 13–17. doi:10.1186/s40659-021-00336-4
He, B., Xu, T., Pan, B., Pan, Y., Wang, X., Dong, J., et al. (2018a). Polymorphisms of TGFBR1, TLR4 are associated with prognosis of gastric cancer in a Chinese population. Cancer Cell. Int. 18, 1–10. doi:10.1186/s12935-018-0682-0
He, B., Yang, X., Li, Y., Huang, D., Xu, X., Yang, W., et al. (2018b). TLR9 (toll-like receptor 9) agonist suppresses angiogenesis by differentially regulating VEGFA (vascular endothelial growth factor a) and sFLT1 (soluble vascular endothelial growth factor receptor 1) in preeclampsia. Hypertension 71 (4), 671–680. doi:10.1161/HYPERTENSIONAHA.117.10510
He, C., Tu, H., Sun, L., Xu, Q., Gong, Y., Jing, J., et al. (2015). SNP interactions of Helicobacter pylori-related host genes PGC, PTPN11, IL1B, and TLR4 in susceptibility to gastric carcinogenesis. Oncotarget 6 (22), 19017–19026. doi:10.18632/oncotarget.4231
He, C., Tu, H., Sun, L., Xu, Q., Li, P., Gong, Y., et al. (2013). Helicobacter pylori-related host gene polymorphisms associated with susceptibility of gastric carcinogenesis: a two-stage case-control study in Chinese. Carcinogenesis 34 (7), 1450–1457. doi:10.1093/carcin/bgt079
Hishida, A., Matsuo, K., Goto, Y., Mitsuda, Y., Hiraki, A., Naito, M., et al. (2009). Toll-like receptor 4+ 3725 G/C polymorphism, helicobacter pylori seropositivity, and the risk of gastric atrophy and gastric cancer in Japanese. Helicobacter 14 (1), 47–53. doi:10.1111/j.1523-5378.2009.00659.x
Hishida, A., Matsuo, K., Goto, Y., Naito, M., Wakai, K., Tajima, K., et al. (2010). No associations of Toll-like receptor 2 (TLR2)-196 to-174del polymorphism with the risk of Helicobacter pylori seropositivity, gastric atrophy, and gastric cancer in Japanese. Gastric cancer official J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 13, 251–257. doi:10.1007/s10120-010-0567-y
Hold, G. L., Rabkin, C. S., Chow, W. H., Smith, M. G., Gammon, M. D., Risch, H. A., et al. (2007). A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology 132 (3), 905–912. doi:10.1053/j.gastro.2006.12.026
Hold, G. L., Rabkin, C. S., Gammon, M. D., Berry, S. H., Smith, M. G., Lissowska, J., et al. (2009). CD14-159C/T and TLR9-1237T/C polymorphisms are not associated with gastric cancer risk in Caucasian populations. Eur. J. cancer Prev. official J. Eur. Cancer Prev. Organ. (ECP) 18 (2), 117–119. doi:10.1097/CEJ.0b013e3283101292
Hu, Y., Hong, Y., Xu, Y., Liu, P., Guo, D.-H., and Chen, Y. (2014). Inhibition of the JAK/STAT pathway with ruxolitinib overcomes cisplatin resistance in non-small-cell lung cancer NSCLC. Apoptosis 19, 1627–1636. doi:10.1007/s10495-014-1030-z
Huang, H., Wu, J., Jin, G., Zhang, H., Ding, Y., Hua, Z., et al. (2010). A 5′-flanking region polymorphism in toll-like receptor 4 is associated with gastric cancer in a Chinese population. J. Biomed. Res. 24 (2), 100–106. doi:10.1016/S1674-8301(10)60017-6
Huang, J., Hang, J.-J., Qin, X.-R., Huang, J., and Wang, X.-Y. (2019). Interaction of H. pylori with toll-like receptor 2-196 to-174 ins/del polymorphism is associated with gastric cancer susceptibility in southern China. Int. J. Clin. Oncol. 24, 494–500. doi:10.1007/s10147-018-1379-z
Huang, L., Wu, R.-L., and Xu, A.-M. (2015). Epithelial-mesenchymal transition in gastric cancer. Am. J. Transl. Res. 7 (11), 2141–2158.
