Abstract
High hydrostatic pressure (HHP, 400 MPa/10 min and 500 MPa/8 min) and thermal processing (TP, 90°C/2 min) were comparatively evaluated by examining their impacts on microorganisms, physicochemical characteristics (TSS, pH, color, ascorbic acid, total phenols, total anthocyanins, and particle size distribution), antioxidant activity, endogenous enzyme activity, and sensory quality of the anthocyanin-rich fruit puree during 20 days of storage at 4°C. The count of total aerobic bacteria (TAB) in HHP treated samples was less than 2.02 log10CFU/mL, and yeasts and molds (Y&M) were not detected during storage. Compared with TP treated samples, the total anthocyanins, total phenols, ascorbic acid, antioxidant capacity, and color of HHP treated ones were better maintained. Principal component analysis (PCA) also proved that the original quality of puree could be better preserved by HHP after treatment and during storage. However, the activity of polyphenol oxidase (PPO) and pectin meth esterase (PME) in HHP treated samples were not inactivated totally. In sum, better quality parameters were observed in HHP treated samples, so HHP was a potential way to be applied to fruit puree.
1 Introduction
Anthocyanin is a kind of natural flavonoid compound making the plants show various colors such as blue, red, and purple. And it mostly exists in the vacuole of plant cells (Collings, 2019). The antioxidant capacity of anthocyanin is quite strong due to the multiple phenolic hydroxyls of its structure (Akhbari et al., 2019). Based on current research, anthocyanin has varieties of biological activities including effects of regulating on body health and preventing on lots of diseases (Mohammadi Pour et al., 2019). Therefore, anthocyanin has broad application prospects in the field of foods, cosmetics, and pharmaceuticals.
In recent years, the compound fruit puree containing anthocyanin is more and more popular among consumers. It has become an important nutritional supplement for children, the elderly, patients, or other people. Among the fruits, strawberry and pitaya are rich in varieties of phenolic compounds with antioxidant properties, especially anthocyanin, which has the potential of preventing in cancer, cardiovascular, and other chronic diseases (Dharmawansa et al., 2020; Chen et al., 2021). Besides, apple is a good basic material of puree, it is not only famous for its taste and flavor, but also famous for its nutrition and pharmacological value as a good source of selected micronutrients (e.g., iron and vitamins C) and polyphenols (e.g., procyanidins and phloridzin) (Oyenihi et al., 2022). Therefore, the combination of the anthocyanin-rich fruit puree and apple puree is a promising product.
Nowadays, thermal processing (TP) is the main processing technology of fruit puree. And the quality deterioration such as nutrition, flavor, and color will be triggered by TP (Huang et al., 2017; Al-juhaimi et al., 2018). Therefore, the demand for betty qualities and more nutritious products highlights the need to develop novel and gentle technologies for fruit puree processing.
High hydrostatic pressure (HHP) is an emerging non-thermal food processing technology. And HHP can inactivate pathogenic and spoilage microorganisms without significantly compromising the nutritional and organoleptic quality of the food. Recently, HHP technology has been used in different branches of the food industry. Some studies have already proved the good quality parameters of HHP treatment, such as microbiological safety (Chen et al., 2016; Zou et al., 2016; Pei et al., 2018), a better retention rate of ascorbic acid, total phenols, total anthocyanins, and antioxidant capacity (He et al., 2018; Zhang et al., 2021; Wang et al., 2022) and so on. However, the suitability of HHP for anthocyanin-rich fruit puree remains systematically investigation due to the complexity caused by the different proportions of ingredients in the compound fruit puree.
The objective of this work was to evaluate the effects of HHP and TP treatments of the anthocyanin-rich fruit puree on microorganisms, physicochemical characteristics (TSS, pH, color, ascorbic acid, total phenols, total anthocyanins, and particle size distribution), antioxidant activity, endogenous enzyme activity, and sensory quality during 20 days of storage at 4°C. Namely, whether HHP has potential application in the processing of anthocyanin-rich fruit puree. This study will provide technical support for the commercial application of the HHP technique in fruit industry.
2 Materials and methods
2.1 Preparation of the anthocyanin-rich fruit puree
In this study, the apple variety “Malus pumila Mill”, strawberry variety “Kamairuosha”, and pitaya variety “Hylocereus undulatus Britt” were harvested at commercial maturity, and obtained from a local market (MerryMart Chain Commerce) in Beijing (China). Raw materials were rinsed in tap water, peeled, and sliced into small pieces. According to the results of pre-experiment, apple, strawberry, and pitaya were mixed at a mass ratio of 3:2:1. The slices were pureed with a juice extractor (Joyong JYL- 610, Joyong Electric Appliance Co., Shandong, China), immediately repacked into a high-temperature retort pouch of 60 g/bag, and kept the mixture at 4°C until use.
2.2 HHP and TP treatments of the anthocyanin-rich fruit puree
The samples were placed into the vessel for HHP processing. HHP treatment was carried out using a hydrostatic pressurization unit (CQC30L-600, Suyuan Zhongtian Co., Ltd., Beijing, China) with a capacity of 30.0 L at ambient temperature (≈18°C). The pressurization rate was about 120 MPa/min and the depressurization was immediate (<3 s). Distilled water was used as the pressure-transmitting fluid. The treatment time reported in this study did not include the pressure-increase time and pressure- release time.
A thermocouple was inserted into the center of the anthocyanin-rich fruit puree and located at the package cold point. When samples achieved a core temperature of 90°C in a water bath (S-HH-W21-Cr600, Changan science and Technology Instrument Factory, China), they were held at this temperature for 2 min. TP treatment was using the same packing material as the ones used for HHP.
According to our previous research studies, after HHP (400 MPa/min, 500 MPa/8 min) and TP (90°C/2 min), yeasts and molds (Y&M) were not detected, and the counts of total aerobic bacteria (TAB) were less than 2.00 log10CFU/mL which met the requirements of Chinese Food Safety Standards-Drinks GB 7101-2015. Therefore, the anthocyanin-rich fruit puree was processed by these treatments in this study.
The treated samples were stored at 4 ± 2°C in the dark. Sample analyses were carried out after 0, 1, 4, 7, 10, 15, and 20 days of storage. Untreated samples were considered as the control and the experiments were conducted at room temperature (25 ± 1°C) in independent triplicates (n = 3).
2.3 Microbial analysis
The total plate count method was used to count viable microorganisms in the anthocyanin-rich fruit puree. An untreated or treated sample was serially diluted with sterile 0.85% NaCl solution, and 1.0 ml of each dilution was plated into duplicate plates of appropriate agar. Nutrient agar (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was used for counting the viable total aerobic bacteria (TAB) cells after incubation at 37°C for 48 ± 2 h. Rose bengal agar (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was used for counting the viable Y&M cells after incubation at 27°C for 72–120 h. After incubation, the colonies were counted.
2.4 Physicochemical characteristics analysis
2.4.1 TSS and pH
Samples were controlled at 25°C to measure total soluble solids (TSS) and pH values by WAY-2Sdigital Abbe refractometer (Shanghai Precision and Scientific Instrument Co., Shanghai, China) and Thermo Orion 868 pH meter (Thermo Fisher Scientific, Inc., MA, United States). The results of TSS were reported as °Brix.
2.4.2 Color assessment
The color assessment was measured by color measurement spectrophotometer (HunterLab Color Quest XE, HunterAssociates Laboratory, Inc., Virginia, United States) in the reflectance mode at 25°C. The color was expressed in L*, a*, and b* values. All measurements were made in triplicate and results were averaged. In addition, the total color difference (ΔE) was calculated using the following equation, where L*0, a*0, and b*0 are the control values for untreated samples.
2.4.3 Determination of ascorbic acid
The method was proposed by Cao et al. (2012) with some modifications to extract and analyze ascorbic acid. 5 g anthocyanin-rich fruit puree was mixed with 25 ml metaphosphoric acid (2.5%) and incubated at 4°C for 2 h, then the mixture was centrifuged using 17300 g for 10 min at 4°C; the supernatant was collected and filtrated with 0.45 μm water system membrane. Chemicals were obtained from Beijing Chemicals Co. (Beijing, China).
Ascorbic acid was separated using liquid chromatography (LC-20AT) equipped with a UV/Vis detector (SPD-20AV), an autosampler (SIL-20A), and a column oven (CTO-20A) from Shimadzu Co., Japan. The separation was performed on an Alltech Alltima TM C18, (4.6 × 250 mm i.d, 5 μm particle size) from the waters. The mobile phase was an isocratic solvent system consisting of 95% monopotassium phosphate (50 mM, pH = 3.0) and 5% acetonitrile. The flow rate was 1 ml/min and aliquots of 20 μL were injected. The analyses were conducted at ambient temperature. The detection was carried out at 245 nm in absorbance mode. Mobile phase solvent purchased from Honeywell (Shanghai, China). Results were expressed as mg of ascorbic acid per 100 g of anthocyanin-rich fruit puree (mg/100 g).
