
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 06 April 2023
Sec. Applied Genetic Epidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fgene.2023.1056186
 Maolin Cao1,2,7†
Maolin Cao1,2,7† Yifei Zhang1,2†
Yifei Zhang1,2† Dan Chen1,2
Dan Chen1,2 Jiaju Zhong3
Jiaju Zhong3 Xiaoli Zhang4
Xiaoli Zhang4 Ling Yang5
Ling Yang5 Xue Li1,2
Xue Li1,2 Liang Fang1,5
Liang Fang1,5 Beizhong Liu1,6
Beizhong Liu1,6 Fang Gong1,2,7*
Fang Gong1,2,7* Chanjuan Zhou1,2,5*
Chanjuan Zhou1,2,5*Background: Dyslipidemia is an independent predictor of ischemic stroke (IS). Genetic variations in lipid-metabolism related genes may increase the risk of IS. Fatty acid-binding protein 1 (FABP1) and fatty acid-binding protein 2 (FABP2) are lipid chaperones responsible for lipid transport and metabolism. The present study aimed to determine the association between FABP1 or FABP2 and ischemic stroke.
Methods: A total of 251 participants were recruited composed of 138 patients with ischemic stroke and 113 healthy subjects. DNA was extracted from peripheral blood samples. The rs2241883 polymorphism in FABP1 and rs1799883 polymorphism in FABP2 were genotyped using polymerase chain reaction-restriction fragment length polymorphism. Generalized multifactor dimensionality reduction (GMDR) was used to find out the interaction combinations between two SNPs and environmental factors.
Results: The GA genotype of FABP2 rs1799883 increased susceptibility to ischemic stroke under overdominant inheritance model (p = 0.042). After adjusting for the risk factors of IS, it was associated with a significantly higher risk of IS in the codominant inheritance model (adjust OR = 3.431, 95%CI = 1.060–11.103, p = 0.04). The interactions of FABP1 rs2241883 and FABP2 rs1799883 were not associated with IS risk (p = 0.172). Moreover, interaction analysis of two genes (rs1799883 and rs2241883) and two environmental factors (smoking and alcohol consumption) was associated with an increased risk of IS (p = 0.011).
Conclusion: The GA genotype of FABP2 rs1799883, interactions between rs1799883, rs2241883 and smoking and alcohol consumption were associated with IS risk in Chinese Han populations.
Stroke is a leading cause of mortality and long-term disability, especially in low- and middle-income countries (Ma et al., 2021). The economic burden of stroke remains severe (GBD, 2016 Stroke Collaborators, 2019; Ma et al., 2021). Strokes are clinically categorized as ischemic stroke (IS), intracerebral hemorrhage, or subarachnoid hemorrhage. Among these, IS accounts for 60%–90% of cases (Liu et al., 2020; Kleindorfer et al., 2021). IS a complex disease that involves multiple genetic and environmental risk factors (Wang et al., 2018; Dichgans et al., 2019). Lipids are the key triggers of atherosclerosis, and dysfunction of lipid metabolism and its related processes play a vital role in the process of IS development (Chow et al., 2020). Several studies reported that lipid-related genes, such as EH domain-binding protein 1 and apolipoprotein E, are associated with an increased risk of IS (Liu et al., 2022; Qiao et al., 2022). Their genetic variation can cause lipid and lipid mediator dysregulation, which contribute to IS pathophysiology and possible mechanisms (Kloska et al., 2020). Hence, it is important to identify susceptibility genes in patients with IS for early screening and individualized treatment.
Fatty acid-binding proteins (FABPs), as lipid chaperones, are small intracellular proteins mediators that absorb, store, and export lipids (Hotamisligil and Bernlohr, 2015). Recently, drugs or antibodies targeting FABPs have provided new insights for managing chronic metabolic diseases (Hotamisligil and Bernlohr, 2015; Huang et al., 2022). Baier et al. first described a common nucleotide transition from guanine (G) to adenine (A) at codon 54 in exon 2 of FABP2 (Ala54Thr, rs1799883) (Baier et al., 1995), which was shown to increase fat oxidation and insulin resistance (Baier et al., 1995; Albala et al., 2004; Albala et al., 2007). Furthermore, Brouillette et al. reported the Thr94Ala of FABP1 (rs2241883), which could influence obesity indices as well as the risk of exhibiting residual hypertriglyceridemia (Brouillette et al., 2004). As a result, both FABP1 and FABP2 are associated with diseases accompanied by dysregulated lipid levels, such as metabolic syndrome, non-alcoholic fatty liver disease, and type 2 diabetes (Liu et al., 2015; Zare-Feyzabadi et al., 2022). However, the association between these genes and the risk of IS in the Chinese Han population remains unclear.
In this study, we validated the FABP1 and FABP2 polymorphisms in IS patients. We aimed to investigate two SNPs (rs1799883 and rs2241883) in the Han population in southwest China. We analyzed the associations between two SNPs(rs2241883 and rs1799883) and the risk of IS, gene-gene, and gene-environment interaction analyse.