Huang, L., Yuan, K., Liu, J., Ren, X., Dong, X., Tian, W., et al. (2014). Polymorphisms of the TLR4 gene and risk of gastric cancer. Gene 537 (1), 46–50. doi:10.1016/j.gene.2013.12.030
Janovec, V., Ryabchenko, B., Škarková, A., Pokorná, K., Rösel, D., Brábek, J., et al. (2021). TLR4-Mediated recognition of mouse polyomavirus promotes cancer-associated fibroblast-like phenotype and cell invasiveness. Cancers 13 (9), 2076. doi:10.3390/cancers13092076
Jiang, C., Xu, M., Kuang, X., Xiao, J., Tan, M., Xie, Y., et al. (2017). Treponema pallidum flagellins stimulate MMP-9 and MMP-13 expression via TLR5 and MAPK/NF-κB signaling pathways in human epidermal keratinocytes. Exp. Cell. Res. 361 (1), 46–55. doi:10.1016/j.yexcr.2017.09.040
Jiang, J., Dong, L., Qin, B., Shi, H., Guo, X., and Wang, Y. (2016). Decreased expression of TLR7 in gastric cancer tissues and the effects of TLR7 activation on gastric cancer cells. Oncol. Lett. 12 (1), 631–636. doi:10.3892/ol.2016.4617
Jing, Y.-Y., Han, Z.-P., Sun, K., Zhang, S.-S., Hou, J., Liu, Y., et al. (2012). Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 10 (1), 98–12. doi:10.1186/1741-7015-10-98
Karapetyan, L., Luke, J. J., and Davar, D. (2020). Toll-like receptor 9 agonists in cancer. OncoTargets Ther. 13, 10039–10060. doi:10.2147/OTT.S247050
Kasurinen, A., Hagström, J., Laitinen, A., Kokkola, A., Böckelman, C., and Haglund, C. (2019). Evaluation of toll-like receptors as prognostic biomarkers in gastric cancer: high tissue TLR5 predicts a better outcome. Sci. Rep. 9 (1), 12553. doi:10.1038/s41598-019-49111-2
Kim, J., Cho, Y. A., Choi, I. J., Lee, Y.-S., Kim, S.-Y., Hwang, J.-A., et al. (2013). Effects of polymorphisms of innate immunity genes and environmental factors on the risk of noncardia gastric cancer. Official J. Korean Cancer Assoc. 45 (4), 313–324. doi:10.4143/crt.2013.45.4.313
Kim, S., and Karin, M. (2011). Role of TLR2-dependent inflammation in metastatic progression. Ann. N. Y. Acad. Sci. 1217 (1), 191–206. doi:10.1111/j.1749-6632.2010.05882.x
Kobayashi, H., Enomoto, A., Woods, S. L., Burt, A. D., Takahashi, M., and Worthley, D. L. (2019). Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterology hepatology 16 (5), 282–295. doi:10.1038/s41575-019-0115-0
Korneev, K. V., Atretkhany, K.-S. N., Drutskaya, M. S., Grivennikov, S. I., Kuprash, D. V., and Nedospasov, S. A. (2017). TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 89, 127–135. doi:10.1016/j.cyto.2016.01.021
Kupcinskas, J., Wex, T., Bornschein, J., Selgrad, M., Leja, M., Juozaityte, E., et al. (2011). Lack of association between gene polymorphisms of Angiotensin converting enzyme, Nod-like receptor 1, Toll-like receptor 4, FAS/FASL and the presence of Helicobacter pylori-induced premalignant gastric lesions and gastric cancer in Caucasians. BMC Med. Genet. 12 (1), 112–119. doi:10.1186/1471-2350-12-112
Kutikhin, A. G., Yuzhalin, A. E., Volkov, A. N., Zhivotovskiy, A. S., and Brusina, E. B. (2014). Correlation between genetic polymorphisms within IL-1B and TLR4 genes and cancer risk in a Russian population: a case-control study. Tumor Biol. 35, 4821–4830. doi:10.1007/s13277-014-1633-6
Landskron, G., De la Fuente, M., Thuwajit, P., Thuwajit, C., and Hermoso, M. A. (2014). Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 1–19. doi:10.1155/2014/149185
