- 1Consistence Microstructure Research Laboratory, Barendrecht, Netherlands
- 2IFFCO Central R&D, Sharjah, United Arab Emirates
Here we present a novel, combined cryo-scanning electron microscopy (cryo-SEM) and confocal laser scanning microscopy (CLSM) method for imaging of chocolate confectionery product microstructures of several millimeters down to about 100 nm. The SEM part of the method is based on cryo-fixation, cryo-polishing, and scanning electron microscopy, at low vacuum and low temperature using the backscattered electron signal. Starting with cryo-fixation of the chocolate sample in a desired state (cooled, ambient, or melted), the sample is cryo-planed in a cryo-ultramicrotome. Once a polished cut is obtained, the sample is analyzed using a cryo-SEM technique, with the unusual combination of low temperature and low vacuum settings, without heavy metal coating. Imaging is done based on material density contrast. Elemental composition of particles is recorded by energy dispersive x-ray spectroscopy (EDS). The combination of imaging contrast and EDS allows identification and measurement of the four main constituents of chocolate (cocoa solids, fat, sugar, and milk solids). Finally, the same cryo-polished sample sections of solid chocolate products are analyzed using a CLSM imaging technique to reveal complementary microstructural details. An obvious application of the method could be the visualization and quantitative analysis of the size, shape, and composition of chocolate confectionery products.
Introduction
Chocolate is a product obtained from the processing of cocoa beans, the main components of Theobroma cacao fruits. Different chocolate variations are made with cocoa beans of different origin: Criollo, Forastero, Nacional, and Trinitarian (Afoakwa et al., 2008), resulting in a broad range of products with different flavor profiles suitable for various applications.
Real chocolate is a processed mixture mainly comprising cocoa butter, cocoa solids, sugar, milk solids and emulsifiers, containing trace amounts of moisture, ca 0.5%–1.5% (Afoakwa et al., 2007). Chocolate compounds (CC) are made through partial or total replacement of cocoa butter by various vegetable fats with appearance, texture and application/sensory properties (e.g., melting behavior and flavor release) resembling real chocolate (Smith, 2012; Talbot, 2009). Cocoa butter substitutes (CBS) are lauric fats originating from palm fruit kernels or coconuts, which are used to design CC primarily due to affordability, but also for the ease of application, as they do not require tempering (Lonchampt and Hartel, 2004; Stewart and Timms, 2002). In this article we use the word “chocolate” only in relation to CBS-based CC compositions.
From a microstructural point of view, chocolate can be regarded as a suspension of semi-solid fine particles in a continuous fat phase (Afoakwa, 2016). To develop desired types of chocolate product textures, optimize processing and deliver tailored sensorial properties of chocolate in product applications, good understanding of chocolate microstructure is required. Chocolate microstructure properties can be studied using particle size analysis, rheology and texture analysis, or imaging with various types of microscopes (Glicerina et al., 2016; Afoakwa et al., 2009). Particle analysis yields a stochastic quantification of particle size distribution, while rheology and texture analysis reveal the response of the system under small or large deformations and help to predict flow, texture and sensory properties of chocolate. Imaging is mainly used for a detailed understanding of individual building blocks of the structure, enabling a detailed interpretation of the orientation, morphology and shape of individual microscopic particles and fat crystals in a macroscopic product matrix (Tan and Balasubramanian, 2017; Awad and Marangoni, 2006).
Imaging of internal chocolate microstructure has been done in the past with several methodologies and with varying success. For regular transmitted light microscopy, a thin specimen needs to be prepared, which is difficult to make from a solid chocolate sample. Therefore, most research has been done using melted (and resolidified) samples, where a droplet of melted chocolate was spread between microscope glasses (Gaikwad, 2012). Furthermore, to discern individual particles, dilution in oil is useful (Feichtinger et al., 2020). Due to limits in resolution and sample preparation options, no satisfactory method seems available for light microscopy imaging of chocolate bulk structure in the intact (not melted) state. With confocal laser scanning microscopy (CLSM), resolution is better than with regular light microscopy, and autofluorescence (Gaikwad, 2012) or fluorescent staining (Gaikwad, 2012; Soltanahmadi et al., 2023) can be used to discern different types of particles. However, published CLSM methods also have significant limitations in sample preparation options. As a non-invasive 3D imaging method, x-ray microtomography (µCT) has many advantages over the already mentioned light microscopy methods, and has proven to be useful e.g., in analyzing air bubbles (Haedelt et al., 2005; Haedelt et al., 2007; Frisullo et al., 2010), sugar content and degree of anisotropy (Frisullo et al., 2010), and cracks (Reinke et al., 2016). However, µCT, even in case of using synchrotron radiation, has limitations regarding small particles, since minimum detectable particle size was estimated to be 6 µm by Reinke et al. (2016).
