- 1College of Animal Science and Technology, Inner Mongolia Minzu University, Tongliao, China
- 2College of Agriculture, Ningxia University, Yinchuan, China
In the present study, we investigated the effects of cellulase and Lactiplantibacillus plantarum (L. plantarum) on the fermentation quality, microbial diversity, gene function prediction, and in vitro rumen fermentation parameters of the Caragana korshinskii (C. korshinskii) silage. The experimental treatment groups included control (CK), cellulase (C), L. plantarum (L), and cellulase + L. plantarum (CL). Triplicate silos were sampled after 14 and 56 days of ensiling. The microbial diversity of C. korshinskii silage at 56 days was analyzed using Illumina MiSeq sequencing technology, and the effects of C. korshinskii silage on rumen fermentation were evaluated by the in vitro artificial rumen gas production method. The results showed that the addition of cellulose and L. plantarum treatments reduced ammonia-nitrogen (NH3-N), dry matter (DM), neutral detergent fiber (NDF), and acid detergent fiber content during ensiling. Compared with the CK group, higher lactic acid content was observed in the silage of the C and L groups, but the CL group had a higher acetic acid (AA) content. Compared with the CK group, the relative abundance of Lactiplantibacillus significantly increased, but that of Enterococcus, Weissella, Enterobacter, and Pediococcus significantly decreased in all other groups except the C group (p < 0.05). The results of gene function prediction were as follows: pyruvate kinase, 1-phosphofructokinase, and fructokinase were lactate production enzymes, which suggested the association of the high AA content in the CL group to the high abundance of 6-phosphate glucose dehydrogenase. The abundance of H + -transporting ATPase and ATP-binding cassette (ABC) transporters in the L and CL groups was higher than that in the CK groups. Metabolism of terpenoids and polyketides, mismatch repair, DNA replication, nucleotide excision repair, and homologous recombination increased in the CL group compared with those in the CK group. Compared with the CK group, NH3-N and microbial protein content and the degradation rates of DM and NDF increased in the L and CL groups. To conclude, the L and CL groups had increased Lactiplantibacillus abundance, improved fermentation quality, and high DM degradation rates compared with the CK group.
Introduction
Recently, the high feeding cost because of the shortage of forage resources and unbalanced seasonal supply is an important factor that restricts the rapid development of ruminant animal farming in China. Therefore, the development of non-conventional forage resources is crucial to solving this problem. Caragana korshinskii (C. korshinskii) is a perennial deciduous shrub with luxuriant branches, developed root systems, rapid growth, high coverage rate, and nitrogen fixation ability (Tian and Mao, 2007; Ren et al., 2015). The artificial plantation areas of C. korshinskii have been growing continuously in the deserted areas of northern China. More than 10,000 hm2 of artificial C. korshinskii have increased every year. Statistically, the total area of C. korshinskii plantations in Inner Mongolia is >4 million hm2, and the annual biological production is estimated to be >6 million tons based on the annual dry weight of 1.5 t/hm2 (Zhang et al., 2010a), which provides sufficient resources for the development of the C. korshinskii feed. However, with the extension of maturity, the degree of lignification increases and the palatability decreases, making it difficult for animals to digest the feed. Therefore, direct feeding often loses its feeding value.
Silage is a common means to improve the quality of roughage. It is beneficial to improve the palatability and nutritional quality of C. korshinskii. Silage additives are widely used, among which microbial inoculation and cell wall degrading enzymes are used to reduce dry matter (DM) losses and nutrient losses during the fermentation process. The dominant bacteria in silage are lactic acid bacteria (LAB), which have competitive or synergistic effects on the other microorganisms (Bai et al., 2021; Wu et al., 2022). The type and numbers of LAB have different effects on the fermentation quality of silage. Lactiplantibacillus plantarum (L. plantarum) is one of the most commonly used LAB in silage. L. plantarum converts soluble carbohydrates in silage into organic acids, reduces the pH value, alters the microbial community, inhibits the growth of harmful bacteria, and prolongs the storage time during ensiling (Wang Q. et al., 2022). Li et al. (2022a) demonstrated that the addition of LAB to natural grass improves the fermentation quality of silage by changing the structure of the bacterial community. Zi et al. (2021) found that the addition of L. plantarum significantly reduces the pH, acetic acid (AA), propionic acid (PA), and ammonia nitrogen content of king grass silage and significantly increases the content of lactic acid (LA). Additionally, cellulase, as a highly efficient enzyme additive, can degrade cellulose in silage into fermented and absorbable sugar, resulting in degraded fiber components and increased silage fermentability. Moreover, cellulase can promote LA production by fermentation in LAB, greatly reducing the pH value, improving the fermentation efficiency, and increasing the nutritional value during fermentation (Muck et al., 2018). Hu et al. (2020) showed that the addition of cellulase improved the fermentation quality and increased the beneficial bacterial (Lactobacillus) abundance in the alfalfa silage. Therefore, the addition of cellulase and L. plantarum can have a positive effect on improving the silage quality.
The application of additives (LAB and cellulase) improved the nutritional value of the silage and the rumen digestion of the ruminants. Interestingly, rumen microorganisms degraded feed substrates to produce large amounts of methane (CH4). CH4 emissions not only lead to global warming but also cause a huge waste of feed energy. The ruminal CH4 production can be reduced by improving the nutrient characteristics of the rumen fermentation substrate and regulating the rumen microbes. Some studies have shown that the palm leaves and alfalfa silage treated with cellulase and L. plantarum reduce ruminal CH4 emissions, improving the degradation rate of ruminal DM and fibers (Zhang et al., 2022a; Kholif et al., 2022). Additionally, Chen et al. (2022) showed that the alfalfa silage treated with L. plantarum could also inhibit the production of ruminal CH4, which is beneficial to improve the rumen energy distribution and reduce feed consumption.
Therefore, in this study, we aimed to investigate the effects of cellulase and L. plantarum on the fermentation quality, microbial diversity, gene prediction functional characteristics of 16S rRNA, in vitro rumen fermentation parameters, and ruminal greenhouse gas emissions from fermenting C. korshinskii silage. These findings provided insights into the bacterial community and the in vitro rumen fermentation process in the C. korshinskii silage.
Materials and methods
Silage preparation
The sampling site was located in the experimental field at China’s Inner Mongolia Minzu University. The C. korshinskii samples used in this study were collected on 12 July 2021. The C. korshinskii samples were wilted to 412.97 g/kg of DM under indoor air ventilation for 4 h and then chopped to 10–20 mm lengths with a crop cutter. The nutrient content was determined after thoroughly mixing the stems and leaves (Table 1). Cellulase (Longkete Ltd., Shandong, China) was obtained from Trichoderma viride with endo-β-1,4-glucanases activity ≥15000 IU/g. L. plantarum (No. 6026) was isolated from the Leymus chinensis silage. The experimental treatment groups included control (CK), cellulase (C), L. plantarum (L), and cellulase + L. plantarum (CL). 20 mg/kg of cellulase was added in the C group (fresh weight basis), the inoculation amount of L. plantarum in the L group was 1 × 106 cfu/g (fresh sample basis), and the CL group was added with 20 mg/kg cellulase + 1 × 106 cfu/g L. plantarum (fresh sample basis). An equal amount of distilled water was added to the CK group. The final mixes from each group (300 g) were packed tightly in the plastic pouches (BN-10, 250 × 350 mm; Wangnuo, Beijing, China) using a commercial vacuum sealer (ZK-320; Ouxin, Beijing, China). Triplicate silages were prepared for each treatment, and silos were stored at room temperature (25°C–27°C) for 14 and 56 days, respectively.
Fermentation products and chemical composition analysis
For determining the pH, organic acid, and NH3-N content, each sample (20 g) was homogenized with sterilized water (180 ml) in a blender for 1 min, followed by filtration using a membrane (0.22 µm). A glass electrode pH meter (SX-620, Sanxin, Shanghai, China) was used to measure the pH of this homogenized mix immediately. A total of 2 ml of filtrate was purified by centrifugation at 12000× g at 4°C for 15 min to determine the organic acid and NH3-N content, and the contents of LA, AA, and PA were determined through high-performance liquid chromatography (Shodex RS Pak KC-811, Showa Denko K.K., Kawasaki, Japan; detector: RID10A, Shimadzu Co., Ltd, Kyoto, Japan; eluent: 0.1% phosphoric acid, 1.0 ml min−1; temperature: 40°C). The NH3-N content was determined using the Broderick and Kang (1980) method.
The fresh material was continuously dried in a drying oven at 65°C for 48 h to constant weight, and DM content was determined. The dried samples were ground using a Wiley mill of a 1-mm sieve (ZM200, Retsch GmbH) to determine the chemical composition. The crude protein (CP) content was determined using the standard Association of Official Analytical Chemists (AOAC, 1990) procedures. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) content were quantified using the method described by Van Soest et al. (1991), and the water-soluble carbohydrate (WSC) content was determined by the phenol-sulfuric acid assay (Wu and Nishino, 2016).
In vitro batch culture
The treated silage samples were oven-dried at 65°C and ground using a Wiley mill of a 1-mm sieve. After weighing, 220 mg of dried silages were added into 100 ml glass bottles with butyl rubber stoppers and Hungate’s screw caps. Five bottles per silage sample and 20 bottles for each treated silage were tested. A volume of 20 ml of buffer solution (pH 6.85, Menke and Steingass, 1988) and 10 ml of filtered rumen fluid collected from three rumen-fistulated beef cattle (Simmental) were added to the bottles 1 h before the morning feeding. The bottles were purged by adding N2 for 5 s and sealed with the butyl rubber stopper and Hungate’s screw caps. All bottles were incubated at 39°C for 0, 2, 4, 8, 12, 24, 36, 48, 60, and 72 h, and the experiment was performed in triplicate. After incubating for 72 h, the fermentation broth was collected to determine the pH and NH3-N and microbial protein (MCP) content. The pH and the NH3-N content of the fermentation broth were determined according to the aforementioned method. MCP levels were determined by Makkar et al. (1982) method. DM degradation rate (DMD), NDF degradation rate (NDFD), and ADF degradation rate (ADFD) were measured using the method described by Zhang et al. (2017). Sampling bags (100 ml) made of aluminum foil were used to continuously collect the gas from each glass bottle. The gas compositions were measured using gas chromatography (Thermo, Trace 1300) fitted with a capillary column (Agilent, HP-INNOWAX, 30 m × 0.25 mm × 0.25 µm). N2 was used as the carrier gas, and the flow rate was set at 1 ml/min.
