
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Food. Sci. Technol. , 30 November 2022
Sec. Food Biotechnology
Volume 2 - 2022 | https://doi.org/10.3389/frfst.2022.1045579
This article is part of the Research Topic Traditionally Produced Fermented Foods and Innovative Technological Processes View all 5 articles
Kuruts are traditional acid-coagulated fermented dairy products in semisolid or dried form. The present study used probiotic Lacticaseibacillus rhamnosus GG (LGG) and Cryptococcus laurentii yeast, a kurut isolate, to make mixed-fermentation kurut. In addition, kurut was fermented with L. rhamnosus GG as starter. Kurut was also fermented with kefir grains representing a traditional way to initiate the fermentation. The viability of probiotic L. rhamnosus GG strain and physicochemical properties of semisolid kurut products were monitored during storage over 22 days at 4°C. The tested probiotic strain showed viability higher than 7 log CFU/mL in both mixed-fermentation and single-strain-fermentation kurut during storage at 4°C. When prepared with probiotic bacteria, the syneresis values of mixed-fermentation kurut were lower (p > 0.05) than single-strain-fermentation kurut. The flavor and overall acceptability scores for kurut prepared using the combination of probiotic LGG and C. laurentii yeast as starter culture were higher than for the other samples at 11 days of storage (p > 0.05). Taking the above into consideration, probiotic kuruts obtained by milk fermentation inoculated with co-culture of L. rhamnosus GG and C. laurentii yeast could be potential probiotic products to be produced in the dairy industry.
Kurut is an acid-coagulated dairy product from China, Turkey, and some Central Asian countries such as Kazakhstan (Kamber, 2008). In Kazakhstan, shipping fresh milk to a milk processing plant is difficult, so ranchers produce kurut in a semisolid or dried form similar to cottage cheese, dried yoghurt, and kashk from the fresh milk to increase its shelf life period. This popular fermented dairy product is known for unique attributes such as its health benefits, yeasty flavor, creamy density, acceptable shelf life, and desirable nutritional value (Kamber, 2008; Jafari et al., 2019; Wang et al., 2020). As a traditional fermented milk product, kurut is mostly produced from yak, cow, sheep, and goat milk by spontaneous fermentation in which lactic acid bacteria (LAB) and some yeast species play roles (Luo et al., 2011; Ispirli and Dertli, 2017). Kurut, which comes from the Turkish word meaning dried, is produced by drying yogurt or Ayran, which is produced in eastern parts of Turkey, in Turkic countries, and Iran (Karabulut et al., 2007; Ispirli and Dertli, 2017). In China, kurut is not only the traditional food, but also a raw material to make mar (a type of butter) and cula (a type of cheese) (Luo et al., 2011).
In order to produce kurut as the same as kashk, different starter cultures, including fermented milk, yoghurt, and kefir is used. Therefore, unique organoleptic sensations, microbial community, and textural characterizations can be different based on the used starter cultures. In the case of applying kefir grains as starter culture, bacterial communities embedded in kefir grain include Lacticaseibacillus, Lactobacillus, Lactococcus, Leuconostoc, and Streptococcus genera while yeast genera include Kluyveromyces, Candida, Saccharomyces, and Pichia, which produces lactic acid and alcohol, respectively, these products can be transferred in kurut products (Ismail et al., 2018; Jafari et al., 2019; Yousefvand et al., 2022). The mixed fermentation of LAB and yeast improve fermented products’ flavor attributes effectively. In addition, the combination of LAB and yeast as a starter culture is often used to manufacture multifunctional fermented foods (Adesulu-Dahunsi et al., 2020). This combination is used to produce aromatic components (Liu et al., 2020), increase the desirable nutritional trait (Luan et al., 2021), and produce functional aspects (Boudaoud et al., 2021).
Nowadays, probiotic fermented dairy products consumption has been increasing due to their health benefits for humans, including alleviation of lactose intolerance symptoms, improvement of stomach and colon health, anti-cholesterolemic, antimicrobial activities against gastrointestinal pathogens, as well as anti-inflammatory, antidiabetic, and antiangiogenic activities (Turroni et al., 2014; Amiri et al., 2021; Ghaderi-Ghahfarokhi et al., 2021). Dairy products sold with probiotic claims should meet at least 107 cfu/ml of viable populations at the time of consumption (Nyanzi et al., 2021).
Among common used probiotic bacteria in food industry, the Lacticaseibacillus rhamnosus GG (LGG) strain has been vastly researched. It has been documented as a non-motile, gram-positive, facultative heterofermentative, anaerobic, catalase-negative, and gram-positive microorganism (Valik et al., 2008; Oliveira et al., 2011). Moreover, It has been reported that the LGG strain can endure the acidic conditions of the gastrointestinal tract, which is known as a harsh environment for probiotics (Kareb and Aïder, 2019; Ghaderi-Ghahfarokhi et al., 2020). Several health benefits have been imputed to the LGG strain, including inhibition of indicator pathogens, modulating host immune system, and treatment of gastrointestinal infections (Nyanzi et al., 2021). Thus, many examinations have already been piloted to produce LGG-enriched products, such as Camembert-type cheese, yoghurt, buttermilk, salad dressing, sausage, and kefir (Rodgers, 2001; Galli et al., 2019; Yousefvand et al., 2022). It has been reported that C. laurentii yeast function as a biocontrol agent against fruit pathogens (Mateo et al., 2020). In addition, this yeast was isolated from Zimbabwean traditional fermented milks (Gadaga et al., 2000).
This study aimed to evaluate the viability of probiotic LGG and sensory attributes in semisolid single-strain-fermentation and mixed-fermentation kurut made with LGG in one-step fermentation and the combination of C. laurentii yeast isolated from kurut and probiotic LGG as starter cultures. Moreover, quality attributes, namely organoleptic, syneresis, and chemical properties of kurut products, were analyzed over 22-day storage at 4°C.
One sample of dried kurut was collected from Zhambyl region, Kazakhstan. The sample was transferred to the food microbiology lab of the University of Helsinki for the isolation and characterization of the yeast strains. Then, twenty yeast strains were isolated. Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, United States). The food sample was serially diluted with physiological saline solution. The pour-plate technique was employed using YPD medium 1% yeast extract (Acros Organics, NJ, United States), 2% peptone (Fisher Scientific, Janssen-Pharmaceuticalaan, Belgium), 2% glucose (Acumedia, Lansing, MI, United States), and the plates were aerobically incubated at 30°C for 2–3 days. Each colony isolate was subcultured in the YPD broth; subsequently, the stocks were prepared using glycerol (20% v/v) and then stored at −80°C.
