- Department of Food Science and Technology, University of Georgia, Athens, GA, United States
This study investigates the antioxidant and physicochemical characteristics of raw ground beef patties and raw ground beef patties treated with varying percentages of roselle (Hibiscus sabdariffa L.) and rose (Rosa canina L.) powders during 7 days of storage at 4°C. The analysis included key parameters such as antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, lipid oxidation through 2-thiobarbituric acid reactive substances (TBARS), protein oxidation markers (carbonyls, Schiff bases, and free thiols), water-holding capacity, pH, color, and texture. Both roselle and rose powders enhanced the antioxidant capacity of the patties, reducing oxidative markers (TBARS, carbonyls, Schiff bases, and free thiols) during storage. Additionally, improved water-holding capacity and reduced pH were observed across all treated patties, with minimal impact on texture. However, while roselle powder showed beneficial effects, patties treated with rose powder exhibited superior overall results. The more favorable outcomes in rose-treated patties, particularly in oxidative stability and physicochemical properties, can be attributed to the higher concentrations of bioactive compounds, such as phenolic acids and flavonoids, present in rose powder. These compounds likely contributed to enhanced free radical scavenging activity, providing stronger protection against lipid and protein oxidation. Furthermore, rose powder maintained more stable color and physicochemical properties, with patties showing acceptable color and minimal texture degradation by the 7th day of storage. These findings highlight the potential of rose powder as a highly effective natural additive for extending the shelf life and preserving the quality of ground beef patties, positioning it as a promising ingredient for future applications in the food industry.
1 Introduction
Beef is a highly nutritious and widely consumed food source (Drouillard, 2018). However, during storage, especially in raw ground beef patties, the meat is prone to oxidative processes that degrade its physicochemical qualities, including flavor, texture, color, and water-holding capacity (Liu et al., 2022). Lipid and protein oxidation in beef patties generate reactive aldehydes such as malondialdehyde (MDA), 4-hydroxy-2-nonenal (4-HNE), and four-oxo-2-nonenal (4-ONE), which negatively impact product quality and reduce shelf life (Love and Pearson, 1971; Mohan et al., 2022). These oxidation by-products pose not only a challenge to food quality but may also have potential health risks due to their toxic nature.
Natural antioxidants, including edible flowers like Hibiscus sabdariffa L. and Rosa canina L., have gained attention for their potential to mitigate these oxidative changes in meat products (Da-Costa-Rocha et al., 2014). Rich in polyphenols, vitamins, and other bioactive compounds, edible flowers offer antioxidant properties that inhibit free radicals and oxidative reactions (Mlcek and Otakar, 2011; Bozkurt and Belibagli, 2009). The use of natural antioxidants aligns with the growing consumer preference for clean-label products without synthetic additives (Chensom et al., 2019). Hibiscus, in particular, has been shown to inhibit lipid oxidation in meat products, performing comparably to synthetic antioxidants like butylated hydroxytoluene (BHT), but at lower concentrations (Bozkurt and Belibagli, 2009). Additionally, R. canina, or rosehip juice, has demonstrated similar antioxidant properties in meat stabilization (Tyburcy et al., 2014).
Previous studies have explored the effectiveness of hibiscus and rose extracts in maintaining the physicochemical stability of meat. For example, research conducted by Gibis and Weiss, (2010) showed the potential of Hibiscus sabdariffa to reduce heterocyclic aromatic amine (HAA) formation in beef products, highlighting the plant’s capacity to prevent oxidative degradation. Similarly, Bozkurt and Belibagli, (2009) demonstrated the significant effect of hibiscus in reducing lipid oxidation in beef patties. Although these results are promising, limited studies have examined the post-cooking impact of these flowers on meat during retail storage, leaving a research gap in the area (Tyburcy et al., 2014; Zhang et al., 2019). Moreover, the specific interactions between phenolic compounds in edible flowers and proteins in raw meat products are not fully understood (Soladoye et al., 2015).
Currently, no known studies have specifically compared the effects of two edible flowers on raw ground beef patties without adding other ingredients or preservatives. This lack of focused research on edible flowers’ standalone impacts on the physicochemical attributes of raw beef patties, such as lipid and protein oxidation, creates a gap in understanding their effectiveness as natural antioxidants.
Therefore, this study aims to investigate the impact of Hibiscus sabdariffa L. and R. canina L. powders on the physicochemical stability of raw ground beef patties during storage. The research will assess various parameters, including lipid and protein oxidation, color stability, texture profile, pH, water-holding capacity, and antioxidant capacity. The study will use seven treatments: raw ground beef patties (control patties), raw ground beef patties treated with 1%, 2%, and 3% of hibiscus powder, and raw ground beef patties treated with 1%, 2%, and 3% of rose powder. This approach will help determine the optimal concentration of these edible flower powders for enhancing the shelf life and quality of raw ground beef patties during refrigerated storage.
2 Materials and methods
2.1 Raw materials and chemicals
Fresh raw ground beef (lean/fat blend ratio of 80/20, serving as the experimental unit) was procured from a local beef supplier, FPL Foods, in Augusta, GA. A certificate of analysis was provided with the purchased ground beef blend, verifying its composition and lean/fat ratio. Food-grade rose, and hibiscus flowers were acquired from organic edible flower selections available from a local purveyor. After lyophilization (freeze-drying), the flowers were stored in vacuum-sealed packets at 4°C to prevent moisture absorption and degradation of bioactive compounds. The ground flower powders had an approximate particle size of <500 µm in order to ensure that ground edible flowers powders were evenly distributed when mixed with raw ground beef patties. Analytical grade chemicals, including Thiobarbituric acid (TBA), trichloroacetic acid (TCA), 1,1,3,3-tetraethoxypropane (TEP), diethylenetriaminepentaacetic acid (DTPA), butylated hydroxytoluene (BHT), 2,4-dinitrophenylhydrazine (DNPH), 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), and bicinchoninic acid (BCA), were sourced from Sigma-Aldrich, Co. (based in St. Louis, MO). Additionally, chemical standards of deuterated 4-oxo-2-nonenal-d3 and the derivatization reagent amino oxyacetic acid (AOA) were purchased from the Cayman Chemical Company, headquartered in Ann Arbor, MI.
2.2 Processing of raw ground beef patties, packaging, and retail storage
The raw ground beef patties were mixed with the treatment ingredients and molded into patties. The ground beef patties were subsequently placed in foam trays on absorbent pads and overwrapped with polyvinyl chloride film (oxygen transmission rate of 14,000 cc/mm2/24 h/L atm; Koch Supplies, Inc., Kansas City, Mo., United States). The ground beef patties were stored and displayed under retail display conditions (4°C ± 1°C; continuous deluxe warm white, fluorescent lighting; 1,600 lx; Phillips, Inc., Somerset, N.J., United States) for 7 days in a retail display case. The experiment was repeated three times independently on three separate occasions. The treatments included mixing raw ground beef with 1%, 2%, and 3% concentrations of hibiscus and rose powders. One control treatment consisted of raw ground beef patties without the addition of any edible flower concentrations (Figure 1). No other ingredients were added to the formulations. This entails dividing the raw ground beef into seven treatments, each consisting of 30 g: raw ground beef patties (control), raw ground beef patties mixed with 1% hibiscus powder, ground beef patties mixed with 2% hibiscus powder, ground beef patties mixed with 3% hibiscus powder, raw ground beef patties mixed with 1% rose powder, ground beef patties mixed with 2% rose powder, and ground beef patties mixed with 3% rose powder. All preparation procedures, including mixing, patty formation, packaging, and storage, were conducted at 4°C ± 1°C. Three replicates of each treatment formulation were prepared per experiment, ensuring reproducibility and statistical power for analysis. Each patty weighed 30 g, with a diameter of approximately 6 cm and a thickness of 1 cm. Although the patties are smaller, this size allows effective packaging and analysis without affecting statistical significance. The packages were rotated daily to minimize any potential effects due to localized conditions. The packages were rotated daily to minimize any potential effects due to localized conditions. For analysis, 5 g of raw ground beef patties were homogenized using an Ultra Turrax homogenizer at 3,000 rpm with 35 mL of 20 mM phosphate buffer containing 0.6 M NaCl and adjusted to a pH of 6.5. These aliquots were then stored at −80°C for future analysis.

Figure 1. The visual appearance of the raw ground beef patties was evaluated on day 0 after preparation and on day 7 at the end of the analysis, considering the seven treatments: Raw ground beef patties (control), Raw ground beef patties treated with 1% Hibiscus sabdariffa L. powder, Raw ground beef patties treated with 2% Hibiscus sabdariffa L. powder, Raw ground beef patties treated with 3% Hibiscus sabdariffa L. powder, Raw ground beef patties treated with 1% Rosa canina L. powder, Raw ground beef patties treated with 2% Rosa canina L. powder, Raw ground beef patties treated with 3% Rosa canina L. powder.
2.3 pH
The pH of raw ground beef patties, including those containing 1%, 2%, and 3% of edible flowers (rose and hibiscus powders), was assessed using a pH meter (Thermo Scientific, Athens, Georgia, United States) equipped with a pierced probe. Measurements were taken after processing the patties and storing them at 4°C for 7 days. The pH readings were recorded three times for each sample and averaged for statistical analysis (Mohan et al., 2016).
2.4 Water holding capacity
The water holding capacity (WHC) of the uncooked product was assessed in triplicate following the method described by (Hughes et al., 1997). Initially, 10 g of the batter (W1), prepared from raw ground beef patties and raw ground beef patties containing 1%, 2%, and 3% of edible flowers (rose and hibiscus powders), was weighed and placed into a glass jar. The jar was heated in a water bath at 90°C for 10 min. After allowing the samples to cool to room temperature, they were wrapped in cotton cheesecloth and centrifuged at 1,400 rpm for 15 min. After centrifugation, the samples were reweighed (W2). WHC was calculated using the following equation:
where T is the amount of water lost after heating and centrifugation, and M is the total moisture content of the sample.
2.5 Instrumental color analysis
The L*, a*, and b* color values were measured using a Hunter Lab colorimeter (McKinley Scientific, Reston, Virginia, United States). L* represents lightness (luminosity), a* represents the red-green spectrum, and b* represents the yellow-blue spectrum, as described by Bumsted et al. (2023). These parameters are standard in the assessment of meat color quality. Color measurements were taken at three random locations on each raw ground beef patty and raw ground beef patties prepared with edible flowers, rose, and hibiscus powders of 1, 2 and 3 percentages. The values were averaged for statistical analysis. The raw ground beef patties, including control samples and those made with edible flowers rose and hibiscus powders 1, 2 and 3 percentages, were rotated daily to minimize positional effects (Mohan et al., 2016).