Scanning Electron Microscopy (SEM) could be applied to overcome restrictions in imaging resolution. Furthermore, cryo-preparation methods could be used to enable imaging of internal (bulk) chocolate structure, without a need for melting. Cryo-fixation of chocolate products is possible both from the melted and the solid state, and, when kept at low temperature, no significant recrystallisation or structure-reordering takes place (Rousseau, 1997). Several groups have reported results on freeze-fracturing of chocolate (e.g., Bikos et al., 2023). Clear drawbacks of freeze-fracturing are a rocky appearance of the created fracture surfaces and a low contrast between the suspended particles, resulting in problematic interpretation of the underlying material microstructure. Instead of freeze fracturing, the method of cryo-planing could be applied, resulting in polished block surfaces. Normally, cryo-planed surfaces require ice sublimation (freeze-etching) and heavy metal sputtercoating to gain stable imaging and a secondary electron imaging contrast of solids in an aqueous matrix (Nijsse and Van Aelst, 1999). However, since chocolate does not contain liquid water, the method of sublimation and sputtercoating would leave just a polished surface without clear microstructural details, but merely knife scratches. This contrast problem could be solved by imaging material contrast instead of surface contrast, now using a backscattered electron detector (Buckman et al., 2020). But, then a heavy metal sputtercoating should be very thin or absent, which in turn would result in imaging artifacts due to electron charging. The charging problem could be overcome by applying low vacuum conditions. Low vacuum, however, would cause water vapour contamination of the cryo-planed sample surface when applying standard cryo-SEM conditions.
Here we present a novel method, making use of a specific combination of low vacuum and low temperature, where chocolate cross sections can be imaged at even sub-micrometer resolution in SEM. In case of solid chocolate products, such samples can be analyzed in combination with CLSM as well.
Materials and methods
Chocolate compound material
A milk chocolate compound (MCC) sample consisting of crystal sugar (Vijayanagar Sugar, Gangapur, India), lauric vegetable CBS fat IFFCO PRO PSCO 32 (IFFCO, Sharjah, UAE) with melting point 34°C ± 1°C, skimmed milk powder (Fonterra, Auckland, New Zealand), whey powder (Agropur, Longueuil, Canada), natural fat-reduced cocoa powder (Olam Cocoa, Singapore), anhydrous milk fat (Amul, Palanpur, India), and soybean lecithin (Sun Nutrafoods, Mandideep, India), with a total fat content of 35.5% was made at IFFCO Central R&D laboratories, using a Bühler pilot plant consisting of a three-roller mill and a conche, to achieve final particle size of 20 ± 2 μm measured using a micrometre (Mitutoyo 293–831). In the micrometre, a diluted sample of chocolate in sunflower oil (1:1) was used to place a small drop on the anvil until getting resistance from the largest particles on the spindle to get the measurement readout. According to different authors, these largest particles are linked to the D90 in a particle size distribution measurement made by laser diffraction (Afoakwa et al., 2009; Tan and Balasubramanian, 2017; Beckett, 2019).
Cryo-fixation
A drop of melted chocolate was added on a rivet sample holder and allowed to solidify. Then the rivet with sample was cryo-fixed by plunging into melting nitrogen. This step ensures that the microstructure does not change during further steps. Cryo-fixation of a solid piece of chocolate was done by cutting out a 5 × 3 × 3 mm piece of chocolate, clamping the piece into a cryo-SEM vice-holder, and plunge-freezing as described above.
Cryo-planing
Using a cryo-ultramicrotome (Leica Ultracut UCT EM-FCS) at a cryogenic temperature (−110°C), the sample was cut with a glass knife, to obtain a cross section of the sample. This cross section was further polished by cutting thin slices with a diamond knife. The slices were discarded, and the polished block face was used for imaging and analysis. The polished area was 2 mm2 for the sample on the rivet and 15 mm2 for the clamped sample.
Cryo-SEM and elemental analysis
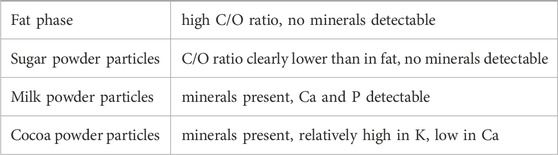
The thus cryo-planed sample was loaded into a Low-Temperature Scanning Electron Microscope (cryo-SEM, Jeol 6490LA with Gatan Alto2500 cryo-system), and analyzed at low vacuum (40 Pa), at a temperature of −40°C. The gas inlet of the low vacuum was fed by a nitrogen tank. Images were made using a Backscattered Electron Detector (Jeol MP-44160BEIW, having a Si P-N type semiconductor detector) with Compositional Contrast settings (BEC). Differences in material density cause differences in BEC contrast, resulting in images showing particles of light grey tones on a darker background of the continuous phase, since the particles (sugar, cocoa solids, milk powder) have a higher specific density than the oil phase. Brightness was adjusted such that the fat phase appeared dark grey, and contrast was adjusted to a grey level differentiation between the different ingredient particles. Elemental composition of the components was analyzed using an in-built Energy Dispersive Spectroscopy (EDS) instrument.
