
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Genet. , 15 April 2020
Sec. Genomic Medicine
Volume 11 - 2020 | https://doi.org/10.3389/fgene.2020.00234
This article is part of the Research Topic Advances in Genomic and Genetic Tools, and Their Applications for Understanding Embryonic Development and Human Diseases View all 33 articles
 Guangyuan Chen1†
Guangyuan Chen1† Cong Hu1†
Cong Hu1† Yuxuan Song2†
Yuxuan Song2† Mengxi Xiu1
Mengxi Xiu1 Wanfeng Liang3
Wanfeng Liang3 Ningjing Ou2
Ningjing Ou2 Xiaoqiang Liu2
Xiaoqiang Liu2 Peng Huang4,5*
Peng Huang4,5*The relationship between urolithiasis and vitamin D receptor (VDR) gene variants is still under debate according to the available published literature. To assess correlations between VDR gene variants ApaI (rs7975232), BsmI (rs1544410), FokI (rs2228570), and TaqI (rs731236) and urolithiasis susceptibility, we performed the present study through meta-analysis. The PubMed, Cochrane Library, China National Knowledge Infrastructure, EMBASE, Web of Science, and Wanfang databases were searched to retrieve qualified case-control studies. Finally, 31 reports were selected for the present meta-analysis. The results demonstrated that the VDR gene TaqI TT genotype was related to decreased risk of urolithiasis in the overall population (TT vs. Tt+tt: P = 0.011, OR = 0.824, 95% CI = 0.709–0.957). In ethnicity subgroup analysis, we found that the TaqI variant was obviously correlated to urolithiasis risk among Asians and Caucasians (P < 0.05). Additionally, significant urolithiasis risk was identified in adults. However, the FokI, BsmI, and ApaI variants did not have an increased risk of developing urolithiasis. Trial sequential analysis results were on a sufficiently large number of participants and did not require more research to confirm associations. Our research suggested that the VDR gene variant TaqI was correlated with urolithiasis susceptibility and that the t-allele might be the risk gene and T-allele the protective gene in VDR TaqI variant.
Urolithiasis is a disease with a prevalence rate of 1 to 5% around the world, and relapse is common after treatment. The 10 year recurrence rate of urolithiasis is up to 50% (Moe and Li, 2018). Epidemiologic research from Europe shows that 4 to 10% of adults get urolithiasis at least one time in their lives, which indicates that urolithiasis is one of the most common diseases threatening human health (Rivers et al., 2000). Causes of urolithiasis are complex and might be related to many factors such as environment, age, habits, genetic factors, metabolic disorders, etc. As more and more mature gene technologies come into use, various genes related to urolithiasis are gradually being discovered.
Vitamin D receptor (VDR) belongs to the superfamily of transcription factor nuclear receptors and is a soluble protein that exists in many nuclei and cell membranes (González-Castro et al., 2019). VDR is mainly distributed in the kidney, small intestine, skin, and bones. In addition, VDR expression is also found in human immune cells (macrophages, monocytes) and many tumor cells (Ou et al., 2014). The high variance of the VDR gene is the most important genetic factor that determines the host's ability to respond to the immune system. VDR gene variants are thought to be related to susceptibility to urolithiasis. So far, at least 25 VDR variants have been found. Among them, FokI (rs2228570), TaqI (rs731236), BsmI (rs1544410), and ApaI (rs7975232) are most intensively studied. VDR gene is located in 12q13.11 on the chromosome. Among the four VDR variants (FokI, ApaI, TaqI, and BsmI), three of them occur in the intron sections: the TaqI, ApaI, and BsmI variants, while only the FokI variant changes the codon (Ou et al., 2014; González-Castro et al., 2019). On account of the location of the gene, the influence of each variant can be different; for example, BsmI and TaqI variants can influence the translation efficiency and/or stability of the RNA, but they do not modify the structure of the VDR protein (Miyazawa and Suzuki, 2011). Variants of VDR have been found to be a significant risk factor for urolithiasis (Rivers et al., 2000; Stamatelou et al., 2003). At present, more and more epidemiologic investigations are centered on the relationship between urolithiasis risk and VDR gene variants, but the conclusions remain controversial (Aji et al., 2012; Cakir et al., 2016; Huang et al., 2019). For the above reasons, this meta-analysis was carried out to investigate whether susceptibility to urolithiasis was correlated to the VDR gene variants on the basis of widely collected investigations.
A comprehensive search of the PubMed, Cochrane Library, China National Knowledge Infrastructure, EMBASE, Web of Science, and Wanfang databases and manual search were carried out to find relevant studies. Search strategies were: (VDR OR vitamin D receptor) AND (kidney stone disease OR calcium kidney stones OR urolithiasis) AND (variant OR SNP OR genotype OR polymorphism). We searched published studies up to Jan 30, 2020. In addition, we also traced back the relevant references of relevant studies and manually searched relevant articles as well as degree papers.
(1) Case-control studies that focused on the relationship of VDR gene variants and urolithiasis; (2) research included VDR gene BsmI, ApaI, TaqI and FokI variants; (3) frequencies of alleles or genotypes in control groups and case groups could be exacted from the articles; (4) the distribution of genotypes of controls were in accord with Hardy-Weinberg equilibrium (HWE).
(1) Reviews, meta-analysis, letters, case reports; (2) studies published repeatedly; (3) results not based on VDR FokI, BsmI, ApaI, and TaqI variants; (4) works with incomplete data or where data were not available.
The Newcastle-Ottawa Scale (NOS) was applied to estimate the quality of relevant articles. If the score was more than or equal to 5 points, the quality of the article was considered to be high. Two people independently extracted data from included documents depending on the formal criteria and then carefully checked the results against each other. If disagreement arose, then the document would be sent to another person for evaluation and review. The data extraction included the first author's name, population ethnicity and area of origin, publication date, sample size of total cases and controls, hypercalciuria in the urolithiasis group, age group, genotype distribution of four VDR gene variants, and genotyping methods. The genetic nomenclatures used in this meta-analysis were according to the recommendations of the Human Genome Variation Society (HGVS) and the American College of Medical Genetics and Genomics (ACMGG) (see Table 1).
