
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Food. Sci. Technol., 22 January 2025
Sec. Food Biotechnology
Volume 5 - 2025 | https://doi.org/10.3389/frfst.2025.1526934
This article is part of the Research TopicBioactive Peptides in Functional Foods: Production, Characterization, and Health BenefitsView all articles
 Roberta Nogueira Pereira da Silva1
Roberta Nogueira Pereira da Silva1 Angelica Priscila Parussolo Tonin2
Angelica Priscila Parussolo Tonin2 Gabriela Soares Macello Ramos1
Gabriela Soares Macello Ramos1 Juliana Furtado Dias3
Juliana Furtado Dias3 Eduardo Cesar Meurer2
Eduardo Cesar Meurer2 Maria Gabriela Bello Koblitz1*
Maria Gabriela Bello Koblitz1*Beer, one of the most widely consumed alcoholic beverages globally, is typically produced from barley and hops, and contains carbohydrates, proteins, vitamins, minerals, ethanol, and bioactive phytochemicals such as phenolic compounds. However, the knowledge of protein content, particularly bioactive peptides in beer, remains limited. Given that beer production involves raw materials rich in both proteins and proteolytic enzymes, which may remain active throughout the product’s shelf life, beer holds potential as a source of bioactive peptides. This study aimed to investigate the presence of bioactive di- and tripeptides in craft beer samples from Pilsner and IPA styles, after 3 or 6 months of storage. LC-MS/MS analysis was performed using the 46 Da neutral loss method and collision-induced dissociation, followed by peptide bioactivity screening through the BIOPEP database. Twelve di- and tripeptides, with masses ranging from 177 to 329 (m/z), were identified, exhibiting potential bioactivities such as dipeptidyl peptidase IV and III inhibition, ACE inhibition, and antioxidative properties. These activities are associated with reduced risk of high blood pressure and metabolic syndrome. After 3 months of storage, peptide intensity decreased in Pilsner samples but increased in IPA samples. Pilsner beers, typically clear due to added chill-proofing proteases, showed reduced peptide intensity over time, whereas IPA, which often remains hazy and lacks such enzymes, exhibited increased peptide levels. These findings suggest that Pilsner beers may benefit from quicker consumption, while IPA may be better suited for longer storage to maximize bioactive peptide intake.
Beer is one of the most popular and ancient alcoholic beverages in the world. It is produced through the mashing of barley malt and/or other cereals such as wheat and corn, with the addition of hops, and yeast for fermentation. Beers are classified into distinct styles, with defined sensorial features, due to differences in raw materials, such as malted cereals with varying degrees of roasting, and hops with diverse aroma and bitterness intensities. Time and temperature of wort preparation; fermentation and maturation, as well as yeast species and strains may also be related to the styles’ special characteristics (Cortacero-Ramı́rez et al., 2003; Kerr et al., 2021).
Beer composition presents carbohydrates, proteins, lipids, vitamins, minerals, ethanol and other phytochemicals related to the raw materials, such as phenolic compounds (Quifer-Rada et al., 2015; Sohrabvandi et al., 2012). Most of the proteins in beer come from barley (hordeins and glutelins) and yeast (Colgrave et al., 2012; Cortacero-Ramı́rez et al., 2003). The extracted proteins undergo changes throughout both the brewing process and storage. Yeasts in turn secrete enzymes into the wort during fermentation and residual cells undergo autolysis throughout storage, also interfering with the protein and peptide profiles of the beverage (Li et al., 2016; Wenhui et al., 2022). The protein and peptide profile of beers have been studied to evaluate their influence on the sensory characteristics of the beverage. However, little is known about the biofunctional activity of these peptides. Bioactive peptides are generally short-chain peptides that may be released from proteins by enzymatic hydrolysis. They are recognized for showing physiological roles in the prevention and control of some diseases, such as diabetes, arterial hypertension and cancer (Aderinola and Duodu, 2022).
The aim of the present study was to investigate and compare the abundance of di- and tripeptides in Pilsner and IPA styles craft beers, after 3- and 6-months storage, and to verify their possible bioactivities, intestinal absorption and oral toxicity by comparison with bioactive peptide databases. This is, to the extent of our knowledge, the first study to present the composition of low molecular mass peptides (di- and tripeptides) in beers.
Bottles were collected at the end of the production line in a craft brewery located in Rio de Janeiro, Brazil. Pilsner and IPA styles from two different batches were randomly sampled and samples consisted of 3,500 mL-bottles of each style and batch. Different batches were considered replicates. Products characteristics, as described by the manufacturer’s labels, are displayed in Table 1.
Sample preparation was performed according to Cheiran et al. (2019), with modifications. The contents of 3,500 mL-bottles of the same style and batch were homogenized for 10 s, in a 2 L beaker, and degassed using a probe ultrasound device (Desruptor 500 W, Eco-sonics), for 7 min, at maximum power (99%), in an ice bath. Samples were filtered through #1Whatmann paper filter; aliquoted and stored at −80°C until use.
The degassed beer samples were ultra filtrated by applying ultra centrifugal filters with a cutoff mass of 3 kDa (Amicon Ultra-4 Filters with Ultracel-3 membrane, Merck). The filtrate (with molecular mass lower than 3 kDa) was freeze-dried and stored at −80°C until use.