CLSM
After investigation in SEM, the sample temperature was adjusted to ambient, and the sample was transferred to a CLSM (Leica SP5), still in the SEM sample holder. The sample was analyzed by detecting autofluorescence (excitation wavelengths: 405 nm and 488 nm; emission channel bands: blue 413–467 nm, green 517–577 nm, red 661–788 nm), using a 10x dry objective lens and a 63x oil immersion objective lens.
Results
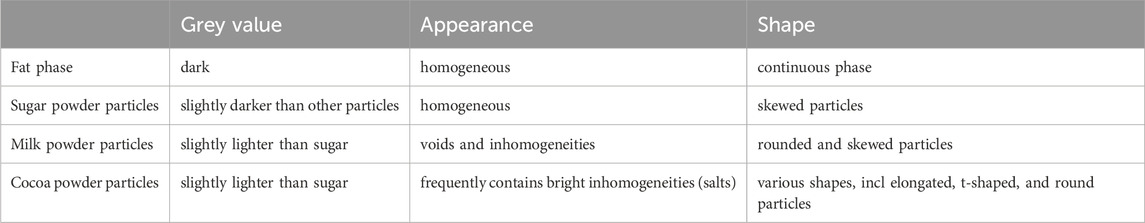
Cryo-planing of the melted and resolidified sample resulted in a flat section through the chocolate material (Figures 1–7). The uncoated specimen was analyzed using a backscattered electron detector, resulting in images with compositional contrast, based on material density (Figure 1). This results in detection of grey particles (milk powder, sugar, cocoa powder) embedded in a dark background of the continuous fat phase, because of the lower specific density of the latter. Low magnification images could be used to study the prevalence of large particles, air bubbles (not in this sample), as well as inhomogeneities of the particle suspension. At increasing magnification (Figures 3–6A), different types of particles become apparent, by differences in grey level, appearance of the internal structure, and the outer shape. Figure 4 shows the image observed at the highest magnification reached with the used method, since higher magnification resulted in blurred and distorted images due to beam damage. Particles of sub-micrometer sizes, and details of particles, such as salt particle inclusions (as identified with EDS, data not shown) can be discerned with this method (Figure 4). Figure 5 shows a cryo-planed milk chocolate before (A) and after (B) extensive imaging and EDS analysis, showing the nature and extent of beam damage.
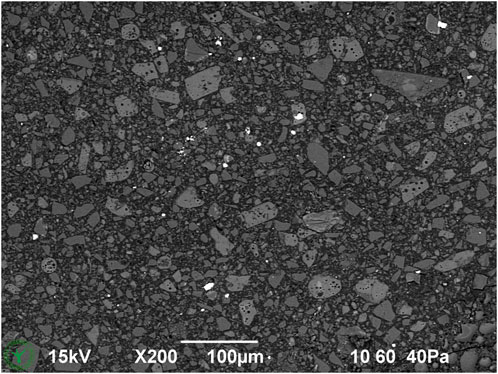
Figure 1. Overview cryo-SEM image of cryo-planed milk chocolate (melted and resolidified during sample preparation).
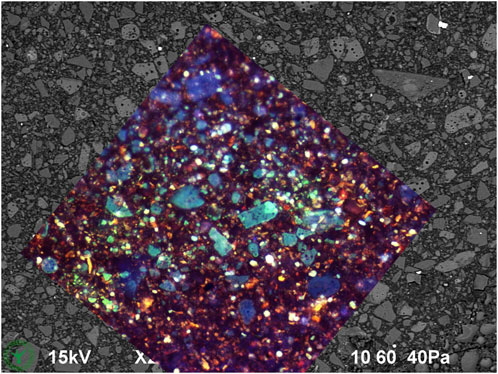
Figure 2. Same cryo-SEM image as in Figure 1, now with overlay of CLSM (10x dry objective) autofluorescence image made at same location after finishing the cryo-SEM investigation.
