- 1Centre for Food, Food Security, and Nutrition Research, Institute of Medical Research and Medicinal Plant Studies, Yaounde, Cameroon
- 2Departement of Industrial Chemistry and Environmental Engineering, National School of Agro-industrial Sciences, University of Ngaoundere, Ngaoundere, Cameroon
This study was focused on the collecting and recycling systems of bottles reused for traditional food packaging in the city of Yaoundé and the presence of biofilms in these bottles. A cross-sectional study approved by the Institutional Review Board was conducted in 43 quarters randomly selected in Yaoundé using a semi-structured questionnaire. The target population was producers and street sellers of traditional foods who used recovered bottles for food packaging and had freely signed the informed consent clearance. Then, the recovered bottles cleaned by the participants and ready to be reused as traditional food packaging were sampled and screened for the presence of biofilms. The results showed that 84% of the 162 participants were women. Bottles reused for traditional food packaging were mainly collected from garbage (70.4%). A total of six different cleaning processes were identified from manufacturers of traditional food products. The cleaning processes were significantly (p < 0.05) dependent on the education level and varied from one manufacturer to another. Amongst the identified cleaning processes, those which included soaking and disinfection unit operations were more efficient in biofilms’ removal. Bottles cleaned according to these processes scored the lowest biofilms’ contents. Independently of the cleaning processes, biofilms were detected in all recovered bottles. This might suggest a potential health risk for consumers.
1 Introduction
Around 50% of the population in developing countries prefers to consume street-vended traditional foods (Rana and Ahirrao, 2016). The main reasons are their low price, the taste and nutritional value of these meals, which are generally sold close to schools and workplaces, and the long commuting distances between the workplace or school and home (FAO, 2016). The street food sector accounts for 15–25% of the total informal employment in African cities (Skinner, 2011). Amongst these foods, those packed in recovered bottles represent a great proportion of those sold and consumed in developing countries like Ghana and Cameroon (Gonzalez-Rivas et al., 2018; Nguendo Yongsi, 2018; Abrokwah et al., 2020). In this country, there are several industries which produce sweet beverages and water in plastic and glass bottles of different volumes. Once they have been consumed by populations, bottles are thrown in the garbage together with several other domestic wastes or recycled for reuse purposes (Abrokwah et al., 2020). Sometimes, they are thrown in drains where they block water circulation leading to flooding (Tabeyang, 2018). A sustainable management system for handling these wastes is not regulated in the country. Due to economic difficulties, many traditional foods such as juices, fermented milk, yoghurt, roasted maize, peanuts, palm wine, traditional beer, beverages, cooking oils, and drinking water, are mainly packed in recovered plastic bottles (collected from garbage bins and drains) before being sold (Ingram and Mala, 2010; Djoulde et al., 2013; Tabeyang, 2018; Abrokwah et al., 2020). These products are mainly consumed by the low and middle class of the population including children and elderly people, and this without any further treatment or processing. The production processes of these traditional foods generally include several unit operations which may represent critical points of microbial contamination. Indeed, the quality of ingredients and the water used, the respect of good hygiene and manufacturing practices, the packaging, conditioning, and storage conditions are known as the parameters responsible for the poor microbiological quality of the final product (Gonzalez-Rivas et al., 2018; Abrokwah et al., 2020). The reused of recovered bottles may definitely represent a serious health hazard as they are quite often collected from garbage and drains where they might have stayed for long and therefore exposed to microbial pathogens. In these conditions, biofilms can easily be formed inside the bottles and become difficult to remove as reported by Gonzalez-Rivas et al. (2018). Biofilms are structured communities of microorganisms encapsulated within a self-developed polymeric matrix and adherent to a living or inert surface (Satpathy et al., 2016). Their formation on plastic matrix has already been reported by several studies (Kregiel, 2015; Oberbeckmann et al., 2016). Brooks and Flint (2008) pointed out the difficulties faced by the food industry to eliminate biofilms on surfaces. The efficiency cleaning processes of these collected bottles are therefore of great importance. In the literature, several strategies for controlling biofilms were reported (Carrascosa et al., 2021). They rely on the dissolution or the break up of the exopolysaccharide matrix that protects the biofilms in order to ease access of disinfectants to the viable cells (Simoes et al., 2010). For that, after an effective cleaning procedure, mechanic actions, high temperatures and pressures, or surfactants are generally used. These methods are always followed with disinfection in view of killing viable cells from the biofilms and preventing microbial growth on the cleaned surfaces (Carrascosa et al., 2021). Amongst antibiofilm disinfectants, peracetic acid due to its strong oxidizing ability was reported as effective for biofilm elimination (Skowron et al., 2018). The use of sodium hypochlorite as an antibiofilm agent with interesting biofilm removal efficiency was noticed by Skowron et al. (2018). The efficiency of the pulverization with a mixture of hydrogen peroxide, sodium hypochlorite, and peracetic acid against biofilms in the food industry was highlighted by Park et al. (2012). Green strategies based on the use of enzyme-based detergents were also reported as efficient against biofilms (Simoes et al., 2010).
Unfortunately, these effective biofilm removal systems remain expensive for traditional food producers and their use also requires some skills. Considering the scarcity of potable water and the general lack of food safety knowledge of populations often reported (Ako et al., 2010; Nguendo Yongsi, 2010; Pouokam et al., 2017), one could therefore question the antimicrobial efficiency of cleaning processes adopted by the traditional food and drink producers. Up to our knowledge, outbreaks of foodborne diseases are generally associated to poor surface hygiene (Gonzalez-Rivas et al., 2018), not to packaging bottles. That new approach has never been reported in the literature and could bring more insights on the diseases associated to the consumption of food packaged in recovered plastic bottles. This study aimed at identifying the collecting and recycling systems of bottles by street food sellers and assessing their antimicrobial efficiency with as target biofilms.
2 Materials and methods
2.1 Survey
A cross-sectional survey was conducted from September to November 2021 in the City of Yaoundé (3° 5′N and 11° 31′ E), the capital of Cameroon. The city has an area of 304 km2, a population of 4.1 million distributed following a density of approximately 13.487 inhabitants/km2. The sector of traditional food products is well developed in the city. At every school, market or street corner, a great variety of traditional food products are daily exposed for selling.
2.2 Study population
The study population consisted of people both producers and street sellers of traditional food and who used recovered bottles for food packaging. A stratified random sampling was applied to select 43 neighborhoods in the city of Yaoundé, Cameroon. In these neighborhoods, participants were selected using a snowball sampling design. The questionnaire was anonymous and volunteer participants were informed of the objectives of the study. The study was approved by the Ethics Committee of the Institute of Medical Research and Medicinal Plants Studies of Yaoundé, Cameroon and the Centre Regional Committee for Human Health Research (CE n 1932/CRERSH/2020).