Samples were analyzed as described by Poliseli et al. (2021), using a Quattro Premier XE triple-quadrupole mass spectrometer (Waters Corporation, Milford, MA, United States) equipped with an electrospray ionization source, a Waters 515 pump and an XBridge (Waters) C18 (4.6 × 50 mm) column. For sample preparation, 0.1 g of the freeze-dried material was dissolved in 1 mL of 50 mM ammonium bicarbonate solution. The solution was mixed and then the first dilution was conducted where 100 µL of this solution was mixed with 900 µL of the mobile phase acetonitrile: water: formic acid (70:30:0.1) (v/v/v) and was centrifuged at 3 × g for 10 min. The sample remained refrigerated at 4°C for 60 min. Then, the second dilution was performed, in which 100 µL of the solution was mixed with 900 µL of mobile phase, followed by vortexing for 1 min. The diluted sample was injected into the valve of the LC-MS/MS system, the injection volume was 5 μL, and the analysis run time was 5 min for each sample. The LC-MS/MS (full scan and fragmentation) experiments were conducted using a conventional electrospray ionization source (ESI). The desolvation and source gas temperatures were 350°C and 110°C, respectively. The electrospray source was operated in positive ionization mode (ESI +) at 4.0 kV. The cone voltage, collision energy and collision gas pressure (argon) were 20 V, 15 V and 3.0 × 10−3 bar respectively.
The spectra obtained were interpreted as described by Cantú et al. (2008) and generated the di- and tripeptides’ sequences used for the database searches and computational analysis.
Peptides’ functionality was evaluated loading the sequences into the BIOPEP-UWM database (https://biochemia.uwm.edu.pl/biopep-uwm/) (Minkiewicz et al., 2019). The AdmetSar web server (http://lmmd.ecust.edu.cn/admetsar2/) was applied to predict human intestinal absorption (HIA) and oral toxicity of the sequenced peptides. This server’s tools can predict chemical properties of Adsorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) of different peptide sequences. For these analyzes the peptides sequence files were converted to a simplified (SMILES) format by PepSMI (https://www.novoprolabs.com/tools/convert-peptideto-smiles-string).
The software GraphPad Prism (v.5 - GraphPad Software, San Diego, CA, United States) was used for statistical comparison of peptides intensities data. A Two-way Analysis of Variance (ANOVA) followed by Bonferroni posttests, was performed with 95% confidence.
MetaboAnalyst version 6.0 (www.metaboanalyst.ca) was used for Hierarchical Cluster Analysis (HCA) and Principal Component Analysis (PCA) statistical evaluation. Before data analysis, Pareto scaling was implemented to make variables more comparable.
Three- and 6-months storage Pilsner and IPA beer samples were analyzed. Ions’ masses and intensities were detected by the LC-MS/MS method applied, enabling the evolution of the peptides in each sample during the bottled aging of beers (Supplementary Material S1).
In Table 2 the masses and intensities of the di- and tripeptides detected in the samples are presented. In both styles, 12 peptides with masses ranging from 177 to 329 m/z were found. The intensity of peptides with m/z 230, 255, 264, 284 and 329 was significantly different (p < 0.05) between the styles. The intensity of masses 230, 284 and 329 (m/z) was higher in the Pilsner style whereas masses 255 and 264 (m/z) intensities were higher in the IPA sample after 6-month storage period.
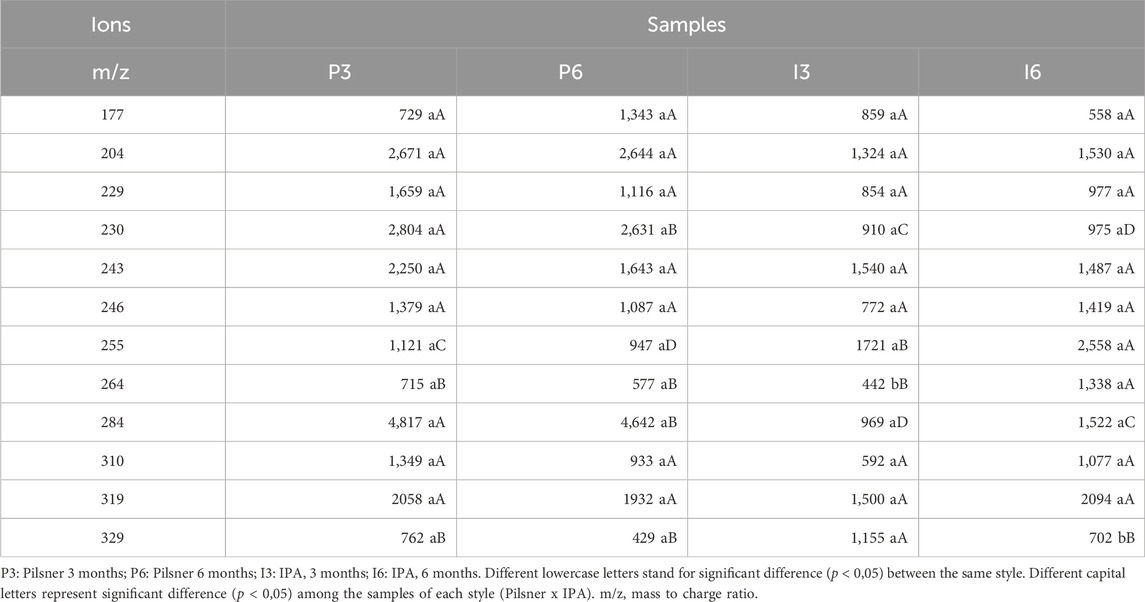
Table 2. Average intensity of peaks (triplicate) in the neutral loss scan of 46 Da (NL46) corresponding to peptides in beers by style and storage time.
