
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Food. Sci. Technol., 05 March 2025
Sec. Food Biotechnology
Volume 5 - 2025 | https://doi.org/10.3389/frfst.2025.1542284
This article is part of the Research TopicEnhancing Functional Dairy Foods: Bioactive Compounds in Fermented MatricesView all 4 articles
Fermentation by lactic acid bacteria has been used for millennia to preserve food and make it more palatable. These microorganisms may also generate bioactive compounds with the potential to serve as components in active edible food packaging or as alternative therapeutics. Dairy waste products, especially whey, provide a substrate for growth of these bacteria, and can be incorporated into the formulations of edible food packaging. This minireview deals with the use of dairy waste to grow lactic acid bacteria to produce bioactive compounds, specifically antimicrobial peptides and immunoregulatory molecules, and their potential use in food and therapeutic applications.
Around 18% of milk is wasted for various reasons, and these byproducts of dairy processing, including curd chunks, spilled and spoiled milk, waste ice cream, and whey, should be considered a serious problem for the environment due to their high chemical oxygen demand levels (Ahmad et al., 2019; Sar et al., 2022). Production of 1 kg of cheese generates at least 9 L of sweet whey (Tunick, 2009) and manufacture of 1 kg of Greek yogurt results in 2–3 kg of acid whey (Erickson, 2017). The major components of whey are water (around 94%), lactose (4%–5%), protein (0.8%–1%), and minerals (0.5%–0.7%) (Tsakali et al., 2010). Whey proteins constitute 20% of the protein in bovine milk and are comprised of approximately 50% β-lactoglobulin, 20% α-lactalbumin, 16% immunoglobulins, and 8% serum albumin, with the balance being designated as minor whey proteins, such as lactoferrin (Deeth and Bansal, 2019). Some whey is processed into more useful and valuable forms including whey protein concentrate (Senan et al., 2016), whey powder (Sabo et al., 2019), and whey permeate (Musatti et al., 2020), but much of the liquid whey generated in the manufacture of cheese and yogurt is discarded or fed to livestock (Tunick, 2009).
Upcycling dairy waste streams into valuable new products is clearly advantageous for the dairy industry and the environment. Fermentation with lactic acid bacteria (LAB) is one way to generate useful products from this waste (Patel and Shukula, 2017). LAB are Gram-positive non-spore formers that metabolize carbohydrates to lactic acid and other compounds. Fermented dairy products have been reported to possess numerous benefits such as anticarcinogenic, antimicrobial, antioxidant, hypocholesterolemic, hypotensive, and immunomodulatory properties due to microbial metabolites formed during the fermentation process (García-Burgos et al., 2020; Ağagündüz et al., 2021; Raman et al., 2022; Dasriya et al., 2023).
Some of the most important bioactive compounds formed by LAB during the fermentation process are bacteriocins (antimicrobial peptides), exopolysaccharides, lactic acid, and peptides, along with amylase, lipase, and protease enzymes (Mathur et al., 2020; Ağagündüz et al., 2021). These compounds may be generated during routine manufacture of fermented dairy products (Akalin, 2014) but can be created from waste streams as well. Sweet whey from cheesemaking, acid whey from Greek yogurt production, and buttermilk from cultured butter preparation have all been cited as sources of bioactives and other useful compounds (Faria et al., 2023).
The valorization of whey and other dairy waste into useful products is a highly active research area. This minireview focuses on the advantages and challenges of fermentation by LAB of waste dairy streams to produce antimicrobial and immunoregulatory compounds, along with suggestions for future research.
Liquid whey and reconstituted whey powder have been used as fermentation substrates with various strains of LAB (Bandara et al., 2023; Vera-Santander et al., 2024). The steps involved usually include the addition of yeast extract, vitamins C and E, and Mg and Mn salts; incubation; and recovery of bioactives by centrifugation. The largest advantage in using this dairy byproduct is its availability and low cost – it is usually obtainable at no charge. Issues involving growth of LAB in whey are shown in Figure 1. Lactose, the carbohydrate in whey, is often supplemented by glucose, which LAB hydrolyze faster (Hayek et al., 2019). Whey protein, peptides, and amino acids serve as nitrogen sources, which may be enhanced with peptone and yeast extract. Mg, Mn, and other minerals are added, as are buffering agents, which maintain the proper pH level, and surfactants, which improve nutrient uptake (Hayek et al., 2019).
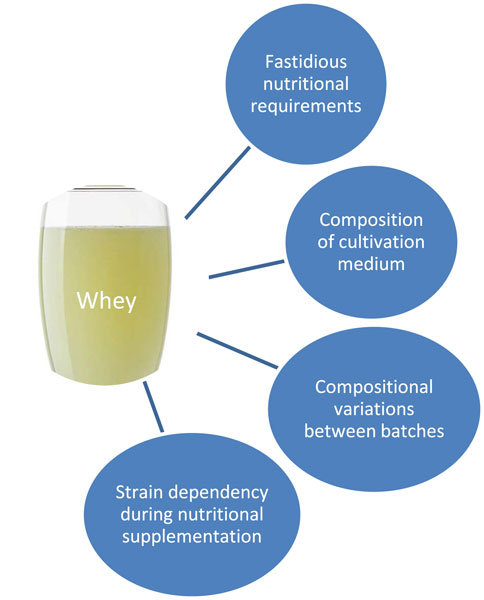
Figure 1. Factors in using liquid whey as a substrate for fermentation by lactic acid bacteria (Hayek et al., 2019; Rama et al., 2019).
LAB use a variety of proteases and peptidases to hydrolyze proteins, producing essential amino acids for their growth and potentially releasing bioactive peptides. The proteolytic activity of Lactobacillus helveticus strains has been extensively studied, with several novel antimicrobial peptides generated from the breakdown of casein (Fan, et al., 2019; Singh et al., 2023). In addition to antimicrobial peptides released during protein degradation, several LAB strains have been reported to naturally produce antimicrobial peptides called bacteriocins.
