
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Food. Sci. Technol. , 09 November 2022
Sec. Food Safety and Quality Control
Volume 2 - 2022 | https://doi.org/10.3389/frfst.2022.1033814
Salmonella enterica contamination of low water activity foods (LWAFs) has resulted in recalls of spices, herbs, and seeds and outbreaks of salmonellosis. To improve the safety of these ready-to-eat products, new treatment methods, including fumigation with chlorine dioxide (ClO2) or hydrogen peroxide (H2O2) gas are being explored, and effectiveness determined. To prevent overestimation of treatment effectiveness, it is vital that recovery methods should accurately quantify all viable cells, even those injured. This study evaluated different media and supplements for the recovery of multiple strains of S. enterica and Enterococcus faecium NRRL B2354, from ClO2 or H2O2 treated black peppercorns, dried basil leaves, and chia seeds. Also, this study aimed to compare the log reduction of these two microorganisms to evaluate E. faecium NRRL B2354, as a surrogate for S. enterica. On average, recovery of S. enterica was improved by 1 log CFU from ClO2 and H2O2 treated LWAFs when a non-selective but differential media containing tryptic soy agar with yeast extract, ammonium iron citrate and sodium thiosulfate (MTSAYE) was used, when compared to plating on XLD (p < 0.05). Furthermore, addition of sodium pyruvate, ferrous sulfate, or 3’3’-thiodiproionate supplements to MTSAYE did not show increased recovery of either S. enterica or E. faecium NRRL B2354 (p > 0.05). On each treatment and LWAF combination tested, there was no significant difference between the log reduction of S. enterica and E. faecium NRRL B2354, indicating its suitability as a surrogate under the test conditions.
Spices, herbs, and edible seeds are minimally processed agricultural products at high risk for bacterial contamination during pre and post handling activities (Gurtler et al., 2019). Salmonella enterica can survive on low water activity foods (LWAF), like spices, for months (Santillana-Farakos and Frank, 2014, Xie et al., 2022). S. enterica contamination of spices have caused several outbreaks and led to costly recalls (Van Doren et al., 2013). Because spices, herbs and seeds are “ready to eat”, processors in the United States must conduct a hazard analysis and implement preventive controls for those hazards (USFDA, 2018).
Interventions that target the reduction of Salmonella and other foodborne pathogens on spices, herbs and seeds include irradiation, steam treatment, or chemical fumigation (ASTA, 2017). Chemical fumigation has the advantage of having a minimal impact on flavor and appearance of the treated spice (Kim et. al, 2016). Gaseous sanitizers are more diffusible than in aqueous form, resulting in better microorganism control on food surfaces. Ethylene oxide fumigation can inactivate Salmonella on spices (ASTA, 2017), however health concerns due to ethylene chlorohydrin byproducts have limited its use in the United States and resulted in a ban in Europe (Schweiggert et. al., 2007). Gaseous chlorine dioxide (ClO2) and hydrogen peroxide (H2O2), used for disinfection of medical equipment and produce, may be a replacement for Salmonella control on LWAF (Rane et. al, 2020). Gaseous ClO2 will not add moisture to low aw foods and has increased penetration ability through the rough surface of the spice product itself, compared to aqueous ClO2 (Wason et al., 2021). Gaseous ClO2 is effective against Salmonella and other foodborne pathogens on almonds and whole peppercorns (Chai et al, 2021; Wei et. al, 2021). Gaseous H2O2 is safer than ethylene oxide or ClO2, as it breaks down into oxygen and water with minimal effect on the sensory characteristics of a food, while inactivating Salmonella and other pathogens on fresh produce (Back et al., 2014).
Reactive oxygen species released from oxidizing agents such as ClO2 and H2O2, alter bacterial proteins, disrupt membranes, and may result in cell injury and death (McDonnell and Russell, 2001). Sub-lethally injured cells may still be pathogenic, but unable to grow on media containing selective agents, like sodium deoxycholate (Gurtler and Kornacki, 2009). Injury can be repaired under the right conditions and provided ample time. Growth promoters and antioxidants may be used to promote repair of sub-lethally injured cells (Wu, 2008). Antioxidants, including sodium thiosulfate, are frequently added to samples that may contain residual sanitizers to prevent additional sub-lethal damage during sample preparation (Lillard, 1979); a similar release of radicals may occur, with chlorine dioxide and hydrogen peroxide. Yeast extract, ferrous sulfate, 3,3-thiodipropionic acid, sodium pyruvate, lactate, mannitol and glycerolphosphate have also been shown to result in increased recovery of heat damaged S. enterica, and may provide a strategy to promote repair of chemically damaged cells (Gurtler and Kornacki, 2009). Demonstration that control strategies for microbial hazards are effective is essential for food safety, and the use of methods that promote the recovery of sub-lethally injured cells is critical.
This study compared different plating media and supplements for the recovery of S. enterica and Enterococcus faecium cells from antimicrobial gas treated LWAF. S. enterica and E. faecium NRRL B2354 inoculated black peppercorn, dried basil leaves, and chia seeds were treated with chlorine dioxide or hydrogen peroxide. S. enterica recovered using XLD, a commonly used selective media, and MTSAYE, a non-selective but differential media were compared. Sodium thiosulfate and ammonium iron citrate were included in the MTSAYE formula to make the resulting media differential for S. enterica and E. faecium. These microorganisms break down thiosulfate into sulfite and H2S gas, H2S reacts with the ferric ions in ammonium iron citrate to produce a black precipitate in the center (McLaughlin and Balaa, 2006). Additionally, different antioxidants were added to recovery media in attempt to further improve recovery of S. enterica and E. faecium cells that have been sub-lethally injured by antimicrobial gas treatment. Recovery of S. enterica was compared with E. faecium NRRL B2354 to determine the latter’s suitability as a surrogate organism.
