- 1Institute of Biosciences and BioResources (IBBR), National Research Council (CNR), Naples, Italy
- 2Department of Industrial Engineering, University of Salerno, Fisciano, Italy
- 3Department of Theoretical and Applied Sciences, e-Campus University, Novedrate, Italy
An exploratory study was carried out to determine the occurrence of aflatoxins B1, B2, G1, and G2 in different aromatic preparations used in the production of bitters, liqueurs and flavored wines. Aflatoxin analysis was performed by liquid-liquid extraction followed by immunoaffinity column for purification of the extracts. The aflatoxins were quantitatively detected using high performance liquid chromatography technique with post-column derivatization and fluorescence detection. After in-house validation, the method was applied to the determination of aflatoxins in 40 samples of aromatic preparations used in the production of aperitifs and bitter drinks, vermouths and aromatized wines, and nut and citrus liqueurs. This method showed good accuracy between days (72%–95% recovery) and precision (3%–13% relative standard deviation). None of the samples analyzed contained detectable levels of aflatoxins. Only in one sample of aromatic extract of vermouth and aromatized wine aflatoxins B1 and G1 were found below the limit of quantification. From the results, it was concluded that these extracts for commercial purposes are safe for human consumption in terms of aflatoxin concentrations. In addition, the general outcome of the study showed that an accurate analysis of AFs can be obtained in a short time with a high sensitivity, even on difficult matrices such as hydro-alcoholic mixtures of different aromatic preparations.
1 Introduction
Alcoholic beverages have always played a central role in human societies, being an essential part of daily life and economy. The first documented evidence of such beverages can be traced back to the Sumerians around 3200 BC (Damerow et al., 2012). Despite this fact, excessive consumption can lead to negative concerns for human health (O'Keefe et al., 2018; Axley et al., 2019).
The spirit drinks sector, which is regulated at the EU level, includes 44 main categories and approximately 250 spirit drinks registered as typical geographical indication (IGP) products (European Commission’s Directorate-General for Agriculture and Rural Development, 2022).
Current European legislation, which sets out criteria for the production and labelling of spirit drinks, defines flavoured spirit drinks as alcoholic beverages of an alcoholic strength by volume of at least 15%, produced by flavoring ethyl alcohol, distillate, or both with flavoring substances and/or flavoring preparations. These beverages include bitters (bitter tasting spirits) and liqueurs. Compared to bitters, liqueurs may also be fortified with sweetening substances with a minimum content, expressed as invert sugar, of at least 70 g per liter (Regulation EU No. 2019).
Deeply rooted in culture and traditions, the Italian production of spirits and distilled beverages, generated a profit of approximately 1.6 billion euros in 2021 (Ozbun, 2022). Italy is one of the largest producers of bitter and herbal liqueurs in the world, with many flavors, traditionally consumed as aperitifs or digestives, commonly known as “amari” or, literally, “bitters”. The latter represents about 20% of total production of spirits, a very important economic reality for Italy (Patini, 2021).
Given the wide variety of herbs and raw materials that can be combined to produce these alcoholic beverages, a wide variety of liqueurs are available on the market today, some of which can reach combinations of 50 or even 100 different herbs or spices (Buglass et al., 2010).
Typically, aromatic formulations used in the production of flavor extracts and alcoholic beverages are quite complex and derived from historical recipes or trade secrets. Spices and herbs have been widely utilized to improve aroma and flavor (Peter and Shylaja, 2012; Śliwińska et al., 2015). According to ethnobotanical research, the most common plant species used in Italy for aromatization of spirits and production of liqueurs and aromatized wines belong to the Rosaceae, Asteraceae and Lamiaceae families (Motti et al., 2022).
Bitter and aromatic plants are typically used dried because dehydrated samples can be stored for long periods of time, without significantly changing the concentration of aromatic compounds. In fact, one of the most crucial factors to consider when evaluating herbs and spices for botanical preparations is residual moisture. It can affect the quality of the final product, the amount of extract or essential oil, and most importantly, it can promote fungal growth, which can lead to the production of mycotoxins (Salgueiro et al., 2010; Tonutti and Liddle, 2010). These toxic molecules are secondary fungal metabolites that can accumulate at all stages of the supply chain. Depending on environmental factors (temperature, humidity, rainfall) and farm management practices (planting, harvesting, and storage conditions), fungal growth and mycotoxin excretion can occur at any stage of the agricultural product life cycle (Wan Ainiza et al., 2015; Kabak and Dobson, 2017; Potortì et al., 2020). The most widespread and dangerous mycotoxins are aflatoxins (AFs) released by certain fungi almost all of which belong to genus Aspergillus, Fusarium, and Penicillium (Frisvad et al., 2019).
Chemically, AF molecules are classified as difuranocoumarins and have a coumarin core to which a difuran moiety is connected on one side and either a pentene ring or a six-membered lactone ring on the other side. Accordingly, aflatoxins can be divided into two main categories: difurocoumarocyclopentenones, which include the aflatoxin B series and its derivatives, and difurocoumarolactones, which consist primarily of the aflatoxin G series, which typically includes AFG1, AFG2, and related compounds (Benkerroum, 2020). The native fluorescence properties of such compounds are due to the highly conjugated and rigid aflatoxin moieties. Furthermore, the above fluorescence properties are drastically affected by the small structural variations that distinguish the aflatoxins. G2 and B2 derivatives are much more fluorescent than their unsaturated homologs B1 and G1.
The ubiquity of aflatoxin-producing fungi and the strong biological activity of their mycotoxins at very low concentrations has stimulated phenomenal research in many areas. AFs have been identified as teratogenic, mutagenic and recognised as carcinogenic by the International Agency for Research on Cancer (B and G series AFs) and are therefore of major concern to human health (IARC - International Agency for Research on Cancer, 2012).