Whey and other waste from dairy processing have been investigated as inexpensive feedstocks for generating antimicrobial peptides, especially bacteriocins (Sar et al., 2022; Hameed et al., 2023; Miller et al., 2024). Bacteriocins, which are synthesized by ribosomes, are categorized into three or four classes and several subclasses based on their characteristics (Alvarez-Siero et al., 2016; Darbandi et al., 2022; Pujato et al., 2024). Bacteriocins are easily produced, sensitive to proteases, non-toxic to eukaryotic cells, and do not influence sensory properties of foods (Peng et al., 2020).
Bacteriocins produced by LAB exhibit antibacterial properties against Gram-positive bacteria, notably the foodborne pathogens including Listeria monocytogenes, Staphylococcus aureus, and species of Clostridium (Silva et al., 2023). Most LAB bacteriocins operate by forming pores within the cell membranes of susceptible bacteria leading to cell lysis, or by degrading bacterial cell walls (Pérez-Ramos et al., 2021). Interest in defining applications for LAB bacteriocins is high due to consumer demand for reducing the number of chemical preservatives within foods, and concern over the emergence of antibiotic-resistant microorganisms (Daba and Elkhateeb, 2023). Nisin remains the only LAB bacteriocin with “generally recognized as safe” status by the US Food and Drug Administration, while pediocin-producing Pediococcus spp. have been given “qualified presumption of safety” by the European Food and Safety Authority (Darbandi et al., 2022; Raman et al., 2022) status; thus, both bacteriocins have been used commercially as food preservatives (Radaic et al., 2020). Nisin is the most well studied Class I bacteriocin, or lantibiotic, which is characterized by presence of the amino acid lanthionine and undergoes post-translational modifications; while pediocin is a Class IIa bacteriocin, defined as a small, heat-stable peptide that does not undergo post-translational modification (Yi et al., 2022). Both nisin and pediocin are used to prevent the growth of L. monocytogenes (Grigore-Gurgu et al., 2024), while other LAB bacteriocins, including lacticin 3,147 (Darbandi et al., 2022), durancin 41D (Renye et al., 2009), and thermophilin 110 (Ceruso et al., 2021), are currently being investigated as alternative anti-listerials. Many other bacteriocins have been identified and are currently being studied for potential applications and approval by the Food and Drug Administration.
Laboratory procedures for isolation and purification of bacteriocins have been established for years (Twomey et al., 2021), but such techniques are not cost-effective on an industrial scale (Kamal et al., 2023). These obstacles could be avoided by using bacteriocin-containing fermentates (Yi et al., 2022). Several companies market purified nisin and several others sell LAB as bioprotective cultures for generating bacteriocins directly in the food (Pujato et al., 2024). Use of dairy byproducts that are rich in carbohydrates and protein would reduce the amount of waste while providing low-cost carbon and nitrogen sources for LAB. Whey is a good choice for this application due to the presence of lactose. Bacteriocins from whey are typically obtained by culturing LAB overnight, centrifuging, and adjusting the pH level. Protein is needed for normal metabolic processes but can also serve as a source of bioactive peptides via proteolysis. In one study, bacteriocin production was maximized in a bench-top fermenter when yeast extract was added to whey containing Bifidobacterium animalis subsp. Lactis, which acts against L. monocytogenes (Balciunas et al., 2016). In another study using goat cheese whey with prebiotic inulin and Lactococcus lactis added, bacteriocin was produced and found to be effective against L. monocytogenes (de Lima et al., 2017). Demineralized whey and deproteinized whey protein fermentation liquor have also been used to produce bacteriocins (Abbasiliasi et al., 2017). Bacteriocins have also been isolated from waste ice cream. The ice cream was shaken and then strained to remove butter grains, and the aqueous phase was inoculated with strains of Streptococcus thermophilus or Lactobacillus lactis. Bacteriocins were produced in amounts comparable to concentrations detected in microbiological growth medium (Miller et al., 2024). The challenges in producing bacteriocins are shown in Figure 2.
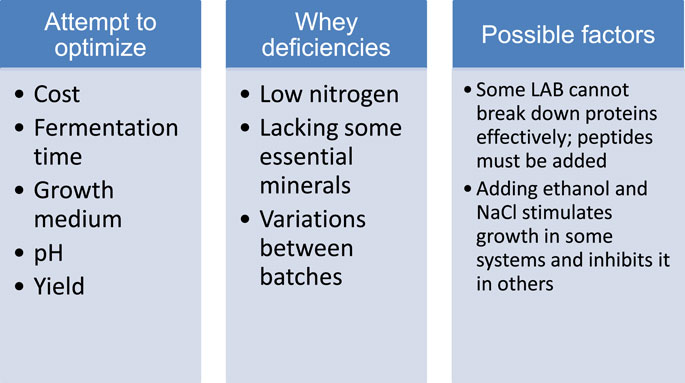
Figure 2. Factors involved in producing bacteriocins from fermentation of whey (Abbasiliasi et al., 2017; Ali et al., 2023).
The ice cream industry also generates a large amount of wasted material during production which cannot be sold commercially but is rich in valuable food grade components including sugar and proteins. Like other dairy waste streams, waste ice cream poses environmental hazards due to its chemical oxygen demand, thus it must be processed prior to disposal or used as animal feed. Disposal of this material can lead to a significant economic burden for ice cream producers, thus finding ways for upcycling this material is high priority for the industry. Recently it was shown that an aqueous product collected after the extraction of fat from melted vanilla ice cream could function as a fermentation substrate for bacteriocin production, specifically nisin and thermophilin 110 (Miller et al., 2024). More studies are needed to confirm that an aqueous stream from industrial, pooled waste ice cream would support the same level of bacterial growth and bacteriocin production.