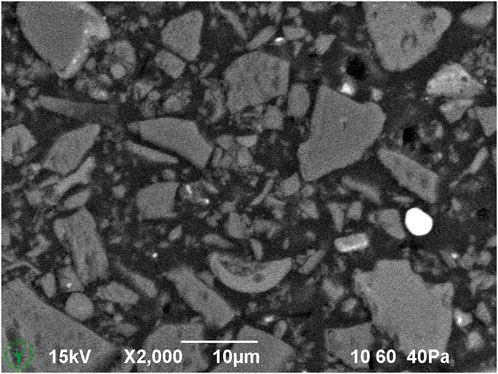
Figure 3. Cryo-SEM detail image of cryo-planed milk chocolate (melted and resolidified during sample preparation).
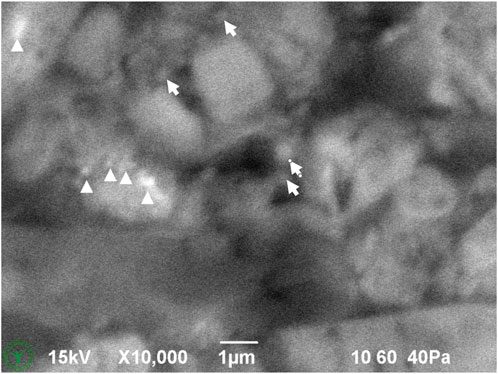
Figure 4. High magnification down to sub-micrometer resolution of upper left part of Figure 3. Some small particles (arrows) and salt inclusions (bright spots, arrow heads) are indicated in the image.

Figure 5. (A) Cryo-SEM image of cryo-planed milk chocolate that was melted and resolidified during sample preparation. (B) Same area imaged after EDS analysis. Based on EDS analysis, the marked particles are: S: Sugar powder; M: milk powder; C: cocoa powder.
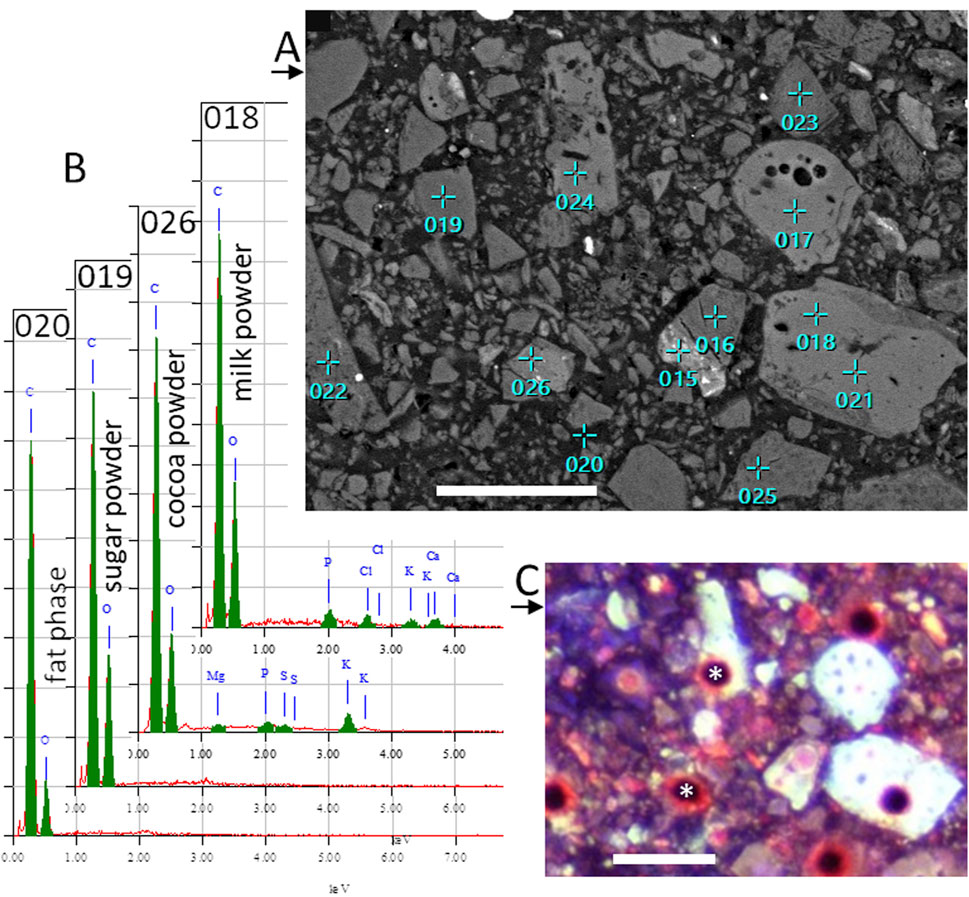
Figure 6. (A) SEM image of cryo-planed milk chocolate, same as in Figure 5, now with locations of EDS point analyses. (B) EDS spectra of some locations indicated in (A). (C) CLSM autofluorescence image, with 10x dry objective lens, of same area, showing holes (*) at some EDS analysis locations. Scale bar represent 30 µm.
