- 1Department of Food Science and Technology, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 2Department of Food, Halal and Agricultural Products, Food Technology and Agricultural Products Research Center, Standard Research Institute (SRI), Karaj, Iran
Sheep and cow butter oils have high consumption and trade rate in the market, but standards regarding type differentiation are lacking. Therefore, the objective of this study was to assess the distinction of the main and molecular components of sheep and cow butter oils. Sanjabi sheep and Holstein cow butters were produced from milk collected in two seasons of summer and autumn via the conventional local method. The main components including fatty acids, triacylglycerols, sterol composition, and PCR test for the evaluation of molecular components were determined in butter oil samples. The most significant differences in fatty acids were for C10:0 and C16:0, and regarding TAGs were for C40, C42, C48, and C50 in sheep butter oils of the two seasons. The sterol composition in cow and sheep butter oils was similar but had differences in the two seasons. PCR tests also showed that cow and sheep butter oils differ in their molecular components. It can be concluded that it is possible to differentiate these two oils using triacylglycerols, fatty acids, and molecular evaluation.
Introduction
The Codex standard 280: 2018 defined butter oil or ghee as a product entirely manufactured from milk, cream, or butter through different processes, which nearly caused a total removal of the nonfat solids and water, with the particularly developed physical structure and flavor. Butter oil, produced by a traditional local method, has high rate in Iran, especially in the Kermanshah province. Kermanshah is where 1,000 tons of butter oil is traditionally produced each year, and also, this product is manufactured in other regions such as Africa, the Middle East, and Asia (Salarabadi et al., 2015).
From the chemical composition viewpoint, butter oil is considered a complex combination of glycerides, phospholipids, sterols, and vitamins (Ali et al., 2020). Triacylglycerols (TAGs), the main portion of lipids, make up about 95–98 percentage of the edible oils. The identification and determination of TAGs are the best way to achieve the composition and purity of edible oils (Piravi vanak, 2018). According to many research studies and international standards (ISO, 2008), the evaluation of milk fat purity should have been carried out by TAG analysis. Fatty acid composition is the main part of the triacylglycerol structure. Based on the nature and level of fatty acids present, the source of edible oils and fats can be recognized. Therefore, this item is considered as the main parameter in international standards such as the Codex standard 210: 2019 (Piravi vanak, 2018). In addition to TAG and fatty acids as the main components of butter oil, there are minor components comprising 0.5–1% of butter oil. Sterols, especially cholesterol, have the higher content in the minor component portion (Ali et al., 2020).
There have been several reports on using the chromatographic techniques for the determination of fraud in dairy products (Naviglio et al., 2017; Raftani Amiri and Salmani, 2017). Previous studies have also described the polymerase chain reaction (PCR) technique for animal species detection in dairy products (Khanzadi et al., 2013; Nooratiny et al., 2013; Hazra et al., 2017).
Compared to cow milk, sheep milk contains varied fatty acids (branch fatty acids and CLA) and has a greater nutritional, industrial, and therapeutical importance (Khudhair Jabir Alrikabi, 2015). Sheep butter oil has high demand by the consumers and is costly due to its favorable flavor and also acceptability. Therefore, sheep butter oil can be adulterated by cow butter oil, and there has to be methods to differentiate these products and also detect adulteration.
There are different methods to produce butter and butter oil. One local and traditional method is producing yoghurt and then shaking and blending the yoghurt in a leather container to separate the fat phase from the water phase as butter. Then, the produced butter can be used for butter oil production mainly via gentle heating and removing water. There is little information regarding the composition and quality of butter oil produced by this traditional method in spite of its popularity and high importance in the market. The objective of this study was to determine the main composition and molecular components of sheep and cow butter oil produced by the traditional local method to draw the distinction. Regarding the high consumption of the two types of butter oils and higher price of sheep butter oil than cow’s butter oil in Iran and lack of national regulations, the results of this research can help set national standards and also detect fraud and adulteration.
Materials and methods
Sample preparation
Milk was collected from a local sheep breed (Sanjabi) and cow breed (Holstein) from a small farm in Kermanshah in two seasons (summer and autumn) (ISO 707: 2008). The starter culture was inoculated into the milk, and after 7 h at 45°C, yogurt was prepared; then the samples were stored overnight at 4°C. Yogurt was diluted with water and treated via shaking in a leather container, which is called mashk locally. Then, butter was separated from the diluted yogurt. The water from the obtained butter was removed by heating to obtain butter oil, as described previously (Najafi et al., 2011).