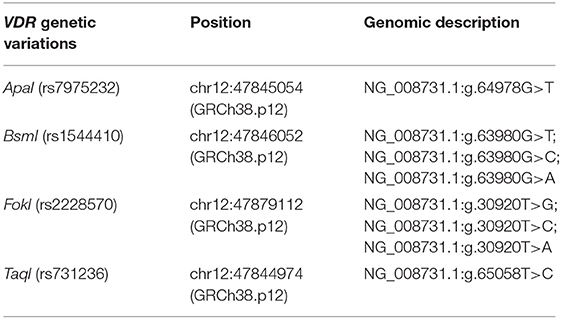
Table 1. Genetic nomenclature used in this meta-analysis according to the recommendations of the Human Genome Variation Society (HGVS) and the American College of Medical Genetics and Genomics (ACMGG).
The odds ratio (OR) and 95% confidence interval (95% CI) were used for the analyses. The Cochran's Q-Test and heterogeneity coefficient I2 were applied to measure the heterogeneity. If I2 < 50% and P > 0.1, it was considered that the studies had no heterogeneity overall, and a fixed-effect model was used; otherwise, a random-effect model was applied. The contrast models were the allele model, dominant model, co-dominant model, homozygote model, and recessive model. Associations of VDR variants and urolithiasis under different genetic models were, respectively, analyzed by STATA 12.0 software.
We used Egger's test to evaluate the publication bias. If the P-value of Egger's test is more than 0.05 and the funnel plot is symmetrical, it can be considered that there is no significant evidence of publication bias.
We performed a sensitivity analysis to assess whether individual studies affected the overall results. In the end, it was found that after removing each one of the studies in turn, the combined odds ratio (OR) of the remaining studies was within the 95% CI in the meta-analysis. The above results showed that the combined OR of this meta-analysis had good stability.
For subgroup analysis, we divided all subject data into different subgroups so that comparisons could be made between subgroups. Subgroup analysis can be performed on different types of subjects (e.g., different ethnic or age groups). Ethnicity was categorized as Caucasian, Asian, or African. In the present study, we performed subgroup analysis by different ethnicity, age, and calciuria level group.
The TSA was performed using TSA v0.9.5.10 Beta software developed by the Copenhagen Clinical Trial Center in Denmark. This study sets the OR reduction to 20%, the first type of error α = 0.05, and the second type of error β = 0.2 to calculate the required information size (RIS). When the size of the population is accumulating but is less than the expected amount, a trial sequential monitoring boundary (TSMB) is set based on the RIS. We performed this analysis according to the RIS and TSMB. When the cumulative Z-value crosses the TSMB, the results are considered statistically significant. At the same time, it can be considered that the sample size is sufficient.
A total of 31 articles (Jackman et al., 1999; Ruggiero et al., 1999; Chen et al., 2001a,b; Nishijima et al., 2002; Ozkaya et al., 2003; Shaogang et al., 2003; Relan et al., 2004; Rendina et al., 2004; Shao-Qun et al., 2004; Bid et al., 2005a,b; Gunes et al., 2006; Liu et al., 2007; Moyano et al., 2007; Seyhan et al., 2007; Wang et al., 2009, 2012; Li Zhengming and Shi, 2010; Mittal et al., 2010; Mossetti et al., 2010; Seo et al., 2010; Aji et al., 2012; Basiri et al., 2012; Ruan et al., 2012; Guha et al., 2015; Aykan et al., 2016; Cakir et al., 2016; Goknar et al., 2016; Subaşi et al., 2017; Huang et al., 2019) reporting the relationship between VDR ApaI, BsmI, FokI, or TaqI gene variants and urolithiasis susceptibility were recruited through the search strategy (Figure 1 and Table 2). The data we needed were extracted fully (Table 2). Of these 31 articles, 12 articles studied the BsmI (rs1544410) variant, 19 articles the FokI (rs2228570) variant, 17 articles the TaqI (rs731236) variant, and 13 articles the ApaI (rs7975232) variant. The distribution of genotypes from these 31 studies were all in accordance with HWE.
The effects of the TaqI variant on urolithiasis susceptibility were investigated in 17 studies, comprising 2,155 cases and 2,326 controls. We found that a significant protective association was observed between urolithiasis susceptibility and the TaqI TT genotype in the overall population (TT vs. Tt+tt: P = 0.011, OR = 0.824, 95% CI 0.709–0.957; Figure 2A and Table 3).