2.3 Questionnaire design and administration
Information’s on the cleaning processes of recovered bottles used for food packaging were collected with the help of a semi-structured questionnaire that has 7 sections and 50 questions developed considering ISO/TS 22002-1:2009 standards relative to food safety related to food manufacturing. Each section included closed-ended questions with categorial/multiple-choice nominal questions. The 7 sections consisted of socio-demographic characteristics (gender, education level, age, marital status, profession, quarter), the practice of traditional food processing involving the use of recovered bottles for packaging (training, type of food products, experience), bottle collection system (type of bottles, supply source, supply mode), cleaning system of bottles (place, cleaning and disinfection processes), storage of food products in bottles (selling points, selling conditions, storage duration), after selling management of products (complaints, loses, management of spoiled products), and knowledge on food contaminants (microorganisms, biofilms). The questionnaire was firstly pretested and validated on 20 participants to adjust open-ended and closed questions. The participants’ responses were confidential and in the signed informed consent clearance it was mentioned that there will be no diverse outcomes for themselves or their businesses if they provided honest responses.
2.4 Samples collection
Recovered bottles were collected from participants’ stock using a convenience sampling design and brought to the laboratory. Three bottles of 1.5 L and three others of 1 L, which were already cleaned and ready to be used by participants, were randomly sampled for each cleaning process identified from the survey. Besides bottles from participants included in the study, some recovered bottles cleaned and sold in different markets of the city of Yaoundé (Mokolo, Acacia) by persons who did not manufacture traditional food products were also collected using the sample sampling design. Bottles of drinking water from three industrial companies which were opened in the laboratory were used as negative controls. All these bottles were made of the same polymer (polyethylene terephthalate).
2.5 Screening of the presence of biofilms in bottle samples
The antimicrobial efficiency of the cleaning processes was identified using a biofilm concept. The presence of biofilms in the sampled bottles was assessed using the method described by Djordjevic et al. (2002) and O’Toole (2011) with slight modifications. The bottles’ inside was immersed with crystal violet 1% (Sigma Aldrich, Germany) and incubated at room temperature (25 ± 1°C) for 45 min. Then, the excess stain was removed and the bottles were gently washed thrice with sterile distilled water. The washed bottles were drained for 20 min at room temperature (25 ± 1°C) and 100 ml of absolute ethanol was introduced to remove the stained biofilms. After 5 min of manual agitation, the optical densities of the obtained ethanolic solutions were read at 595 nm (UVmini-1240, UV-Vis Spectrophotometer, Shimadzu, Japan) against the blank. The instrument accuracy was ±0.003 abs. The quantity of biofilms was expressed in terms of absorbance at 595 nm. Experiments were performed in triplicate. The method was calibrated during preliminary investigations using sixty bottles of drinking water of different volumes from three industrial companies which were opened in the laboratory. Experiments were performed 20 times and the optical density values obtained were not significantly different (p > 0.05) even after several days while repeating the same experiments (non-published data).
2.6 Statistical analyses of data
Data collected from the survey were edited, coded, and exported to the Statistical Package for Social Science 20.0.0 (SPSS Inc., IBM Corporation, Chicago, United States). Descriptive statistics such as frequencies and percentages were used to present the findings. Cross-tabulation and Khi square were used to establish relationships between factors and variables assessed in this study. A p-value lower than 0.05 (p < 0.05) was used to define the significant difference between variables. Data obtained from the screening of biofilms in bottle samples were analyzed using Statgraphic Centurion XVI version 16.1.18 (StatPoint Technologies, Inc., Virginia, United States) and expressed as mean ± standard deviation. One-way ANOVA and Duncan multiple range tests were used to compare means at p < 0.05.
3 Results
3.1 Sociodemographic characteristics of the study population
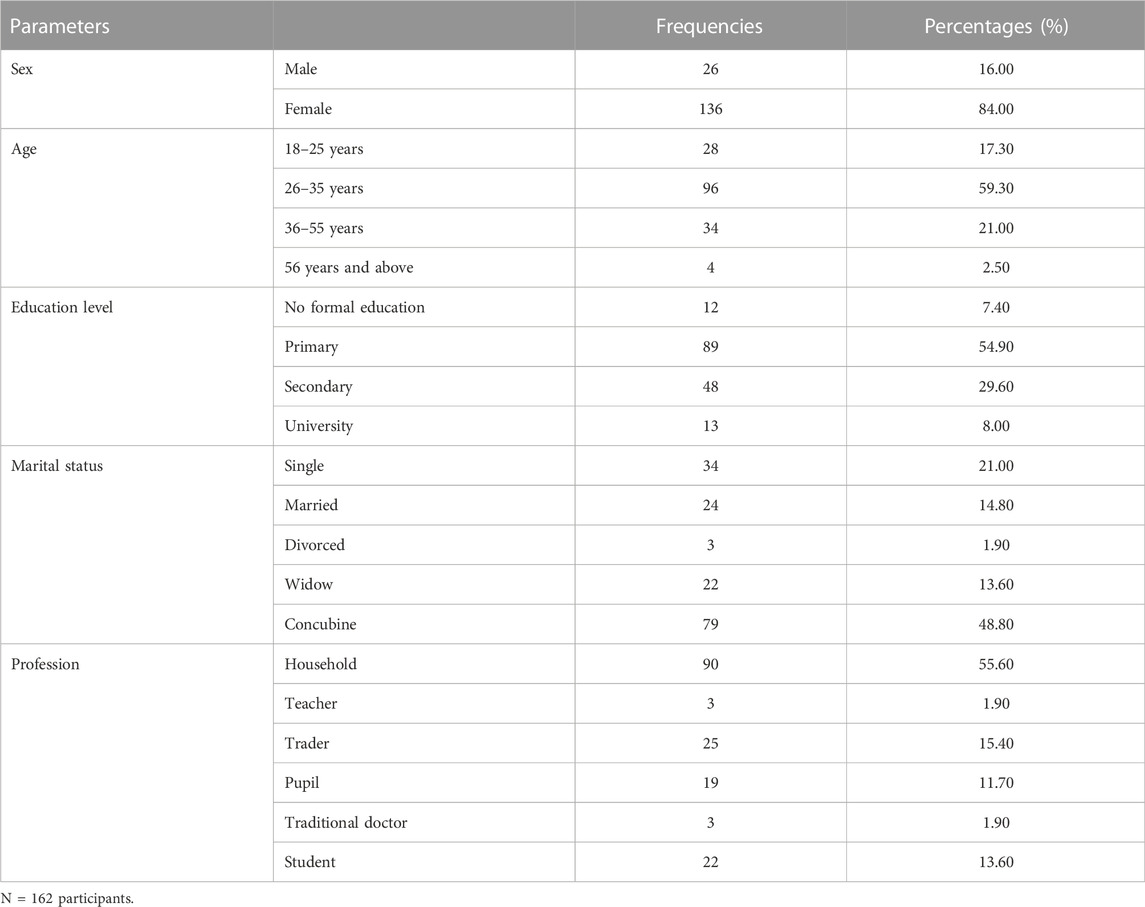
Table 1 presents the socio-demographic characteristics of the study population. A total of 162 persons involved in the use of recovered bottles for food packaging were included in the present study. Females represented the majority of the studied population (84%). Participants were mainly aged between 26 and 35 years (59.30%), while the age group corresponding to the elderly person (more than 55 years old) scored the lowest percentage (2.50%). Among this studied population, 62.3% had an education level lower than secondary (54.90% for primary education level +7.40% for no formal education. Concubine persons were mostly represented in this study (48.80%), followed by single (21.0%), married (14.80%), and widow (13.60%) persons. Only 1.9% of the population was divorced. The majority of the women involved in this study were housewives (55.60%).