Microbial community analysis
The refrigerated silage (10 g) was blended for 2 h at 120 rpm with sterile phosphate-buffered saline (40 ml; pH 7.4) using an electronic oscillator. The samples were then filtered using double-gauze masks. The filtrate was centrifuged for 10 min at 4°C and 13,000 × g. The supernatant was removed, and the pellet was kept on dry ice. The metagenomic sequencing, including DNA extraction and polymerase chain reaction amplification, followed by Illumina MiSeq sequencing and final sequencing data processing, was performed at Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The UPARSE version 7.1 was used to cluster the operational taxonomic units (OTUs) at the 97% similarity threshold (Edgar, 2013). Following the identification and elimination of the chimeric sequences, the Ribosomal Database Project Classifier (version 2.2) was used for the taxonomic analysis of the typical OTU sequences against a 16S rRNA database, such as Silva v138, with a confidence level of 0.7 (Yang et al., 2019). The metabolic potential of the bacterial community and their composition of functional genes were postulated by assigning the 16S rRNA marker gene sequences to the functional annotations of the sequenced metagenomes based on the Kyoto encyclopedia of genes and genomes (KEGG) on the first, second, and third pathway levels, using Tax4Fun (version 0.3.1), as described by Aßhauer et al. (2015). The sequencing data generated in this study have been deposited in the National Center for Biotechnology Information sequence read archive database under the accession number PRJNA909496.
Statistical analysis
The data were analyzed statistically using the John’s Macintosh Project version 13 software (SAS Institute, Japan) and two-way analysis of variance (ANOVA), with additive and storage time as primary factors. To evaluate the treatment effects, the one-way ANOVA and subsequent Tukey’s test-based multiple comparisons were performed. The data of total gas production, CO2 production, CH4 production, and in vitro fermentation were used to perform one-way ANOVA at p < 0.05.
Results
Chemical composition and fermentation products of C. korshinskii silage after 14 and 56 days of ensiling
The effects of additives and ensiling time on the chemical composition of C. korshinskii silage are shown in Table 2. Compared with the CK group, DM content significantly decreased in the C and CL groups after 56 days of ensiling (p < 0.05). After 14 and 56 days of ensiling, the CP content of the L and CL groups increased significantly (p < 0.05) more than that of the CK group. After 14 days of ensiling, compared with the CK group, the NDF and ADF contents in the C and L groups did not differ significantly (p > 0.05), whereas that in the CL group decreased significantly (p < 0.05). After 56 days of ensiling, the NDF and ADF contents in the L and CL groups significantly decreased compared with those in the CK group (p < 0.05). The WSC content was the highest in the CL group after 56 days of ensiling.
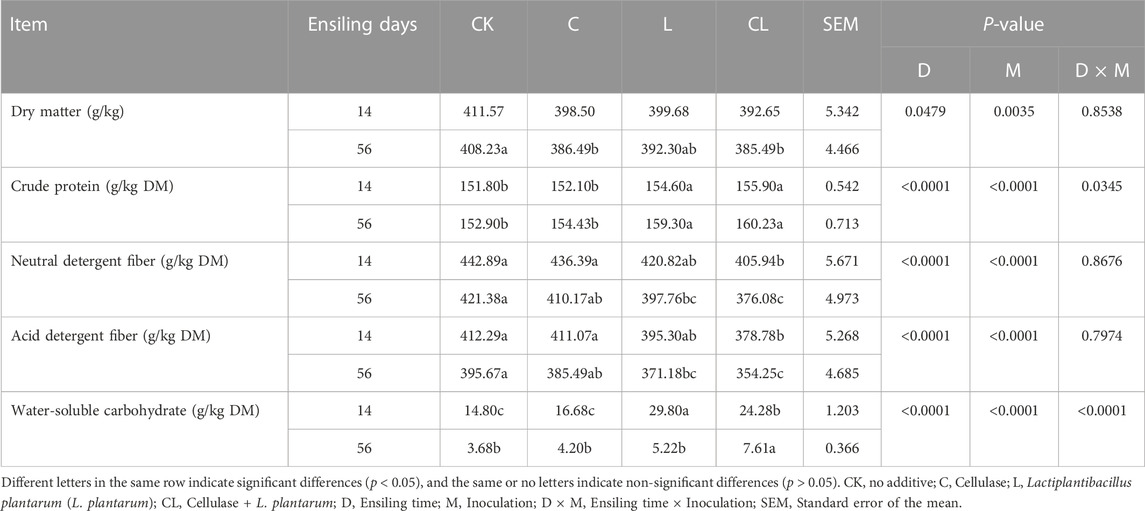
TABLE 2. Effect of cellulase and Lactiplantibacillus plantarum on the chemical composition of Caragana korshinskii silage.
The effects of additives and ensiling time on the fermentation products of C. korshinskii silage are shown in Table 3. The pH and NH3-N content decreased significantly during ensiling (p < 0.05). After 14 days, compared with the CK group, the LA content increased significantly in all the other groups (p < 0.05), and after 56 days, the LA content of the C and L groups significantly increased (p < 0.05), whereas it decreased significantly in the CL group (p < 0.05). Interestingly, the AA content was highest in the CL group after 56 days of ensiling.
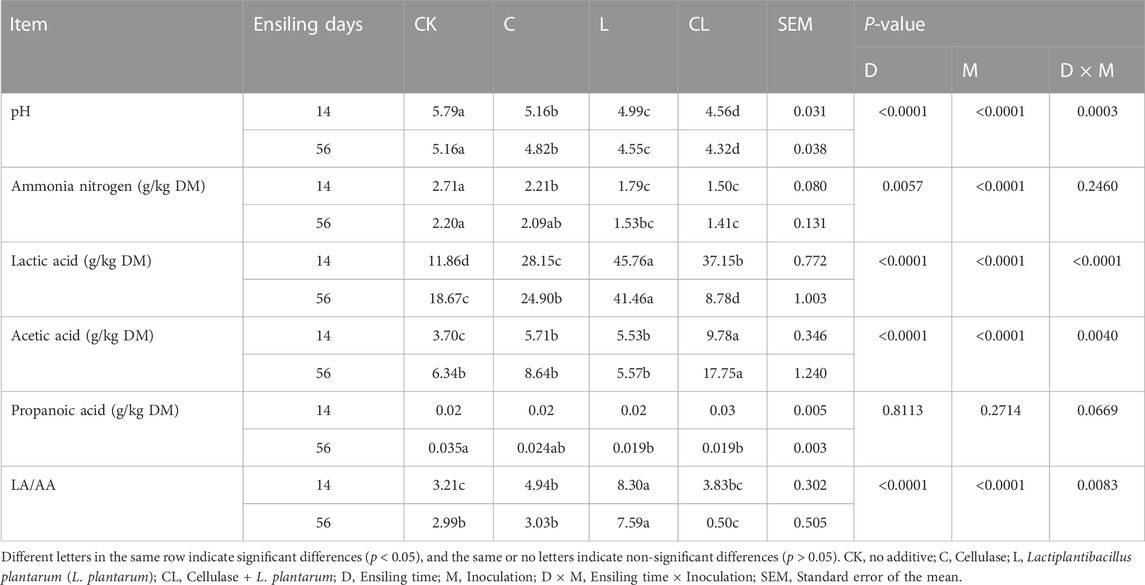
TABLE 3. Effect of cellulase and Lactiplantibacillus plantarum on the fermentation products of Caragana korshinskii silage.
Effects of cellulase and L. plantarum on the microbial diversity in C. korshinskii silage
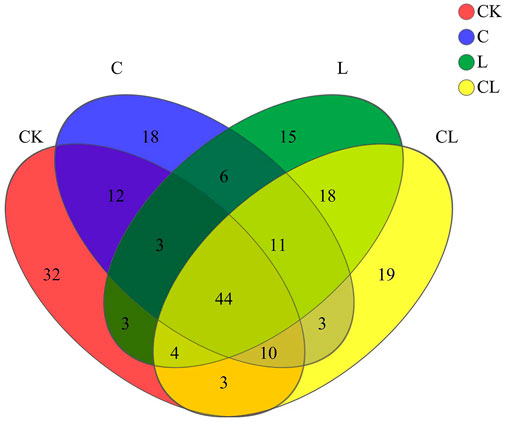
High-throughput sequencing analysis of the V3 and V4 regions of the 16S rRNA gene for 12 samples generated 639,131 valid sequences. According to Figure 1, based on the number of OTUs, the descending order of the four groups was CL > CK > C > L. The core OTUs was 44, and the number of unique OTUs in the L, C, CL, and CK groups were 15, 18, 19, and 32, respectively.
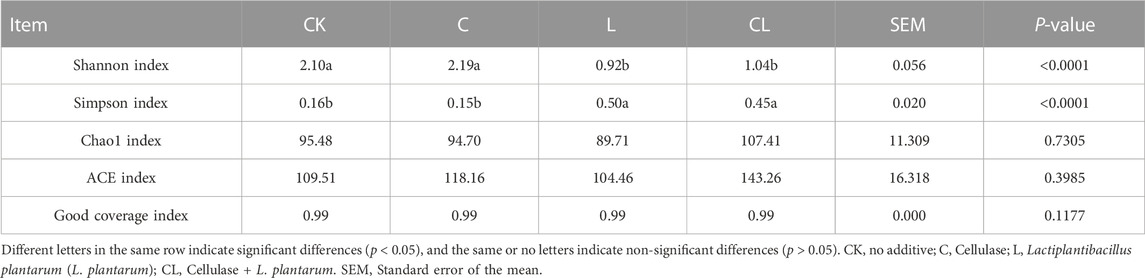
In Table 4, all samples had coverage values of around 0.99, indicating that the sequencing results accurately reflected the characteristics of the microbial community. The Shannon index in the L and CL groups decreased compared with those in the CK group, whereas the Simpson index significantly increased (p < 0.05). The Chao and ACE indexes had no significant difference in all the groups (p > 0.05).
Principal Component Analysis (PCA) showed the variability in the bacterial community composition among different treatment groups (Figure 2). PC1 and PC2 accounted for 24.38% and 14.55% of the total variation, respectively. Microbial communities clustered significantly in the CK, C, L, and CL groups but more closely in the L and CL groups compared with the CK group (based on the Bray-Curtis distance ANOSIM, R = 0.69, p = 0.003). Thus, cellulase and L. plantarum affected the β-diversities and the community structure of C. korshinskii silage.
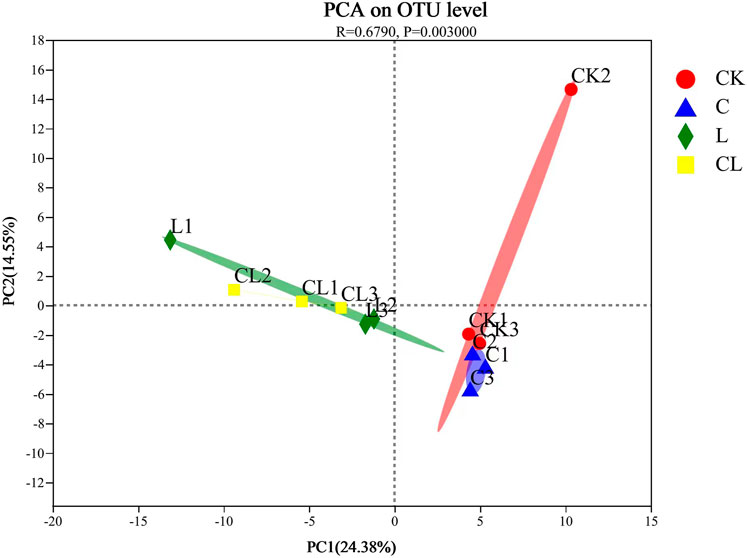
FIGURE 2. Principal coordinate anaysis (PCoA) of microbial community in Caragana korshinskii silage with different treatements.