PCA (Figure 1A) identified differences between samples based on peptide intensity and allowed separation into two groups, one with Pilsner samples and the other with IPA samples. Separation was explained mainly based on PC1, which accounted for 55.8% of the variation among samples. The sum of the two components (PC1 and PC2) was able to explain 76.3% of the variance among groups.
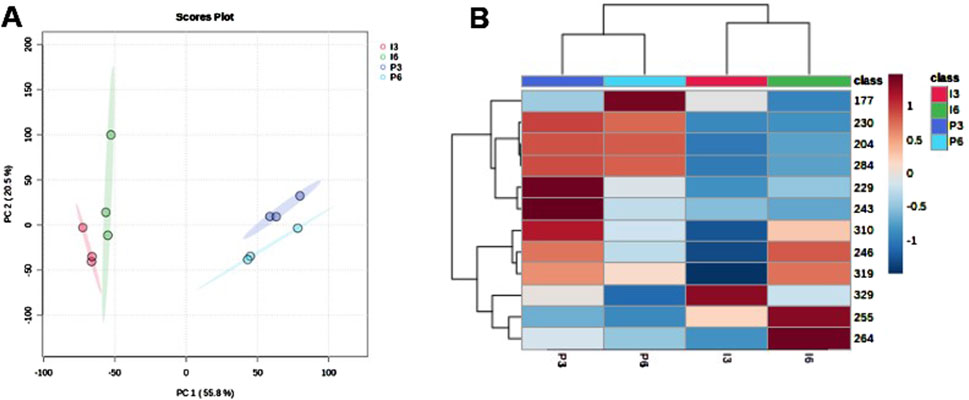
Figure 1. (A) Principal component analysis (PCA) for the intensities of the peptides identified in each sample. (B) Heatmap representing the behavior of the peptide’s intensities.
In Figure 1B, the heatmap allows for the observation of the behavior trend of the peptides after storage. For the Pilsner style, there was a small reduction in intensity, while for IPA, the opposite occurred, with an increase in intensity for most of the peptides.
Table 3 shows the sequences of the different di- and tripeptides along with their attributed bioactivities, according to BIOPEP'S database; their calculated human intestinal absorption (HIA) and acute oral toxicity (AOT).
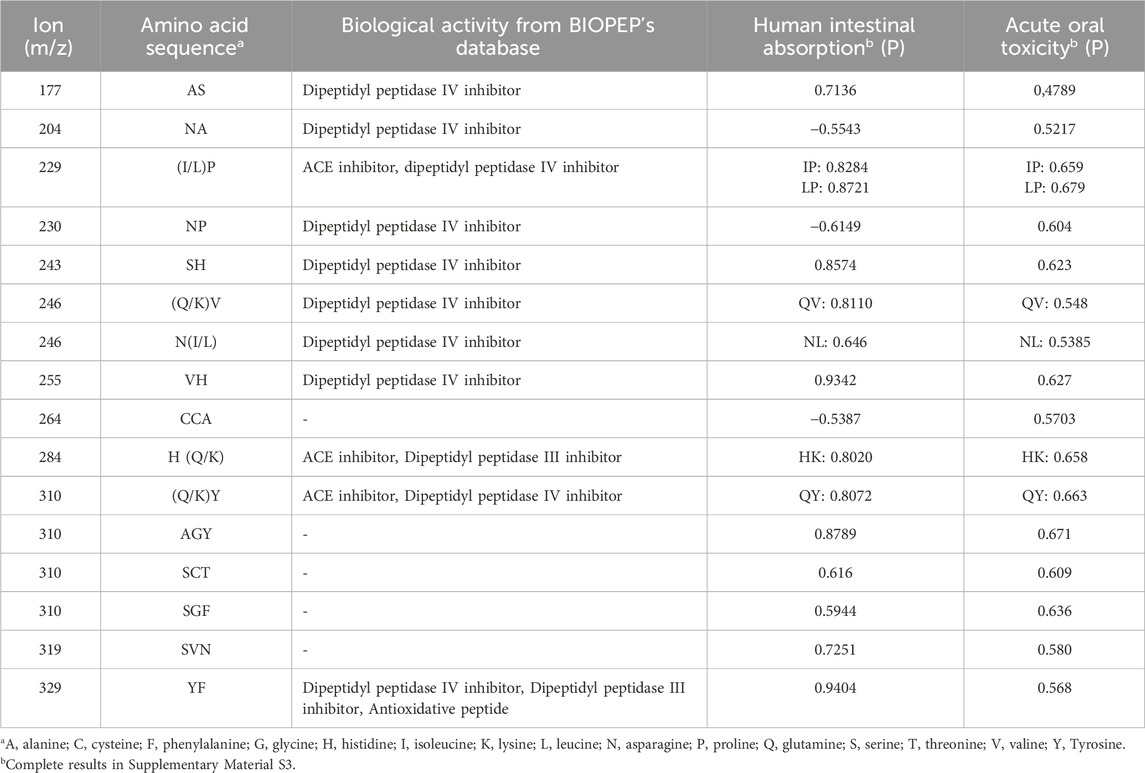
Table 3. Peptide amino acid sequences identified by LC-MS/MS and absorption and toxicity prediction of beer peptides.