Liquid chromatography coupled with fluorescent and/or MS detection is a common and official method to detect AF in food and beverages (Shephard, 2009; Mahfuz et al., 2020; Miklós et al., 2020; Zhang and Banerjee, 2020; Caldeirão et al., 2021). A number of immunological techniques, such as ELISA and other rapid antibody-based assays, are typically used for screening purposes (Beyene et al., 2019). However, confirmatory analyses using more robust methods are often required for these methods (Shephard, 2009; Mahfuz et al., 2020).
The occurrence of AFs and other mycotoxins has already been studied in some alcoholic beverages such as wine, beer and cider (Pizzuti et al., 2014; Carballo et al., 2021), as well as in spices and herbal products (Boonzaaijer et al., 2008; Ashiq et al., 2014; Dubey et al., 2014; Kabak and Dobson, 2017; Zhang et al., 2018; Potortì et al., 2020; Caldeirão et al., 2021; Oztekin and Karbancioglu-Guler, 2022; Palma et al., 2022).
Aflatoxin contamination of flavoring preparations may occur during the maceration process, as aflatoxins are readily soluble in polar organic solvents such as ethanol (O'Neil, 2001) and can be transferred from contaminated raw materials into alcoholic solutions.
The aim of this study is to analyse for the presence of AFB1 and total AFs in selected flavouring preparations used in the production of alcoholic beverages, as literature data on AFs levels in flavouring preparations for alcoholic beverages are scarce (Boonzaaijer et al., 2008). Since the quantification of mycotoxins falls under trace analysis, the analytical determination of AFs requires sample cleaning and preconcentration procedures to remove matrix compounds and improve sensitivity. Extraction of AFs from aromatic preparations is not straightforward due to the presence of phenolic compounds and aromatic volatiles (Johnson et al., 2015; Rodríguez-Solana et al., 2016; Montero et al., 2020), which can be co-extracted with AFs and interfere with analytical determination. Montero et al. (2020) found phenolic compounds in eight different commercial herbal liqueurs using LC-MS. The main phenolic groups were phenolic acids and flavonoids. Coumarins, lignans and, to a lesser extent, chalcones, terpenes, stilbenes and chromones were also detected.
In this work, AFs analysis was performed using liquid-liquid extraction and then immunoaffinity column clean-up followed by quantification by HPLC analysis, with post-column derivatization and fluorescence detection, making some modifications to the official procedure of the Association of Official Analytical Chemists International (AOAC 991.31), to improve the method’s efficiency in these matrices. Specifically, this paper reports the optimal conditions of the sample clean-up step to ensure the adsorption of AFs on immunoaffinity columns to remove matrix components and improve sensitivity prior to chromatographic analysis.
Subsequently, the variations were validated for linearity, sensitivity, accuracy, recovery and repeatability. The method was successfully applied to evaluate the natural presence of AFs in flavoring preparations. The use of the validated simple, repeatable, and highly sensitive method for the simultaneous determination of AFs B1, B2, G1, G2 in aromatic preparations used to produce liqueurs and bitters is of great importance to continue the toxicological assessments to better understand the mycotoxin exposure and thus determine a more detailed “risk assessment”. This will serve as a reference to help the authorities review the regulations.
2 Materials and methods
2.1 Chemicals and reagents
A mixed aflatoxin stock standard solution (CRM46304) with a purity > of 99%, was purchased from Sigma Aldrich (St Louis, MO, United States). The mixed aflatoxin stock standard solution was stored at −20°C until use. It was stored at room temperature in the dark for 30 min before use.
Deionized water was obtained using a Milli-Q (Merck Millipore, MA United States) laboratory water purification system. All analytical grade solvents and reagents, as well as HPLC grade methanol and acetonitrile, were purchased from Sigma Chemical Co. (St. Louis, MO, United States).
2.2 Flavoring preparation samples
Forty samples of aromatic extracts were used in this study. All samples were kindly provided by some Italian companies that produce semifinished aromatic preparations from raw materials of European and non-European origin, consisting of hydroalcoholic infusions obtained by maceration of herbs and spices or parts of plants. The complete list of the aromatic plants used as ingredients, as well as the maceration conditions used to produce the hydroalcoholic aromatic preparations analyzed, were not disclosed because they are covered by trade secrets.
The samples were grouped into four categories of flavorings according to the type of alcoholic beverage for which they were used. For each category, the main ingredients were known but not the exact proportions. The four types of aromatic preparations consisted of.
- Aperitif and Bitters liqueurs aromatic preparation (10 samples), obtained by maceration of various herbs, spices, roots, seeds in variable proportions including bitter and sweet oranges (Citrus aurantium L.; Citrus sinensis) peels, wormwood (Artemisia pontica, Artemisia absinthium) flowers and leaves, cloves (Syzygium aromaticum), cardamom (Elettaria cardamomum) seeds, gentian (Gentiana lutea) roots, juniper (Juniperus Communis) berries, mint (Menta Piperita) leaves, rhubarb (Rheum palmatum L.) roots, sage (Salvia officinalis) roots, and other herbs and spices covered by industrial secret
- Vermouth and aromatized wine aromatic preparation (10 samples), obtained by maceration of the leaves of two wormwood variety (A. pontica L. and A. absinthium L.) flowers and other leaves and other botanicals in variable proportions including elderberry flowers (Sambucus nigra L.), nutmeg (Myristica fragrans Houtt.), Ceylon cinnamon queen bark (Cinnamomum zeylanicum) as well as other spices and herbs not indicated because they are covered by industrial secrecy
- Nut-flavored liqueurs (10 samples) consisting of natural hydroalcoholic extracts of pistachio (Pistacia vera), and hazelnut (Corylus avellana L.) nuts, or coffee (Coffea arabica and Coffea canephora), cocoa (Theobroma cacao) seeds.
- Citrus flavored liqueurs (10 samples) consisting of natural hydroalcoholic extracts of citrus peels including lemon (Citrus limon), orange (Citrus sinensis), mandarin (Citrus reticulata), and bergamot (Citrus bergamia).