2.4.4 Quantification of total phenols
The total phenols were determined using the Folin-Ciocalteu method described by Cao et al. (2011) with some modifications. 10 g anthocyanin-rich fruit puree was mixed with 20 ml methanol, and then the mixture was kept in the dark for 30 min at 4°C, centrifuged at 17300 g for 20 min at 4°C (CF16RXⅡ Hitachi, Japan). 0.1 ml extract was diluted to 0.4 ml and mixed with 2 ml Folin-Ciocalteu reagent (previously diluted 10-fold with distilled water) and set for 1 h in the dark at room temperature. After that, 1.8 ml sodium carbonate solution (7.5%) was added to the mixture and reacted for 15 min, then the mixture was immediately measured at 765 nm using a spectrophotometer (UV-726, Shimadzu, Shanghai, China). Chemicals were obtained from Beijing Chemicals Co. (Beijing, China). Results were expressed as mg of total phenols per 100 g of anthocyanin-rich fruit puree (mg/100 g).
2.4.5 Quantification of total anthocyanins
The spectrophotometric pH differential method (Xu et al., 2010) was used to quantify anthocyanins in the extracts in this study. 0.025 M potassium chloride buffer (pH = 1) and 0.4 M sodium acetate buffer (pH = 4.5) were prepared to dilute the samples, respectively. The absorbance values of the sample dilutions at 520 and 700 nm were measured by spectrophotometer (UV-726, Shimadzu, Shanghai, China) after equilibrating for 15 min. Distilled water in buffer was as control. Chemicals were obtained from Beijing Chemicals Co. (Beijing, China). Anthocyanin content was calculated by the following equation.where A = ApH1.0−ApH4.5, Mw is the molecular weight of cyanidin-3-glucoside (449.2 g/mol), DF, the dilution factor, ε, the molar absorptivity (26, 900 L/cm/mol) and L, the path length (1 cm). Total anthocyanin content was reported as mg of total anthocyanins per 100 g of anthocyanin-rich fruit puree (mg/100 g).
2.4.6 Particle size distribution
The Hydro LV module of the laser particle size analyzer (Malvern, Mastersizer 3,000, United Kingdom) was used to determine the particle size distribution of the samples (Gao et al., 2015). The refractive index parameter, particle density, absorbance and refractive index were 1.59, 1.05, 0.00 and 1.33, respectively. The analysis model selected the general Mile model. The instrument would cycle 5 times automatically.
2.5 Antioxidant capacity measurements
2.5.1 Antioxidant capacity determined by stable radical method (DPPH)
Radical scavenging activity of the anthocyanin-rich fruit puree against stable DPPH was determined according to the method proposed by Miller et al. (1995) with some modifications. 100 µL of the extract was added to 4 ml methanol (Honeywell, Shanghai, China) and 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma Aldrich, St. Louis, United States) solution (0.14 mM). And the absorbance was measured at 517 nm after keeping in the dark for 45 min at room temperature. 100 µL of methanol was added to 4 ml methanolic DPPH solution as a control. Determinations were performed using a spectrophotometer (UV-726, Shimadzu, Shanghai, China). The radical scavenging activity was calculated from the (±)6-hydroxy-2,5,7,8-tetramethylchroma-n-2-carboxylic acid (Trolox, Sigma Aldrich, St. Louis, United States) standard curve, which was expressed as the μmol of DPPH reduction per 1 g of anthocyanin-rich fruit puree (μmol/g).
2.5.2 Antioxidant capacity determined by ferric reducing antioxidant power (FRAP)
The FRAP assays were performed as described by Aljadi and Kamaruddin (2004) with some modifications. 10 mM 2,4,6-tri-2-pyridyl-1,3,5-triazine (TPTZ, Sigma Aldrich, St. Louis, United States) were prepared by dissolving in 40 mM HCl. Freshly prepared FRAP solution contained 25 ml 0.3 M acetate buffer (pH 3.6), 2.5 ml 10 mM TPTZ, and 2.5 ml 20 mM ferric chloride. 100 μL of the extract was added to 4 ml FRAP solution. And the absorbance was measured at 593 nm after keeping in the dark for 10 min at 37°C. 100 µL of distilled water was added to 4 ml FRAP solution as a control. Other chemicals were obtained from Beijing Chemicals Co. (Beijing, China). Determinations were performed using a spectrophotometer (UV-726, Shimadzu, Shanghai, China). The ferric reducing antioxidant power was calculated from the Trolox standard curve, which was expressed as the μmol of ferric reduction per 1 g of anthocyanin-rich fruit puree (μmol/g).
2.6 Enzyme activity assay
2.6.1 PPO activity
The extraction of polyphenol oxidase (PPO) was carried out according to the procedure described by Liu et al. (2012) with some modifications. 5 g anthocyanin-rich fruit puree was mixed with 20 ml of 0.2 M phosphate buffer (pH 6.5) and 4% polyvinylpolypyrrolidone (PVPP), pulped for 2 min and extracted for 1 h at 4°C. The mixture was centrifuged at 6,155 g for 10 min at 4°C, and the supernatant obtained was collected and analyzed for enzyme activity. The reaction mixture for PPO was 0.5 ml of enzyme extract and 2.5 ml of 0.07 M catechol in 0.2 M sodium phosphate buffer (pH 6.5) solution. Chemicals were obtained from Beijing Chemicals Co. (Beijing, China). The mixture was incubated at 30°C before recording the absorbance with a UV-1800 UV VIS Spectrophotometer (Unico Co., Ltd., Shanghai, China). PPO activity (1/min) was calculated based on the slope of the linear portion of the reaction curve of ΔA420.
2.6.2 PME activity
10 g anthocyanin-rich fruit puree was added to 0.2 M Tris-chloride buffer (pH 7.5) with 0.1 M NaCl for 12 h at 4°C, and centrifuged at 10,000 g for 10 min. The activity of PME was measured at pH 7.5 according to the method proposed by Rouse et al. (1965). The solution was adjusted to pH 7.5 with 0.01M NaOH. Chemicals were obtained from Beijing Chemicals Co. (Beijing, China). The PME activity expressed in pectin methyl esterase units (PMEU) was calculated by the following equation:where [NaOH] is NaOH concentration, VNaOH is the volume of NaOH used, Vsample is the volume of sample used, and t′ is the time (in minutes) needed for pH to return to 7.5 after the addition of NaOH.
2.6.3 Determination of enzyme residual activity
A first order kinetic model could often describe the inactivation of enzymes, whereby enzyme activity decreased linearly as a function of time. Results expressed in residual enzyme activity A/A0, where A0 is the mean initial activity of the enzyme, and A the mean residual activity after treatment.
2.7 Sensory analysis
A 4-point scale was used to evaluate: color, organizational status, aroma, taste, and an overall quality assessment was conducted using a 9-point hedonic scale (Chandra et al., 2015). Assessments were made by a trained sensory panel (5 women and 5 men). All samples were evaluated independently in a test room complying with the requirements of ISO 8589:2007. These sensory qualities were evaluated on the processing day and at every storage interval until the end of shelf life during storage at 4°C.
2.8 Statistical analysis
Principal component analysis (PCA) was used to simplify the complexity of data by transforming it into a few principal components (Lever et al., 2017). In order to differentiate the quality of untreated, HHP treated, and TP treated samples, the raw data of physicochemical characteristics and sensory quality were subjected to PCA analysis. PCA was supported by MetaboAnalyst, which was accessible at http://www.metaboanalyst.ca (Xia et al., 2009). The raw data were summarized into much fewer variables called scores which are weighted average of the original variables. The PCA analysis was performed using the prcomp package. The calculation was based on singular value decomposition. The Rscript chemometrics. R is required.
Each experiment was carried out in triplicate. Statistical Program for Social Sciences (SPSS 21.0, Chicago, IL, United States) for windows statistical software was used for t test analysis and the Duncan’s multiple range test between groups, and p < 0.05 indicated significant difference. Graphics were produced using the Origin Pro 2015 software (OriginLab, Northampton, MA, United States).
3 Results
3.1 Microbiological analysis
The number of surviving cells of the anthocyanin-rich fruit puree before or after treatment was determined by monitoring the TAB and Y&M counts. As shown in Table 1, the initial counts of TAB and Y&M in untreated samples were 4.13 and 3.25 log10CFU/g, respectively. And the counts of TAB in anthocyanin-rich fruit puree after treatments were significantly reduced to 0.70, 1.69, and 1.38 log10CFU/g at 90°C/2 min, 400 MPa/10 min, and 500 MPa/8 min, respectively. Although the counts of HHP was higher than that of TP at the beginning, the counts of TP increased significantly during 20 days of storage until reaching 2.44 log10CFU/g. In contrast, the counts of HHP did not exceed 2.02 log10CFU/g. It proved the microbiological safety of HHP treatment. Many studies also observed similar results in mulberry juice and Hami lemon juice during 28 days of storage (Zou et al., 2016; Pei et al., 2018).