A previously described cocktail of S. enterica (Agona 447967, Reading Moff 180418, Tennessee K4643, Montevideo 488275, Mbandaka 698538) strains chosen because of their association in low moisture food outbreaks or for their high thermal resistance properties was used (Wei et al., 2021). E. faecium NRRL B2354 was evaluated as a potential non-pathogenic surrogate for the S. enterica cocktail, as the microorganism has showed promise as a surrogate for S. enterica under similar treatments (Wei et al., 2021), and was obtained from the US Department of Agriculture, Agricultural Research Service (USDA, ARS) in Peoria, IL.
Strains were grown overnight individually in trypticase soy broth with added 0.6% (w/w) yeast extract, then lawns were prepared by spread plating 0.1 ml on tryptic soy agar with added 0.6% (w/w) yeast extract, and incubated at 37°C for 24 h. The bacterial lawns were harvested using 3 ml of 0.1% (w/w) buffered peptone water. The S. enterica cocktail was produced by mixing in equal proportions cells from each preparation yielding 10.50 ± 0.10 log CFU/ml.
Black peppercorns and dried basil leaves originating from three different lots were obtained pre-sterilized from McCormick Inc. (Baltimore, MD, United States). A mix of black and white chia seeds (Organic chia seeds, BetterBody foods, Utah, United States) originating from different lots were purchased. Initial water activity of the samples was determined using a dew point water activity meter (Model: 4TE, Meter Group; 25°C).
Inoculation procedures of black peppercorns, basil leaves, and chia seeds has been previously described by (Wei et al, 2021; Verma et al., 2022; Lau et al, 2021) respectively. Briefly, 6 ml of S. enterica cocktail or E. faecium inoculum were individually sprayed over a thin layer of sample (300 g). Bags containing inoculated samples were hand massaged for 5 min and then hand shaken for 5 min to ensure uniformity of bacterial inoculum. All samples were dried in a custom designed relative humidity (RH) equilibrium chamber (Lau and Subbiah, 2020). RH set to 55% for black peppercorn and dried basil leaves, and 53% for chia seeds. After drying the average log CFU/g was: 7.41 ± 0.23 (Sal) and 7.74 ± 0.27 (EF) on black peppercorns, 7.81 ± 0.03 (Sal) and 7.93 ± 0.11 (EF) on dried basil leaves and 7.96 ± 0.17 (Sal) and 8.01 ± 0.23 (EF) in chia seeds.
LWAF samples were ClO2 gas treated as described by (Verma et. al., 2022) at the University of Nebraska (Lincoln, NE). A Minidox-M system (ClorDisys) was used to generate ClO2 gas, monitor and maintain concentration, and maintain RH throughout treatment. A polypropylene chamber with dimensions (L × W × H = 0.73 m × 0.44 m × 0.68 m) was obtained from ClorDiSys Solutions, Inc., (Branchburg, NJ). A temperature and RH inducer (Model 6621, Testo, Titisee-Neustadt, Germany) was installed on the chamber to monitor temperature and RH. Humid air was added to the chamber via pipe using an ultrasonic humidifier (EE-5301, Crane, Itasca, IL). Two fans (38HX82; Grainger, China) were placed at opposite ends of the chamber to circulate ClO2 gas.
Two grams of inoculated sample were placed in the chamber packed in heat sealed paper bags. Treatment was conducted with 70% RH and a gas concentration of 10 mg/L with a 2-h exposure. Following aeration, samples were removed from the chamber, sealed in sterile bags, and sent to Virginia Tech (Blacksburg, VA) on the same day as processing. Enumeration occurred within 24–36 h post processing.
H2O2 treatment of dried basil leaves were performed at the University of Nebraska. A treatment chamber made of polystyrene with the dimensions (L × W × H = 0.35 m × 0.30 m × 0.27 m) was fitted with a vaporized H2O2 (VHP) generator (Bioquell L-2, Horsham, PA, United States). A closed loop was made by attaching the inlet and outlet hoses of the generator to opposite sides of the chamber with a camlock connection. H2O2 gas sterilization process has a conditioning phase, a gassing phase, a dwelling phase, and an aeration phase. In the conditioning phase, temperature and RH are conditioned to stable target values. After a pre-set conditioning time, the gassing phase begins. Liquid H2O2 is pumped at a set rate onto a hot surface, producing VHP. VHP is circulated with the airstream into and out of the treatment chamber continuously by the supply hoses for a set amount of time. A dwell phase of no injection of VHP was set to increase residence time of VHP in the chamber. Finally, filtered air was circulated in the chamber for at least 1 h to remove VHP in the aeration phase. Following this, the chamber could be safely opened to retrieve samples.
Treatment was conducted with an injection rate of 3 g/min and an air flow rate of 10 m3/h at a temperature of 40°C. Dried basil leaf samples were exposed for 5 min with a dwell time of 30 min. Following removal from treatment chamber, dried basil leaf samples were packed and shipped to Virginia Tech for enumeration within 24–36 h of processing.
The selective media Xylose Lysine Deoxycholate (XLD) (Becton Dickinson, Franklin Lakes, NJ) was prepared per the manufacturer’s instruction. MTSAYE was created by combining Tryptic Soy Agar (TSA) (Becton Dickinson) powder (40 g/L), with 6 g/L of yeast extract (Remel Inc., San Diego, CA), 0.75 g/L ammonium iron citrate (Sigma-Aldrich, St. Louis, MO), and 0.3 g/L sodium thiosulfate (Fisher Scientific, Kansas City, MO) in 1 L of deionized water. This solution boiled 1 min before autoclaved at 121°C for 15 min. Supplements previously shown by (Gurtler and Kornacki, 2009) to improve recovery were chosen. MTSAYE-NP media was made using the components of MTSAYE and 1 g/L sodium pyruvate (Fisher Scientific) then mixed in 1 L of deionized water before boiling and autoclaving. MTSAYE-FS media was made using the components of MTSAYE and 1 g/L ferrous sulfate (Fisher Scientific). MTSAYE-TDP media was made using the components of MTSAYE and 1 g/L of 3’3’- thiodipropionate (Acros Organics, Carlsbad, CA).