All samples were stored in sealed glass vials below 4°C and in the dark for further analysis.
2.3 Preparation of samples and extraction of AFs
The extraction and purification of AFs from aromatic extracts was performed by a modification of the official AOAC method 991.31 according to with Weaver and Trucksess (2010), who reported the results of the method validation study for the quantification of AFs in botanical roots by immunoaffinity column clean-up and subsequent chromatographic analysis by HPLC with fluorescence detection. Vicam AflaTest™ immunoaffinity columns (Milford, MA United States) were used for cleanup total and individual AFs.
Briefly, 5 g of the sample were accurately weighed, 1 g of sodium chloride was added, and the mixture was made up to a volume of 25 mL with a water-methanol mixture (3:7 v/v). The mixture was sonicated for 30 min and then filtered through Whatman No. One paper. Then, 15 mL of the mixture was taken and added to 30 mL of water in a 50 mL centrifuge tube. The mixture was stirred on a vortex mixer for 5 min and then filtered through a glass wool filter. Fifteen milliliters of the filtrate (equivalent to 1 g of test sample) was immediately used for the immunoaffinity column chromatography.
The diluted extract was cleaned by passage through an AflaTest™ immunoaffinity column (Vicam, Milford, United States) at a flow rate of approximately one-drop per second. Before eluting the aflatoxins with 1 mL of analytical grade methanol in a clean vial, the column was washed with 10 mL of water and dried by pushing 3 mL of air through a syringe. The HPLC analysis was performed immediately.
2.4 HPLC-FL analysis
The analysis was performed by reverse-phase high performance liquid chromatography (HPLC) employing a Waters 2,690 instrument equipped with a 474-fluorescence detector and a post-column reaction module (Milford, MA, United States). The reactor volume used for post-column derivatization was 1 mL. The post-column iodine derivatization agent was prepared daily by dissolving 25 mg of Iodine in 10 mL of methanol. The mixture was mixed, 90 mL of water was added, and then it was filtered through a 0.45 μm filter.
Compounds were separated on a 250 mm × 4.6 mm i.d. LiChrospher® RP-18 HPLC Column (Merck Darmstadt, Germany). Isocratic mobile phase with a combination of acetonitrile–methanol–water (20:20:60, v/v/v) at a flow rate of 1.0 mL/min was utilized. The post-column reagent was used at a flow rate of 0.5 mL/min. The excitation and emission wavelengths of the fluorescence detector were 360 and 450 nm, respectively. The injection volume was 50 mL. The column and post-column reactor temperatures were set 20°C and 70°C, respectively. The aflatoxins were identified based on the retention time and quantified by comparison of the sample peak area with the calibration curve.
2.5 Validation of the analytical method
The method was validated in accordance with the consolidated EU Regulation No. 401/2006 on the use of analytical methods for the official control of mycotoxin levels in foodstuffs. The validation parameters investigated were linearity, limits of detection and quantification, recovery, and precision of the method.
Linearity was analyzed preparing six-point calibration standards by dilution of the mixed aflatoxins stock standard solution with methanol. Working standard solutions cover the ranges of 0.50–4 ng/mL for AFB1 and AFG1, and 0.125–1 ng/mL for AFB2 and AFG2.
Each calibration standard was analyzed in triplicate and calibration curves were prepared separately for each aflatoxin by plotting the mean peak area against its concentration and determining the slope and intercept by the least-squares method. Linearity was evaluated by considering a correlation coefficient greater than 0.990 and a slope of the linear calibration curve that was statistically different from zero at the 95% confidence level.
The limits of detection (LOD) and limits of quantitation (LOQ) were calculated using the standard deviation of the response and the slope of the calibration curve, as specified in the International Conference on Harmonization (ICH) guideline for the validation of analytical techniques (ICH, 2005) using an ethanol solution (70% v/v) as a blank sample. The LOD and LOQ were stated as 3 and 10 times the ratio of the standard deviation of the response to the blank (six replicates) and the slope of the calibration curve, respectively.
The recovery and precision of the method was evaluated using an aromatic alcohol extract sample that was found to be free of aflatoxins. Fortified samples were prepared by pipetting different volumes of aflatoxin stock solution onto 5 g of sample to obtain the appropriate low, medium, and high spike levels for each aflatoxin, corresponding to 0.5, 1, and 1.5 times the maximum level of aflatoxin B1 (2 μg/kg) according to the European Pharmacopoeia in herbal products (European Pharmacopoeia, 2016). The concentrations of aflatoxin in the fortified samples were: 1, 2 and 3 μg/kg for AFB1 and AFG1, and 0.25, 0.5, and 0.75 μg/kg for AFB2 and AFG2.
The fortified samples were allowed to stand for at least 30 min before aflatoxin extraction to simulate natural contamination. The entire analytical procedure was carried out in triplicate for the evaluation of the precision, while the recovery was calculated as the ratio between the obtained concentration and the nominal concentration in %. The precision was evaluated based on the relative standard deviation RDS (%) calculated from the results obtained under repeatability conditions.
2.6 Statistical analysis
The results were expressed as mean ± standard deviation (M ± SD). Calibration curves and linear regression analyses (R2) were determined using SigmaPlot 13.0 (Systat Software Inc., California, United States). Statistical data analysis was performed using the Student’s t-test with p < 0.05 considered significant.
3 Results and discussion
Analytical methods based on immunoaffinity column cleaning and subsequent quantification by liquid chromatography are a well-established and validated tool for the determination of mycotoxins in numerous food matrices (Mahfuz et al., 2020; Miklós et al., 2020; Zhang and Banerjee, 2020). This procedure has been successfully applied to the analysis of AFs in herbs, spices, nuts, and other foods (Campos et al., 2017; el Darra et al., 2019; Mahfuz et al., 2020; Omar et al., 2020; Oztekin and Karbancioglu-Guler, 2022; Palma et al., 2022).