In the composition of the peptides, the most frequent nonpolar amino acids were phenylalanine (F), isoleucine (I), leucine (L), proline (P), valine (V) and alanine (A). Among the neutral polar amino acids were serine (S), cysteine (C) and asparagine (N); the only positive polar amino acid was lysine (K) and the negative polar amino acid was glutamine (K). No peptides containing the amino acids methionine (M), arginine (R) and tryptophan (W) were found.
The bioactivities found were dipeptidyl peptidase IV (DPP-IV) inhibitor, dipeptidyl peptidase III (DPP-III) inhibitor, Angiotensin I-converting enzyme (ACE) inhibitor and antioxidative. The peptides human intestinal absorption and their respective acute oral toxicity are present in Table 3 according to AdmetSar web server and the complete list for the 21 possible peptide sequences in the samples are available in Supplementary Material S3.
Of the most abundant peptides in each style, the 284 (m/z) dipeptide, with amino acid sequence H (Q/K), was found in the Pilsner style samples, presenting the highest intensity of all. According to the BIOPEP database, it presents ACE inhibitor and DPP-III inhibitor activities. Other abundant peptides in this same style also presented bioactivities according to this database: 230 (m/z) (NP) - dipeptidyl peptidase IV inhibitor and 329 (m/z) (YF) – DPP-IV inhibitor, DPP-III inhibitor, and was the only peptide identified with antioxidant activity. In the IPA style, the highest intensity was of the VH dipeptide with a mass of 255 (m/z) that presents DPP-IV inhibitor activity, whereas the 264 (m/z) tripeptide (CCA) did not match any bioactivities in BIOPEP (Tables 2, 3; Figure 1B).
According to AdmetSar, negative values of HIA indicate absorption inferior to 30% and, among the positive values, the closer to 1, the greater the absorption of the evaluated peptide. As may be seen in Table 3, most peptides showed positive HIA values. One noteworthy exception was the dipeptide NP, abundant in the Pilsner samples, which showed DPP-IV inhibitor activity, but negative HIA, indicating low predicted absorption. Another exception was the tripeptide CCA, one of the most abundant in the IPA samples. This peptide, however, did not match any bioactivities. The acute oral toxicity is determined by the median lethal dose (LD50) and classified into categories according to the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). The identified peptides were classified in categories III (50 and 300 mg/kg) and IV (300 and 2000 mg/kg) and are thus considered of low toxicity (United Nations, 2023) (Table 3 and Supplementary Material S3).
Barley malt is the main ingredient that provides proteins for the composition of beer. The genetic variety of barley as well as malting - with different temperatures and process times - may influence the proteome of the malted grain (Li et al., 2014). The malt proteins are extracted in the initial stage of wort preparation, in which malt is added to water. Barley malt contains around 10% of protein, of which about one-third is extracted during mashing (Colgrave et al., 2012). As the temperature increases (55°C), proteases act to generate peptides and amino acids. At the end of the process, aggregation or precipitation may occur due to the increase in temperature (78°C–100°C). Proteins and peptides that resist proteolysis and boiling may be found in the beverage (Kerr et al., 2019; 2021).
Hops contain between 14% and 21% protein by dry matter, with proteins ranging from 5 to 45 kDa on average. However, the amount of hops added to the beverage is quite small, which means that their proteins have little influence on the peptide and protein composition of beer (Neugrodda et al., 2014). The study by Narziß and Roettger (1973) determined that when 300 g of powdered hops are used to produce 1 hL of wort, approximately 5 mg Nitrogen.100 mL-1 of wort can be recovered, most of which with a molecular mass of less than 2.6 kDa.
Different yeast strains can also influence the beer proteome (Kerr et al., 2021). The Pilsner style is produced with strains of Saccharomyces carlsbergensis or S. pastorianus that operate at temperatures of 5°C–10°C and perform a lager-type fermentation. IPA is produced with S. cerevisiae and ale-type fermentation at a higher temperature of 15°C–25°C (Joubert et al., 2000; Tafulo et al., 2010). In the study by Schulz et al. (2018), the proteomes of sweet and hopped wort and bright beer were compared. The proteins found were attributed to malt and yeast. The latter consisted basically of metabolic enzymes, especially glycosyl hydrolases, and made up the main difference between the wort and beer proteomes. This same study identified several peptides with m/z ranging from 527.3 to 762.37 (with z = 2), which were attributed to the hydrolysis of malt and yeast proteins during the malting, mashing and fermentation processes.
Liquid chromatography coupled with mass spectrometry (LC-MS/MS) has been commonly used for protein and peptide identification in beer (Picariello et al., 2015; 2017; Schulz et al., 2018; Tian et al., 2024; Wenhui et al., 2022). These methods generally detect peptides comprised of above four or five amino acids, and di- and tripeptides are overlooked. In the present study, a rapid LC-MS/MS method based on neutral loss (NL) of 46 Da (CO and H2O or formic acid) was used. In this approach, the protonated molecules of di- and tripeptides were selected through selective neutral loss (46 Da) due to the carboxylic acid moiety of the peptides and were also screened from collision-induced dissociation (CID) fragments. Afterwards the spectra were interpreted to generate the peptide sequences by the de novo sequencing method. (Poliseli et al., 2021; Ribeiro et al., 2019). This study was the first to identify the di- and tripeptide profile of Pilsner and IPA beer samples and to monitor changes in the intensities of these peptides during 3-months bottled storage.