Black peppercorns, dried basil leaves and chia samples (5 g) were weighed into a stomacher bag containing 45 ml of neutralizing buffer (BD Life Sciences) for black peppercorn and basil leaves samples, and 145 ml of neutralizing buffer for chia seed samples (Lau et al., 2021). For whole black peppercorn samples treated with chlorine dioxide plate counts were performed using neutralizing buffer or 0.1% sterile peptone (Sigma). Samples were processed for 1 min at a speed setting of 1 in a lab blender (Bagmixer 400, Interscience, Guelph, Ontario). The liquid diluent for all samples, except chia seed samples, were then vacuum filtered through #4 qualitative paper to remove spice particles. The filtrate was then serially diluted in sterile neutralizing buffer and 100 μl plated onto MTSAYE, MTSAYE-NP, MTSAYE-FS, MTSAYE-TDP, and XLD (Becton Dickinson), in duplicate on final plate dilutions ranging from 10−1 through 10−7. XLD media was only used for S. enterica inoculated samples. All plates were incubated at 37°C for 48 h before enumeration. The limit of detection was 1 log CFU/g.
The two treatment methods were repeated in triplicate for each spice using freshly prepared spices and plated on five media types. For each replicate, duplicate plating was performed, and the average reported for each media type. Bacterial counts were log transformed, and for each spice/treatment/media combination the log CFU/g of treated samples were subtracted from log CFU/g of untreated samples to obtain a log CFU/g reduction for use in statistical analysis. The untreated controls were inoculated on the same day as the treated product and enumerated on the day of receipt of the treated product to account for any differences.
To compare the effect of different media types on S. enterica recovery, the log reduction CFU/g values of S. enterica plated on MTSAYE, MTSAYE-NP, MTSAYE-FS, MTSAYE-TDP, and XLD from each spice and treatment type were compared. Log reduction CFU/g of E. faecium NRRL B2354 plated on MTSAYE, MTSAYE-NP, MTSAYE-FS, and MTSAYE-TDP from each spice type were compared. Within each spice type, but not between spice types. Spice type and treatment were considered as main factors and media type as the split factor. ANOVA, followed by pairwise comparisons [Tukey’s Honestly Significant Difference (HSD)] was used to determine significant differences between the log reductions calculated for each treatment and media type. Spice type and treatment were considered as main factors and media type as the split factor. The significance level of p < 0.05 was used. Analysis was performed using JMP (version 14, SAS, Cary, NC).
To compare the log reductions of the two microorganisms, the log reduction CFU/g of S. enterica plated on MTSAYE was compared to the log reduction CFU/g of E. faecium NRRL B2354 plated on MTSAYE from each spice and treatment. An ANOVA was performed followed by Tukey’s HSD test to determine if there was a significant (p < 0.05) difference in the log reduction of each microorganism associated with the two different treatments.
On whole black peppercorn samples treated with ClO2 the average log reductions of S. enterica plated on each media type were comparable when plated on MTSAYE, MTSAYE-NP, MTSAYE-FS, MTSAYE-TDP (Figure 1A). On average, the log reduction of S. enterica was 1.0 log greater when plated on XLD, compared to the non-selective media types (Figure 1A) (p = 0.04). Comparable recoveries were seen when neutralizing buffer and 0.1% sterile peptone were used as a diluent (results not shown). Average log reductions of E. faecium NRRL B2354 plated on the different media were not significantly different (p = 0.18) from each other regardless of media type (Figure 1B).
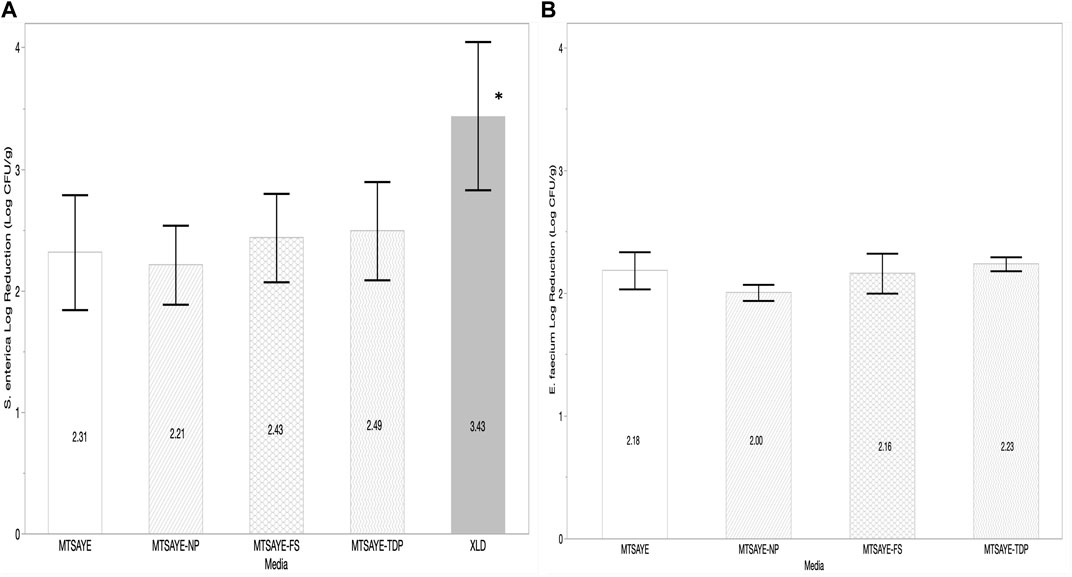
FIGURE 1. Comparison of the mean log reduction of S. enterica (log CFU/g) (A) and E. faecium (log CFU/g) (B) plated on different media formulations recovered from whole black peppercorns treated with ClO2 fumigation (n = 3). Each error bar represents one standard deviation from the mean. Figure abbreviations: Modified TSA with Yeast extract (MTSAYE), MTSAYE with added Sodium Pyruvate (MTSAYE-NP), MTSAYE with added ferrous sulfate (MTSAYE-FS), MTSAYE with added 3’3’-thiodipropionate (MTSAYE-TDP), Xylose Lysine Deoxycholate (XLD). The * indicates statistical difference p < 0.05.