Fatty acid analysis
Fatty acid methyl esters were prepared according to ISO (ISO 15884: 2002). Then, 100 mg of the sample was dissolved in 5 ml of normal heptane and methylated with 0.2 ml of 2 M sodium methoxide. A measure of 1 µl of fatty acid methyl ester (FAME) was injected into a (YL Instrument, 6500, Korea) GC system equipped with a capillary column (60 m × 0.25 mm) and a flame ionization detector (FID) using hydrogen as the carrier gas with a flow rate of 2 ml/min. The injector and detector temperatures were adjusted to 250°C and 260°C, respectively. The relative retention time of the obtained results was compared to that of the standards, and the fatty acids were identified (ISO 15885: 2002).
Triacylglycerol analysis
Butter oil was analyzed by gas chromatography to determine triacylglycerols, separated by the total carbon numbers (ISO 17678: 2010). A GC (Shimadzu, Nexis-2030, Japan) system equipped with a capillary column (30 m × 0.53 mm) and a flame ionization detector (FID) using hydrogen as the carrier gas with a flow rate of 4 ml/min was used. The injection volume was 0.5 µl, and the injector and detector temperatures were adjusted to 370°C.
Sterol analysis
The sterol content of ghee was determined according to EC (EC 273: 2008). The sterols were transformed to trimethyl-silyl ethers and were analyzed by gas chromatography with reference to an internal standard/botulin. A YL6500 GC (YL Instrument, 6500, Korea) system equipped with a capillary column (30 m × 0.25 mm) and a flame ionization detector (FID) using hydrogen as the carrier gas with a flow rate of 1.5 ml/min was used. The injection volume was 1 μl, and the injector and detector temperatures were adjusted to 300°C and 310°C, respectively.
Molecular analysis
The molecular method consists of a lysis step, followed by removing contaminants and nucleases from the DNA-containing aqueous phase using phenol and chloroform, respectively, final DNA precipitation with ethanol concentrating the DNA, and eliminating salts and residual chloroform (ISO 21571: 2005). The PCR technique was performed according to ASTM, 1973 (ASTM E1873: 2006).