TABLE 1
| Storage time (day) | Log10CFU/g | ||||
|---|---|---|---|---|---|
| Untreated | 90°C/2 min | 400 MPa/10 min | 500 MPa/8 min | ||
| TAB | 0 | 4.13 ± 0.01A | 0.70 ± 0.56dC | 1.69 ± 0.08cB | 1.38 ± 0.29cB |
| 4 | — | 1.43 ± 0.12cB | 1.90 ± 0.07aA | 1.51 ± 0.15bcB | |
| 7 | — | 1.89 ± 0.09bA | 1.80 ± 0.13bAB | 1.68 ± 0.09bB | |
| 10 | — | 2.04 ± 0.06bA | 1.85 ± 0.11bB | 1.71 ± 0.07abB | |
| 15 | — | 2.22 ± 0.03abA | 1.89 ± 0.12bB | 1.80 ± 0.11abB | |
| 20 | — | 2.44 ± 0.03aA | 2.02 ± 0.08aB | 1.91 ± 0.08aB | |
| Y&M | 0 | 3.25 ± 0.01 | ND | ND | ND |
| 1–20 | — | ND | ND | ND | |
Variations of total aerobic bacteria (TAB) and yeasts and molds (Y&M) in anthocyanin-rich fruit puree during 20 days of storage at 4°C.
—, not tested. ND, no detected (detection limit <1 CFU/g). TAB, total aerobic bacteria. Y&M, yeasts and molds. Values with different lower case letters within one column and different capital letters within one row are significantly different (p < 0.05).
Moreover, Both TP and HHP treatments resulted in the inactivation of Y&M to a level below the detection limit, and the counts of Y&M were not detected in all treated samples during 20 days of storage at 4°C. Therefore, the growth of Y&M could be effectively inhibited by TP and HHP treatment during storage. Similar to many studies that Y&M are not detected during storage after both treatments (Pei et al., 2018; Ates et al., 2021).
3.2 Physicochemical characteristics
3.2.1 TSS, pH, and color assessment
The changes in TSS, pH, and color of samples during storage were shown in Table 2. TSS and pH of the untreated sample were 10.73°Brix and 3.90, respectively. Their values increased slightly after TP and HHP treatments on day 0 but decreased significantly during the storage of 20 days at 4°C.
TABLE 2
| Storage time (day) | Untreated | 90°C/2 min | 400 MPa/10 min | 500 MPa/8 min | |
|---|---|---|---|---|---|
| TSS | 0 | 10.73 ± 0.12B | 10.80 ± 0.01abB | 10.80 ± 0.01abB | 10.87 ± 0.01aA |
| 4 | — | 10.87 ± 0.12aA | 10.77 ± 0.06abA | 10.83 ± 0.05abA | |
| 7 | — | 10.67 ± 0.06bB | 10.87 ± 0.06aA | 10.80 ± 0.10bA | |
| 10 | — | 10.73 ± 0.06bA | 10.80 ± 0.01abA | 10.77 ± 0.06bA | |
| 15 | — | 10.53 ± 0.06cA | 10.67 ± 0.06cA | 10.63 ± 0.05cA | |
| 20 | — | 10.30 ± 0.10dA | 10.37 ± 0.06dA | 10.43 ± 0.06dA | |
| pH | 0 | 3.90 ± 0.03C | 3.99 ± 0.01aA | 3.96 ± 0.02aAB | 3.96 ± 0.01aB |
| 4 | — | 3.96 ± 0.01bA | 3.96 ± 0.01aA | 3.95 ± 0.02aA | |
| 7 | — | 3.98 ± 0.01aA | 3.95 ± 0.01aB | 3.96 ± 0.02aAB | |
| 10 | — | 3.94 ± 0.01bA | 3.92 ± 0.01bA | 3.92 ± 0.02bA | |
| 15 | — | 3.91 ± 0.01cA | 3.92 ± 0.01bA | 3.92 ± 0.02bA | |
| 20 | — | 3.88 ± 0.01dA | 3.89 ± 0.02dA | 3.87 ± 0.01dA | |
| L* | 0 | 26.68 ± 0.18C | 27.88 ± 0.13bA | 27.33 ± 0.08bB | 27.37 ± 0.15bB |
| 4 | — | 28.06 ± 0.47bA | 27.38 ± 0.25bA | 27.68 ± 0.17bA | |
| 7 | — | 28.55 ± 0.34bA | 27.52 ± 0.35bB | 27.47 ± 0.22bB | |
| 10 | — | 29.10 ± 0.61bA | 27.60 ± 0.17abB | 27.62 ± 0.13abB | |
| 15 | — | 30.80 ± 0.40abA | 27.95 ± 0.10abB | 27.91 ± 0.24abB | |
| 20 | — | 31.92 ± 0.11aA | 28.07 ± 0.25aB | 28.02 ± 0.28aB | |
| a* | 0 | 4.95 ± 0.11B | 5.18 ± 0.22cB | 6.34 ± 0.13bcA | 6.41 ± 0.21bA |
| 4 | — | 5.18 ± 0.20cB | 6.36 ± 0.09bA | 6.39 ± 0.17bA | |
| 7 | — | 5.31 ± 0.27cC | 6.36 ± 0.13bB | 6.64 ± 0.13aA | |
| 10 | — | 5.83 ± 0.29bC | 6.45 ± 0.07aB | 6.64 ± 0.08aA | |
| 15 | — | 6.03 ± 0.16bA | 6.27 ± 0.15cA | 6.39 ± 0.24bA | |
| 20 | — | 6.61 ± 0.16aA | 6.31 ± 0.24cA | 6.49 ± 0.27bA | |
| b* | 0 | 0.44 ± 0.21B | 1.52 ± 0.24dA | 0.22 ± 0.13cB | 0.18 ± 0.10cB |
| 4 | — | 1.84 ± 0.19bA | 0.23 ± 0.16aB | 0.29 ± 0.09cB | |
| 7 | — | 1.62 ± 0.23cdA | 0.27 ± 0.05cB | 0.42 ± 0.20bB | |
| 10 | — | 1.71 ± 0.24cA | 0.46 ± 0.16bB | 0.46 ± 0.06bB | |
| 15 | — | 1.93 ± 0.20abA | 0.66 ± 0.11bB | 0.44 ± 0.18bB | |
| 20 | — | 1.98 ± 0.26aA | 0.99 ± 0.21aB | 0.64 ± 0.36aB | |
| ΔE | 0 | 0 | 1.46 | 1.54 | 1.64 |
| 4 | — | 1.55 | 1.61 | 1.75 | |
| 7 | — | 2.24 | 1.65 | 1.87 | |
| 10 | — | 2.87 | 1.76 | 1.94 | |
| 15 | — | 4.52 | 1.85 | 1.90 | |
| 20 | — | 5.71 | 2.02 | 2.06 |
Changes of total soluble solids (TSS), pH, and color parameters in anthocyanin-rich fruit puree during 20 days of storage at 4°C.
—, Not tested. Values with different lower case letters within one column and different capital letters within one row are significantly different (p < 0.05).
Color differences between the samples were determined after processing and during storage by measuring their L*, a*, and b* values. The L*, a*, and b* values increased after TP and HHP treatments at day 0, which might be attributed to that the treatments promoted the dissolution of colored substances in tissue cells. During the storage of 20 days at 4°C, the L* and b* values increased significantly (p < 0.05), but the change of the HHP treated samples was always smaller than that of TP. And the a* values of TP treated samples were increased with the storage time, but that of HHP treated ones showed good stability. This result agreed with that obtained by Chen et al. (2016), the change of the L*, a*, and b* values of HHP treated samples in cloudy ginger juice were smaller than that of TP during 40 days storage at 4°C.
It has been considered that ΔE over three would be a noticeable visual difference for a number of situations (Hu et al., 2020). The △E value of the samples after TP and HHP treatment were 1.46, 1.54 and 1.64 at day 0, respectively, indicating that TP and HHP treatments would not cause visual distinguishable changes. But the ΔE values increased in all treated samples after 20 days of storage, and that of TP treated samples were much higher than in HHP treated samples. This result was similar to the studies that HHP treatment could better maintain the original color of the pumpkin and BlackBerry puree during storage (Pei et al., 2018; Hu et al., 2020).
3.2.2 Ascorbic acid, total phenols and anthocyanins
As shown in Figure 1A, compared with untreated samples, the ascorbic acid degraded by 63.74% after TP treatment, but only 11.61% (400 MPa/10 min) and 22.71% (500 MPa/8 min) after HHP treatment. During 20 days of storage at 4°C, all treated samples showed a reduction in ascorbic acid, but the content of ascorbic acid in TP treated samples was significantly lower than HHP treated ones. Moussa-Ayoub et al. (2017) also reported that the HHP treatment of 600 MPa for 10 min resulted in better retention of ascorbic acid than TP treatment of 95°C for 3 min in cactus juice.