The yeast identification systems API 20C AUX (BioMerieux, France) were used to assess the strain-specific pattern of carbon compound assimilation and other phenotypic assays. The API strips were prepared as described by the manufacturer’s instructions. The system contains 20 cupules of dehydrated reagents for biochemical tests and cupules, including negative control and glucose, giving a positive control. Freshly subcultured yeast strains on SDA plates were taken with a loop and added into the medium, which the manufacturer provided. The density of the suspension was standardized according to the standard of MacFarland 2. All cupules on the disposable plastic strip were filled with the suspension, incubated at 30°C for 48–72 h, and then inspected for growth daily.
Kurut was produced as described by Kök-Tas et al. (2013) (Erdogan et al., 2019) and Irigoyen et al. (2005). Kefir grains were obtained from prebiotic company (Tehran, Iran). The kefir grains were stored at −18°C and used after reactivation in commercial low-fat ultra-high temperature (UHT) milk [1.5% fat, 10.7% total solids (TS) content, and pH of 6.67] to obtain high amounts of the kefir grain biomass. The grains were inoculated in UHT milk at room temperature and kept for short periods; the medium was replaced with fresh UHT milk daily to maintain the grains’ viability. The grains were activated to obtain high amounts of the kefir grain biomass. Kefir grains were used to ferment milk for kefir production and for fermentation of kurut.
Before the kurut preparation, L. rhamnosus GG strain (LGG; ATCC53103) was routinely cultured in de Man, Rogosa, Sharpe (MRS; Oxoid, Basingstok, Hampshire, United Kingdom) medium at 37°C for 24 h under anaerobic conditions. Yeast strain, C. laurentii, was grown at 37°C for 24–48 h in an YPD medium. Following incubation, 50 μl of LGG and C. laurentii strain were sub-cultured in plastic tubes containing 50 ml of MRS and YPD broth, which were incubated at 37°C overnight anaerobically and 24–48 h aerobically, respectively. Next, the bacteria and C. laurentii biomass were harvested by centrifugation at 4,000 × g for 10 min at 20°C, and the cell sediment was washed twice with sterilized standard saline solution, resuspended in 10 ml of UHT milk, and used as LGG and C. laurentii cultures to produce kurut products. Three kurut formulations, including Control (with kefir grains), LGG-Cont (containing LGG), and LGG-CL (containing LGG and C. laurentii) were prepared by the procedure shown in Figure 1. Kurut samples containing kefir grains were prepared using 2% kefir grains at 37°C for 19 h. Then, samples were filtered by a cloth sieve to filter out the grains. Kurut samples containing LGG were prepared using 1% probiotic LGG with 3% glucose at 37°C for 19 h to reach the pH of ∼4.6. Kurut samples containing LGG and C. laurentii strain were prepared by two-step fermentation; in the first step, LGG was inoculated into UHT milk with 3% glucose and fermenting at 37°C for 19 h, and in the second step, C. laurentii strain was inoculated into fermented milk and incubated overnight at 37°C. After kurut production, semisolid samples were drained at room temperature for 1 and 3 days, respectively. Next, the kurut samples, cooled to 4°C and stored for 22 days, were then analysed for their probiotic LGG count and physicochemical properties on days 1, 5, 10, 15, and 22 of storage.
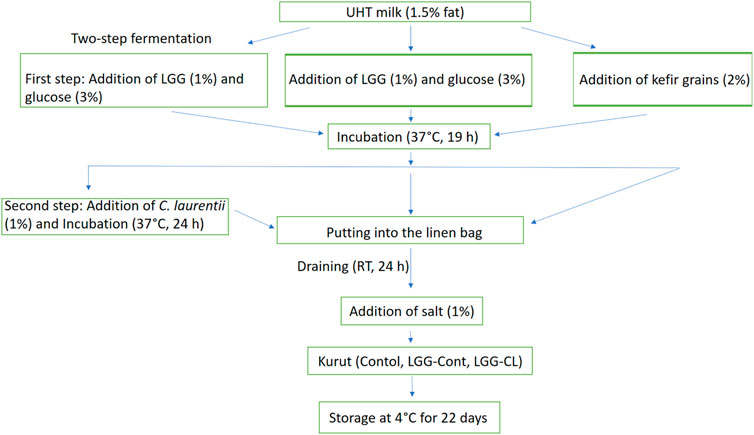
FIGURE 1. Manufacturing flowchart of different formulations of kurut. Control, Kurut produced with kefir grains; LGG-Cont, Kurut containing LGG; LGG-CL, Kurut containing LGG and C. laurentii; LGG, Lacticaseibacillus rhamnosus GG; RT, Room temperature.
The pH values of the kurut were measured using a pH-meter electrode (Thermo Orion Model-420A). Additionally, total titratable acidity (TTA) was determined by the AOAC official method (Horwitz and Latimer, 2005).
The syneresis values of the kurut samples were measured as recommended by Aryana (Aryana, 2003). Briefly, 100 g of each kurut batch was weighted on a fine mesh screen (14 μm) placed on top of a funnel. Syneresis is expressed as the amount of whey separated from the samples under the force of gravity at room temperature after 2 h of drainage into a flask of known weight, divided by the initial kurut mass.
The moisture content of the kurut samples was measured according to the AOAC official method (AOAC, 1995). Each kurut product (10 g) was placed in an oven at 105°C for 3 h. Next, reading was taken at a constant weight. The moisture content was then expressed as the percentage (%) of the dry weight of sample. Based on the weight of the residue obtained from moisture content analysis, the total solids of each of kurut samples was measured and expressed as the percentage (%) according to AOAC (AOAC International, 2006). The ash content of each of kurut samples was measured at 550°C according to AOAC (AOAC, 1995) and expressed as the inorganic residue left as a percentage of the total weight of kurut incinerated. The water activity of kurut samples were measured at 25°C using an aw-meter (Novasina LabMaster, Novasina, Switzerland).
The cell population of LGG was counted in produced kurut samples during storage at 4°C and expressed as log colony-forming units (CFU) per gram of the product (log CFU/g). First, 1 g of each sample was transferred into 9 ml of physiological saline solution and homogenized using a vortex mixer for 30 s. Samples were then serially diluted; using the spread-plate technique in MRS medium supplemented with 0.01% of cycloheximide, then, the plates were incubated at 37°C for 24–48 h in an anaerobic jar (Ghaderi Ghahfarokhi et al., 2020; Abouloifa et al., 2021).