Figure 3 shows the microbial communities in C. korshinskii silage at the phylum, family, and genus levels. Firmicutes and Proteobacteria were the dominant phyla in C. korshinskii silage (Figure 3A). The CK and C groups contained mainly Firmicutes (75.20% and 76.74%, respectively) and Proteobacteria (24.69% and 23.17%, respectively). Firmicutes in the L and CL groups accounted for more than 97% of all detected sequences. The dominant family in C. korshinskii silage was Lactobacillaceae (Figure 3B). The CK and C groups mainly consisted of Lactobacillaceae (51.17% and 53.72%, respectively), Enterobacteriaceae (24.52% and 23.05%, respectively), Enterococcaceae (21.62% and 17.81%, respectively), and Leuconostocaceae (1.85% and 2.86%, respectively). Lactobacillaceae abundance in the L and CL groups was 98.25% and 97.27%, respectively. At the genus level, all the groups of C. korshinskii silage were dominated by Lactiplantibacillus (Figure 3C). The CK and C groups mainly consisted of Lactiplantibacillus (40.75% and 40.76%, respectively), Enterococcus (21.51% and 17.84%, respectively), Pediococcus (10.44% and 12.90%, respectively), Weissella (1.85% and 2.80%, respectively), and Enterobacter (23.87% and 22.11%, respectively). The L and CL groups were mainly dominated by Lactiplantibacillus (98.10% and 97.19%, respectively). The LEfSe multilevel species difference analysis (LDA = 4) of the microbial community in C. korshinskii silage revealed that two phyla, two orders, two classes, seven families, and eight genera differed significantly in abundance among the groups (Figure 4).
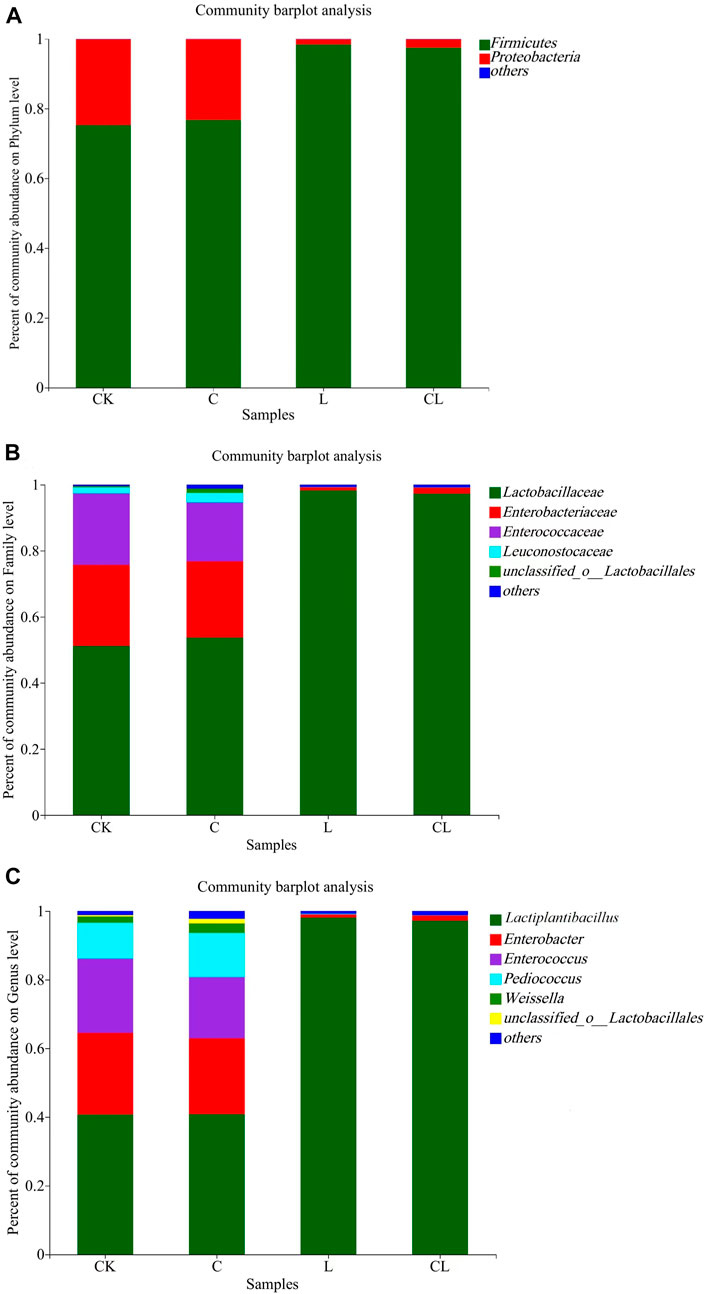
FIGURE 3. (A) Phylum, (B) Family, and (C) Genus-level microbial communities in Caragana korshinskii silage; CK, no additive; C, cellulase; L, Lactiplantibacillus plantarum (L. plantarum); and CL, cellulase + L. plantarum.
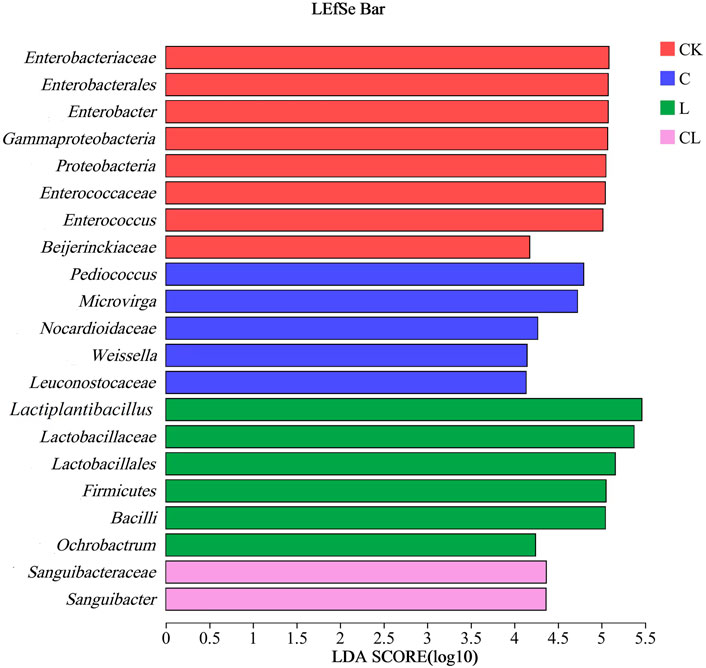
FIGURE 4. Discriminant analysis of LEfSe multilevel difference in Caragana korshinskii silage; CK, no additive; C, cellulase; L, Lactiplantibacillus plantarum (L. plantarum); and CL, cellulase + L. plantarum.
KEGG metabolic pathway and key enzyme activities of the microbial communities in C. korshinskii silage
Figure 5 illustrates the functional mapping of 16S rRNA gene predictions for the first (Figure 5A) and second (Figure 5B) pathway levels and carbohydrate (Figure 5C) and amino acid (Figure 5D) metabolisms. After 56 days of ensiling, metabolism and environmental and genetic information processing in the KEGG metabolic pathways were significantly higher than other metabolic pathways (Figure 5A). The metabolic pathways of membrane transport and the metabolisms of carbohydrates, nucleotides, and amino acids were much higher than the other metabolic pathways (Figure 5B). Carbohydrate metabolic pathways were specifically analyzed at the third pathway level. Tricarboxylic acid cycle (TCA cycle) and pyruvate, glyoxylate, and dicarboxylate metabolism in the CL group were significantly higher than those in the CK group (Figure 5C). The amino acid metabolic pathways were specifically analyzed at the third pathway level, as shown in Figure 5D. The alanine, aspartate, and glutamate metabolisms, lysine biosynthesis and degradation, tyrosine metabolism, and tryptophan metabolism in the L and CL groups were upregulated compared with those in the CK group.
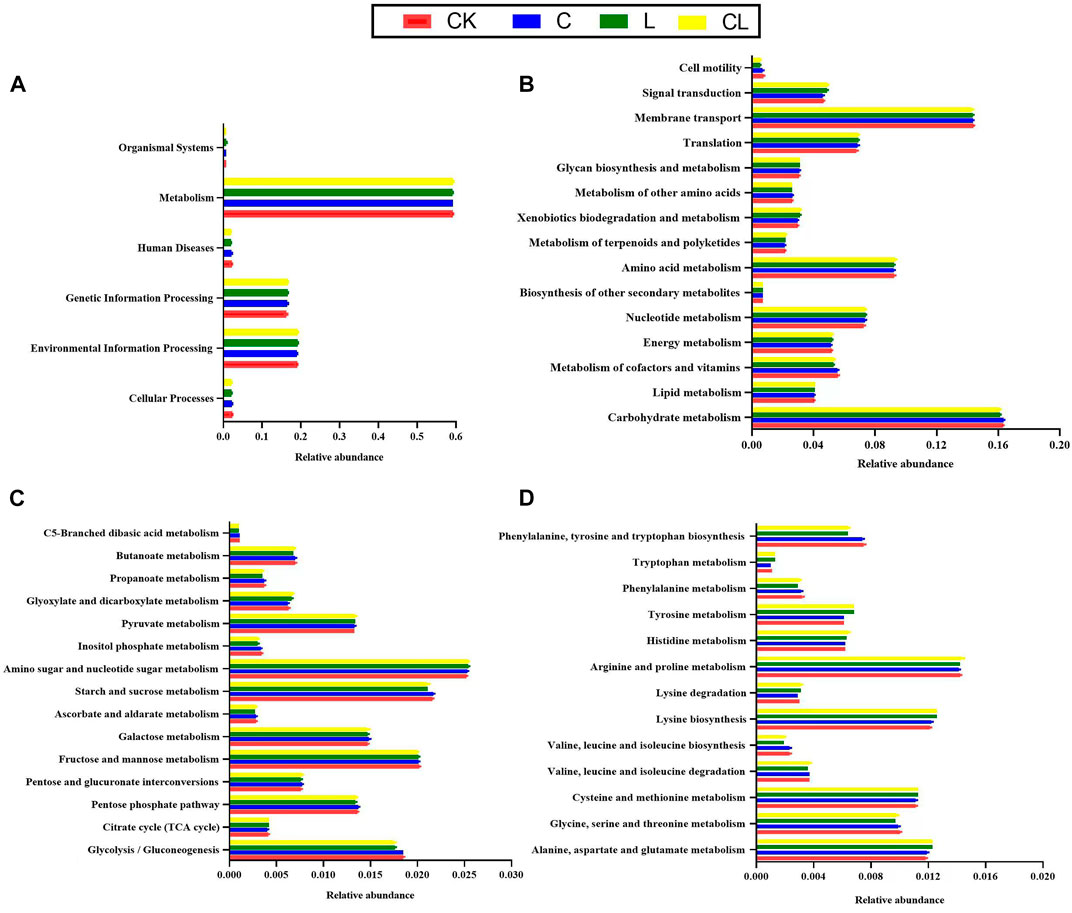
FIGURE 5. Predicted functional profiles of the 16S rRNA genes of the Kyoto encyclopedia of genes and genomes metabolic pathway in the first (A) and second (B) metabolic pathways, carbohydrate metabolism (C), and amino acid metabolism (D) in different groups as determined by Tax4Fun; CK, no additive; C, cellulase; L, Lactiplantibacillus plantarum (L. plantarum); and CL, cellulase + L. plantarum.
