
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Food. Sci. Technol., 19 October 2022
Sec. Food Packaging and Preservation
Volume 2 - 2022 | https://doi.org/10.3389/frfst.2022.1011445
Rice bran is one of the main byproducts of rice-processing industries, making approximately 10% of the total mass of rice kernels. It is rich in bioactive phytochemicals, which have several health benefits. Among others, rice bran contains 10%–23% oil, constituting the major bioactive elements of the bran. The aim of this work is therefore to evaluate rice bran oil obtained from Ethiopian small-scale rice-milling plants for suitability of human consumption. The rice bran was stabilized using microwave heating to inactivate endogenous lipase enzymes that would otherwise cause rancidity of the oil and render it inedible. The oil was then extracted and evaluated for its physicochemical properties, fatty acid composition, and antioxidant activity using standard methods. The result of the work confirmed that the rice bran oil from Ethiopian rice-milling plants has physicochemical characteristics that satisfy the acceptable threshold values set by various organizations. Interestingly, the oil contained a substantial fatty acid profile (high amount of unsaturated fatty acids) and a high content of unsaponifiable matter which contributes to the claimed health benefits of the oil. In addition, it exhibited a strong antioxidant activity (95.319%) at a concentration of 18 mg/ml, which also essentially contributes to its health benefits. Proper integration of rice milling with byproduct utilization such as rice bran oil production will contribute to the efforts to alleviate the oil scarcity in the country.
Rice (Oryza sativa L.) is a main source of food for more than 3 billion world population annually. The 2022/23 rice production in the global market is predicted to be 514.6 million tons on the basis of milled rice which is 1.8 million tons from last year (Childs and Lebeau, 2022). Rice is also one of the important cereal food crops grown in different parts of Africa including Ethiopia. In 2010, 18.4 million tons of rice were produced in sub-Saharan Africa, and this value increased to 46.8 million tons in 2020 (Cen, 2020). In Ethiopia, the potential rice production area is estimated to be about 5.4 million hectares. Even though rice is a relatively new crop for Ethiopia, it has attracted attention for agricultural production and is said to help ensure the food security of the country (Mesfin and Zemedu, 2018), especially since 2006, rice production has been increasing rapidly. In 2020, rice production in Ethiopia was 189,649 tonnes. Though Ethiopia rice, paddy production fluctuated substantially in recent years, it tended to increase through 2001–2020 period ending at 189,649 tonnes in 2020 (World Data Atlas, 2020).
A rice grain consists of 20% husk, 11% bran, and 69% endosperm (Dhankhar, 2014). When rice is processed, byproducts such as rice bran and rice husks are generated. Rice bran, which is obtained after removing the outer layer of the rice kernel during milling, contains pericarp, aleurone, and subaleurone fractions. It is estimated that 63 to 76 million tons of rice bran are produced each year in the world (Chiou et al., 2013). Rice bran is nutritionally rich and an important source of rice bran oil bioactive compounds (Friedman, 2013). Rice bran contains 15%–22% lipids, 34%–52% carbohydrates, 7%–11% fiber, 6%–10% ash, 8%–12% moisture, and 10%–16% protein (Bodie et al., 2019).
Rice bran oil (RBO) is a rich source of antioxidants and useful bioactive phytochemicals, including γ-oryzanol, phytosterols, tocopherols, and tocotrienols (Gul et al., 2015). Along with other edible oils, rice bran oil has gained popularity due to its superior cooking qualities, longer shelf life, balanced fatty acid composition, and presence of many antioxidants (Ali and Devarajan, 2017) (Res et al., 2015). The phytochemical constituents of rice bran oil exhibit high antioxidant, anti-inflammatory, hypocholesterolemic, anti-diabetic, and anti-cancer properties (Ali and Devarajan, 2017). Rice bran oil is widely used as premium edible oil in most Asian countries (Japan, Korea, China, Taiwan, Thailand, and Indonesia) (Ghosh, 2007; Res et al., 2015).
Rice bran contains endogenous lipase enzymes that hydrolyze oil into glycerol and free fatty acids in the presence of high temperatures, oxygen, and metal ions, making it unsuitable for human consumption. To overcome this problem, rice bran has to be stabilized before 6 hours of milling (Hoogenkamp, 2008). Various stabilization techniques, including moist heating, low temperature storage, chemical treatment, controlling storage relative humidity, simultaneous milling and extraction, and microwave heating, have been used to inactivate the lipase enzyme (Ramezanzadeh et al., 2000; Lakkakula et al., 2004). Edible oil can be successfully extracted from stabilized rice bran. Solvent extraction is the most frequently used technique for oil extraction (Hoogenkamp, 2008).
