
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 20 June 2016
Sec. Pharmacogenetics and Pharmacogenomics
Volume 7 - 2016 | https://doi.org/10.3389/fgene.2016.00108
 Xiao-Li Zang1,2,3,4
Xiao-Li Zang1,2,3,4 Wei-Qing Han1,2,3,4
Wei-Qing Han1,2,3,4 Feng-Ping Yang5
Feng-Ping Yang5 Kai-Da Ji1,2,3,4
Kai-Da Ji1,2,3,4 Ji-Guang Wang1,2,3,4
Ji-Guang Wang1,2,3,4 Ping-Jin Gao1,2,3,4
Ping-Jin Gao1,2,3,4 Guang He5*
Guang He5* Sheng-Nan Wu1,2,3,4*
Sheng-Nan Wu1,2,3,4*
A recent study suggested that SLC35F3 which encoded a thiamine transporter was a new candidate gene for hypertension. The goal of this study was to investigate the association between the single-nucleotide polymorphisms (SNPs) in the SLC35F3 gene and hypertension in a Chinese population. Sanger sequencing was performed in 93 samples to find SNPs in coding regions and intron–exon boundaries in the SLC35F3 gene. We found eight genetic variants in the coding regions of SLC35F3 and subsequently genotyped a non-synonymous variant rs34032258 (C > G) in 1060 hypertension patients and 1467 controls. After adjusting for age and gender, multivariate analysis of covariance showed that the variant was associated with hypertensive traits. In detail, diastolic blood pressure (DBP) was 8 mmHg higher, blood urea nitrogen was 12 mmol/L higher, and creatinine was 15 mmol/L lower in G/G group compared with C/C group (p = 0.007; 0.012 and 0.029, respectively). Further study suggested that C/G+G/G had higher DBP than C/C genotype in those whose DBP ≥ 90 mmHg (98.02 mmHg vs. 94.04 mmHg, p = 0.021). No significant difference has been found in systolic blood pressure between different genotypes. Additionally, in the subgroup of obesity, allele distribution of this variant has shown significant difference between hypertensive patients and normotensive controls (p = 0.018). In conclusion, we found that the rs34032258 in the SLC35F3 gene was associated with high blood pressure and may increase the risk of hypertension. The new hypertension-susceptibility locus may involve in the pathogenesis of hypertension and indicate some novel treatment implications.
The prevalence of hypertension is increasing in most countries and hypertension is an important risk factor for the development of cardiac-cerebral vascular diseases. However, the cause still remains largely enigmatic (Evans et al., 2003; D’Agostino et al., 2008). Growing evidence showed that genetic and environmental factors played a crucial role in the onset of hypertension (Zhang et al., 2010; Munroe et al., 2011). According to single pressure value, the hypertensive heritability was 31–34%. In addition, average value from more than three measurements showed a higher heritability of 56–57% and it could reach as high as 63–68% based on 24 h-ambulatory blood pressure monitory (Kupper et al., 2006). The heritable trait remains the most potent and crucial risk factor for cardiovascular diseases, although details of its genetic determination are poorly understood (Munroe et al., 2011).
The solute carrier (SLC) group of transporters transports organic or inorganic molecules across cell or organelle membranes (Ishida et al., 2005; Zhang et al., 2012). Nearly 400 SLC members are organized into 52 families (Ishida and Kawakita, 2004). Members of the human solute carrier 35 (SLC35) transporter family, which encode for nucleotide sugar transporters, have been divided into six subfamilies (A–F; Ishida and Kawakita, 2004; Saier et al., 2015)and are predominately expressed in the lumen of the endoplasmic reticulum (ER) and the Golgi apparatus (Goda et al., 2006). Genetic mutations in SLC35 transporter family have been found associated with cardiovascular diseases. In Zhang et al. (2014) have found the SLC35F3 was associated with blood pressure in North America and Western Europe. SLC35F3 sequence homolog to a putative yeast thiamine (vitamin B1) transporter is located at 1q42.2 with 9 exons. The SLC35F3 mRNA was expressed at the highest levels in the adult cerebellum (Nishimura et al., 2008). Up to now, the effect of SLC35F3 genetic variants on blood pressure has not been studied in Chinese populations. In this study, we explored whether variants in the coding regions of the SLC35F3 gene contributed to blood pressure variation and hypertension.