Optimum conditions were established for extraction of aflatoxin from alcoholic samples, experiments were conducted in which the initial and/or final composition of solvent systems (mixtures of methanol/water) were selected as described in materials and methods section. When these systems were used, the recovery of aflatoxin B1 and G1 from samples was satisfactory. These results are shown in Table 1 and also suggest that solvent system used in extraction and clean-up procedures reduced the time required for aflatoxin analyses, which were completed in approximately 30 min.

TABLE 1. Validation parameters for the HPLC-FL method of aflatoxin B1; B2, G1, and G2 determination in natural flavoring preparation and extracts for spirits drinks production. R2 = linear correlation coefficient; SD = Standard deviation; LOD = Limit of detection, LOQ = Limit of quantification.
Due to the complexity of aromatic extracts used in the production of liqueurs and bitter beverages (mainly composed of phenolic and volatile compounds in addition to alcohol) (Johnson et al., 2015; Rodríguez-Solana et al., 2016; Montero et al., 2020), the extraction of aflatoxins from aromatic preparations is not straightforward because of the highly colored contaminants that can be co-extracted with AFs. Therefore, to improve its performance for the quantification of aflatoxins in these matrices, the AOAC-IUPAC analytical method No. 991.31 has been adapted and validated. This method included an initial extraction step with MeOH-H2O (70:30) and subsequent recovery of AFs with immunoaffinity columns. The chromatographic procedure with fluorescence detection and post-column derivatization (HPLC-FL) was carried out and validated and then applied to study the AFs content in flavorings.
3.1 Method validation
The chromatographic resolution obtained for each aflatoxin was satisfactory under the experimental conditions of the method, as shown in Figure 1. All aflatoxins were well separated in less than 20 min and eluted sequentially in the following order: aflatoxin G2, G1, B2 and finally B1.
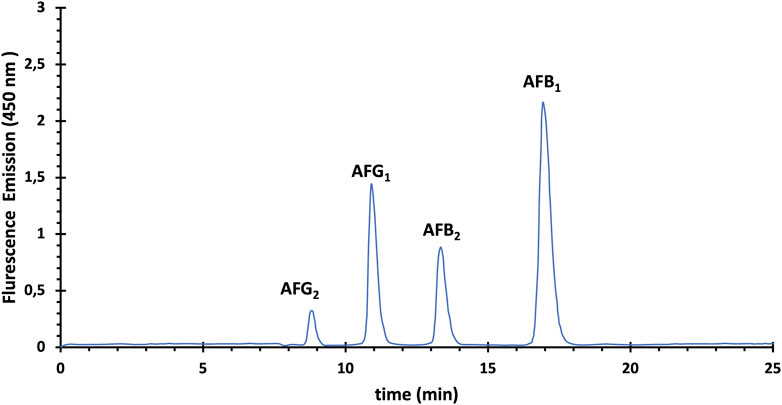
FIGURE 1. HPL Chromatogram of aflatoxins (AFs) standard solution containing 2 ng/mL for AFB1 and AFG1, and 0.5 ng/mL for AFB2 and AFG2.
The first step was to determine if there were any compounds that could be inferred from the aflatoxin peaks by examining an aflatoxin-free sample as a blank. Indeed, it was verified that no peaks or matrix effects affected the AF peaks. The immunoaffinity columns used in this study, due to their high specificity, provided a quick and easy solution to remove contaminants that may co-extract with AFs, thus providing analytical samples free of interfering peaks that could be inferred from the AF peaks.
The method was also validated for linearity, precision, accuracy, and specificity (Table 1). Good linearity of response was observed for all analytes in the concentration range tested, 0.50–4 ng/mL for AFB1 and AFG1, and 0.125–1 ng/mL for AFB2 and AFG2, with correlations greater than 0.99 (Table 1). The limits of detection (LOD) and limits of quantitation (LOQ) of the method are presented in Table 1, along with the recoveries and repeatability values. The LOD and LOQ for AFB1 and AFG1 were 0.10 and 0.30 μg/kg, and 0.04 and 0.12 μg/kg for AFB2 and AFG2, respectively.
Recovery experiments were used to evaluate the accuracy and precision of the method ((Table 1). The mean recovery values of all fortified samples ranged from 72.1% to 94.5%, and the relative standard deviation (RSD %), calculated from the results obtained under repeatability conditions, varied from 2.8% to 13.2%, indicating a good accuracy of the method in accordance with the official standards, which set an RSD <15% as the upper limit of acceptability (EC Commission Regulation No. 401/2006).
To assess whether the different matrices adversely affected the recovery of AFs during the extraction process, recovery tests were also performed on each sample by adding known amounts of the standard and evaluating the average extraction yields for each type of matrix. The results of the recovery tests (Figure 2A–D) show that the recoveries for all categories of aromatic extracts were within the range of 70% and 110% as required by European Commission Regulation (EC) No. 401/2006.