Sensory evaluations were performed on the 11th day of storage using a panel of 15 semi-trained and experienced members (students, academic staff and faculty members at the University of Helsinki, Helsinki, Finland). Kurut samples were served to the panelists in 100-ml polyethylene cups bearing 3-digit random codes. Each sample was scored for flavor, body, texture, color, appearance, and overall acceptability individually on a 5-point hedonic scale ranging from 1 (dislike very much or unacceptable) to 5 (like very much or acceptable) on the eleventh day of storage. Evaluators were instructed to rinse their mouths with drinking water before tasting each sample.
All physicochemical measurements and microbial enumeration were carried out in triplicates. The data obtained for the physicochemical, microbial and organoleptic evaluation of kuruts were submitted to ANOVA using the General Linear Model procedure and were then reported as mean ± standard deviations. Tukey’s test was, in turn, used to compare the means, and significant differences were considered based on a p < 0.05. All statistical analyses were performed using Minitab 16 program (Minitab Inc., State College, PA, United States).
Several chemical attributes of kurut are presented in Table 1. As seen, average total solid values of the samples were 15.43%, 14.26%, and 14.73% respectively for kurut produced from kefir grains (Control), kurut containing LGG (LGG-Cont), and kurut containing LGG and C. laurentii (LGG-CL). As results of statistical analysis, there were no differences observed between them (p > 0.05). It was apparent to see that the kurut formulations had higher total solids than the yoghurt products reported by Karaca et al. (2019), displaying higher nutrient density in the kurut. Our results were in line with those of Zhang et al. (2008), who reported that the total solid contents of kurut were 14.2%. In another study, Kök-Taş et al. (2013) found the total solid content of kefir samples to range from 7.81 to 8.21, which was less than the total solid contents of Control kurut in our study. Therefore, the draining of whey may be the reason for higher total solid contents in kurut formulations compared to kefir produced by Kök-Taş et al. (2013). In the current study, the average ash content produced in LGG-CL, LGG-Cont and Control samples fermented were 1.76%, 1.72% and 1.68%, respectively, and there were no differences observed among them (p > 0.05) (Table 1). These values are similar to those reported by Montanuci et al. (2012) for Kes cheeses (1.1%), higher than the results reported by Amatayakul et al. (2006) for yoghurts enriched casein and whey protein (0.45%), and lower than the results reported by Kamber (2008) for dried kurut (9.9%). Ash content of LGG-CL was significantly higher than Control samples (p < 0.05). Longer fermentation periods may be associated with the higher ash content of LGG-CL than LGG-Cont and Control samples.
In our study, the moisture content of LGG-CL, LGG-Cont and Control samples were 27.06%, 28.3% and 28.6%, respectively, and showed non-significant differences among them (p > 0.05) (Table 1). The moisture values are similar to those reported by Seçkin et al. (2010) for dried yoghurt, higher than the findings reported by Kamber (2008) for kurut, and Jafari et al. (2019) for kashk. The moisture content of Control samples was slightly higher than LGG-CL and LGG-Cont samples (p > 0.05). Water activity level of LGG-CL, LGG-Cont and Control samples were found as 0.92, 0.91 and 0.93, respectively (Table 1). The aw values of all kurut samples steadily decreased during 22 days of storage at 4°C (results not included). Also, aw values of the kurut formulations made with kefir grains were significantly different (p < 0.05) from those with the added LGG probiotic, but no significant difference (p > 0.05) in aw contents between the LGG-CL and LGG-Cont formulations was observed. The ratio of aw levels obtained in this study was higher than the findings of Seçkin et al. (2010), who found 0.50 at first day of drying of dried yoghurt. In another research, Mollabashi and Atasever (2018) observed that the aw content of Kuruts was 0.97, which was higher than our findings. No significant difference (p > 0.05) in chemical properties was observed between the kurut made with probiotic LGG bacteria and the co-culture of LGG and C. laurentii.
By the API 20C system, a physiological characterization of the strain was carried out (Table 2).
With reference to API 20C AUX strip, the data were submitted into the Apiweb identification system, and then the isolated yeast was identified as C. laurentii. API Zym strip has not been designed for identification objectives (no option has been included in Apiweb software). The only purpose of this strip is to propose an enzymatic profile of the microorganism assayed.
The lactose fermentation and decreasing pH are associated with developing the basic structure and texture of fermented dairy products and breaking down the milk proteins (Sah et al., 2016; Yousefvand et al., 2022). The pH indexes of the semisolid kurut samples were assessed after 1, 5, 10, 15, and 22 days of storage at 4°C. The pH value of all kurut samples varied between 3.8 and 3.99 on day 1; these values dropped all over the storage duration, as expressed in other products such as yoghurt (Karaca et al., 2019; Ghaderi-Ghahfarokhi et al., 2021). pH values of Control, LGG-Cont, and LGG-CL kurut samples varied from 3.8 to 3.74, 3.94 to 3.72, and 3.99 to 3.75 throughout storage time, respectively (Figure 2A). These values were between those ratios reported by Kamber (2008), who noted the pH value from 3.6 to 4.9 for kurut samples.
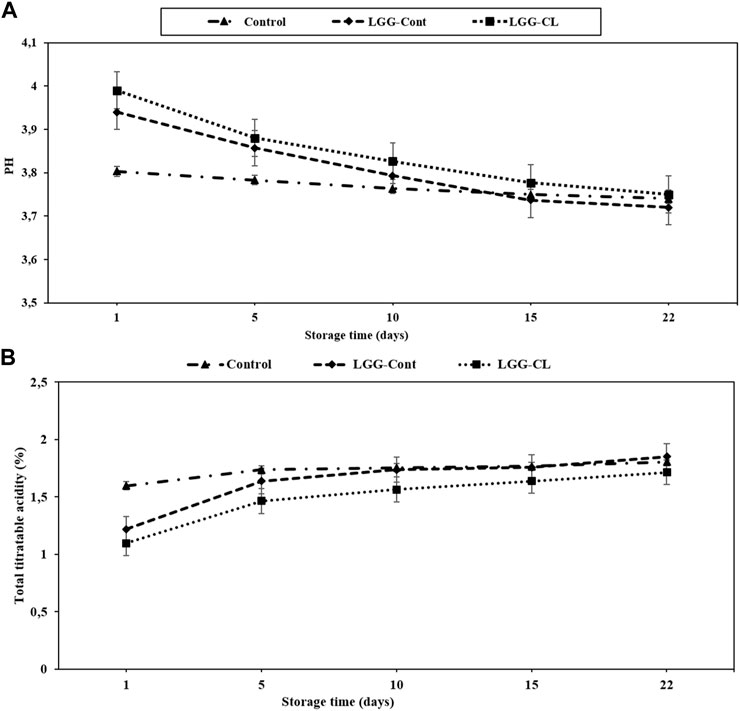
FIGURE 2. pH (A) and total titratable acidity (as lactic acid %) (B) of different formulations of kurut during storage at 4°C. Control, Kurut produced from kefir grains; LGG-Cont, Kurut containing LGG; LGG-CL, Kurut containing LGG and C. laurentii. Error bars represent the mean (n = 3) ± standard deviation (SD).