We conducted a two-stage strategy in this study. First, we scanned all the exons of SLC35F3 gene in 93 hypertensive patients by Sanger sequencing. Subsequently, the most suspicious variants were further genotyped by TaqMan-MGB assays in a total of 2527 participants, including 1060 hypertensive patients and 1467 normotensive controls. All of the participants were examined at Shanghai Ruijin Hospital. Every participant signed a consent form, and the study was approved by the hospital’s ethics committee.
Age, gender, and medication usage were obtained from Shanghai Institute of Hypertension, Ruijin hospital. Height and weight were measured and BMI (kg/m2) was calculated. Blood pressure was measured using a calibrated mercury sphygmomanometer with appropriate adult cuff size by well-trained examiners. Diagnosis of hypertension was based on a mean SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg on two occasions, and/or current usage of anti-hypertensive treatment.
During different analytic processes, the samples selected were diverse and the details are showed in Supplementary Table S1. The characteristics of all the 2527 samples are showed in Table 1. All the hypertensive patients (n = 1060) were selected in order to analyze the effect of the SNP on hypertensive traits (Table 2). Based on the results in Table 2, we selected 578 patients whose DBP ≥ 90 mmHg to further verify the relationship between the SNP and DBP (Figure 2). In addition, allelic frequencies of rs34032258 in different BMI levels were analyzed between 1467 controls and 1035 cases excluding 25 unavailable BMI data in case groups (Table 3). The effect of gender on SBP and DBP is shown in Table 4 including all the participants. Besides, the plasma of 344 hypertensive patients was available for ELISA test in order to verify the effect of this gene on blood thiamine in patients (Figure 3).
DNA was extracted from peripheral venous blood by lyzing red blood cells (RBCs) and digesting the remaining white cell pellet with proteinase K in accordance with the protocol (TIANamp Genomic DNA kit, Tiangen, China). DNA samples were stored at –80°C until additional analysis was finished.
Sanger sequencing of 9 exons of the SLC35F3 gene in 93 people was performed by Mapbioo Technology Company of China. Variant calling was carried out using Sequencher 5.1 and the annotation of the detected variants was checked on National Center for Biotechnology Information. We selected missense variant and designed primers for further genotyping.
Genotypes were determined by pre-designed TaqMan Allele Discrimination Assay (Cat. #4351379, primer forward, 5′-AGCGTGCGTCACTGAATGA-3′; reverse, 5′-ACACCCCCATGACTCAAGTG-3′, Life Technologies, USA). TaqMan polymerase chain reaction (PCR) was performed on a 7900 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) in total volume of 2.5 μL consisting of 2.4 μL TaqMan PCR Master Mix (Life Technologies, USA) and 0.03 μL genomic DNA. Cycling conditions were 95°C for 30 s, and 50 cycles of 95°C for 15 s, and 60°C for 1 min.
Thiamine B1 concentration was determined by using ELISA kit (E-EL-0007c, Elabscience).
Continuous variables expressed as mean ± standard error (SE) were compared between two groups by unpaired t-test. Relations between categorical variables were examined by χ2 test. The association of examined SNPs with hypertension as a binary trait and BP as a continuous trait was done by Logistic and linear regression analyses, respectively, after adjusting for age, gender, and BMI. MANCOVA was used to compare the differences of BP, BMI, BUN, and Cr across the genotypes of rs34032258 after treating age and gender as covariates. The frequencies of genotypes between patients and controls were estimated by SHE1. Considering the impact of antihypertensive regimens, SBP and DBP were added by a fixed value of 10 and 5 mmHg, respectively, according to a previous report (Cui et al., 2003). BMI was classified according to the guidelines released by the Ministry of Health of China in 2010. Statistical analyses were performed with SPSS version 13.0 for Windows. Two-sided p < 0.05 was considered to be significant.