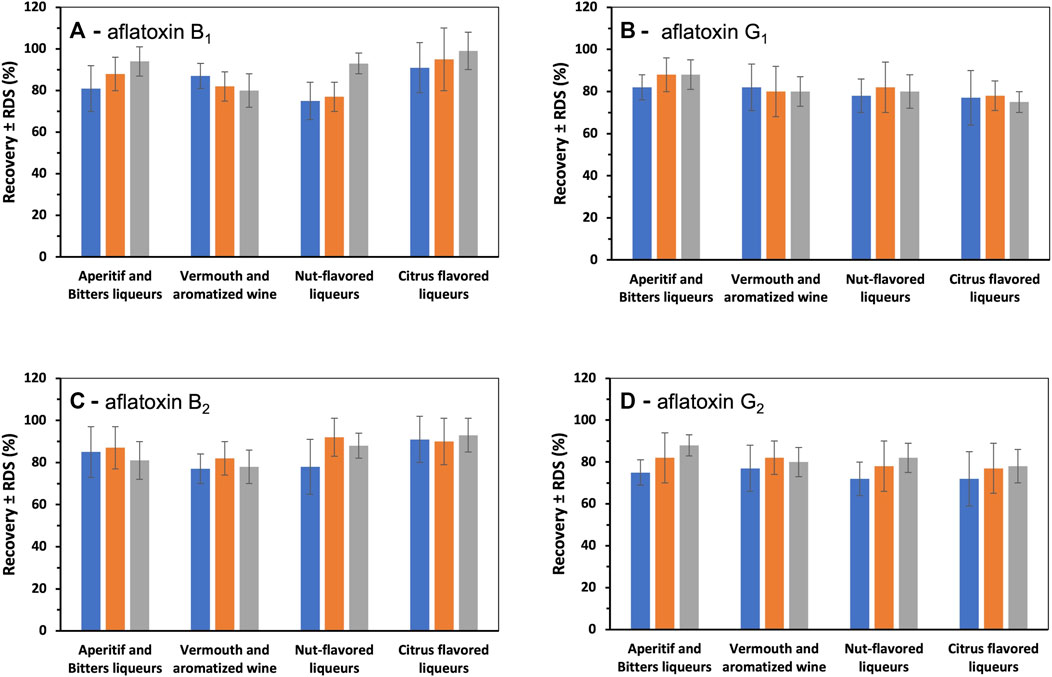
FIGURE 2. Average recovery of AFB1 (A), AFG1 (B), AFB2 (C) and AFG2 (D) at three different spiking level corresponding to 0.5 (blue histogram); 1 (orange histogram), and 1.5 (gray histogram) times the AFB1 maximum level (2 μg/kg) according to the European Pharmacopeia in herbal products (European Pharmacopoeia, 2016). Relative standard deviation (RDS; %) is represented as error bar.
For all classes of flavoring preparations examined and for all three levels of spiking of AFs, the average recoveries of AFs were greater than 75% (Figure 2D).
The relative standard deviation (RSD) values obtained for all AFs in the different aromatic extracts were also less than15%, regardless of the level of spiking, indicating that the accuracy of the adopted and validated procedure for these matrices was in compliance with European Commission Regulation 401/2006 (Figure 2D).
3.2 Occurrence of aflatoxins in aromatic preparations
Several plant species can be contaminated with AFs due to inappropriate pre- and post-processing conditions, and contamination can affect different parts according to a recent review by Qin et al. (2020). Botanicals (various herbs, spices, rhizomes, roots, fruits and seeds) used in extracts for flavoring aperitifs, bitters, liqueurs, vermouths and aromatized wines are extremely diverse. In addition, climatic conditions and the time and duration of storage have a strong influence on the quality of the raw material used in flavoring (Morata et al., 2019). Hence, due to the high solubility in hydroalcoholic solutions of AFs, contaminated raw materials could carry over into flavorings during the infusion. Therefore, the content of AFs in different types of semi-finished products, obtained by hydroalcoholic infusion of spices, herbs and other aromatic plant parts, was screened using the validated method defined in Section 3.1.
The results of the exploratory survey on the presence of AFs in several aromatic preparations used in the production of bitters, liqueurs and aromatized wines indicate that the contamination levels found are not a cause for concern. No detectable amounts of AFs were found in any of the samples analyzed. Levels below the limit of quantification for AFB1 and AFG1 were found only in one sample of flavoring preparation for vermouth and aromatized wine. The lack of quantification of the individual ingredients used in such flavorings complicates the investigation of analyte contamination in this type of product.
Herbal liqueurs are made from the alcoholic maceration of medicinal and aromatic plants and are rich in aromatic volatiles that provide the aroma and flavor of alcoholic beverages. Rodríguez -Solana et al. (2016) identified up to 32 volatile compounds responsible for the characteristic flavours and aromas of these products in samples of commercial herbal liqueurs from different companies belonging to the geographical denomination “Spirits and traditional liqueurs from Galicia”. They included terpenes, alcohols, carbonyl compounds, volatile phenols and lactones, and some of them, such as menthol, thymol and linalool among the terpenes, and eugenol and trans-anethole were found in relatively high concentrations (Śliwińska et al., 2015). Volatile compounds such as aldehydes, acetate esters and alcohols had an inhibitory effect on fungal growth and hence AF formation. Liang et al. (2015) observed inhibitory effects of cinnamaldehyde, citral and eugenol on AF biosynthesis.
Surprisingly, the flavoring preparations for nut-based liqueurs were also found to be aflatoxin free, probably due to strict control of AFs levels in raw materials. Dried fruits such as pistachios, hazelnuts and almonds, which have a high incidence of AFs contamination, are the most frequently rejected foods by border controls in Europe due to non-compliance with the maximum allowable limits for these contaminants (Gallo et al., 2021).
Several studies have suggested that certain plant extracts can effectively degrade AFs by removing the double bond in the terminal furan ring and modifying the AF lactone ring (Vijayanandraj et al., 2014; Iram et al., 2015; Ponzilacqua et al., 2019). In addition to a decrease in toxicity, researchers have also observed a loss of fluorescence of the molecule, as the lactone ring is crucial for the fluorescence of the AFs molecule (Lee et al., ;1981). Although the processes by which plant extracts modify mycotoxin molecules are unknown, there is evidence that the longer the incubation time with plant extracts, the more modifications to the molecular structure of AFs occur, reducing their toxicity (Vijayanandraj et al., 2014). Herbal liqueurs are extracts rich in phenolic compounds, with huge chemical complexity due to the large number of different herbs and spices used in their preparation (Montero et al., 2020).
Regarding the aromatic preparations used in the production of citrus-based liqueurs, the results are consistent with those reported by Boonzaaijer et al. (2008). Citrus peels are a rich source of flavanones and many polymethoxylated flavones. In vitro studies have suggested that some flavonoids isolated from orange and grapefruit fruits reduce the growth of Penicillium digitatum, supporting the idea that all of these compounds play an active role in protecting fruits from pathogen attack (Ortuño et al., 2006).