A diversity of traditional foods was recorded as manufactured and packed in recovered bottles. It consisted of H. sabdariffa juice (49.40%), drinking water (47.50%), cooking oil (22.80%), palm wine (20.40%), lemon juice (18.50%), Zingiber officinale juice (14.80%), peanuts (14.80%), baobab juice (9.30%), honey (9.90%), fermented milk (6.20%), and traditional medicine (4.30%).
3.2 Practice of traditional food processing
Amongst the study population, only 9.9% had more than 10 years of practice. The categories 6–10 years, 3–5 years, and less than 2 years of experience counted 30.90%, 37.70%, and 21.60% of this population, respectively. A total of 84.60% of the population declared to have been trained by their parents (49.40% of them), friends (33.30% of them), or through more formal training (17.30% of them). The majority of traditional food manufacturers involved in this study had less than two employees (58.60%). Few of them had three to five employees (30.20%) or more than five employees (11.10%).
3.3 Bottles collection system
Plastic and glass bottles were found to be used for traditional food packaging, but plastic bottles appeared as the most used ones (98.10%). Bottles of small volumes (0.35 and 0.5 L) are generally used for traditionally manufactured juices (H. sabdariffa, Z. officinale, lemon, and baobab) and fermented milk, and those of 1 or 1.5 L mainly for drinking water, cooking oil, peanut, honey, traditional medicine, and palm wine. On the other side, glass bottles were used only by manufacturers of peanuts which included grilled peanuts, peanuts enrobed with barley, peanuts enrobed with sugar commonly called caramel, and grilled peanuts mixed with grilled maize.
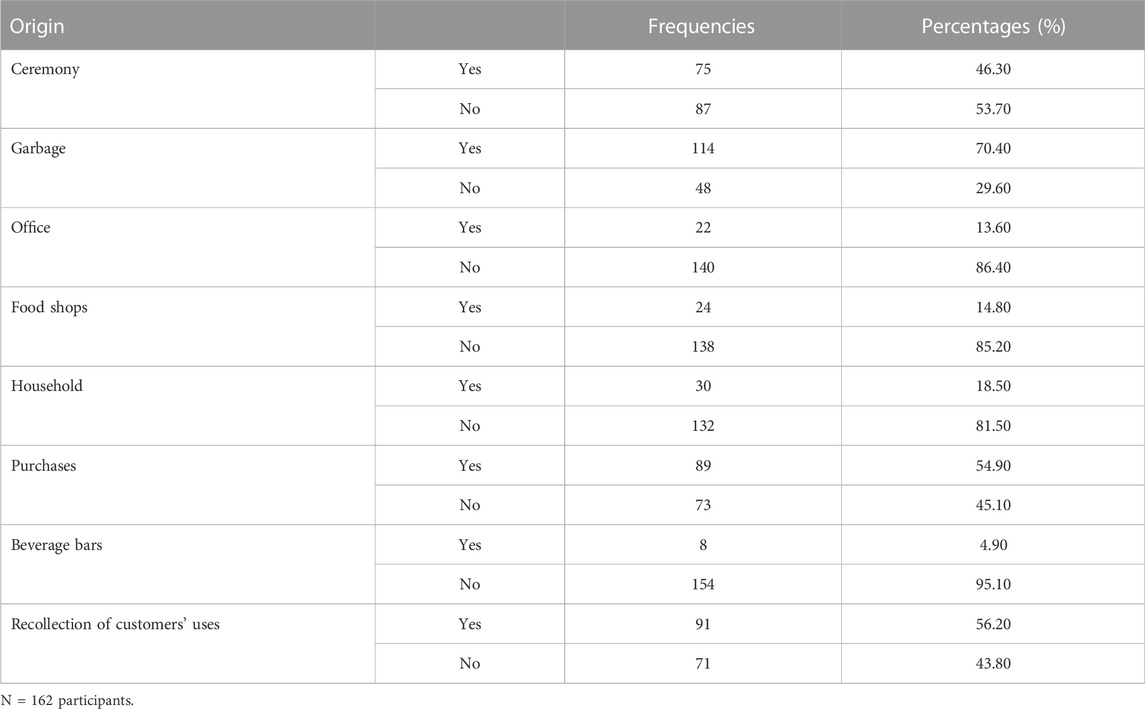
Table 2 presents the origin of the bottles used as food packaging by the manufacturers of traditional products in the city of Yaoundé. Glass bottles come from the recovery of alcoholic beverage bottles such as whisky, while plastic bottles come from the recovery of industrial juice bottles as well as drinking water bottles. For 70.4% of participants, garbage represented a place where bottles are collected. Collections directly from initial product consumers or after ceremonies were also reported (56.20% and 46.30%, respectively). Some also purchased recycled bottles directly from markets (54.90%).
3.4 Cleaning system of bottles
Different unit operations in the cleaning process of recovered bottles were reported by participants. The first one was sorting, which was followed by superficial cleaning (applied by 92.00% of participants). Then comes pre-rinsing (applied by 94.40% of participants) and soaking in detergent solution (applied by 31.50% of participants). For the latter, powder detergents were used by 90.70% of the population while only 9.30% used liquid ones. Regarding water, 59.30% used well water, 13.60% borehole water, 5.60% source water and 67.30% used tap water when available. Amongst those who soaked bottles, the soaking durations were different. Indeed, 12.30% soaked bottles from 1 to 15 min, 21% from 16 to 30 min, 11.70% from 31 to 45 min, and 26.50% from 46 to 60 min. As cleaning materials, it was noticed that the bottles inside are cleaned with a brush (2.50%), sponge (77.20%), or sprinkler (23.50%). However, 73.50% of respondents reported using only manual shaking to remove contaminants present inside the bottles. The next operation unit consisted of rinsing, which was directly followed by disinfection (applied by 6.8% of respondents) before the drying unit operation (applied by 93.2% of respondents). Bottles are dried through shade (72.80%) or sunlight (16.00%) exposure, wiping (3.10%) and draining (3.70%). Disinfection also appears to be rather applied after this drying step by 45.10% of the participants. It consisted of the use of chlorine (30.90%) with hot water (14.20%) or not. It was done for 1–15 min (5.60%), 16–30 min (24.10%), and 31–60 min (15.10%) with cold or fresh water (7.40%), lukewarm (23.50%), and hot water (14.20%). For those performing disinfection, it was observed that 32.10% rinsed the bottles before utilization. It was mainly those who used sodium hypochlorite (chlorine) as a disinfectant and a few parts of those who performed disinfection with hot water. The cleaned bottles were conditioned in cleaned bags (77.80%), basins (14.80%), and buckets (3.7%). Few participants declared not applying conditioning as they directly used cleaned bottles.