In 93 hypertension patients, eight genetic variants were found in the coding regions of SLC35F3. Among them, a missense variant, rs34032258, was detected with an allele frequency of 3.73% (Figure 1B, 466C > G). However, SNPs previously reported to be associated with hypertension, either rs16842784 or rs17514104 was not found in the 93 samples (Figure 1A).
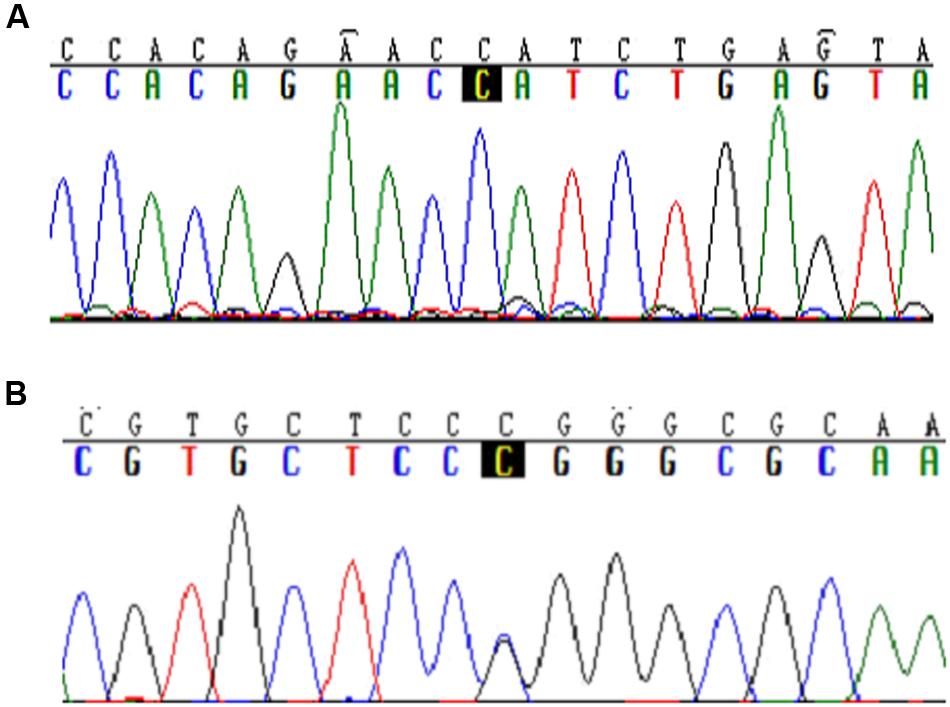
FIGURE 1. Typical sequences of specific mutations in the SLC35F3 SNPs in hypertensive patients. (A) Rs16842784 was not found mutation in Chinese people. (B) C > G mutation in rs34032258.
The comparisons of the demographic and clinical features between hypertensive patients and normotensive controls are shown in Table 1. The distributions of age (p = 0.16) and gender (p = 0.24) did not differ significantly between patients and controls. BMI (p < 0.001), blood pressures (both p < 0.001) and Cr were significantly higher in patients than in controls as expected.
The associations of rs34032258 with BP, BMI, BUN, and Cr are shown in Table 2. After adjusting for age and gender, the result from MANCOVA showed that rs34032258 was associated with DBP, BUN, and Cr. In detail, DBP was 8 mmHg higher, BUN was 12 mmol/L higher, and Cr was 15 mmol/L lower in G/G group compared with C/C group. In contrast, there was no difference in SBP and BMI between the two groups. Further study suggested that C/G+G/G had higher DBP than C/C genotype in those whose DBP ≥ 90 mmHg (98.02 mmHg vs. 94.04 mmHg, p = 0.021; Figure 2). Notably, we found that G allele frequency was significantly lower in hypertensive patients compared with controls in obesity group (OR = 1.34, p = 0.018; Table 3). No significant difference was found between controls and cases in underweight, normal weight, or overweight subjects.