FIGURE 1
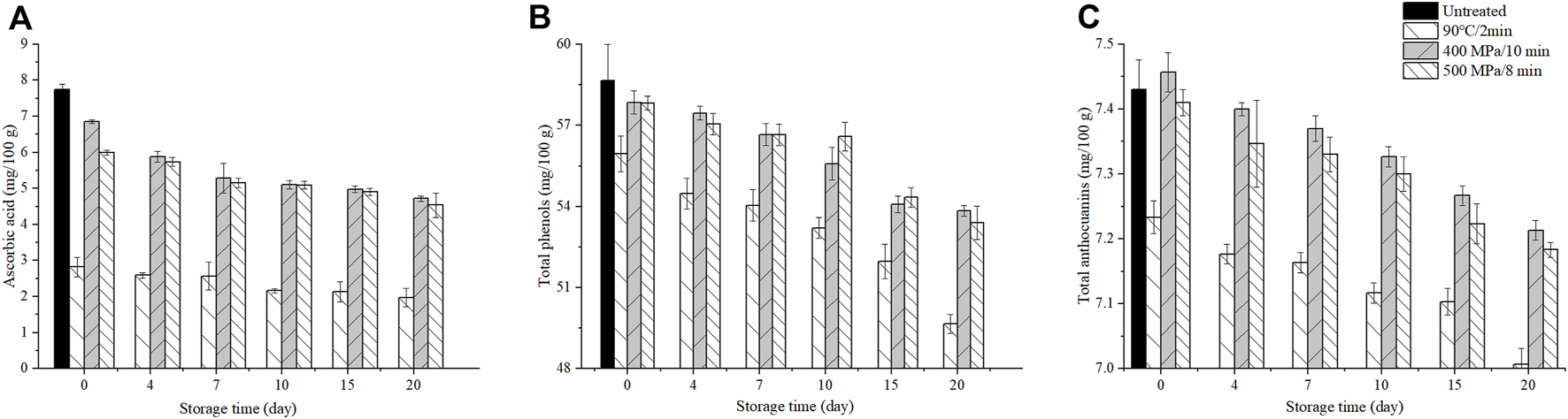
Changes of ascorbic acid (A), total phenols (B), and anthocyanins (C) content in the anthocyanin-rich fruit puree during 20 days of storage at 4°C (Untreated samples did not test after day 0 due to a sample rot).
Similarly, the content of total phenols also degraded after TP treatments, but HHP treated samples showed no significant reduction at day 0. After 20 days of storage, the contents of total phenols were decreased from 55.96 to 49.66 mg/100 g in TP treatment, from 57.86 to 53.84 mg/100 g in 400 MPa/10 min treatment and from 57.83 to 53.39 mg/100 g in 500 MPa/8 min treatment, respectively (Figure 1B). It suggested that HHP treatment cold retain more total phenols. This result was in accord with that found by Feng et al. (2020) in strawberry-apple-lemon juice blend, they observed that the content of total phenols in TP treated samples (86°C/1 min) were significantly lower than HHP treated ones (500 MPa/15 min) after 10 days of storage at 4°C (p < 0.05).
Compared with the untreated samples, the content of total anthocyanins showed no significant change in HHP treated samples at day 0, but that of TP treatment decreased significantly. After 20 days of storage, the total anthocyanins decreased from 7.23 to 7.01 mg/100 g in TP treatment, from 7.45 to 7.21 mg/100 g in 400 MPa/10 min treatment and from 7.41 to 7.18 mg/100 g in 500 MPa/8 min treatment, respectively (Figure 1C). This result indicated that HHP treatment had less effect on anthocyanins than TP treatment. Feng et al. (2020) and Zou et al. (2016) also reported that better maintenance of total anthocyanins was observed in the HHP treated juice (500 MPa/15 min and 500 MPa/5 min, respectively) than TP treatment (86°C/1 min and 110°C/8.6 s, respectively) during the storage.
3.2.3 Particle size distribution
The particle size distribution of the anthocyanin-rich fruit puree was shown in Figure 2 and Table 3. It could be seen that there was no significant difference in particle size distribution between TP treated and untreated samples, d43 and d32 also indicated that there was no significant change.
FIGURE 2
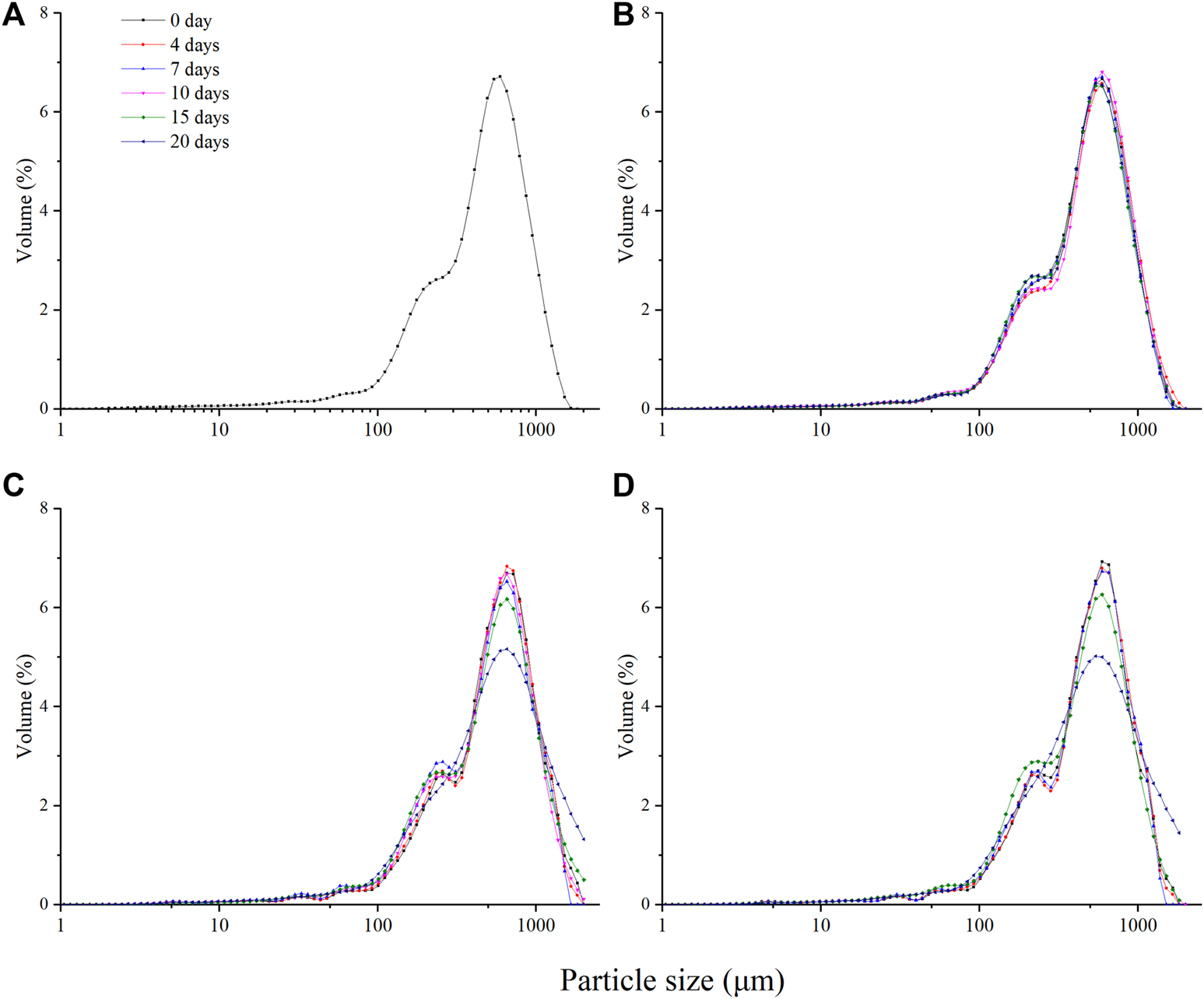
Changes of particle size distribution of the anthocyanin-rich fruit puree during 20 days of storage at 4°C. [Untreated (A), 90°C/2 min (B), 400 MPa/10 min (C) and 500 MPa/8 min (D); Untreated samples did not test after day 0 due to a sample rot].
TABLE 3
| Storage time (day) | Untreated | 90°C/2 min | 400 MPa/10 min | 500 MPa/8 min | |
|---|---|---|---|---|---|
| d 43 | 0 | 933.5 ± 20.21A | 924.11 ± 15.40aA | 911.90 ± 11.85cA | 928.6 ± 13.23cA |
| 4 | — | 922.7 ± 10.35aC | 982.81 ± 16.78bcA | 975.57 ± 32.5bcB | |
| 7 | — | 915.2 ± 15.51dB | 978.36 ± 22.89bcA | 999.28 ± 24.26bcA | |
| 10 | — | 916.8 ± 7.53cC | 1025.55 ± 6.40bB | 1061.9 ± 18.44bA | |
| 15 | — | 920.4 ± 9.07bC | 1257.30 ± 18.97aA | 1157.25 ± 22.56bB | |
| 20 | — | 917.4 ± 7.72cC | 1171.07 ± 16.73bB | 1232.42 ± 21.82aA | |
| d 32 | 0 | 819.3 ± 12.56A | 815.20 ± 10.40bA | 810.01 ± 9.69cA | 826.87 ± 9.42cA |
| 4 | — | 823.4 ± 5.23aB | 848.88 ± 9.81cA | 851.62 ± 19.83cA | |
| 7 | — | 818.5 ± 2.98bB | 882.77 ± 18.48cA | 871.27 ± 15.56cA | |
| 10 | — | 809.9 ± 4.64cC | 860.48 ± 4.95cB | 1052.93 ± 16.34aA | |
| 15 | — | 818.3 ± 15.90bB | 1021.74 ± 12.14bA | 1007.11 ± 18.35bA | |
| 20 | — | 826 ± 13.24aC | 1086.16 ± 17.30aA | 1008.93 ± 19.46bB |
Changes of particle size d43 and d32 in anthocyanin-rich fruit puree during 20 days of storage at 4°C.