In addition to bacteriocin production, caseins are known sources of antimicrobial peptides, such as casocidin, isracidin, kappacin, and lactenin. Casocidin and isracidin, for instance, are generated during fermentation of milk and can be used to ward off bacterial contamination (Somkuti and Paul, 2010). Any dairy waste stream containing caseins, including wasted cheese curd or ice cream, could be a source for generating milk-derived antimicrobial peptides via fermentation.
Microbial fermentation of whey produces bioactive peptides that have been associated with antioxidant, antihypertensive, antidiabetic, anticancer, opioid, and immunomodulatory activity along with muscle protein synthesis (Minj and Anand, 2020). Bacteriocins are responsible for other Therapeutic use of health benefits. Macedocin, produced from Streptococcus macedonicus, kills oral pathogens and can be incorporated in toothpaste and mouthwash (Zoumpopoulou et al., 2013). PLNC8, a bacteriocin produced by Lactiplantibacillus plantarum, interacts with phospholipids in the cell membrane of Helicobacter pylori, which has been implicated in ulcers and other gastric diseases (Liang et al., 2022). More recently, it was reported that thermophilin 110, produced by the dairy starter culture S. thermophilus, can inhibit the growth of Streptococcus mutans and Cutibacterium acnes, suggesting potential applications for oral and skin care respectively (Renye and Steinberg, 2021; Renye et al., 2023). These bacteriocins could presumably be produced on an industrial scale using whey as a substrate.
Recombinant DNA technology may be employed to alter the genome of certain strains of LAB with the goal of increasing the yield of bioactive compounds in milk and generating new ones. Developments in this area will allow amino acids to be inserted or removed, which could increase the efficiency and stability of the compounds produced (Kamal et al., 2023).
Edible coatings and films have been explored for preventing oxidation reactions that could negatively affect food quality and shelf life (Gamze, 2024). In addition, it has been shown that bioactive materials, such as bacteriocins, can been incorporated into these packaging materials resulting in active films and coatings which can be used to further prevent the growth of spoilage microorganisms and pathogens (Silva et al., 2023). The bacteriocins may be encapsulated as nanoparticles, nanofibers, or liposomes (Chandrakasan et al., 2019). Much of the research reported on biopreservation of dairy products has dealt with films based on whey protein and containing bacteriocins, especially nisin, or lysozymes (Esmer and Sahin, 2017; Kandasamy et al., 2021).
To take advantage of differing film properties, conventional materials may be used for outer packaging, with protein-based films containing bioactive compounds for inner packaging. Multilayer films containing antimicrobials usually have control, matrix, barrier, and outer layers. The bacteriocins are embedded in the matrix layer and the control layer regulates their release into the product (Rejeesh and Anto, 2023). Using protein-based films as inner packaging may lead to some shortcomings in technical properties in some cases but provides sustainable and so-called chemical-free food contact surfaces (Chen et al., 2019). Improvement of barrier properties, elongation at break, and tensile strength edible coatings based on whey protein will be helpful in the acceptance of this product (Chaudhary et al., 2022). Few studies have reported on the inclusion of fermentation-generated antimicrobials in edible packaging (Kandasamy et al., 2021), so further development in this area will help the dairy industry meet sustainability goals while enhancing food protection.
A fermentate is defined as a product of a microbial fermentation. Fermentation has been used for many centuries to preserve and enhance the flavor of food. By optimizing the types of microorganisms and fermentation conditions, the process can generate compounds such as bioactive peptides, enzymes, and vitamins (Hernandez-Figueroa et al., 2024). This section deals with fermentates containing bioactive molecules other than bacteriocins.
Fermentates contain LAB cells, either viable or inactivated, along with growth metabolites, and may be used as alternatives to antimicrobial agents such as benzoates, propionates, and sorbates (Hernandez-Figueroa et al., 2024). In addition to bacteriocins, fermentates may contain acetoin, antifungal peptides, diacetyl, ethanol, hydrogen peroxide, and organic acids, all of which act as antimicrobials (Silva et al., 2018). Bovine lactoferrin, one of the minor whey proteins, acts as an antimicrobial agent by sequestering iron from bacterial pathogens, and two peptides produced by its digestion, lactoferrocin and lactoferrampin, exhibit antibacterial and antifungal properties (Superti, 2020). Novel antimicrobials have been identified from LAB species isolated from raw milk, including a strain of Lacticaseibacillus paracasei isolated from a popular acid curd cheese in the Baltic region. This strain exhibits antifungal activity and could be grown using the whey and waste cheese (Vasiliauskaite et al., 2023).
Genetic factors, aging, lifestyle, and environmental factors can influence immune function (Kim et al., 2022). Some bioactive compounds in LAB fermentates have been shown to positively influence and play a crucial role in modulating the immune response (Mathur et al., 2020). Probiotics and LAB have been studied for their immunomodulatory properties, as they can stimulate immune cells and modulate the gut microbiota (Rocha-Ramírez et al., 2021; Saleena et al., 2023). The immunoregulatory properties of fermented dairy products are strain-dependent (Dasriya et al., 2023; Zou et al., 2024). Many studies have investigated the immunomodulatory properties of fermented dairy products like yogurt, kefir, milk, and cheese (Marco et al., 2017; Farag et al., 2020; Saleem et al., 2024). Research has shown that the consumption of specific functional foods can stimulate the activity of immune cells and provide protection against cancer, viruses, and bacteria (Hachimura et al., 2018; Kim et al., 2022).