Some metabolic pathways with significant differences at the second level were further analyzed at the third metabolic pathway level, as shown in Figure 6. The degradations of geraniol, limonene, and pinene, biosynthesis of the siderophore group, non-ribosomal peptides, non-ribosomal peptide structures, ABC transporters, and two-component system were upregulated in the L and CL groups compared with those in the CK group (p < 0.05), and the biosynthesis of ansamycins and bacterial secretion systems in the L and CL groups were downregulated compared with those in the CK group (p < 0.05). Figure 7 illustrates that the relative abundance of H+-transporting ATPase was significantly increased in the L and CL groups compared with those in the CK and C groups, whereas the relative abundances of 1-phosphofructokinase, fructokinase, and pyruvate kinase decreased significantly (p < 0.05).
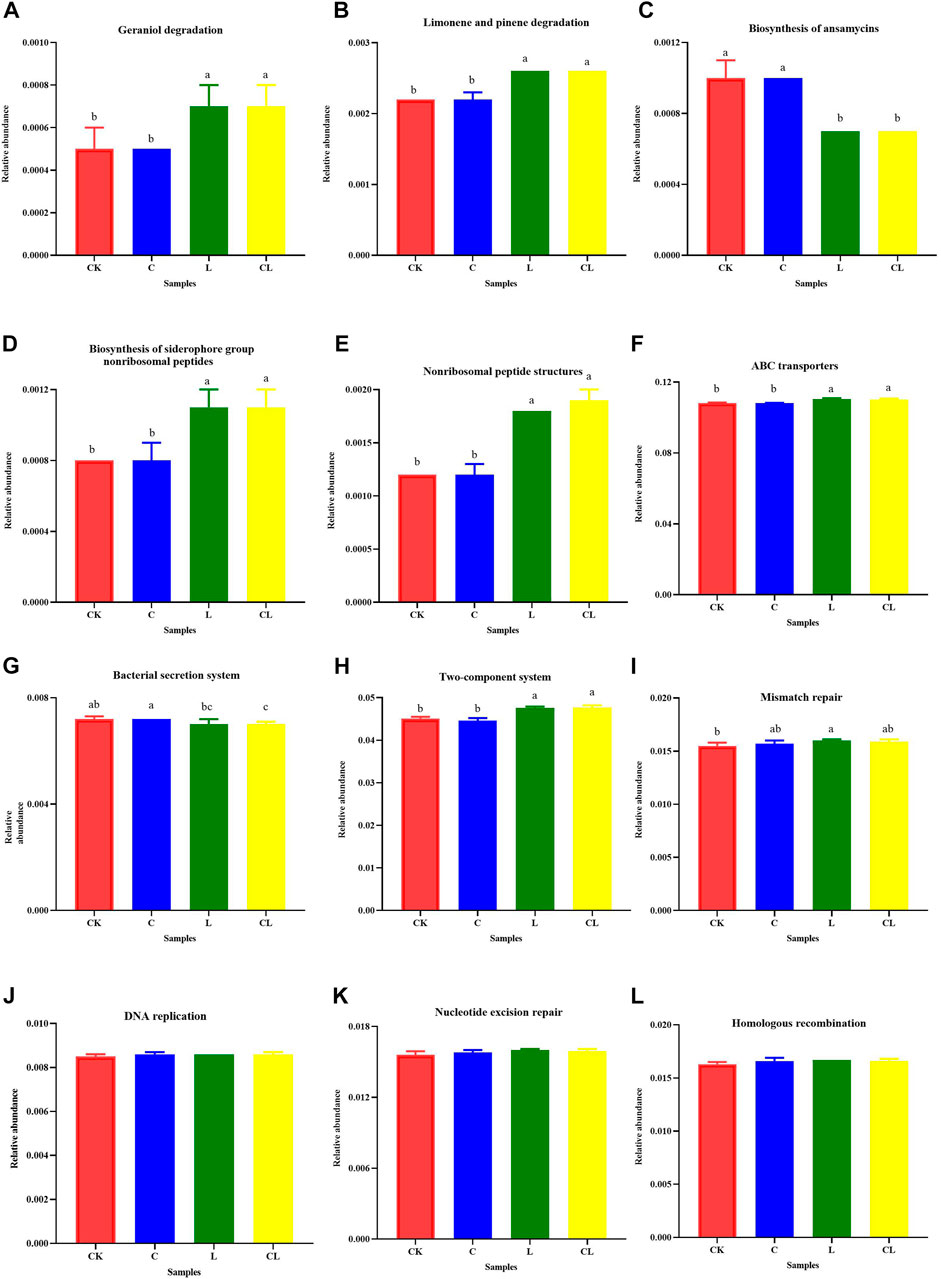
FIGURE 6. Effects of some Kyoto encyclopedia of genes and genomes metabolic pathways obtained with Tax4Fun in different groups on the metabolism of terpenoids and polyketides (A–E), membrane transport (F, G), signal transduction (H), and replication and repair (I–L). Different letters in the same column indicate significant differences (p < 0.05). CK, no additive; C, cellulase; L, Lactiplantibacillus plantarum (L. plantarum); and CL, cellulase + L. plantarum.
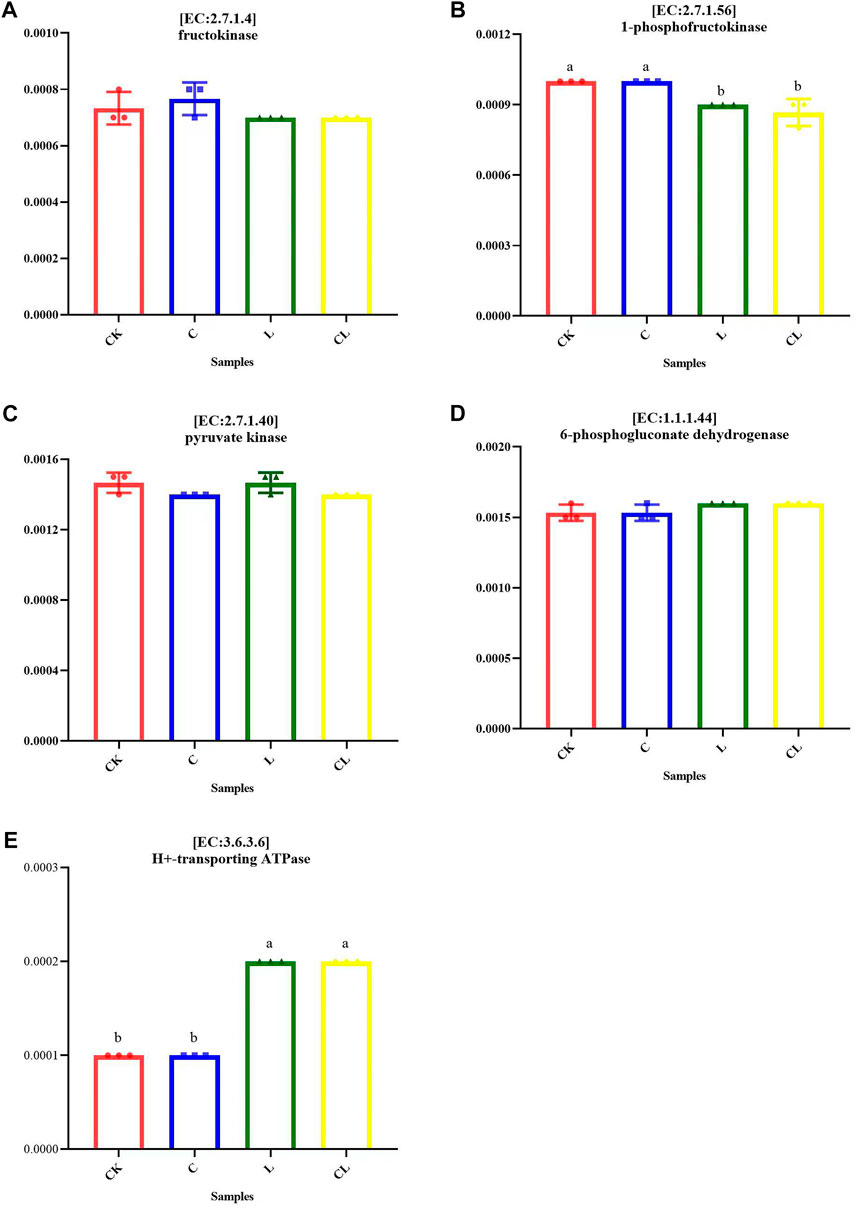
FIGURE 7. Abundance changes of key enzymes (A–E) involved in some metabolic pathways in Caragana korshinskii silage. Different letters in the same column indicate a significant difference (p < 0.05). CK, no additive; C, cellulase; L, Lactiplantibacillus plantarum (L. plantarum); and CL, cellulase + L. plantarum.
Gas production and in vitro rumen fermentation of C. korshinskii silage
The in vitro gas production of C. korshinskii silage after 56 days of ensiling is shown in Table 5. After 72 h of in vitro rumen fermentation, the gas production of each group was stable. Compared with the CK group, the in vitro gas production in the L and CL groups increased to different degrees, but the cumulative gas production was the highest in the CL group, and the gas production in the CL group was also significantly higher than that in the other groups in the first 2–12 h and 48 h (p < 0.05). The gas production rate in the CL group increased significantly compared with the other groups (p < 0.05). The theoretical maximum of the in vitro fermentation gas production from fermenting C. korshinskii silage predicted by the model was similar to the actual gas production. According to Table 6, the differences in the yields of CH4 and CO2 among different treatment groups were not significant (p > 0.05), but the yields of CH4 and CO2 in the C group showed a decreasing trend compared with the other groups. Compared with the CK group, the NH3-N and MCP contents were significantly increased in the L and CL groups (p < 0.05).