—, Not tested. Values with different lower case letters within one column and different capital letters within one row are significantly different (p < 0.05).
Meanwhile, there was no significant change in particle size distribution in HHP treated samples during the early storage period, but the particle size gradually increased during later storage. Accordingly, it could be observed that d43 and d32 had a significant increase after 20 days (Table 3).
3.3 Antioxidant capacity
As shown in Figure 3, there was no significant difference in DPPH values among untreated, TP treated and HHP treated samples at day 0. Compared to untreated samples, FRAP values decreased in TP treated samples, did not change significantly in 400 MPa/10 min treated samples, and increased slightly on day 0 in 500 MPa/8 min treated samples at day 0.
FIGURE 3
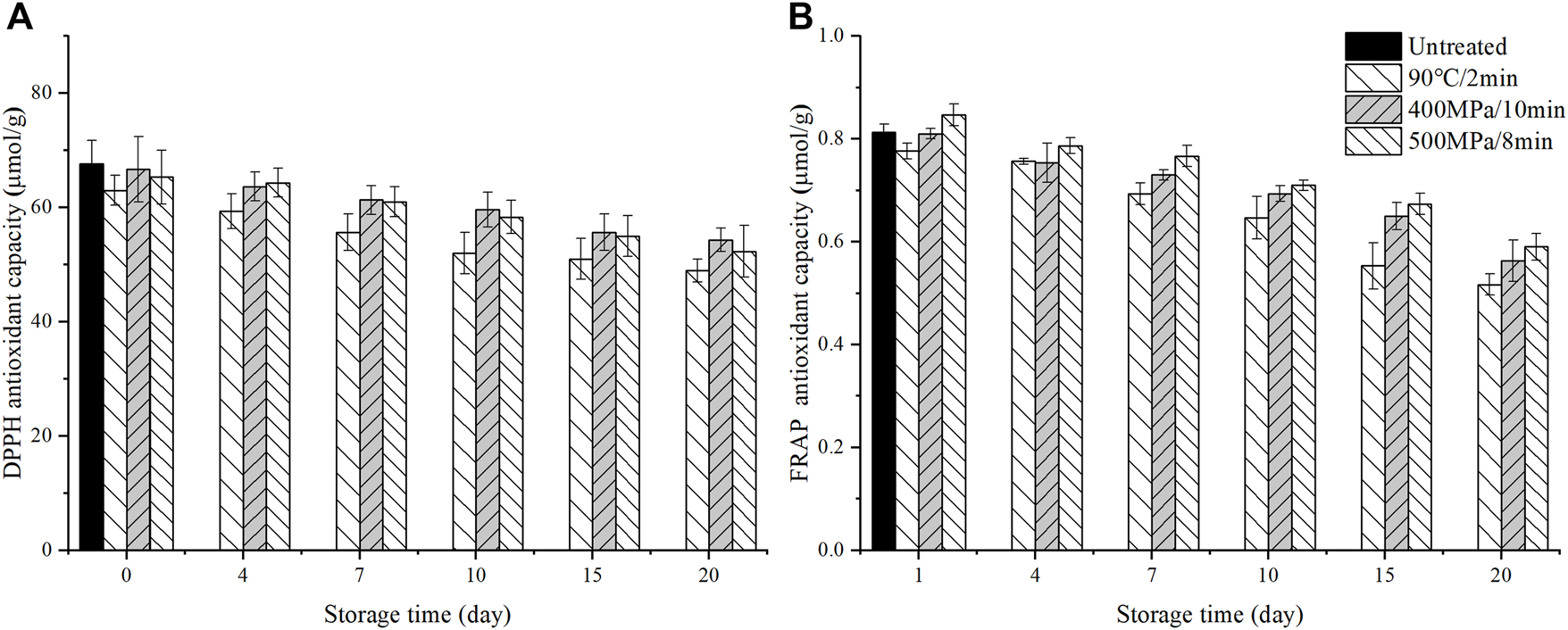
Changes of DPPH (A) and FRAP (B) antioxidant capacity in the anthocyanin-rich fruit puree during 20 days of storage at 4°C (Untreated samples did not test after day 0 due to a sample rot).
During storage, the DPPH and FRAP values of all samples kept decreasing. After 20 days of storage, the DPPH values decreased by 18–20%, the FRAP values decreased by 31–33%, respectively. However, the DPPH and FRAP values in TP treated samples were always lower than HHP treated ones.
3.4 PPO and PME activity
The changes in PPO and PME activity after different treatments are shown in Figure 4. TP treatment completely inactivated the PPO activity while HHP treatment partially reduced the PPO activity. 400 MPa/10 min and 500 MPa/8 min treatment reduced the PPO activity to 16.6 and 17.4%, respectively, and with the prolongation of the storage days, the PPO activity decreased to 0 on the 15th day of storage. It showed that HHP treatment could effectively inactivate the PPO activity. The same phenomenon was described by Soto-Caballero et al. (2021) that HHP had a significant effect on PPO inactivation.
FIGURE 4
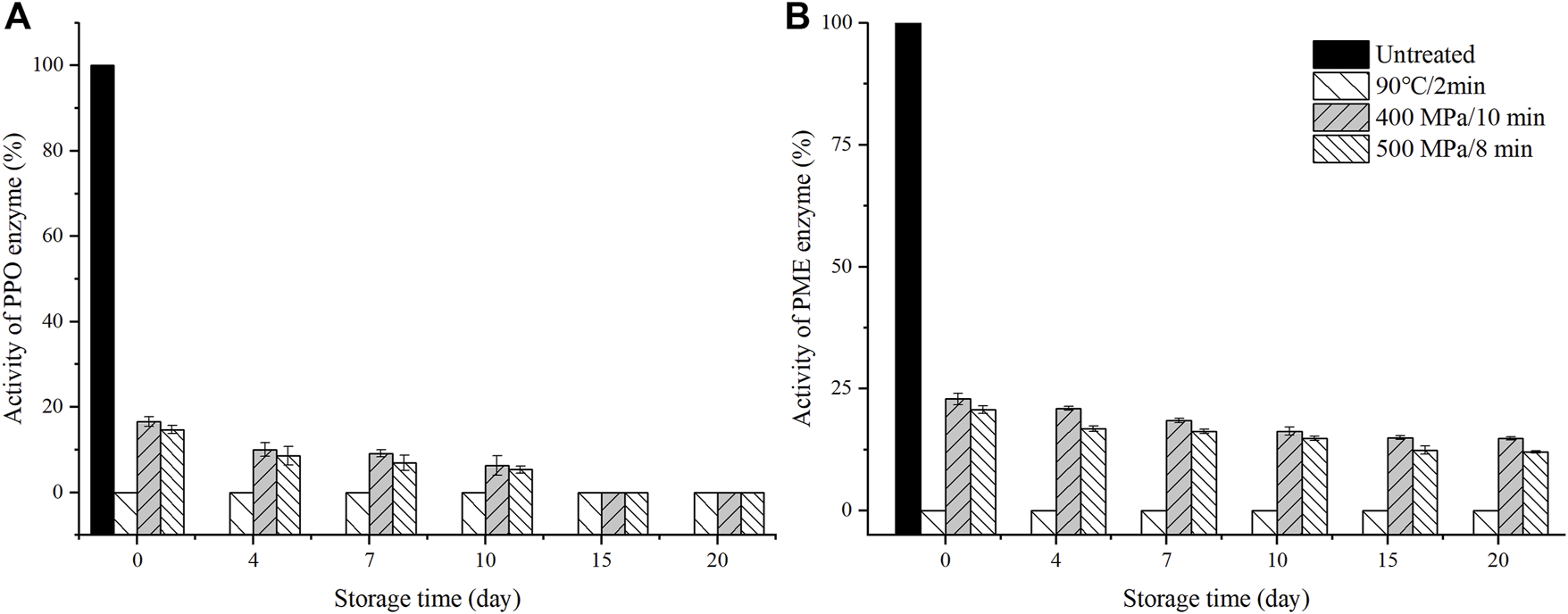
Changes of activity of polyphenol oxidase (PPO) (A) and pectin meth esterase (PME) (B) in anthocyanin-rich fruit puree during 20 days of storage at 4°C (Untreated samples did not test after day 0 due to a sample rot).