An example of an illness where conventional medical management is supplemented with fermented dairy products is inflammatory bowel disease (IBD), which primarily consists of ulcerative colitis and Crohn’s disease. The etiology of IBD is still unknown (El-Salhy, 2023). Ulcerative colitis is a type of IBD that manifests as non-specific chronic inflammation of the colonic mucosa with alternating cycles of remission and exacerbation (Huang et al., 2023). An estimated 0.4 percent of people in the US suffer from IBD (Gros and Kaplan, 2023). Colonic inflammation, remission, and relapse also afflict those with Crohn’s disease, which affects 0.2 percent of people in the US (Gajendran et al., 2018). Research indicates that imbalance of intestinal flora, along with genetic and environmental factors, reduces the proper functioning of the intestinal epithelial barrier, leading to excessive immune responses and eventually to IBD (Jakubczyk et al., 2020). Current treatment of IBD relies on anti-inflammatory and immunosuppressive medications or surgery (Gros and Kaplan, 2023). The use of fermented foods containing probiotics may improve the composition and activity of the intestinal flora, which would regulate the intestinal immune response and inhibit adhesion of pathogenic bacteria to intestinal epithelial cells. Several genera of LAB have been investigated as potential probiotics for IBD treatment (Chlebicz-Wójcik and Śliżewska, 2021). Sweet whey supplemented with whey protein concentrate and fermented with a strain of Limosilactobacillus fermentum was found to reduce ulcerative colitis in mice by increasing the production of anti-inflammatory cytokine while suppressing the secretion of inflammatory mediators (Kaur et al., 2022). In another mouse study, sweet whey fermented with a strain of Lacticaseibacillus rhamnosus also increased cytokine creation and stimulated the intestinal immune system (García et al., 2020). A recent meta-analysis showed that the remission effect induced by probiotics on ulcerative colitis was significantly higher than that induced by other drugs. These authors recommend that patients take probiotics as an adjunct to their usual treatment of IBD and that a personalized treatment plan should be employed (Chen et al., 2021).
Angiotensin-converting enzyme (ACE) is a component of the system that controls blood pressure in the body. ACE converts the hormone angiotensin to a vasoconstrictor, which increases blood pressure; ACE inhibitors are used to treat hypertension by suppressing this conversion and increasing the production of a vasodilator (Khurana and Goswami, 2022). Food-derived ACE inhibitors are safe and specific and have not been shown to result in side effects, which is an advantage over synthetic inhibitors (Li et al., 2023). An in vitro study of acid and sweet whey from cow, sheep, and goat milk indicated that the ACE inhibitory properties of fermentates was significantly higher than those of non-digested whey (Dalaka et al., 2024). A study of whey protein concentrate fermented by Lacticaseibacillus paracasei and Saccharomyces cerevisiae showed that peptides with antioxidant and ACE-inhibitory properties were generated (Chopada et al., 2023).
Complex culture media that includes nutrient-rich mixtures such as brain heart infusion, glucose extract, and soy broth are often employed to generate high levels of bioactive compounds, but their cost and possible toxicity limits their use (Hernandez-Figueroa et al., 2024). Bioactive compounds produced using this media would require further purification, which would significantly increase production costs. The protein matrix in whey has been found to be a good substrate for generation of bioactive peptides (Venegas-Ortega et al., 2019) and would be a means for promoting a circular economy by upcycling a waste material.
Whey and other dairy waste have been used as substrates for producing other useful materials. These are depicted in Figure 3.
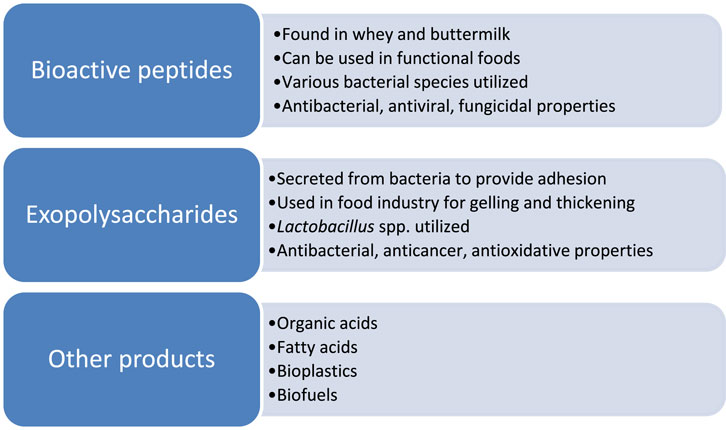
Figure 3. Other applications of dairy waste fermentation (Ahmad et al., 2019; Hameed et al., 2023; Sar et al., 2022).
• More research is needed on reducing inefficiencies in scaling up the production of useful bioactive compounds from dairy waste. Whey is usually utilized as a substrate for this, and other dairy waste streams such as leftover milk, buttermilk, and ice cream should be targeted.
• Discovery of additional antimicrobial peptides in milk and how they affect humans is another area that requires investigation.
• Incorporation of bioactive compounds from milk into edible coatings is an area that should be explored.
• Antimicrobial resistance is a growing problem, and more work is needed to investigate the potential for antimicrobial peptides to act as alternate therapeutics to prevent resistance development to clinical antibiotics.
• Research should continue into identifying LAB capable of producing immunoregulatory metabolites, optimizing their production, and investigating applications for the prevention or treatment of disease.
MT: Writing–original draft, Writing–review and editing. JR: Writing–review and editing. RG: Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the in-house project 8072-41000-114-00D, “Reclaiming Value from Coproducts of Dairy Food Manufacture.”