This study retrospectively included 138 patients with IS and 113 healthy controls (HCs) between October 2019 and June 2022 at Yongchuan Hospital of Chongqing Medical University. All participants were of Chinese Han descent and lived in southwest China. This study was approved by Yongchuan Hospital of Chongqing Medical University Research Ethics Committee, China. We followed the ethical guidelines of the Declaration of Helsinki and obtained written informed consent from all participants in our study.
The inclusion criteria for IS comprised age >18 years, symptoms of new-onset neurological deficits diagnostic confirmation by neurological examination and brain computed tomography (CT) or magnetic resonance imaging (MRI) according to the guidelines (Powers et al., 2019). The exclusion criteria comprised intracerebral or post-infarction hemorrhage, a history of liver, kidney, or autoimmune diseases, pregnant or breastfeeding women, or incomplete clinical data. Healthy persons were recruited from the Physical Examination Center of Yongchuan Hospital of Chongqing Medical University according to the following inclusion criteria: age >40 years, normal blood lipid and blood glucose values and liver and kidney functions.
General information, including sociodemographic data, lifestyle characteristics, medical history, and laboratory data of all participants, was collected through face-to-face interviews or extracted from their medical records. Smoking was defined as at least one cigarette per day for half a year before admission, and alcohol consumption was defined as drinking any type of alcoholic beverage at least once a week for more than 6 months before admission. Serum total cholesterol (TC), hypertriglyceridemia (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein (Apo) A1, Apo B, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), direct bilirubin (DBIL), albumin (ALB), total protein (TB), fasting blood glucose, creatinine, white blood cell (WBC), red blood cell (RBC), hemoglobin (Hb), and platelets (PLT) levels were measured using standard laboratory methods.
Blood was obtained from all participants using an anticoagulant tube containing EDTA and quickly frozen at −80°C until use. Genomic DNA was extracted from the blood samples using TIANamp Blood DNA Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocol. The DNA samples were quantified using a NanoDrop-2000 instrument (Thermo Fisher Scientific, USA). All samples were genotyped in duplicate.
DNA nucleic acid PCR amplification was performed using a TaKaRa PCR Amplification Kit (Takara Bio, Japan) according to the manufacturer’s protocol. Briefly, DNA samples (0.5 μg) were amplified in a 50 μL volume consisting of 5 μL 10x PCR Buffer (1x), 4 μL dNTP mix (200 μM), 0.25 μL Taq enzyme (1.25 U),0.5 μL forward primer (20 μM), 0.5 μL reverse primer (20 μM) and 39.75 μL nuclease-free water. The primers for FABP1 were: forward primer, 5′-CAGTTGGAAGGTGACAATAAACTGTGACA-3′; reverse primer, 5′-GAGGGGTGGCATTAGGGTATGTGAG-3′. The primers of FABP2 were: forward primer, 5′-ACAGGTGTTAATATAGTGAAAAG-3′; reverse primer, 5′-TACCCTGAGTTCAGTTCCGTC-3′. The PCR products obtained were analyzed on a 2% agarose gel stained with 4S Green Nucleic Acid Stain (Sangon Biotech, Shanghai, China) to certify amplification.
The PCR products of FABP1 were digested by Hind III endonuclease (Takara Bio, Japan), and PCR products of FABP2 were digested by Hha I endonuclease (Takara Bio, Japan) according to the manufacturer’s protocol. Digestion products were electrophoresed on a 4% agarose gel and treated with 4S Green Nucleic Acid Stain. The bands were detected using the ChemiDoc XRS + System (Bio-Rad, USA). We detected the following FABP2 genotypes: Ala/Ala genotype with 2 fragments at 99 and 81bp; Ala/Thr genotype with three fragments at 180, 99, and 81bp; and Thr/Thr genotype with one 180bp fragment (Figure 1). Moreover, the FABP1 Thr/Thr genotype corresponded to 182bp; Thr/Ala genotype with three fragments at 182, 153, and 29bp, whereas Ala/Ala genotype was characterized by and two fragments of 153 and 29bp (Figure 2).
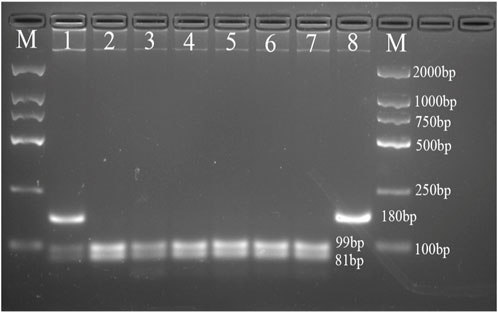
FIGURE 1. Agarose gel picture showing digested PCR products for FABP2 gene polymorphism. M, marker; 1 shows Ala/Thr genotype; 2, 3, 4, 5, 6, and 7 show Ala/Ala genotype; 8 shows Thr/Thr genotype.