Based on the application or not of the above-mentioned unit operations, six different cleaning processes could be defined (Figure 1). The cleaning process A (Sorting-superficial cleaning-internal-washing-rinsing-drying-conditioning) was used by 30.20% of the participants; the cleaning process B (Sorting-superficial cleaning-internal washing-rinsing-disinfection-rinsing-drying-conditioning) by 26.50%, the cleaning process C (Sorting-soaking-superficial cleaning-internal washing-rinsing-disinfection-rinsing-drying-conditioning) by 15.00%, the cleaning process D (Sorting-soaking-superficial cleaning-internal washing-rinsing-drying-conditioning) by 12.80%, the cleaning process E (Sorting-internal washing-superficial cleaning-rinsing-drying-conditioning) by 9.30% and finally the cleaning process F (Sorting-superficial cleaning-rinsing-drying-conditioning) by 6.20%. A cleaning process with similar unit operations as process A was identified for cleaned recovered bottles sold on markets by persons who do not manufacture artisanal foods.
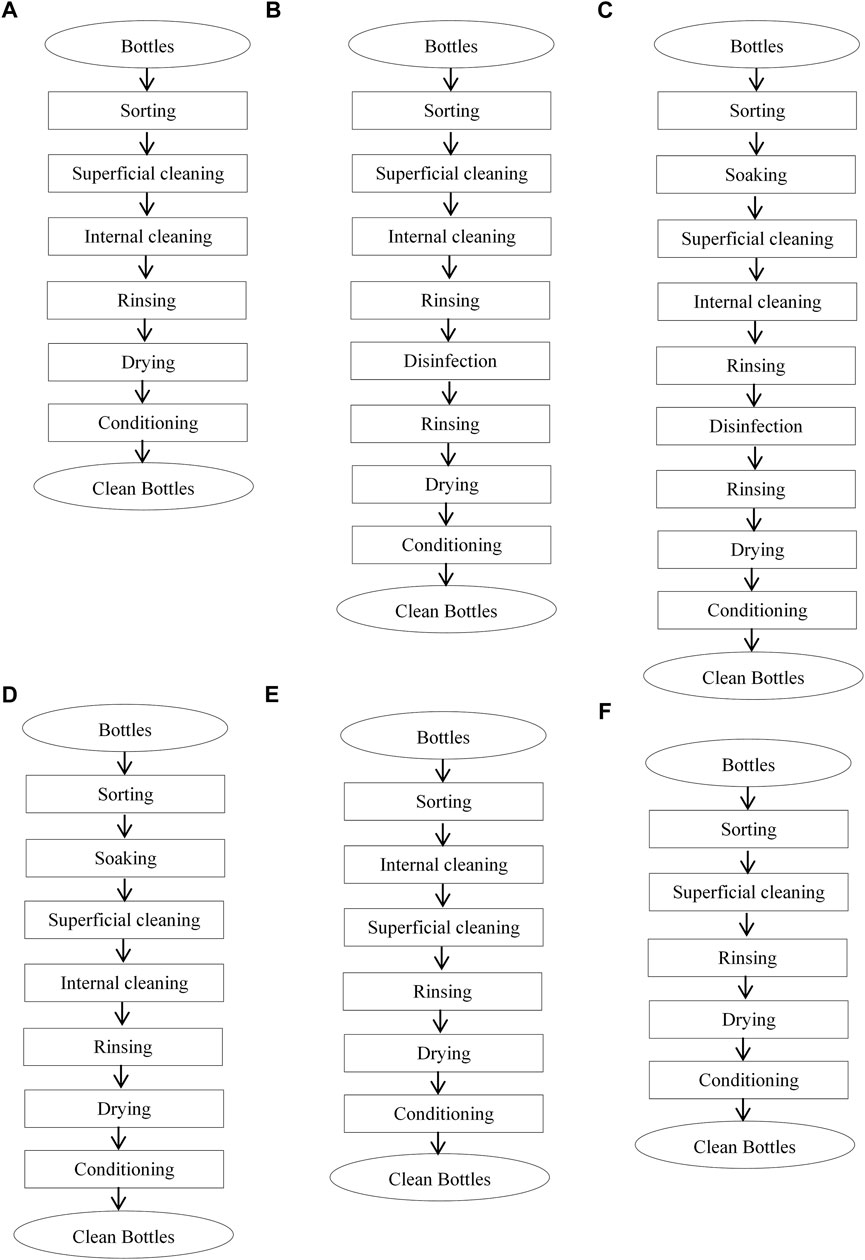
FIGURE 1. The six different cleaning processes of recovered bottles (A–F) obtained from the manufacturers of traditional food products.
3.5 Association between the cleaning processes and the education level of participants
As observed in Table 3, the highest proportion of participants using the cleaning process A was of primary education level (41.60%), while those using process F had no formal education (66.70%). Processes B (31.20%), D (20.80%), and E (16.70%) were mainly used by participants of secondary education level. Participants with a university education level used at 46.20% process C. Statistical analyzes revealed that processes A (χ2 = 12.01, p < 0.01), C (χ2 = 10.18, p < 0.010) and F (χ2 = 81.98, p ≤ 0.001) were significantly associated with the education level of participants.
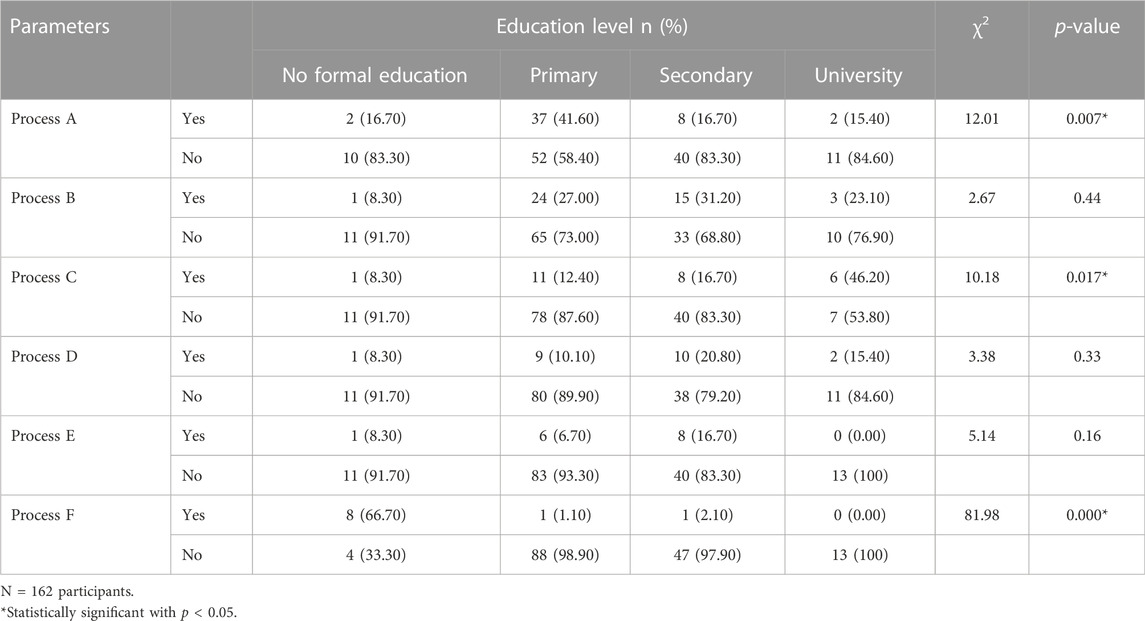
TABLE 3. Correlation between education level and cleaning process of recovered plastic bottles in the city of Yaoundé.