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbasiliasi, S., Tan, J. S., Ibrahim, T. A. T., Bashokouh, F., Ramakrishnan, N. R., Mustafa, S., et al. (2017). Fermentation factors influencing the production of bacteriocins by lactic acid bacteria: a review. RSC Adv. 7, 29395–29420. doi:10.1039/c6ra24579j
Ağagündüz, D., Yılmaz, B., Şahin, T. Ö., Güneşliol, B. E., Ayten, Ş., Russo, P., et al. (2021). Dairy lactic acid bacteria and their potential function in dietetics: the food-gut-health axis. Foods 10, 3099. doi:10.3390/foods10123099
Ahmad, T., Aadil, R. M., Ahmed, H., ur Rahman, U., Soares, B. C., Souza, S. L., et al. (2019). Treatment and utilization of dairy industrial waste: a review. Trends Food Sci. Technol. 88, 361–372. doi:10.1016/j.tifs.2019.04.003
Akalin, A. S. (2014). Dairy-derived antimicrobial peptides: action mechanisms, pharmaceutical uses and production proposals. Trends Food Sci. Technol. 36, 79–95. doi:10.1016/j.tifs.2014.01.002
Ali, A., Tahir, A., Khadid, W., Khan, A., Zeng, X.-A., Jani, R., et al. (2023). “Bacteriocins production using whey,” in Whey valorization. Editors A. Poonia,, and A. Trajkovska Petkoska (Singapore: Springer), 259–283. doi:10.1007/978-981-99-5459-9_13
Alvarez-Sieiro, P., Montalbán-López, M., Mu, D., and Kuipers, O. P. (2016). Bacteriocins of lactic acid bacteria: extending the family. Appl. Microbiol. Biotechnol. 100, 2939–2951. doi:10.1007/s00253-016-7343-9
Balciunas, E. M., Al Arni, S., Converti, A., Leblanc, J. G., and Oliveira, R. P. (2016). Production of bacteriocin-like inhibitory substances (BLIS) by Bifidobacterium lactis using whey as a substrate. Int. J. Dairy Technol. 69, 236–242. doi:10.1111/1471-0307.12247
Bandara, T. A., Munasinghe-Arachchige, S. P., and Gamlath, C. J. (2023). Fermented whey beverages: a review of process fundamentals, recent developments and nutritional potential. Int. J. Dairy Technol. 76, 737–757. doi:10.1111/1471-0307.12993
Ceruso, M., Liu, Y., Gunther IV, N. W., Pepe, T., Anastasio, A., Qi, P. X., et al. (2021). Anti-listerial activity of thermophilin 110 and pediocin in fermented milk and whey. Food control. 125, 107941. doi:10.1016/j.foodcont.2021.107941
Chandrakasan, G., Rodríguez-Hernández, A. I., del Rocío López-Cuellar, M., Palma-Rodríguez, H. M., and Chavarría-Hernández, N. (2019). Bacteriocin encapsulation for food and pharmaceutical applications: advances in the past 20 years. Biotechnol. Lett. 41, 453–469. doi:10.1007/s10529-018-02635-5
Chaudhary, V., Kajla, P., Kumari, P., Bangar, S. P., Rusu, A., Trif, M., et al. (2022). Milk protein-based active edible packaging for food applications: an eco-friendly approach. Front. Nutr. 9, 942524. doi:10.3389/fnut.2022.942524
Chen, H., Wang, J., Cheng, Y., Wang, C., Liu, H., Bian, H., et al. (2019). Application of protein-based films and coatings for food packaging: a review. Polymers 11, 2039. doi:10.3390/polym11122039
Chen, M., Feng, Y., and Liu, W. (2021). Efficacy and safety of probiotics in the induction and maintenance of inflammatory bowel disease remission: a systematic review and meta-analysis. Ann. Palliat. Med. 10, 11821–11829. doi:10.21037/apm-21-2996
Chlebicz-Wójcik, A., and Śliżewska, K. (2021). Probiotics, prebiotics, and synbiotics in the irritable bowel syndrome treatment: a review. Biomolecules 11, 1154. doi:10.3390/biom11081154
Chopada, K., Basaiawmoit, B., Sakure, A. A., Maurya, R., Bishnoi, M., Kondepudi, K. K., et al. (2023). Purification and characterization of novel antihypertensive and antioxidative peptides from whey protein fermentate: in vitro, in silico, and molecular interactions studies. J. Am. Nutr. Assoc. 42, 598–617. doi:10.1080/27697061.2022.2110966
Daba, G. M., and Elkhateeb, W. A. (2023). Ribosomally synthesized bacteriocins of lactic acid bacteria: simplicity yet having wide potentials–A review. Int. J. Biol. Macromol. 256, 128325. doi:10.1016/j.ijbiomac.2023.128325
Dalaka, E., Stefos, G. C., Politis, I., and Theodorou, G. (2024). Evaluation of in vitro antihypertensive and anti-inflammatory properties of dairy by-products. Appl. Sci. 14, 6885. doi:10.3390/app14166885
Darbandi, A., Asadi, A., Ari, M. M., Ohadi, E., Talebi, M., Zadeh, M. H., et al. (2022). Bacteriocins: properties and potential use as antimicrobials. J. Clin. Lab. Anal. 36, e24093. doi:10.1002/jcla.24093
Dasriya, V., Ranveer, S., Bajaj, R., Sharma, A., Dasriya, Y., and Dhillon, H. S. (2023). “Role of fermented dairy products in enhancing the immunity,” in Nutritional science and technology: concept to application. Editors T. Dhewa, A. K. Puniya, and A. Panghal (Hoboken, NJ: Scrivener Press), 117–133. doi:10.1002/9781394229116.ch6
Deeth, H., and Bansal, N. (2019). “Whey proteins: an overview,” in Whey proteins: from milk to medicine. Editors H. Deeth,, and N. Bansal (London, UK: Academic Press), 1–50. doi:10.1016/B978-0-12-812124-5.00001-1