On basil leave samples treated with ClO2, average S. enterica log reductions were similar when plated on non-selective media with supplements (Figure 2A). S. enterica log reduction was 0.5–0.6 log greater when plated on XLD, compared to the non-selective media types (p = 0.01). The average log reduction of E. faecium NRRL B2354 plated on the different media were not significantly different (p = 0.82) from each other (Figure 2B).
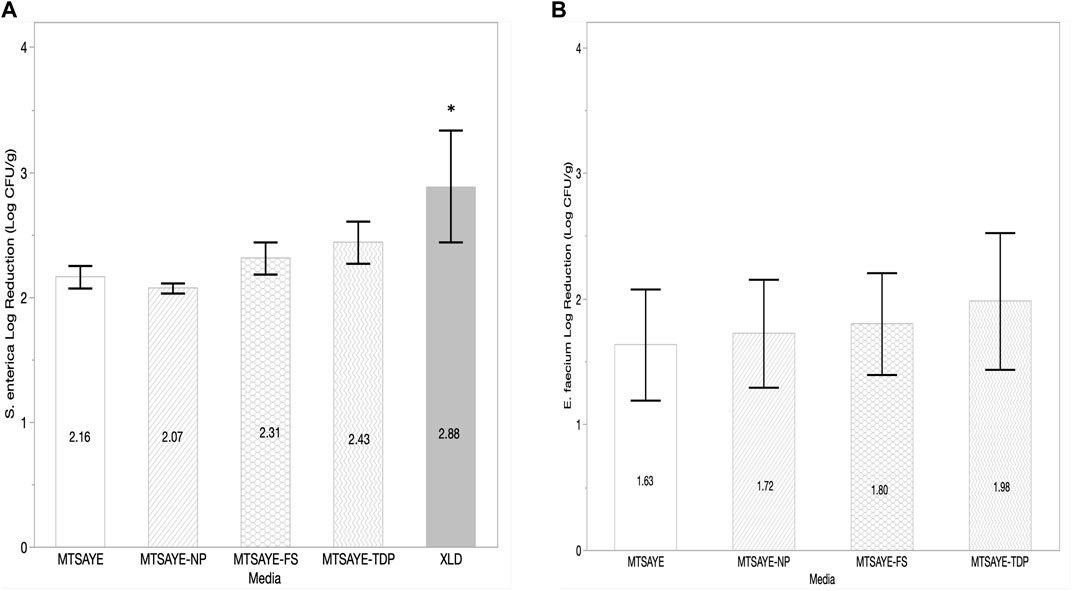
FIGURE 2. Comparison of the mean log reduction of S. enterica (log CFU/g) (A) and E. faecium (log CFU/g) (B) plated on different media formulations recovered from basil leaves treated with ClO2 fumigation (n = 3). Each error bar represents one standard deviation from the mean. Figure abbreviations: Modified TSA with Yeast extract (MTSAYE), MTSAYE with added Sodium Pyruvate (MTSAYE-NP), MTSAYE with added ferrous sulfate (MTSAYE-FS), MTSAYE with added 3’3’-thiodipropionate (MTSAYE-TDP). The * indicates statistical difference p < 0.05.
For chia seed samples treated with ClO2 the average log reduction of S. enterica was not significantly different between any of the media types (p = 0.41) between the media types (Figure 3A). E. faecium NRRL B2354 log reductions were not significantly different (p = 0.96) between any of the media types (Figure 3B).
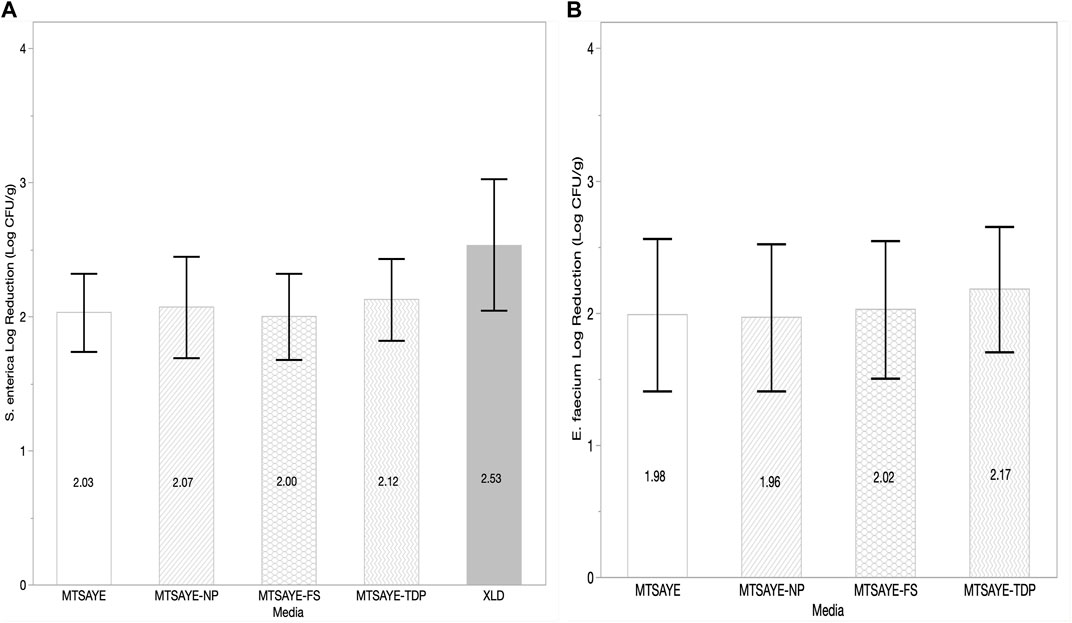
FIGURE 3. Comparison of the mean log reduction of S. enterica (log CFU/g) (A) and E. faecium (log CFU/g) (B) plated on different media formulations recovered from chia seeds treated with ClO2 fumigation (n = 3). Each error bar represents one standard deviation from the mean. Figure abbreviations: Modified TSA with Yeast extract (MTSAYE), MTSAYE with added Sodium Pyruvate (MTSAYE-NP), MTSAYE with added ferrous sulfate (MTSAYE-FS), MTSAYE with added 3’3’-thiodipropionate (MTSAYE-TDP).