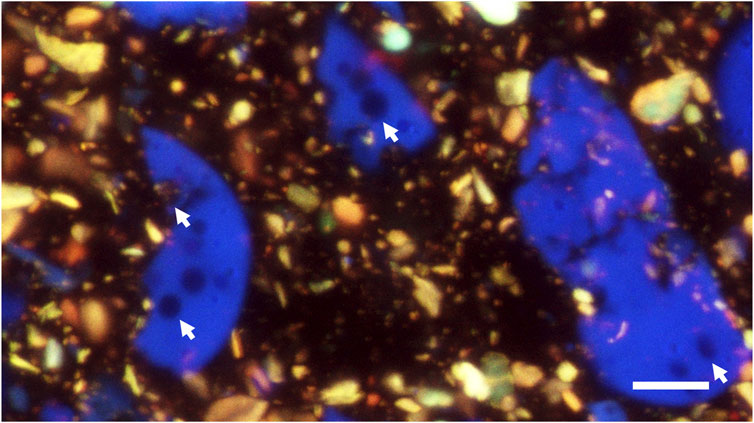
Figure 7. CLSM autofluorescence image of a cryo-planed cross section of chocolate (melted and resolidified during sample preparation). A 63x objective lens was used with immersion oil between lens and sample. This image is a max-projection of a focal series of 15 images with z-steps of 1 µm. Arrows indicate voids in particles. Scale bar: 10 µm.
Figure 6A shows the same cryo-planed chocolate cross section with locations of EDS point analyses. Examples of the typical EDS measurements are given in Figure 6B. The identity of the measured particles is indicated in Figure 5A. After SEM imaging and EDS analysis, the sample location was analyzed with CLSM as well (Figure 6C), where most points of EDS analysis are visible as holes. Correlation of SEM and CLSM imaging was made by comparison of Figure 1 with Figure 2.
The best CLSM resolution of cryo-planed samples was obtained using an oil immersion lens with the immersion oil applied directly between the sample and the lens (Figure 7). Based on size and shape, and especially the presence of voids within the particles, which were noticed already with SEM, it can be concluded that the blue particles in Figure 7 are milk powder. Sugar and fat are not visible because of lack of autofluorescence, and therefore it can be concluded that the yellowish particles are cocoa powder. It should be noted that false colours were used, and these depend on the used lasers, detected fluorescence wavelengths, and the choice of colours per detection channel.
Another sample of the same milk chocolate was kept solid during sample preparation and clamped in a SEM-holder, cryoplaned, imaged in SEM (Figure 8), and subsequently imaged with CLSM (Figure 9). This solid-kept sample shows dark areas (Figure 8), which indicate fat phase in absence of ingredient particles. Such dark areas were not found in the same chocolate material that was melted and resolidified during sample preparation (Figure 5A), where particles show a more homogeneous spatial distribution.
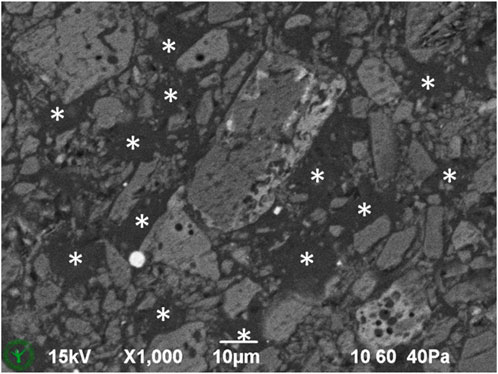
Figure 8. Detail of cryo-SEM image of cryo-planed milk chocolate, sampled from solid chocolate and kept solid. Asterisks indicate dark areas with fat phase only.
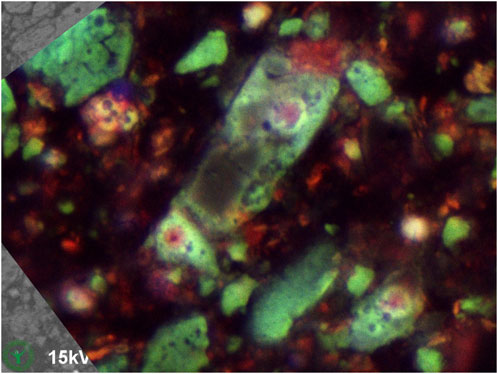
Figure 9. Same cryo-SEM image as in Figure 8, now with overlay of CLSM (10x dry objective) autofluorescence image made at same location after finishing the cryo-SEM investigation.