This study aimed to investigate whether rice bran oil from small-scale rice-processing plants in Ethiopia is suitable for human consumption. To this end, we extracted oil from microwave-stabilized rice bran and evaluated its physicochemical properties, fatty acid composition, and antioxidant activity. Proper use of rice bran, a byproduct of rice processing, can also maximize resource utilization efficiency and increase industry income while meeting the country’s urgent oil needs. Furthermore, the authors believe that the output of this work could be input for the government and the rice-processing plants to give emphasis to rice-milling byproduct utilization to recover all possible benefits from resources. It could also be a starting point for fellow researchers who are interested in further studying the health effects and other aspects of rice bran oil.
Rice bran used in this study was collected from a rice-milling plant located in Wereta District in the Amhara Regional State of Ethiopia. Relatively good quality rice was purchased, and the bran was collected through the bran outlet pipe. The rice bran was immediately packed in polypropylene zipper top bags and kept in ice box (2–5°C) and microwave-stabilized. Oil extraction and characterization were then carried out. Oil samples were also kept in a refrigerator for analysis of physicochemical properties, fatty acid composition and determination of antioxidant activity.
Rice bran was stabilized by microwave heating using the method described in Tao et al. (1993). A consumer microwave oven (Microwave Oven EG823AEL, China) with a frequency of 2,450 MHz and 800 W maximum output power was used as the microwave energy source. About 150 g of bran with 21% moisture content was exposed to a microwave energy of 340 W for about 4 min. The moisture content of the bran was adjusted to 21% from an initial value of 12% by adding water.
The oil from the stabilized rice bran was extracted using the conventional Soxhlet extraction method (Luque de Castro and Priego-Capote, 2010), which is used as a standard procedure. The solvent used to obtain the oil was n-hexane with a hexane-to-bran ratio (w/w) of 5:1 and extraction time of 1 h. The hexane was later removed using a rotary vacuum evaporator (Buchi Rotavapor R-300 Evaporation Systems) at 50°C. The flasks were placed in a desiccator compartment for 1 h, and the oil yield was calculated. The maximum crude oil recovered from microwave-stabilized rice bran during this study was 16.85%.
Physicochemical properties of the oil were determined using standard procedures (Firestone and Yurawecz, 2002; Srigley, 2017; AOAC, 2000). The analysis was performed in triplicate, and the values are represented as the mean values and standard deviation.
The moisture content, pH value, specific gravity, and viscosity of the crude rice bran oil were determined using the methods described in the following paragraphs.
The moisture content of crude rice bran oil was determined using the Association of Official Analytical Chemists (AOAC) Official Methods 926.12 (AOAC, 2000).
To measure the pH value of the oil, the pH electrode was standardized with the buffer solution, and the electrode was immersed into the sample in a beaker, and the pH value was recorded.
The specific gravity of crude rice bran oil was determined using the Association of Official Analytical Chemists (AOAC) Official Methods 920.212 (AOAC, 2000).
The vibro viscometer (A and D Co., Ltd. Japan), which measures viscosity by vibrating its sensor plates immersed into a sample at a relatively low frequency of sine-wave, was used for measuring the viscosity. The oil sample was filtered to remove dust and other solid material. Then, approximately 150 ml of oil sample was poured into a 200-ml beaker. The height of the sensor unit of the viscometer was adjusted to fix the sensor plates at proper place in the oil sample. Then, the viscosity of the oil was then recorded at 30°C using a laboratory vibro viscometer (A and D Limited Company, Japan).
Other physicochemical properties are determined using official methods of analysis which have been systematically evaluated and approved by a method-endorsing organization for routine use in regulatory and contract laboratories (Srigley, 2017). In this work, we used methods adopted by the Association of Official Analytical Chemists (AOAC) for oils and fats (Firestone and Yurawecz, 2002). The measured properties and the respective used method numbers from the AOAC method in parenthesis are: saponification value (920.160), unsaponifiable matter (933.08), free fatty acid (940.28a), iodine value (993.20), and peroxide value (965.33).