Generally, the mean pH values were similar between kurut with and without probiotic strain (p > 0.05) (Figure 2A), following results reported by Yousefvand et al. (2022), who also monitored a slight but insignificant decrease in pH values in kefir samples enriched with LGG strain. In our study, after 1 day of cold storage, pH values of the kurut samples made with kefir grains were significantly different (p < 0.05) from those with the added LGG probiotic and C. laurentii but during the storage period, the decrease in pH values was not significant (p > 0.05). Notably, the pH of kurut products was correlated with their acidity (Kök-Taş et al., 2013; Mitra and Ghosh, 2019; Yousefvand et al., 2022). Lactic acid is the most rampant acid produced by probiotic bacteria (Gunenc et al., 2016). In the current study, the TTA (%) of Control, LGG-Cont, and LGG-CL samples varied from 1.59 to 1.8, 1.21 to 1.85, and from 1.09 to 1.71, respectively, during the 22 days of storage period (Figure 2B). In a similar study but modified preparation procedure of kurut in terms of longer draining time, Kamber (2008) found the lactic acid content of kurut samples to range from 1.90 to 3.8. In our study, the TTA values of LGG-Cont samples were significantly higher than LGG-CL samples (p < 0.05) (Figure 2B). These differences likely take place owing to the yeast content restricting the propagation of LAB (Collar, 1996).
Regarding syneresis in the kurut samples for up to 22 days at 4°C (Figure 3), it was observed that the syneresis of all samples increased. Previous studies have demonstrated the extent of syneresis in different fermented dairy products such as yoghurt and kefir during storage time (Sah et al., 2016; Ghaderi-Ghahfarokhi et al., 2020; Yousefvand et al., 2022). In the current study, the syneresis indexes of Control samples ranged from 1.05 to 1.57, and hence a greater whey separation than LGG-Cont and LGG-CL kurut samples, whose syneresis values ranged from 0.24 to 0.39 and 0.24 to 0.36 at 5, 10, 15, and 22 days at 4°C (p < 0.05). These findings are in line with Yousefvand et al. (2022) and Montanuci et al. (2012), who reported more extensive syneresis of a fermented milk with kefir grains compared to the fermented milk with LGG strain and starter culture, respectively.
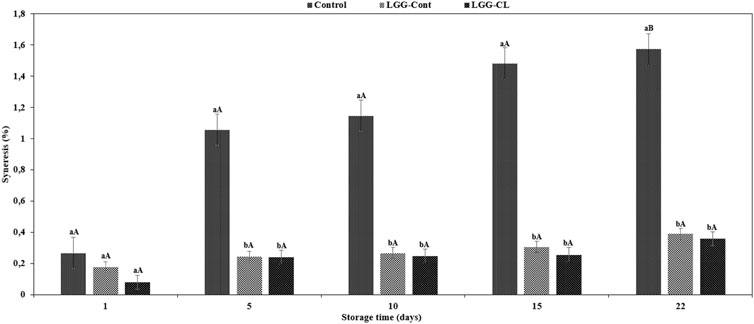
FIGURE 3. Syneresis (%) of different formulations of kurut during storage at 4°C. Control, Kurut produced from kefir grains; LGG-Cont, Kurut containing LGG; LGG-CL, Kurut containing LGG and C. laurentii. Different lowercase letters indicate significant differences (p < 0.05) between samples at the same storage time point, and same uppercase letters indicate non-significant differences (p > 0.05) between the storage days of each kefir sample. Error bars represent the mean (n = 3) ± standard deviation (SD).
Intriguingly, it was observed that the syneresis values of LGG-Cont and LGG-CL products exhibited non-significant differences during storage (p > 0.05). Nevertheless, on days 1, 10, 15, and 22 of the storage period, LGG-CL samples showed lower whey separation than LGG-Cont. In fact, it has been known that the C. laurentii produce exopolysaccharides, which are of interest due to their potential industrial use (Breierová et al., 2005). Therefore, the lower whey separation of LGG-CL samples may be contributed to higher exopolysaccharide amounts produced by the C. laurentii yeast compared to kurut made with LGG. It is generally recognized that several elements can contribute to this variable, namely accumulation of organic acids, post-acidification (Montanuci et al., 2012), kefir concentration (Delgadillo et al., 2017), kefiran concentration (Moradi and Kalanpour, 2019), total solid, and milk composition (Vareltzis et al., 2016).
The survival of LGG bacteria in fermented dairy products has already been reported (Mitra and Ghosh, 2019; Yousefvand et al., 2022). Despite the health benefits proposed for probiotic-enriched dairy products, the primary challenge is maintaining the survival rate and viability of probiotic bacteria above the critical threshold throughout cold storage and in the gut environment, which means that dairy products marketed as fermented probiotics should contain at least 107 cfu/ml of viable probiotic cells (Innocente et al., 2016; Fazilah et al., 2018).