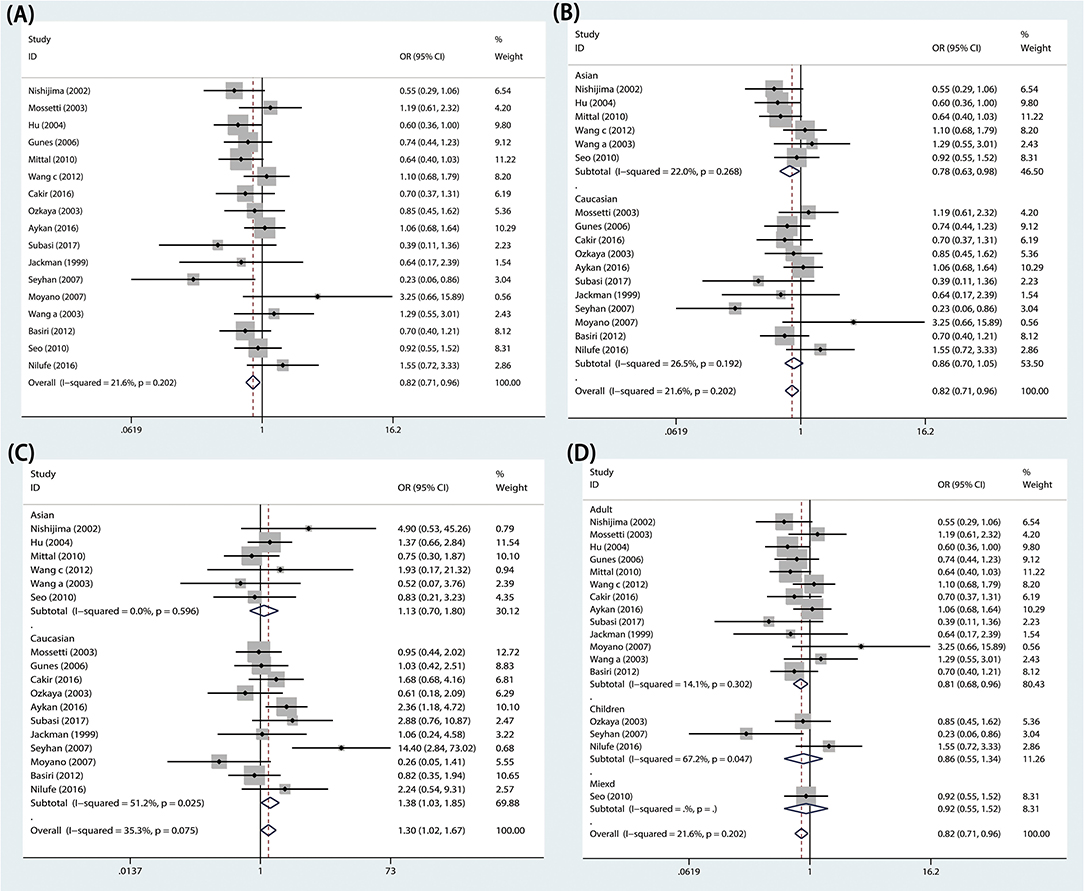
Figure 2. Forest plots of VDR TaqI variant and urolithiasis: (A) overall for tt vs. TT, (B) Asian population for TT vs. Tt+tt, (C) Caucasian population for tt vs. TT, and (D) adults for TT vs. Tt+tt.
In subgroup analyses, significant urolithiasis risk was identified among Asians (Tt vs. TT: P = 0.016, OR = 1.332, 95% CI = 1.055-1.683; TT vs. Tt+tt: P = 0.030, OR = 0.782, 95% CI = 0.626–0.977; Figure 2B) and among Caucasians (t vs. T: P = 0.032, OR = 1.162, 95% CI = 1.013–1.332; tt vs. TT: P = 0.032, OR = 1.380, 95% CI 1.029–1.852; Figure 2C). Furthermore, lower urolithiasis risk was observed among adults (OR = 0.809, 95% CI = 0.684–0.957, P = 0.013; Figure 2D). However, patients with hypercalciuria did not exhibit a significant risk of urolithiasis.
The ApaI variant was investigated in 13 articles. The numbers of cases and controls were 1946 and 2004, respectively. In this meta-analysis, we observed that VDR ApaI variant had no association with risk of urolithiasis in the overall population (AA vs. aa: P = 0.970, OR = 0.996, 95% CI 0.807–1.229; Figure 3A and Table 3).
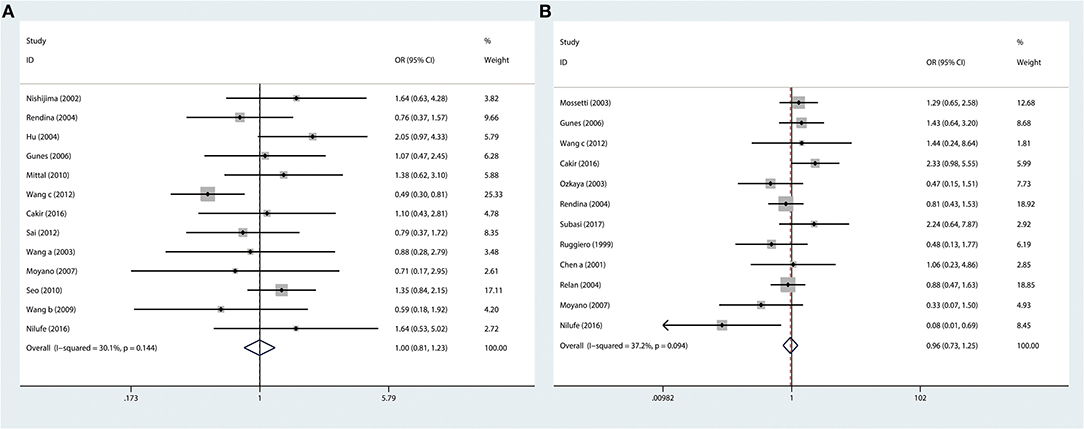
Figure 3. Forest plots of VDR TaqI variant and urolithiasis: (A) overall for ApaI AA vs. aa and (B) overall for BsmI BB vs. bb.
In ethnicity subgroup analysis, no relationship was observed among Asians and among Caucasians. In the subgroup of calciuria level, we did not find significant urolithiasis susceptibility among hypercalciuric cases (aa vs. AA+Aa: P = 0.966, OR = 0.994, 95% CI 0.745–1.325; Table 3). In subgroup analyses by age, we did not identify any correlation among adult patients (aa vs. AA+Aa: P = 0.179, OR = 1.127, 95% CI 0.947–1.342; Table 3).
Twelve studies (1,481 cases and 1,477 controls) considering the relationship between susceptibility to urolithiasis and VDR BsmI variant were selected for this study. In this meta-analysis, we found that VDR BsmI variant was not correlated with urolithiasis susceptibility in the overall population (BB vs. bb: P = 0.745, OR = 0.957, 95% CI 0.734–1.247; Figure 3B and Table 3).
Among Caucasian and Asian populations, VDR BsmI was again not correlated with urolithiasis susceptibility (Caucasian: BB vs. bb: P = 0.729, OR = 0.916, 95% CI 0.557–1.506; Asian: BB vs. bb: P = 0.828, OR = 0.941, 95% CI 0.544–1.628; Table 3), and neither were there any obvious correlations in subgroup analysis of age group and calciuria level group (Adult: bb vs. BB+Bb: P = 0.988, OR = 1.002, 95% CI 0.761–1.320; Hypercalciuria group: bb vs. BB+Bb: P = 0.223, OR = 1.411, 95% CI 0.811–2.458; Table 3).