2.6 Texture analysis
This experiment used a TAXT2i texture analyzer (Stable Micro Systems Ltd., Surrey, United Kingdom) equipped with a 40 mm diameter glass probe. The objective was to assess the texture of raw ground beef patties and patties containing 1%, 2%, and 3% edible flowers (hibiscus and rose powders) under specified conditions. The sample was positioned directly beneath the instrument on a plate, and a Texture Profile Analysis (TPA) compression test was conducted using a 50 kg load cell and a 2 kg weight for calibration purposes. The samples were divided into three equal parts and placed in the center of the compression plate for texture measurement. Two consecutive compressions were performed, with the instrument set at 50% strain and a crosshead speed of 250 mm/min. A platen probe with a diameter of 100 mm was utilized to compress the sample and evaluate various textural parameters between the two compressions, including force (N)-distance (mm) curve, hardness (N), springiness (%), resilience (%), cohesiveness (%), gumminess (%) and chewiness(N). The test was conducted in duplicate to ensure accuracy, with three measurements taken for each replicate. Subsequently, the data collected from the three measurements were averaged to mitigate any potential anomalies or measurement errors (Mohan et al., 2016).
2.7 Determination of TBARS
The MDA measurement in raw ground beef patties and patties containing 1%, 2%, and 3% edible flowers (hibiscus and rose powders), as free MDA equivalents, was conducted following the procedures outlined by Reitznerova et al. (2017) with modifications. Homogenates were prepared by blending 5 g of ground beef with 20 mL of 20 mM phosphate buffer (pH 6.5) containing 0.6 M NaCl. Standard solutions were prepared using 1,1,3,3-tetraethoxypropane (TEP) as the source for the MDA standard curve.
Initially, 400 μL of homogenate or standard solution was transferred to a 1.5 mL microtube, and the volume was adjusted to 1 mL using a 7.5% (w/v) trichloroacetic acid (TCA) solution. The samples were vortexed and sonicated for 5 min to release the MDA from the matrix and precipitate the proteins. After centrifugation at 3,000 g for 5 min, 500 μL of the supernatant was mixed with 500 μL of thiobarbituric acid (TBA) solution (40 mM, prepared in glacial acetic acid), vortexed, and heated in a water bath at 90°C for 45 min. Following cooling in an ice bath for 10 min and centrifuging at 3,000 g for 1 min, the absorbance was measured at 532 nm using a UV1800 Spectrophotometer. MDA levels were quantified using a standard TEP curve (0–10 μmol), and the results were expressed as mg MDA per kg of sample.
2.8 Determination of protein carbonyl content
Total protein carbonyls were measured using the DNPH method as described by Levine et al. (1994), with some modifications. First, 400 μL of raw ground beef samples and raw ground beef patties containing 1%, 2%, and 3% edible flowers (hibiscus and rose powders) were mixed with 1 mL of ice-cold 10% trichloroacetic acid (TCA) and stored at 4°C for 15 min. From these homogenized samples, 400 μL of the thawed digest was transferred to 1.5-mL Eppendorf tubes, combined with 1 mL of ice-cold 10% TCA, and incubated at 40°C for 15 min to release the digested proteins from the lipid particles. One set of samples was then treated with 2,4-dinitrophenylhydrazine (DNPH) for derivatization, while blank samples were treated with 2.0 M HCl instead of DNPH. The carbonyl concentration (nmol/mg protein) was determined using absorbance values at 280 nm and 370 nm, applying the equation:
where εhydrazone,370 is 22,000 M−1 cm−1 and the carbonyl concentrations obtained from the blanks will be subtracted from the corresponding treated sample.
2.9 Determination of free thiols concentration
Raw ground beef patties and those containing 1%, 2%, and 3% edible flowers (hibiscus and rose powders) were homogenized using an Ultra Turrax homogenizer at 3,000 rpm with 35 mL of 20 mM phosphate buffer containing 0.6 M NaCl, adjusted to a pH of 6.5. The resulting ground beef homogenates (5 mL) were centrifuged at 10,000 g for 15 min to remove insoluble proteins, and the liquid portion obtained after centrifugation was used as the digest. A modified Ellman’s method, using 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), was then applied to measure the concentration of thiol oxidation. Subsequently, 0.5 mL of 10 mM DTNB was added to 4.5 mL aliquots of the supernatant. All mixtures were shielded from light and allowed to react at room temperature for 30 min. A reagent blank, consisting of 0.5 mL of 20 mM phosphate buffer (pH 6.5), was also prepared. Absorbance was measured spectrophotometrically at 412 nm, and the thiol concentration was calculated using the Lambert-Beer law (ε412 = 14,000 M–1 cm–1), expressed in nmol of thiol per milligram of protein. Protein content was measured spectrophotometrically at 280 nm using a BSA standard curve (Hu et al., 2018).
2.10 Determination of schiff bases
The fluorescence emission of Schiff bases was evaluated following the protocol outlined by Sobral et al. (2020) with minor adjustments. Initially, raw ground beef samples and patties containing 1%, 2%, and 3% of edible flowers (hibiscus and rose powders), weighing 1 g, were homogenized for 30 s with 5 mL of phosphate buffer solution (20 mM, NaCl 0.6 M, pH 6.5). Subsequently, 2 mL of the extract was diluted with 8 mL of solvent (dichloromethane: ethanol in a 2:1 v/v ratio) and vortexed for 30 s. After centrifugation at 4,000 g for 10 min, the upper phase was collected, and 200 µL of the supernatant was transferred to a cuvette to measure the fluorescence intensities (FI). The fluorescence emission was measured using a Cary Eclipse Fluorimeter (Agilent, United States) set to an excitation wavelength of 360 nm. Emission spectra were recorded from 390 to 600 nm. All measurements were performed in triplicate, and the fluorescence intensities were expressed in arbitrary units (AU).
2.11 Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay
Samples were prepared, and the DPPH radical scavenging activity was assessed following the protocol outlined by Soriano et al. (2018) with minor adjustments. Raw ground beef patties and patties containing 1%, 2%, and 3% of edible flowers (hibiscus and rose powders) were used for the analysis. Initially, 3 g of the refrigerated stored patty was weighed using an analytical balance and homogenized in 6 mL of methanol: water (80:20, v/v) using a homogenizer at 10,000 rpm for 1 min. Subsequently, the mixture was centrifuged at 9,840 g for 10 min using a refrigerated centrifuge. The supernatant was filtered through Whatman filter paper No. 1. An aliquot (200 μL) of the supernatant was mixed with 800 μL of distilled water and 1 mL of 0.2 mM methanolic DPPH solution, followed by vortex using a test tube shaker at high speed for 2 min. The mixture was left in the dark for 20 min before the absorbance was measured at 517 nm using a UV-Vis spectrophotometer. The percentage of DPPH radical scavenging activity was calculated using the following equation:
3 Statistical analysis
The findings were evaluated using descriptive statistical analysis (mean ± SD), one-way ANOVA, and post hoc comparison using the Tukey honest significant difference (HSD) test to discover substantial changes between experiments (p-value ≤ 0.05). All analyses were carried out by using JMP analytic software (SAS Institute Inc., Cary, NC, United States).
4 Results
4.1 pH
The influence of rose and hibiscus edible flower powders on the pH of raw ground beef patties is depicted in Figure 2. The pH values of raw ground beef patties (control) and those treated with rose powder at 1% and 2% concentrations exhibited significant differences on days 0 and 7 of storage (p < 0.05). However, there were no significant differences (p > 0.05) in pH values among the raw ground beef patties treated with 1%, 2%, and 3% hibiscus powders and 3% rose powder between the 0th and 7th day of storage.
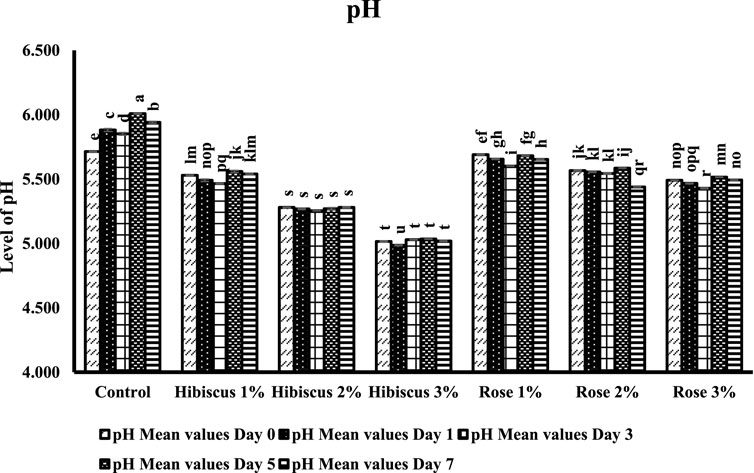
Figure 2. pH level measurements from day 0 to day 7 across different treatments. Significant differences indicated by letters (p < 0.05).
On day 0, the pH of raw ground beef patties (control) was 5.7, which increased to 5.9 by the day (p < 0.05). In contrast, patties treated with 1% hibiscus powder showed a pH of 5.5 on day 0, which remained the same at 5.5 by day 7 with no significant difference (p > 0.05). Also, patties treated with 2% hibiscus powder had a pH of 5.3 on day 0, which remained almost unchanged at 5.3 on day 7 (p > 0.05). Likewise, those treated with 3% hibiscus powder maintained a pH of 5.0 on days 0, 5, and 7 (p > 0.05). On the other hand, patties treated with 2% rose powder exhibited similar pH results on both days, 0 and 7. Patties treated with 1% rose powder showed a pH of 5.6 on day 0, which remained the same as 5.6 by day 7 (p > 0.05). However, those treated with 2% rose powder had a pH of 5.5 on day 0, which decreased to 5.4 on day 7 (p < 0.05). Also, patties treated with 3% rose powder had the lowest pH on day 0 compared to control and patties treated with 1% and 2% rose powders, with pH values of 5.4 on day 0 remaining the same which was 5.4 on day 7 (p > 0.05).
4.2 Water holding capacity
The impact of rose and hibiscus on raw ground beef patties is illustrated in Figure 3. Water holding capacity (WHC) values of raw ground beef patties (control) and those treated with 2% and 3% rose powder exhibited significant differences on both the 0th and 7th day of storage (p < 0.05).
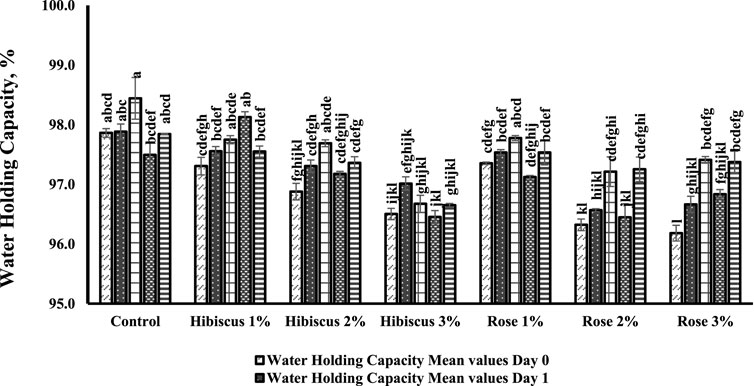
Figure 3. Water holding capacity measurements from day 0 to day 7 under various treatments. Significant differences indicated by different letters (p < 0.05).