The combination of LAB and yeasts during fermentation of non-dairy products is well documented (Santos et al., 2014; Ai et al., 2015; Freire et al., 2015; Menezes et al., 2018). It has been reported that yeasts are successfully employed as starter cultures for non-dairy beverage elaboration, producing compounds that confer sensory attributes such as satisfying aroma and flavor (Santos et al., 2014; Freire et al., 2015). To our knowledge, little is known about the combination of LAB and yeasts in dairy products. Hence, in the present study, we employed C. laurentii and LGG as starter culture to develop novel kurut and evaluated their viability during a storage time of 22 days at 4°C (Figure 4). After the first storage day at 4°C, LGG counts of LGG-Cont and LGG-CL samples were 7.75 and 7.98 log cfu/ml, respectively (Figure 4). As shown in Figure 4, LGG counts in LGG-Cont and LGG-CL samples indicated a constant increase up to 5 days of storage and the highest counts of probiotics were observed after 5 days in both LGG-Cont and LGG-CL samples. At this stage, the LGG counts of LGG-CL samples were a slight but insignificantly higher than in the LGG-Cont samples (8.54 and 8.37 log cfu/ml, respectively) (p > 0.05; Figure 4). These findings are in agreement with Menezes et al. (2018) who reported that L. paracasei LBC-81 counts in a cocktail culture with L. paracasei LBC-81 and S. cerevisiae CCMA 0731 were higher than in pure culture in maize-based beverages. The coexistence of LAB and yeasts in fermented foods is well known since yeasts provide some compounds such as amino acids and vitamins to LAB, and vice versa; their growth is favored by the lactic acid production imposed by LAB. In accordance with our LGG counts after 5 and 10 days of storage, Oliveira et al. (2001) and Yousefvand et al. (2022) similarly reported that the viability of L. rhamnosus GG bacteria ranged between 8 and 9 log cfu/ml in casein hydrolysate and milk protein, and kefir samples, respectively, after 7 days of storage. In another study, after 7 days of cold storage, researchers reported that LGG counts were between 7 and 8 log cfu/ml in co-culture with Streptococcus thermophilus (Oliveira et al., 2009). On the contrary, de Souza Oliveira et al. (2012) reported that the LGG viability decreased from 6.88 to 6.70 log cfu/ml in a co-culture with S. thermophilus, Lactobacillus acidophilus, Lactobacillus delbrueckii ssp. bulgaricus, and Bifidobacterium lactis from days 1 to 7 at 4°C.
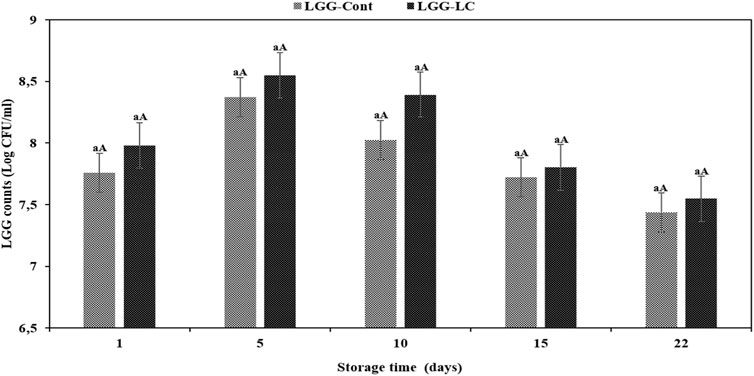
FIGURE 4. Viability of LGG in different formulations of kurut during storage at 4°C. LGG-Cont, Kurut containing LGG; LGG-CL, Kurut containing LGG and C. laurentii. The same lowercase letters are not significant differences (p > 0.05) between samples at the same storage time point; the same uppercase letters are not significant differences (p > 0.05) between the storage days of each kefir sample. Error bars represent the mean (n = 3) ± standard deviation (SD).
After 5 days of storage, LGG counts gradually decreased until 22 days of storage (p > 0.05), where the LGG final counts in LGG-Cont and LGG-CL formulations reached 7.43 and 7.54 log cfu/ml, respectively (Figure 4). LGG counts were similar between LGG-Cont and LGG-CL samples during storage (p > 0.05). It is supposed that pH reduction and post-acidification during storage of kefir samples (Figure 2) may adversely affect the survival rate of probiotic bacteria (Ghaderi-Ghahfarokhi et al., 2020; Nejati et al., 2020).
It is already well established that kurut can increase dietary protein intake with a traditional product with an extended shelf life after drying, as reported in several works. For instance, the protein content of kurut from fermented yak in three regions of Tibet was estimated to be 4.93 g/100 g (Chen et al., 2009), the mass fraction of protein in another studied kurut samples from Kyrgyz republic, Kyrgyzstan averaged 14.6% (Kochkorova and Kitarova, 2021) while another study reported protein ratio at (51%–60%) which was double what was earlier obtained (25.53%) in rural communities of Turkey (Kamber, 2008; Atasever and Atasever, 2018). Promoting the sensory acceptance and consumption of kurut will be critical to achieving its nutritional and commercial benefits. The scores given for organoleptic assessment of kurut samples are presented in Table 3. Applying of LGG and in co-culture with C. laurentii in kurut formulations was shown to not significantly change all of the investigated sensory properties of LGG-Cont and LGG-CL formulations compared to Control samples (p > 0.05). These results are in agreement with Mitra and Ghosh (2019), who reported that sensory characteristics of kefir did not change significantly after adding LGG bacteria in kefir products. However, Yousefvand et al. (2022) declared that the addition of probiotic LGG bacteria influenced the flavor of the final product. The flavor score of LGG-CL samples was higher than LGG-Cont and Control samples, indicating that the panelists favored lower acidity and yeasty flavor in kurut made from the co-culture of C. laurentii and LGG (Table 3). The superior flavor of LGG-CL may assumable be associated with lower acidity compared to the other kurut samples, which is evident from the pH and TTA parameters (Figure 2). In addition, yeasty flavor and higher acidity may lead to a lower flavor score in the Control samples; LGG-Cont samples were evaluated to have the lowest flavor scores. In agreement with our results, Yousefvand et al. (2022) reported a similar statement in LGG-enriched kefir products. Similarly, Hekmat and Reid (2006) stated that probiotic yoghurt samples enriched with Lactobacillus reuteri and L. rhamnosus bacteria also received lower flavor scores. It was shown that probiotic LGG bacteria can produce lactic acid, pyruvic acid, and orotic acid in probiotic products (Østlie et al., 2003; Østlie et al., 2005). In our study, the flavor scores in LGG-Cont samples were likely affected by organic acids produced by LGG bacteria.
Body and texture scores of all the kuruts showed no significant difference (p > 0.05) among the samples, although these scores for LGG-Cont and LGG-CL samples were lower than Control samples. The lower body and texture scores of kurut with probiotic LGG may be contributed to lower exopolysaccharide amounts produced by the LGG bacteria compared to kurut made with kefir grain containing a high amount of exopolysaccharide by a consortium of many bacteria (Kiekens et al., 2019; Moradi and Kalanpour, 2019). However, the color and appearance of all products did not differ significantly (p > 0.05), and they had a creamy consistency and viscous characteristics. The same observation was found for the overall acceptability index among the samples (p > 0.05). Studies on the effect of adding probiotic bacteria on sensory characteristics in different fermented products such as yoghurt and camembert-type cheese enriched with Bifidobacterium bifidum and LGG bacteria, respectively, showed no significant differences in appearance, body and texture, and overall acceptability compared to control samples (Galli et al., 2019; Ghaderi-Ghahfarokhi et al., 2021).