Discussion
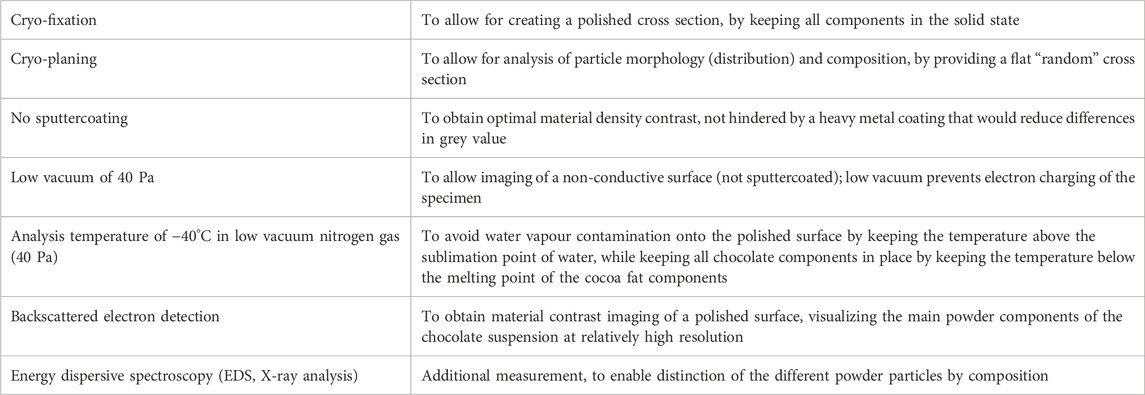
The imaging of chocolate microstructure has always been complicated due to many limitations and methodological hurdles. Due to displacement of liquid oil and displacement of the suspended particles while sectioning, it is impossible to obtain a good cross section of chocolate at ambient conditions. Cryofixation in combination with subsequent cryo-planing overcomes this difficulty, yielding high-resolution polished planes right through particles, even of sub-micrometer sizes. Cryo-planed surfaces of biological and food materials are usually imaged in cryo-SEM using sputtercoating and secondary electron imaging, but in that case the contrast is created by sublimation of water-containing voids. In chocolate, where no free water is present, regular imaging with cryo-SEM would just reveal a flat area with very low contrast. By applying an unusual combination of low temperature SEM and low vacuum SEM, all conditions were met for suitable imaging of a cryo-planed chocolate cut surface, while the low-contrast issue was overcome by applying backscattered electron detection. Table 1 summarizes the reasons for using each step/setting of the method.
The imaging detail of the here presented method is limited by blur due to the required large spotsize, as well as beam damage caused by the intense electron bundle necessary for low-vacuum imaging, to sub-micrometer level (Figure 4), by far not reaching the limits of the microscope (<10 nm). Future research should focus on further improving imaging resolution of chocolate, e.g., by adding a thin conductive layer on top of the cryo-planed surface, while still enabling material contrast, optimizing working distance, or using a more sensitive backscatter detector. However, current results already provide much finer imaging details than those reported in open literature, where to our knowledge only Fib-SEM work provides some insight in the in-situ chocolate structure to sub-micrometer details within the particles (Hitachi Electron Microscope, 2019), but of a restricted area and with curtaining artifacts.
The here described novel method allows for imaging and analysis (morphology, element composition) of the various particles in the original spatial context of the product. Although many conditions need to be met during sample preparation and imaging, in our hands the method allowed SEM imaging of several cryoplaned cross-sections in 1 day.
The three main particles present in milk chocolate can be distinguished according to features in SEM images (Table 2) and in the EDS spectra (Table 3). Whether the slight grey value differences between the particles (sugar powder, milk powder, cocoa powder) could be enhanced further and whether a combined analysis of the features from imaging and EDS analysis could enable automated recognition of individual ingredient particles requires further research. It should be noted that EDS analysis of such fine particle dispersions should be judged with care, since the interaction volume of the EDS analysis could be larger (in three dimensions) than the targeted particle and even qualitative analysis implies several artifacts (Newbury and Ritchie, 2013). Still EDS has proven to be a powerful method to distinguish different composition of particles. Furthermore, the size and shape and cross-sectional details of milk powder particles may vary between different sources of origin and processing, and might therefore have different discriminating features, but it can be foreseen that the notable presence of calcium and phosphorus will always discriminate milk powder from other ingredient particles.