Conversely, there were no significant differences (p > 0.05) in WHC values among patties treated with 1%, 2%, and 3% hibiscus powders and 1% rose powder between the 0th and 7th day of storage. On day 0, raw ground beef patties (control) had a WHC of 97.8%, which remained at 97.8% by the 7th day (p > 0.05). However, patties treated with 1% hibiscus powder, which contained 1.70% dietary fiber, exhibited a WHC of 97.3% on day 0, slightly increasing to 97.5% by the 7th day, with no significant difference (p < 0.05). Similarly, those treated with 2% hibiscus powder, which contained 1.92% dietary fiber, had a WHC of 96.8% on day 0% and 97.3% on the 7th day, showing no significant difference (p > 0.05).
Likewise, patties treated with 3% hibiscus powder, which contained 2.10% dietary fiber, maintained a consistent WHC with values of 96.5% on day 0% and 96.6% on day 7 (p < 0.05). But patties treated with 2% and 3% rose powder exhibited significant increases in water-holding capacity (WHC) on days 0 and 7. This improvement in WHC can be linked to the higher dietary fiber content of the rose powder, with 2% and 3% treatments having dietary fiber contents of 1.92% and 2.52%, respectively.
The elevated fiber content contributed to enhanced water retention in the raw ground beef patties, thus improving WHC. In contrast, the 1% rose powder treatment, which had a lower dietary fiber content of 1.61%, did not result in a significant change in WHC. For example, patties treated with 2% rose powder had a WHC of 96.3% on day 0, increasing to 97.2% by day 7 (p > 0.05). Similarly, those treated with 3% rose powder had the lowest initial WHC of 96.1% on day 0, which increased to 97.3% by day 7 (p < 0.05).
4.3 Color
The L* -mean values of raw ground beef patties (control) and those treated with hibiscus powders 1%, 2%, and 3%, and rose powders 1%, 2%, and 3%, are presented in Table 1. On day 0, the L* value for raw ground beef patties (control) was 45.27 ± 0.82, which increased to 50.11 ± 0.02 on day 7, indicating a significant difference (p < 0.05). Similarly, raw ground beef patties treated with hibiscus powder (1%) exhibited a lightness of 40.95 ± 0.97 on day 0, which increased to 46.57 ± 0.65 on day 7, showing a significant difference (p < 0.05). Comparable trends were observed for raw ground beef patties treated with hibiscus powder 2% and 3%, with initial lightness values of 39.11 ± 0.62 and 33.95 ± 0.68 on day 0, respectively, increasing to 43.63 ± 0.12 and 40.81 ± 0.3 on day 7, respectively (p < 0.05).
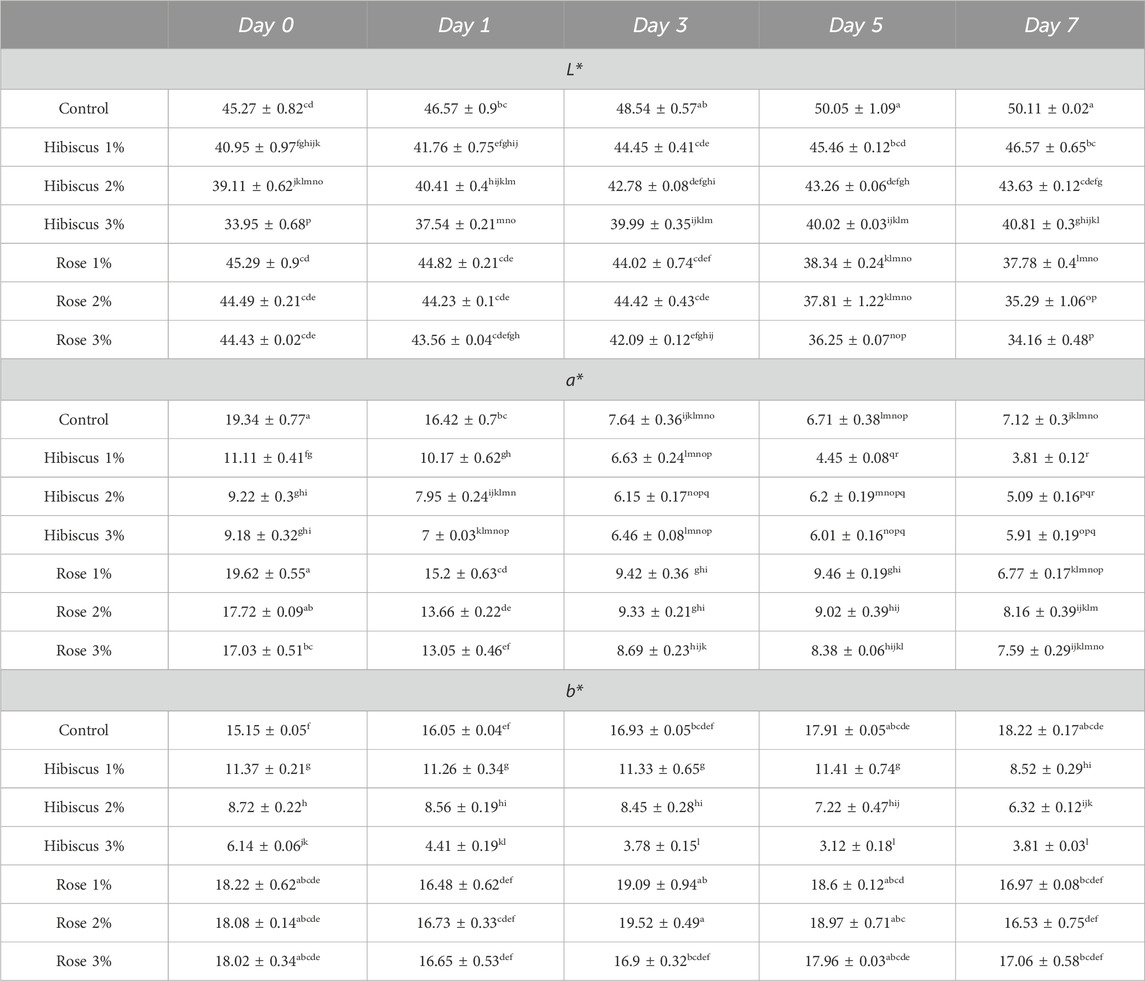
Table 1. Lightness (L*), Red/Green Coordinate (a*), and Yellow/Blue coordinate (b*) from day 0 to day 7 across treatments. Significant differences indicated by different letters (p < 0.05).
In contrast, raw ground beef patties treated with rose powder 1%, 2%, and 3% displayed different results. For instance, those treated with rose powder 1% had a lightness of 45.29 ± 0.9 on day 0, which decreased to 37.78 ± 0.4 on day 7 (p > 0.05). Similarly, patties treated with rose powder 2% exhibited a lightness of 44.49 ± 0.21 on day 0, which decreased to 35.29 ± 1.06 on day 7 (p > 0.05). Likewise, patties treated with rose powder 3% showed a lightness of 44.43 ± 0.02 on day 0, decreasing to 34.16 ± 0.48 on day 7 (p > 0.05). Thus, patties treated with rose powder 3% on days 0 and 7 also displayed significant differences (p < 0.05), like raw ground beef patties (control) and those treated with hibiscus 1%, 2%, and 3%, as well as rose 1% and 2%. Consequently, this experiment suggests that lightness was higher on day 7 in raw ground beef patties (control) compared to those treated with hibiscus 1%, 2%, and 3%, and rose 1%, 2%, and 3% powder percentages.
The a* -mean values of raw ground beef patties (control) and those treated with hibiscus powders 1%, 2%, and 3%, as well as rose powders 1%, 2%, and 3%, are presented in Table 1. On day 0, the a* value for raw ground beef patties (control) was 19.34 ± 0.77, which decreased to 7.12 ± 0.3 on day 7, indicating a significant difference (p > 0.05). Similarly, raw ground beef patties treated with hibiscus powder 1% exhibited a redness of 11.11 ± 0.41 on day 0, which decreased to 3.81 ± 0.12 on day 7, showing a significant difference (p < 0.05). Comparable trends were observed for raw ground beef patties treated with hibiscus powder 2% and 3%, with initial redness values of 9.22 ± 0.3 and 9.18 ± 0.32 on day 0, respectively, decreasing to 5.09 ± 0.16 and 5.91 ± 0.19 on day 7, respectively (p < 0.05).
In addition, raw ground beef patties treated with Rose powder 1%, 2%, and 3% displayed comparable results. For instance, those treated with rose powder 1% had a redness of 19.62 ± 0.55 on day 0, which decreased to 6.77 ± 0.17 on day 7 (p > 0.05). Similarly, patties treated with rose powder 2% exhibited a redness of 17.72 ± 0.09 on day 0, which decreased to 8.16 ± 0.39 on day 7 (p > 0.05). Likewise, patties treated with rose powder 3% showed a redness of 17.03 ± 0.51 on day 0, decreasing to 7.59 ± 0.29 on day 7 (p < 0.05). Thus, patties treated with rose powder 3% on days 0 and 7 also displayed significant differences, like raw ground beef patties (control) and those treated with hibiscus 1%, 2%, and 3%, as well as rose 1% and 2%.
Consequently, this experiment suggests that retention of redness on day 7 was higher in raw ground beef patties treated with rose powder 3% compared to raw ground beef patties (control) and those treated with hibiscus 1%, 2%, 3%, and rose 1%, 2% powders.
The b* -mean values of raw ground beef patties (control) and those treated with hibiscus powders 1%, 2%, and 3%, as well as rose powders 1%, 2%, and 3%, are presented in Table 1. On day 0, the b* value for raw ground beef patties (control) was 15.15 ± 0.05, which increased to 18.22 ± 0.17 on day 7, indicating a significant difference (p < 0.05). Similarly, raw ground beef patties treated with hibiscus powder 1% exhibited a yellowness of 11.37 ± 0.21 on day 0, which decreased to 8.52 ± 0.29 on day 7, showing a significant difference (p < 0.05). Comparable trends were observed for raw ground beef patties treated with hibiscus powder 2% and 3%, with initial yellowness values of 8.72 ± 0.22 and 6.14 ± 0.06 on day 0, respectively, decreasing to 6.32 ± 0.12 and 3.81 ± 0.03 on day 7, respectively (p > 0.05).
In contrast, raw ground beef patties treated with rose powder 1%, 2%, and 3% showed different results. For instance, those treated with rose powder 1% had a yellowness of 18.22 ± 0.62 on day 0, which slightly decreased to 16.97 ± 0.08 on day 7, with no significant difference observed between the 2 days (p > 0.05). Similarly, patties treated with rose powder 2% exhibited a yellowness of 18.08 ± 0.14 on day 0, which slightly decreased to 16.53 ± 0.75 on day 7, with no significant difference observed (p > 0.05). Likewise, patties treated with rose powder 3% showed a yellowness of 18.02 ± 0.34 on day 0, which slightly decreased to 17.06 ± 0.58 on day 7, with no significant difference observed (p > 0.05).
Therefore, this experiment suggests that yellowness was lower on day 7 in raw ground beef patties treated with hibiscus powder 3% compared to raw ground beef patties (control) and those treated with hibiscus powder 1%, 2%, and rose powder 1%, 2%.