Although the levels of AFs contamination found by several authors in herbs and spices produced in humid and tropical climates may exceed the maximum levels for AFB1 and total AFs established by European regulations and/or other international standards (Salgueiro et al., 2010; Kabak and Dobson, 2017; Potort et al., 2020), they contribute to minimizing AFs contamination in flavor preparations (Kabak and Dobson, 2017). Furthermore, given the integrated application of Good Agricultural Practices (GAP) with Good Manufacturing Practices (GMP), and given the current monitoring programs for the raw materials used, the contamination of aflatoxins that can be found in various aromatic preparations will not be a cause for concern or a hazard (Gallo et al., 2021). However, the lack of many data does not allow us to conclude at this stage on the need for strict measures to protect public health. At least for the regulation of mycotoxins, further representative studies on different types of spices and herbal extracts will be necessary.
The use of a validated analytical method on these matrices enables accurate results of AFs analysis in a short time and with high sensitivity could be used in the quality control of raw materials within the scope of the activities reported in the company’s GMP.
4 Conclusion
This study describes a simple, reliable, and highly robust method for the simultaneous quantification of AFB1, AFB2, AFG1, and AFG2 in aromatic formulations using liquid-liquid extraction and immunoaffinity column clean-up followed by the quantification by HPLC analysis, with post-column derivatization and fluorescence detection. HPLC with fluorescence detection has several advantages over other analytical methods and has become the most widely accepted method for the determination of aflatoxins. However, in this case it was necessary to modify the official method (AOAC 991.31) validated according to the consolidated EU Regulation No 401/2006, on the use of analytical methods for the official control of the levels of mycotoxins in food. This is due to the nature of the semi-manufactured products used, which are obtained by hydroalcoholic infusion of spices, herbs and other aromatic plant parts and are rich in phenolic and volatile aromatic compounds that may interfere with the determination of AFs.
Levels of AFs were not detectable in any of the samples analyzed. Levels of AFB1 and AFG1 below the limit of quantification were observed in only one sample of a flavoring preparation for vermouth and aromatized wines.
To control the presence of AFs in such ingredients, a combination of good agricultural practices, proper processing procedures, regular monitoring and analysis and strict quality control measures are important.
Data availability statement
The datasets presented in this study can be found in online repositories. The data sets can be found at https://figshare.com/articles/dataset/rawdata_ID1213980_xlsx/23566266.
Author contributions
BL and DC performed the investigation, developed the methodology, wrote the original draft of the paper. SC, BL and DC performed critical review and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ashiq, S., Hussain, M., and Ahmad, B. (2014). Natural occurrence of mycotoxins in medicinal plants: A review. Fungal Genet. Biol. 66, 1–10. doi:10.1016/j.fgb.2014.02.005
Axley, P. D., Richardson, C. T., and Singal, A. K. (2019). Epidemiology of alcohol consumption and societal burden of alcoholism and alcoholic liver disease. Clin. Liver Dis. 23 (1), 39–50. doi:10.1016/j.cld.2018.09.011
Benkerroum, N. (2020). Aflatoxins: Producing-Molds, structure, health issues and incidence in southeast asian and sub-saharan african countries. Int. J. Environ. Res. Public Health. 17 (4), 1215. doi:10.3390/ijerph17041215
Beyene, A. M., Du, X., E Schrunk, D., Ensley, S., and Rumbeiha, W. K. (2019). High-performance liquid chromatography and enzyme-linked immunosorbent assay techniques for detection and quantification of aflatoxin B1 in feed samples: A comparative study. BMC Res. notes 12 (1), 492–496. doi:10.1186/s13104-019-4538-z
Boonzaaijer, G., van Osenbruggen, W., Kleinnijenhuis, A., and van Dongen, W. (2008). An exploratory investigation of several mycotoxins and their natural occurrence in flavour ingredients and spices, using a multi-mycotoxin LC-MS/MS method. World Mycotoxin J. 1 (2), 167–174. doi:10.3920/wmj2008.x016
Buglass, A. J., McKay, M., and Gook Lee, C. (2010). Liqueurs and their flavorings. Handb. Alcohol. Beverages Tech. Anal. Nutr. Aspects 1, 615–627. doi:10.1002/9780470976524.ch25
Caldeirão, L., Sousa, J., Nunes, L. C., Godoy, H. T., Fernandes, J. O., and Cunha, S. C. (2021). Herbs and herbal infusions: Determination of natural contaminants (mycotoxins and trace elements) and evaluation of their exposure. Food Res. Int. 144, 110322. doi:10.1016/j.foodres.2021.110322
Campos, W. E. O., Rosas, L. B., Neto, A. P., Mello, R. A., and Vasconcelos, A. A. (2017). Extended validation of a senstive and robust method for simultaneous quantification of aflatoxins B1, B2, G1 and G2 in Brazil nuts by HPLC-FLD. J. Food Compos. Anal. 60, 90–96. doi:10.1016/j.jfca.2017.03.014
Carballo, D., Fernández-Franzón, M., Ferrer, E., Pallarés, N., and Berrada, H. (2021). Dietary exposure to mycotoxins through alcoholic and non-alcoholic beverages in valencia, Spain. Toxins 13 (7), 438. doi:10.3390/toxins13070438
Damerow, P. (2012). Sumerian beer: The origins of brewing Technology in ancient mesopotamia.Cuneif. Digital Libr. J. Available at: https://cdli.mpiwg-berlin.mpg.de/articles/cdlj/2012-2 el Darra, N.
Dubey, N., Mishra, P., Kedia, A., and Prakash, B. (2014). “Fungal and mycotoxin contamination of herbal raw materials and prospects of higher plant products as plant-based preservatives during post-harvest processing,” in Microbial diversity and biotechnology in food security. Editors R. Kharwar, R. Upadhyay, N. Dubey, and R. Raghuwanshi (New Delhi: Springer). doi:10.1007/978-81-322-1801-2_45
European Pharmacopoeia (2016). in Determination of aflatoxin B1 in herbal drugs. Editor Ph. Eur 9th Edition (Strasbourg, France: Council of Europe), 289. 2.8.18, 1.