Statistical analysis
To examine the identification of primary and molecular components of sheep and cow butter oils and their possibility of differentiation, a completely randomized design was incorporated and four independent samples were considered. All values were expressed as the mean ± standard deviation (SD). The results were analyzed in SPSS software 22.0 for Windows (SPSS Inc., Chicago, IL, United States). The means were compared using Duncan‚s multiple range test at the significance level of 95%.
Results
Differentiation in fatty acid composition of butter oils
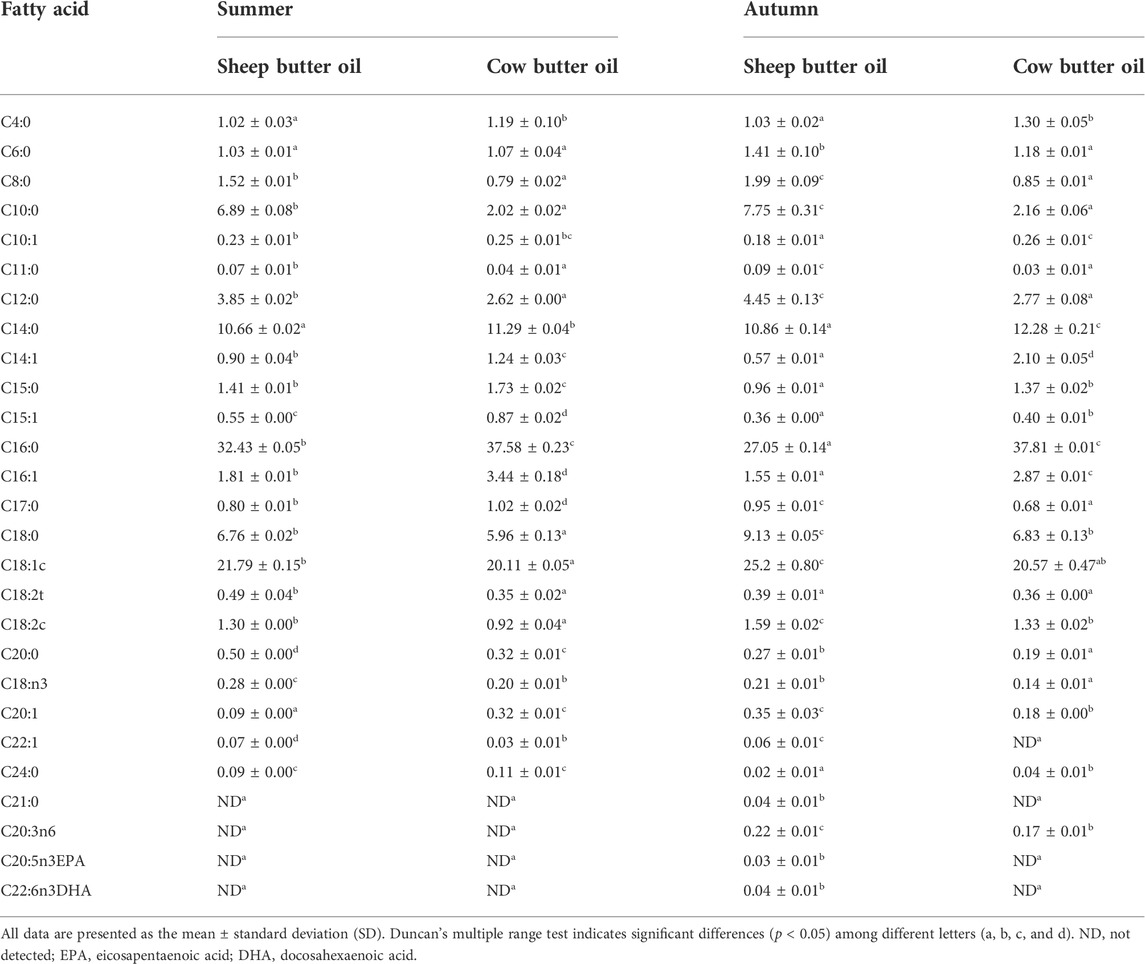
A significant difference (p < 0.05) between the fatty acids of cow and sheep butter oils was found (Table 1). The content of C10:0 in sheep butter oils in the two seasons (6.89–7.75%) was much more than that of the cow type (2.02–2.16%). Palmitic acid, as the predominant saturated fatty acid for the two seasons, in cow butter oils (37.58–37.81%) was much higher than sheep butter oils (27.05–32.43%). There were also many differences (p < 0.05) between fatty acids of cow and sheep butter oils in terms of animal type and seasons such as C4:0, C8:0, C10:0, C12:0, C16:0, C16:1, C17:0, C18:0, and C18:1 (Table 1). In addition to these differences, on short- and medium-chain saturated fatty acid (C4-C14) levels in two butter oils, their sum (25.04–27.58%) for sheep in comparison to cow (19.02–20.57%) in the two seasons can also be considered as a differentiating indicator.
Differentiation in triacylglycerol composition of butter oils
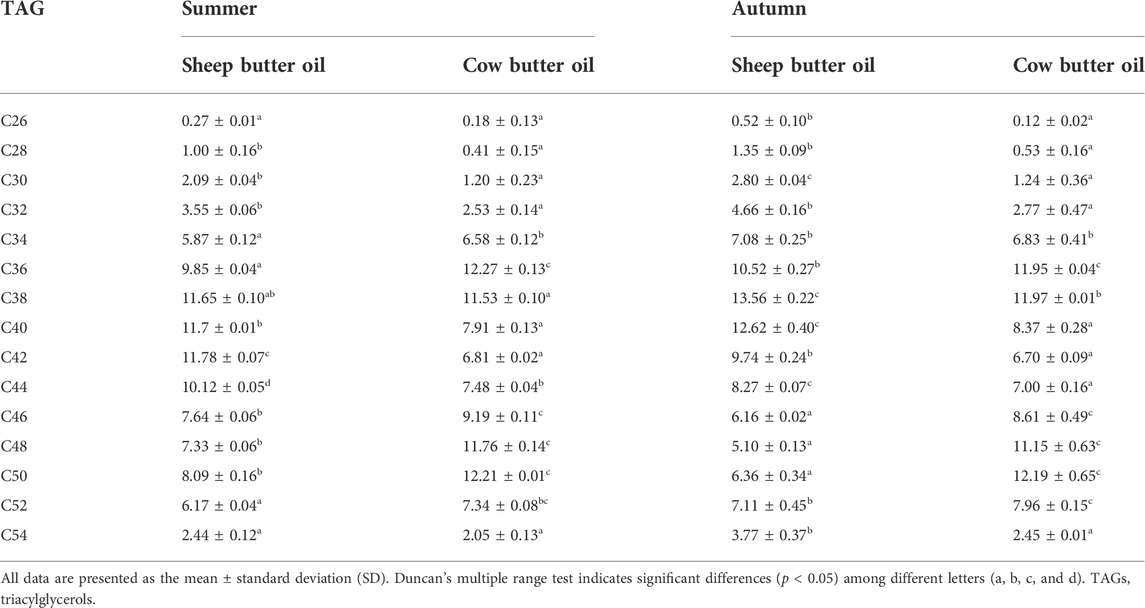
The results of comparing cow and sheep butter oil triacylglycerols in summer and autumn showed that there is a significant difference (p < 0.05). Sheep butter oil triacylglycerols in the two seasons including C40 (11.70–12.62%), C42 (9.74–11.78%), C48 (5.10–7.33%), and C50 (6.36–8.09%) had differences with the corresponding range of triacylglycerols in cow butter oil [C40 (7.91–8.37%), C42 (6.70–6.81%), C48 (11.15–11.76%), and C50 (12.19–12.21%)] (Table 2; Figures 1A–E).