The technique of GC-FID has been widely applied to the quantitative determination of fatty acid derivatives from foods and food ingredients (Srigley, 2017). Gas chromatography–mass spectrometry (GC-MS) is a well-known method and is widely used for the characterization of volatile organic mixtures (Xie et al., 2013). The GC-MS analysis of microwave-stabilized rice bran oil was performed to determine its fatty acid composition. According to the AOAC 996.061 method, it is necessary to change the oil sample to fatty acid methyl esters (FAMEs) before the analysis to make them well-matched with the stationary phase of the column. In this study, the rice bran oil was digested using 8.3 M HCl and ethanol in a shaking water bath set at 70–80°C for 40 min (Chromatography, 2006; Srigley, 2017). GC-MS analysis was performed using an Agilent Technologies 7820 A GC-FID (GC with flame-ionization detection) system (Santa Clara, CA, United States ) equipped with an HP-5 capillary column (30 m × 250 μm; coating thickness, 0.25 μm) coupled to an Agilent Technologies 5977 E mass spectroscope detector (MSD). Analytical conditions selected based on the method described in Conti et al. (2013) were as follows: injector and transfer line temperature, 220 and 260°C, respectively; oven temperature, programmed from 60 to 240°C at 3°C/min; carrier gas, helium at 1 ml/min; injection, 5 μL (10% hexane solution); and split ratio, 1:30. Identification of the constituents was based on the comparison of the retention time along with their percentage peak areas, searching through mass\hunter\library\NIST11. L and mass\hunter\library\W9N11, Llibrary database systems, and comparing with literature data.
The antioxidant activity of the crude rice bran oil was determined by its scavenging activity on the free radical DPPH (2,2-diphenyl-1-picrylhydrazyl) using a method described by Kirby and Schmidt (1997). The DPPH assay measures hydrogen atom (or one electron)-donating activity and hence provides a measure of free-radical-scavenging antioxidant activity. DPPH is a purple-colored stable free radical; it undergoes reduction and becomes yellow-colored diphenyl picrylhydrazine (Kirby and Schmidt, 1997).
A measure of 0.004% DPPH methanol solution was prepared. Different concentration samples (2–24 mg/ml) of rice bran oil in methanol were prepared. Similarly, 6–60 µ g/ml of ascorbic acid was prepared for use as a standard antioxidant. Then, 2 ml of 0.004% DPPH solution was added to the test tubes containing rice bran oil and ascorbic acid samples and mixed using a vortex mixer for 20 s All the samples were incubated for 30 min in the dark at room temperature to allow the reaction to happen between the samples and DPPH. Finally, the absorbance of each sample was measured at 517 nm using a UV-visible spectrophotometer, and the inhibition of free radical DPPH in percent (I%) was calculated using the following equation:
where A control is the absorbance of the control and A sample is the absorbance of DPPH along with different concentrations of samples.
The inhibition effect of antioxidants on DPPH free radicals is reported as an IC50 value, which means the concentration of the antioxidant needed to scavenge 50% of DPPH present in the test solution. The IC50 value for crude rice bran oil was calculated using linear regression by plotting the percentage disappearance of DPPH as a function of the sample concentrations.
The physicochemical properties of crude oil extracted from microwave-stabilized rice bran oil are summarized in Table 1. The result shows that the specific gravity of crude rice bran oil was 0.909 g/ml at 26°C, which is in close agreement with the previous work (Orthoefer, 1996). Codex Alimentarius International, (2005) recommended the specific gravity of rice bran oil to be in the range of 0.910–0.920 g/ml at 30°C. Other physical properties such as moisture content (0.32%), pH value (5.5), and viscosity (28.1 mpa s at 30°C) are also in the acceptable ranges as suggested by different standards and literature works.
The free fatty acid (FFA) content is commonly used to determine the quality of edible oils. It is suggested that the FFA content must not exceed 5% according to Codex Alimentarius International, (2011) (De Almeida et al., 2013). The amount of free fatty acids depends on several factors, including method of extraction, storage conditions, and lipase activity in rice bran. Rice bran has to be stabilized immediately during production to avoid the hydrolysis of oil forming free fatty acid and glycerol, which renders the oil inedible (Orthoefer, 1996). Microwave stabilization is a well-established and effective method (Ramezanzadeh et al., 2000). In this work, the rice bran was stabilized by microwave heating, and the oil was extracted. The free fatty acid content of oleic acid was found to be 3.40%. This result agrees with previous works such as Louisiana and Unive (2000), which reported that the free fatty acids in microwave-heat-stabilized bran increased from an initial value of 3.2%–3.9% in an 8-week storage period.