H2O2 treated basil leaves, showed comparable average log reductions of S. enterica when plated on non-selective media, but a significant decrease in recovery when plated XLD (p = 0.01) (Figure 4A). E. faecium NRRL B2354 log reductions were not significantly different (p = 0.98) between any of the media types (Figure 4B).
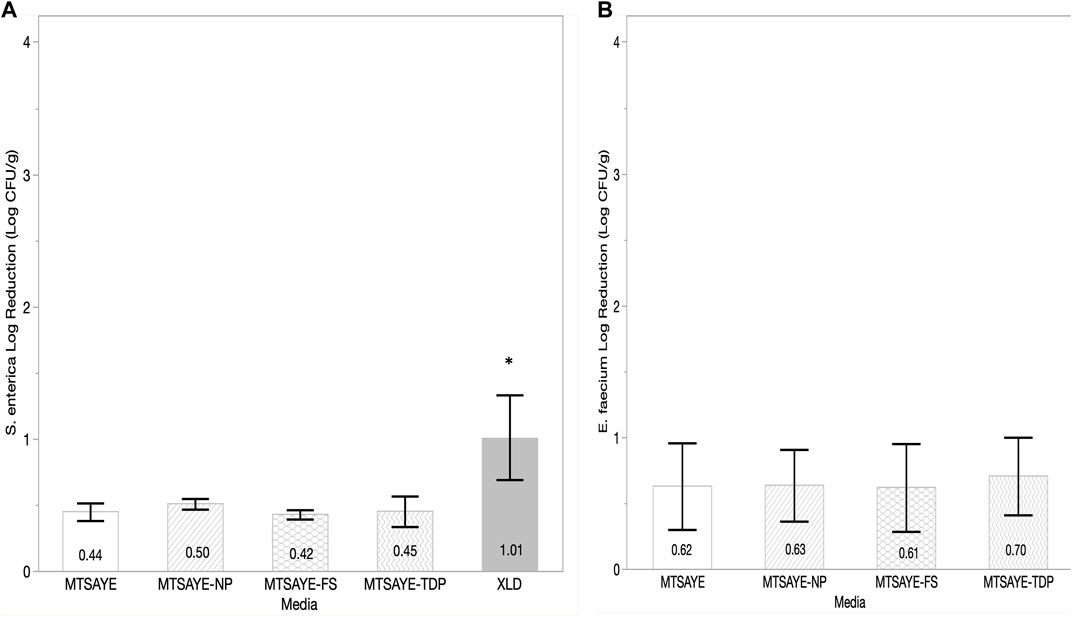
FIGURE 4. Comparison of the mean log reduction of S. enterica (A) and E. faecium (B) (CFU/g) plated on different media formulations recovered from basil leaves treated with H2O2 fumigation (n = 3). Each error bar represents one standard deviation from the mean. Figure abbreviations: Modified TSA with Yeast extract (MTSAYE), MTSAYE with added Sodium Pyruvate (MTSAYE-NP), MTSAYE with added ferrous sulfate (MTSAYE-FS), MTSAYE with added 3’3’-thiodipropionate (MTSAYE-TDP), Xylose Lysine Deoxycholate (XLD). The * indicates statistical difference p < 0.05.
The average water activity of black peppercorns was 0.59 ± 0.01, dried basil leaves was 0.55 ± 0.01, and chia seeds was 0.53 ± 0.003 and was not significantly impacted by treatment.
Log reductions of both microorganisms on ClO2 treated black peppercorns, basil leaves, chia seeds, and H2O2 treated basil leaves were not significantly different when plated on the most permissive media (p = 0.67, 0.11, 0.91, and 0.41, respectively).
Antimicrobial gas residuals on LWAF could inhibit the ability of sub-lethally injured S. enterica cells to repair and grow. When validating a treatment process the ability to recover sub-lethally injured target microorganisms is critical to accurately assess effectiveness. Standard methods for detecting Salmonella in spices and herbs include enrichment in non-selective pre-enrichment broth, isolation on bismuth sulfite agar, XLD and Hektoen enteric agar, and confirmation (Andrews et. al., 2018). While the enrichment procedure facilitates repair and is useful for qualitative analyses, the incubation will allow for microbial growth preventing utility for quantifying surviving bacteria. While process validation experiments frequently inoculate sterilized products to reduce background microbiota, this may not be feasible for all products due to undesirable changes in product or presence of difficult to kill spores. For validation studies on products containing large numbers of spore-forming bacteria, such as spices (Mathot et al., 2021), or other bacteria that may survive in high numbers, it is necessary to use a selective and/or differential media for the target microorganism. Conventional methods for selective and differential enumeration of Salmonella use harsh selective media that can inhibit the growth of injured cells (D’Aoust, 1978). Neglecting these cells can lead to an underestimation of Salmonella populations since they can repair themselves, if returned to favorable conditions (Wu, 2008). This study was designed to determine if the use of a common selective and differential media, XLD, would result in the underestimation of surviving S. enterica after antimicrobial gas treatment. The black peppercorns and basil leaves used for these studies were purchased pre-treated with steam to reduce background microbiota below the limit of detection before inoculation. Physical changes to chia seeds prevented pre-treatment. Sodium thiosulfate and ammonium iron citrate were included in the MTSAYE formula to make the resulting non-selective media differential for S. enterica and E. faecium. These microorganisms break down thiosulfate into sulfite and H2S gas, H2S reacts with the ferric ions in ammonium iron citrate to produce a black precipitate in the center (McLaughlin and Balaa, 2006). While non-inoculated chia seeds did contain 2-3 log CFU/g, no colonies with black centers were seen on MTSAYE (results not shown). Overlay approaches have previously been used to differentiate Salmonella from background microbiota on low water activity foods including spices (whole black peppercorns and cumin seeds), nuts (cashews and macadamia nuts) and dried fruits (raisins) (Saunders et al., 2018; Acuff et al., 2020), however these approaches are more time and resource intensive in comparison to plating directly on a non-selective but differential media.