4.4 Texture
The textural property of hardness in beef patties is illustrated by the mean values presented in Table 2. Table 2 compares the hardness of raw ground beef patties (control) with patties treated with various percentages of hibiscus and rose powders (1%, 2%, 3%). On day 0, raw beef patties (control) exhibited a hardness of 2,696.34 ± 574.62 N, which increased to 4,734.1 ± 576.55 N by day 7 (p < 0.05), indicating a significant difference over the storage period. In contrast, raw ground beef patties treated with 1% hibiscus powder showed a decrease in hardness from day 0 to day 7, with values decreasing from 5,150.17 ± 531.04 N to 4,278.76 ± 763.02 N (p > 0.05). Similarly, patties treated with hibiscus powder 2% and 3% exhibited a decrease in hardness over the same period, with values decreasing from 5,853.81 ± 874.01 N to 4,841.29 ± 201.56 N and from 6,837.73 ± 341.99 N to 5,150.17 ± 531.04 N, respectively (p > 0.05).
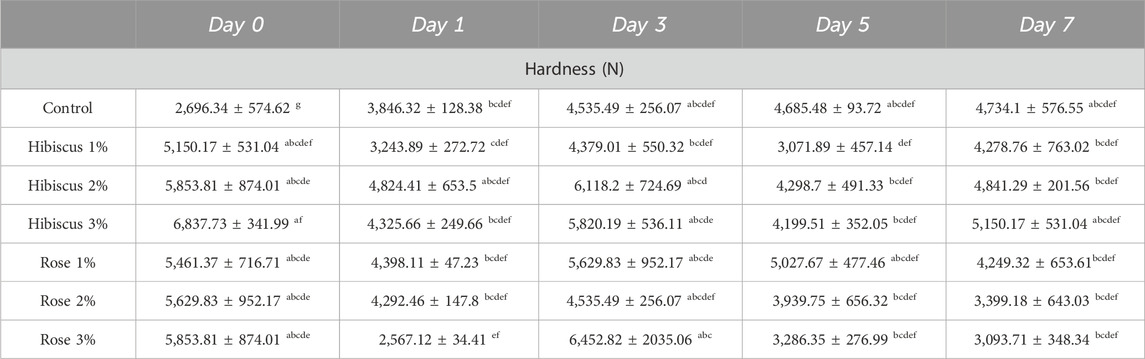
Table 2. Hardness (N) measurements from day 0 to day 7 across treatments. Significant differences indicated by different letters (p < 0.05).
In comparison, raw ground beef patties treated with rose powder 1% did not exhibit a significant difference, with hardness values decreasing from 5,461.37 ± 716.71 N on day 0–4,249.32 ± 653.61 N on day 7 (p > 0.05). Similarly, patties treated with rose powder 2% and 3% showed similar trends, with hardness values decreasing from 5,629.83 ± 952.17 N to 3,399.18 ± 643.03 N and from 5,853.81 ± 874.01 N to 3,093.71 ± 348.34 N, respectively (p > 0.05).
Overall, the hardness analysis indicates that raw ground beef patties treated with rose powder 3% had the lowest hardness value on day 7 compared to raw ground beef patties (control) and those treated with hibiscus and rose powders (2%, 3%). However, no significant difference was observed between raw ground beef patties (control) and those treated with hibiscus and rose powders (1%, 2%, 3%) on both day 0 and day 7 of the analysis.
Table 3 presents the mean cohesiveness values in beef patties, indicating the cohesion percentage in raw ground beef patties (control) and patties treated with hibiscus and rose powders (1%, 2%, 3%). Cohesiveness increased in raw ground beef patties compared to those treated with hibiscus powders 1%, 2%, 3%, and rose powder 2%, 3%.
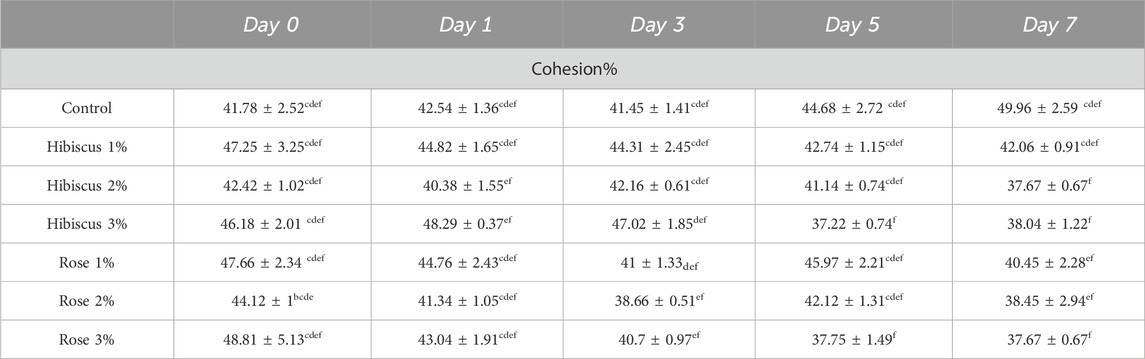
Table 3. Cohesion % measurements from day 0 to day 7 across treatments. Significant differences indicated by different letters (p < 0.05).
On day 0, raw beef patties (control) exhibited a cohesiveness of 41.78% ± 2.52%, which increased slightly to 49.96% ± 2.59% on the 7th day, showing no significant difference (p < 0.05). However, raw ground beef patties treated with hibiscus powder 1% showed a decrease in cohesiveness from 47.25% ± 3.25% on day 0%–42.06% ± 0.91% on day 7 (p > 0.05). Similarly, patties treated with hibiscus powder 2% decreased cohesiveness from 42.42% ± 1.02% on day 0%–37.67% ± 0.67% on day 7 (p > 0.05).
Comparable results were observed for patties treated with hibiscus powder 3%, with cohesiveness decreasing from 46.18% ± 2.01% to 38.04% ± 1.22% from day 0 to day 7 (p > 0.05), raw ground beef patties treated with rose powder 1% showed a significant decrease in cohesiveness from 47.66% ± 2.34% on day 0%–40.45% ± 2.28% on day 7 (p > 0.05). Likewise, patties treated with rose powder 2% and 3% exhibited comparable results to those treated with rose powder 1%, with cohesiveness decreasing from 44.12% ± 1% on day 0%–38.45% ± 2.94% on day 7 (p > 0.05) and from 48.81% ± 5.13% on day 0%–37.67% ± 0.67% on day 7 (p > 0.05), respectively. In conclusion, the analysis of cohesiveness suggests that raw ground beef patties (control) and those treated with hibiscus and rose powder 1,2 and 3 percentages were not significantly different when observed from day 0 to day 7.
The mean value in Table 4 indicates the springiness percentage in raw ground beef patties (control) and patties raw ground beef patties treated with hibiscus and rose powders 1,2,3 percentages. Springiness increased in raw ground beef patties (control) than in raw ground beef patties treated with hibiscus powders 1,2,3 and rose powders 1,2,3 percentages. On the day of 0, raw ground beef patties (control) had (42.26% ± 1.11% of springiness, which increased to 54.54% ± 1.04% on the 7th day, which also showed that the springiness in raw ground beef patties was significantly not different; p < 0.05).
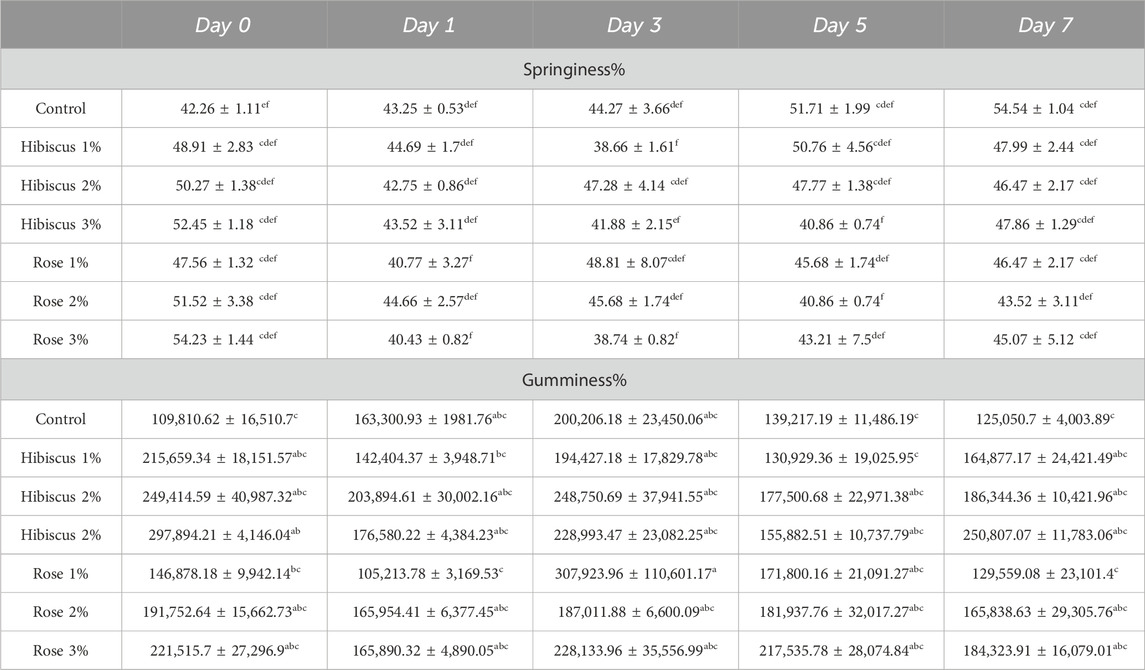
Table 4. Springiness and Gumminess % measurements from day 0 to day 7 across treatments. Significant differences indicated by different letters (p < 0.05).
Contrast results were obtained in raw ground beef patties treated with hibiscus 1 percentage showed different results to that of raw ground beef patties as the springiness decreased from day 0–7 (48.91% ± 2.83% to 47.99% ± 2.44%; p > 0.05) in raw ground beef patties treated with hibiscus powder 1 percentage; p > 0.05) were not significantly different. Whereas patties treated with hibiscus powder two percent resulted in (50.27% ± 1.38% on the 0th day and 46.47% ± 2.17% springiness on the 7th day; p > 0.05) also showed that patties treated with hibiscus powder 2 percent were not significantly different.
Similar results were obtained from raw ground patties treated with hibiscus powder three percentage as the results showed (52.45% ± 1.18% on the 0th day and 47.86% ± 1.29% on the 7th day; p > 0.05). For raw ground beef patties treated with rose powder, one percentage was not significantly different from day 0–7 because the results on the 0th day was 47.56% ± 1.32% which decreased to 46.47% ± 2.17% on the 7th day; p > 0.05). Raw ground beef patties treated with rose powder two percentage showed results of (51.52% ± 3.38% on the 0 days, which decreased to 43.52% ± 3.11% on the seventh day; p > 0.05) was also not significantly different, whereas raw ground beef patties treated with rose powder three percentage showed 54.23% ± 1.44% on 0th day increased to 45.07% ± 5.12% on 7th day; p > 0.05) was also not significantly different. Hence, through this experiment, according to the mean values of springiness, we can conclude that raw ground beef patties (control) and patties treated with hibiscus and rose powder 1,2 and 3 percentages were not significantly different when seen on the 0th to 7th day of analysis.