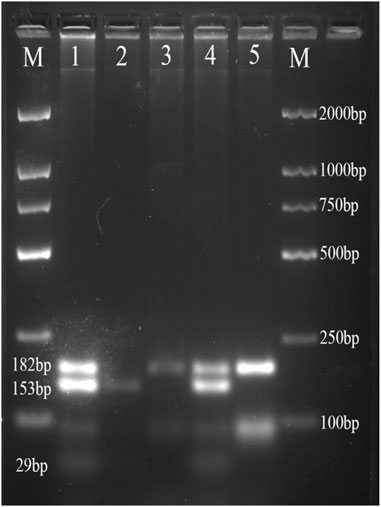
FIGURE 2. Agarose gel picture showing digested PCR products for FABP1 gene polymorphism. M, marker; 1 and 4 show Thr/Ala genotype; 2 shows Ala/Ala genotype; 3 and 5 shows Thr/Thr genotype.
Data were analyzed using the Kolmogorov Smirnov test to determine their distributions. Normally distributed variables are presented as mean standard deviation (SD) and compared using the Student’s t-test. Non-normally distributed variables are expressed as median (interquartile range), and differences between groups were calculated using the Mann-Whitney U test. Categorical variables are presented as numbers (percentages) and were analyzed using the χ2 test or Fisher’s exact test. The Hardy Weinberg equilibrium (HWE) test of genotype distribution was performed using the χ2 test for goodness of fit. Logistic regression analysis was performed to determine the association between genetic polymorphisms and the risk of stroke and other risk factors. Gene-gene and gene-environmental interactions were analyzed using generalized multifactor dimensionality reduction (GMDR). Statistical significance was set at p < 0.05. Statistical analyses were performed using the SPSS version 25.0 software (IBM SPSS Inc., USA) and GMDR software version 09 (http://sourceforge.net/projects/gmdr/). The sample size was caculated by Quanto 1.2.4 software (https://quanto.software.informer.com/download/#downloading) with the following parameters: the prevalence of ischemic stroke was 1,255.9 per 100,000 population (Wang et al., 2022), the allele frequency of the FABP1 genes and the FABP2 genes in the East Asian was 0.2136 and 0.685 in the National Center of Biotechnology Information (NCBI) dbSNP database, α was 0.05, 1-β was 0.8, and expected Odd Rate value was 2–4 according to previous studies (Yamada et al., 2008; Mansego et al., 2012; Peng et al., 2012; Garcés Da Silva et al., 2018). The predicted results showed that 35 to 133 and 161 to 484 samples were needed for the FABP1 and FABP2 gene, respectively.
The baseline characteristics of the 251 participants are presented in Table 1. There were 138 people in the IS group and 113 people in the HCs group. The mean age of all participants was 63.6 ± 13.7 years, and 53% were male. Patients with IS had a higher prevalence of hypertension, diabetes, dyslipidemia, coronary artery disease, atrial fibrillation, smoking, and alcohol consumption (p < 0.05). Compared to the HCs, the patients with IS were older, and had higher blood pressure, AST, ALT, DBIL, fasting blood glucose, and WBC (p < 0.05). However, the level of ALB, creatinine, RBC, and Hb were lower than those in HCs. There were no significant differences in the levels of TBIL, TB, or PLT between two groups (p > 0.05). For the blood lipid testing, the plasma levels of Apo B were higher in patients with IS (p < 0.05), while the levels of TC, HDL-C, and LDL-C were lower in patients with IS (p < 0.05). No difference of TG level was found (p > 0.05).
The genotype frequency distributions of SNPs in HWE and mutation frequencies of the alleles are shown in Table 2.