For VDR FokI variant, 19 research papers, including 2,847 cases and 2,919 normal controls, were selected. In our meta-analysis, urolithiasis risk had no relationship with VDR FokI variant in the overall population (ff vs. FF: P = 0.720, OR = 1.064, 95% CI 0.759–1.490; Table 3).
We performed stratification analyses by ethnicity, but no association was observed among Caucasians and Asians (Caucasian: ff vs. FF: P = 0.569, OR = 0.740, 95% CI 0.263–2.084; Asian: ff vs. FF: P = 0.277, OR = 1.200, 95% CI 0.864–1.666; Table 3). In subgroup analyses by age and calciuria level group, we found no correlation between urolithiasis susceptibility and the FokI variant (Adult: FF vs. Ff+ff: P = 0.340, OR = 0.861, 95% CI 0.633–1.171; Hypercalciuria group: FF vs. Ff+ff: P = 0.819, OR = 0.923, 95% CI 0.511–1.701; Table 3).
Sensitivity analyses were implemented by removing each one investigation from the meta-analysis at a time. Moreover, the pooled ORs did not change markedly [1.01 (Lower Limit) < OR < 1.37 (Upper Limit)], which suggested that our results were reliable (Figure 4).
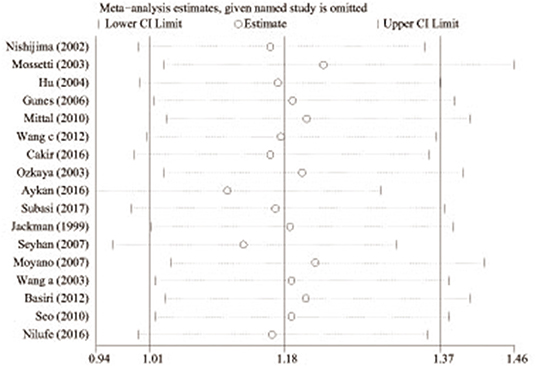
Figure 4. Sensitivity analysis of the pooled ORs and 95% CIs for TaqI variant (tt allele vs. TT allele).
Egger's test and Begg's funnel plots were applied to assess publication bias (dominant contrast of pooled analysis: P = 0.661 for ApaI; P = 0.836 for BsmI; P = 0.953 for FokI; P = 0.464 for TaqI). The final results indicated no publication bias for results relating urolithiasis risk to VDR gene variants in the included studies.
We carried out TSA to reduce the risk of type I error and to assess the RIS. The final results based on the TaqI variant suggested that the size of the 11th study crossed the TSA boundary, and the positive conclusion was obtained in advance, which is consistent with the above meta-analysis results, even though the sample size did not reach the required information size (Figure 5; TaqI: 4,702 cases). Therefore, it can be asserted that TaqI variant TT carriers had a lower risk of urolithiasis than tt carriers and that the evidence was reliable. However, for the ApaI, BsmI, and FokI variants, the sample size did not reach the required information size (Figure 6A; BsmI: 4,306 cases; Figure 6B; ApaI: 4,074 cases; Figure 6C; FokI: 5,819 cases). Hence, more case-control studies are still needed to confirm the relationship between the BsmI, ApaI, and FokI variants and urolithiasis susceptibility.
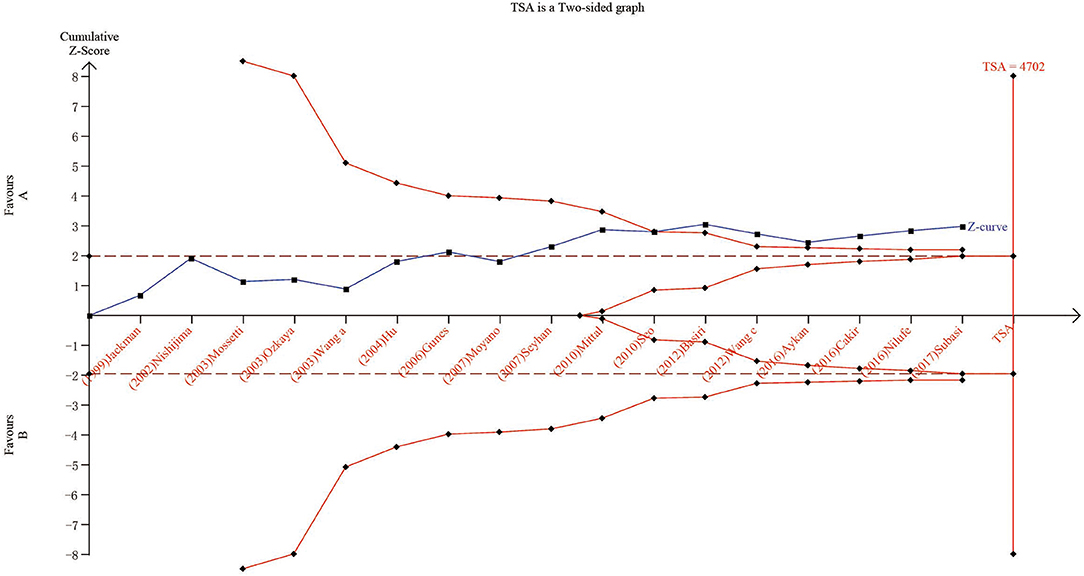
Figure 5. Results of TSA with TaqI variant. The required information size was calculated based on a two-sided α = 5%, β = 15% (power 80%), and a relative risk reduction of 20%.