European Commission (2006). Commission Regulation (EC) No. 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 70, 12.
European Commission's Directorate-general for agriculture and rural development - DG AGRI. Bruxelles/brussel. Available at: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/spirit-drinks_en (accessed December, 2022).
Frisvad, J. C., Hubka, V., Ezekiel, C. N., Hong, S. B., Nováková, A., Chen, A. J., et al. (2019). Taxonomy ofAspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 93, 1–63. doi:10.1016/j.simyco.2018.06.001
Gallo, P., Imbimbo, S., Alvino, S., Castellano, V., Arace, O., et al. (2021). Contamination by aflatoxins B/G in food and commodities imported in southern Italy from 2017 to 2020: A risk-based evaluation. Toxins 13 (6), 368. doi:10.3390/toxins13060368
Gambacorta, L., and Solfrizzo, M. (2019). Multimycotoxins occurrence in spices and herbs commercialized in Lebanon. Food control 95, 63–70. doi:10.1016/j.foodcont.2018.07.033
IARC - International Agency for Research on Cancer (2012). Aflatoxins. Monographs on the evaluation of carcinogenic risks to humans. Chemical agents and related occupations: A review of human carcinogens. Proc. Int. Agency Res. Cancer 100F, 225–244.
ICH Expert Working Group (2006). ICH guideline Q2(R1) validation of analytical procedures: Text and methodology. BMJ Clin. Res. Ed) 333, 873. doi:10.1136/bmj.333.7574.873-a
Iram, W., Anjum, T., Iqbal, M., Ghaffar, A., and Abbas, M. (2015). Mass spectrometric identification and toxicity assessment of degraded products of aflatoxin B1 and B2 by Corymbia citriodora aqueous extracts. Sci. Rep. 5, 14672. doi:10.1038/srep14672
Johnson, A. J., Heymann, H., and Ebeler, S. E. (2015). Volatile and sensory profiling of cocktail bitters. Food Chem. 179, 343–354. doi:10.1016/j.foodchem.2015.01.114
Kabak, B., and Dobson, A. D. W. (2017). Mycotoxins in spices and herbs–An update. Crit. Rev. Food Sci. Nutr. 57 (1), 18–34. doi:10.1080/10408398.2013.772891
Lee, L. S., Dunn, J. J., DeLucca, A. J., and Ciegler, A. (1981). Role of lactone ring of aflatoxin B1 in toxicity and mutagenicity. Experientia 37 (1), 16–17. doi:10.1007/BF01965543
Liang, D., Xing, F., Selvaraj, J. N., Liu, X., Wang, L., Hua, H., et al. (2015). Inhibitory effect of cinnamaldehyde, citral, and eugenol on aflatoxin biosynthetic gene expression and aflatoxin B1 biosynthesis in Aspergillus flavus. J. Food Sci. 80 (12), M2917–M2924. doi:10.1111/1750-3841.13144
Mahfuz, M., Gazi, M. A., Hossain, M., Islam, M. R., Fahim, S. M., and Ahmed, T. (2020). General and advanced methods for the detection and measurement of aflatoxins and aflatoxin metabolites: A review. Toxin Rev. 39, 123–137. doi:10.1080/15569543.2018.1514638
Miklós, G., Angeli, C., Ambrus, Á., Nagy, A., Kardos, V., Zentai, A., et al. (2020). Detection of aflatoxins in different matrices and food-chain positions. Front. Microbiol. 11, 1916. doi:10.3389/fmicb.2020.01916
Montero, L., Schmitz, O. J., and Meckelmann, S. W. (2020). Chemical characterization of eight herbal liqueurs by means of liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 1631, 461560. doi:10.1016/j.chroma.2020.461560
Morata, A., Vaquero, C., Palomero, F., Loira, I., Bañuelos, M. A., and Suárez-Lepe, J. A. (2019). Technology of vermouth wines. InAlcoholic Beverages Vol. 7, 35–63. doi:10.1016/B978-0-12-815269-0.00002-7
Motti, R., Bonanomi, G., and de Falco, B. (2022). Wild and cultivated plants used in traditional alcoholic beverages in Italy: An ethnobotanical review. Eur. Food Res. Technol. 248, 1089–1106. doi:10.1007/s00217-021-03948-y
O'Keefe, E. L., DiNicolantonio, J. J., O'Keefe, J. H., and Lavie, C. J. (2018). Alcohol and CV health: Jekyll and hyde J-curves. Prog. Cardiovasc Dis. 61 (1), 68–75. doi:10.1016/j.pcad.2018.02.001
O'Neil, M. J. (2001). The merck index: An encyclopedia of chemicals drugs and biologicals. 13th ed. USA: Merck.
Omar, S. S., Haddad, M. A., and Parisi, S. (2020). Validation of HPLC and Enzyme-Linked Immunosorbent Assay (ELISA) techniques for detection and quantification of aflatoxins in different food samples. Foods 9 (5), 661. doi:10.3390/foods9050661
Ortuño, A., Báidez, A., Gómez, P., Arcas, M., Porras, I., García-Lidón, A., et al. (2006). Citrus paradisi and Citrus sinensis flavonoids: Their influence in the defence mechanism against Penicillium digitatum. Food Chem. 98 (2), 351–358. doi:10.1016/j.foodchem.2005.06.017
Ozbun, T. (2022). Spirits market in Italy - statistics and facts. Available at: https://www.statista.com/topics/9544/spirits-market-in-italy/#topicOverview (Accessed January 21, 2023).