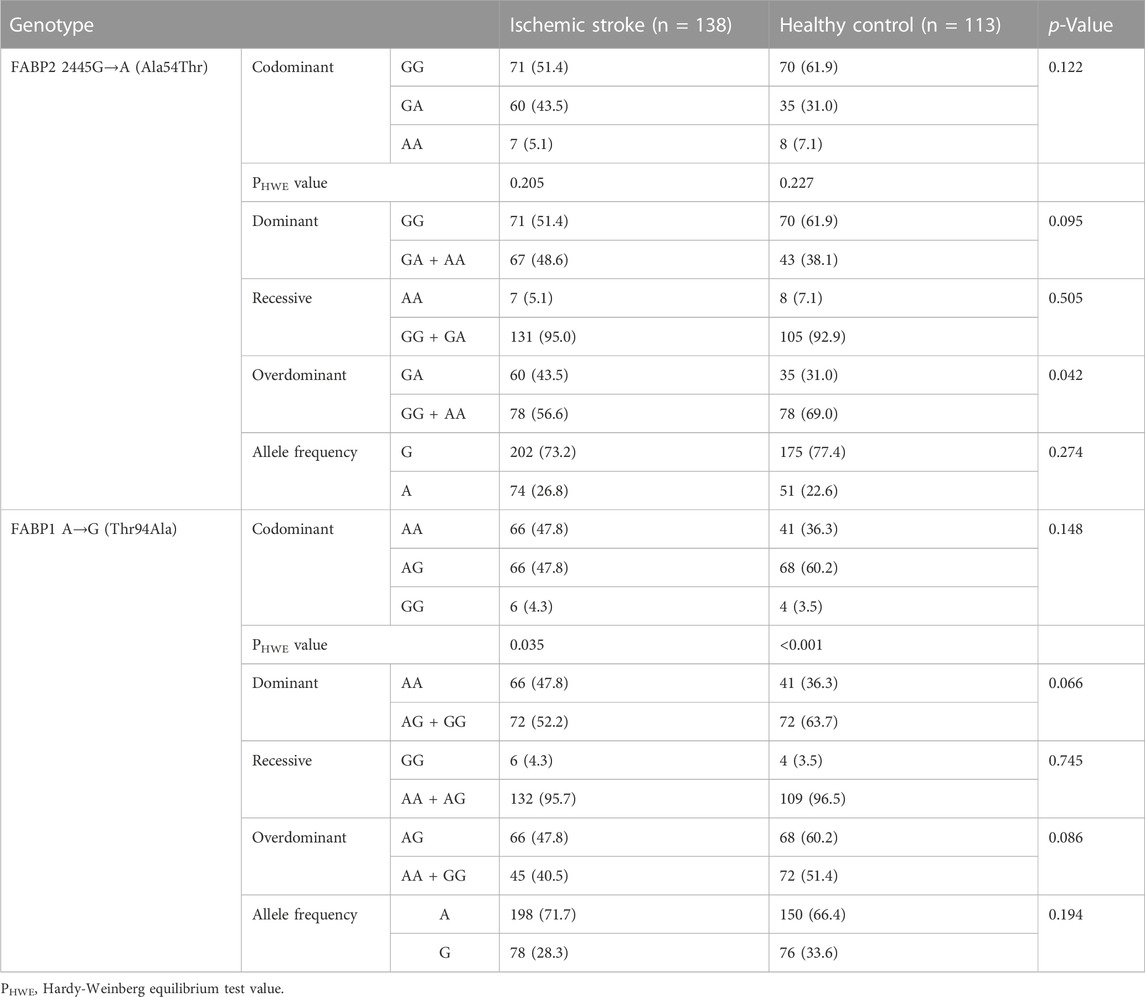
TABLE 2. Genotype frequency and Hardy-Weinberg equilibrium distribution of SNPs and mutation frequency of alleles.
FABP2 re1799883: In the IS group, FABP2 genotype frequencies were GG (51.4%), GA (43.5%), and AA (5.1%), and those in HCs were 61.9%, 31%, and 7.1%, respectively. In the overdominant inheritance model (GG + AA vs GA), the GA frequency of FABP2 rs1799883 in IS patients was significantly higher than that in HCs (p = 0.042). No significant difference has not been reached for codominant, dominant, or recessive inheritance model.
FABP1 rs2241883: FABP1 genotype frequencies were AA (47.8%), AG (47.8%), and GG (4.3%), and those in HCs were 36.3%, 60.2%, and 3.5%, respectively. The rs2241883 polymorphism did not differ between IS patients and HCs under all genotype models (p > 0.05).
Logistic regression analysis was performed to screen the risk for associations between candidate SNPs and IS risk (Table 3). Neither FABP2 rs1799883 nor FABP1 rs2241883 of the FABP1 in four different inheritance models had significant effect on the risk of IS (p > 0.05). After using multivariable logistic recessive adjusted risk factors for IS, we found that patients with the FABP2 rs1799883 GA genotype had a significantly higher risk of stroke in the codominant inheritance model (adjusted odds ratio (OR):3.431; 95%Confidence interval (CI):1.060–11.103; p = 0.04).
To evaluate the association between genotype polymorphisms and risk factors for IS, we found that patients with ischemic stroke who carried GG in FABP2 had a higher prevalence of atrial fibrillation (p = 0.032), and those patients who carried AG in FABP1 were older than those with other genotypes (p = 0.048). There were no significant statistical differences in sex, blood pressure, hypertension, diabetes, dyslipidemia, coronary artery disease, stroke, and blood lipid levels in both FABP2 (Table 4) and FABP1 (Table 5) genotypes.
Lastly, gene-gene and gene-environment interactions were analyzed to further explore genotype polymorphisms and environmental impact in the risk of IS. As shown in Table 6 and Figure 3, the rs1799883-rs2241883 interaction was not associated with IS risk. In the gene-environment interaction analysis, rs1799883, rs2241883, and smoking and alcohol consumption were correlated with an increased risk of IS (p = 0.011), and the model had good cross-validation consistency and training balance accuracy (Table 6). The optimal interaction model of gene-gene and gene-environment interactions is shown in Figure 3 and Figure 4.