Microbial populations may also be further reduced by residual exposure to antimicrobial compounds that may be dispersed during the rehydration process. Addition of neutralizing agents including sodium thiosulfate and lecithin are included in diluents used for poultry carcass sampling and environmental sampling, as they act to bind residual sanitizers (Mohammed et al., 2018). Sodium thiosulfate was required to allow for growth of different S. enterica serotypes when challenged with peracetic acid, which decomposes into acetic acid and peroxide. Use of commercially formulated neutralizing buffers with sodium thiosulfate and aryl sulphonate improved recovery of S. enterica and E. coli on chlorine dioxide treated lettuce (Mahmoud and Linton, 2008). While there was no significant difference in the recovery of S. enterica from chlorine dioxide treated whole peppercorns using 0.1% peptone, compared to commercial neutralizing buffer as a diluent, there was higher variability in counts with 0.1% sterile peptone (results not shown). As a result, neutralizing buffer containing sodium thiosulfate and aryl sulphonate was selected for a diluent. It is interesting to note that significant differences in S. enterica recovered on XLD and MTSAYE were noted only for whole black peppercorns and basil leaves but not chia seeds. Chia seeds are reported to contain a number of phenolic antioxidants that prolong shelf-life by reducing lipid peroxidation (Tepe et al., 2006). It is possible that these phenolic compounds or mucilage produced during rehydration may be binding to remaining sanitizers.
In addition to presence of antioxidants in the diluent, the inclusion of different growth promotors and scavengers were supplemented in the non-selective media. Addition of antioxidants such as 3’3’-thiodipropionate, sodium pyruvate, and ferrous sulfate could scavenge oxidative compounds and may prevent further stress to the cells (Gholamin-Dehkordi, et al., 2017). Sodium pyruvate and ferrous sulfate additionally provide glucose and iron respectively for the injured cells to use. Iron availability has been shown to increase S. enterica growth potential (Kortman et al., 2012). These supplements were selected because they individually proved effective at increasing recovery of heat injured S. enterica from egg albumen between 0.25 and 0.50 CFU/g, compared to TSA alone (Gurtler and Kornacki, 2009). In this study, addition of the same selected antioxidant supplements did not significantly increase the recovery, compared to MTSAYE. It is possible that the yeast extract, in addition to providing additional nutrients, is also acting as an antioxidant (Tofalo and Suzzi, 2016). Yeast extract, present in each of the media formulations, may work to neutralize residual chlorine dioxide or hydrogen peroxide on the treated product and reduce stress on injured cells so they can repair easier. The addition of antioxidants to media used for recovery of acid-stressed Salmonella from beef has also been reported to not improve recovery (Karolenko et al, 2020).
Surrogate organisms are non-pathogenic substitutes for the pathogenic target organism, and should be more robust than the pathogen under target conditions (U.S. Department of Agriculture, National Advisory Committee On Microbiological Criteria For Foods, 2010). E. faecium NRRL B2354 has been considered a suitable surrogate for S. enterica in LWAF subjected to different inactivation treatments. However, due to differences in food product and treatment processes, it is important to evaluate surrogate capability on a case-by-case basis. Surrogate comparisons using antimicrobial gases are limited. S. enterica and E. faecium NRRL B2354 reductions were not significantly different on ethylene oxide treated whole black peppercorns, cumin seeds, or propylene oxide treated cashews and macadamia nuts (Saunders et al, 2018; Chen et al., 2020). In this experiment, there was no significant difference between the log reduction of microorganisms for any treatment and spice combination tested, with the log reduction of E. faecium NRRL B2354 being lower than S. enterica for all chlorine dioxide treatments. The results of this experiment support the capability of E. faecium NRRL B2354 to function as a S. enterica surrogate for chlorine dioxide treated spices. This finding agrees with prior studies that have found E. faecium NRRL B2354 has lower inactivation levels, than S. enterica after chlorine dioxide treatment on inoculated black peppercorns and cumin seeds (Wei et al, 2021). The D-values of E. faecium NRRL B2354 are 1.2–1.9 times greater than that of S. enterica for chlorine dioxide treated basil leaves (Verma et al., 2022), further supporting the conclusion that the microorganism is a suitable surrogate. There is little information evaluating E. faecium NRRL B2354 as a surrogate for S. enterica on hydrogen peroxide treated spices. In this study, there was no significant difference between the log reductions of the two microorganisms on hydrogen peroxide treated basil leaves. This result indicates that the E. faecium NRRL B2354 would be acceptable as a surrogate under these treatment conditions.
This research indicates that the recovery of sub-lethally injured S. enterica cells are inhibited by selective agents in XLD media during enumeration from whole black peppercorns and basil leaves but not chia seeds. Results of this study showed an average of a 1-log difference in recovery between the selective media and the non-selective media. Considering the reported low infectious dose of some strains of S. enterica reported in low water activity foods (Blaser and Newman, 1982), this underestimate may result in the release of a potentially hazardous product. Researchers studying the effect of antimicrobial gas fumigation should use a non-selective, but differential media or incorporate a recovery period including use of an overlay protocol when enumerating for the most accurate results. While antioxidant supplement had little effect in the study reported here, further research should be performed to ascertain if differing concentrations of supplements would improve recovery depending on the amount of residual antimicrobial remaining on the product. This study also indicates that E. faecium NRRL B2354 is suitable as a surrogate for S. enterica when inoculated on whole black peppercorns, basil leaves and chia seeds processed using chlorine dioxide or hydrogen peroxide gasses. Further study on its use as a surrogate for hydrogen peroxide treated spices would be encouraged, as these trials failed to result in a 5-log CFU/g reduction, which is frequently suggested as a target reduction for S. enterica on low water activity foods. Santillana Farakos and Frank, 2014; Duncan et al., 2017; Mathot et al., 2021; U.S. Department of Agriculture, National Advisory Committee On Microbiological Criteria For Foods, 2021; U.S. Department of Agriculture, National Advisory Committee On Microbiological Criteria For Foods, 2010; Wei et al., 2021.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
JG, SW, JS, JE, LS, and MP contributed conception and design of the study. SW performed inoculation and antimicrobial gas experiments. JG performed enumeration experiments and statistical analysis. JG wrote the first draft of the manuscript. MP wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was provided in part, by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2020-67017-33256, and by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture. Funds for open access publication were provided under award number 2020-67017-33256.