In addition, Table 4 also includes the mean values of gumminess % in raw ground beef patties, including both the control group and patties treated with various percentages of hibiscus and rose powders (1%, 2%, 3%). Gumminess increased in raw ground beef patties (control) compared to those treated with hibiscus 1%, 2%, 3%, and rose 1%, 2%, and 3% powder percentages. On day 0, raw beef patties (control) exhibited a gumminess of 109,810.62 ± 16,510.7 N, which increased to 125,050.7 ± 4,003.89 N on the 7th day, showing no significant difference (p < 0.05). However, raw ground beef patties treated with hibiscus 1% powder showed a decrease in gumminess from 215,659.34 ± 18,151.57 N on day 0–164,877.17 ± 24,421.49 N on day 7 (p > 0.05), which was not significantly different. Similarly, patties treated with hibiscus 2% powder decreased gumminess from 249,414.59 ± 40,987.32 N on day 0–186,344.36 ± 10,421.96 N on day 7 (p > 0.05), also not significantly different. The same trend was observed for patties treated with 3% of hibiscus powder, with gumminess decreasing from 297,894.21 ± 4,146.04 N on day 0–250,807.07 ± 11,783.06 N on day 7 (p < 0.05), also not significantly different. Raw ground beef patties treated with rose 1% powder showed similar results to those treated with hibiscus powder 1%, 2%, and 3% when observed from day 0 to day 7, with gumminess decreasing from 146,878.18 ± 9,942.14 N on day 0–129,559.08 ± 23,101.4 N on day 7 (p > 0.05). Likewise, patties treated with rose 2% powder exhibited a decrease in gumminess from 191,752.64 ± 15,662.73 N on day 0–165,838.63 ± 29,305.76 N on day 7 (p > 0.05), not significantly different.
Similarly, patties treated with rose 3% powder showed gumminess decreasing slightly from 221,515.7 ± 27,296.9 N on day 0–184,323.91 ± 16,079.0 N on day 7 (p > 0.05), also not significantly different. In conclusion, according to the mean gumminess values, there was no significant difference observed between raw beef patties (control) and those treated with hibiscus powders 1%, 2%, 3%, and rose powders 1%, 2%, 3% when observed on day 0 and day 7 of analysis.
Table 5 presents the mean chewiness values in raw ground beef patties, including the control group and patties treated with various percentages of hibiscus and rose powders (1%, 2%, 3%). Chewiness increased from the 0th day to the 7th day of analysis in raw ground beef patties and in those treated with hibiscus powders, 1%, 2%, 3%, and rose 1%, 2%, and 3% percentages.
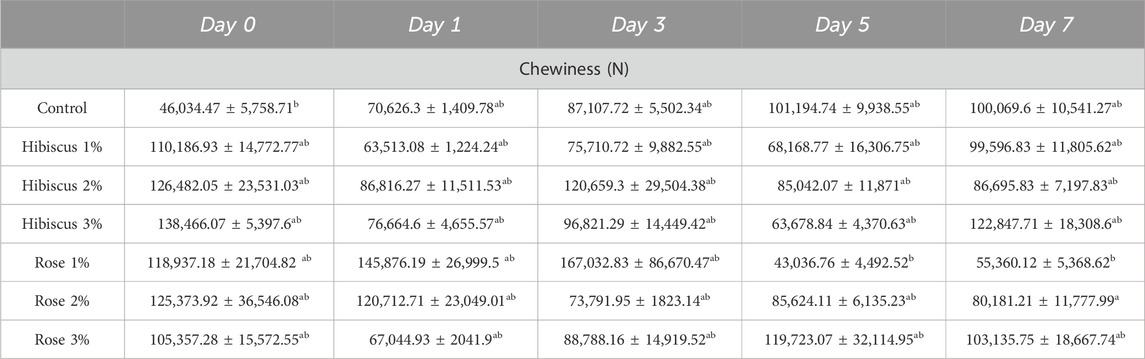
Table 5. Chewiness (N) measurements from day 0 to day 7 across treatments. Significant differences indicated by different letters (p < 0.05).
On the 0th day, raw ground beef patties (control) exhibited a chewiness of 46,034.47 ± 5,758.71 N, which increased to 100,069.6 ± 10,541.27 N on the 7th day, indicating that raw beef patties were significantly different (p < 0.05). Similarly, raw ground beef patties treated with hibiscus 1% powder showed a similar increase in chewiness from 99,596.83 ± 11,805.62 N to 110,186.93 ± 14,772.77 N on the 7th day (p < 0.05). However, patties treated with hibiscus 2% powder decreased chewiness from 126,482.05 ± 23,531.03 N on the 0th day to 86,695.83 ± 7,197.83 N on the 7th day (p > 0.05). The same trend was observed for patties treated with hibiscus 3% powder, with chewiness decreasing from 138,466.07 ± 5,397.6 N on the 0th day to 122,847.71 ± 18,308.6 N on the 7th day (p > 0.05).
In contrast, raw ground beef patties treated with rose 1% powder did not show a significant difference when observed from the 0th to the 7th day, with values increasing from 55,360.12 ± 5,368.62 N on the 0th day to 118,937.18 ± 21,704.82 N on the 7th day (p < 0.05). Likewise, patties treated with rose 2% powder exhibited an increase in chewiness from 80,181.21 ± 11,777.99 N on the 0th day to 125,373.92 ± 36,546.08 N on the 7th day (p < 0.05), not significantly different. Similarly, patties treated with rose 3% powder showed chewiness values decreasing from 105,357.28 ± 15,572.55 N on the 0th day to 103,135.75 ± 18,667.74 N on the 7th day (p > 0.05).
In conclusion, according to the mean values of chewiness, raw ground beef patties (control) and raw ground beef patties treated with hibiscus and rose powders 1%, 2%, and 3%, were not significantly different when observed from the 0th to the 7th day of analysis.
4.5 MDA
Figure 4 illustrates the impact of MDA development in raw ground beef patties (control) compared to those treated with various percentages of hibiscus and rose powders over 0, 1, 3, 5, and 7 days of analysis. Throughout the analysis period, oxidative changes in lipids occurred in all raw beef patties, resulting in notably higher MDA values in raw ground beef patties (control) compared to those treated with hibiscus and rose powders 1,2 and 3 percentages.
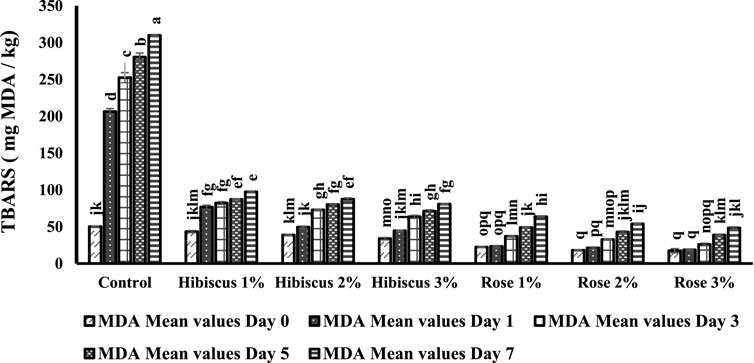
Figure 4. Concentrations of MDA (TBARS, nmol MDA/g) from days 0–7 across different treatments. Results presented as mean ± SD of three independent determinations. Significant differences indicated by different letters (p < 0.05).
MDA equivalents per µg/kg in raw ground beef patties on the 0th day were 50.0 per µg/kg, escalating to 309.8 per µg/kg on day 7 (p < 0.05), significantly different from those treated with hibiscus 1% powder, with MDA equivalents per µg/kg starting at 43.2 and gradually increasing to 97.2 on the 7th day (p < 0.05). The results on days 0 and 7 were significantly different. Similarly, patties treated with hibiscus 2% powder showed MDA equivalents of 38.8 per µg/kg on day 0, which increased to 87.2 on the 7th day (p < 0.05), also significantly different.
Likewise, raw ground beef patties treated with hibiscus 3% powder exhibited significant differences on days 0 and 7, with MDA equivalents of 33.7 per µg/kg on day 0 and 80.7 on day 7 (p < 0.05). Raw ground beef samples treated with rose 1%, 2%, and 3% powders also displayed notably lower MDA values. Rose 1% powder resulted in MDA equivalents per µg/kg of 22.3 on the 0th day, increasing to 63.4 on the 7th day (p < 0.05). Similarly, rose 2% powder showed lower MDA equivalents, with values of 18.0 on the 0th day and 53.8 on the 7th day (p < 0.05). Likewise, patties treated with rose 3% powder showed lower MDA equivalents per µg/kg on days 0 and 7, with values of 17.4 and 48.3, respectively (p < 0.05), as depicted in Figure 4.
In conclusion, this experiment establishes that raw ground beef patties (control) and those treated with hibiscus 1%, 2%, and 3% and rose 1%, 2%, and 3% were significantly different when observed on days 0 and 7 of the analysis, as indicated alphabetically.
4.6 Carbonyls
Figure 5 presents the findings regarding protein carbonylation in raw ground beef patties and those treated with varying percentages of hibiscus and rose powders (1%, 2%, and 3%). Protein carbonyl levels increased significantly in raw ground beef patties (control) compared to those treated with powders.
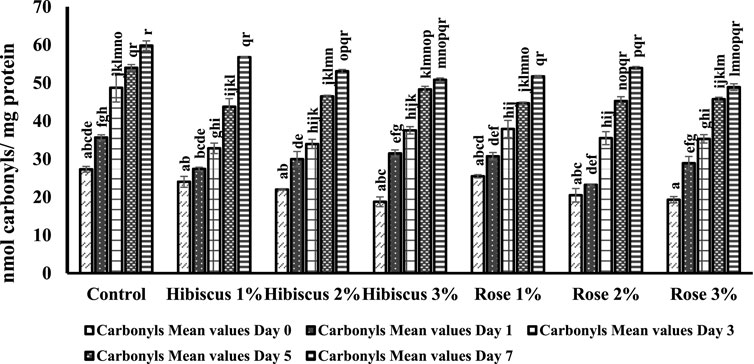
Figure 5. Carbonyl concentrations from day 0 to day 7 across various treatments. Significant differences indicated by different letters (p < 0.05).
On day 0, raw ground beef patties (control) exhibited 27.3 nmol per mg of protein carbonyls, which significantly increased to 59.7 nmol per mg on the 7th day (p > 0.05). Figure 4 indicates different alphabets on days 0 and 7, representing significant differences in protein carbonyl levels. Raw ground beef patties treated with hibiscus 1% powder displayed results like the control group, with a similar increase in protein carbonyl content on both days 0 and 7 (24.01 nmol per mg protein on day 0 and 56.76 nmol per mg protein on day 7; p > 0.05). Similarly, patties treated with hibiscus 2% powder showed increased protein carbonyl content from 21.98 nmol per mg protein on day 0–53.03 nmol per mg protein on day 7 (p > 0.05). Comparable results were observed for raw ground beef patties treated with hibiscus 3% powder, with levels increasing from 18.7 nmol per mg protein on day 0–50.8 nmol per mg protein on day 7 (p > 0.05).