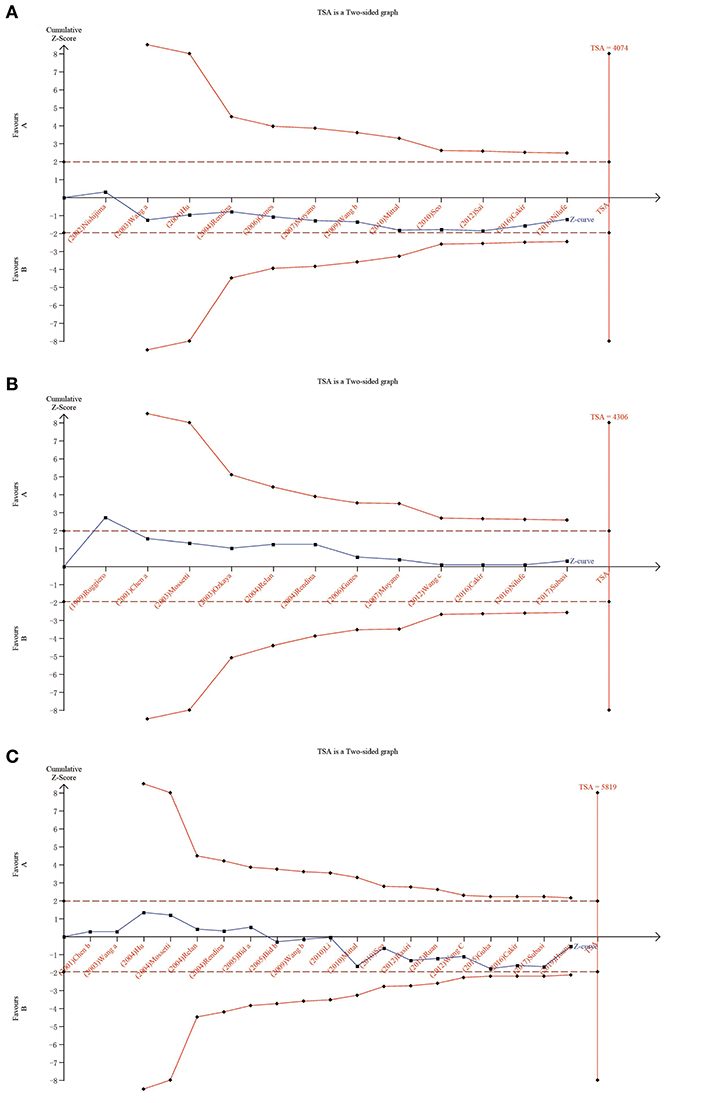
Figure 6. Results of TSA with BsmI, ApaI, and FokI variants. The required information size was calculated based on a two-sided α = 5%, β = 15% (power 80%), and a relative risk reduction of 20%. (A) for ApaI; (B) for BsmI; and (C) for FokI.
Urolithiasis is a common urological disease. It is affected by a variety of genetic and external factors, including natural factors, social environment, dietary habits, local diseases of the urinary system, and systemic metabolic disorders. Many of these are difficult to control, such as climate, latitude and sunlight, water quality, sex, etc. (Imamverdiev and Guseinzade, 2015). Studies have shown that hot conditions, reduced dietary fiber intake, and high intake of animal protein are important risk factors for urolithiasis (Gouru et al., 2018).
There is a direct link between diet and urolithiasis, and studies have found that excessive intake of high-fat foods has a catalytic effect on the formation of urolithiasis (Shimizu et al., 2012). Because of the high protein content of high-fat foods, it is easy to convert into excreted uric acid, and the appearance of high uric acid can lead to urolithiasis (Lee et al., 2008). Additionally, the amino acids in high-fat foods tend to lead to more obvious urine calcium. Due to a large intake of high-fat food, there will be a large amount of calcium excreted in the urine, which will cause the appearance of urolithiasis (Rotily et al., 2000).
Vitamin D (VD) is a fat-soluble substance that is produced due to exposure of the skin to ultraviolet rays from sunlight and is therefore called a “sunshine vitamin” (Philippa et al., 2009). In fact, VD is also a steroid hormone in the human body. Normal people can produce VD from sunlight exposure when they exercise outdoors. However, some groups of people, such as the white-collar workers, especially those living in the Northern hemisphere, have limited exposure to the sun, and their main source of VD is food (Nelson et al., 2015).
VDR is a pronuclear protein and belongs to a member of a superfamily that is composed of steroid hormone/thyroid hormone receptors (Ergon et al., 2017). Additionally, it is a nuclear macromolecule that mediates the biological effects of 1,25-(OH) D, which is a type of activated vitamin D substance (Shamran et al., 2017). As we all know, 1,25-(OH) D has a variety of biological functions in our body, such as regulating immune response, controlling cell proliferation and differentiation, and maintaining a mineral balance (Ruggiero et al., 1999). The most important function of 1,25-(OH) D is adjusting the metabolism of phosphorus and calcium in kidney, intestine, and bone, and it is mainly mediated by VDR (Ergon et al., 2017; Shamran et al., 2017). In urolithiasis, oxalate, phosphate, and calcium salt are the main components of urinary stones, and 85% are from calcium oxalate (Jurutka et al., 2000). Therefore, urolithiasis is closely related to calcium. As a regulatory gene for the metabolism of calcium, VDR is likely to be associated with susceptibility to urolithiasis.
Our meta-analysis systematically investigated correlations between urolithiasis susceptibility and VDR variants. For the TaqI variant, the TT genotype was significantly associated with decreased urolithiasis risk, while the Tt and tt genotypes elevated urolithiasis susceptibility. Therefore, the t-allele might be the risk gene, and the T-allele might be the protective gene. For the ApaI, BsmI, and FokI variants, we did not find any associations with urolithiasis risk.
In the age subgroup analysis, we found a decreased risk of susceptibility to urolithiasis associated with the VDR TaqI variant. The results suggested that TT genotype carriers were at a lower risk compared to tt genotype and Tt genotype carriers in adults. Thus, the TT genotype might be the proactive factor for urolithiasis susceptibility, while the Tt and tt genotype might be risk factors in urolithiasis.
In terms of the mechanism, the TaqI variant does not modify the VDR protein structure but can influence the translation efficiency and/or stability of the RNA, which might affect the development of urolithiasis (Jurutka et al., 2000; Uitterlinden et al., 2004). Yamagata et al. (1999) found that in peripheral blood mononuclear cell (PBMC) the VDR mRNA levels of allele t were significantly higher than those of allele T. Furthermore, Carling et al. (1998) found that individuals exhibiting the tt genotype had significantly higher VDR mRNA levels than those with the TT genotype (Carling et al., 1998). Therefore, it can be considered that people with the t alleles might have increased susceptibility to urolithiasis.