The peroxide value is a measure of oxidative degradation of oil and expressed as milliequivalent of peroxide oxygen combined with 1 kg of fat/oil (meq O2/kg) (Patterson, 2011). Oils rich in polyunsaturated fatty acids are more susceptible to oxidation when exposed to light, moisture, or heat. The results in Table 1 show that the peroxide value of crude rice bran oil samples was 1 meq O2/kg. This value is in agreement with CODEX 210, (2011) values, which recommend less than 15 meq O2/kg oil (Codex Alimentarius International, 2011). According to AOCS, Cd 8b-90, the maximum peroxide value of crude oil that can be tolerated should be less than 25 meqO2/kg of the sample oil to resist oxidation that makes the oils rancid (Farhoosh, R, Tavassoli-Kafrani, M. H., Sharif, 2013). The peroxide value of crude rice bran oil obtained during this work was less than the maximum possible value reported by the Codex Alimentarius and the American Oil Chemists’ Society (AOCS), indicating that the oil is not susceptible to oxidation that facilitates rancidity.
The saponification value (SV) is the milligrams of potassium hydroxide required to completely saponify 1 g of oil/fat. This covers neutral fat and FFA present and obviously relates to the molecular weights of the fatty acids involved (Patterson, 2011). Fat or oil with low molecular weight fatty acids has a higher saponification value. The saponification value of crude rice bran oil was approximately 185.13 mg/g oil (Table 1). This value agrees with the standard saponification value of oils. The Codex Alimentarius Commission recommends the saponification value of rice bran oil for human consumption to be in the range of 180–199 mg KOH/g (Codex Alimentarius International, 2011).
Rice bran oil has a unique composition of unsaponifiable matter such as oryzanol, squalene, sterols, tocopherols, and fatty alcohols (Sahu et al., 2018). These are bioactive components with functional health benefits such as hypocholesterolemic activity (Sugano et al., 2017), growth promotion, improved blood circulation, stimulation of hormonal secretions, prevention and management of cardiovascular disease, relieving menopausal symptoms, increasing cognitive function, and lowering the incidence of allergic reactions (Ali and Devarajan, 2017). The results in Table 1 elucidate that the unsaponifiable content of the crude rice bran oil sample was between 4.05 and 4.45%. Previous works by Raghuram and Rukmini (1995) and Mccaskill and Zhang (1999) show that crude rice bran oil contains about 4.2% unsaponifiable lipids, which is rich in minor constituents such as phytosterols, triterpene alcohols, tocopherols, and tocotrienols. Rice bran oil’s claimed health benefits are mainly attributed to its high content of unsaponifiable matter (Punia et al., 2021). Wilson et al. (2007) also reported that rice bran oil contains a high content of unsaponifiable matter (4.4%) which is several times greater than that of commonly used vegetable oils.
The iodine value of an oil is the number of grams of iodine absorbed by 100 g of oil (Gatade, 2020). The iodine value is an index of the unsaturation as iodine adds across double bonds (He and Liu, 2019). A higher iodine value indicates a higher degree of unsaturation and determines the stability of oil. The iodine value of microwave-stabilized rice bran oil in this study was 106.09 g I2/100 g. This value agrees with the Codex Alimentarius Commission, which recommends the iodine value of rice bran oil for human consumption to be in the range of 90–15 g I2/100 g (Codex Alimentarius International, 2011). This value was also comparable with values reported in the literature and lies within the range of corn, cottonseed, mustard seed, high erucic acid, and sesame seed oils.
The rice bran oil samples were analyzed for fatty acid profiles using gas chromatography–mass spectrometry (GC/MS), and the results are presented in Table 2. The chromatogram of microwave-stabilized crude rice bran oil is also depicted in Figure 1. The results indicate that rice bran oil contains a relatively high level of monounsaturated fatty acids such as oleic acid (C18:1). The amounts of saturated and unsaturated fatty acids in this analysis were comparable to the values reported in the literature, including the value reported in the Codex standard for named vegetable oils (Codex Alimentarius International, 2011). Raghuram and Rukmini (1995) reported that rice bran oil has an excellent fatty acid profile—unsaturated fatty acids [oleic acid (38.4%) and linoleic acid 34.4%] and linolenic acid (2.2%) and saturated fatty acids [palmitic (21.5%) and stearic acid (2.9%)]. The fatty acid composition reported in Hemavathy and Prabhakar (1987) is in close agreement with the result of this analysis. In addition to providing a roughly similar amount of fatty acid composition to other vegetable oils, rice bran oil supplies unique fatty acids that provide several health benefits.