The identified peptides presented different biological activities according to the composition and amino acid sequence (Table 3) (Lemes et al., 2016). DPP-IV inhibitor was the main bioactivity identified. The enzyme dipeptidyl peptidase IV (DPP-IV) acts on incretin hormones, mainly GLP-1 (glucagon-like peptide-1) and GIP (gastric inhibitory peptide), related to the regulation of glycemia, inducing an increase in insulin secretion after food ingestion. Inhibition of this enzyme helps glycemic control in type 2 diabetes (Nongonierma and FitzGerald, 2019). The ACE inhibitor, a bioactivity also found among the sequenced peptides, helps control blood pressure, preventing the conversion of angiotensin I to angiotensin II - a vasoconstrictor that raises blood pressure - by inhibiting the angiotensin I-converting enzyme (Aluko, 2019; Fan et al., 2019). Peptides with dipeptidyl peptidase III (DPP III) inhibition activity, such as HQ, HK and, YF, can also act in the regulation of the renin-angiotensin system with an antihypertensive or hypotensive effect (Abramić and Agić, 2022; Malovan et al., 2023). The peptide 329 (m/z) - YF (tyrosine - phenylalanine) was the only among those identified with antioxidant activity. The endogenous composition of antioxidant compounds in beer may have a major influence on the stability of the beer’s flavor characteristics during storage (Li et al., 2016). The amino acids proline, tyrosine, histidine, cysteine, lysine, glycine, leucine, isoleucine, phenylalanine, threonine, and valine have already been identified as capable of conferring antioxidant capacity (Görgüç et al., 2020). In the human body, reactive oxygen species (ROS) play essential roles in modulating cell cycle progression and, when produced in regular amounts, are neutralized and eliminated naturally from the body. However, pathological or environmental conditions can cause an imbalance leading to oxidative damage, linked to the development of chronic diseases. Antioxidant peptides, as well as other antioxidant substances, are capable of assisting the body’s defense system, reducing the risk of developing this type of disease (Nwachukwu and Aluko, 2019; Tok et al., 2021).
The peptides with the sequences AS, IP, LP, NP, QV, KV, VH, QY, and YF, found in the beers in this study, have also been obtained previously from hordeins and globulins from barley (Colgrave et al., 2012). In these studies, the main bioactivities observed were also DPP-IV inhibitor and ACE inhibitor. Since they could be identified in bottled beverages, it is possible to state that these peptides resisted the different conditions of the beer production process (Kerr et al., 2021; Tok et al., 2021).
To exert their bioactivities, peptides must resist gastrointestinal digestion and be absorbed (Zhu et al., 2017). These characteristics tend to be more frequent in low molecular mass peptides. Wenhui et al. (2021), when studying Chinese beers, identified DPP-IV inhibitor and ACE inhibitor peptides, of which the highest HIA value was 0.6805, for the sequence PPPVHDFNME. In the present study, much higher HIA values were found. The highest HIA value was 0.9404 for the YF sequence, which presented DPP-IV inhibitor, DPP-III inhibitor and antioxidant activity. The higher values found in the present study may probably be attributed to the lower mass of the peptides evaluated, compared to the study by Wenhui et al. (2022).
Changes that occur in the structure of food proteins and give rise to peptides, such as proteolysis and fermentation, characteristic to beer production, can influence the toxic features of these peptides, due to the exposure of allergenic sequences, for instance. However, information on the formation of toxic peptides derived from food is still limited (Liu et al., 2020). Khan et al. (2018), in a review on the toxicity of bioactive peptides, pointed out the following toxic effects as the most frequent concerns in the consumption of these compounds: intestinal wall disruption, erythrocytes and lymphocytes toxicity, free radical production, enzymopathic and immunopathic tissue damage and cytotoxicity. However, toxicity assessment in the wet lab is considered laborious, expensive and time-consuming. Thus, several in silico assessment tools have already been developed to try and predict the toxicity of peptides of interest for food or other forms of human consumption (Ebrahimikondori et al., 2024). In the present study, the ADMETSAR 3.0 (absorption, distribution, metabolism, excretion and toxicity) tool was applied to predict the toxicity of the detected peptides. This application was able to efficiently point out the human health toxicity of peptides when compared to other bioinformatics tools available for the same purpose (Gu et al., 2024). The results, presented as concentration ranges of orally ingested doses, in mg of peptides per kg of body weight, placed the peptides in this study in categories III and IV proposed by the GHS. This guide, coordinated by the United Nation, proposes a total of 5 categories, the 1st with the most toxic substances and the 5th with the least toxic. This latter, however, is reserved for substances or mixtures of substances of low acute toxicity, but that under specific circumstances may be hazardous to vulnerable populations (United Nation, 2023). Therefore, the toxicity prediction for the beer peptides sequenced in the present study indicated that the low-mass bioactive peptides, di- and tripeptides, found in Pilsner and IPA beers presented low potential toxicity, regardless of the storage time.