This meta-analysis included 31 case-control studies with 4,144 controls and 3,782 patients, while a previous study by Zhang et al. (2013) only had 23 articles with 2,303 controls and 2,046 cases. Zhang et al. found a decreased risk of urolithiasis associated with the ApaI and TaqI variants. Nevertheless, we did not find any significant association between urolithiasis susceptibility and the ApaI variant. Even in the racial subgroup analysis, there was no significant correlation among the Asian and Caucasian populations. Our meta-analysis included more studies than Zhang's study. Furthermore, in TSA, the cumulative Z-curve crossed the boundary of the TSA, which showed that our meta-analysis had a sufficient number of cases and controls. This may cause the difference between our results and those of Zhang.
Moreover, our meta-analysis also had some advantages. Firstly, our research carried out TSA to analyze the results of the study and the adequacy of the evidence. The TSA results showed that the results of the effect tests on the whole population, Asians, and Caucasians under different genetic models were based on sufficient evidence. Secondly, we carried out subgroup analysis on the basis of a range of related factors. Thirdly, we included more than 30 articles in our meta-analysis. Compared with previous studies, this meta-analysis contained a larger sample size, containing 7,926 subjects, with 3,782 urolithiasis patients and 4,144 non-urolithiasis participants, which was sufficient to draw a reliable conclusion. Fourthly, the subgroup analysis was sufficient. In addition, after Begg's test and sensitivity analysis, the pooled results and conclusions are proved to be credible to evaluate the relationship between urolithiasis risk and VDR variants.
Heterogeneity is very important and may affect meta results and result in error. In the present meta-analysis, the contrast model of ApaI and FokI variants had significant heterogeneity. Thus, we carried out the subgroup analysis of other related factors in order to decrease the sources of heterogeneity. The heterogeneity decreased in subgroup analysis by ethnicity and age.
There were still some limitations to this study that cannot be avoided. First, some unpublished studies with negative results might not be included. Second, this study did not reveal gene–environment and gene–gene interactions due to a lack of enough original information from included studies. Therefore, more multicenter investigations with a large sample are still needed in future to study the association between urolithiasis risk and VDR variants.
In conclusion, the results from this systematic review and meta-analysis indicated that urolithiasis susceptibility was associated with the TaqI variant. The Tt and tt genotypes could elevate the incidence of urolithiasis, while the TT genotype decreased urolithiasis risk. Therefore, the t-allele might be the risk gene and T-allele the protective gene in the VDR gene variant TaqI. However, no associations were observed in the ApaI, BsmI, and FokI variants. Our results also remind us of the necessity of early screening for urolithiasis in TaqI variant t-allele carriers.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Zhengqi Chen, Nanchang University, for her valuable comments.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.00234/full#supplementary-material
Supplementary Table 1. PRISMA Checklist.
Aji, K., Song, G. L., Yasen, A., Azad, B., and Tursun, H. (2012). Association of vitamin d receptor gene polymorphisms with urolithiasis in Uyghur children from southern Xinjiang, china. Chinese J. Contemp. Pediatr. 14, 956–959.
Aykan, S., Tuken, M., Gunes, S., Akin, Y., Ozturk, M., Seyhan, S., et al. (2016). ApaL1 urokinase and Taq1 vitamin D receptor gene polymorphisms in first-stone formers, recurrent stone formers, and controls in a Caucasian population. Urolithiasis 44, 109–115. doi: 10.1007/s00240-015-0813-1
Basiri, A., Shakhssalim, N., Houshmand, M., Kashi, A. H., Azadvari, M., Golestan, B., et al. (2012). Coding region analysis of vitamin D receptor gene and its association with active calcium stone disease. Urol. Res. 40, 35–40. doi: 10.1007/s00240-011-0399-1
Bid, H. K., Chaudhary, H., and Mittal, R. D. (2005a). Association of vitamin-D and calcitonin receptor gene polymorphism in paediatric nephrolithiasis. Pediatr. Nephrol. 20, 773–776. doi: 10.1007/s00467-005-1846-4
Bid, H. K., Kumar, A., Kapoor, R., and Mittal, R. D. (2005b). Association of vitamin d receptor-gene (FokI) polymorphism with calcium oxalate nephrolithiasis. J. Endourol. 19, 111–115. doi: 10.1089/end.2005.19.111
Cakir, O. O., Yilmaz, A., Demir, E., Incekara, K., Kose, M. O., and Ersoy, N. (2016). Association of the BsmI, ApaI, TaqI, Tru9I and FokI polymorphisms of the vitamin D receptor gene with nephrolithiasis in the Turkish population. Urol. J. 13, 2509–2518.