Special thanks to Kim Waterman and Auja Bywater for providing technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acuff, J. C., Wu, J., Marik, C., Waterman, K., Gallagher, D., Huang, H., et al. (2020). Thermal inactivation of Salmonella, Shiga toxin-producing Escherichia coli, Listeria monocytogenes, and a surrogate (Pediococcus acidilactici) on raisins, apricot halves, and macadamia nuts using vacuum-steam pasteurization. Int. J. Food Microbiol. 333, 108814. doi:10.1016/j.ijfoodmicro.2020.108814
American Spice Trade Association, (2017). Clean, safe spices guidance document. Available at: http://www.astaspice.org (Accessed July 7, 2022).
Andrews, W. H., Wang, H., Jacobson, A., Ge, B., Zhang, G., and Hammack, T. (2018). Bacteriological analytical manual. Association of Official Analytical Chemists. Available at: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-5-salmonella
Back, K.-H., Ha, J.-W., and Kang, D. (2014). Effect of hydrogen peroxide vapor treatment for inactivating Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes on organic fresh lettuce. Food control. 44, 78–85. doi:10.1016/j.foodcont.2014.03.046
Blaser, M. J., and Newman, L. (1982). A review of human salmonellosis: I. Infective dose. Rev. Infect. Dis. 4, 1096–1106. doi:10.1093/clinids/4.6.1096
Chai, H.-E., Hwang, C.-A. L., HuangWu, V., and Sheen, L. (2021). Efficacy of gaseous chlorine dioxide for decontamination of Salmonella, Shiga Toxin-Producing Escherichia coli, and Listeria monocytogenes on almonds and peppercorns. Food control. 132, 108556. doi:10.1016/j.foodcont.2021.108556
Chen, L., Wei, X., Chaves, B., Jones, D., Ponder, M., and Subbiah, K. (2020). Inactivation of Salmonella enterica and Enterococcus faecium NRRL B2354 on cumin seeds using gaseous ethylene oxide. Food Microbiol. 94, 103656. doi:10.1016/j.fm.2020.103656
D'Aoust, J. Y. (1978). Recovery of sublethally heat-injured Salmonella typhimurium on supplemented plating media. Appl. Environ. Microbiol. 35, 483–486. doi:10.1128/AEM.35.3.483-486.1978
Duncan, S. E., Moberg, K., Amin, K. N., Wright, M., Newkirk, J. J., Ponder, M. A., et al. (2017). Processes to preserve spice and herb quality and sensory integrity during pathogen inactivation. J. Food Sci. 82, 1208–1215. doi:10.1111/1750-3841.13702
Gholamian-Dehkordi, N., Luther, T., Asadi-Samani, M., and Mahmoudian-Sani, M. (2017). An overview on natural antioxidants for oxidative stress reduction in cancers; a systematic review. Immunopathol. Persa 3, e12. doi:10.15171/ipp.2017.04
Gurtler, J. B., and Keller, S. (2019). Microbiological safety of dried spices. Annu. Rev. Food Sci. Technol. 10, 409–427. doi:10.1146/annurev-food-030216-030000
Gurtler, J. B., and Kornacki, J. (2009). Comparison of supplements to enhance recovery of heat-injured Salmonella from egg albumen. Lett. Appl. Microbiol. 49, 503–509. doi:10.1111/j.1472-765X.2009.02695.x
Karolenko, C. E., Bhusal, A., Gautam, D., and Muriana, P. (2020). Selenite cystine agar for enumeration of inoculated Salmonella serovars recovered from stressful conditions during antimicrobial validation studies. Microorganisms 8, 338. doi:10.3390/microorganisms8030338
Kim, H., Yum, B., Yoon, S.-S., Song, K.-J., Kim, J.-R., Myeong, D., et al. (2016). Inactivation of Salmonella on eggshells by chlorine dioxide gas. Korean J. Food Sci. Anim. Resour. 36, 100–108. doi:10.5851/kosfa.2016.36.1.100
Kortman, G. A. M., Boleij, A., Swinkels, D. W., and Tjalsma, H. (2012). Iron availability increases the pathogenic potential of Salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS One 7, e29968. doi:10.1371/journal.pone.0029968
Lau, S. K., Panth, R., Chaves, B. D., Weller, C. L., and Subbiah, J. (2021). Thermal inactivation kinetics of Salmonella and Enterococcus faecium NRRL-B2354 on whole chia seeds ( Salvia hispanica L.). J. Food Prot. 84, 1357–1365. doi:10.4315/JFP-20-468
Lau, S. K., and Subbiah, J. (2020). HumidOSH: A self-contained environmental chamber with controls for relative humidity and fan speed. HardwareX 8, e00141. doi:10.1016/j.ohx.2020.e00141
Lillard, H. S. (1979). Levels of chlorine and chlorine dioxide of equivalent bactericidal effect in poultry processing water. J. Food Sci. 44, 1594–1597. doi:10.1111/j.1365-2621.1979.tb09097.x
Mahmoud, B., and Linton, R. (2008). Inactivation kinetics of inoculated Escherichia coli 0157:H7 and Salmonella enterica on lettuce by chlorine dioxide gas. Food Microbiol. 25, 244–252. doi:10.1016/j.fm.2007.10.015
Mathot, A. G., Postollec, F., and Leguerinel, I. (2021). Bacterial spores in spices and dried herbs: The risks for processed food. Compr. Rev. Food Sci. Food Saf. 20, 840–862. doi:10.1111/1541-4337.12690
McDonnell, G., and Russell, A. D. (2001). Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 14, 147–179. doi:10.1128/CMR.12.1.147
McLaughlin, M. R., and Balaa, M. (2006). Enhanced contrast of bacteriophage plaques in Salmonella with ferric ammonium citrate and sodium thiosulfate (FACST) and Tetrazolium Red (TZR). J. Microbiol. Methods 65, 318–323. doi:10.1016/j.mimet.2005.08.008
Mohammed, Z. H., Hasan, A., Kerth, C., Riley, D., and Taylor, T. (2018). Increased effectiveness of microbiological verification by concentration-dependent neutralization of sanitizers used in poultry slaughter and fabrication allowing Salmonella enterica survival. Foods 7, 32. doi:10.3390/foods7030032
Rane, B., Bridges, D. F., and Wu, V. (2020). Gaseous antimicrobial treatments to control foodborne pathogens on almond kernels and whole black peppercorns. Food Microbiol. 92, 103576. doi:10.1016/j.fm.2020.103576
Santillana Farakos, S. M., and Frank, J. (2014). “Challenges in the control of foodborne pathogens in low-water activity foods and spices,” in The microbiological safety of low water activity foods and spices. Editors J. B. Gurtler, M. P. Doyle, and J. L. Kornacki (New York, NY: Springer), 15–34.