Raw ground beef patties treated with rose 1% powder exhibited an increase in protein carbonyl content from 25.4 nmol per mg protein on day 0–51.7 nmol per mg protein on day 7 (p > 0.05). Similarly, patties treated with rose 2% and rose 3% powders showed similar trends, with protein carbonyl levels from day 0 to day 7 (20.4 nmol per mg protein to 53.91 nmol per mg protein for rose 2% powder and 19.3 nmol per mg protein to 48.8 nmol per mg protein for rose 3% powder; p > 0.05).
In conclusion, based on the mean values of protein carbonyls, raw ground beef patties and raw ground beef patties treated with hibiscus and rose powders (1%, 2%, and 3%) exhibited similar trends on both days 0 and 7, except for the control group. The protein carbonyl retention in raw ground beef patties (control) on the 7th day was comparatively lower than in those treated with hibiscus and rose powders.
4.7 Schiff bases
Figure 6 illustrates another marker of protein oxidation in raw ground beef patties (control) and those treated with varying percentages of hibiscus and rose powders (1%, 2%, and 3%). Schiff bases showed a significant increase in raw ground beef patties (control) compared to those treated with powders.
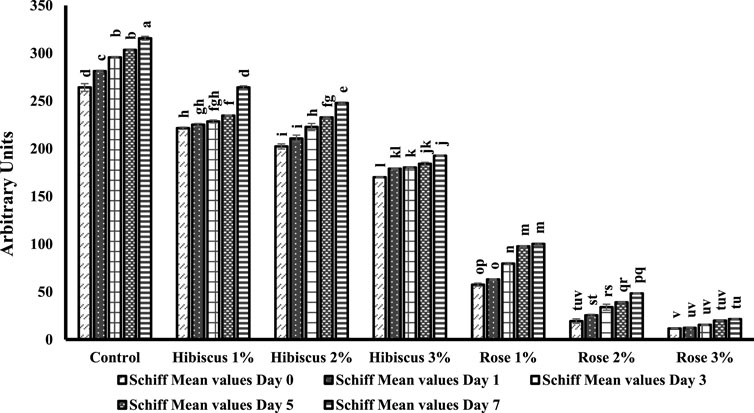
Figure 6. Schiff base concentrations from day 0 to day 7 across various treatments. Significant differences indicated by different letters (p < 0.05).
On day 0, raw ground beef patties (control) exhibited 264.0 nmol per mg of protein of Schiff bases, which significantly increased to 315.6 nmol per mg on the 7th day (p < 0.05). The alphabets in Figure 6 denote significant differences in Schiff bases from day 0 to day 7. Raw ground beef patties treated with hibiscus 1% powder displayed results like the control group, significantly increasing Schiff bases content from day 0–7 (221.3–264.0 nmol per mg protein; p < 0.05). Similarly, patties treated with hibiscus 2% powder showed an increase from 202.2 to 247.8 nmol per mg protein (p < 0.05), and those treated with hibiscus 3% powder increased from 169.9 to 192.5 nmol per mg protein (p < 0.05).
Raw ground beef patties treated with rose 1% powder exhibited similar results to those treated with hibiscus powders, with increased Schiff bases content from 57.4 on day 0–100.0 nmol per mg protein on day 7 (p < 0.05). Additionally, patties treated with rose 2% and rose 3% powders showed comparable trends, with increases from 19.2 to 48.2 nmol per mg protein (p < 0.05) for rose 2% powder and from 11.5 to 21.3 nmol per mg protein (p < 0.05) for rose 3% powder. In conclusion, based on the mean values of Schiff bases, raw ground beef patties (control) and those treated with hibiscus and rose powders (1%, 2%, and 3%) exhibited alphabetical differences on both days 0 and 7, as indicated in Figure 6. Additionally, the Schiff base content increased daily during the analysis week in raw ground beef patties (control) and those treated with hibiscus and rose powders. Notably, patties treated with these powders showed lower Schiff base content on the seventh day than raw ground beef patties (control).
4.8 Free thiols
Figure 7 illustrates another aspect of protein oxidation in raw ground beef patties and those treated with varying percentages of hibiscus and rose powders (1%, 2%, and 3%). Free thiol levels significantly decreased in raw ground beef patties (control) compared to the treated samples.
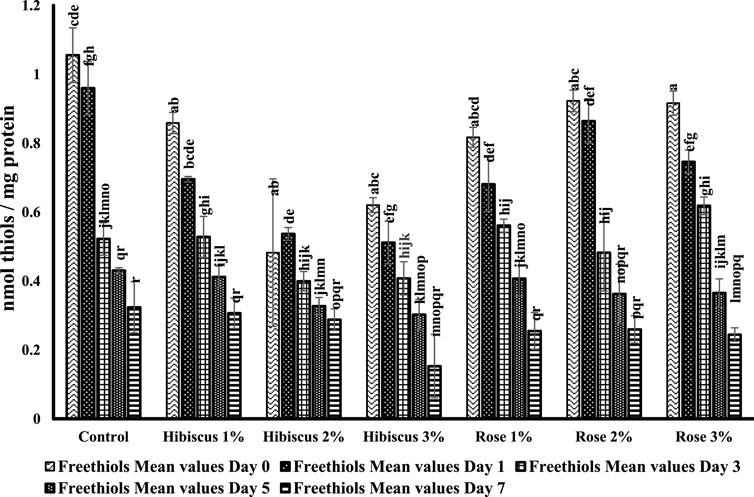
Figure 7. Free thiol concentrations from day 0 to day 7 across various treatments. Significant differences indicated by different letters (p < 0.05).
On day 0, raw ground beef patties had a free thiol level of 1.0 nmol per mg protein, significantly decreasing to 0.3 nmol per mg on the 7th day. The alphabets in the figure indicate that the free thiols from day 0–7 were significantly different (p > 0.05). Raw ground beef patties treated with hibiscus 1% powder showed comparable results to the control, with a significant decrease in free thiol content from day 0–7 (0.8–0.3 nmol per mg protein; p > 0.05). This difference is also evident in the figure, showing that raw ground beef patties treated with hibiscus 1% were significantly different from day 0–7.
Similarly, patties treated with hibiscus 2% powder decreased from 0.4 to 0.2 nmol per mg protein from day 0 to day 7 (p > 0.05). The same trend was observed in patties treated with hibiscus 3% powder, decreasing from 0.6 to 0.1 nmol per mg protein (p > 0.05). Raw ground beef patties treated with rose 1% powder also showed similar trends to those treated with hibiscus powders, decreasing from 0.8 to 0.2 nmol per mg protein on days 0–7 (p > 0.05). Likewise, patties treated with rose 2% and rose 3% powders exhibited similar trends, with decreases from 0.9 to 0.2 nmol per mg protein (p > 0.05) and from 0.9 to 0.2 nmol per mg protein (p > 0.05), respectively.
In conclusion, based on the mean values of free thiols, raw ground beef patties treated with hibiscus and rose powders (1%, 2%, and 3%) showed similar trends on days 0 and 7, except for the control. This indicates that the retention of free thiols in raw ground beef patties (control) on the 7th day was lower, suggesting higher protein oxidation than those treated with hibiscus and rose powders.
4.9 Radical scavenging activity
Figure 8 depicts an assessment of antioxidant capacity in both raw ground beef patties and those treated with varying percentages of hibiscus and rose powders (1%, 2%, and 3%). Radical scavenging activity exhibited a significant decrease in raw ground beef patties (control) compared to the treated samples.
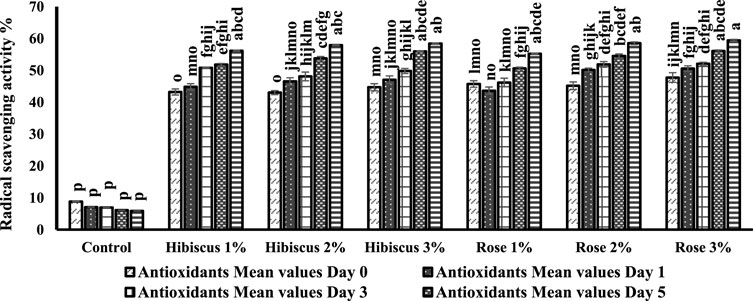
Figure 8. Free radical scavenging activity assay results from day 0 to day 7 across various treatments. Significant differences indicated by different letters (p < 0.05).
On day 0, raw ground beef patties (control) displayed a radical scavenging activity of 8.8, which significantly decreased to 5.8 on the 7th day (p > 0.05). The alphabets, in the figure denotes that the radical scavenging activity from day 0–7 did not significantly differ. In contrast, raw ground beef patties treated with hibiscus 1% powder showed significantly different results than the control, with radical scavenging activity increasing from 43.2 on day 0–56.1 on day 7 (p < 0.05).
Similarly, patties treated with hibiscus 2% powder exhibited an increase from 43.0 to 57.8 (p < 0.05), and those treated with hibiscus 3% powder showed an increase from 44.6 to 58.3 (p < 0.05). Raw ground beef patties treated with rose 1% powder also showed comparable results to those treated with hibiscus powders, with radical scavenging activity increasing from 45.7 on day 0–55.2 on day 7 (p < 0.05).
Additionally, patties treated with rose 2% and rose 3% powders demonstrated similar trends to those treated with rose 1% powder, with radical scavenging activity increasing from 45.1 to 58.5 (p < 0.05) for rose 2% powder and from 47.7 to 59.4 (p < 0.05) for rose 3% powder.
In conclusion, based on the mean values of radical scavenging activity, raw ground beef patties treated with hibiscus and rose powders (1%, 2%, and 3%) significantly differed from days 0–7 during the analysis. Notably, the radical scavenging activity content in raw ground beef patties (control) on the 7th day was comparatively lower than that of the treated samples.
5 Discussion
This study investigates the degradation of physicochemical properties and the formation of lipid and protein oxidation products (LOPs and POPs) in raw ground beef patties treated with hibiscus and rose powders at 1%, 2%, and 3% concentrations, stored at 4°C for intervals of 0, 1, 3, 5, and 7 days. Several parameters such as pH, WHC, textural attributes, and color were measured, alongside oxidative degradation markers such as MDA, protein carbonyls, Schiff bases, and free thiols. The antioxidant capacity was assessed using the DPPH assay.
During storage, the pH levels of patties showed notable changes. Untreated patties began at a pH of 5.7 on day 0, increasing to 5.9 by day 7. Hibiscus-treated patties, however, showed marginal changes. For instance, 1% hibiscus-treated patties had a stable pH of 5.5, 2% hibiscus had a pH of 5.3, and 3% hibiscus remained at pH 5.0 throughout the study. Similarly, rose-treated patties showed a varied pH response, with 1% rose at pH 5.6% and 2% rose dropping from 5.5 to 5.4. The 3% rose treatment maintained a stable pH of 5.4 during storage.