Li, P., He, C.-Y., Xu, Q., Sun, L.-P., Ha, M.-W., and Yuan, Y. (2014). Effect of the− 2081G/A polymorphism of the TLR4 gene and its interaction with Helicobacter pylori infection on the risk of gastric cancer in Chinese individuals. Genet. Test. Mol. biomarkers 18 (9), 610–615. doi:10.1089/gtmb.2014.0047
Li, Z., Gao, H., Liu, Y., Wu, H., Li, W., Xing, Y., et al. (2021). Genetic variants in the regulation region of TLR4 reduce the gastric cancer susceptibility. Gene 767, 145181. doi:10.1016/j.gene.2020.145181
Liu, S., Wang, X., Shi, Y., Han, L., Zhao, Z., Zhao, C., et al. (2015). Toll-like receptor gene polymorphisms and susceptibility to Epstein–Barr virus-associated and-negative gastric carcinoma in Northern China. Saudi J. gastroenterology official J. Saudi Gastroenterology Assoc. 21 (2), 95–103. doi:10.4103/1319-3767.153832
Mitamura, Y., Ogulur, I., Pat, Y., Rinaldi, A. O., Ardicli, O., Cevhertas, L., et al. (2021). Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermat. 85 (6), 615–626. doi:10.1111/cod.13959
Mohamed, F. E.-Z. A., Hammad, S., Luong, T. V., Dewidar, B., Al-Jehani, R., Davies, N., et al. (2020). Expression of TLR-2 in hepatocellular carcinoma is associated with tumour proliferation, angiogenesis and Caspase-3 expression. Pathology-Research Pract. 216 (8), 152980. doi:10.1016/j.prp.2020.152980
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1), 1–9. doi:10.1186/2046-4053-4-1
Mukherjee, D., Devi, K. R., Deka, M., Malakar, M., Kaur, T., Barua, D., et al. (2016). Association of toll-like receptor 2∆ 22 and risk for gastric cancer considering main effects and interactions with smoking: a matched case-control study from Mizoram, India. Tumor Biol. 37, 10821–10826. doi:10.1007/s13277-016-4982-5
Nazari, M., Khorshidian, A., Alizadeh, S., Falahati, A. M., Haghparast, A., Ghasemifar, S., et al. (2022). Association between peroxisome proliferator activated receptor gamma coactivator 1 gene with overweight and obesity risk: case–control study and meta-analysis. Hum. Gene 34, 201123. doi:10.1016/j.humgen.2022.201123
Paulos, C. M., Kaiser, A., Wrzesinski, C., Hinrichs, C. S., Cassard, L., Boni, A., et al. (2007). Toll-like receptors in tumor immunotherapy. Clin. Cancer Res. 13 (18), 5280–5289. doi:10.1158/1078-0432.CCR-07-1378
Qadri, Q., Rasool, R., Afroze, D., Naqash, S., Gulzar, G., Yousuf, A., et al. (2014). Study of TLR4 and IL-8 gene polymorphisms in H. pylori-induced inflammation in gastric cancer in an ethnic Kashmiri population. Immunol. Investig. 43 (4), 324–336. doi:10.3109/08820139.2013.854378
Rastogi, S., Aldosary, S., Saeedan, A. S., Ansari, M., Singh, M., and Kaithwas, G. (2023). NF-κB mediated regulation of tumor cell proliferation in hypoxic microenvironment. Front. Pharmacol. 14, 1108915. doi:10.3389/fphar.2023.1108915
Ravishankar Ram, M., Goh, K. L., Leow, A. H. R., Poh, B. H., Loke, M. F., Harrison, R., et al. (2015). Polymorphisms at locus 4p14 of Toll-like receptors TLR-1 and TLR-10 confer susceptibility to gastric carcinoma in Helicobacter pylori infection. PloS one 10 (11), e0141865. doi:10.1371/journal.pone.0141865
Rigoli, L., Di Bella, C., Fedele, F., Procopio, V., Amorini, M., Giudice, G. L., et al. (2010). TLR4 and NOD2/CARD15 genetic polymorphisms and their possible role in gastric carcinogenesis. Anticancer Res. 30 (2), 513–517.