As displayed by the heatmap in Figure 1B, the behavior of the peptides over 3 months of bottled storage in the Pilsner and IPA styles was opposite. The peptide intensity of Pilsner samples tended to reduce between 3- and 6-months storage while the peptide intensity of IPA peptides tended to increase. Table 1 shows the ingredients and some characteristics of the samples of the present study. As may be observed, although they contain the same ingredients, the Pilsener and IPA samples show very different characteristics, in terms of color, alcohol content and bitterness. This is due to the differences in the treatment of the malts used; the amounts and quality of hops added, and the fermentation methods applied (yeast strains and temperature), among other characteristics. According to the BJCP (Beer Style Guidelines - Beer Judge Certification Program) (Strong and England, 2021), Pilsner style beer shows a clear appearance and bright yellow color and must be produced using Pilsner malt, which has a light color due to the low roasting temperature (Strong and England, 2021). To maintain clarity, proteases may be added. This prevents the formation of protein-polyphenol complexes, which cause turbidity at low temperatures, known as chill-haze (Cimini and Moresi, 2018; Królak et al., 2023; Wang and Ye, 2021). The continuous activity of these proteases during the storage period can lead to a reduction in peptide intensity over storage time, as observed in Pilsner-style samples (Figure 1B). The IPA style is characterized by an amber-red color, may present slight turbidity, and strong hop flavor and aroma (Strong and England, 2021). These features are responsible by the high EBC (28) and IBU (60) values (Table 1) shown by these samples. Chill-proofing enzymes are very seldomly added in the production of this beer style (Królak et al., 2023). Differences in the composition of the malts, yeasts, and hops applied may also explain the variability found in the behavior of the intensity of low-mass peptides between the two styles, after storage. According to Pferdmenges et al. (2022) who analyzed the nutritional composition of 5 different styles of German beers, there can be significant differences in nutrient content, even among samples with similar ingredients and fermentation pattern. The increase in the intensity of low-mass peptides in IPA samples may also be related to the chemical, rather than enzymatic, degradation of beverage proteins. The dissolved oxygen content in beer may contribute to changes in the structural characteristics of proteins such as LTP1 (lipid transport proteins) that are degraded during storage. Their thiol groups act as antioxidants and help stabilize the sensory characteristics of the beverage (Wu et al., 2011). Zong et al. (2020) found that during storage, due to the increase in oxygen content, beer samples showed an increase in the concentration of low-molecular-weight proteins (<3 kDa) and a reduction in the proportion of high-molecular-weight proteins (>10 kDa).
Based on these results, it might be suggested that Pilsner-type beers should be consumed quickly after production, while IPA-type beers should preferably be consumed after longer storage periods, with the aim of enhancing the intake of low-mass bioactive peptides.
In the present study, in samples of Pilsner and IPA craft beers stored for 3 and 6 months, di- and tripeptides were identified by the LC-MS/MS method based on the neutral loss of 46 Da. These peptides showed high intestinal absorption capacity and low predicted toxicity, as well as bioactive potential of DPP-IV inhibitor, DPP-III inhibitor, ACE inhibitor and antioxidative. During bottled storage the intensity of the peptides increased in IPA and decreased in Pilsner samples. Therefore, the consumption of Pilsner beer soon after production and IPA after longer storage periods might be suggested for enhanced intake of low-mass bioactive peptides.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RS: Data curation, Formal Analysis, Investigation, Writing–original draft. AT: Data curation, Formal Analysis, Writing–original draft. GR: Formal Analysis, Writing–original draft. JD: Funding acquisition, Writing–original draft. EM: Data curation, Formal Analysis, Supervision, Writing–original draft. MK: Conceptualization, Funding acquisition, Project administration, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. CAPES (001 PhD scholarship); FAPERJ (E-26/200.105/2020-01 training and technical qualification scholarship); UNIRIO (INOVA 2021).
The authors would like to thank chemist Ricardo Schwartz for his technical advice and support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frfst.2025.1526934/full#supplementary-material
Abramić, M., and Agić, D. (2022). Survey of dipeptidyl peptidase III inhibitors: from small molecules of microbial or synthetic origin to aprotinin. Molecules 27 (9), 3006. doi:10.3390/molecules27093006