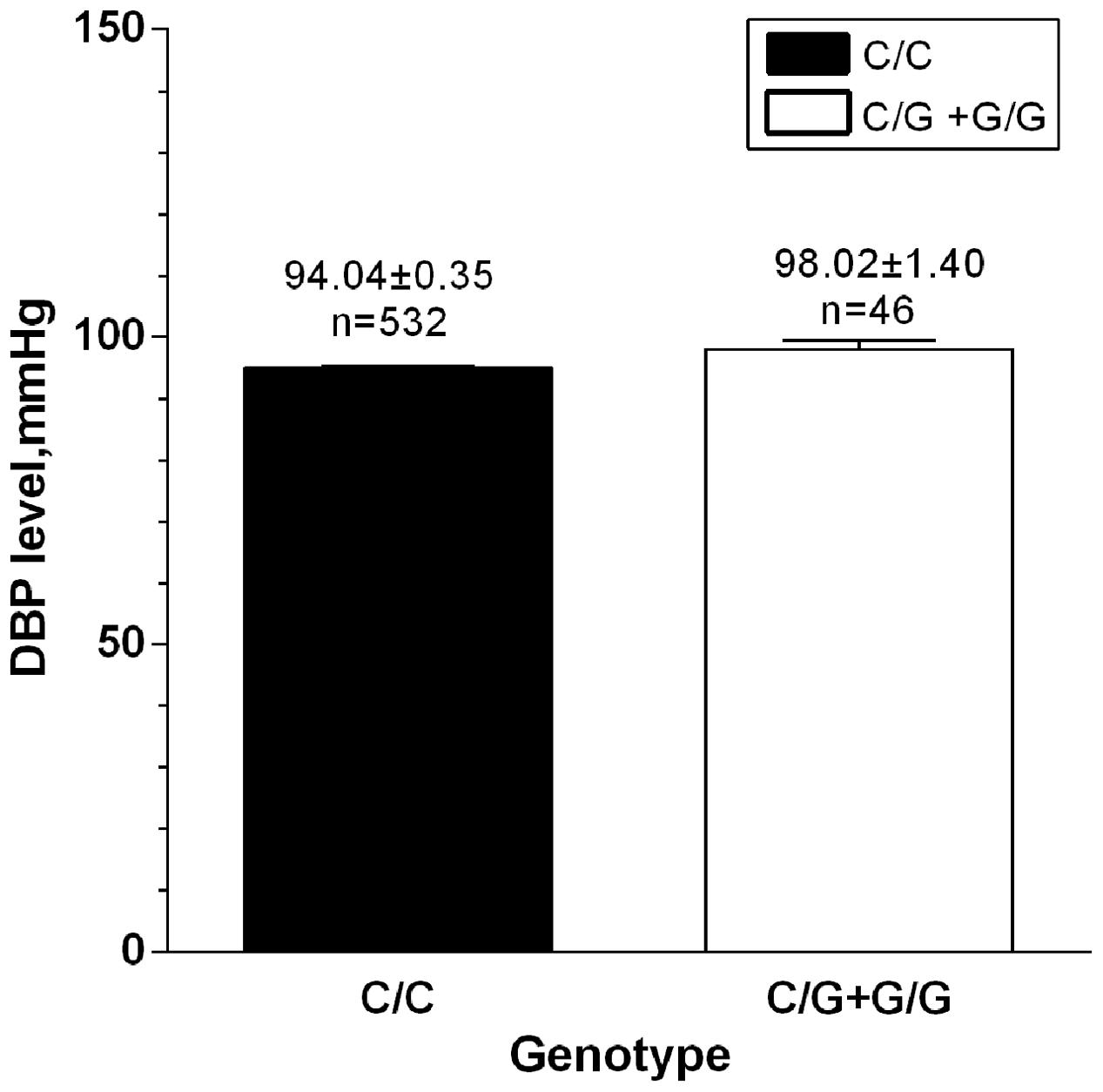
FIGURE 2. DBP level between G-carriers and wild type in those whose DBP ≥ 90 mmHg. n = 578, p = 0.021.