3.6 Storage of traditional food products packed in recovered bottles
The main selling points of traditional food products packed in recovered bottles were schools (88.30%), street corners (79%), markets (65.40%), and living houses (64.80%). The traditional food products packed in recovered bottles were almost air exposed for selling. During the selling, 33.30% of participants stored products in buckets containing ice depending on the nature of the products, 31.50% simply exposed the products on tables or other supports, 24.10% in iceboxes, and 17.90% in the paper box. It is important to highlight that 36.40% of participants stored their products in a refrigerator during sales. This was mainly the case in selling points such as shops, living houses, and offices. Depending on the nature of the products, 51.90% of traditional food products packed in recovered bottles were sold at room temperature while 48.10% were sold at cold temperature using buckets containing ice, iceboxes or refrigerator. Unsold products, depending on their nature, were stored at room temperature (69.70%) or refrigerated temperature (43.20%). Some unsold products were frozen. The storage duration varied from 0 to 1 week (32.70%), 2–4 weeks (15.40%), 4–8 weeks (21.60%), and 9 weeks and more (30.2%).
3.7 Complaints associated with the consumption of traditional products packed in recovered bottles
Among the 162 participants, 58.60% declared that they received customer complaints regarding their products. They include diarrhoea (36.00%), vomiting (6.20%), headaches (3.10%), unpleasant odours (21.00%), modification of the product taste (25.30%), and the presence of particles (2.50%). Some participants affirmed that the products spoiled during storage (39.50%) and the spoilage attributed to microbial fermentation at 57.40% was noticed through an acid taste (47.50%), modification of odour (22.80%) and colour (22.80%) of the products. Regarding the management of spoiled products, it was declared to be thrown by 84.60% of the participants and recycled by 15.40%. The recycled products were consumed by manufacturers’ families (32.10%) or sold again at a low price.
To improve the flavour and taste of their products (mainly juices from H. sabdariffa, lemon, Z. officinale, and baobab), 59.90% of participants used aromatic plants and spices. These plants were citronella (17.30%) and mint (12.30%), and the spice used was clove (39.50%).
3.8 Knowledge on microorganisms and biofilms
The manufacturers’ knowledge on bacteria, viruses, yeasts, and moulds as well as biofilms was also assessed. The results showed that 57.40% were aware of the existence of bacteria, yeasts, virus, and moulds and 46.30% of participants knew that these microorganisms can be found in their products. Very few proportions of them were aware of microbial biofilms (7.40%).
3.9 Presence of biofilms in selected recovered plastic bottles used for food packaging
Biofilms were detected in all analyzed samples as observed in Table 4. Globally, the bottles of 1.5 L contained more biofilms compared to those of 1 L except the bottles cleaned according to processes B and D where the contrary was noticed. Regarding bottles cleaned and sold in markets by participants who did not manufacture traditional food products, the biofilms’ contents tended to vary from one bottle to another with an absorbance at 595 nm that ranged from 2.96 to 6.31 depending on the market and the volume of the bottle. Besides, comparing the effect of the cleaning processes, it clearly appears that it significantly (p < 0.05) determines the biofilms’ content. Process C was the most efficient in biofilms removal as it led to the lowest biofilms content (1.00 ± 0.10), while process F was the least efficient one with the highest biofilms’ content (10.92 ± 0.12). Considering the bottle volume, no significant difference (p > 0.05) was observed for processes A, B, and E while for processes C, D, and F, biofilms’ content was significantly (p < 0.05) high in bottles of 1.5 L compared to those of 1 L.
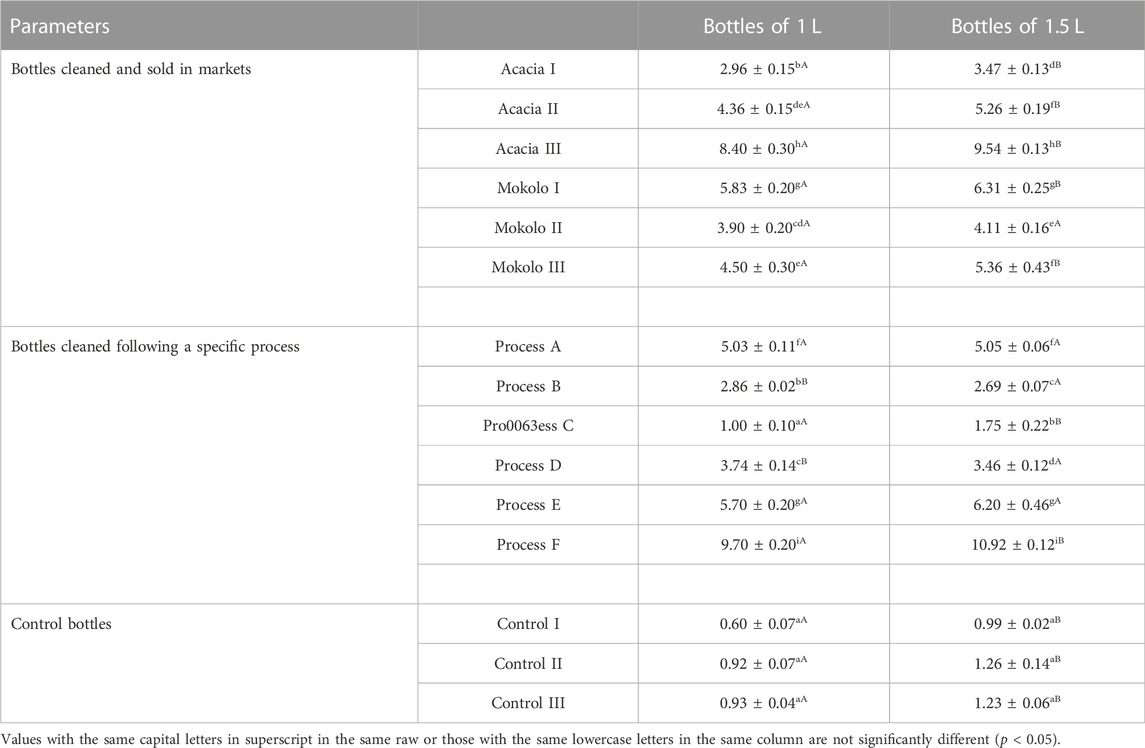
TABLE 4. Quantity of destained biofilms (in terms of absorbance at 595 nm) present in recovered plastic bottle samples collected in the city of Yaoundé.
4 Discussion
The diagnostic of the collecting and cleaning system of recovered bottles used as traditional food packaging in the city of Yaoundé was performed in this study. The traditional food manufacturers involved in the study were mainly female. This could be explained by the fact that in sub-Saharan Africa and particularly in Cameroon, the informal sectors involving cooking practices are mainly dominated by women. Indeed, that practice which is accessible to housewives as it requires a relatively small capital base, allows women to do household chores such as child care besides vending. Amongst these women, the great majority was households, in concubine and with primary or no education level. This observation justifies their involvement in activities requiring a low capital base to supplement their husbands’ lower wages. Drinking water and traditional juices were the most reported products packaged in recovered plastic bottles. This could arise from the fact that they do not require a significant capital base and the cost/profit margins are important. Moreover, these products do not spend much times before being sold compared to others such as cooking oil, honey, peanuts and traditional medicine.