Important is the possibility to analyze cross sections of chocolate in the original solid state, without a melting step. This allows the investigation of confectionery products without altering the spatial distribution of the particles. Apparently, the here studied milk chocolate product contains areas where powder particles (sugar, cocoa, milk) are not present. These areas, having sizes in the order of 10 µm, might contain fat crystal networks that have excluded the powder particles during fat crystal growth. We did not encounter these areas in the same material with a melting step during sample preparation. This indicates the value and the new possibility of studying chocolate microstructure directly from the original solid state.
In addition, we here prove that cryo-planed samples can be studied with CLSM as well (Figure 2). The CLSM imaging of cryo-planed samples delivers high-quality cross-sectional views, providing additional compositional information, still at quite high resolution. While the used SEM method at −40°C could be directly implemented for both liquid and soft chocolate products (such as confectionery spread, or chocolate in the melted state), this would not work with CLSM at ambient temperatures, due to the flow of liquid oil and the presence of suspended particles therein. Still, CLSM imaging of cryo-planed samples could be very useful in “solid chocolates” research, either with or without preceding correlative cryo-SEM analysis of the same sample. Areas with beam damage from SEM show altered autofluorescence as can be concluded from Figure 6C, where locations of prior EDS analysis are visible as spots and holes in CLSM images. The highest CLSM resolution of cryo-planed cross sections was obtained when viewing with immersion oil directly applied between the lens and the specimen. In future studies it may be possible to identify the visible particles (only milk powder and cocoa powder) by finding separate excitation and emission bandwidths for milk vs. cocoa powder.
Practical value of this research for food industry is linked to (i) understanding the interchangeability of ingredients in compound chocolate recipe, as all ingredients are natural materials with high degree of variability, (ii) understanding the influence of inclusions on product microstructure and (iii) understanding the effect of compound chocolate processing on size, shape and spatial distribution of recipe constituents. We believe that our novel combined cryo-SEM/CLSM method can be used for two-dimensional and, possibly, three-dimensional geometry reconstruction during modelling of confectionery mass processing. While this paper mainly deals with the development of microscopic analysis methodology, our future research will be devoted to the identification of correlation between (i) confectionery mass processing conditions, (ii) numerical particle size and aspect ratio values of individual confectionery mass ingredients and (iii) microstructural and sensory properties of confectionery product applications.
A protocol for the here described methods can be found in “Supplementary Material”.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JN: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing–original draft. AC: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Writing–review and editing, Writing–original draft. SM: Conceptualization, Writing–review and editing, Supervision, Resources, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research results from in-kind contributions of IFFCO and Consistence employees.
Conflict of interest
Authors AC and SM were employed by IFFCO Central R&D.
Author JN was employed by Consistence.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frfst.2024.1464882/full#supplementary-material
References
Afoakwa, E. O. (2016). Chocolate science and technology. John Wiley and Sons. doi:10.1002/9781118913758.refs
Afoakwa, E. O., Paterson, A., and Fowler, M. (2007). Factors influencing rheological and textural qualities in chocolate–a review. Trends Food Sci. Technol. 18 (6), 290–298. doi:10.1016/j.tifs.2007.02.002
Afoakwa, E. O., Paterson, A., Fowler, M., and Ryan, A. (2008). Flavor formation and character in cocoa and chocolate: a critical review. Crit. Rev. Food. Sci. Nutr. 48, 840–857. doi:10.1080/10408390701719272
Afoakwa, E. O., Paterson, A., Fowler, M., and Vieira, J. (2009). Microstructure and mechanical properties related to particle size distribution and composition in dark chocolate. Int. J. Food Sci. Technol. 44 (1), 111–119. doi:10.1111/j.1365-2621.2007.01677.x
Awad, T. S., and Marangoni, A. G., (2006). Ingredient interactions affecting texture and microstructure of confectionery chocolate. Marcel Dekker, 423–476. doi:10.1201/9781420028133.ch13
Beckett, S. T. (2019). The science of chocolate. 3rd edn. Croydon, United Kingdom: Royal Society of Chemistry. doi:10.1039/BK9781788012355-FP001
Bikos, D., Samaras, G., Cann, P., Masen, M., Hardalupas, Y., Vieira, J., et al. (2023). Destructive and non-destructive mechanical characterisation of chocolate with different levels of porosity under various modes of deformation. J. Mater. Sci. 58, 5104–5127. doi:10.1007/s10853-023-08324-7
Buckman, J., Aboussou, A., Esegbue, O., Wagner, T., and Gambacorta, G. (2020). Fine-scale heterogeneity of pyrite and organics within mudrocks: scanning electron microscopy and image analysis at the large scale. Minerals 10, 354. doi:10.3390/min10040354
Feichtinger, A., Scholten, E., and Sala, G. (2020). Effect of particle size distribution on rheological properties of chocolate. Food Funct. 11, 9547–9559. doi:10.1039/D0FO01655A
Frisullo, P., Licciardello, F., Muratore, G., and Del Nobile, M. A. (2010). Microstructural characterization of multiphase chocolate using X-Ray microtomography. J. Food Sci. 75 (7), E469–E476. doi:10.1111/j.1750-3841.2010.01745.x
Gaikwad, V. (2012). Oral processing of dark and milk chocolate. Palmerston North, New Zealand: The Riddet Institute, Massey University. Available at: http://hdl.handle.net/10179/4702.