Aderinola, T. A., and Duodu, K. G. (2022). Production, health-promoting properties and characterization of bioactive peptides from cereal and legume grains. BioFactors 48 (5), 972–992. doi:10.1002/biof.1889
Aluko, R. E. (2019). Food protein-derived renin-inhibitory peptides: in vitro and in vivo properties. J. Food Biochem. 43 (1), e12648. doi:10.1111/jfbc.12648
Cantú, M. D., Carrilho, E., Wulff, N. A., and Palma, M. S. (2008). Seqüenciamento de peptídeos usando espectrometria de massas: um guia prático. Quím. Nova 31 (3), 669–675. doi:10.1590/S0100-40422008000300034
Cheiran, K. P., Raimundo, V. P., Manfroi, V., Anzanello, M. J., Kahmann, A., Rodrigues, E., et al. (2019). Simultaneous identification of low-molecular weight phenolic and nitrogen compounds in craft beers by HPLC-ESI-MS/MS. Food Chem. 286, 113–122. doi:10.1016/j.foodchem.2019.01.198
Cimini, A., and Moresi, M. (2018). Combined enzymatic and crossflow microfiltration process to assure the colloidal stability of beer. LWT 90, 132–137. doi:10.1016/j.lwt.2017.12.008
Colgrave, M. L., Goswami, H., Howitt, C. A., and Tanner, G. J. (2012). What is in a beer? Proteomic characterization and relative quantification of hordein (gluten) in beer. J. Proteome Res. 11 (1), 386–396. doi:10.1021/pr2008434
Cortacero-Ramı́rez, S., Hernáinz-Bermúdez de Castro, M., Segura-Carretero, A., Cruces-Blanco, C., and Fernández-Gutiérrez, A. (2003). Analysis of beer components by capillary electrophoretic methods. Trends Anal. Chem. 22 (7), 440–455. doi:10.1016/S0165-9936(03)00704-0
Ebrahimikondori, H., Sutherland, D., Yanai, A., Richter, A., Salehi, A., Li, C., et al. (2024). Structure-aware deep learning model for peptide toxicity prediction. Protein Sci. 33 (7), e5076. doi:10.1002/pro.5076
Fan, H., Liao, W., and Wu, J. (2019). Molecular interactions, bioavailability, and cellular mechanisms of angiotensin-converting enzyme inhibitory peptides. J. Food Biochem. 43 (1), e12572. doi:10.1111/jfbc.12572
Görgüç, A., Gençdağ, E., and Yılmaz, F. M. (2020). Bioactive peptides derived from plant origin by-products: biological activities and techno-functional utilizations in food developments – a review. Food Res. Int. 136, 109504. doi:10.1016/j.foodres.2020.109504
Gu, Y., Yu, Z., Wang, Y., Chen, L., Lou, C., Yang, C., et al. (2024). admetSAR3.0: a comprehensive platform for exploration, prediction and optimization of chemical ADMET properties. Nucleic Acids Res. 52 (W1), W432–W438. doi:10.1093/nar/gkae298
Joubert, R., Brignon, P., Lehmann, C., Monribot, C., Gendre, F., and Boucherie, H. (2000). Two-dimensional gel analysis of the proteome of lager brewing yeasts. Yeast Chichester, Engl. 16 (6), 511–522. doi:10.1002/(SICI)1097-0061(200004)16:6<511::AID-YEA544>3.0.CO;2-I
Kerr, E. D., Caboche, C. H., and Schulz, B. L. (2019). Posttranslational modifications drive protein stability to control the dynamic beer brewing proteome. Mol. and Cell. Proteomics 18 (9), 1721–1731. doi:10.1074/mcp.RA119.001526
Kerr, E. D., Fox, G. P., and Schulz, B. L. (2021). “Grass to glass: better beer through proteomics,” in Comprehensive foodomics (Elsevier), 407–416. doi:10.1016/B978-0-08-100596-5.22869-2
Khan, F., Niaz, K., and Abdollahi, M. (2018). Toxicity of biologically active peptides and future safety aspects: an update. Curr. Drug Discov. Technol. 15 (3), 236–242. doi:10.2174/1570163815666180219112806
Królak, K., Kobus, K., and Kordialik-Bogacka, E. (2023). Effects on beer colloidal stability of full-scale brewing with adjuncts, enzymes, and finings. Eur. Food Res. Technol. 249 (1), 47–53. doi:10.1007/s00217-022-04131-7
Lemes, A., Sala, L., Ores, J., Braga, A., Egea, M., and Fernandes, K. (2016). A review of the latest advances in encrypted bioactive peptides from protein-rich waste. Int. J. Mol. Sci. 17 (6), 950. doi:10.3390/ijms17060950
Li, H., Zhao, M., Cui, C., Sun, W., and Zhao, H. (2016). Antioxidant activity and typical ageing compounds: their evolutions and relationships during the storage of lager beers. Int. J. Food Sci. and Technol. 51 (9), 2026–2033. doi:10.1111/ijfs.13173
Li, X., Jin, Z., Gao, F., Lu, J., Cai, G., Dong, J., et al. (2014). Comparative proteomic analysis of dan’er malts produced from distinct malting processes by two-dimensional fluorescence difference in gel electrophoresis (2D-DIGE). J. Agric. Food Chem. 62 (38), 9310–9316. doi:10.1021/jf5030483
Liu, L., Li, S., Zheng, J., Bu, T., He, G., and Wu, J. (2020). Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. and Technol. 96, 199–207. doi:10.1016/j.tifs.2019.12.022
Malovan, G., Hierzberger, B., Suraci, S., Schaefer, M., Santos, K., Jha, S., et al. (2023). The emerging role of dipeptidyl peptidase 3 in pathophysiology. FEBS J. 290 (9), 2246–2262. doi:10.1111/febs.16429
Minkiewicz, P., Iwaniak, A., and Darewicz, M. (2019). BIOPEP-UWM database of bioactive peptides: current opportunities. Int. J. Mol. Sci. 20 (23), 5978. doi:10.3390/ijms20235978
Narziß, L., and Roettger, W. (1973). Über den Gehalt des Hopfens an verschiedenen Stickstofffraktionen - Veränderung der Eiweißgegebenheiten während des Hopfenkochens. Brauwissenschaft 26, 302–307.