We also performed logistic regression analysis to assess the association between hypertension and rs34032258 after adjusting confounding factors such as age, gender, and BMI. The result exhibited that compared to C/C genotype, C/G and G/G genotypes were not statistically associated with the risk of hypertension, with the corresponding odds ratio of being 1.18 (95% CI: 0.64–2.12) and 1.25 (95% CI: 0.60–2.52), respectively (both p > 0.05). Moreover, we examined the association of rs34032258 with SBP and DBP on a continuous scale by using linear regression analysis with age, gender, and BMI as covariates, and as expected, this variant was significantly associated with SBP and DBP (p = 0.033 and 0.010), especially for the latter (data not shown).
To discover the relation between sex and BP, one-way ANOVA analysis was performed and the result showed that male had higher DBP both in case group (93.57 mmHg vs. 89.43 mmHg, p < 0.000; Table 4) and in control group (74.62 mmHg vs. 71.50 mmHg, p < 0.000; Table 4).
No significant difference was detected in Thiamine B1 concentration between rs34032258 genotypes (C/C 5.40 ± 2.19 mmol/L vs. C/G+G/G 4.92 ± 2.82 mmol/L, p = 0.551; Figure 3).
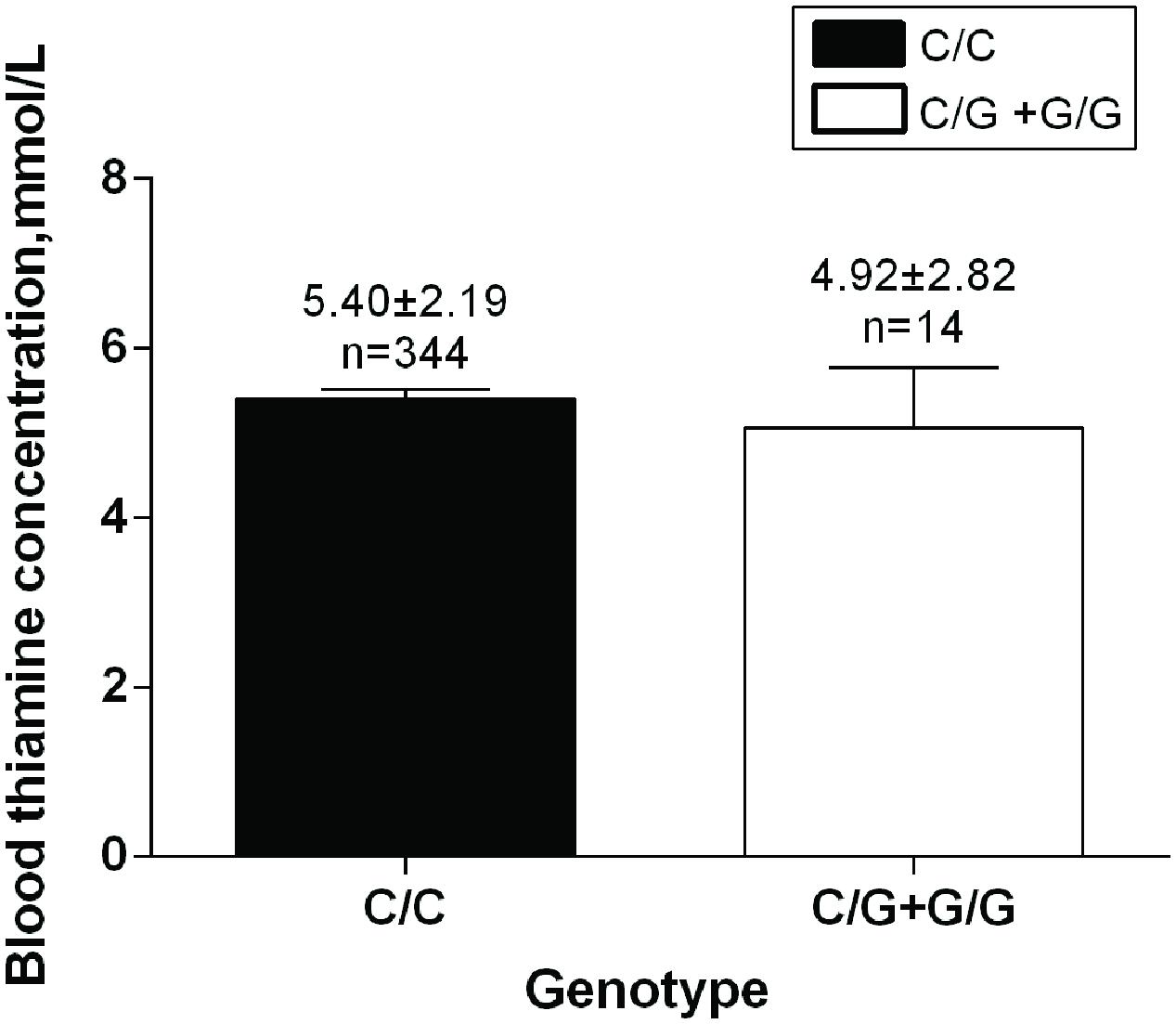
FIGURE 3. SLC35F3 genotypes effect on blood thiamine in patients. C/C genotype: n = 344; C/G +G/G genotype: n = 14, p = 0.551.
Zhang et al. (2014) found that rs17514104 and rs16842784 in the SLC35F3 gene were associated with BP in subjects from North America and Western Europe, through the use of phenotypic extremes and genomic DNA pooling (Zhang et al., 2014). However, we detected neither rs17514104 nor rs16842784 in 93 Chinese people, because allele frequency might be variable in different ethnic population.
We found that rs34032258 was associated with DBP, BUN and Cr, but not with SBP, BMI. This finding was consistent with previous studies showing that DBP but not SBP was substantially heritable (Snieder et al., 2003; Kupper et al., 2005). In addition, in those patients whose DBP ≥ 90 mmHg, G-carriers had higher