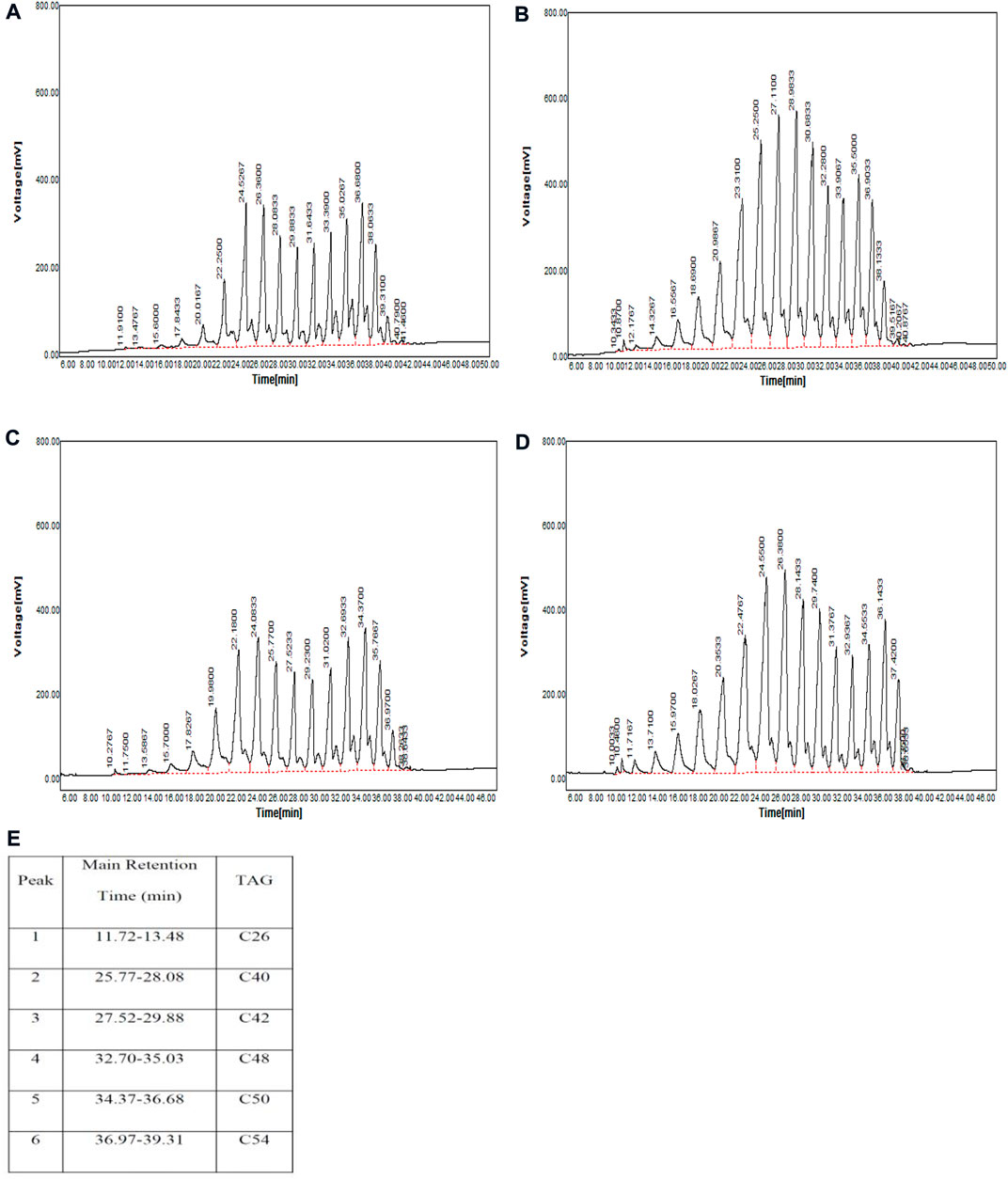
FIGURE 1. TAG chromatograms (three experiments). (A) Summer sheep butter oil, (B) summer cow butter oil, (C) autumn sheep butter oil, (D) autumn cow butter oil, and (E) guide.