de Lima, E. D. L. C., de Moura Fernandes, J., and Cardarelli, H. R. (2017). Optimized fermentation of goat cheese whey with Lactococcus lactis for production of antilisterial bacteriocin-like substances. LWT Food Sci. Technol. 84, 710–716. doi:10.1016/j.lwt.2017.06.040
El-Salhy, M. (2023). Intestinal bacteria associated with irritable bowel syndrome and chronic fatigue. Neurogastroenterol. Motil. 35, e14621. doi:10.1111/nmo.14621
Erickson, B. E. (2017). Acid whey: is the waste product an untapped goldmine? Chem. Eng. News 95(6), 26. doi:10.1021/cen-09506-cover
Esmer, O. K., and Sahin, B. (2017). “Active packaging applied to dairy products,” in Advances in dairy products. Editors F. Contò, M. A. Del Nobile, M. Faccia, A. V. Zambrini, and A. Conte (Hoboken, NJ: Wiley Blackwell), 295–313. doi:10.1002/9781118906460.ch3c
Fan, M., Guo, T., Li, W., Chen, J., Li, F., Wang, C., et al. (2019). Isolation and identification of novel casein-derived bioactive peptides and potential functions in fermented casein with Lactobacillus helveticus. Food Sci. Hum. Wellness 8, 156–176. doi:10.1016/j.fshw.2019.03.010
Farag, M. A., Jomaa, S. A., Abd El-Wahed, A., and El-Seedi, R., H. (2020). The many faces of kefir fermented dairy products: quality characteristics, flavour chemistry, nutritional value, health benefits, and safety. Nutrients 12, 346. doi:10.3390/nu12020346
Faria, D. J., Carvalho, A. P. A. D., and Conte-Junior, C. A. (2023). Valorization of fermented food wastes and byproducts: bioactive and valuable compounds, bioproduct synthesis, and applications. Fermentation 9, 920. doi:10.3390/fermentation9100920
Gajendran, M., Loganathan, P., Catinella, A. P., and Hashash, J. G. (2018). A comprehensive review and update on Crohn's disease. Dis. Mon. 64, 20–57. doi:10.1016/j.disamonth.2017.07.001
Gamze, Ü. (2024). “Bacteriocins incorporated into edible films and coatings: a sustainable approach to enhance food preservation,” in Studies in natural products chemistry. Editor Atta-ur-Rahman (Amsterdam: Elsevier), 305–339. doi:10.1016/B978-0-443-22214-6.00013-2
García, G., Agosto, M. E., Cavaglieri, L., and Dogi, C. (2020). Effect of fermented whey with a probiotic bacterium on gut immune system. J. Dairy Res. 87, 134–137. doi:10.1017/S0022029919000980
García-Burgos, M., Moreno-Fernández, J., Alférez, M. J., Díaz-Castro, J., and López-Aliaga, I. (2020). New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 72, 104059. doi:10.1016/j.jff.2020.104059
Grigore-Gurgu, L., Bucur, F. I., Mihalache, O. A., and Nicolau, A. I. (2024). Comprehensive review on the biocontrol of Listeria monocytogenes in food products. Foods 13, 734. doi:10.3390/foods13050734
Gros, B., and Kaplan, G. G. (2023). Ulcerative colitis in adults: a review. J. Am. Med. Assn. 330, 951–965. doi:10.1001/jama.2023.15389
Hachimura, S., Totsuka, M., and Hosono, A. (2018). Immunomodulation by food: impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 82, 584–599. doi:10.1080/09168451.2018.1433017
Hameed, A., Anwar, M. J., Perveen, S., Amir, M., Naeem, I., Imran, M., et al. (2023). Functional, industrial and therapeutic applications of dairy waste materials. Int. J. Food Prop. 26, 1470–1496. doi:10.1080/10942912.2023.2213854
Hayek, S. A., Gyawali, R., Aljaloud, S. O., Krastanov, A., and Ibrahim, S. A. (2019). Cultivation media for lactic acid bacteria used in dairy products. J. Dairy Res. 86, 490–502. doi:10.1017/S002202991900075X
Hernandez-Figueroa, R. H., López-Malo, A., and Mani-López, E. (2024). Antimicrobial activity and applications of fermentates from lactic acid bacteria–a review. Sustain. Food Technol. 2, 292–306. doi:10.1039/d3fb00241a
Huang, C., Hao, W., Wang, X., Zhou, R., and Lin, Q. (2023). Probiotics for the treatment of ulcerative colitis: a review of experimental research from 2018 to 2022. Front. Microbiol. 14, 1211271. doi:10.3389/fmicb.2023.1211271
Jakubczyk, D., Leszczyńska, K., and Górska, S. (2020). The effectiveness of probiotics in the treatment of inflammatory bowel disease (IBD)—a critical review. Nutrients 12, 1973. doi:10.3390/nu12071973
Kamal, I., Ashfaq, U. A., Hayat, S., Aslam, B., Sarfraz, M. H., Yaseen, H., et al. (2023). Prospects of antimicrobial peptides as an alternative to chemical preservatives for food safety. Biotechnol. Lett. 45, 137–162. doi:10.1007/s10529-022-03328-w
Kandasamy, S., Yoo, J., Yun, J., Kang, H.-B., Seol, K.-H., Kim, H.-W., et al. (2021). Application of whey protein-based edible films and coatings in food industries: an updated overview. Coatings 11, 1056. doi:10.3390/coatings11091056
Kaur, H., Gupta, T., Kapila, S., and Kapila, R. (2022). Lactobacillus fermentum (MTCC-5898) based fermented whey renders prophylactic action against colitis by strengthening the gut barrier function and maintaining immune homeostasis. Microb. Pathog. 173, 105887. doi:10.1016/j.micpath.2022.105887
Khurana, V., and Goswami, B. (2022). Angiotensin converting enzyme (ACE). Clin. Chim. Acta 524, 113–122. doi:10.1016/j.cca.2021.10.029