As shown in this study, the recovered bottles are generally used for the packaging of a diversity of traditional foods/drinks which are widely consumed in the city of Yaoundé. The assessment of the potential health risk therefore, becomes of huge importance. The case of diarrhea (χ2 = 0.30, p > 0.05), vomiting (χ2 = 0.97, p > 0.05), and headache (χ2 = 1.30, p > 0.05) reported by these product sellers as well as the change of taste or smell suggest the presence of pathogens or spoilage microorganisms in the sold products. This observation could be explained by the non-respect of good hygiene and manufacturing practices which leads to the contamination of the final products as reported by Mouafo et al. (2020). The contamination may certainly happen at many steps during the process, but the use of recovered bottles for packaging appears as a critical step. Bottles were reported to be mainly collected from garbage (70.40%), which is known as a place of important microbial activity and diversity. The presence of pathogens such as coliforms in plastic bottles collected from garbage and reused as traditional food packaging in Ghana was noticed by Abrokwah et al. (2020). Moreover, it is well known that several pathogens belonging to the group of coliforms deserve biofilms’ formation ability. Hence, the possible presence of microbial biofilm in bottles collected from garbage is more important than in other reported collecting points.
Previous studies showed that biofilms can be easily formed on inert materials such as plastic bottles (Kregiel, 2015; Oberbeckmann et al., 2016), and they are very difficult to remove from these surfaces even if disinfection is applied (Gonzalez-Rivas et al., 2018). Whatever the origin of the bottles, the cleaning process adopted by the food manufacturers shall be able to remove biofilms that could be present.
Biofilms were present in all bottle samples. The ability of some microorganisms such as S. aureus to form biofilms on PVC materials was also demonstrated by Baomog et al. (2020). Regarding bottles cleaned and sold in markets, it was noticed that the quantity of biofilms varies significantly (p < 0.05) from one cleaning site to another. Among the six cleaning processes identified in this study (Figure 1), process A (Sorting-superficial cleaning-internal cleaning-rinsing-drying-conditioning) was the most used (30.20%). This could be due to the fact that it seems to be rapid as it does not include soaking and disinfection. This disinfection step is clearly determinant since the cleaning process C (Sorting-soaking-superficial cleaning-internal cleaning-rinsing-disinfection-rinsing-drying-conditioning) appears as the most efficient in biofilms’ removal, while process F (Sorting-superficial cleaning-rinsing-drying-conditioning) was the least effective. In this process F, the bottles inside were only rinsed with water without any ripping and wiping or use of detergents or disinfectants which can remove biofilms. Rubbing and wiping have yet been demonstrated as effective in biofilms’ removal because, during these unit operations, fingers and tissue applied mechanical friction and shearing forces on the surface, thus improving biofilms’ reduction (Wu et al., 2010). Unfortunately, 73.50% of participants reported using only manual shaking to remove contaminants present inside the bottles. Processes B and C which were characterized by their lower biofilms’ contents include soaking and disinfection unit operations. Andoh et al. (2013) highlighted that soaking represents an important step of a cleaning process because it enhances dirt removal and thus the cleaning efficiency. Moreover, the bottles were disinfected with hot water and chlorine. The ability of hot water to remove biofilms was reported by Baomog et al. (2020). Del Carpio-Perochena et al. (2011) showed that sodium hypochlorite might dissolve biofilms when applied in a direct contact test. Stojicic et al. (2012) noticed that the use of a sodium hypochlorite solution at 1–2% destroyed biofilms derived from Enterococcus faecalis. Sodium hypochlorite was reported by Köse and Yapar (2017) as the most effective disinfectant against biofilms formed by some microorganisms. Despite the use of disinfectants such as chlorine (sodium hypochlorite), biofilms were not completely removed. This observation could be justified by the fact that alkaline hypochlorite has a longer penetration time into biofilms because active chlorine is neutralized in the outermost region of the biofilms (Stewart et al., 2001). Indeed, the maximum disinfection duration noticed in the present study was between 31 and 60 min (15.10%). The ability of biofilms to resist to cleaning products as well as antimicrobials was demonstrated by Srey et al. (2013) and Colagiorgi et al. (2017). Taking into consideration the different unit operations involved in the identified cleaning processes of recovered bottles, a standard process which included all these unit operations was proposed as shown in Figure 2. Under reservation of its validation, it could be suggested to participants as the best cleaning system.
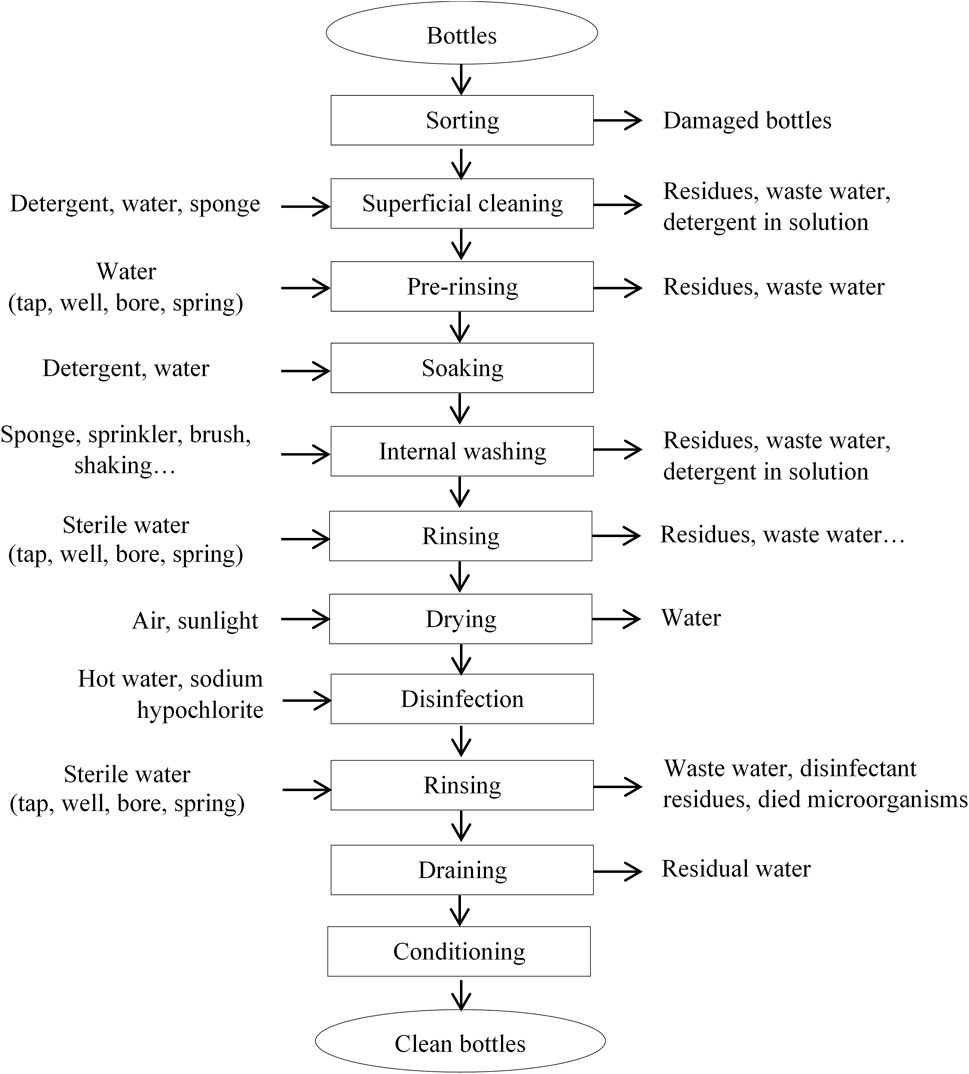
FIGURE 2. Standard flow chart proposed for the cleaning process of recovered bottles used for traditional food packaging in the city of Yaoundé.