Differentiation in the sterol content of butter oils
The results showed that while there was a significant difference between summer and autumn sterol contents (1.30–1.78%), there was no difference regarding the animal types.
Differentiation in molecular components of butter oils
Figure 2A,B showed that the molecular components of cow and sheep butter oils are significantly different. The B samples loaded at positions 3 and 4 genetically matched with the cow sample at position 5, and it was determined to be bovine butter oil in Figure 2A. The A samples loaded at positions 8 and 9 had a genetic match with the sheep sample at position 13, and it was determined to be sheep butter oil in Figure 2B.

FIGURE 2. PCR of different butter oils with a specific primer on the agarose gel (three experiments). (A) Cow butter oil and (B) sheep butter oil.
In this study, the PCR test results along with fatty acid and triacylglycerol analyses could be applicable methods for the differentiation of sheep and cow butter oils.
Discussion
The current findings showed that fatty acids of cow and sheep butter oils in the two seasons were different. Markiewicz-Keszycka et al. (2013) and Balthazar et al. (2017) reported that sheep milk contains more short and medium fatty acids than cow milk, such as caproic, caprylic, capric, and lauric acids, which create a unique aroma in the milk of these small ruminants. Our finding in terms of short and medium fatty acids of sheep butter oil, particularly capric acid, was consistent with these results. Djordjevic et al. (2019) concluded in their research that sheep milk has less palmitic acid content than cow milk and the profiles of milk fatty acids of different animal species are different. This result also corresponds to our result with respect to palmitic acid as the predominant saturated fatty acid for the two seasons in cow butter oils that was much higher than sheep butter oils. Revila et al. (2017) reported that seasonal changes strongly affect fatty acids due to changes in the composition of forage that animals feed. In various studies, it was found that sheep milk is richer in conjugated linoleic acid (CLA) than cow milk, perhaps due to the semi-extensive nature of the system in which small ruminants are usually raised. Our findings also showed that there are many differences between fatty acids of cow and sheep butter oils in terms of the kind of animal and season.
Cow and sheep butter oils in triacylglycerols determined in the two seasons were different. Fontecha et al. (2005) revealed that the most significant difference between triacylglycerol compounds between sheep and cow milk is triacylglycerols that contain C40, C42, and C44. Also, the levels of triacylglycerols of C50 and C52 in sheep milk fat are lower than those in cow milk fat. The most significant difference in our results in triacylglycerols was related to these four triacylglycerols. Liu et al. (2017) reported that the profile of milk TAGs is not constant during the lactation season and varies with the change of seasons. Although the seasonal variation of most TAG groups is evident, the causative factors for such a variation remain unclear. Our findings were consistent with this issue in terms of the kind of animal and season.
The sterol of cow and sheep butter oils in the two seasons determined that the animal types did not affect their sterol content, but this component is different in summer and autumn.
Moatsou and Sakkas (2019), in their research, showed that the cholesterol content of sheep milk is similar to cow milk, although there is a significant difference between their fat content. According to the research studies, the concentration of cholesterol in sheep and cow milk fats is affected by lactation and season (Strzałkowska et al., 2010; Mayer and Flechter, 2012). These findings also correspond to our result in terms of the cholesterol content.