FIGURE 4. The optimal interaction model of genes and environmental factors. (A) Interaction between two genes and smoking. (B) interaction between two genes and smoking and alcohol consumption.
Lipids play a fundamental role in the pathogenesis atherosclerosis, which can contribute to disease development and mortality, including IS. As lipid chaperones, FABPs mediate both lipid-related biological processes and systemic metabolic homeostasis by regulating multiple lipid signals (Furuhashi and Hotamisligil, 2008). Therefore, understanding the relationship between FABPs and ischemic stroke is important. Here we evaluated the associations between alleles of FABP1 and FABP2 genes and ischemic stroke risk in 251 Chinese Han patients.
We uncovered significantly different genotype frequencies of FABP2 in the overdominant inheritance model compared to those in HCs. The Ala54Thr polymorphism of FABP2 is associated with metabolic or cardiovascular diseases (Wanby et al., 2005; Abbas et al., 2015; Liu et al., 2015). Similar to Panby’s work (Wanby et al., 2007), we found that patients carrying FABP2 rs1799883 GA genotype might have increased IS risk. This indicated that the Ala54 site may be a risk predictor for IS in Han population. The FABP2 rs1799883 is a common SNP at codon 54 in exon 2(Ala54Thr) located at Chr4q28-q31 (Furuhashi and Hotamisligil, 2008), which could lead to changed gene expression and biofunctions of FABP2(Formanack and Baier, 2004). Compared to the alanine (Ala)-containing protein in FABP2, the threonine (Thr)-containing protein has a twofold higher binding affinity for long-chain fatty acids in human (Baier et al., 1995). An vitro study also showed that Thr54 mutation enabled FABP2 to transport more long-chain fatty acids, resulting in enhanced triglycerides secrete (Baier et al., 1996). Besides, the Ala54Thr polymorphism of FABP2 could increase the small intestinal lipid absorption (Levy et al., 2001). Increasing evidence has observed the changes in blood fatty acid metabolisms in patients with acute IS (Au, 2018; Wang et al., 2020). Therefore, one possible explanation of our findings is that people carrying rs1799883 GA genotype may have dysregulated fatty acids metabolisms, which contribute to the high risk of IS. As we did not measure the fatty acid levels in patients with ischemic stroke, the relationship between fatty acid levels and the FABP2 Ala54Thr genotype requires further validation. Notably, the present findings of the relationship between Ala54Thr polymorphism and IS differ from those of an earlier study (Yamada et al., 2008). Ischemic stroke is a complex disease caused by various genetic and environmental factors. Different diets, geographical environments, and genetic backgrounds can lead to altered susceptibilities.
The Thr94Ala of FABP1 is a polymorphism at condon 94 in exon 3 at Chr2p12-q11. One study reported that FABP1 Thr94Ala variant affected TG accumulation in human hepatocytes (McIntosh et al., 2014). Although FABP1 Thr94Ala polymorphisms are also likely related to influence plasma lipid levels (Fisher et al., 2007; Tian et al., 2015; Valizadeh et al., 2021), we found that blood lipid levels were not related to stroke risk under all genotype models. We did not find an association between FABP1 Thr94Ala and IS either, suggesting that the Thr94Ala polymorphism of FABP1 may not be an independent risk factor for IS in this Han population. The Thr94Ala polymorphism in FABP1 is a susceptibility site for atherothrombotic cerebral infarction among Japanese patients with metabolic syndrome (Yamada et al., 2008). However, we included all types of IS rather than IS patients who were diagnosed with metabolic syndrome before, pervious study might overestimation risk of stroke. In addition, we found that genotype frequencies of FABP1 deviate from HWE in HCs. Since selection, mutations, linkage disequilibrium, population stratification and inbreeding could cause deviations from HWE in a population (Chen et al., 2009), it would be difficult for us to find out the potential origins of the FABP1 deviation in the included populations. Lager cohort study may need to be further validated.
We assessed the impact of FABP1-FABP2, and gene-environmental interaction on IS risk. Genetic and environmental factors, as well as gene-gene and gene-environment interactions, are related to the incidence of IS (Ferreira et al., 2019; Zhang et al., 2019). In our study, FABP1-FABP2 interactions did not contribute to IS risk, indicating that FABP1 and FABP2 interaction cannot adequately explain interindividual variability in patients with IS. Notably, the interaction between two FABPs (rs2241883 in FABP1 and rs1799883 in FABP2) and two environmental factors (smoking and alcohol consumption) significantly increased the risk of IS. Smoking and alcohol consumption have previously been shown to increase the risk of IS (Ma et al., 2021; Roerecke, 2021). Thus, patients carrying FABP1 rs2241883 or FABP2 rs17799883 should reduce smoking and alcohol consumption to reduce the risk of IS. In addition, our study also indicated that FABP1 and FABP2 polymorphisms can be potential early biomarkers for IS risk, providing crucial information for identifying its underlying mechanisms.