Similarly, the PME activity was completely inactivated by TP treatment but partially inactivated by HHP treatment to 20.7%. With the prolongation of the storage days, the PME activity of HHP treated samples continued to decline, but the lowest activity was still over 12%. Therefore, the residual activity of PME was related to the change of particle size distribution. Fernandez et al. (2019) also reported that the PME activity of a fruit and vegetable smoothie decremented up to 83.9% at 630 MPa/5 min initially, and showed a tendency to reduce insignificantly during storage.
3.5 Sensory quality
According to Table 4, compared to untreated samples, the sensory assessment demonstrated the significant change in sensory quality occurred in the anthocyanin-rich fruit puree after TP treatment. However, the taste, color, organizational status, flavor and total score of samples treated with HHP showed no significant differences compared to untreated samples at day 0, which suggested that HHP treatment could better maintain the original sensory of the anthocyanin-rich fruit puree. This result was similar to Inada et al. (2018) that HHP treatment had the advantage of preserving the sensory qualities than TP treatment in jabuticaba juice. During storage, the sensory qualities became worse and worse in TP treated samples. However, HHP treated samples still exhibited stable sensory qualities at day 14, and showed a slight decrease at day 20. Quiroz-Gonzalez et al. (2020) also reported that the sensorial acceptability of pitaya juice was not affected by HHP treatment during 60 days of storage.
TABLE 4
| Storage time (day) | Project | |||||
|---|---|---|---|---|---|---|
| Taste | Color | Organizational status | Flavor | Total score | ||
| Untreated | 0 | 32.20 ± 4.87a | 17.10 ± 0.99a | 15.40 ± 1.58b | 16.60 ± 1.96a | 81.30 ± 7.10a |
| 14 | — | — | — | — | — | |
| 20 | — | — | — | — | — | |
| 90°C/2 min | 0 | 23.15 ± 4.34cd | 13.65 ± 3.52c | 15.49 ± 1.63b | 13.92 ± 1.24ab | 66.21 ± 10.73bc |
| 14 | 25.81 ± 5.31c | 11.32 ± 1.98d | 14.32 ± 1.57bc | 10.09 ± 3.58c | 61.54 ± 12.44c | |
| 20 | 22.45 ± 1.25d | 11.54 ± 3.21ab | 12.21 ± 1.48c | 9.54 ± 1.05c | 55.74 ± 6.99d | |
| 400 MPa/10 min | 0 | 32.21 ± 2.42a | 15.31 ± 2.21b | 16.31 ± 0.97a | 15.04 ± 1.25a | 78.87 ± 6.85a |
| 14 | 34.57 ± 2.11a | 15.15 ± 1.57b | 17.33 ± 1.24a | 15.56 ± 1.75a | 82.61 ± 6.67a | |
| 20 | 30.48 ± 4.36bc | 14.48 ± 0.68bc | 16.88 ± 1.87a | 13.25 ± 1.75ab | 75.09 ± 8.66ab | |
| 500 MPa/8 min | 0 | 33.68 ± 1.33a | 16.98 ± 1.34a | 15.02 ± 2.54b | 16.98 ± 0.99a | 82.66 ± 6.20a |
| 14 | 33.44 ± 1.58a | 15.90 ± 1.05a | 17.10 ± 1.04a | 15.39 ± 0.58a | 81.83 ± 4.25a | |
| 20 | 30.61 ± 1.25b | 12.32 ± 2.57c | 14.54 ± 3.24bc | 12.58 ± 1.28b | 70.05 ± 8.34b | |
Sensory evaluation of anthocyanin-rich fruit puree during 20 days of storage at 4°C.
—, Not tested. Values with different letters within one column are significantly different (p < 0.05).
3.6 Principal component analysis (PCA)
PCA analysis was performed among the untreated, TP and HHP treatments during 20 days of storage at 4°C based on physicochemical characteristics and sensory quality to provide a better and more direct understanding of the difference of samples. The two principal components [PC1 (73.7%) and PC2 (13.2%)] explained the variation and cumulatively accounted for 86.9% of the total variance.
As shown in Figure 5, untreated and HHP treated samples distributed in the positive direction of the PC1 axis, while TP was in the negative direction of the PC1 axis away from them. Sun et al. (2019) also reported that the samples of traditional thermal method (65°C/3 min) were extremely discriminated from control and HPP method (500 MPa/3 min) in PCA analysis. Moreover, the crossover was observed between two HHP treatments.
FIGURE 5
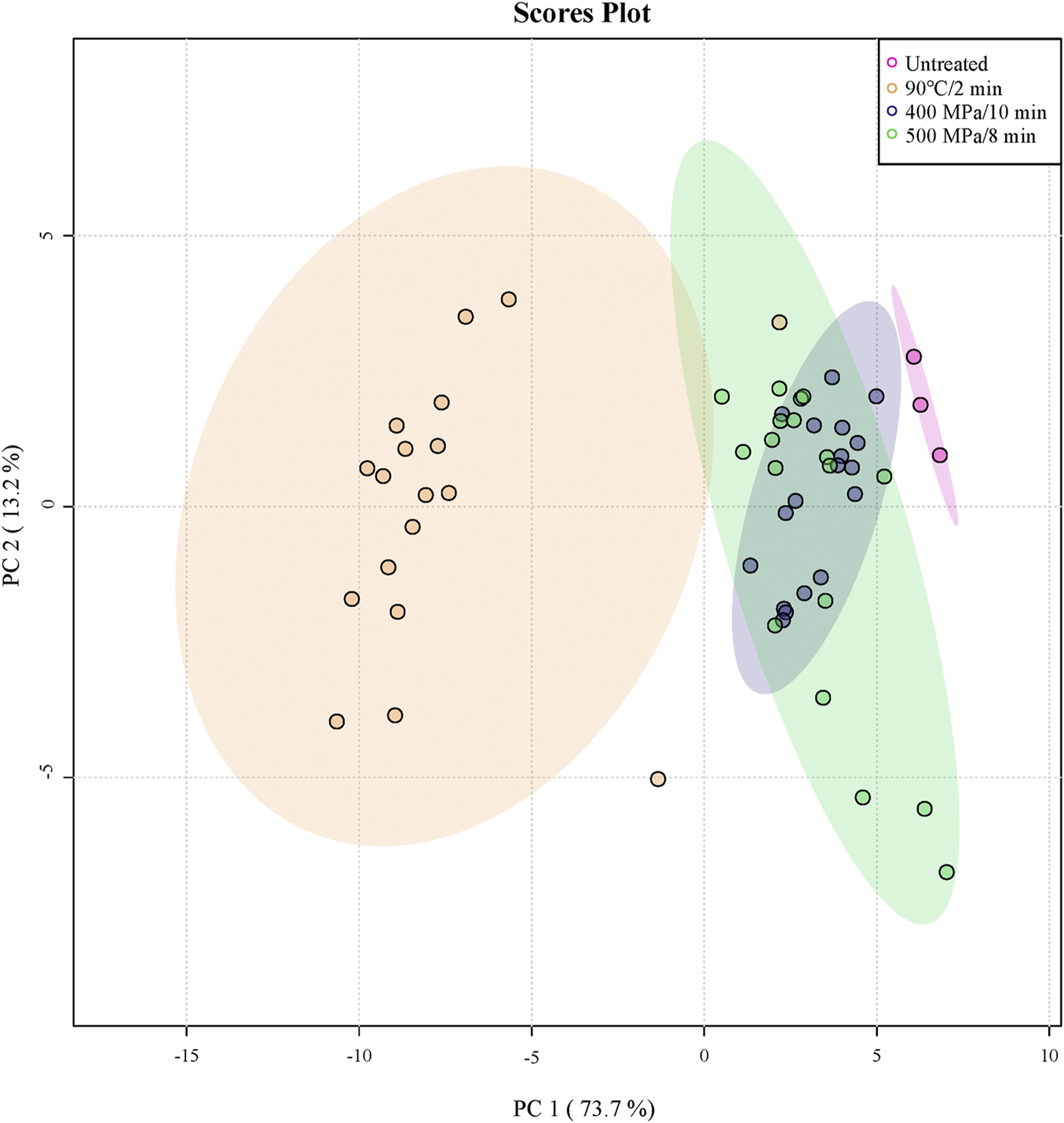
Principal component analysis (PCA) scores plot of the anthocyanin-rich fruit puree during 20 days of storage at 4°C (Untreated (Pink), 90°C/2 min (Yellow), 400 MPa/10 min (Blue) and 500 MPa/8 min (Green); Untreated samples did not test after day 0 due to a sample rot).