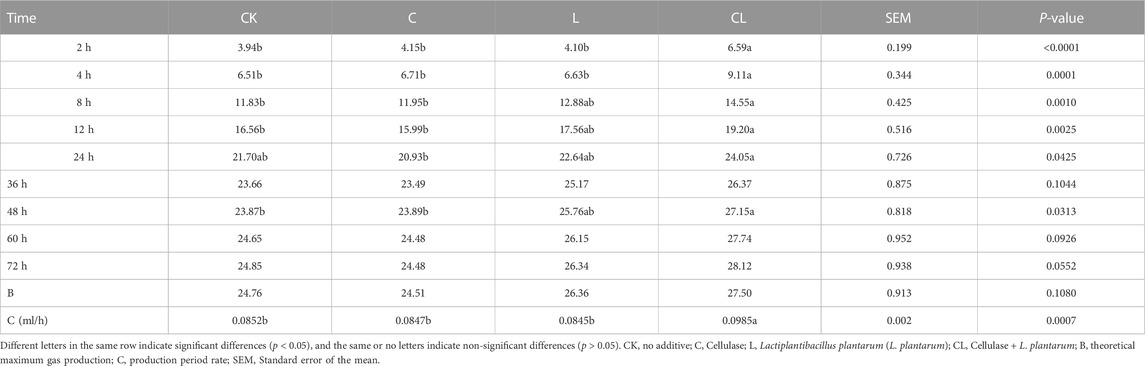
TABLE 5. Cumulative gas production from Caragana korshinskii silage fermented in vitro for 72 h (ml/220 mg DM).
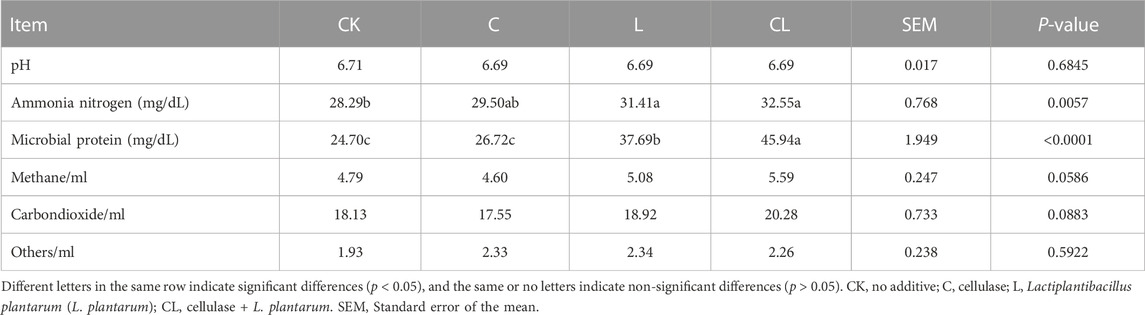
TABLE 6. In vitro fermentation parameters and ruminal gas production from Caragana korshinskii silage fermented in vitro for 72 h.
Figure 8 the DMD, NDFD, and ADFD in the C. korshinskii silage after 56 days of ensiling. After 72 h of in vitro fermentation, DMD in the CL group was significantly higher than that in the other groups (p < 0.05). NDFD was significantly increased in the L and CL groups compared with the CK group (p < 0.05).
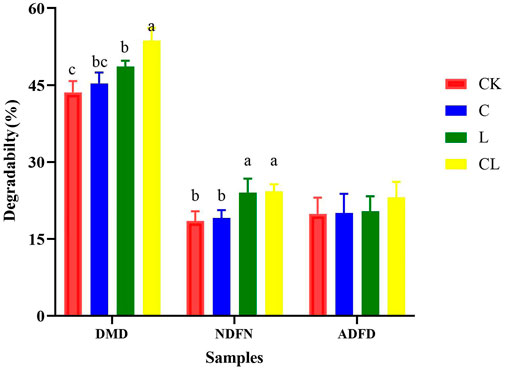
FIGURE 8. The nutrient degradation rate in Caragana korshinskii silage after 72 h in vitro rumen fermentation.
Discussion
Chemical composition and fermentation production of C. korshinskii silage
The nutrient compositions of C. korshinskii are affected by many factors, such as plant cultivation conditions, climatic conditions, soil fertility, growth period, and harvesting period. Furthermore, the nutrient composition of C. korshinskii is a major factor in determining the fermentation quality of silage, especially the DM content (Xu et al., 2020). In this study, we observed that the DM content of each group decreased with the extension of ensiling. During the ensiling process, the nutrient contents are continuously consumed because of the respiration of plant cells and active aerobic microorganisms, which produces water, carbon dioxide, and free ammonia, thereby decreasing the DM content in C. korshinskii silage (Borreani et al., 2018). Generally, the high contents of NDF and ADF in silage decrease the intake and digestibility of ruminants (Grant and Ferraretto, 2018). During the entire ensiling period, the NDF and ADF contents of C. korshinskii silage in the C group were slightly different from those in the CK group. It is not easily degraded because C. korshinskii belongs to shrub cellulose, which forms a compact structure with lignin and other substances. Li et al. (2017) demonstrated that adding cellulase and L. plantarum can significantly decrease NDF and ADF contents in stylo silage, which is consistent with the results of this study. WSC is the main energy source for the growth of LAB (Guan et al., 2018). In this study, the WSC content was higher in untreated silage than in fresh material, which was consistent with the results reported by Dunière et al. (2013). At day 56, CL had higher WSC content compared with that in the other groups because L. plantarum degraded digestible plant cell walls (cellulose, hemicellulose, and lignin) to produce organic acids during ensiling, and cellulase increased the WSC content by degrading silage plant cell walls (Xing et al., 2009; Ogunade et al., 2018).
NH3-N in silage is mainly produced by hydrolysis of plant protease and MCP by Clostridium and Enterobacter, which indicates the decomposition degree of protein in silage (Su et al., 2019). In this study, the NH3-N concentrations were higher in the CK and C groups, which was mainly associated with the breakdown of proteins in raw materials by Enterobacter (Clostridium was not detected) (Wang Y. L. et al., 2022). Grabber et al. (1995) demonstrated that the addition of LAB prevented the degradation of CP in silage. The CP content in the L and CL group was higher than that in the other two groups, whereas the NH3-N concentration was lower, indicating that the addition of L. plantarum inhibits the CP degradation in C. korshinskii silage. Furthermore, a rapid decrease in pH inhibited protein hydrolysis caused by various plant enzymes and decreased the rate of NH3-N oxidation to nitrite and nitrate (Li et al., 2020). In this study, we found that the CL had the lowest pH and NH3-N and the highest CP. These results indicated that the addition of cellulase decreased the pH and increased the LA content in silage, which was consistent with the result of Chen et al. (2017), Sun et al. (2012), but different from the results of Liu et al. (2016a), Zhang et al. (2022b). This difference can be because the enzyme activity is affected by various factors, such as the composition and source of the enzyme, raw material ingredient, the additional amount of enzyme, and the conditions of enzyme survival (Martínez et al., 2020).
pH and organic acids are crucial indicators to determine the quality of silage. Most harmful bacteria cannot survive in an acidic environment. Generally, the lower the pH (<4.2), the better quality of the silage (Liu et al., 2019). In this study, the pH was above 5.0 in CK at days 14 and 56 mainly because of the less number of LAB attached to the C. korshinskii. Although the pH of the other additive groups decreased significantly compared with CK, they were still above 4.2. Furthermore, this phenomenon was related to a higher crude protein content and pH buffering capacity in C. korshinskii. The lignification degree of C. korshinskii is high, and the degradation rate of C. korshinskii by LAB and cellulase is slow, resulting in a slow decrease in pH. An appropriate concentration of AA (30–40 g/kg DM) inhibits the growth of harmful pathogenic microorganisms, such as yeast and molds, in silage (Kung et al., 2018), which contributes to the success of silage. At day 56, the AA content in the CL and C groups was higher than that in the CK and L groups, especially in the CL group (17.75 g/kg DM), which might be because of the following two reasons: the addition of cellulase can hydrolyze hemicellulose to free pentose in silage, and pentose can be converted into D-xylose-5-phosphate, and then fermented into the mixture of LA and AA (Li et al., 2018; Li et al., 2019). Besides, when the DM is low, and the buffered energy is high, the LA produced in the silage can be converted to AA under anaerobic conditions (Pahlow et al., 2003). Therefore, the AA content is higher in the C and CL groups. LA is the ideal fermentation product, which is the major factor in decreasing the pH of silage. This study showed that the LA content in L is significantly higher than in all the other groups, which indicated that the addition of L. plantarum can promote multiple substrates to produce LA and inhibit AA; these findings are consistent with those of Liu et al. (2016b). Furthermore, the LA/AA ratio responded to the type of LAB fermentation. When LA/AA was >3.0, it was homozygous LAB fermentation, whereas when LA/AA was <3.0, it was heterozygous (Zhang et al., 2010b). The LA/AA in L ranged from 8.30 to 7.59 during the whole ensiling process, which was more than twice as many as the other groups. This indicated that L was dominated by homozygous LAB fermentation. Moreover, the fermentation type of LAB changed from homozygous to heterozygous during different stages of silage fermentation. In this study, we showed that LA/AA was higher in the silage of CL at the early stage of silage but decreased in CL at the end stage of silage. This might be associated with the intraspecific competition between L. plantarum and cellulase in the late stage of silage, leading to a shift from homozygous to heterozygous LAB fermentation in C. korshinskii silage.
Typically, the addition of L. plantarum or cellulase alone affects the quality, but the best quality of C. korshinskii silage was observed with cellulase + L. plantarum because of the synergistic effect of L. plantarum and cellulase on silage fermentation.
Microbial community of C. korshinskii silage
Silage is a community succession process involving various microorganisms. Therefore, the composition of microbial communities is crucial for understanding the complex fermentation process in silage (Lv et al., 2020). In this study, On the 56th day, CK had a high Shannon index and low Simpson index, indicating high microbial diversity. The addition of L. plantarum and cellulase + L. plantarum decreased the microbial diversity in C. korshinskii silage. It is mainly because of the rapidly lower pH in L and CL, which restrained the growth of Enterobacter, and promoted the growth of Lactiplantibacillus. When the abundance of dominant bacteria in silage was higher, the diversity of the bacterial community was lower. Similar results were shown by Jiang et al. (2020) and Wang et al. (2019) in the whole-plant corn and mulberry leaf silage. Moreover, PCA revealed a significant separation and difference between the bacterial community in CK, L, and CL, indicating that the microbial community changed in the L. plantarum and cellulase + L. plantarum treatment during the ensiling process. This difference in silage quality can be attributed to changes in the microbial community. Therefore, based on the α and β diversity analyses, it was concluded that cellulase and L. plantarum affected the microbial diversity and community structure of C. korshinskii silage.
At the phylum level, the microbial community structure difference is smaller between different silage, which were mainly Firmicutes and Proteobacteria. The majority of lactic acid bacteria belong to the Firmicutes, which secrete various lipases, cellulases, and proteases and participate in the degradation of biological macromolecules (cellulose, starch, and protein), while the Proteobacteria consist of several detrimental bacteria, such as Escherichia coli, Salmonella, and Helicobacter pylori, which decompose organic matter in the feed, leading to the degradation of silage quality (Yuan et al., 2020). In this study, the relative abundance of Firmicutes was 75.20%, 76.74%, 98.39%, and 97.44% in CK, C, L, and CL, respectively, and the relative abundance of Proteobacteria was 24.69%, 23.17%, 1.50%, and 2.40%, respectively. Zhang et al. (2022c) demonstrated that Proteobacteria (54.50%) were predominant, followed by Actinobacteria (30.06%), Bacteroidetes (9.57%), and Firmicutes (3.26%) in untreated C. korshinskii silage. These results indicated that higher Firmicutes richness was beneficial to improve the quality of silage.