DBP. This finding indicated that SLC35F3 may be associated with the regulation of BP and kidney function. We did not observe any association between rs34032258 and BMI. However, we found that there was a lower frequency of G allele of rs34032258 in cases compared with normotensive controls in obesity group. This result suggested that rs34032258 may be involved in obesity-related hypertension.
SLC35F3 encoded vitamin B1 transporter. According to the prediction using online software2, the mutation of SLC35F3 in rs34032258 (Supplement Figure S1) induced the loose of protein tertiary structure of vitamin B1 transporter, then probably decreased the concentration of vitamin B1 in plasma.
Vitamin B1, a water-soluble vitamin, played an important role in intracellular glucose metabolism. As a coenzyme for α-ketoglutarate-dehydrogenase, it was implicated in the tricarboxylic acid (TCA) cycle, catalyzing the oxidation of ketoglutaric acid to succinyl-CoA. In addition, it was a coenzyme for the pyruvate dehydrogenase complex (PDHC), converting pyruvate to acetyl-CoA. Clinical studies have shown that vitamin B1 might be associated with cardiovascular diseases (Wilkinson et al., 1997). Vitamin B1 supplementation was reported to reduce blood pressure, especial in patients combined with hyperglycemia (Alaei-Shahmiri et al., 2015). In accordance with the result, thiamine repletion could relieve the symptoms of hypertension and hyperinsulinemia in spontaneously hypertensive rats (SHR; Tanaka et al., 2007). However, the underlying mechanisms are still unknown.
One of the mechanisms might be that vitamin B1 could ameliorate the endothelium-dependent vasodilation (Subodh Arora et al., 2006). Routine administration of thiamine might improve endothelial function and therefore slowed the progression of atherosclerosis, especially in patients with impaired glucose tolerance (IGT) who were prone to develop accelerated atherosclerosis (Subodh Arora et al., 2006). The SLC35F3 mutation could result in the shortage of vitamin B1, then increased the blood pressure.