4 Discussion
4.1 Microbiological safety
As shown in Table 1, some surviving bacteria still existed in treated samples, while their growth was effectively inhibited by the low pH 3.9 of puree. With the prolong of the storage, the counts of TAB in HHP treated samples were significantly lower than that in TP. In addition, Y&M were not detected in all treated samples during 20 days of storage at 4°C. It was due to the special cell wall structure and cell morphology, Y&M are more sensitive than gram-negative bacteria to high pressure and low acidity (Feng et al., 2016; Shen et al., 2016; Yucui et al., 2019). Therefore, the environment of low pH could ensure the microbiological safety of samples during storage. The microbiological safety of HHP treatment was better than TP.
4.2 Physicochemical characteristics, antioxidant capacity, and endogenous enzyme activity
It could be observed that TSS and pH decreased during storage. TP could promote the decomposition and oxidation of nutrients such as sugars, and HHP could destroy the cell walls and cell membranes to release cell contents (Rinaldi et al., 2020). The reduction in nutrients content caused by TP and HHP treatments resulted in lower TSS of samples. Meanwhile, the decrease in pH values was caused by factors including the pressure may affected the ionization equilibrium of the aqueous solution, and more acidic substances dissolved from destroyed cells (Pei et al., 2018).
In addition, the dissolution of colored substances such as anthocyanins in the TP and HHP treatments resulted in color differences. During HHP treatment, colored substances such as anthocyanins were retained to some extent, and there was no noticeable visual difference in HHP treated samples. In contrast, the process of TP treatment led to the degradation and oxidation of colored substances, which resulted in greater color change (Wibowo et al., 2019).
Besides, higher retention of ascorbic acid, total phenols, and anthocyanins were observed in HHP treatment. These results may be attributed to that HHP treatment does not affect the covalent bonds (Morata and Guamis, 2020), which has limited effect on ascorbic acid, total phenols and anthocyanins. And TP treatment causes the degradation and oxidation of them (Wibowo et al., 2019).
The higher retention of ascorbic acid, total phenols, and anthocyanins could also account for higher antioxidant capacity in HHP treated samples than that in TP (Feng et al., 2020). Similarly, Blaszczak et al. (2021) reported that HHP treatment (450, 550, or 650 MPa for 5 or 15 min) of kiwi berry fruits resulted in increases in FRAP values which also be attributed to the promoted release of polyphenols from the plant tissue by HHP treatment. These results were also in accord with those found by Marengo-Orozco et al. (2020) in tropical beverages processed at 500 MPa/250 s during storage.
Another reason that could explain the high retention was the good passivation effect of HHP treatment on PPO activity. The effect was helpful to maintain the quality of total phenols, anthocyanins and color during storage. Besides, the fact that the PME activity was not completely inactivated by HHP led to the formation of large pectin polymer in low pH conditions (Szczepanska et al., 2021). And the particle size gradually increased during storage. However, the PME was completely passivated by TP treatment, and the particle size of TP treated samples showed little change during storage (Illera et al., 2018).
Overall, these results showed that HHP treated samples exhibited better physicochemical characteristics stability and antioxidant capacity than TP treated samples. However, the PME activity could not be well passivated by HHP treatments, which requires further research.
4.3 Comparation in samples
The scores plot of the samples in Figure 5 could indicate the TP treatment differentiated from untreated and HHP treatment. And the HHP treatment could better retain the original quality of anthocyanin-rich fruit puree after treatment and during storage. The crossover of the two HHP treated samples could not be differentiated well. However, the samples of 400 MPa/10 min were closer to the untreated samples than that of 500 MPa/8 min, which was more suitable for the anthocyanin-rich puree.
5 Conclusion
HHP treated samples exhibited a high microbial reduction after processing and better microbiological stability during 20 days of storage at 4°C, which could promise the microbiological safety. In addition, less change in color, more retained ascorbic acid, total anthocyanins and total phenols, as well as better sensory quality were observed in HHP treated samples. PCA differentiated TP treatment from untreated and HHP treatment according to the physicochemical characteristics and sensory quality during storage. It indicated that HHP treatment could better maintain the original quality of the samples.
In summary, HHP may be a good processing technology for the anthocyanin-rich purees due to its stability on good physicochemical characteristics and sensory quality, which could provide a guideline for the fruit industry. However, PME cannot be well passivated by HHP treatments, which requires further research.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
XY: Methodology, Investigation, Data curation, Writing—original draft, HD:Conceptualization, Methodology, SL: Formal analysis, Validation, XS: Conceptualization, Supervision, NW: Data curation, YW: Project administration, Funding acquisition, Writing—review and editing.
Funding
This research was supported by the 2115 Talent Development Program of China Agricultural University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HHP, High hydrostatic pressure; PCA, principal component analysis; PME, pectin meth esterase; PPO, polyphenol oxidase; TP, thermal processing; TAB, total aerobic bacteria; Y&M, yeasts and molds; TSS, total soluble solids.
References
1
Akhbari M. Hamedi S. Aghamiri Z. S. (2019). Optimization of total phenol and anthocyanin extraction from the peels of eggplant (Solanum melongena L.) and biological activity of the extracts. J. Food Meas. Charact.13 (4), 3183–3197. 10.1007/s11694-019-00241-1
2
Al-juhaimi F. Ghafoor K. Özcan M. M. Jahurul M. H. A. Babiker E. E. Jinap S. et al (2018). Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. J. Food Sci. Technol.55 (10), 3872–3880. 10.1007/s13197-018-3370-0
3
Aljadi A. M. Kamaruddin M. Y. (2004). Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem.85 (4), 513–518. 10.1016/s0308-8146(02)00596-4
4
Ates C. Akdemir Evrendilek G. Uzuner S. (2021). High-pressure processing of shalgam with respect to quality characteristics, microbial inactivation, and shelflife extension. J. Food Process. Preserv.45 (7), e15598. 10.1111/jfpp.15598
5
Blaszczak W. Latocha P. Jez M. Wiczkowski W. (2021). The impact of high-pressure processing on the polyphenol profile and anti-glycaemic, anti-hypertensive and anti-cholinergic activities of extracts obtained from kiwiberry (Actinidia arguta) fruits. Food Chem.343, 128421. 10.1016/j.foodchem.2020.128421
6
Cao X. Bi X. Huang W. Wu J. Hu X. Liao X. et al (2012). Changes of quality of high hydrostatic pressure processed cloudy and clear strawberry juices during storage. Innov. Food Sci. Emerg. Technol.16, 181–190. 10.1016/j.ifset.2012.05.008
7
Cao X. Zhang Y. Zhang F. Wang Y. Yi J. Liao X. et al (2011). Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric.91 (5), 877–885. 10.1002/jsfa.4260
8
Chandra D. Choi A. J. Kim Y. P. Kim J. G. (2015). Physicochemical, microbial and sensory quality of fresh-cut red beetroots in relation to sanization method and storage duration. Ital. J. Food Sci.27 (2), 208–220. 10.14674/1120-1770/ijfs.v188
9
Chen D. Pan S. Chen J. Pang X. Guo X. Gao L. et al (2016). Comparing the effects of high hydrostatic pressure and ultrahigh temperature on quality and shelf life of cloudy ginger juice. Food Bioproc. Tech.9 (10), 1779–1793. 10.1007/s11947-016-1759-1
10
Chen J. Xu B. Sun J. Jiang X. Bai W. (2021). Anthocyanin supplement as a dietary strategy in cancer prevention and management: a comprehensive review. Crit. Rev. Food Sci. Nutr.19, 1–13. 10.1080/10408398.2021.1913092
11
Collings D. A. (2019). Anthocyanin in the vacuole of red onion epidermal cells quenches other fluorescent molecules. Plants8 (12), 596. 10.3390/plants8120596
12
Dharmawansa K. V. S. Hoskin D. W. Rupasinghe H. P. V. (2020). Chemopreventive effect of dietary anthocyanins against gastrointestinal cancers: a review of recent advances and perspectives. Int. J. Mol. Sci.21 (18), 6555. 10.3390/ijms21186555
13
Feng H. Yi J. Bi J. Li J. (2016). Effect of high hydrostatic pressure on qualities of prepared green asparagus spears. Sci. Technol. Food Ind.37 (13), 101–106. 10.13386/j.issn1002-0306.2016.13.012
14
Feng X. P. Zhou Z. Y. Wang X. Q. Bi X. F. Ma Y. Xing Y. G. et al (2020). Comparison of high hydrostatic pressure, ultrasound, and heat treatments on the quality of strawberry-apple-lemon juice blend. Foods9 (2), 218. 10.3390/foods9020218
15
Fernandez M. V. Denoya G. I. Jagus R. J. Vaudagna S. R. Aguero M. V. (2019). Microbiological, antioxidant and physicochemical stability of a fruit and vegetable smoothie treated by high pressure processing and stored at room temperature. LWT--Food Sci. Technol.105, 206–210. 10.1016/j.lwt.2019.02.030
16
Gao G. Zhao L. Ma Y. Wang Y. T. Sun Z. J. Liao X. J. et al (2015). Microorganisms and some quality of red grapefruit juice affected by high pressure processing and high temperature short time. Food Bioproc. Tech.8 (10), 2096–2108. 10.1007/s11947-015-1556-2
17
He Y. Wen L. Yu H. Zheng F. Wang Z. Xu X. et al (2018). Effects of high hydrostatic pressure-assisted organic acids on the copigmentation of Vitis amurensis Rupr anthocyanins. Food Chem.268, 15–26. 10.1016/j.foodchem.2018.06.052
18
Hu X. N. Ma T. Ao L. Kang H. Hu X. S. Song Y. et al (2020). Effect of high hydrostatic pressure processing on textural properties and microstructural characterization of fresh-cut pumpkin (Cucurbita pepo). J. Food Process Eng.43 (4), e13379. 10.1111/jfpe.13379
19
Huang Y. Hsieh S. Hsu C. (2017). Application of non-thermal atmospheric plasma for blanching on sliced apple and banana. Taiwan. J. Agric. Chem. Food Sci.55 (5/6), 270–282.