Several limitations should be addressed in our study. Firstly, although the sample was small. We observed the associations between FABP2 rs1799883 polymorphisms and ischemic stroke. Nonetheless studies with larger sample sizes form different ethnicities are helpful to confirm our findings. Secondly, numerous environmental factors are associated with ischemic stroke. Yet, owing to limited clinical data, only the two most important environmental risk factors were analyzed. Thirdly, although we discovered potential interactions between genes and environmental conditions for IS, the connecting mechanisms between stroke risk, smoking, and alcohol consumption remain unclear. Finally, etither FABP1 or FABP2 protein involves in the absorption and transportation of lipids. As a result, further studies are needed to identify the impacts of FABPs’ gene polymorphisms on lipid-related metabolites. This will facilitate a better understanding of the relationship among FABP polymorphisms, lipid metabolism, and the IS risk.
This study examined the associations of FABP1 and FABP2, gene-gene, and gene-environment, with IS in a Han population in Southwest China. Rs2241883 in FABP1 and rs1799883 in FABP2 were not associated with IS. After adjusting for risk factors, the GA genotype of FABP2 might increase the risk of IS in the codominant inheritance model. Our results indicate that gene-environment interactions may increase IS risk.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Yongchuan Hospital of Chongqing Medical University Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MC, YZ, and DC conducted experiments. YZ performed the statistical analysis of the data. MC drafted the manuscript. MC, YZ, DC, JZ, XZ, LY, and XL collected blood samples and clinical data. Juan Liao, LF, and BL provided statistical and experimental methodology assistance. CZ reviewed and edited the paper. FG and CZ designed the experiments, funding acquisition, project administration, resources, and supervised the study. All authors have read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Grant No. 81701361); the Key Project of the Natural Science Foundation of Yongchuan District, Chongqing, China (Grant No.2022yc-jckx20007, 2022yc-jckx20009); the Science Foundation funded project, Yongchuan Hospital of Chongqing Medical University (Grant No. YJRC202102, YJSJ202136).
The authors thank all the participants in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, S., Raza, S. T., Chandra, A., Rizvi, S., Ahmed, F., Eba, A., et al. (2015). Association of ACE, FABP2 and GST genes polymorphism with essential hypertension risk among a North Indian population. Ann. Hum. Biol. 42 (5), 461–469. doi:10.3109/03014460.2014.968206
Albala, C., Santos, J. L., Cifuentes, M., Villarroel, A. C., Lera, L., Liberman, C., et al. (2004). Intestinal FABP2 A54T polymorphism: Association with insulin resistance and obesity in women. Obes. Res. 12 (2), 340–345. doi:10.1038/oby.2004.42
Albala, C., Villarroel, A., Santos, J. L., Angel, B., Lera, L., Liberman, C., et al. (2007). FABP2 Ala54Thr polymorphism and diabetes in Chilean elders. Diabetes Res. Clin. Pract. 77 (2), 245–250. doi:10.1016/j.diabres.2006.12.006
Au, A. (2018). Metabolomics and lipidomics of ischemic stroke. Adv. Clin. Chem. 85, 31–69. doi:10.1016/bs.acc.2018.02.002
Baier, L. J., Sacchettini, J. C., Knowler, W. C., Eads, J., Paolisso, G., Tataranni, P. A., et al. (1995). An amino acid substitution in the human intestinal fatty acid binding protein is associated with increased fatty acid binding, increased fat oxidation, and insulin resistance. J. Clin. Invest. 95 (3), 1281–1287. doi:10.1172/jci117778
Baier, L. J., Bogardus, C., and Sacchettini, J. C. (1996). A polymorphism in the human intestinal fatty acid binding protein alters fatty acid transport across Caco-2 cells. J. Biol. Chem. 271 (18), 10892–10896. doi:10.1074/jbc.271.18.10892
Brouillette, C., Bossé, Y., Pérusse, L., Gaudet, D., and Vohl, M. C. (2004). Effect of liver fatty acid binding protein (FABP) T94A missense mutation on plasma lipoprotein responsiveness to treatment with fenofibrate. J. Hum. Genet. 49 (8), 424–432. doi:10.1007/s10038-004-0171-2