In addition, vitamin B1 might be related to the cardiac function (Han et al., 1995). Reduction of vitamin B1 was found to be involved the accumulation of intermediate products in glucose metabolism such as pyruvic acid and lactic acid, which could stimulate expansion of peripheral arterial, reduce peripheral resistance, and increase venous return, cardiac output and blood pressure (Zenuk et al., 2003).
The present study may have some limitations. Although our study sample size was considerable, we might not be powerful enough to capture some potential rare but functional causal variants in the SLC35F3 gene. These disease-causing variants could be in linkage disequilibrium with rs34032258.
In summary, this study is the first to report the association between a missense variant, rs34032258, in the SLC35F3 gene and hypertension in a Chinese Han population. Further study is needed to discover the molecular mechanisms of SLC35F3 to blood pressure in order to improve clinical treatment. Our results may have implications for the pathogenesis and treatment of systemic hypertension.
All the authors participated in the whole work. However, X-LZ and W-QH took on great work about design of the work and analysis, or interpretation of data. F-PY, K-DJ made contributions to perform the experiment and select samples. GH, S-NW took on drafting the work or revising it critically for important intellectual content. J-GW, P-JG agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This research is supported by grants from the National Natural Science of China (No. 30600363 and No. 81270344).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to all participants in this study. Especially, thanks Tao-Yu for his contribution to proofreading.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fgene.2016.00108
ANOVA, analysis of variance; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; Cr, creatinine; DBP, diastolic blood pressure; F, female; HR, heart rate; MAF, minor allele frequency; SBP, systolic blood pressure; MANCOVA, multivariate analysis of covariance; SNP, single-nucleotide polymorphism; TC, total cholesterol; TG, triglyceride; WC, waist circumference.
Alaei-Shahmiri, F., Soares, M. J., Zhao, Y., and Sherriff, J. (2015). The impact of thiamine supplementation on blood pressure, serum lipids and C-reactive protein in individuals with hyperglycemia: a randomised, double-blind cross-over trial. Diabetes Metab. Syndr. 9, 213–217. doi: 10.1016/j.dsx.2015.04.014
Cui, J. S., Hopper, J. L., and Harrap, S. B. (2003). Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension 41, 207–210. doi: 10.1161/01.HYP.0000044938.94050.E3
D’Agostino, R. B. Sr., Vasan, R. S., Pencina, M. J., Wolf, P. A., Cobain, M., Massaro, J. M., et al. (2008). General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117, 743–753. doi: 10.1161/CIRCULATIONAHA.107.699579
Evans, A., Van Baal, G. C., McCarron, P., DeLange, M., Soerensen, T. I., De Geus, E. J., et al. (2003). The genetics of coronary heart disease: the contribution of twin studies. Twin Res. 6, 432–441. doi: 10.1375/136905203770326439
Goda, E., Kamiyama, S., Uno, T., Yoshida, H., Ueyama, M., Kinoshitatoyoda, A., et al. (2006). Identification and characterization of a novel Drosophila 3’-phosphoadenosine 5’-phosphosulfate transporter. J. Biol. Chem. 281, 28508–28517. doi: 10.1074/jbc.M605045200
Han, S., Almog, S., Vered, Z., Seligmann, H., Shefi, M., Peleg, E., et al. (1995). Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am. J. Med. 98, 485–490. doi: 10.1016/S0002-9343(99)80349-0
Ishida, N., and Kawakita, M. (2004). Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflügers Archiv. Eur. J. Physiol. 447, 768–775. doi: 10.1007/s00424-003-1093-0