Saunders, T., Wu, J., Williams, R., Huang, H., and Ponder, M. (2018). Inactivation of Salmonella and surrogate bacteria on cashews and macadamia nuts exposed to commercial propylene oxide processing conditions. J. Food Prot. 81 (3), 417–423. doi:10.4315/0362-028X.JFP-17-252
Schweiggert, U., Carle, R., and Schieber, A. (2007). Conventional and alternative processes for spice production - a Review. Trends Food Sci. Technol. 18, 260–268. doi:10.1016/j.tifs.2007.01.005
Tepe, B., Sokmen, M., Akpulat, A. H., and Sokmen, A. (2006). Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. x. 95, 200–204. doi:10.1016/j.foodchem.2004.12.031
Tofalo, R., and Suzzi, G. (2016). “Yeasts,” in Encyclopedia of food and health. Editors B. Caballero, P. M. Finglas, and F. Toldrá (Oxford: Academic Press), 593–599.
U.S. Department of Agriculture, National Advisory Committee On Microbiological Criteria For Foods (2021). Microbiological testing by industry of ready-to-eat foods under fda’s jurisdiction for pathogens (or appropriate indicator organisms): verification of preventive controls. Available at:https://www.fsis.usda.gov/sites/default/files/media_file/2021-07/NACMCF_2018-2020_RTETesting.pdf.
U.S. Department of Agriculture, National Advisory Committee On Microbiological Criteria For Foods (2010). Parameters for determining inoculated pack/challenge study protocols. J. Food Prot. 73, 140–202. doi:10.4315/0362-028x-73.1.140
U.S. Food and Drug Administration (2018). Current good manufacturing practice, hazard analysis, and risk-based preventive controls for human food. Fed. Regist.. Available at: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-117 21CFR117, 135.
Van Doren, J. M., Neil, K. P., Parish, M., Gieraltowski, L., Gould, L. H., and Gombas, K. (2013). Foodborne illness outbreaks from microbial contaminants in spices, 1973–2010. Food Microbiol. 36, 456–464. doi:10.1016/j.fm.2013.04.014
Verma, T., Wei, X., Chaves, B. D., Howell, T., and Subbiah, J. (2022). Antimicrobial efficacy of gaseous chlorine dioxide for inactivation of Salmonella and Enterococcus faecium NRRL B-2354 on dried basil leaves. LWT 153, 112488. doi:10.1016/j.lwt.2021.112488
Wason, S., Verma, T., and Subbiah, J. (2021). Validation of process technologies for enhancing the safety of low-moisture foods: A review. Compr. Rev. Food Sci. Food Saf. 20, 4950–4992. doi:10.1111/1541-4337.12800
Wei, X., Chen, L., Chaves, B., Ponder, M. A., and Subbiah, J. (2021). Modeling the effect of temperature and relative humidity on the ethylene oxide fumigation of Salmonella and Enterococcus faecium in whole black peppercorn. LWT 140, 110742. doi:10.1016/j.lwt.2020.110742
Wei, X., Verma, T., Danao, M. G. C., Ponder, M. A., and Subbiah, J. (2021). Gaseous chlorine dioxide technology for improving microbial safety of spices. Innov. Food Sci. Emerg. Technol. 73, 102783. doi:10.1016/j.ifset.2021.102783
Wu, V. C. H. (2008). A review of microbial injury and recovery methods in food. Food Microbiol. 25 6, 735–744. doi:10.1016/j.fm.2008.04.011
Keywords: spices, whole spices, low water activity foods, black pepper, fumigation, chlorine dioxide, hydrogen peroxide
Citation: Garcia JO, Wason S, Subbiah J, Eifert J, Strawn LK and Ponder MA (2022) Media impacts recovery of Salmonella enterica and Enterococcus faecium NRRL B2354 from whole black peppercorns, basil leaves, and chia seeds treated with antimicrobial gasses. Front. Food. Sci. Technol. 2:1033814. doi: 10.3389/frfst.2022.1033814
Received: 31 August 2022; Accepted: 26 October 2022;
Published: 09 November 2022.
Edited by:
Evgenia Spyrelli, Agricultural University of Athens, GreeceReviewed by:
Veerachandra Kranti Yemmireddy, The University of Texas Rio Grande Valley, United StatesCopyright © 2022 Garcia, Wason, Subbiah, Eifert, Strawn and Ponder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica A. Ponder, bXBvbmRlckB2dC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.