Santini, D., Angeletti, S., Ruzzo, A., Dicuonzo, G., Galluzzo, S., Vincenzi, B., et al. (2008). Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms in gastric cancer of intestinal and diffuse histotypes. Clin. Exp. Immunol. 154 (3), 360–364. doi:10.1111/j.1365-2249.2008.03776.x
Schmidt, H. M. A., Ha, D. M., Taylor, E. F., Kovach, Z., Goh, K. L., Fock, K. M., et al. (2011). Variation in human genetic polymorphisms, their association with Helicobacter pylori acquisition and gastric cancer in a multi-ethnic country. J. gastroenterology hepatology 26 (12), 1725–1732. doi:10.1111/j.1440-1746.2011.06799.x
Semlali, A., Almutairi, M., Parine, N. R., Al Amri, A., Shaik, J. P., Al Naeem, A., et al. (2017). No genetic relationship between TLR2 rs4696480, rs3804100, and rs3804099 gene polymorphisms and female breast cancer in Saudi populations. OncoTargets Ther. 10, 2325–2333. doi:10.2147/OTT.S121618
Shi, J., Li, Q., Sheng, M., Zheng, M., Yu, M., and Zhang, L. (2016). The role of TLR4 in M1 macrophage-induced epithelial-mesenchymal transition of peritoneal mesothelial cells. Cell. Physiology Biochem. 40 (6), 1538–1548. doi:10.1159/000453204
Simawaranon, T., Wattanawongdon, W., and Tongtawee, T. (2017). Toll-like receptors are associated with Helicobacter pylori infection and gastric mucosa pathology. Jundishapur J. Microbiol. 10 (12). doi:10.5812/jjm.58351
Sindhava, V. J., Oropallo, M. A., Moody, K., Naradikian, M., Higdon, L. E., Zhou, L., et al. (2017). A TLR9-dependent checkpoint governs B cell responses to DNA-containing antigens. J. Clin. investigation 127 (5), 1651–1663. doi:10.1172/JCI89931
Sohani, Z. N., Meyre, D., de Souza, R. J., Joseph, P. G., Gandhi, M., Dennis, B. B., et al. (2015). Assessing the quality of published genetic association studies in meta-analyses: the quality of genetic studies (Q-Genie) tool. Bmc Genet. 16 (1), 50–58. doi:10.1186/s12863-015-0211-2
Song, E.-J., Kang, M.-J., Kim, Y.-S., Kim, S.-M., Lee, S.-E., Kim, C.-H., et al. (2011). Flagellin promotes the proliferation of gastric cancer cells via the Toll-like receptor 5. Int. J. Mol. Med. 28 (1), 115–119. doi:10.3892/ijmm.2011.656
Stubljar, D., Jeverica, S., Jukic, T., Skvarc, M., Pintar, T., Tepes, B., et al. (2015). The influence of cytokine gene polymorphisms on the risk of developing gastric cancer in patients with Helicobacter pylori infection. Radiology Oncol. 49 (3), 256–264. doi:10.2478/raon-2014-0041
Sultan, A. M., Shenouda, R., Sultan, A. M., Shehta, A., and Nabiel, Y. (2022). The relation between host TLR9-1486t/C, rs187084 gene polymorphisms and Helicobacter pylori cagA, sodB, hsp60, and vacA virulence genes among gastric cancer patients. Pol. J. Microbiol. 71 (1), 35–42. doi:10.33073/pjm-2022-003
Sun, Y., Liu, W.-Z., Liu, T., Feng, X., Yang, N., and Zhou, H.-F. (2015). Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 35 (6), 600–604. doi:10.3109/10799893.2015.1030412
Susi, M. D., Rasmussen, L. T., Payão, S. L. M., Rossi, A. F. T., Silva, A. E., de Oliveira-Cucolo, J. G., et al. (2019). Toll-like receptor 9 polymorphisms and Helicobacter pylori influence gene expression and risk of gastric carcinogenesis in the Brazilian population. World J. Gastrointest. Oncol. 11 (11), 998–1010. doi:10.4251/wjgo.v11.i11.998
Tahara, T., Arisawa, T., Wang, F., Shibata, T., Nakamura, M., Sakata, M., et al. (2007). Toll-like receptor 2–196 to 174del polymorphism influences the susceptibility of Japanese people to gastric cancer. Cancer Sci. 98 (11), 1790–1794. doi:10.1111/j.1349-7006.2007.00590.x