Oztekin, S., and Karbancioglu-Guler, F. (2022). Simultaneous detection of ochratoxin A and aflatoxins in industrial and traditional red and isot pepper flakes along with dietary exposure risk assessment. ACS omega 7 (36), 31756–31766. doi:10.1021/acsomega.2c02236
Palma, P., Godoy, M., Vidal, M., Rivera, A., and Calderón, R. (2022). Adaptation, optimization, and validation of a sensitive and robust method for the quantification of total aflatoxins (B1, B2, G1, and G2) in the spice merkén by HPLC-FLD with post-column derivatization. Microchem. J. 178, 107342. doi:10.1016/j.microc.2022.107342
Patini, D. (2021). I consumi di bevande alcoliche in Italia e il ruolo degli spirits nell’era post-Covid. Available at: https://www.nomisma.it/dati-mercato-spirits-osservatorio-nomisma (Accessed January 21, 2023).
Peter, K. V., and Shylaja, M. R. (2012). “1 - introduction to herbs and spices: Definitions, trade and applications BT - handbook of herbs and spices,” in Woodhead publishing series in food science, Technology and nutrition. Second edition (New York: Woodhead Publishing), 1–24.
Pizzutti, I. R., de Kok, A., Scholten, J., Righi, L. W., Cardoso, C. D., Rohers, G. N., et al. (2014). Development, optimization and validation of a multimethod for the determination of 36 mycotoxins in wines by liquid chromatography-tandem mass spectrometry. Talanta 129, 352–363. doi:10.1016/j.talanta.2014.05.017
Ponzilacqua, B., Rottinghaus, G. E., Landers, B. R., and Oliveira, C. A. F. (2019). Effects of medicinal herb and Brazilian traditional plant extracts on in vitro mycotoxin decontamination. Food control 100, 24–27. doi:10.1016/j.foodcont.2019.01.009
Potortì, A. G., Tropea, A., Lo Turco, V., Pellizzeri, V., Belfita, A., Dugo, G., et al. (2020). Mycotoxins in spices and culinary herbs from Italy and Tunisia. Nat. Prod. Res. 34 (1), 167–171. doi:10.1080/14786419.2019.1598995
Qin, L., Jiang, J. Y., Zhang, L., Dou, X. W., Ouyang, Z., Wan, L., et al. (2020). Occurrence and analysis of mycotoxins in domestic Chinese herbal medicines. Mycology 11 (2), 126–146. doi:10.1080/21501203.2020.1727578
Regulation (EU) No (2019). The European Parliament and of the Council of 17 April 2019 on the definition, description, presentation and labelling of spirit drinks, the use of the names of spirit drinks in the presentation and labelling of other foodstuffs, the protection of geographical indications for spirit drinks, the use of ethyl alcohol and distillates of agricultural origin in alcoholic beverages, and repealing Regulation (EC) No 110/2008. Official Journal of the European Union L 130/1, 62.
Rodríguez-Solana, R., Salgado, J. M., Domínguez, J. M., and Cortés-Diéguez, S. (2016). Phenolic compounds and aroma-impact odorants in herb liqueurs elaborated by maceration of aromatic and medicinal plants in grape marc distillates. J. Inst. Brew. 122 (4), 653–660. doi:10.1002/jib.377
Salgueiro, L., Martins, A. P., and Correia, H. (2010). Raw materials: The importance of quality and safety. A review. Flavour Fragr. J. 25, 253–271. doi:10.1002/ffj.1973
Shephard, G. S. (2009). Aflatoxin analysis at the beginning of the twenty-first century. Anal. Bioanal. Chem. 395, 1215–1224. doi:10.1007/s00216-009-2857-y
Śliwińska, M., Wiśniewska, P., Dymerski, T., Wardencki, W., and Namieśnik, J. (2015). The flavour of fruit spirits and fruit liqueurs: A review. Flavour Fragr. J. 30, 197–207. doi:10.1002/ffj.3237
Tonutti, I., and Liddle, P. A. (2010). Aromatic plants in alcoholic beverages. A review. Flavour Fragr. J. 25, 341–350. doi:10.1002/ffj.2001
Vijayanandraj, S., Brinda, R., Kannan, K., Adhithya, R., Vinothini, S., Senthil, K., et al. (2014). Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica Nees. Microbiol. Res. 169 (4), 294–300. doi:10.1016/j.micres.2013.07.008
Wan Ainiza, W. M., Jinap, S., and Sanny, M. (2015). Simultaneous determination of aflatoxins and ochratoxin A in single and mixed spices. Food control 50, 913–918. doi:10.1016/j.foodcont.2014.10.051
Weaver, C. M., and Trucksess, M. W. (2010). Determination of aflatoxins in botanical roots by a modification of AOAC official MethodSM 991.31: Single-laboratory validation. J. AOAC Int. 93 (1), 184–189. doi:10.1093/jaoac/93.1.184
Zhang, K., and Banerjee, K. (2020). A review: Sample preparation and chromatographic technologies for detection of aflatoxins in foods. Toxins 12, 539. doi:10.3390/toxins12090539
Keywords: aflatoxins, flavoring preparations, high-performance liquid chromatography, method validation, aromatic plants, alcoholic beverage
Citation: Laratta B, Carpentieri S and Cautela D (2023) Investigation of aflatoxins occurrence in flavoring preparations for the alcoholic beverage industry. Front. Food. Sci. Technol. 3:1213980. doi: 10.3389/frfst.2023.1213980
Received: 28 April 2023; Accepted: 26 June 2023;
Published: 05 July 2023.
Edited by:
Luana Izzo, University of Naples Federico II, ItalyReviewed by:
Luigi Castaldo, University of Naples Federico II, ItalyAnca Ioana Nicolau, Dunarea de Jos University, Romania
Copyright © 2023 Laratta, Carpentieri and Cautela. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico Cautela, ZG9tZW5pY28uY2F1dGVsYUB1bmllY2FtcHVzLml0
†These authors have contributed equally to this work
 Bruna Laratta
Bruna Laratta Serena Carpentieri
Serena Carpentieri Domenico Cautela
Domenico Cautela