At the genus level, the microbial community structure was different between different silages. Corn, sorghum, and rice were dominated by Weissella and Lactiplantibacillus, and alfalfa was dominated by Leuconostoc (Pang et al., 2011). Zhang et al. (2022c) found that the raw material of C. korshinskii contains Rhodococcus (13.73%), Sphingomonas (12.70%), Pantoea (7.09%), Hymenobacter (7.06%), Burkholderia-Caballeronia-Paraburk-holderia (2.37%), and a large number of other bacteria (56.23%). In the present study, the CK and C groups mainly consisted of Lactiplantibacillus, Enterobacter, Enterococcus, Pediococcus, and Weissella, whereas Lactiplantibacillus was predominant in the L and CL groups at an abundance of up to 98.10% and 97.19%, respectively. This suggests that the bacterial community changed significantly after ensiling. Enterobacter decreased significantly in the L and CL groups compared with the CK group. This result showed that the acid environment produced by adding L. plantarum and L. plantarum + cellulase ferment to C. korshinskii silage could effectively inhibit the growth of Enterobacter, thus improving silage quality.
Lactiplantibacillus is dominant in silage fermentation, promoting the production of organic acids, lowering the pH, and restraining the reproduction of harmful bacteria. Cheng et al. (2022) demonstrated that LA content was positively correlated with the relative abundance of Lactiplantibacillus. In the present study, Lactiplantibacillus was slightly different in the L and CL groups; however, LA content in the CL group was significantly lower than that in the L group. It indicates that adding cellulase + L. plantarum inhibits LA production. According to the LEfSe analysis, the most dominant bacteria were Enterobacter and Enterococcus in the CK group. Enterobacter is a harmful microorganism in the silage, which competes with beneficial bacteria, including LAB, for fermentation substrates, producing undesirable fermentation products such as butyric acid, succinic acid, and ammonia nitrogen, preventing LAB growth and destroying silage quality (Graf et al., 2016). Enterococcus is a parthenogenic anaerobic lactic acid bacterium that can be isolated from silage. Cai (1999) used five strains of Enterococcus as inoculants and found that it had less effect on silage quality. Enterobacter and Enterococcus were 23.87% and 21.51% in the CK group, respectively, which might be the reason for the slow pH decrease and poor silage quality. The most dominant bacteria were Pediococcus, Weissella, and Microvirga in the C group. Pediococcus and Weissella are heterofermentative LAB. They produce a mixture of LA and AA by metabolizing WSC, which plays a critical role in the early stage of ensiling; however, its activity is restrained by decreasing pH in the late stage of ensiling (Xu et al., 2021). Microvirga belongs to Proteobacteria, mostly grows in arid or semi-arid soils, and exhibits nitrogen fixation (Veyisoglu et al., 2016). However, Microvirga is rarely found in silage, and its role in silage needs further study. Furthermore, Ochrobactrum and Sanguibacter in the L and CL groups are rarely reported in silage, and their roles need further exploration.
KEGG metabolic pathway and key enzyme activities of C. korshinskii silage
KEGG is a bioinformatics resource for understanding the role of cells and organisms from a genomic perspective. In fermentation, bacteria mainly transform fermentable substrates into different metabolites via different metabolic pathways. Therefore, we assessed C. korshinskii silage metabolism pathways based on KEGG analysis using Tax4Fun. After 56 days, the abundance of cellular processes and human diseases was higher in the CK and C groups than in the L and CL groups, whereas the abundance of environmental information processing and organismal systems was significantly lower under pathway level 1. The results showed that exogenous microorganisms obtained by adding L. plantarum and L. plantarum + cellulase to C. korshinskii silage could improve the metabolic level of beneficial bacteria and the fermentation quality of C. korshinskii silage by changing cell characteristics and inhibiting membrane transport and signal transmission in undesirable bacteria. Under pathway level 2, Bai et al. (2021) demonstrated that carbohydrate, amino acid, nucleotide, cofactor, vitamin, and energy metabolisms were closely related to the metabolic pathways during ensiling. Carbohydrate metabolism mainly includes glycoisomerization and glycolysis (Kanehisa and Goto, 2000). In the present study, the relative abundance of the TCA cycle was higher in the CL group than in the CK group, indicating that LA produced was lower in the CL group. As the TCA cycle requires aerobic conditions, removed hydrogen ions cannot enter the respiratory chain for complete oxidation under anaerobic conditions (Banfalvi, 1991). Furthermore, the abundance of pyruvate, glyoxylate, and dicarboxylate metabolisms was higher in the CL group than in the CK group. Pyruvate can be directly oxidized into AA and CO2 under certain conditions, further explaining the reason behind the lower content of LA than that of AA in the CL group. Amino acids, essential substances in plants, are crucial in promoting primary metabolism and plant protein synthesis. After 56 days of ensiling, no significant changes were detected in amino acid metabolism in each treatment group under pathway level 2. Under pathway level 3, alanine, aspartate, and glutamate metabolisms, lysine biosynthesis, lysine degradation, and tyrosine and tryptophan metabolisms in the L and CL groups were significantly increased than that in the C and CK groups, indicating that these amino acid metabolisms are associated with NH3-N formation in C. korshinskii silage. Therefore, the appropriate inhibition of these amino acid metabolic pathways can be a potential measure to reduce NH3-N concentration in C. korshinskii silage.
Metabolic pathways with significant differences were analyzed under pathway level 3 to further understand the function of bacterial communities in C. korshinskii silage. These metabolic pathways mainly included the metabolism of terpenoids and polyketides, membrane transport, signal transduction, replication, and repair. After 56 days of ensiling, relative abundance of geraniol, limonene, and pinene degradation was higher in the L and CL groups than in the CK and C groups, indicating that the metabolism of terpenoids and polyketides was increased in the L and CL groups. The relative abundance of ABC transporters increased, whereas the relative abundance of the bacterial secretion system decreased in the CL groups than in the CK group. This indicated that membrane transport in bacterial communities fermented with cellulase + L. plantarum was mainly via ABC transporters, and ABC transporters were more efficient than the bacterial secretion system in the silage environment. Furthermore, the abundance of mismatch repair, DNA replication, nucleotide excision repair, and homologous recombination in the L and CL groups was slightly higher than that in the CK group, indicating that biochemical reactions involved in DNA replication and repair are mainly reflected by the rapid growth and increment of Lactiplantibacillus in the L and CL groups. The relative abundance of the two-component system was lower in the CK and C groups than in the L and CL groups, indicating that the signaling was inhibited in the CK and C group.
Key enzymes play a critical role in forming the final fermentation products of silage. In homofermentative LAB fermentation, glucose is metabolized via the Embden-Meyerhof pathway (EMP) to exclusively produce LA, whereas heterofermentative LAB fermentation via the pentose phosphate pathway (PPP) produces AA, ethanol, and CO2 in addition to LA (Abdel-Rahman et al., 2011). 1-phosphofructokinase, fructokinase, and pyruvate kinase are major enzymes involved in EMP. The relative abundance of 1-phosphate dehydrogenase, fructokinase, and pyruvate kinase in the CK group was higher than that in the CL group, indicating that enzymes involved in lactic acid fermentation were higher in the CK group than in the CL group, thereby decreasing LA content in the CL group. 6-phosphate glucose dehydrogenase was mainly involved in the PPP. In the present study, the relative abundance of 6-phosphate and 6-phosphoglucose dehydrogenase was higher in the CL group than in the CK group, indicating that heterofermentation was promoted in the CL group. Furthermore, the PPP was decreased in the CL group than in the CK group under pathway level 3. This phenomenon can be attributed to the complex variety of enzymes involved in the PPP, whereas 6-phosphoglucose dehydrogenase does not represent this pathway. The relative abundance of H+-transporting ATPase was much higher in the L and CL groups than in the CK and C groups, and the relative abundance of ABC transporters was higher in the L and CL groups, indicating their stronger membrane transport capacity.
Gas production and in vitro rumen fermentation of C. korshinskii silage
Rumen pH is one of the important indices that reflect a rumen environment, and its normal range is 5.5–7.5 (Calsamiglia et al., 2008). In the present study, rumen fermentative pH (6.69–6.71) was within this normal range and did not adversely affect the normal growth and metabolism of microorganisms. A higher NH3-N concentration increased the degradation rate of proteins in the rumen or decreased the utilization rate of NH3-N by rumen microorganisms. A lower NH3-N concentration inhibited cellulose degradation and MCP synthesis (Hristov et al., 2002). Satter and Slyter. (1974) demonstrated that the most suitable NH3-N concentration for rumen microbial growth was 20–50 mg/dL in vitro. NH3-N content was within the normal range in all groups, indicating that NH3-N concentration did not affect the growth of rumen microorganisms. In the rumen, protein or non-protein nitrogen was degraded to NH3-N, which could be activated by microorganisms with peptides and amino acids to synthesize MCP (Reynal et al., 2007). Therefore, MCP can reflect the growth rate and the number of microbial cells (Chen et al., 2020). In the present study, increased MCP content indirectly implied that adding cellulase and L. plantarum benefitted the growth of rumen microorganisms in the L and CL groups.
In vitro gas production is an important index to evaluate the fermentation degree of the feed, which can reflect the degradation characteristics of the feed for ruminants (Chen et al., 2016). Gas production amount is related to feed nutritional value, rumen microbial activity, and feed utilization. In the present study, the cumulative total gas production in the CL group increased significantly in the first 48 h compared with that in the CK group, probably because of the high quality of C. korshinskii silage in the CL group, in which WSC content was positively, and NDF content was negatively correlated with gas production (Li et al., 2022b). However, no significant difference was observed in gas production among the groups within 72 h, which may be because of the difference in the DM degradation of C. korshinskii silage treated with different additives by rumen microorganisms, which was similar to the results of Xiong et al. (2022). The theoretical maximum gas production was close to the actual gas production in each group; however, the difference in the theoretical maximum gas production between different treatment groups was not significant. However, the gas production rate was significantly higher in the CL group than in the other groups. CH4 and CO2 are greenhouse gases produced during rumen fermentation in ruminants. We found that adding LAB to whole-plant maize and sorghum silages reduced ruminal methane emissions (Khota et al., 2017; Wang Q. et al., 2022). However, Ellis et al. (2016) found no significant changes in CH4 levels when Holstein cows were fed silage treated with LAB for long and short periods, and the present study also showed the same result.
Silage digestibility is one of the key indicators that reflect silage quality (Hao et al., 2021). Du et al. (2016) studied the correlation between the chemical composition of silage and the degradation rate of rumen nutrients, and the results showed that DMD and NDFD were positively correlated with CP content and negatively correlated with NDF and ADF contents. In the present study, DMD and NDFD were the highest in the CL group and significantly different from those in the CK group, which might be related to high CP and low NDF contents in the CL group, which was consistent with the results of Liu et al. (2016a). Therefore, in the present study, cellulase + L. plantarum addition positively affected the digestibility of C. korshinskii silage.