Chen, A. Q., Zang, J., Feng, Y. X., and Wang, K. J. (2009). [Influential factors of Hardy-Weinberg equilibrium on the study of association between gene polymorphism and disease]. Zhonghua Liu Xing Bing Xue Za Zhi 30 (11), 1203–1206. doi:10.3760/cma.j.issn.0254-6450.2009.11.027
Chow, Y. L., Teh, L. K., Chyi, L. H., Lim, L. F., Yee, C. C., and Wei, L. K. (2020). Lipid metabolism genes in stroke pathogenesis: The atherosclerosis. Curr. Pharm. Des. 26 (34), 4261–4271. doi:10.2174/1381612826666200614180958
Dichgans, M., Pulit, S. L., and Rosand, J. (2019). Stroke genetics: Discovery, biology, and clinical applications. Lancet Neurol. 18 (6), 587–599. doi:10.1016/s1474-4422(19)30043-2
Ferreira, L. E., Secolin, R., Lopes-Cendes, I., Cabral, N. L., and França, P. H. C. (2019). Association and interaction of genetic variants with occurrence of ischemic stroke among Brazilian patients. Gene 695, 84–91. doi:10.1016/j.gene.2019.01.041
Fisher, E., Weikert, C., Klapper, M., Lindner, I., Möhlig, M., Spranger, J., et al. (2007). L-FABP T94A is associated with fasting triglycerides and LDL-cholesterol in women. Mol. Genet. Metab. 91 (3), 278–284. doi:10.1016/j.ymgme.2007.03.002
Formanack, M. L., and Baier, L. J. (2004). Variation in the FABP2 promoter affects gene expression: Implications for prior association studies. Diabetologia 47 (2), 349–351. doi:10.1007/s00125-003-1289-z
Furuhashi, M., and Hotamisligil, G. S. (2008). Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 7 (6), 489–503. doi:10.1038/nrd2589
Garcés Da Silva, M. F., Guarin, Y. A., Carrero, Y., Stekman, H., Núñez Bello, M. L., Hernández, C., et al. (2018). Postprandial hypertriglyceridemia is associated with the variant 54 threonine FABP2 gene. J. Cardiovasc Dev. Dis. 5 (3), 47. doi:10.3390/jcdd5030047
GBD 2016 Stroke Collaborators (2019). Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 18 (5), 439–458. doi:10.1016/s1474-4422(19)30034-1
Hotamisligil, G. S., and Bernlohr, D. A. (2015). Metabolic functions of FABPs-mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 11 (10), 592–605. doi:10.1038/nrendo.2015.122
Huang, X., Zhou, Y., Sun, Y., and Wang, Q. (2022). Intestinal fatty acid binding protein: A rising therapeutic target in lipid metabolism. Prog. Lipid Res. 87, 101178. doi:10.1016/j.plipres.2022.101178
Kleindorfer, D. O., Towfighi, A., Chaturvedi, S., Cockroft, K. M., Gutierrez, J., Lombardi-Hill, D., et al. (2021). 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American heart association/American stroke association. Stroke 52 (7), e364–e467. doi:10.1161/str.0000000000000375
Kloska, A., Malinowska, M., Gabig-Cimińska, M., and Jakóbkiewicz-Banecka, J. (2020). Lipids and lipid mediators associated with the risk and pathology of ischemic stroke. Int. J. Mol. Sci. 21 (10), 3618. doi:10.3390/ijms21103618
Levy, E., Ménard, D., Delvin, E., Stan, S., Mitchell, G., Lambert, M., et al. (2001). The polymorphism at codon 54 of the FABP2 gene increases fat absorption in human intestinal explants. J. Biol. Chem. 276 (43), 39679–39684. doi:10.1074/jbc.M105713200
Liu, Y., Wu, G., Han, L., Zhao, K., Qu, Y., Xu, A., et al. (2015). Association of the FABP2 Ala54Thr polymorphism with type 2 diabetes, obesity, and metabolic syndrome: A population-based case-control study and a systematic meta-analysis. Genet. Mol. Res. 14 (1), 1155–1168. doi:10.4238/2015.February.6.19
Liu, L., Chen, W., Zhou, H., Duan, W., Li, S., Huo, X., et al. (2020). Chinese stroke association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc. Neurol. 5 (2), 159–176. doi:10.1136/svn-2020-000378
Liu, C. X., Yin, R. X., Cao, X. L., Shi, Z. H., Huang, F., Wei, B. L., et al. (2022). EHBP1, TUBB, and WWOX SNPs, gene-gene and gene-environment interactions on coronary artery disease and ischemic stroke. Front. Genet. 13, 843661. doi:10.3389/fgene.2022.843661
Ma, Q., Li, R., Wang, L., Yin, P., Wang, Y., Yan, C., et al. (2021). Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: An analysis for the global burden of disease study 2019. Lancet Public Health 6 (12), e897–e906. doi:10.1016/s2468-2667(21)00228-0
Mansego, M. L., Martínez, F., Martínez-Larrad, M. T., Zabena, C., Rojo, G., Morcillo, S., et al. (2012). Common variants of the liver fatty acid binding protein gene influence the risk of type 2 diabetes and insulin resistance in Spanish population. PLoS One 7 (3), e31853. doi:10.1371/journal.pone.0031853