Education level appeared as a determining parameter in the cleaning process used. The highest proportion of participants who used process C was of university education level (46.20%). In contrast, the worst cleaning process (process F) was significantly adopted by participants with no formal education level (χ2 = 81.89, p ≤ 0.001). Most of those collecting bottles from garbage had no formal education (83.30%) or primary education level (76.40%). This can be ascribed to their poor knowledge of the risks that consumers will be exposed to. A significant relationship between the education level of participants and their knowledge on microorganisms (χ2 = 70.40, p ≤ 0.001) and biofilms (χ2 = 148.54, p ≤ 0.001) was observed. Besides, the variation in the storage duration of food products stored in the recovered bottles was recorded. This can result from microbial activity as a significant correlation between the disinfection of bottles and the storage duration (χ2 = 10.40, p < 0.05) was noticed. Furthermore, the storage was reported to be often long (9 weeks and more at 30.2%) or done at room temperature (69.70%), conditions which are suitable for microbial growth. A similar observation was highlighted by String et al. (2020) with household water storage containers. This lack of good hygiene and manufacturing practices also originated from the lack of proper training, considering that 84.60% of the respondents declared to have been trained by their parents, friends, or through other informal training (17.30% of them).
With regards to persons who sold cleaned recovered plastic bottles on markets, the cleaning process did not vary whatever the sales volume. Indeed, they purchased bottles from children independent of their collection origins, cleaned them using the same process (process A) and sold them at prices that allow a profit. The family income and education level might be the reasons which induced the vendors to purchase cleaned bottles on markets or scavenge bottles from landfill. Indeed, vendors who purchased bottles imputed its cost on the final price of the product so that it becomes costly. They were not worries about the selling duration as they have facilities to store the products for a long period. However, vendors with low family income prefer to scavenge bottles from landfill and choose fast selling products with rapid profits such as drinking water and traditional juices. Although the traditional juices and water were reported to be sterilized respectively through boiling and boiling/chlorination, they are packaged in recovered plastic bottles for which the cleaning process is not always appropriate. This is liable to result in the contamination of these products. These observations suggest the formation and sensitization of traditional food producers as well as vendors of cleaned recovered plastic bottles on markets as the targets of policymakers.
5 Conclusion
The present study described the collecting and cleaning systems of recovered bottles used for packaging by local manufacturers of traditional food products. Bottles are mainly collected from garbage and six different cleaning processes are used by the manufacturers of traditional food products. The cleaning processes vary from one manufacturer to another according to some unit operations. The cleaning process was positively and significantly associated with the education level. Based on biofilms removal, the most efficient cleaning process is the process C (Sorting-soaking-superficial cleaning-internal cleaning-rinsing-disinfection-rinsing-drying-conditioning). The overall cleaning systems of recovered bottles used for traditional food packaging in the city of Yaoundé do not lead to complete removal of biofilms. This study suggests that recovered bottles used for food packaging might represent a potential health risk for consumers. Hence, further studies on the microbial profile of the biofilms found in these recovered plastic bottles should be performed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HM: conceptualization, experimentation, data curation, statistical analyses, writing–review and editing, project administration, and funding acquisition. JA: conceptualization, investigation, writing and review. RH: conceptualization, visualization, writing and review. AB: conceptualization, writing and review. AT: review and editing. JK: data collection and experimentation. LM: review and editing. PB: review and editing. RB: validation, review and editing. GM: resources, review and editing, supervision.
Funding
This research was funded by the International Foundation for Science (IFS) under grant reference No. I-3-E-6577-1.
Acknowledgments
Authors acknowledge the cooperation of the participants involved in this study who use recovered bottles to package their traditional food products. The authors thank the Director of the Institute of Medical Research and Medicinal Plant Studies for providing facilities for the successful completion of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frfst.2022.1060880/full#supplementary-material
References
Abrokwah, S., Ekumah, B., and Abrokwah, F. (2020). Microbial assessment of plastic bottles reused for packaging food products in Ghana. Food control. 109, 106956. doi:10.1016/j.foodcont.2019.106956
Ako, A. A., Shimada, J., Eyong, G. E. T., and Fantong, W. Y. (2010). Access to potable water and sanitation in Cameroon within the context of Millennium Development Goals (MDGS). Water Sci. Technol. 61 (5), 1317–1339. doi:10.2166/wst.2010.836
Andoh, P. Y., Fiagbe, Y. A. K., Davis, F., and Asaana, S. (2013). A mathematical model to predict the quantity of defective bottles in an automated bottle washer using factorial design technique. Int. J. Sci. Technol. Res. 2 (10), 56–62.
Baomog, A. M. B., Tchuenchieu, A. D., Nkoudou, Z. N., Sado, S. L. K., and Essia-Ngang, J. J. (2020). Impact of PVC and stainless-steel materials on Staphylococcus aureus biofilms production and their thermodeactivation as affected by pH, time and NaCl. Int. Res. J. Adv. Eng. Sci. 5 (2), 107–112.
Brooks, J. D., and Flint, S. H. (2008). Biofilms in the food industry: Problems and potential solutions. Int. J. Food Sci. Technol. 43, 2163–2176. doi:10.1111/j.1365-2621.2008.01839.x
Carrascosa, C., Raheem, D., Ramos, F., Saraiva, A., and Raposo, A. (2021). Maltitol: Analytical determination methods, applications in the food industry, metabolism and health impacts. Int. J. Environ. Res. Public Health 18, 5227. doi:10.3390/ijerph17145227
Colagiorgi, A., Bruini, I., Ciccio, P. A. D., Zanardi, E., Ghidini, S., and Ianieri, A. (2017). Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens 6, 41–49. doi:10.3390/pathogens6030041
Del Carpio-Perochena, A. E., Bramante, C. M., Duarte, M. A., Cavenago, B. C., Villas-Boas, M. H., Graeff, M. S., et al. (2011). Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J. Endod. 37 (8), 1134–1138. doi:10.1016/j.joen.2011.04.013
Djordjevic, D., Wiedmann, M., and McLandsborough, L. A. (2002). Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68 (6), 2950–2958. doi:10.1128/AEM.68.6.2950-2958.2002
Djoulde, D. R., Lendzemo, V., Essia-Ngang, J. J., and Etoa, F. X. (2013). Processing of “Kossam” an African sour fermented milk beverage from northern Cameroon. Ann. Food Sci. Technol. 14 (2), 261–269.