Conclusion
The addition of additives reduced the NH3-N, DM, NDF, and ADF contents during ensiling. Higher LA content was observed in the silage treated with cellulase and L. plantarum, but the CL group had the higher AA content. The relative abundance of Lactiplantibacillus was significantly increased, but that of Enterococcus, Weissella, Enterobacter, and Pediococcus were significantly decreased in all the groups except for the C group compared with the CK group. The high AA content in the CL group could be related to the high abundance of 6-phosphate glucose dehydrogenase. The abundance of H+-transporting ATPase and ABC transporters was higher in the L and CL groups. Both L and CL groups increased NH3-N and MCP content and the degradation rates of dry matter and neutral detergent fiber. L. plantarum and cellulase addition improved the fermentation parameters, microbial community composition, and DM degradation in vitro of C. korshinskii silage, and the combination of L. plantarum and cellulase had the best effects.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI Sequence Read Archive database under BioProject PRJNA909496.
Ethics statement
The animal study was reviewed and approved by Ethics Committee of Medical School of Inner Mongolia Minzu University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JJ, MW, and BW designed the experiment. MX, RZ, YZ, and MW conducted the experiments. CD and LD analyzed the data. JJ wrote the manuscript. GZ, MW, and BW were involved in the revision of the manuscript. All the authors read and approved the manuscript.
Funding
Ministry of Agriculture and Rural Affairs “Integrated Demonstration Project of Key Technologies of Cattle and Sheep Husbandry and Breeding in the Transitional Zone of Agriculture and Animal Husbandry” (No. 16190050, No. 16200158, No. 16210096); Inner Mongolia Science and Technology Support Project (No. 2022YFXZ0015); Inner Mongolia Natural Science Foundation (No. 2022MS03072, No. 2022QN03027).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Rahman, M. A., Tashiro, Y., and Sonomoto, K. (2011). Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 156, 286–301. doi:10.1016/j.jbiotec.2011.06.017
Aßhauer, K. P., Wemheuer, B., Daniel, R., and Meinicke, P. (2015). Tax4fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31, 2882–2884. doi:10.1093/bioinformatics/btv287
Bai, J., Ding, Z., Ke, W., Xu, D., Wang, M., Huang, W., et al. (2021). Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: Ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb. Biotechnol. 14, 1171–1182. doi:10.1111/1751-7915.13785
Banfalvi, G. (1991). Conversion of oxidative energy to reductive power in the citrate cycle. Biochem. Educ. 19, 24–26. doi:10.1016/0307-4412(91)90138-X
Borreani, G., Tabacco, E., Schmidt, R. J., Holmes, B. J., and Muck, R. E. (2018). Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 101, 3952–3979. doi:10.3168/jds.2017-13837
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63, 64–75. doi:10.3168/jds.S0022-0302(80)82888-8
Cai, Y. (1999). Identification and characterization of Enterococcus species isolated from forage crops and their influence on silage fermentation. J. Dairy Sci. 82 (11), 2466–2471. doi:10.3168/jds.S0022-0302(99)75498-6
Calsamiglia, S., Cardozo, P. W., Ferret, A., and Bach, A. (2008). Changes in rumen microbial fermentation are due to a combined effect of type of diet and pH. J. Dairy Sci. 86, 702–711. doi:10.2527/jas.2007-0146
Chen, L., Bao, X., Guo, G., Huo, W., Li, Q., Xu, Q., et al. (2022). Evaluation of gallnut tannin and Lactobacillus plantarum as natural modifiers for alfalfa silage: Ensiling characteristics, in vitro ruminal methane production, fermentation profile and microbiota. J. Appl. Microbiol. 132, 907–918. doi:10.1111/jam.15246
Chen, L., Guo, G., Yuan, X., Zhang, J., Li, J., and Shao, T. (2016). Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat-common vetch intercrop on the Tibetan Plateau. J. Sci. Food Agric. 96, 1678–1685. doi:10.1002/jsfa.7271
Chen, X., Li, W., Gao, C., Zhang, X., Weng, B., and Cai, Y. (2017). Silage preparation and fermentation quality of kudzu, sugarcane top and their mixture treated with lactic acid bacteria, molasses and cellulase. Anim. Sci. J. 88, 1715–1721. doi:10.1111/asj.12840
Chen, Y. Y., Wang, Y. L., Wang, W. K., Zhang, Z. W., Si, X. M., Cao, Z. J., et al. (2020). Beneficial effect of Rhodopseudomonas palustris on in vitro rumen digestion and fermentation. Benefic. Microbes. 11, 91–99. doi:10.3920/BM2019.0044
Cheng, Q., Chen, L., Chen, Y., Li, P., and Chen, C. (2022). Effects of LAB inoculants on the fermentation quality, chemical composition, and bacterial community of oat silage on the qinghai-Tibetan plateau. Microorganisms 10, 787. doi:10.3390/microorganisms10040787
Du, S., Xu, M., and Yao, J. (2016). Relationship between fibre degradation kinetics and chemical composition of forages and by-products in ruminants. J. Appl. Anim. Res. 44, 189–193. doi:10.1080/09712119.2015.1031767
Dunière, L., Sindou, J., Chaucheyras-Durand, F., Chevallier, I., and Thévenot-Sergentet, D. (2013). Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 182, 1–15. doi:10.1016/j.anifeedsci.2013.04.006
Edgar, R. C. (2013). Uparse: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 10, 996–998. doi:10.1038/nmeth.2604
Ellis, J. L., Hindrichsen, I. K., Klop, G., Kinley, R. D., Milora, N., Bannink, A., et al. (2016). Effects of lactic acid bacteria silage inoculation on methane emission and productivity of Holstein Friesian dairy cattle. J. Dairy Sci. 99, 7159–7174. doi:10.3168/jds.2015-10754
Grabber, J. H., Hatfield, R. D., Ralph, J., Zoń, J., and Amrhein, N. (1995). Ferulate cross-linking in cell walls isolated from maize cell suspensions. Phytochemistry 40, 1077–1082. doi:10.1016/0031-9422(95)00413-2
Graf, K., Ulrich, A., Idler, C., and Klocke, M. (2016). Bacterial community dynamics during ensiling of perennial ryegrass at two compaction levels monitored by terminal restriction fragment length polymorphism. J. Appl. Microbiol. 120, 1479–1491. doi:10.1111/jam.13114
Grant, R. J., and Ferraretto, L. F. (2018). Silage review: Silage feeding management: Silage characteristics and dairy cow feeding behavior. J. Dairy Sci. 101, 4111–4121. doi:10.3168/jds.2017-13729
Guan, H., Yan, Y., Li, X., Li, X., Shuai, Y., Feng, G., et al. (2018). Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 265, 282–290. doi:10.1016/j.biortech.2018.06.018
Hao, Y., Huang, S., Liu, G., Zhang, J., Liu, G., Cao, Z., et al. (2021). Effects of different parts on the chemical composition, silage fermentation profile, in vitro and in situ digestibility of paper mulberry. Animals 11, 413. doi:10.3390/ani11020413
Hristov, A. N., Ropp, J. K., and Hunt, C. W. (2002). Effect of barley and its amylopectin content on ruminal fermentation and bacterial utilization of ammonia-N in vitro. Anim. Feed Sci. Technol. 99, 25–36. doi:10.1016/S0377-8401(02)00076-7
Hu, Z., Niu, H., Tong, Q., Chang, J., Yu, J., Li, S., et al. (2020). The microbiota dynamics of alfalfa silage during ensiling and after air exposure, and the metabolomics after air exposure are affected by Lactobacillus casei and cellulase addition. Front. Microbiol. 11, 519121. doi:10.3389/fmicb.2020.519121
Jiang, F. G., Cheng, H. J., Liu, D., Wei, C., An, W. J., Wang, Y. F., et al. (2020). Treatment of whole-plant corn silage with lactic acid bacteria and organic acid enhances quality by elevating acid content, reducing pH, and inhibiting undesirable microorganisms. Front. Microbiol. 11, 593088. doi:10.3389/fmicb.2020.593088
Kanehisa, M., and Goto, S. (2000). Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi:10.1093/nar/28.1.27
Kholif, A. E., Gouda, G. A., Morsy, T. A., Matloup, O. H., Fahmy, M., Gomaa, A. S., et al. (2022). Dietary date palm leaves ensiled with fibrolytic enzymes decreased methane production, and improved feed degradability and fermentation kinetics in a ruminal in vitro system. Waste Biomass Valorization 13, 3475–3488. doi:10.1007/s12649-022-01752-7
Khota, W., Pholsen, S., Higgs, D., and Cai, Y. (2017). Fermentation quality and in vitro methane production of sorghum silage prepared with cellulase and lactic acid bacteria. Asian-Australas. J. Anim. Sci. 30, 1568–1574. doi:10.5713/ajas.16.0502
Kung, L., Shaver, R. D., Grant, R. J., and Schmidt, R. J. (2018). Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 101, 4020–4033. doi:10.3168/jds.2017-13909
Li, F., Ding, Z., Ke, W., Xu, D., Zhang, P., Bai, J., et al. (2019). Ferulic acid esterase-producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: Ensiling characteristics, carbohydrates composition and enzymatic saccharification. Bioresour. Technol. 282, 211–221. doi:10.1016/j.biortech.2019.03.022
Li, F., Ke, W., Ding, Z., Bai, J., Zhang, Y., Xu, D., et al. (2020). Pretreatment of Pennisetum sinese silages with ferulic acid esterase-producing lactic acid bacteria and cellulase at two dry matter contents: Fermentation characteristics, carbohydrates composition and enzymatic saccharification. Bioresour. Technol. 295, 122261. doi:10.1016/j.biortech.2019.122261
Li, J., Yuan, X., Dong, Z., Mugabe, W., and Shao, T. (2018). The effects of fibrolytic enzymes, cellulolytic fungi and bacteria on the fermentation characteristics, structural carbohydrates degradation, and enzymatic conversion yields of Pennisetum sinese silage. Bioresour. Technol. 264, 123–130. doi:10.1016/j.biortech.2018.05.059
Li, M., Zhou, H., Zi, X., and Cai, Y. (2017). Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim. Sci. J. 88, 1531–1537. doi:10.1111/asj.12795
Li, X., Chen, F., Xu, J., Guo, L., Xiong, Y., Lin, Y., et al. (2022b). Exploring the addition of herbal residues on fermentation quality, bacterial communities, and ruminal greenhouse gas emissions of paper mulberry silage. Front. Microbiol. 12, 820011. doi:10.3389/fmicb.2021.820011
Li, Y., Du, S., Sun, L., Cheng, Q., Hao, J., Lu, Q., et al. (2022a). Effects of lactic acid bacteria and molasses additives on dynamic fermentation quality and microbial community of native grass silage. Front. Microbiol. 13, 830121. doi:10.3389/fmicb.2022.830121
Liu, B., Huan, H., Gu, H., Xu, N., Shen, Q., and Ding, C. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 273, 212–219. doi:10.1016/j.biortech.2018.10.041
Liu, Q. H., Li, X. Y., Desta, S. T., Zhang, J. G., and Tao, S. H. A. O. (2016a). Effects of Lactobacillus plantarum and fibrolytic enzyme on the fermentation quality and in vitro digestibility of total mixed rations silage including rape straw. J. Integr. Agric. 15, 2087–2096. doi:10.1016/S2095-3119(15)61233-3
Liu, Q. H., Shao, T., and Bai, Y. F. (2016b). The effect of fibrolytic enzyme, Lactobacillus plantarum and two food antioxidants on the fermentation quality, alpha-tocopherol and beta-carotene of high moisture napier grass silage ensiled at different temperatures. Anim. Feed Sci. Technol. 221, 1–11. doi:10.1016/j.anifeedsci.2016.08.020
Lv, H., Pian, R., Xing, Y., Zhou, W., Yang, F., Chen, X., et al. (2020). Effects of citric acid on fermentation characteristics and bacterial diversity of Amomum villosum silage. Bioresour. Technol. 307, 123290. doi:10.1016/j.biortech.2020.123290
Makkar, H. P., Sharma, O. P., Dawra, R. K., and Negi, S. S. (1982). Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 65, 2170–2173. doi:10.3168/jds.S0022-0302(82)82477-6
Martínez, J. R. P. F., Huerta, A. G., López, D. D. J. P., Cuevas, R. S., Zeidan, A., Salem, M., et al. (2020). Effect of xylanase, cellulase and natural maguey extract on the chemical composition of corn silage and in vitro rumen gas production. Int. J. Agric. Nat. Resour. 47, 23–34. doi:10.7764/ijanr.v47i1.2128
Menke, K. H., and Steingass, H. (1988). Estimation of the energetic feed value obtained by chemical analysis and in vitro gas production using rumen fluid. Animal Res. Dev. 28, 7–55.