We manufactured a novel kurut by two-step fermentation using LGG and C. laurentii yeast. Single-strain-fermentation using LGG and mixed-fermentation kurut showed an average LGG counts of 7.86 and 8.05 log cfu/mL during 22 days of storage at 4°C, respectively. Along with this benefit, in sensory analysis at 11 days of storage, although kurut containing LGG and C. laurentii acquired the highest overall acceptability score near the top, other kurut products were assessed as having satisfactory sensorial acceptance. Based on the superior flavor, sensory attributes, and the viability of LGG observations, we suggest that mixed-fermentation kurut could potentially be used as a fermented dairy product, while the addition of LGG to kurut products did not significantly affect the body and texture attributes. Taken together, using mixed LGG and C. laurentii as potential starter culture is more convenient and applicable than retaining kefir grains and old kurut products in large-scale industrial production. This fermented dairy product has the potential to be developed as a novel food. Although more work is needed on rheological characteristics and organic acid profile of kurut.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
PS designed the conception of the study and arranged resources for research material via Magnus Ehrnrooth’s Foundation. AY and AT performed the experimentation and wrote the first draft of the manuscript. PS and AY edited the manuscript. AY performed the analysis and organized the database. All authors contributed to manuscript revision, read, and approved the submitted version.
AT is the recipient of the Bolashak International Scholarship of the President of the Republic of Kazakhstan. The Magnus Ehrnrooth’s Foundation is acknowledged for the stipend to support Per Saris research group. The authors thank the University of Helsinki for providing open access funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
LGG, Lacticaseibacillus rhamnosus GG; UHT, ultra-high temperature; Aw, water activity; TS, total solids; LAB, lactic acid bacteria; TTA, total titratable acidity; CFU, colony-forming units.
Abouloifa, H., Rokni, Y., Bellaouchi, R., Hasnaoui, I., Gaamouche, S., Ghabbour, N., et al. (2021). Technological properties of potential probiotic Lactobacillus strain isolated from traditional fermenting green olive. J. Microbiol. Biotechnol. Food Sci. 9 (5), 884–889. doi:10.15414/jmbfs.2020.9.5.884-889
Adesulu-Dahunsi, A. T., Dahunsi, S. O., and Olayanju, A. (2020). Synergistic microbial interactions between lactic acid bacteria and yeasts during production of Nigerian indigenous fermented foods and beverages. Food Control. 110, 106963. doi:10.1016/j.foodcont.2019.106963
Ai, J., Li, A. L., Su, B. X., and Meng, X. C. (2015). Multi-Cereal beverage fermented by Lactobacillus helveticus and Saccharomyces cerevisiae. J. Food Sci. 80 (6), M1259–M1265. doi:10.1111/1750-3841.12859
Amatayakul, T., Halmos, A. L., Sherkat, F., and Shah, N. P. (2006). Physical characteristics of yoghurts made using exopolysaccharide-producing starter cultures and varying casein to whey protein ratios. Int. Dairy J. 16 (1), 40–51. doi:10.1016/j.idairyj.2005.01.004
Amiri, S., Rezazadeh-Bari, M., Alizadeh-Khaledabad, M., Rezaei-Mokarram, R., and Sowti-Khiabani, M. (2021). Fermentation optimization for co-production of postbiotics by Bifidobacterium lactis BB12 in cheese whey. Waste Biomass Valorization 12 (11), 5869–5884. doi:10.1007/s12649-021-01429-7
AOAC (1995). in Official methods of analysis of the association of official analytical chemists. Vol II. 16th ed. (Arlington, VA: AOAC).
AOAC International (Editor) (2006). Official methods of analysis. 18th ed. (Arlington, VA: AOAC International).
Aryana, K. J. A. R. (2003). Folic acid fortified fat-free plain set yoghurt. Int. J. Dairy Technol. 56 (4), 219–222. doi:10.1046/j.1471-0307.2003.00105.x
Atasever, M. A., and Atasever, M. (2018). Some quality properties of kurut, a traditional dairy product in Turkey. Manas J. Agr. Vet. Life Sci. 8 (1), 68–74. Available at: https://dergipark.org.tr/en/pub/mjavl/issue/43031/521015.
Boudaoud, S., Aouf, C., Devillers, H., Sicard, D., and Segond, D. (2021). Sourdough yeast-bacteria interactions can change ferulic acid metabolism during fermentation. Food Microbiol. 98, 103790. doi:10.1016/j.fm.2021.103790
Breierová, E., Hromádková, Z., Stratilová, E., Sasinková, V., and Ebringerová, A. (2005). Effect of salt stress on the production and properties of extracellular polysaccharides produced by Cryptococcus laurentii. Z. Naturforsch. C J. Biosci. 60 (5–6), 444–450. doi:10.1515/znc-2005-5-613
Chen, Y., Sun, T., Wang, J., Airden, C., Bai, M., and Zhang, H. (2009). Comparison of nutrition and microbiological compositions between two types of fermented milk from Tibet in China. Int. J. Food Sci. Nutr. 60 (7), 243–250. doi:10.1080/09637480903005540
Collar, C. (1996). Review: Biochemical and technological assessment of the metabolism of pure and mixed cultures of yeast and lactic acid bacteria in breadmaking applications/Revisión: Aspectos bioquímicos y tecnológicos del metabolismo de cultivos puros y mixtos de levaduras y bacterias ácido lácticas en panificación. Food Sci. Technol. Int. 2 (6), 349–367. doi:10.1177/108201329600200601
de Souza Oliveira, R. P., Perego, P., de Oliveira, M. N., and Converti, A. (2012). Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT 47 (2), 358–363. doi:10.1016/j.lwt.2012.01.031
Delgadillo, J. O. V., de Jesús Luna Lara, M., Santillan, C. K. L., Sampieri, C. B., Micloth, L., and del Castillo, M. L. (2017). Physicochemical and rheological characterization of an acidic milk product: Kefir concentration effect. J. Food Sci. Eng. 7 (2), 86–92. doi:10.17265/2159-5828/2017.02.003