Ishida, N., Kuba, T., Aoki, K., Miyatake, S., Kawakita, M., and Sanai, Y. (2005). Identification and characterization of human Golgi nucleotide sugar transporter SLC35D2, a novel member of the SLC35 nucleotide sugar transporter family. Genomics 85, 106–116. doi: 10.1016/j.ygeno.2004.09.010
Kupper, N., Ge, D., Treiber, F. A., and Snieder, H. (2006). Emergence of novel genetic effects on blood pressure and hemodynamics in adolescence: the Georgia Cardiovascular Twin Study. Hypertension 47, 948–954. doi: 10.1161/01.HYP.0000217521.79447.9a
Kupper, N., Willemsen, G., Riese, H., Posthuma, D., Boomsma, D. I., and De Geus, E. J. (2005). Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension 45, 80–85. doi: 10.1161/01.HYP.0000149952.84391.54
Munroe, G. B., Rice, P. B., Bochud, K. M., Johnson, M., Chasman, A. D., Smith, D. I., et al. (2011). Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109. doi: 10.1038/nature10405
Nishimura, M., Suzuki, S., Satoh, T., and Naito, S. (2008). Tissue-Specific mRNA Expression Profiles of Human Solute Carrier 35 Transporters. Drug Metabol. Pharmacokinet. 23, 22–44. doi: 10.2133/dmpk.23.22
Saier, M. H., Reddy, V. S., Tsu, B. V., Ahmed, M. S., Li, C., and Morenohagelsieb, G. (2015). The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res. 44, D372–D379. doi: 10.1093/nar/gkv1103
Snieder, H., Harshfield, G. A., and Treiber, F. A. (2003). Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension 41, 1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D
Subodh Arora, M. D., Anne Lidor, M. D., Abularrage, C. J., Weiswasser, J. M., Eric Nylen, M. D., Dwight Kellicut, M. D., et al. (2006). Thiamine (Vitamin B 1 ) Improves Endothelium-Dependent Vasodilatation in the Presence of Hyperglycemia. Ann. Vasc. Surg. 20, 653–658. doi: 10.1007/S10016-006-9055-6
Tanaka, T., Sohmiya, K., Kono, T., Terasaki, F., Horie, R., Ohkaru, Y., et al. (2007). Thiamine attenuates the hypertension and metabolic abnormalities in CD36-defective SHR: uncoupling of glucose oxidation from cellular entry accompanied with enhanced protein O -GlcNAcylation in CD36 deficiency. Mol. Cell. Biochem. 299, 23–35. doi: 10.1007/s11010-005-9032-3
Wilkinson, T. J., Hanger, H. C., Elmslie, J., George, P. M., and Sainsbury, R. (1997). The response to treatment of subclinical thiamine deficiency in the elderly. Am. J. Clin. Nutr. 66, 925–928.
Zenuk, C., Healey, J., Donnelly, J., Vaillancourt, R., Almalki, Y., and Smith, S. (2003). Thiamine deficiency in congestive heart failure patients receiving long term furosemide therapy. Can. J. Clin. Pharmacol. 10, 184–188.
Zhang, K., Huentelman, M. J., Rao, F., Sun, E. I., Corneveaux, J. J., Schork, A. J., et al. (2014). Genetic Implication of a novel thiamine transporter in human hypertension. J. Am. Coll. Cardiol. 63, 1542–1555. doi: 10.1016/j.jacc.2014.01.007
Zhang, K., Weder, A. B., Eskin, E., and O’Connor, D. T. (2010). Genome-wide case/control studies in hypertension: only the ‘tip of the iceberg’. J. Hypertens. 28, 1115–1123. doi: 10.1097/HJH.0b013e328337f6bc
Keywords: SLC35F3, association, hypertension, susceptibility, SNP
Citation: Zang X-L, Han W-Q, Yang F-P, Ji K-D, Wang J-G, Gao P-J, He G and Wu S-N (2016) Association of a SNP in SLC35F3 Gene with the Risk of Hypertension in a Chinese Han Population. Front. Genet. 7:108. doi: 10.3389/fgene.2016.00108
Received: 19 February 2016; Accepted: 30 May 2016;
Published: 20 June 2016.
Edited by:
Wanqing Liu, Purdue University, USAReviewed by:
Guanglong Jiang, Indiana University School of Medicine, USACopyright © 2016 Zang, Han, Yang, Ji, Wang, Gao, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng-Nan Wu, c253dUBzaWJzLmFjLmNu; Guang He, aGVndWFuZ0BzanR1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.