The results showed that the molecular components of cow and sheep butter oils are different. Khanzadi et al (2013) reported that PCR research is a suitable method to prevent fraud and differentiation of milk in dairy products of animals such as cow and sheep by targeting the DNA sequences with adequate species diversity. Tafavizi and Helalat (2014) found that healthy mammary glands of ruminant milk contain many cells, including somatic cells, leukocytes, and epithelial cells containing DNA. Milk somatic cells remain intact during cheese production and are suitable for DNA extraction and tracing of animal species from its products. In this study, they detected the adulteration of cow milk in sheep cheese by PCR, which was proven and can be generalized to other dairy products.
Conclusion
Regarding cow and sheep butter oils produced by the traditional local method, the predominant fatty acids for discrimination were C10:0 and C16:0, and capric acid in sheep milk fat was more than three times as much as in cow milk fat, and C16:0 was much more in cow milk fat. C40, C42, C48, and C50, as important TAGs, were different between the two kinds of butter oils so that C40 and C42 were much more in sheep butter oils in the two seasons than cow butter oils, and cow butter oils in the two seasons had C48 and C50 higher than sheep kinds. The sterol composition in cow and sheep butter oils is similar but was different in the two seasons. PCR tests showed that cow and sheep butter oils differ in their molecular components. Therefore, it can be concluded that it is possible to differentiate these two oils using triacylglycerols and fatty acids, as well as molecular evaluation, and to detect adulteration. The obtained data can also be used to set a national standard for cow and sheep butter oils and can be a dataset for international standards.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors have contributed to the research and writing of the manuscript, and it has been approved by all of them. Conceptualization: ZP. Methodology: ZP, AD, and MG. Investigation: AD. Writing—original draft: ZP and AD.
Acknowledgments
The authors are grateful for the collaboration with Haj Ghasemi as a statistician.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, A. H., Wei, W., and Wang, X. (2020). Characterisation of bovine and buffalo anhydrous milk fat fractions along with infant formulas fat: Application of differential scanning calorimetry, Fourier transform infrared spectroscopy, and colour attributes. LWT-Food Sci. Technol. 129, 109542. doi:10.1016/j.lwt.2020.109542
Balthazar, C. F., Pimentel, T. C., Ferrão, L. L., Almada, C. N., Santillo, A., Albenzio, M., et al. (2017). Sheep milk: Physicochemical characteristics and relevance for functional Food development. Compr. Rev. Food Sci. Food Saf. 16, 247–262. doi:10.1111/1541-4337.12250
Polymerase Chain Reaction Technique Chromatographic analysis of triglycerides (Reference method). Geneva: International Organization for Standardization.
Djordjevic, J., Ledina, T., Baltic, M. Z., Trbovic, D., Babic, M., and Bulajic, S. (2019). Fatty acid profile of milk. Conf. Ser. Earth Environ. Sci., 333. doi:10.1088/1755-1315/333/1/012057
EC (2008). Luxembourg: Europen Commission. Commission Regulation-Annex VIII (Article 5), Determining Sitosterol or Stigmasterol in Butter or Concentrated Butter by Capillary-Column Gas Chromatography.
Fontecha, J., Goudjil, H., Rios, J. J., Fraga, M. J., and Juarez, M. (2005). Identity of the major triacylglycerols in ovine milk fat. Int. Dairy J. 15, 1217–1224. doi:10.1016/j.idairyj.2004.11.013
Hazra, T., Sharma, V., Sharma, R., and Arora, S. (2017). A species specific simplex polymerase chain reaction-based approach for detection of goat tallow in heat clarified milk fat (ghee). Int. J. Food Prop. 20, S69–S75. doi:10.1080/10942912.2017.1289542
ISO (2005). Foodstuffs — methods of analysis for the detection of genetically modified organisms and derived products — nucleic acid extraction. Geneva: International Organization for Standardization, 21571.
ISO (2008). Milk and milk products – guidance on sampling. Geneva: International Organization for Standardization.
ISO (2002a). Milk fat — determination of the fatty acid composition by gas-liquid chromatography. Geneva: International Organization for Standardization, 15885.
ISO (2002b). Milk fat — preparation of fatty acid methyl esters. Geneva: International Organization for Standardization, 15884.
Khanzadi, S., Jamshidi, A., Razmyar, J., and Mohsenzadeh, M. (2013). PCR-based detection of cow and goat milk in sheep milk and dairy products marketed in Mashhad city of Iran. Iran. J. Vet. Med. 7, 257–262. doi:10.22059/ijvm.2013.36285
Khudhair Jabir Alrikabi, A. (2015). Characterization of Iraqi sheep milk fat. J. Biol. Agric. Healthc. 5, 85–93.