Glicerina, V., Balestra, F., Dalla Rosa, M., and Romani, S. (2016). Microstructural and rheological characteristics of dark, milk and white chocolate: a comparative study. J. Food Eng. 169, 165–171. doi:10.1016/j.jfoodeng.2015.08.011
Haedelt, J., Beckett, S. T., and Niranjan, K. (2007). Bubble-included chocolate: relating structure with sensory response. J. Food Sci. 72 (3), 138–142. doi:10.1111/j.1750-3841.2007.00313.x
Haedelt, J., Pyle, L. D., Beckett, S. T., and Niranjan, K. (2005). Vacuum-induced bubble formation in liquid-tempered chocolate. Food Eng. Phys. Prop. 70 (2), 160–164. doi:10.1111/j.1365-2621.2005.tb07090.x
Hitachi Electron Microscope (2019). [Life] Cryo ion milling and low voltage SEM of chocolate. Available at: https://youtu.be/n6LgQr0GSpk?si=Rt-Bvnx4DWwUpp63 (Accessed September 25, 2024).
Lonchampt, P., and Hartel, R. W. (2004). Fat bloom in chocolate and compound coatings. Eur. J. Lipid Sci. Technol. 106 (4), 241–274. doi:10.1002/ejlt.200400938
Newbury, D. E., and Ritchie, N. W. (2013). Is scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS) quantitative? Scanning 35 (3), 141–168. doi:10.1002/sca.21041
Nijsse, J., and van Aelst, A. C. (1999). Cryo-planing for cryo-scanning electron microscopy. Scanning 21, 372–378. doi:10.1002/sca.4950210603
Reinke, S. K., Wilde, F., Kozhar, S., Beckmann, F., Vieira, J., Heinrich, S., et al. (2016). Synchrotron X-Ray microtomography reveals interior microstructure of multicomponent food materials such as chocolate. J. Food Eng. 174, 37–46. doi:10.1016/j.jfoodeng.2015.11.012
Rousseau, D. (1997). Modification of the physical and compositional properties of butter fat-canola oil blends by chemical and enzymatic interesterification. Doctoral dissertation (Guelph: University of Guelph).
Smith, K. W. (2012). “Confectionery fats,” in Cocoa butter and related compounds. Bedford, United Kingdom: AOCS Press, 475–495. doi:10.1016/B978-0-9830791-2-5.50022-0
Soltanahmadi, S., Bryant, M., and Sarkar, A. (2023). Insights into the multiscale lubrication mechanism of edible phase change materials. ACS Appl. Mater. Interfaces 15 (3), 3699–3712. doi:10.1021/acsami.2c13017
Stewart, I. M., and Timms, R. E. (2002). Fats for chocolate and sugar confectionery. Sheffield: Sheffield Academy Press, 159–191.
Talbot, G. (2009). “Fats for confectionery coatings and fillings,” in Science and technology of enrobed and filled chocolate, confectionery and bakery products (Cambridge, United Kingdom: Woodhead Publishing), 53–79. doi:10.1533/9781845696436.1.53
Keywords: (compound) chocolate, cocoa, confectionery, confocal laser scanning microscopy (CLSM), cryo-scanning electron microscopy (cryo-SEM), cryo-polishing, energy dispersive x-ray spectroscopy (EDS)
Citation: Nijsse J, Cruz Serna AF and Melnikov SM (2024) Microstructure of confectionery masses revealed by cryo-planing. Front. Food. Sci. Technol. 4:1464882. doi: 10.3389/frfst.2024.1464882
Received: 15 July 2024; Accepted: 16 October 2024;
Published: 28 October 2024.
Edited by:
Jose Bonilla, University of Southern Denmark, DenmarkReviewed by:
Miek Schlangen, University of Southern Denmark, DenmarkDimitrios Bikos, Imperial College London, United Kingdom
Copyright © 2024 Nijsse, Cruz Serna and Melnikov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaap Nijsse, amFhcEBjb25zaXN0ZW5jZS5ubA==
 Jaap Nijsse
Jaap Nijsse Adriana Fernanda Cruz Serna
Adriana Fernanda Cruz Serna Sergey M. Melnikov
Sergey M. Melnikov