Kim, J. H., Kim, D. H., Jo, S., Cho, M. J., Cho, Y. R., Lee, Y. J., et al. (2022). Immunomodulatory functional foods and their molecular mechanisms. Exp. Mol. Med. 54, 1–11. doi:10.1038/s12276-022-00724-0
Li, R., Zhuang, Y., Lin, L., Li, L., Fan, X., and Sun, L. (2023). In vitro simulated gastrointestinal digestion stability and in vivo antihypertensive effect of the peptide KYPHVF and its network pharmacology. J. Funct. Foods 107, 105672. doi:10.1016/j.jff.2023.105672
Liang, Y., Yan, J., Chen, Z., Gu, Q., and Li, P. (2022). Antibacterial effects of bacteriocin PLNC8 against Helicobacter pylori and its potential mechanism of action. Foods 11, 1235. doi:10.3390/foods11091235
Marco, M. L., Heeney, D., Binda, S., Cifelli, C. J., Cotter, P. D., Foligné, B., et al. (2017). Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 44, 94–102. doi:10.1016/j.copbio.2016.11.010
Mathur, H., Beresford, T. P., and Cotter, P. D. (2020). Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 12, 1679. doi:10.3390/nu12061679
Miller, A. L., Renye, J. A., Oest, A. M., Liang, C., Garcia, R. A., Plumier, B. M., et al. (2024). Bacteriocin production by lactic acid bacteria using ice cream co-product as the fermentation substrate. J. Dairy Sci. 107, 3468–3477. doi:10.3168/jds.2023-24249
Minj, S., and Anand, S. (2020). Whey proteins and its derivatives: bioactivity, functionality, and current applications. Dairy 1, 233–258. doi:10.3390/dairy1030016
Musatti, A., Cavicchioli, D., Mapelli, C., Bertoni, D., Hogenboom, J. A., Pellegrino, L., et al. (2020). From cheese whey permeate to sakacin a: a circular economy approach for the food-grade biotechnological production of an anti-listeria bacteriocin. Biomolecules 10, 597. doi:10.3390/biom10040597
Patel, S., and Shukla, S. (2017). “Fermentation of food wastes for generation of nutraceuticals and supplements,” in Fermented foods in health and disease prevention. Editors J. Frias, C. Martinez-Villanuenga, and E. Peñas (London, UK: Academic Press), 707–734. doi:10.1016/B978-0-12-802309-9.00030-3
Peng, K., Koubaa, M., Bals, O., and Vorobiev, E. (2020). Recent insights in the impact of emerging technologies on lactic acid bacteria: a review. Food Res. Int. 137, 109544. doi:10.1016/j.foodres.2020.109544
Pérez-Ramos, A., Madi-Moussa, D., Coucheney, F., and Drider, D. (2021). Current knowledge of the mode of action and immunity mechanisms of LAB-bacteriocins. Microorganisms 9, 2107. doi:10.3390/microorganisms9102107
Pujato, S. A., Mercanti, D. J., Briggiler Marcó, M., Capra, M. L., Quiberoni, A., and Guglielmotti, D. M. (2024). Bacteriocins from lactic acid bacteria: strategies for the bioprotection of dairy foods. Front. Food Sci. Technol. 4, 1439891. doi:10.3389/frfst.2024.1439891
Radaic, A., de Jesus, M. B., and Kapila, Y. L. (2020). Bacterial anti-microbial peptides and nano-sized drug delivery systems: the state of the art toward improved bacteriocins. J. Control. Release 321, 100–118. doi:10.1016/j.jconrel.2020.02.001
Rama, G. R., Kuhn, D., Beux, S., Maciel, M. J., and de Souza, C. F. V. (2019). Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int. Dairy J. 98, 25–37. doi:10.1016/j.idairyj.2019.06.012
Raman, J., Kim, J. S., Choi, K. R., Eun, H., Yang, D., Ko, Y. J., et al. (2022). Application of lactic acid bacteria (LAB) in sustainable agriculture: advantages and limitations. Int. J. Mol. Sci. 23, 7784. doi:10.3390/ijms23147784
Rejeesh, C. R., and Anto, T. (2023). Packaging of milk and dairy products: approaches to sustainable packaging. Mater. Today Proc. 72, 2946–2951. doi:10.1016/j.matpr.2022.07.467
Renye, J. A., and Steinberg, D. H. (2021). Thermophilin 110 inhibits growth and biofilm formation of Streptococcus mutans. Biotechnol. Rep. 16, e00647. doi:10.1016/j.btre.2021.e00647
Renye, Jr. J. A., Mendez-Encinas, M. A., White, A. K., Miller, A. L., McAnulty, M. J., Yadav, M. P., et al. (2023). Antimicrobial activity of thermophilin 110 against the opportunistic pathogen Cutibacterium acnes. Biotechnol. Lett. 45, 1365–1379. doi:10.1007/s10529-023-03419-2
Renye, Jr. J. A., Somkuti, G. A., Paul, M., and Van Hekken, D. L. (2009). Characterization of antilisterial bacteriocins produced by Enterococcus faecium and Enterococcus durans isolates from Hispanic-style cheeses. J. Ind. Microbiol. Biotechnol. 36, 261–268. doi:10.1007/s10295-008-0494-7
Rocha-Ramírez, L. M., Hernández-Chiñas, U., Moreno-Guerrero, S. S., Ramírez-Pacheco, A., and Eslava, C. A. (2021). Probiotic properties and immunomodulatory activity of Lactobacillus strains isolated from dairy products. Microorganisms 9, 825. doi:10.3390/microorganisms9040825
Sabo, S. S., Converti, A., Ichiwaki, S., and Oliveira, R. P. (2019). Bacteriocin production by lactobacillus plantarum ST16Pa in supplemented whey powder formulations. J. Dairy Sci. 102, 87–99. doi:10.3168/jds.2018-14881