Erdogan, F. S., Ozarslan, S., Guzel-Seydim, Z. B., and Kök Taş, T. (2019). The effect of kefir produced from natural kefir grains on the intestinal microbial populations and antioxidant capacities of Balb/c mice. Food Res. Int. 115, 408–413. doi:10.1016/j.foodres.2018.10.080
Fazilah, N. F., Ariff, A. B., Khayat, M. E., Rios-Solis, L., and Halim, M. (2018). Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J. Funct. Foods 48, 387–399. doi:10.1016/j.jff.2018.07.039
Freire, A. L., Ramos, C. L., and Schwan, R. F. (2015). Microbiological and chemical parameters during cassava based-substrate fermentation using potential starter cultures of lactic acid bacteria and yeast. Food Res. Int. 76, 787–795. doi:10.1016/j.foodres.2015.07.041
Gadaga, T. H., Mutukumira, A. N., and Narvhus, J. A. (2000). Enumeration and identification of yeasts isolated from Zimbabwean traditional fermented milk. Int. Dairy J. 10 (7), 459–466. doi:10.1016/S0958-6946(00)00070-4
Galli, B. D., Baptista, D. P., Cavalheiro, F. G., and Gigante, M. L. (2019). Lactobacillus rhamnosus GG improves the sensorial profile of Camembert-type cheese: An approach through flash-profile and CATA. LWT 107, 72–78. doi:10.1016/j.lwt.2019.02.077
Ghaderi-Ghahfarokhi, M., Yousefvand, A., Ahmadi Gavlighi, H., Zarei, M., and Farhangnia, P. (2020). Developing novel synbiotic low-fat yogurt with fucoxylogalacturonan from tragacanth gum: Investigation of quality parameters and Lactobacillus casei survival. Food Sci. Nutr. 8 (8), 4491–4504. doi:10.1002/fsn3.1752
Ghaderi-Ghahfarokhi, M., Yousefvand, A., Ahmadi Gavlighi, H., and Zarei, M. (2021). The effect of hydrolysed tragacanth gum and inulin on the probiotic viability and quality characteristics of low-fat yoghurt. Int. J. Dairy Technol. 74 (1), 161–169. doi:10.1111/1471-0307.12742
Gunenc, A., Khoury, C., Legault, C., Mirrashed, H., Rijke, J., and Hosseinian, F. (2016). Seabuckthorn as a novel prebiotic source improves probiotic viability in yogurt. LWT - Food Sci. Technol. 66, 490–495. doi:10.1016/j.lwt.2015.10.061
Hekmat, S., and Reid, G. (2006). Sensory properties of probiotic yogurt is comparable to standard yogurt. Nutr. Res. 26 (4), 163–166. doi:10.1016/j.nutres.2006.04.004
Horwitz, W., and Latimer, G. W. (2005). Official methods of analysis. 18th ed. Washington, DC, USA: AOAC, 7.
Innocente, N., Biasutti, M., Rita, F., Brichese, R., Comi, G., and Iacumin, L. (2016). Effect of indigenous Lactobacillus rhamnosus isolated from bovine milk on microbiological characteristics and aromatic profile of traditional yogurt. LWT - Food Sci. Technol. 66, 158–164. doi:10.1016/j.lwt.2015.10.031
Irigoyen, A., Arana, I., Castiella, M., Torre, P., and Ibáñez, F. C. (2005). Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. x. 90 (4), 613–620. doi:10.1016/j.foodchem.2004.04.021
Ismail, Y. S., Yulvizar, C., and Mazhitov, B. (2018). Characterization of lactic acid bacteria from local cows milk kefir. IOP Conf. Ser. Earth Environ. Sci. 130 (1), 012019. doi:10.1088/1755-1315/130/1/012019
Ispirli, H., and Dertli, E. (2017). Isolation and characterisation of lactic acid bacteria from traditional koumiss and kurut. Int. J. Food Prop. 20, S2441–S2449. doi:10.1080/10942912.2017.1372473
Jafari, M., Rezaei, M., Gheisari, H. R., Abhari, K., Khaniki, G. J., Noori, N., et al. (2019). Application of cultivable lactic acid bacteria isolated from Iranian traditional dairy products for the production of liquid and dried kashks. LWT 116, 108519. doi:10.1016/j.lwt.2019.108519
Kamber, U. (2008). The manufacture and some quality characteristics of kurut, a dried dairy product. Int. J. Dairy Technol. 61 (2), 146–150. doi:10.1111/j.1471-0307.2008.00362.x
Karabulut, I., Adnan Hayaloglu, A., and Yildirim, H. (2007). Thin-layer drying characteristics of kurut, a Turkish dried dairy by-product. Int. J. Food Sci. Technol. 1 (9), 421080–421086. doi:10.1111/j.1365-2621.2006.01351.x
Karaca, O. B., Güzeler, N., Tangüler, H., Yaşar, K., and Akın, M. B. (2019). Effects of apricot fibre on the physicochemical characteristics, the sensory properties and bacterial viability of nonfat probiotic yoghurts. Foods 8 (1), E33–E15. doi:10.3390/foods8010033
Kareb, O., and Aïder, M. (2019). Whey and its derivatives for probiotics, prebiotics, synbiotics, and functional foods: A critical review. Probiotics Antimicrob. Proteins 11 (2), 348–369. doi:10.1007/s12602-018-9427-6
Kiekens, S., Vandenheuvel, D., Broeckx, G., Claes, I., Allonsius, C., De Boeck, I., et al. (2019). Impact of spray-drying on the pili of Lactobacillus rhamnosus GG. Microb. Biotechnol. 12 (5), 849–855. doi:10.1111/1751-7915.13426
Kochkorova, F. A., and Kitarova, G. S. (2021). Nutrional value of the national dairy product kurut and its place in the nutrition of adolescents of the Kyrgyz Republic. Vopr. Pitan. 90 (5), 87–95. doi:10.33029/0042-8833-2021-90-5-87-95
Kök-Taş, T., Seydim, A. C., Özer, B., and Guzel-Seydim, Z. B. (2013). Effects of different fermentation parameters on quality characteristics of kefir. J. Dairy Sci. 96 (2), 780–789. doi:10.3168/jds.2012-5753
Liu, N., Miao, S., and Qin, L. (2020). Screening and application of lactic acid bacteria and yeasts with L-lactic acid-producing and antioxidant capacity in traditional fermented rice acid. Food Sci. Nutr. 8 (11), 6095–6111. doi:10.1002/fsn3.1900
Luan, C., Zhang, M., Devahastin, S., and Liu, Y. (2021). Effect of two-step fermentation with lactic acid bacteria and Saccharomyces cerevisiae on key chemical properties, molecular structure and flavor characteristics of horseradish sauce. LWT 147, 111637. doi:10.1016/j.lwt.2021.111637