FAO (2016). Street food in urban Ghana: A desktop review and analysis of findings and recommendations from existing literature. Food and Agriculture Organization of the United Nations Accra, 91p Available at: http://www.fao.org/3/a-i5804e.pdf.
Gonzalez-Rivas, F., Ripolles-Avilan, C., Fontecha-Umanan, F., Ros-Castillon, A. G., and Rodriguez-Jerez, J. J. (2018). Biofilms in the spotlight: Detection, quantification, and removal methods. Compr. Rev. Food Sci. Food Saf. 17, 1261–1276. doi:10.1111/1541-4337.12378
Ingram, V. J., and Mala, A. W. (2010). Apiculture products in Cameroon. Mobilization and capacity building of small and medium enterprises in non-wood forest products value chains in Central Africa. Apiculture 1–6. doi:10.17528/cifor/004638
Köse, K., and Yapar, N. (2017). The comparison of various disinfectants’ efficacy on Staphylococcus aureus and Pseudomonas aeruginosa biofilm layers. Turk. J. Med. Sci. 47, 1287–1294. doi:10.3906/sag-1605-88
Kregiel, D. (2015). Health safety of soft drinks: Contents, containers, and microorganisms. Biomed. Res. Int. 2015, 1–15. doi:10.1155/2015/128697
Mouafo, T. H., Baomog, A. M. B., Adjele, J. J. B., Sokamte, T. A., Mbawala, A., and Ndjouenkeu, R. (2020). Microbial profile of fresh beef sold in the markets of ngaoundéré, Cameroon, and antiadhesive activity of a biosurfactant against selected bacterial pathogens. J. Food Qual. 2020, 1–10. doi:10.1155/2020/5989428
Nguendo Yongsi, H. B. (2018). Eating to live or eating to damage one’s health: Microbiological risks associated with street-vended foods in a subtropical urban setting (Yaoundé-Cameroon). Nutr. Food Sci. Int. J. 6 (4), 001–0013. doi:10.19080/NFSIJ.2018.06.555695
Nguendo Yongsi, H. B. (2010). Suffering for water, suffering from water: Access to drinking-water and associated health risks in Cameroon. J. Health Popul. Nutr. 28 (5), 424–435. doi:10.3329/jhpn.v28i5.6150
Oberbeckmann, S., Osborn, A. M., and Duhaime, M. B. (2016). Microbes on a bottle: Substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 11 (8), 1–24. doi:10.1371/journal.pone.0159289
O’Toole, G. A. (2011). Microtiter dish biofilm formation assay. J. Vis. Exp. 47, e2437. doi:10.3791/2437
Park, S. H., Cheon, H. L., Park, K. H., Chung, M. S., Choi, S. H., Ryu, S., et al. (2012). Inactivation of biofilm cells of foodborne pathogen by aerosolized sanitizers. Int. J. Food Microbiol. 154, 130–134. doi:10.1016/j.ijfoodmicro.2011.12.018
Pouokam, G. B., Foudjo, B. U. S., Samuel, C., Yamgai, P. F., Silapeux, A. K., Sando, J. T., et al. (2017). Contaminants in foods of animal origin in Cameroon: A one health vision for risk management “from farm to fork”. Front. Pub. Health 5, 197. doi:10.3389/fpubh.2017.00197
Rana, V. S., and Ahirrao, M. (2016). A study on street food preparation practices in Jalgaon City. IBMRD’s J. Manag. Res. 5, 40–32. doi:10.17697/ibmrd/2016/v5i2/100477
Satpathy, S., Sen, S. K., Pattanaik, S., and Raut, S. (2016). Review on bacterial biofilm: An universal cause of contamination. Biocatal. Agric. Biotechnol. 7, 56–66. doi:10.1016/j.bcab.2016.05.002
Simoes, M., Simoes, L. C., and Vieira, M. J. (2010). A review of current and emergent biofilm control strategies. LWT - Food Sci. Technol. 43, 573–583. doi:10.1016/j.lwt.2009.12.008
Skinner, C. (2011). AAPS planning education toolkit: The informal economy, 26p. Cape Town, South Africa: African Association of Planning Schools.
Skowron, K., Hulisz, K., Gryń, G., Olszewska, H., Wiktorczyk, N., and Paluszak, Z. (2018). Comparison of selected disinfectants efficiency against Listeria monocytogenes biofilm formed on various surfaces. Int. Microbiol. 21, 23–33. doi:10.1007/s10123-018-0002-5
Srey, S., Jahid, I. K., and Ha, S. D. (2013). Biofilm formation in food industries: A food safety concern. Food control. 31, 572–585. doi:10.1016/j.foodcont.2012.12.001
Stewart, P. S., Rayner, J., Roe, F., and Rees, W. M. (2001). Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl. Microbiol. 91, 525–532. doi:10.1046/j.1365-2672.2001.01413.x
Stojicic, S., Shen, Y., Qian, W., Johnson, B., and Haapasalo, M. (2012). Antibacterial and smear layer removal ability of a novel irrigant, QMiX. Int. Endod. J. 45, 363–371. doi:10.1111/j.1365-2591.2011.01985.x
String, G., Domini, M., Mirindi, P., Brodsky, H., Kamal, Y., Tatro, T., et al. (2020). Efficacy of locally-available cleaning methods in removing biofilms from taps and surfaces of household water storage containers. npj Clean. Water 3 (13), 13–11. doi:10.1038/s41545-020-0061-y
Tabeyang, E. (2018). Managing single-use land-based plastics in Cameroon: Recommendation drawn from global experiences. Master thesis. (Malmö, Sweden: World Maritime University), 78.
Keywords: recovered bottles, traditional food packaging, collection system, cleaning system, biofilms
Citation: Mouafo HT, Adjele JJB, Hell RH, Baomog AMB, Tchuenchieu AD, Kamgnia JAN, Manet L, Bonny P, Baleba RMM and Medoua GN (2022) Popular cleaning systems of bottles reused for traditional food packaging in the city of Yaoundé (Cameroon) and study of their prospective effectiveness on biofilms. Front. Food. Sci. Technol. 2:1060880. doi: 10.3389/frfst.2022.1060880
Received: 03 October 2022; Accepted: 29 November 2022;
Published: 14 December 2022.
Edited by:
Olivier Vitrac, INRA Centre Versailles-Grignon, FranceCopyright © 2022 Mouafo, Adjele, Hell, Baomog, Tchuenchieu, Kamgnia, Manet, Bonny, Baleba and Medoua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hippolyte T. Mouafo, aGlwcG9seXRlLnRlbmVAZ21haWwuY29t
 Hippolyte T. Mouafo
Hippolyte T. Mouafo Jorelle J. B. Adjele1
Jorelle J. B. Adjele1