20
Illera A. E. Sanz M. T. Trigueros E. Beltran S. Melgosa R. (2018). Effect of high pressure carbon dioxide on tomato juice: Inactivation kinetics of pectin methylesterase and polygalacturonase and determination of other quality parameters. J. Food Eng.239, 64–71. 10.1016/j.jfoodeng.2018.06.027
21
Inada K. O. P. Torres A. G. Perrone D. Monteiro M. (2018). High hydrostatic pressure processing affects the phenolic profile, preserves sensory attributes and ensures microbial quality of jabuticaba (Myrciaria jaboticaba) juice. J. Sci. Food Agric.98 (1), 231–239. 10.1002/jsfa.8461
22
Lever J. Krzywinski M. Atman N. (2017). Points of significance: principal component analysis. Nat. Methods14 (7), 641–642. 10.1038/nmeth.4346
23
Liu J. Hu Z. Su Y. (2012). Activity and browning inhibition of polyphenol oxidase from purple sweet potato. Food Sci.33 (17), 207–211.
24
Marengo-Orozco C. Patricia Tarazona-Diaz M. Ines Rodriguez L. (2020). Formulation of a tropical beverage by applying heat treatment and high hydrostatic pressure. Food Technol. Biotechnol.58 (3), 239–248. 10.17113/ftb.58.03.20.6459
25
Miller N. J. Diplock A. T. Riceevans C. A. (1995). Evaluation of the total antioxidant activity as a marker of the deterioration of apple juice on storage. J. Agric. Food Chem.43 (7), 1794–1801. 10.1021/jf00055a009
26
Mohammadi Pour P. Fakhri S. Asgary S. Farzaei M. H. Echeverria J. (2019). The signaling pathways, and therapeutic targets of antiviral agents: Focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol.10, 1207. 10.3389/fphar.2019.01207
27
Morata A. Guamis B. (2020). Use of UHPH to obtain juices with better nutritional quality and healthier wines with low levels of SO2. Front. Nutr.7, 598286. 10.3389/fnut.2020.598286
28
Moussa-Ayoub T. E. Jaeger H. Knorr D. El-Samahy S. K. Kroh L. W. Rohn S. et al (2017). Impact of pulsed electric fields, high hydrostatic pressure, and thermal pasteurization on selected characteristics of opuntia dillenii cactus juice. LWT - Food Sci. Technol.79, 534–542. 10.1016/j.lwt.2016.10.061
29
Oyenihi A. B. Belay Z. A. Mditshwa A. Caleb O. J. (2022). An apple a day keeps the doctor away": The potentials of apple bioactive constituents for chronic disease prevention. J. Food Sci.87, 2291–2309. 10.1111/1750-3841.16155
30
Pei L. Hou S. Wang L. Chen J. (2018). Effects of high hydrostatic pressure, dense phase carbon dioxide, and thermal processing on the quality of Hami melon juice. J. Food Process Eng.41 (6), 12828. 10.1111/jfpe.12828
31
Quiroz-Gonzalez B. Rodriguez-Martinez V. Welti-Chanes J. Garcia-Mateos M. D. Corrales-Garcia J. Ybarra-Moncada M. C. et al (2020). Refrigerated storage of high hydrostatic pressure (HHP) treated pitaya (Stenocereus pruinosus) juice. Rev. Mex. Ing. Quim.19 (1), 387–399. 10.24275/rmiq/Alim588
32
Rinaldi M. Littardi P. Ganino T. Aldini A. Rodolfi M. Barbanti D. et al (2020). Comparison of physical, microstructural, antioxidant and enzymatic properties of pineapple cubes treated with conventional heating, ohmic heating and high-pressure processing. Lwt--Food Sci. Technol.134, 110207. 10.1016/j.lwt.2020.110207
33
Rouse A. H. Atkins C. D. Moore E. L. (1965). Seasonal changes occurring in pectinesterase activity and pectic constituents of component parts of citrus fruits. 3. silver cluster grapefruit. Food Technol.19 (4), 673.
34
Shen G. Chen A. Chen S. Zhang Z. (2016). Inactivation and kinetics analysis of microorganisms in pickled radish processed by high hydrostatic pressure. Food Sci.37 (5), 67–71.
35
Soto-Caballero M. C. Cano-Monge E. E. Cano-Monge S. M. Welti-Chanes J. Escobedo-Avellaneda Z. (2021). Effect of high hydrostatic pressures on microorganisms, total phenolic content and enzyme activity of mamey (Pouteria sapota) nectar. J. Food Sci. Technol.59, 2599–2604. 10.1007/s13197-021-05278-z
36
Sun L. C. Sridhar K. Tsai P. J. Chou C. S. (2019). Effect of traditional thermal and high-pressure processing (HPP) methods on the color stability and antioxidant capacities of Djulis (Chenopodium formosanum Koidz.). LWT--Food Sci. Technol.109, 342–349. 10.1016/j.lwt.2019.04.049
37
Szczepanska J. Skapska S. Marszalek K. (2021). Continuous high-pressure cooling-assisted homogenization process for stabilization of apple juice. Food Bioproc. Tech.14 (6), 1101–1117. 10.1007/s11947-021-02611-4
38
Wang X. H. Chen F. Ma L. J. Liao X. J. Hu X. S. (2022). Non-volatile and volatile metabolic profiling of tomato juice processed by high-hydrostatic-pressure and high-temperature short-time. Food Chem.371, 131161. 10.1016/j.foodchem.2021.131161
39
Wibowo S. Essel E. A. De Man S. Bernaert N. Van Droogenbroeck B. Grauwet T. et al (2019). Comparing the impact of high pressure, pulsed electric field and thermal pasteurization on quality attributes of cloudy apple juice using targeted and untargeted analyses. Innov. Food Sci. Emerg. Technol.54, 64–77. 10.1016/j.ifset.2019.03.004
40
Xia J. G. Psychogios N. Young N. Wishart D. S. (2009). MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res.37, 652–660. 10.1093/nar/gkp356
41
Xu Z. Wu J. Zhang Y. Hu X. Liao X. Wang Z. et al (2010). Extraction of anthocyanins from red cabbage using high pressure CO2. Bioresour. Technol.101 (18), 7162–7168. 10.1016/j.biortech.2010.04.004
42
Yucui W. Fan Z. Yi F. Dequan W. Hong X. (2019). Efficiency and kinetic analysis of microbial inactivation in soft packaged litchi processed by high hydrostatic pressure combined with lemon juice as a hurdle technology. Food Sci.40 (23), 117–122. 10.7506/spkx1002-6630-20181109-090
43
Zhang F. Chai J. Zhao L. Wang Y. Liao X. (2021). The impact of N2-assisted high-pressure processing on the microorganisms and quality indices of fresh-cut bell peppers. Foods10 (3), 508. 10.3390/foods10030508
44
Zou H. Lin T. Bi X. Zhao L. Wang Y. Liao X. et al (2016). Comparison of high hydrostatic pressure, high-pressure carbon dioxide and high-temperature short-time processing on quality of mulberry juice. Food Bioproc. Tech.9 (2), 217–231. 10.1007/s11947-015-1606-9
Summary
Keywords
high hydrostatic pressure (HHP), thermal processing (TP), quality, anthocyanin, puree
Citation
Yang X, Ding H, Luo S, Sun X, Wang N and Wang Y (2022) Comparison of high hydrostatic pressure and thermal processing on microorganisms and quality of anthocyanin-rich fruit puree. Front. Food. Sci. Technol. 2:911283. doi: 10.3389/frfst.2022.911283
Received
02 April 2022
Accepted
29 June 2022
Published
22 July 2022
Volume
2 - 2022
Edited by
Mariana Morales-de la Peña, Monterrey Institute of Technology and Higher Education (ITESM), Mexico
Reviewed by
Oluwafemi James Caleb, Stellenbosch University, South Africa
Alifdalino Sulaiman, Putra Malaysia University, Malaysia
Updates
Copyright
© 2022 Yang, Ding, Luo, Sun, Wang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongtao Wang, wangyongtao102@cau.edu.cn
This article was submitted to Food Process Design and Engineering, a section of the journal Frontiers in Food Science and Technology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.