The variation in pH responses is linked to the unique chemical compositions of hibiscus and rose powders, particularly the presence of acids and non-extractable polyphenols (Villasante et al., 2020). Additionally, the isoelectric point (pI) of meat proteins typically occurs at pH 5.0–5.2, where protein solubility and WHC decrease significantly due to a minimal net charge (Pergande and Cologna, 2017). This pH stabilization aligns with the 3% hibiscus-treated patties, which maintained a pH of 5.0 throughout, indicating reduced protein solubility near the pI.
The WHC values also exhibited differences. While untreated patties maintained a consistent WHC, rose-treated patties, especially at 2% and 3%, demonstrated an increase in WHC. This effect could be attributed to the lower pH and higher fiber content of rose powders (Yıldız-Turp and Serdaroglu, 2010). Conversely, hibiscus-treated patties did not exhibit significant changes in WHC compared to untreated samples, implying that hibiscus powders did not restrict water movement within the patties.
The complex interaction between pH, WHC, and the polyphenol content of these powders is a critical component of food preservation. The acids and polyphenols in hibiscus and rose could have significantly contributed to the observed effects, showcasing the multifaceted impact of botanical additives on meat products (Gómez-Cortés et al., 2018). Further research is warranted to explore the mechanisms driving these interactions and to optimize the application of edible flower powders for enhanced sensory and preservation attributes.
In addition to this finding by Youssef and Srivastava, (2017) also highlights similar findings related to natural coatings, demonstrating that flaxseed gum in combination with lemongrass essential oil, when applied to meat, could improve oxidative stability and extend shelf life by reducing lipid oxidation. This further supports the antioxidant potential seen in the hibiscus and rose powder treatments.
Color and texture are crucial attributes of meat quality. This study revealed that hibiscus-treated patties showed reduced yellowness compared to both the untreated and rose-treated patties, with the most pronounced color preservation observed in patties treated with 3% hibiscus. This color stability can be attributed to the presence of anthocyanins in hibiscus, which impart red, purple, and blue hues and contribute to the overall visual appeal of food products (Zhang et al., 2019). The antioxidative properties of hibiscus, reflected by lower levels of lipid oxidation markers like MDA, play a significant role in maintaining meat color by inhibiting metmyoglobin formation, a primary cause of meat discoloration (Augustynska-Prejsnar et al., 2018; Viana et al., 2017; Wang et al., 2021).
Adding hibiscus and rose powders effectively reduced the hardness of the patties, especially at higher concentrations. After 7 days, treatments with 3% hibiscus and rose powders led to a significant decrease in hardness, aligning with findings from Jung et al. (2013), who reported similar softening effects in meat products with plant-based additives. The observed reduction in hardness may be attributed to the lower pH of the treated patties, which affects protein gel formation and matrix stability, ultimately leading to a softer texture (Banerjee et al., 2020; Perez-Baez et al., 2021). Additionally, the higher fiber retention and improved WHC of patties treated with hibiscus and rose powders likely contributed to the softer texture. Fiber content helps retain moisture within the matrix, enhancing juiciness and tenderness, consistent with studies on fiber-enriched meat products by Kurt and Gençcelep (2018). This effect is also supported by a recent study conducted by Cerón-Guevara et al. (2020), which showed that 5% Pleurotus (Pd5) concentrations led to the lowest hardness values in meat products due to the enhanced water retention properties of these natural additives. Similar findings were observed in studies on sausages, where higher doses of roselle extract (above 6%) achieved comparable texture softening effects, further validating the moisture-retaining capabilities of hibiscus and rose powders in meat applications (Bermúdez et al., 2023). The minimal reduction in springiness and cohesiveness observed in hibiscus- and rose-treated patties were not significant, owing to common outcomes in meat products containing non-meat additives, as described by Akesowan (2016) and Bermúdez et al. (2023). Overall, hibiscus and rose powders appear to be effective in reducing hardness while maintaining acceptable springiness and cohesiveness in beef patties, supporting their potential use as natural additives to improve texture and moisture retention in meat products.
The study also explored the antioxidant activity, lipid oxidation, and protein oxidation in the patties. The DPPH assay confirmed that hibiscus and rose powders improved antioxidant capacity, as evidenced by decreased lipid oxidation and lower TBARS values in treated patties .3% rose treatments exhibited the highest antioxidant capacity, which remained consistent throughout storage (Bozkurt and Belibagli, 2009; Malelak et al., 2017). These effects are supported by the rich phenolic content of hibiscus and rose powders (Mohamed et al., 2007). Lower levels of MDA in treated patties further indicated the antioxidative capacity of these powders in mitigating lipid oxidation (Zhang et al., 2016).
Protein oxidation, assessed through protein carbonylation and Schiff base formation, showed significant reductions in hibiscus- and rose-treated patties compared to the control group. The preservation of free thiol groups in treated patties is likely due to the presence of phenolic-rich extracts in these powders, which protect against protein oxidation (Estévez and Cava, 2006; Jongberg et al., 2011). The strong antioxidant properties of hibiscus and rose contribute to maintaining the structural integrity and quality of meat during storage (Stadtman and Levine, 2003; Haak et al., 2009; Utrera et al., 2015; Ganhão et al., 2010; Vossen et al., 2012). Infact the reduction in protein carbonylation could be influenced by cold storage conditions, emphasizes the importance of considering storage conditions when evaluating protein oxidation in beef products (Filgueras et al., 2010; Lindahl et al., 2010; Rowe et al., 2004).
In conclusion, the results of this study demonstrate that hibiscus and rose powders significantly contribute to reducing both lipid and protein oxidation in raw ground beef patties, improving their color, texture, and overall quality (Kurt and Gençcelep, 2018). The distinct chemical compositions of these powders, including their polyphenolic content, dietary fiber, and acids, play a crucial role in their antioxidative properties and effects on meat preservation (Jia et al., 2012). This study underscores the potential of edible flower powders in extending the shelf life and enhancing the sensory attributes of meat products, warranting further investigation into their application as natural additives in food preservation.
6 Conclusion
The incorporation of hibiscus and rose powders at concentrations of 1%, 2%, and 3% into raw ground beef patties has shown notable benefits in reducing lipid and protein oxidation. These edible flowers significantly enhance the oxidative stability of meat by boosting free radical scavenging activity, improving water-holding capacity, and reducing oxidative markers such as TBARS values and protein carbonyls, while retaining essential compounds like free thiols and Schiff bases. Among the tested concentrations, hibiscus and rose powders at 3% demonstrated the highest efficacy in mitigating lipid and protein oxidation compared to lower concentrations and untreated patties. However, it is crucial to carefully regulate the powder content to avoid negatively impacting the flavor and color of the patties, ensuring the product remains suitable for industrial applications and consumer preferences.
7 Future research
Further research is required to understand the post-cooking effects of hibiscus and rose powders on ground beef patties. This includes conducting sensory analysis to gauge consumer acceptance, microbial activity assessments to ensure product safety, and studying the interactions between phenolic compounds from the powders and meat proteins to gain insight into their antioxidant mechanisms. Additionally, exploring advanced technologies and different methods for incorporating edible flowers into meat products will provide a more comprehensive understanding of their benefits. Filling these research gaps could lead to innovative strategies for utilizing edible flowers in meat processing, offering enhanced product quality while meeting consumer expectations for both taste and safety.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
KD: Writing–original draft, Formal Analysis, Writing–review and editing, Methodology, Investigation. GM: Formal Analysis, Writing–original draft, Writing–review and editing. FK: Supervision, Writing–review and editing. AM: Visualization, Validation, Supervision, Resources, Project administration, Funding acquisition, Data curation, Conceptualization, Writing–review and editing, Methodology, Investigation.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Georgia Beef Commission Grant (RGABF000176260A), Georgia, United States.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akesowan, A. (2016). Influence of soy protein isolate on quality of light pork sausages. Asian J. Animal Sci. 10 (2), 50–58.