Neugrodda, C., Gastl, M., and Becker, T. (2014). Protein profile characterization of hop (humulus lupulus L.) varieties. J. Am. Soc. Brew. Chem. 72 (3), 184–191. doi:10.1094/ASBCJ-2014-0629-01
Nongonierma, A. B., and FitzGerald, R. J. (2019). Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 43 (1), e12451. doi:10.1111/jfbc.12451
Nwachukwu, I. D., and Aluko, R. E. (2019). Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 43 (1), e12761. doi:10.1111/jfbc.12761
Pferdmenges, L. E., Schröter, A., Lohmayer, R., Striegel, L., Rychlik, M., Müller, A., et al. (2022). Characterization of the nutrient composition of German beer styles for the German nutrient database. J. Food Compos. Analysis 105, 104181. doi:10.1016/j.jfca.2021.104181
Picariello, G., Mamone, G., Cutignano, A., Fontana, A., Zurlo, L., Addeo, F., et al. (2015). Proteomics, peptidomics, and immunogenic potential of wheat beer (weissbier). J. Agric. Food Chem. 63 (13), 3579–3586. doi:10.1021/acs.jafc.5b00631
Picariello, G., Mamone, G., Nitride, C., and Ferranti, P. (2017). “Proteomic analysis of beer,” in Proteomics in food science (Elsevier), 383–403. doi:10.1016/B978-0-12-804007-2.00023-0
Poliseli, C. B., Tonin, A. P. P., Martinez, F. C., Nascimento, N. C. do, Braz, V., Maluf, J., et al. (2021). Tri- and dipeptides identification in whey protein and porcine liver protein hydrolysates by fast LC–MS/MS neutral loss screening and de novo sequencing. J. Mass Spectrom. 56 (2), e4701. doi:10.1002/jms.4701
Quifer-Rada, P., Vallverdú-Queralt, A., Martínez-Huélamo, M., Chiva-Blanch, G., Jáuregui, O., Estruch, R., et al. (2015). A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 169, 336–343. doi:10.1016/j.foodchem.2014.07.154
Ribeiro, M. A. S., Tonin, A. P. P., Poliseli, C. B., Lima, B. B., Castro, L. E. N., Silva, V. M., et al. (2019). Teaching venturi electrospray mass spectrometry with amino acid analysis. Int. J. Mass Spectrom. 444, 116183. doi:10.1016/j.ijms.2019.116183
Schulz, B. L., Phung, T. K., Bruschi, M., Janusz, A., Stewart, J., Meehan, J., et al. (2018). Process proteomics of beer reveals a dynamic proteome with extensive modifications. J. Proteome Res. 17 (4), 1647–1653. doi:10.1021/acs.jproteome.7b00907
Sohrabvandi, S., Mortazavian, A. M., and Rezaei, K. (2012). Health-related aspects of beer: a review. Int. J. Food Prop. 15 (2), 350–373. doi:10.1080/10942912.2010.487627
Strong, G., and England, K. (2021). Guide of styles 2021 beer Judge certification Program (BJCP). Available at: https://www.bjcp.org/bjcp-style-guidelines/.
Tafulo, P. A. R., Queirós, R. B., Delerue-Matos, C. M., and Sales, M. G. F. (2010). Control and comparison of the antioxidant capacity of beers. Food Res. Int. 43 (6), 1702–1709. doi:10.1016/j.foodres.2010.05.014
Tian, W., Zhang, C., Zheng, Q., Hu, S., Yan, W., Yue, L., et al. (2024). In silico screening of bioactive peptides in stout beer and analysis of ACE inhibitory activity. Foods 13 (13), 1973. doi:10.3390/foods13131973
Tok, K., Moulahoum, H., Kocadag Kocazorbaz, E., and Zihnioglu, F. (2021). Bioactive peptides with multiple activities extracted from Barley (Hordeum vulgare L.) grain protein hydrolysates: biochemical analysis and computational identification. J. Food Process. Preserv. 45 (1). doi:10.1111/jfpp.15024
United Nations (2023). Globally harmonized system of classification and labeling of chemicals (GHS). Available at: https://unece.org/sites/default/files/2023-07/GHS%20Rev10e.pdf (ST/SG/AC.10/30/Rev.10.
Wang, Y., and Ye, L. (2021). Haze in beer: its formation and alleviating strategies, from a protein–polyphenol complex angle. Foods 10 (12), 3114. doi:10.3390/foods10123114
Wenhui, T., Shumin, H., Yongliang, Z., Liping, S., and Hua, Y. (2022). Identification of in vitro angiotensin-converting enzyme and dipeptidyl peptidase IV inhibitory peptides from draft beer by virtual screening and molecular docking. J. Sci. Food Agric. 102 (3), 1085–1094. doi:10.1002/jsfa.11445
Wu, M. J., Clarke, F. M., Rogers, P. J., Young, P., Sales, N., O’Doherty, P. J., et al. (2011). Identification of a protein with antioxidant activity that is important for the protection against beer ageing. Int. J. Mol. Sci. 12 (9), 6089–6103. doi:10.3390/ijms12096089
Zhu, Q., Chen, X., Wu, J., Zhou, Y., Qian, Y., Fang, M., et al. (2017). Dipeptidyl peptidase IV inhibitory peptides from Chlorella vulgaris: in silico gastrointestinal hydrolysis and molecular mechanism. Eur. Food Res. Technol. 243, 1739–1748. doi:10.1007/s00217-017-2879-1
Keywords: DDP-IV inhibitor, ACE-inhibitor, antioxidant, dipeptide, tripeptide, LC-MS, neutral loss, DDP-III inhibitor
Citation: da Silva RNP, Tonin APP, Ramos GSM, Dias JF, Meurer EC and Koblitz MGB (2025) Bioactive potential and storage behavior of low molecular mass peptides in Pilsner and IPA style craft beers. Front. Food. Sci. Technol. 5:1526934. doi: 10.3389/frfst.2025.1526934
Received: 12 November 2024; Accepted: 03 January 2025;
Published: 22 January 2025.
Edited by:
Tomás García-Cayuela, Tecnologico de Monterrey, MexicoCopyright © 2025 da Silva, Tonin, Ramos, Dias, Meurer and Koblitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Gabriela Bello Koblitz, bWFyaWEua29ibGl0ekB1bmlyaW8uYnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.