McIntosh, A. L., Huang, H., Storey, S. M., Landrock, K. K., Landrock, D., Petrescu, A. D., et al. (2014). Human FABP1 T94A variant impacts fatty acid metabolism and PPAR-α activation in cultured human female hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 307 (2), G164–G176. doi:10.1152/ajpgi.00369.2013
Peng, X. E., Wu, Y. L., Lu, Q. Q., Hu, Z. J., and Lin, X. (2012). Two genetic variants in FABP1 and susceptibility to non-alcohol fatty liver disease in a Chinese population. Gene 500 (1), 54–58. doi:10.1016/j.gene.2012.03.050
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 50 (12), e344–e418. doi:10.1161/str.0000000000000211
Qiao, S. Y., Shang, K., Chu, Y. H., Yu, H. H., Chen, X., Qin, C., et al. (2022). Apolipoprotein E ε4 polymorphism as a risk factor for ischemic stroke: A systematic review and meta-analysis. Dis. Markers 2022, 1407183. doi:10.1155/2022/1407183
Roerecke, M. (2021). Alcohol's impact on the cardiovascular System. Nutrients 13 (10), 3419. doi:10.3390/nu13103419
Tian, Y., Li, H., Wang, S., Yan, J., Chen, Z., Li, Z., et al. (2015). Association of L-FABP T94A and MTP I128T polymorphisms with hyperlipidemia in Chinese subjects. Lipids 50 (3), 275–282. doi:10.1007/s11745-015-3990-3
Valizadeh, M., Aghasizadeh, M., Nemati, M., Hashemi, M., Aghaee-Bakhtiari, S. H., Zare-Feyzabadi, R., et al. (2021). The association between a Fatty Acid Binding Protein 1 (FABP1) gene polymorphism and serum lipid abnormalities in the MASHAD cohort study. Prostagl. Leukot. Essent. Fat. Acids 172, 102324. doi:10.1016/j.plefa.2021.102324
Wanby, P., Palmquist, P., Brudin, L., and Carlsson, M. (2005). Genetic variation of the intestinal fatty acid-binding protein 2 gene in carotid atherosclerosis. Vasc. Med. 10 (2), 103–108. doi:10.1191/1358863x05vm609oa
Wanby, P., Nilsson, I., Brudin, L., Nyhammar, I., Gustafsson, I., and Carlsson, M. (2007). Increased plasma levels of asymmetric dimethylarginine in patients with carotid stenosis: No evidence for the role of the common FABP2 A54T gene polymorphism. Acta Neurol. Scand. 115 (2), 90–96. doi:10.1111/j.1600-0404.2006.00764.x
Wang, Y., Liu, J., Wu, H., and Cai, Y. (2018). Combined biomarkers composed of environment and genetic factors in stroke. Biosci. Trends 12 (4), 360–368. doi:10.5582/bst.2018.01150
Wang, X., Zhang, L., Sun, W., Pei, L. L., Tian, M., Liang, J., et al. (2020). Changes of metabolites in acute ischemic stroke and its subtypes. Front. Neurosci. 14, 580929. doi:10.3389/fnins.2020.580929
Wang, Y. J., Li, Z. X., Gu, H. Q., Zhai, Y., Zhou, Q., Jiang, Y., et al. (2022). China stroke statistics: An update on the 2019 report from the national center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, national center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and Institute for global neuroscience and stroke collaborations. Stroke Vasc. Neurol. 7 (5), 415–450. doi:10.1136/svn-2021-001374
Yamada, Y., Kato, K., Oguri, M., Yoshida, T., Yokoi, K., Watanabe, S., et al. (2008). Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int. J. Mol. Med. 21 (6), 801–808. doi:10.3892/ijmm.21.6.801
Zare-Feyzabadi, R., Mozaffari, M., Ghayour-Mobarhan, M., and Valizadeh, M. (2022). FABP1 gene variant is associated with risk of metabolic syndrome. Comb. Chem. High. Throughput Screen 25 (8), 1355–1360. doi:10.2174/1386207324666210603114434
Keywords: fatty acid-binding protein 1 (FABP1), fatty acid-binding protein 2 (FABP2), single nucleotide polymorphism (SNPs), ischemic stroke (IS), susceptibility
Citation: Cao M, Zhang Y, Chen D, Zhong J, Zhang X, Yang L, Li X, Fang L, Liu B, Gong F and Zhou C (2023) Polymorphism in genes encoding two fatty acid binding proteins increases risk of ischemic stroke in a Chinese Han population. Front. Genet. 14:1056186. doi: 10.3389/fgene.2023.1056186
Received: 12 October 2022; Accepted: 28 March 2023;
Published: 06 April 2023.
Edited by:
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), MexicoReviewed by:
Ali H. Ad’hiah, University of Baghdad, IraqCopyright © 2023 Cao, Zhang, Chen, Zhong, Zhang, Yang, Li, Fang, Liu, Gong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Gong, gflinda@163.com; Chanjuan Zhou, chanjuanzhou@hospital.cqmu.edu.cn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.