Muck, R. E., Nadeau, E. M. G., McAllister, T. A., Contreras-Govea, F. E., Santos, M. C., and Kung, L. (2018). Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 101, 3980–4000. doi:10.3168/jds.2017-13839
Ogunade, I. M., Jiang, Y., Pech Cervantes, A. A., Kim, D. H., Oliveira, A. S., Vyas, D., et al. (2018). Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 101, 2048–2059. doi:10.3168/jds.2017-12876
Pahlow, G., Muck, R. E., Driehuis, F., Elferink, S. J. O., and Spoelstra, S. F. (2003). Microbiology of ensiling. Silage Sci. Technol. 42, 31–93. doi:10.2134/agronmonogr42.c2
Pang, H., Qin, G., Tan, Z., Li, Z., Wang, Y., and Cai, Y. (2011). Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 34, 235–241. doi:10.1016/j.syapm.2010.10.003
Ren, Y. Y., Gao, C. H., Zhao, Y. X., Yan, X. B., Lu, L. N., and Zhang, X. J. (2015). Study on nutritional characteristics and silage technology of Caragana korshinskii for feeding. Feed Res. 5, 1–2+34. doi:10.13557/j.cnki.issn1002-2813.2015.05.001
Reynal, S. M., Ipharraguerre, I. R., Liñeiro, M., Brito, A. F., Broderick, G. A., and Clark, J. H. (2007). Omasal flow of soluble proteins, peptides, and free amino acids in dairy cows fed diets supplemented with proteins of varying ruminal degradabilities. J. Dairy Sci. 90, 1887–1903. doi:10.3168/jds.2006-158
Satter, L. D., and Slyter, L. L. (1974). Effect of ammonia concentration of rumen microbial protein production in vitro. Br. J. Nutr. 32, 199–208. doi:10.1079/BJN19740073
Su, R., Ni, K., Wang, T., Yang, X., Zhang, J., Liu, Y., et al. (2019). Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage. PeerJ 7, e7712. doi:10.7717/peerj.7712
Sun, Q., Gao, F., Yu, Z., Tao, Y., Zhao, S., and Cai, Y. (2012). Fermentation quality and chemical composition of shrub silage treated with lactic acid bacteria inoculants and cellulase additives. Anim. Sci. J. 83 (4), 305–309. doi:10.1111/j.1740-0929.2011.00962.x
Tian, S. F., and Mao, K. Z. (2007). Research advances on feeding value of Caragana microphylla to herbivores. Anhui Nongye Kexue 25, 7836–7837. doi:10.13989/j.cnki.0517-6611.2007.25.039
Van Soest, P. V., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi:10.3168/jds.S0022-0302(91)78551-2
Veyisoglu, A., Tatar, D., Saygin, H., Inan, K., Cetin, D., Guven, K., et al. (2016). Microvirga makkahensis sp. nov., and Microvirga arabica sp. nov., isolated from sandy arid soil. Antonie Leeuwenhoek 109, 287–296. doi:10.1007/s10482-015-0631-z
Wang, Q., Wang, R., Wang, C., Dong, W., Zhang, Z., Zhao, L., et al. (2022). Effects of Cellulase and Lactobacillus plantarum on fermentation quality, chemical composition, and microbial community of mixed silage of whole-plant corn and peanut vines. Appl. Biochem. Biotechnol. 194, 2465–2480. doi:10.1007/s12010-022-03821-y
Wang, Y., Chen, X., Wang, C., He, L., Zhou, W., Yang, F., et al. (2019). The bacterial community and fermentation quality of mulberry (Morus alba) leaf silage with or without Lactobacillus casei and sucrose. Bioresour. Technol. 293, 122059. doi:10.1016/j.biortech.2019.122059
Wang, Y. L., Wang, W. K., Wu, Q. C., Zhang, F., Li, W. J., Yang, Z. M., et al. (2022). The effect of different lactic acid bacteria inoculants on silage quality, phenolic acid profiles, bacterial community and in vitro rumen fermentation characteristic of whole corn silage. Fermentation 8, 285. doi:10.3390/fermentation8060285
Wu, B., Hu, Z., Wei, M., Yong, M., and Niu, H. (2022). Effects of inoculation of Lactiplantibacillus plantarum and Lentilactobacillus buchneri on fermentation quality, aerobic stability, and microbial community dynamics of wilted Leymus chinensis silage. Front. Microbiol. 13, 928731. doi:10.3389/fmicb.2022.928731
Wu, B., and Nishino, N. (2016). Identification and isolation of Lactobacillus fructivorans from wilted alfalfa silage with and without molasses. J. Appl. Microbiol. 120, 543–551. doi:10.1111/jam.13031
Xing, L., Chen, L. J., and Han, L. J. (2009). The effect of an inoculant and enzymes on fermentation and nutritive value of sorghum straw silages. Bioresour. Technol. 100 (1), 488–491. doi:10.1016/j.biortech.2008.06.017
Xiong, Y., Xu, J., Guo, L., Chen, F., Jiang, D., Lin, Y., et al. (2022). Exploring the effects of different bacteria additives on fermentation quality, microbial community and in vitro gas production of forage oat silage. Animals 12, 1122. doi:10.3390/ani12091122
Xu, D., Ding, Z., Wang, M., Bai, J., Ke, W., Zhang, Y., et al. (2020). Characterization of the microbial community, metabolome and biotransformation of phenolic compounds of sainfoin (Onobrychis viciifolia) silage ensiled with or without inoculation of Lactobacillus plantarum. Bioresour. Technol. 316, 123910. doi:10.1016/j.biortech.2020.123910
Xu, D., Wang, N., Rinne, M., Ke, W., Weinberg, Z. G., Da, M., et al. (2021). The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb. Biotechnol. 14, 561–576. doi:10.1111/1751-7915.13623
Yang, L., Yuan, X., Li, J., Dong, Z., and Shao, T. (2019). Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 275, 280–287. doi:10.1016/j.biortech.2018.12.067
Yuan, X., Dong, Z., Li, J., and Shao, T. (2020). Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass Forage Sci. 75, 37–44. doi:10.1111/gfs.12455
Zhang, H., Zhu, H. F., Yan, Y. H., and Zhang, G. J. (2022c). Effects of rice bran and lactic acid bacteria preparation on fermentation quality and microbial diversity of Caragana korshinskii silage. Chin. J. Anim. Nutr. 03, 1800–1808.
Zhang, Q., Zhao, M., Wang, X., Yu, Z., Na, R., Wang, Z., et al. (2017). Factors associated with hand joint destruction in Chinese patients with rheumatoid arthritis. Grassl. Sci. 63, 211–217. doi:10.1186/s12891-017-1548-7
Zhang, X., Ke, W., Ding, Z., Xu, D., Wang, M., Chen, M., et al. (2022a). Microbial mechanisms of using feruloyl esterase-producing Lactobacillus plantarum A1 and grape pomace to improve fermentation quality and mitigate ruminal methane emission of ensiled alfalfa for cleaner animal production. J. Environ. Manage. 308, 114637. doi:10.1016/j.jenvman.2022.114637
Zhang, Y. C., Wang, X. K., Lin, Y. L., Zheng, Y. L., Ni, K. K., and Yang, F. Y. (2022b). Effects of Microbial Inoculants on fermentation quality and aerobic stability of Paper Mulberry silages prepared with molasses or cellulase. Fermentation 8, 167. doi:10.3390/fermentation8040167
Zhang, X. J., Sheng, J. H., and Zhao, H. P. (2010a). Advanced in feeding conversion technology on Caragana and its prospect of Caragana feed industry in Inner Mongolia. Animal Husb. Feed Sci. 5, 21–23. doi:10.16003/j.cnki.issn1672-5190.2010.05.012
Zhang, Y. G., Xin, H. S., and Hua, J. L. (2010b). Effects of treating whole-plant or chopped rice straw silage with different levels of lactic acid bacteria on silage fermentation and nutritive value for lactating Holsteins. Asian-Australas. J. Anim. Sci. 23, 1601–1607. doi:10.5713/ajas.2010.10082
Keywords: Caragana korshinskii silage, lactiplantibacillus plantarum, microbial diversity, In vitro rumen fermentation, cellulase
Citation: Ju J, Zhang G, Xiao M, Dong C, Zhang R, Du L, Zheng Y, Wei M, Wei M and Wu B (2023) Effects of cellulase and Lactiplantibacillus plantarum on the fermentation quality, microbial diversity, gene function prediction, and in vitro rumen fermentation parameters of Caragana korshinskii silage. Front. Food. Sci. Technol. 2:1108043. doi: 10.3389/frfst.2022.1108043
Received: 25 November 2022; Accepted: 30 December 2022;
Published: 12 January 2023.
Edited by:
Ramesh C. Ray, Central Tuber Crops Research Institute (ICAR), IndiaReviewed by:
Song Wang, Northeast Agricultural University, ChinaQing Zhang, South China Agricultural University, China
Copyright © 2023 Ju, Zhang, Xiao, Dong, Zhang, Du, Zheng, Wei, Wei and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manlin Wei, d2VpbWFubGluQDE2My5jb20=; Baiyila Wu, YmFpeWlsYXdAaW11bi5lZHUuY24=
 Ji Ju
Ji Ju Guijie Zhang2
Guijie Zhang2