Tallant, T., Deb, A., Kar, N., Lupica, J., De Veer, M. J., and DiDonato, J. A. (2004). Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-κB and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 4 (1), 33–24. doi:10.1186/1471-2180-4-33
Tang, K., McLeod, L., Livis, T., West, A. C., Dawson, R., Yu, L., et al. (2022). Toll-like receptor 9 promotes initiation of gastric tumorigenesis by augmenting inflammation and cellular proliferation. Cell. Mol. gastroenterology hepatology 14 (3), 567–586. doi:10.1016/j.jcmgh.2022.06.002
Tongtawee, T., Simawaranon, T., Wattanawongdon, W., Dechsukhum, C., and Leeanansaksiri, W. (2019). Toll-like receptor 2 and 4 polymorphisms associated with Helicobacter pylori susceptibility and gastric cancer. Turkish J. Gastroenterology 30 (1), 15–20. doi:10.5152/tjg.2018.17461
Trejo-de la, O. A., Torres, J., Pérez-Rodríguez, M., Camorlinga-Ponce, M., Luna, L. F., Abdo-Francis, J. M., et al. (2008). TLR4 single-nucleotide polymorphisms alter mucosal cytokine and chemokine patterns in Mexican patients with Helicobacter pylori-associated gastroduodenal diseases. Clin. Immunol. 129 (2), 333–340. doi:10.1016/j.clim.2008.07.009
Trejo-de la, O. A., Torres, J., Sánchez-Zauco, N., Pérez-Rodríguez, M., Camorlinga-Ponce, M., Flores-Luna, L., et al. (2015). Polymorphisms in TLR9 but not in TLR5 increase the risk for duodenal ulcer and alter cytokine expression in the gastric mucosa. Innate Immun. 21 (7), 706–713. doi:10.1177/1753425915587130
Tsujimura, H., Tamura, T., Kong, H. J., Nishiyama, A., Ishii, K. J., Klinman, D. M., et al. (2004). Toll-like receptor 9 signaling activates NF-kappaB through IFN regulatory factor-8/IFN consensus sequence binding protein in dendritic cells. J. Immunol. 172 (11), 6820–6827. doi:10.4049/jimmunol.172.11.6820
Verstrepen, L., Bekaert, T., Chau, T.-L., Tavernier, J., Chariot, A., and Beyaert, R. (2008). TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell. Mol. life Sci. 65, 2964–2978. doi:10.1007/s00018-008-8064-8
Vivarelli, M. S., McDonald, D., Miller, M., Cusson, N., Kelliher, M., and Geha, R. S. (2004). RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J. Exp. Med. 200 (3), 399–404. doi:10.1084/jem.20040446
Wang, F., Wen, X., Wen, T., and Liu, Z. (2023). Association of TLR4 gene 2026A/G (rs1927914), 896A/G (rs4986790), and 1196C/T (rs4986791) polymorphisms and cancer susceptibility: meta-analysis and trial sequential analysis. Medicine 102 (8), e33040–e. doi:10.1097/MD.0000000000033040
Wang, X., Xu, Z., and Miao, C. (2015b). Pooled analysis of association between a genetic variant in the 3′-untranslated region of toll-like receptor 4 and cancer risk. Genet. Mol. Res. GMR 14 (4), 17847–17855. doi:10.4238/2015.December.22.9
Wang, X., Xue, L., Yang, Y., Xu, L., and Zhang, G. (2013). TLR9 promoter polymorphism is associated with both an increased susceptibility to gastric carcinoma and poor prognosis. PloS one 8 (6), e65731. doi:10.1371/journal.pone.0065731
Wang, X.-D., Gao, N.-N., Diao, Y.-W., Liu, Y., Gao, D., Li, W., et al. (2015a). Conjugation of toll-like receptor-7 agonist to gastric cancer antigen MG7-Ag exerts antitumor effects. World J. Gastroenterology WJG 21 (26), 8052–8060. doi:10.3748/wjg.v21.i26.8052
Wang, Y. W., Zhang, C. H., and He, X. (2019). Minor allele of rs1057317 polymorphism in TLR4 is associated with increased risk of Helicobacter pylori-induced gastric cancer. J. Cell. Biochem. 120 (2), 1969–1978. doi:10.1002/jcb.27493
Wei, M., Liu, S., Liu, S., and Wang, X. (2015). TLR4 gene polymorphisms and susceptibility to gastric carcinoma and Epstein-Barr virus-associated gastric carcinoma in Northern China. Int. J. Sci. 4 (10), 22–30.