Luo, F., Feng, S., Sun, Q., Xiang, W., Zhao, J., Zhang, J., et al. (2011). Screening for bacteriocin-producing lactic acid bacteria from kurut, a traditional naturally-fermented yak milk from Qinghai-Tibet plateau. Food control. 22 (1), 50–53. doi:10.1016/j.foodcont.2010.05.006
Mateo, J. J., Garcerà, P., and Maicas, S. (2020). Unusual non-Saccharomyces yeasts isolated from unripened grapes without antifungal treatments. Fermentation 6, 41. doi:10.3390/fermentation6020041
Menezes, A. G. T., Ramos, C. L., Dias, D. R., and Schwan, R. F. (2018). Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 111, 187–197. doi:10.1016/j.foodres.2018.04.065
Mitra, S., and Ghosh, B. C. (2019). Quality characteristics of kefir as a carrier for probiotic Lactobacillus rhamnosus GG. Int. J. Dairy Technol. 70, 384–391. doi:10.1111/1471-0307.12664
Mollabashi, N. M., and Atasever, M. A. (2018). Microbiological and chemical properties of kurut (Kishk) samples collected from Iranian. Ataturk Univ. Vet. Bilim. Derg. 13 (1), 70–76. doi:10.17094/ataunivbd.292589
Montanuci, F. D., Pimentel, T. C., Garcia, S., and Prudencio, S. H. (2012). Effect of starter culture and inulin addition on microbial viability, texture, and chemical characteristics of whole or skim milk kefir. Food Sci. Technol. 32 (4), 580–865. doi:10.1590/s0101-20612012005000119
Moradi, Z., and Kalanpour, N. (2019). Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 223 (1), 115100. doi:10.1016/j.carbpol.2019.115100
Nejati, F., Junne, S., and Neubauer, P. (2020). A big world in small grain: A review of natural milk kefir starters. Microorganisms 8 (2), E192. doi:10.3390/microorganisms8020192
Nyanzi, R., Jooste, P. J., and Buys, E. M. (2021). Invited review: Probiotic yogurt quality criteria, regulatory framework, clinical evidence, and analytical aspects. J. Dairy Sci. 104 (1), 1–19. doi:10.3168/jds.2020-19116
Oliveira, M. N., Sodini, I., Remeuf, F., and Corrieu, G. (2001). Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. Int. Dairy J. 11 (11–12), 935–942. doi:10.1016/S0958-6946(01)00142-X
Oliveira, R. P., Perego, P., Converti, A., and De Oliveira, M. N. (2009). The effect of inulin as a prebiotic on the production of probiotic fibre-enriched fermented milk. Int. J. Dairy Technol. 62 (2), 195–203. doi:10.1111/j.1471-0307.2009.00471.x
Oliveira, R. P., Perego, P., De Oliveira, M. N., and Converti, A. (2011). Effect of inulin as a prebiotic to improve growth and counts of a probiotic cocktail in fermented skim milk. LWT - Food Sci. Technol. 44 (2), 520–523. doi:10.1016/j.lwt.2010.08.024
Østlie, H. M., Helland, M. H., and Narvhus, J. A. (2003). Growth and metabolism of selected strains of probiotic bacteria in milk. Int. J. Food Microbiol. 87 (1–2), 17–27. doi:10.1016/S0168-1605(03)00044-8
Østlie, H. M., Treimo, J., and Narvhus, J. A. (2005). Effect of temperature on growth and metabolism of probiotic bacteria in milk. Int. Dairy J. 15 (10), 989–997. doi:10.1016/j.idairyj.2004.08.015
Rodgers, S. (2001). Preserving non-fermented refrigerated foods with microbial cultures - a review. Trends Food Sci. Technol. 12 (8), 276–284. doi:10.1016/S0924-2244(01)00093-0
Sah, B. N. P., Vasiljevic, T., McKechnie, S., and Donkor, O. N. (2016). Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT - Food Sci. Technol. 65, 978–986. doi:10.1016/j.lwt.2015.09.027
Santos, C. C. A. do A., Libeck, B. da S., and Schwan, R. F. (2014). Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int. J. Food Microbiol. 186, 32–41. doi:10.1016/j.ijfoodmicro.2014.06.011
Seçkin, A. K., Ergönül, B., Tosun, H., and Ergönül, P. G. (2010). Effects of prebiotics (inulin and fructooligosaccharide) on quality attributes of dried yoghurt (kurut). Food Sci. Technol. Res. 15 (6), 605–612. doi:10.3136/fstr.15.605
Turroni, F., Duranti, S., Bottacini, F., Guglielmetti, S., van Sinderen, D., and Ventura, M. (2014). Bifidobacterium bifidum as an example of a specialized human gut commensal. Front. Microbiol. 5, 437–438. doi:10.3389/fmicb.2014.00437
Valik, L., Medvedova, A., and Liptakova, D. (2008). Characterization of the growth of Lactobacillus rhamnosus GG in milk at suboptimal temperatures. J. Food Nutr. Res. 47 (2), 60–67.
Vareltzis, P., Adamopoulos, K., Stavrakakis, E., Stefanakis, A., and Goula, A. M. (2016). Approaches to minimise yoghurt syneresis in simulated tzatziki sauce preparation. Int. J. Dairy Technol. 69 (2), 191–199. doi:10.1111/1471-0307.12238
Wang, B., Wang, J., Xu, L. Y., Zhang, J. H., Ai, N. S., and Cao, Y. P. (2020). Characterization of the key odorants in kurut with aroma recombination and omission studies. J. Dairy Sci. 103 (5), 4164–4173. doi:10.3168/jds.2019-17521
Yousefvand, A., Huang, X., Zarei, M., and Saris, P. E. J. (2022). Lacticaseibacillus rhamnosus GG survival and quality parameters in kefir produced from kefir grains and natural kefir starter culture. Foods 11 (4), 523. doi:10.3390/foods11040523
Keywords: Cryptococcus laurentii, kefir grain, traditional food, kashk, qurt, khuruud, aaruul
Citation: Tuganbay A, Yousefvand A and Saris PEJ (2022) Production of kurut (kurt) using probiotic Lacticaseibacillus rhamnosus GG strain in combination with a yeast isolated from Kazakhstan kurut. Front. Food. Sci. Technol. 2:1045579. doi: 10.3389/frfst.2022.1045579
Received: 15 September 2022; Accepted: 21 November 2022;
Published: 30 November 2022.
Edited by:
Dele Raheem, University of Lapland, FinlandReviewed by:
Ömer Şimşek, Yıldız Technical University, TurkeyCopyright © 2022 Tuganbay, Yousefvand and Saris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Per Erik Joakim Saris, cGVyLnNhcmlzQGhlbHNpbmtpLmZp
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.