Carling, T., Rastad, J., Åkerström, G., and Westin, G. (1998). Vitamin D receptor (VDR) and parathyroid hormone messenger ribonucleic acid levels correspond to polymorphic VDR alleles in human parathyroid tumors. J. Clin. Endocrinol. Metab. 83, 2255–2259. doi: 10.1210/jc.83.7.2255
Chen, W. C., Chen, H. Y., Hsu, C. D., Wu, J. Y., and Tsai, F. J. (2001a). No association of vitamin D receptor gene BsmI polymorphisms with calcium oxalate stone formation. Mol. Urol. 5, 7–10. doi: 10.1089/109153601750124203
Chen, W. C., Chen, H. Y., Lu, H. F., Hsu, C. D., and Tsai, F. J. (2001b). Association of the vitamin D receptor gene start codon Fok I polymorphism with calcium oxalate stone disease. BJU Int. 87, 168–171. doi: 10.1046/j.1464-410x.2001.02074.x
Ergon, E. Y., Akil, I. O., Taneli, F., Oran, A., and Ozyurt, B. C. (2017). Etiologic risk factors and vitamin D receptor gene polymorphisms in under one-year-old infants with urolithiasis. Urolithiasis 46:349. doi: 10.1007/s00240-017-1009-7
Goknar, N., Öktem, F., Torun, E., Gok, O., Demir, A. D., Kucukkoc, M., et al. (2016). The role of vitamin D receptor gene polymorphisms in Turkish infants with urolithiasis. Ren. Fail. 38, 545–551. doi: 10.3109/0886022X.2016.1148557
González-Castro, T. B., Blachman-Braun, R., Hernández-Díaz, Y., Tovilla-Zárate, C. A., Pérez-Hernández, N., Moscardi, P. R. M., et al. (2019). Association of vitamin D receptor polymorphisms and nephrolithiasis: a meta-analysis. Gene 711:143936. doi: 10.1016/j.gene.2019.06.026
Gouru, V. R., Pogula, V. R., Vaddi, S. P., Manne, V., Byram, R., Kadiyala, L. S., et al. (2018). Metabolic evaluation of children with urolithiasis. Urol. Ann. 10:94. doi: 10.4103/UA.UA_98_17
Guha, M., Bankura, B., Ghosh, S., Pattanayak, A. K., Ghosh, S., Pal, D. K., et al. (2015). Polymorphisms in CASR and CLDN14 genes associated with increased risk of kidney stone disease in patients from the eastern part of India. PLoS ONE 10:e0130790. doi: 10.1371/journal.pone.0130790
Gunes, S., Bilen, C. Y., Kara, N., Asci, R., Bagci, H., and Yilmaz, A. F. (2006). Vitamin D receptor gene polymorphisms in patients with urolithiasis. Urol. Res. 34, 47–52. doi: 10.1007/s00240-005-0033-1
Huang, Y., Peng, Q., Bao, M., Liu, C., Wu, K., and Zhou, S. (2019). Biochemical metabolic levels and vitamin D receptor FokI gene polymorphisms in Uyghur children with urolithiasis. PLoS ONE 14:e0212183. doi: 10.1371/journal.pone.0212183
Imamverdiev, S. B., and Guseinzade, R. T. (2015). Possible influence of epidemiological risk factors on the development of urolithiasis. 88, 68–72. doi: 10.17116/terarkh201688368-72
Jackman, S. V., Kibel, A. S., Ovuworie, C. A., Moore, R. G., Kavoussi, L. R., and Jarrett, T. W. (1999). Familial calcium stone disease: TaqI polymorphism and the vitamin D receptor. J. Endourol. 13, 313–316. doi: 10.1089/end.1999.13.313
Jurutka, P. W., Remus, L. S., Whitfield, G. K., Thompson, P. D., Hsieh, J. C., Zitzer, H., et al. (2000). The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol. Endocrinol. 14, 401–420. doi: 10.1210/mend.14.3.0435
Lee, S. C., Kim, Y. J., Kim, T. H., Yun, S. J., Lee, N. K., and Kim, W. J. (2008). Impact of obesity in patients with urolithiasis and its prognostic usefulness in stone recurrence. J. Urol. 179, 570–574. doi: 10.1016/j.juro.2007.09.040
Li Zhengming, R. L., and Shi, G. (2010). Association between single nucleofide polymorphism of vitamin D receptor start codon Fok I and urinary calcium level. Chin. J. Biomed. Eng. 16, 38–41.
Liu, C. C., Huang, C. H., Wu, W. J., Huang, S. P., Chou, Y. H., Li, C. C., et al. (2007). Association of vitamin D receptor (Fok-I) polymorphism with the clinical presentation of calcium urolithiasis. BJU Int. 99, 1500–1505. doi: 10.1111/j.1464-410X.2007.06792.x
Mittal, R. D., Mishra, D. K., Srivastava, P., Manchanda, P., Bid, H. K., and Kapoor, R. (2010). Polymorphisms in the vitamin D receptor and the androgen receptor gene associated with the risk of urolithiasis. Indian J. Clin. Biochem. 25, 119–126. doi: 10.1007/s12291-010-0023-0
Miyazawa, K., and Suzuki, K. (2011). Gene expression and its role on urolithiasis. Clin. Calcium 21, 1473–1479.
Moe, O. W., and Li, H. R. X. (2018). Hyperuricosuric calcium urolithiasis. J. Nephrol.. 215, 1–8. doi: 10.1007/s40620-018-0469-3
Mossetti, G., Vuotto, P., Rendina, D., Numis, F. G., Viceconti, R., Giordano, F., et al. (2010). Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J. Intern. Med.. 253, 194–200. doi: 10.1046/j.1365-2796.2003.01086.x
Moyano, M. J., Gómez de Tejada, M. J., García Lozano, R., Moruno, R., Ortega, R., Martí, V., et al. (2007). Alterations in bone mineral metabolism in patients with calcium kidney stone disease and polymorphism of vitamin D receptor. Preliminary results. Nefrología Publicación Oficial De La Sociedad Espaola Nefrologia. 27, 694–703.
Nelson, D. A., Agarwal, S. C., and Darga, L. L. (2015). Bone health from an evolutionary perspective: development in early human populations. Nutr. Health 1, 3–20. doi: 10.1007/978-1-4939-2001-3_1
Nishijima, S., Sugaya, K., Naito, A., Morozumi, M., Hatano, T., and Ogawa, Y. (2002). Association of vitamin D receptor gene polymorphism with urolithiasis. J. Urol. 167, 2188–2191. doi: 10.1016/S0022-5347(05)65126-9
Ou, C., Zhao, H. L., Zhu, B., Huang, L. S., Li, P. Z., and Lao, M. (2014). Association of vitamin d receptor gene polymorphism with the risk of renal cell carcinoma: a meta-analysis. J. Recept. Res. 34, 6–12. doi: 10.3109/10799893.2014.919593
Ozkaya, O., Söylemezoglu, O., Misirlioglu, M., Gönen, S., Buyan, N., and Hasanoglu, E. (2003). Polymorphisms in the vitamin D receptor gene and the risk of calcium nephrolithiasis in children. Eur. Urol. 44, 150–154. doi: 10.1016/S0302-2838(03)00206-9
Philippa, H. Y., Janda, M., and Kimlin, M. (2009). Vitamin D and sun protection: The impact of mixed public health messages in Australia. International Journal of Cancer. 124, 1963–1970. doi: 10.1002/ijc.24154
Relan, V., Khullar, M., Singh, S. K., and Sharma, S. K. (2004). Association of vitamin d receptor genotypes with calcium excretion in nephrolithiatic subjects in northern India. Urol. Res. 32, 236–240. doi: 10.1007/s00240-004-0414-x
Rendina, D., Mossetti, G., Viceconti, R., Sorrentino, M., Castaldo, R., Manno, G., et al. (2004). Association between vitamin D receptor gene polymorphisms and fasting idiopathic hypercalciuria in recurrent stone-forming patients. Urology 64, 833–838. doi: 10.1016/j.urology.2004.05.013
Rivers, K., Shetty, S., and Menon, M. (2000). When and how to evaluate a patient with nephrolithiasis. Urol. Clin. North Am. 27, 203–213. doi: 10.1016/S.0094-0143(05)70251-2
Rotily, M., Léonetti, F., Iovanna, C., Berthezene, P., Dupuy, P., Vazi, A., et al. (2000). (Effects of low animal protein or high-fiber diets on urine composition in calcium nephrolithiasis. Kidney Int. 57, 1115–1123. doi: 10.1046/j.1523-1755.2000.00939.x
Ruan, L., Li, Z. M., Zheng, R. G., Huang, W. S., Shi, G. Q., Li, G., et al. (2012). Relationship between vitamin D receptor FokI polymorphism and calcium oxalate stone disease in Guangzhou Chinese patients. Guangdong Med. J. 33, 84–85.