Augustynska-Prejsnar, A., Ormian, M., and Sokolowicz, Z. (2018). Physicochemical and sensory properties of broiler chicken breast meat stored frozen and thawed using various methods. J. Food Qual. 2018, 1–9. doi:10.1155/2018/6754070
Banerjee, D. K., Das, A. K., Banerjee, R., Pateiro, M., Nanda, P. K., Gadekar, Y. P., et al. (2020). Application of enoki mushroom (Flammulina Velutipes) stem wastes as functional ingredients in goat meat nuggets. Foods 9 (4), 432. doi:10.3390/foods9040432
Bermúdez, R., Rangel-Vargas, E., Lorenzo, J. M., Rodríguez, J. A., Munekata, P. E. S., Teixeira, A., et al. (2023). Effect of partial meat replacement by Hibiscus sabdariffa by-product and Pleurotus djamor powder on the quality of beef patties. Foods 12 (2), 391. doi:10.3390/foods12020391
Bozkurt, H., and Belibaglı, K. B. (2009). Use of rosemary and Hibiscus sabdariffa in production of kavurma, a cooked meat product. J. Sci. Food Agric. 89 (7), 1168–1173. doi:10.1002/jsfa.3570
Bumsted, J., Ford, E., Blair, A., Underwood, K., and Zuelly, S. M. S. (2023). Instrumental color measurements have relationships to fat smearing in fresh sausage. Foods 12 (14), 2813. doi:10.3390/foods12142813
Cerón-Guevara, M. I., Rangel-Vargas, E., Lorenzo, J. M., Bermúdez, R., Pateiro, M., Rodriguez, J. A., et al. (2020). Effect of the addition of edible mushroom flours (Agaricus bisporus and Pleurotus ostreatus) on physicochemical and sensory properties of cold-stored beef patties. J. Food Process. Preserv. 44 (3), e14351. doi:10.1111/jfpp.14351
Chensom, S., Okumura, H., and Mishima, T. (2019). Primary screening of antioxidant activity, total polyphenol content, carotenoid content, and nutritional composition of 13 edible flowers from Japan. Prev. Nutr. Food Sci. 24 (2), 171–178. doi:10.3746/PNF.2019.24.2.171
Da-Costa-Rocha, I., Bonnlaender, B., Sievers, H., Pischel, I., and Heinrich, M. (2014). Hibiscus sabdariffa L.–A phytochemical and pharmacological review. Food Chem. 165, 424–443. doi:10.1016/j.foodchem.2014.05.002
Drouillard, J. S. (2018). Current situation and future trends for beef production in the United States of America—a review. Asian-Australasian J. Animal Sci. 31 (7), 1007–1016. doi:10.5713/ajas.18.0428
Estévez, M., and Cava, R. (2006). Effectiveness of rosemary essential oil as an inhibitor of lipid and protein oxidation: contradictory effects in different types of frankfurters. Meat Sci. 72 (2), 348–355. doi:10.1016/j.meatsci.2005.08.005
Filgueras, R. S., Gatellier, P., Aubry, L., Thomas, A., Bauchart, D., Durand, D., et al. (2010). Colour, lipid and protein stability of Rhea americana meat during air- and vacuum-packaged storage: influence of muscle on oxidative processes. Meat Sci. 86 (3), 665–673. doi:10.1016/j.meatsci.2010.06.003
Ganhão, R., Morcuende, D., and Estévez, M. (2010). Protein oxidation in emulsified cooked burger patties with added fruit extracts: influence on colour and texture deterioration during chill storage. Meat Sci. 85 (3), 402–409. doi:10.1016/j.meatsci.2010.02.008
Gibis, M., and Weiss, J. (2010). Inhibitory effect of marinades with hibiscus extract on formation of heterocyclic aromatic amines and sensory quality of fried beef patties. Meat Sci. 85, 735–742. doi:10.1016/j.meatsci.2010.03.034
Gómez-Cortés, P., Guerra-Rivas, C., Gallardo, B., Lavín, P., Mantecón, A. R., De la Fuente, M. A., et al. (2018). Grape pomace in ewes diet: effects on meat quality and the fatty acid profile of their suckling lambs. Food Res. Int. 113, 36–42. doi:10.1016/j.foodres.2018.06.052
Haak, L., Raes, K., Van Dyck, S., and De Smet, S. (2009). Effect of dietary antioxidant and fatty acid supply on the oxidative stability of fresh and cooked pork. Meat Sci. 82 (3), 323–329. doi:10.1016/j.meatsci.2009.02.005
Hu, L., Ren, S., Shen, Q., Ye, X., Chen, J., and Ling, J. (2018). Protein oxidation and proteolysis during roasting and in vitro digestion of fish (Acipenser gueldenstaedtii). J. Sci. Food Agric. 98 (14), 5344–5351. doi:10.1002/JSFA.9075
Hughes, E., Cofrades, S., and Troy, D. J. (1997). Effects of fat level, oat fibre and carrageenan on frankfurters formulated with 5, 12 and 30% fat. Meat Sci. 45 (3), 273–281. doi:10.1016/S0309-1740(96)00109-X
Jia, N., Kong, B., Liu, Q., Diao, X., and Xia, X. (2012). Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 91 (4), 533–539. doi:10.1016/j.meatsci.2012.03.010
Jongberg, S., Skov, S. H., Tørngren, M. A., Skibsted, L. H., and Lund, M. N. (2011). Effect of white grape extract and modified atmosphere packaging on lipid and protein oxidation in chill stored beef patties. Food Chem. 128 (2), 276–283. doi:10.1016/j.foodchem.2011.03.015
Jung, E., Kim, Y., and Nami, J. (2013). Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.). N J. Sci. Food Agric. 93 (15), 3769–3776. doi:10.1002/jsfa.6256
Kurt, A., and Gençcelep, H. (2018). Enrichment of meat emulsion with mushroom (Agaricus bisporus) powder: impact on rheological and structural characteristics. J. Food Eng. 237, 128–136. doi:10.1016/j.jfoodeng.2018.05.028
Levine, R. L., Williams, J. A., Stadtman, E. P., and Schacter, E. (1994). Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 233, 346–357. doi:10.1016/s0076-6879(94)33040-9
Lindahl, G., Lagerstedt, Å., Ertbjerg, P., Sampels, S., and Lundström, K. (2010). Ageing of large cuts of beef loin in vacuum or high oxygen modified atmosphere–Effect on shear force, calpain activity, desmin degradation and protein oxidation. Meat Sci. 85 (1), 160–166. doi:10.1016/j.meatsci.2009.12.020
Liu, J., Elliesoury, M. P., Stoyanchev, T., and Hocquette, J. F. (2022). Consumer perception of beef quality and how to control, improve and predict it? Focus on eating quality. Foods 11 (12), 1732. doi:10.3390/FOODS11121732
Love, J. D., and Pearson, A. M. (1971). Lipid oxidation in meat and meat products—a review. J. Am. Oil Chemists’ Soc. 48 (10), 547–549. doi:10.1007/BF02544559
Malelak, G. E. M., Lalel, H. J. D., Kale, P. R., and Jelantik, I. G. N. (2017). The Sensory properties, color, microbial, lipid oxidation, and residual nitrite of sei marinated with lime and roselle calyces extracts. Media Peternak. 40 (3), 194–201. doi:10.5398/medpet.2017.40.3.194
Mlcek, J., and Otakar, R. (2011). Fresh edible flowers of ornamental plants–A new source of nutraceutical foods. Trends Food Sci. and Technol. 22 (10), 561–569. doi:10.1016/j.tifs.2011.04.006
Mohamed, R., Fernandez, J., Pineda, M., and Aguilar, M. (2007). Roselle (Hibiscus sabdariffa) seed oil is a rich source of γ-tocopherol. J. Food Sci. 72 (3), S207–S211. doi:10.1111/j.1750-3841.2007.00285.x
Mohan, A., Jaico, T., Kerr, W., and Singh, R. (2016). Functional properties of bicarbonates on physicochemical attributes of ground beef. LWT 70, 333–341. doi:10.1016/J.LWT.2016.02.053
Mohan, A., Roy, A., Duggirala, K., and Klein, L. (2022). Oxidative reactions of 4-oxo-2-Nonenal in meat and meat products. LWT 165, 113747. doi:10.1016/J.LWT.2022.113747
Perez-Baez, A. J., Camou, J. P., Gonzalez-Aguilar, G., Lucas-Gonzalez, R., Valenzuela-Melendres, M., and Viuda-Martos, M. (2021). Roselle (Hibiscus sabdariffa L.) extracts added to Frankfurt-type sausages: effects on chemical, physicochemical, and sensorial properties. J. Food Process. Preserv. 45 (10), e15782. doi:10.1111/jfpp.15782
Pergande, M. R., and Cologna, S. M. (2017). Isoelectric point separations of peptides and proteins. Proteomes 5 (1), 4. doi:10.3390/proteomes5010004
Reitznerova, A., Ulekova, M., Nagy, J., Marcincak, S., Semjon, B., Certik, M., et al. (2017). Lipid peroxidation process in meat and meat products: a comparison study of malondialdehyde determination between modified 2-thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules 22 (11), 1988. doi:10.3390/MOLECULES22111988
Rowe, L. J., Maddock, K. R., Lonergan, S. M., and Huff-Lonergan, E. (2004). Influence of early postmortem protein oxidation on beef quality. J. Animal Sci. 82 (3), 785–793. doi:10.2527/2004.823785x
Sobral, M. C., Casal, S., Faria, M. A., Cunha, S. C., and Ferreira, I. M. P. L. V. O. (2020). Influence of culinary practices on protein and lipid oxidation of chicken meat burgers during cooking and in vitro gastrointestinal digestion. Food Chem. Toxicol. Int. J. Publ. Br. Industrial Biol. Res. Assoc. 141, 111401. doi:10.1016/J.FCT.2020.111401
Soladoye, O. P., Juarez, M. L., Aalhus, J. L., Shand, P., and Estévez, M. (2015). Protein oxidation in processed meat: mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 14 (2), 106–122. doi:10.1111/1541-4337.12127
Soriano, A., Alanon, M. E., Alarcon, M., García-Ruíz, A., Díaz-Maroto, M. C., and Perez-Coello, M. S. (2018). Oak wood extracts as natural antioxidants to increase shelf life of raw pork patties in modified atmosphere packaging. Food Res. Int. 111, 524–533. doi:10.1016/j.foodres.2018.05.055
Stadtman, E. R., and Levine, R. L. (2003). Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25, 207–218. doi:10.1007/s00726-003-0011-2
Tyburcy, A., Scibisz, I., Rostek, E., Pasierbiewicz, A., and Florowski, T. (2014). Przeciwutleniające własciwosci soków zurawiny i z rozy w produktach z mięsa rozmrożonego. Zywnosc. Nauka. Technol. Jakosc/Food. Sci. Technol. Qual. 21 (5), 72–84. doi:10.15193/ZNTJ/2014/96/072-084
Utrera, M., Morcuende, D., Ganhão, R., and Estévez, M. (2015). Role of phenolics extracting from Rosa canina L. on meat protein oxidation during frozen storage and beef patties processing. Food Bioprocess Technol. 8, 854–864. doi:10.1007/s11947-014-1450-3
Viana, F. M., Canto, A., Costa-Lima, B. R. C., Salim, A., and Junior, C. A. C. (2017). Color stability and lipid oxidation of broiler breast meat from animals raised on organic versus non-organic production systems. Poult. Sci. 96 (3), 747–753. doi:10.3382/ps/pew331
Villasante, J., Ouerfelli, M., Bobet, A., Metón, I., and Almajano, M. P. (2020). The Effects of pecan shell, roselle flower and red pepper on the quality of beef patties during chilled storage. Foods 9 (11), 1692. doi:10.3390/foods9111692
Vossen, E., Utrera, M., De Smet, S., Morcuende, D., and Estévez, M. (2012). Dog rose (Rosa canina L.) as a functional ingredient in porcine frankfurters without added sodium ascorbate and sodium nitrite. Meat Sci. 92 (4), 451–457. doi:10.1016/j.meatsci.2012.05.010
Wang, X., Wang, Z., Zhuang, H., Nasiru, M. M., Yuan, Y., Zhang, J., et al. (2021). Changes in color, myoglobin, and lipid oxidation in beef patties treated by dielectric barrier discharge cold plasma during storage. Meat Sci. 176, 108456. doi:10.1016/j.meatsci.2021.108456
Yıldız-Turp, G., and Serdaroglu, M. (2010). Effects of using plum puree on some properties of low fat beef patties. Meat Sci. 86 (4), 896–900. doi:10.1016/j.meatsci.2010.07.009
Yousuf, B., and Srivastava, A. K. (2017). Flaxseed gum in combination with lemon grass essential oil as an effective edible coating for ready-to-eat pomegranate arils. Int. J. Biol. Macromol. 104, 1030–1038. doi:10.1016/j.ijbiomac.2017.07.025
Zhang, H., Wu, J., and Guo, X. (2016). Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci. Hum. Wellness 5 (1), 39–48. doi:10.1016/j.fshw.2015.11.003
Keywords: antioxidant, lipid and protein oxidation, TBARS, DPPH, textural properties
Citation: Duggirala KB, Mummaleti G, Kong F and Mohan A (2024) Influence of edible flowers on the physicochemical and oxidative stability of raw ground beef patties. Front. Food. Sci. Technol. 4:1487336. doi: 10.3389/frfst.2024.1487336
Received: 27 August 2024; Accepted: 21 November 2024;
Published: 18 December 2024.
Edited by:
Aris E. Giannakas, University of Patras, GreeceReviewed by:
Cristiane Canan, Universidade Tecnológica Federal do Paraná, BrazilYing Chen, Yangzhou University, China
Copyright © 2024 Duggirala, Mummaleti, Kong and Mohan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anand Mohan, YW5hbmRtb2hhbkB1Z2EuZWR1
 Krishna Brunda Duggirala
Krishna Brunda Duggirala Gopinath Mummaleti
Gopinath Mummaleti Fanbin Kong
Fanbin Kong Anand Mohan
Anand Mohan