Xu, T., Fu, D., Ren, Y., Dai, Y., Lin, J., Tang, L., et al. (2017). Genetic variations of TLR5 gene interacted with Helicobacter pylori infection among carcinogenesis of gastric cancer. Oncotarget 8 (19), 31016–31022. doi:10.18632/oncotarget.16050
Yang, C. A., Scheibenbogen, C., Bauer, S., Kleinle, C., Wex, T., Bornschein, J., et al. (2013). A frequent toll-like receptor 1 gene polymorphism affects NK-and T-cell IFN-γ production and is associated with helicobacter pylori-induced gastric disease. Helicobacter 18 (1), 13–21. doi:10.1111/hel.12001
Zeng, H., Wu, H., Sloane, V., Jones, R., Yu, Y., Lin, P., et al. (2006). Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am. J. Physiology-Gastrointestinal Liver Physiology 290 (1), G96-G108–G108. doi:10.1152/ajpgi.00273.2005
Zeng, H.-M., Pan, K.-F., Zhang, Y., Zhang, L., Ma, J.-L., Zhou, T., et al. (2011a). The correlation between polymorphisms of Toll-like receptor 2 and Toll-like receptor 9 and susceptibility to gastric cancer. Zhonghua yu Fang yi xue za zhi Chin. J. Prev. Med. 45 (7), 588–592.
Zeng, H.-M., Pan, K.-F., Zhang, Y., Zhang, L., Ma, J.-L., Zhou, T., et al. (2011b). Genetic variants of toll-like receptor 2 and 5, helicobacter pylori infection, and risk of gastric cancer and its precursors in a Chinese population. Cancer Epidemiol. biomarkers Prev. 20 (12), 2594–2602. doi:10.1158/1055-9965.EPI-11-0702
Zhao, X., Kang, S., Liu, L., and Zhang, D. (2013). Correlation of Asp299Gly and Thr399Ile polymorphisms in toll-like receptor 4 gene with digestive cancer risk: a meta-analysis. Biomed. Rep. 1 (2), 294–302. doi:10.3892/br.2012.32
Zhou, Q., Wang, C., Wang, X., Wu, X., Zhu, Z., Liu, B., et al. (2014). Association between TLR4 (+896A/G and+ 1196C/T) polymorphisms and gastric cancer risk: an updated meta-analysis. PloS one 9 (10), e109605. doi:10.1371/journal.pone.0109605
Keywords: gastric cancer, toll-like receptors, polymorphisms, meta-analysis, TLR-4
Citation: Al Othaim A, Al-Hawary SIS, Alsaab HO, Almalki SG, Najm MAA, Hjazi A, Alsalamy A, Firras Almulla A and Alizadeh H (2023) Common variants in toll-like receptor family genes and risk of gastric cancer: a systematic review and meta-analysis. Front. Genet. 14:1280051. doi: 10.3389/fgene.2023.1280051
Received: 19 August 2023; Accepted: 07 November 2023;
Published: 28 November 2023.
Edited by:
Qing Lin, Johns Hopkins University, United StatesReviewed by:
Yang Yuan, Johns Hopkins University, United StatesRocio Gomez, Center for Research and Advanced Studies (CINVESTAV), Mexico
Copyright © 2023 Al Othaim, Al-Hawary, Alsaab, Almalki, Najm, Hjazi, Alsalamy, Firras Almulla and Alizadeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamzeh Alizadeh, aGFtYWxpZ3VpbGFuQGdtYWlsLmNvbQ==
 Ayoub Al Othaim1
Ayoub Al Othaim1 Hamzeh Alizadeh
Hamzeh Alizadeh