Ruggiero, M., Pacini, S., Amato, M., Aterini, S., and Chiarugi, V. (1999). Association between vitamin d receptor gene polymorphism and nephrolithiasis. Miner. Electrolyte Metab. 25, 185–190. doi: 10.1159/000057443
Seo, I. Y., Kang, I. H., Chae, S. C., Park, S. C., Lee, Y. J., Yang, Y. S., et al. (2010). Vitamin D receptor gene Alw I, Fok I, Apa I, and Taq I polymorphisms in patients with urinary stone. Urology 75, 923–927. doi: 10.1016/j.urology.2009.10.006
Seyhan, S., Yavascaoglu, I., Kilicarslan, H., Dogan, H. S., and Kordan, Y. (2007). Association of vitamin D receptor gene Taq I polymorphism with recurrent urolithiasis in children. Int. J. Urol. 14, 1060–1062. doi: 10.1111/j.1442-2042.2007.01899.x
Shamran, H. A., Ali, S. H., Ali, M. A., Al-Mayah, Q. S., and Jasim, E. A. (2017). E-cadherin gene polymorphisms and susceptibility to urolithiasis in iraqi children. Nephrology 24, 17–20. doi: 10.1111/nep.13184
Shaogang, W., Jihong, L., Shaoqun, H., and Zhangqun, Y. (2003). Association of vitamin D receptor gene polymorphisms with calcium oxalate calculus disease. J. Huazhong Univ. Sci. Technol. 23, 38–41. doi: 10.1007/BF02829458
Shao-Qun, H. U., Ji-Hong, L., and Shao-Gang, W. (2004). Relationship between vitamin D receptor allele polymorphism and calcium oxalate stone disease. Chin. J. Urol. 25, 155–158. doi: 10.3760/j:issn:1000-6702.2004.03.003
Shimizu, H., Ichikawa, D., Tamagaki, K., Komatsu, S., Kubota, T., Okamoto, K., et al. (2012). Evaluation of postoperative nephrolithiasis and renal dysfunction in gastric cancer patients. Gastric Cancer 16, 338–344. doi: 10.1007/s10120-012-0192-z
Stamatelou, K. K., Francis, M. E., Jones, C. A., Nyberg, L. M., and Curhan, G. C. (2003). Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 63, 1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x
Subaşi, B., Gökçe, I., Delil, K., and Alpay, H. (2017). Vitamin d receptor gene polymorphisms in children with kidney stone disease. Turk. J. Pediatr. 59, 404–409. doi: 10.24953/turkjped.2017.04.006
Uitterlinden, A. G., Fang, Y., Van Meurs, J. B., Pols, H. A., and Van Leeuwen, J. P. (2004). Genetics and biology of vitamin D receptor polymorphisms. Gene 338, 143–156. doi: 10.1016/j.gene.2004.05.014
Wang, Q. Z., Qian, B., and Guo-Fu, D. (2009). Vitamin D receptor gene polymorphisms in Chinese uygur patients with urohthiasis in south Xinjian. J. Pract. Med. 25, 2805–2807.
Wang, S., Wang, X., Wu, J., Lin, Y., Chen, H., Zheng, X., et al. (2012). Association of vitamin D receptor gene polymorphism and calcium urolithiasis in the Chinese Han population. Urol. Res. 40, 277–284. doi: 10.1007/s00240-011-0438-y
Yamagata, M., Nakajima, S., Tokita, A., Sakai, N., Yanagihara, I., Yabuta, K., et al. (1999). Analysis of the stable levels of messenger RNA derived from different polymorphic alleles in the vitamin D receptor gene. J. Bone Miner. Res. 17, 164–170. doi: 10.1007/s007740050080
Keywords: urolithiasis, variant, vitamin D receptor, meta-analysis, trial sequential analysis
Citation: Chen G, Hu C, Song Y, Xiu M, Liang W, Ou N, Liu X and Huang P (2020) Relationship Between the ApaI (rs7975232), BsmI (rs1544410), FokI (rs2228570), and TaqI (rs731236) Variants in the Vitamin D Receptor Gene and Urolithiasis Susceptibility: An Updated Meta-Analysis and Trial Sequential Analysis. Front. Genet. 11:234. doi: 10.3389/fgene.2020.00234
Received: 14 October 2019; Accepted: 27 February 2020;
Published: 15 April 2020.
Edited by:
Ahmed Rebai, Centre of Biotechnology of Sfax, TunisiaReviewed by:
Lukasz Laczmanski, Ludwik Hirszfeld Institute of Immunology and Experimental Therapy (PAN), PolandCopyright © 2020 Chen, Hu, Song, Xiu, Liang, Ou, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Huang, cGVuZ2hAbmN1LmVkdS5jbg==; aHVhbmdwZW5nbmN1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.