Saleem, G. N., Gu, R., Qu, H., Bahar Khaskheli, G., Rashid Rajput, I., Qasim, M., et al. (2024). Therapeutic potential of popular fermented dairy products and its benefits on human health. Front. Nutr. 11, 1328620. doi:10.3389/fnut.2024.1328620
Saleena, L. A. K., Teo, M. Y. M., How, Y. H., In, L. L. A., and Pui, L. P. (2023). Immunomodulatory action of Lactococcus lactis. J. Biosci. Bioeng. 135, 1–9. doi:10.1016/j.jbiosc.2022.10.010
Sar, T., Harirchi, S., Ramezani, M., Bulkan, G., Akbas, M. Y., Pandey, A., et al. (2022). Potential utilization of dairy industries by-products and wastes through microbial processes: a critical review. Sci. Total Environ. 810, 152253. doi:10.1016/j.scitotenv.2021.152253
Senan, S., El-aal, H. A., Dave, R., and Hassan, A. (2016). Production and stability of nisin in whey protein concentrate. LWT Food Sci. Technol. 71, 125–129. doi:10.1016/j.lwt.2016.03.031
Silva, C. C. G., Silva, S. P. M., and Ribeiro, S. C. (2018). Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 9, 594. doi:10.3389/fmicb.2018.00594
Silva, S. P., Teixeira, J. A., and Silva, C. C. (2023). Recent advances in the use of edible films and coatings with probiotic and bacteriocin-producing lactic acid bacteria. Food Biosci. 56, 103196. doi:10.1016/j.fbio.2023.103196
Singh, A., Duche, R. T., Wandhare, A. G., Sian, J. K., Singh, B. P., Sihag, M. K., et al. (2023). Milk-derived antimicrobial peptides: overview, applications, and future perspectives. Probiotics Antimicrob. Proteins 15, 44–62. doi:10.1007/s12602-022-10004-y
Somkuti, G. A., and Paul, M. (2010). Enzymatic fractionation of the antimicrobial peptides casocidin and isracidin by Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus. Appl. Microbiol. Biotechnol. 87, 235–242. doi:10.1007/s00253-009-2433-6
Superti, F. (2020). Lactoferrin from bovine milk: a protective companion for life. Nutrients 12, 2562. doi:10.3390/nu12092562
Tsakali, E., Petrotos, K., D’Allessandro, A., and Goulas, P. (2010). “A review on whey composition and the methods used for its utilization for food and pharmaceutical products,” in 6th International conference on simulation and modelling in the food and bio-industry, 195–201. doi:10.1051/IUFoST:20060639
Tunick, M. H. (2009). “Whey protein production and utilization: a brief history,” in Whey processing, functionality and health benefits. Editors C. I. Onwulata,, and P. J. Huth (Ames, IA: Wiley-Blackwell), 1–13. doi:10.1002/9780813803845.ch1
Twomey, E., Hill, C., Field, D., and Begley, M. (2021). Recipe for success: suggestions and recommendations for the isolation and characterisation of bacteriocins. Int. J. Microbiol. 2021, 9990635. doi:10.1155/2021/9990635
Vasiliauskaite, A., Mileriene, J., Kasparaviciene, B., Aleksandrovas, E., Songisepp, E., Rud, I., et al. (2023). Screening for antifungal indigenous lactobacilli strains isolated from local fermented milk for developing bioprotective fermentates and coatings based on acid whey protein concentrate for fresh cheese quality maintenance. Microorganisms 11, 557. doi:10.3390/microorganisms11030557
Venegas-Ortega, M. G., Flores-Gallegos, A. C., Martínez-Hernández, J. L., Aguilar, C. N., and Nevárez-Moorillón, G. V. (2019). Production of bioactive peptides from lactic acid bacteria: a sustainable approach for healthier foods. Compr. Rev. Food Sci. Food Saf. 18, 1039–1051. doi:10.1111/1541-4337.12455
Vera-Santander, V. E., Mani-López, E., López-Malo, A., and Jiménez-Munguía, M. T. (2024). Use of whey for a sustainable production of postbiotics with potential bioactive metabolites. Sustain. Food Technol. 2, 1101–1112. doi:10.1039/d4fb00061g
Yi, Y., Li, P., Zhao, F., Zhang, T., Shan, Y., Wang, X., et al. (2022). Current status and potentiality of class II bacteriocins from lactic acid bacteria: structure, mode of action and applications in the food industry. Trends Food Sci. Technol. 120, 387–401. doi:10.1016/j.tifs.2022.01.018
Zou, H., Wang, H., Zhang, Z., Lin, H., and Li, Z. (2024). Immune regulation by fermented milk products: the role of the proteolytic system of lactic acid bacteria in the release of immunomodulatory peptides. Crit. Rev. Food Sci. Nutr. 64, 10498–10516. doi:10.1080/10408398.2023.2225200
Keywords: dairy, waste products, lactic acid bacteria, fermentation, whey, upcycling, bacteriocin, immune response
Citation: Tunick MH, Renye JA Jr. and Garcia RA (2025) Conversion of whey and other dairy waste into antimicrobial and immunoregulatory compounds by fermentation. Front. Food Sci. Technol. 5:1542284. doi: 10.3389/frfst.2025.1542284
Received: 09 December 2024; Accepted: 20 February 2025;
Published: 05 March 2025.
Edited by:
María Vélez, CONICET Instituto de Lactología Industrial (INLAIN), ArgentinaReviewed by:
Silvina Pujato, National University of Littoral, ArgentinaCopyright © 2025 Tunick, Renye and Garcia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael H. Tunick, bWljaGFlbC50dW5pY2tAdXNkYS5nb3Y=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.