Liu, Z., Wang, J., Cocks, B. G., and Rochfort, S. (2017). Seasonal variation of triacylglycerol profile of bovine milk. Metabolites 7, 24–35. doi:10.3390/metabo7020024
Markiewicz-Keszycka, M., Czyzak-Runowska, G., Lipinska, P., and Wójtowski, J. (2013). Fatty acid profile of milk - a review. Bull. Vet. Inst. Pulawy. 57, 135–139. doi:10.2478/bvip-2013-0026
Mayer, H. K., and Fiechter, G. (2012). Physical and chemical characteristics of sheep and goat milk in Austria. Int. Dairy J. 24, 57–63. doi:10.1016/j.idairyj.2011.10.012
Moatsou, G., and Sakkas, L. (2019). Sheep milk components: Focus on nutritional advantages and biofunctional potential. Small Rumin. Res. 180, 86–99. doi:10.1016/j.smallrumres.2019.07.009
Najafi, T., Eghtesadi, Sh, Rezaei, M., and Daneshvar, K. (2011). The effect of kermanshahi animal oil on serum lipid profile in healty men. J. Kermanshah Univ. Med. Sci. 14, 290–294.
Naviglio, D., Dellagreca, M., Ruffo, F., Andolfi, A., and Gallo, M. (2017). Rapid analysis procedures for triglycerides and fatty acids as pentyl and phenethyl esters for the detection of butter adulteration using chromatographic techniques. J. Food Qual., 11. 2017, doi:10.1155/2017/9698107
Nooratiny, I., Sahilah, A. M., Alif Alfie, A. R., and MohdFarouk, M. Y. (2013). DNA extraction from ghee and beef species identification using polymerase chain reaction (PCR) assay. Int. Food Res. J. 20, 2959–2961.
Piravi vanak, z. (2018). An overview on edible oils with integrity approach. J. Food Biosci. Technol. 8, 11–18.
Raftani Amiri, Z., and Salmani, S. (2017). Detection of vegetable oils in industrial dairy products of kermanshah using chromatography methods. Iran. J. Food Sci. Technol., 205–214.
Revilla, I., Escuredo, O., González-Martín, M. I., and Palacios, C. (2017). Fatty acids and fat-soluble vitamins in Ewe’s milk predicted by near infrared reflectance spectroscopy. Determination of seasonality. Food Chem. 214, 468–477. doi:10.1016/j.foodchem.2016.07.078
Salarabadi, A., Arbabi Bidgoli, S., and Madani, S. H. (2015). Roles of kermanshahi oil, animal fat, dietary and non-dietary vitamin D and other nutrients in increased risk of premenopausal breast cancer: A case control study in Kermanshah, Iran. Asian pac. J. Cancer Prev. 16, 7473–7478. doi:10.7314/APJCP.2015.16.17.7473
Strzałkowska, N., Jóźwik, A., Bagnicka, E., Krzyżewski, J., Cooper, R. G., and Horbańczuk, J. O. (2010). Factors affecting the cholesterol content of milk of cows fed conserved feeds in a TMR system throughout the year. Mljekarstvo 60, 273–279.
Keywords: chemical composition, cow butter oil, molecular properties, season, sheep butter oil
Citation: Dasht Peyma A, Piravi Vanak Z and Ghavami M (2022) Differences in the main composition and molecular components of sheep and cow butter oils produced by the local traditional method. Front. Food. Sci. Technol. 2:962529. doi: 10.3389/frfst.2022.962529
Received: 06 June 2022; Accepted: 14 September 2022;
Published: 05 October 2022.
Edited by:
Iuliana Vintila, Dunarea de Jos University, RomaniaReviewed by:
Sodeif Azadmard Damirchi, University of Tabriz, IranJesús Rodríguez-Miranda, Instituto Tecnológico de Tuxtepec, Mexico
Copyright © 2022 Dasht Peyma, Piravi Vanak and Ghavami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zahra Piravi Vanak, enBpcmF2aUBnbWFpbC5jb20=
 Atefeh Dasht Peyma
Atefeh Dasht Peyma Zahra Piravi Vanak
Zahra Piravi Vanak Mehrdad Ghavami1
Mehrdad Ghavami1