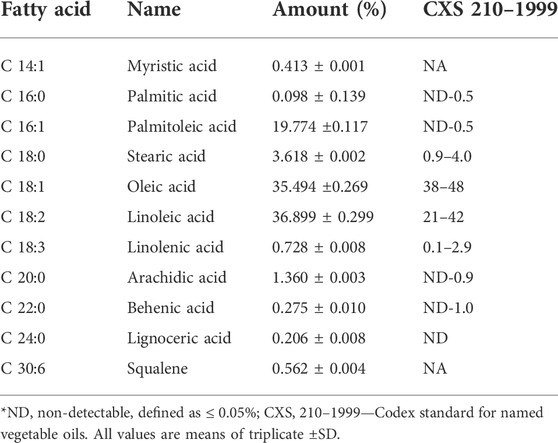
TABLE 2. Fatty acid composition of microwave-stabilized rice bran oil as determined by the GC-MS method.
The antioxidant activity of crude rice bran oil was determined by its free radical-scavenging activity on the stable DPPH radical (Heinonen et al., 1998). Ascorbic acid was used as the standard for the determination of the antioxidant activity by the DPPH method. A maximum scavenging activity of 95.319% was observed for crude rice bran oil samples at a concentration of 18 mg/ml. Arab et al. (2011) reported a maximum scavenging activity of 93.91% for the extract of the Fajr rice bran variety. For ascorbic acid used in this study, the maximum inhibition effect of 96.06% was observed at a concentration of 54 μg/ml. This result is close to the result reported in Nariya et al. (2013) in which the authors found an inhibition effect of 52.74% and 99.86% at concentrations of 10 and 60 μg/ml, respectively. The rice bran oil used in this assay showed appreciable antioxidant activity compared to the standard ascorbic acid. The high antioxidant effect of rice bran oil is mainly because of its high content of total tocopherols (Farhoosh, R, Tavassoli-Kafrani, M. H., Sharif, 2013). Tocopherols have antioxidant properties, which contribute essentially to their health benefits. Sesame oil and rice bran oil are considered to be two of the most oxidatively stable edible oils due to their high content of total tocopherols (Farhoosh, R and M. H., Sharif, 2013). The IC50 concentration of crude rice bran oil was 3.8 mg/mL as calculated using the data plotted in Figure 2. Similarly, for ascorbic acid (Figure 3), the IC50 value was 15.5 μg/ml. The lower IC50 value reflects better DPPH radical-scavenging activity and has strong antioxidant potential.
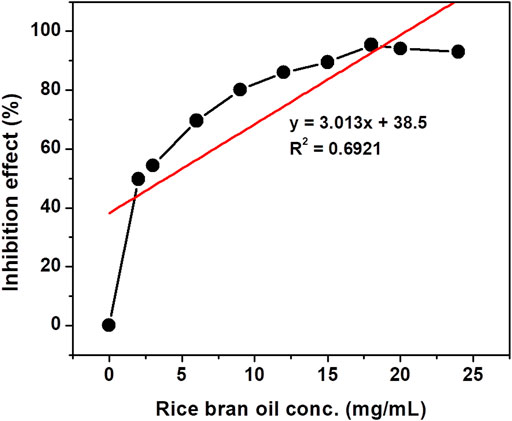
FIGURE 2. Inhibition effect of different concentrations of crude rice bran oil on the DPPH free radical at 517 nm.
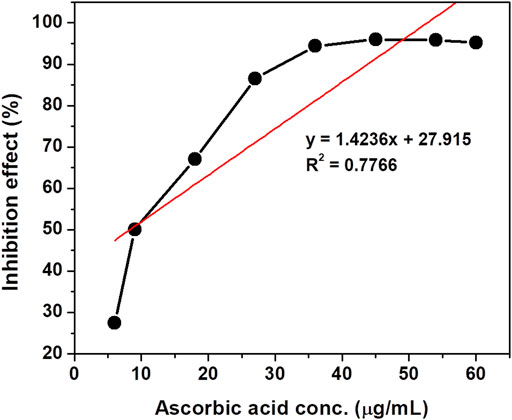
FIGURE 3. Effect of different concentrations of ascorbic acid with the DPPH free radical on absorbance at 517 nm.
In this research, we investigated the suitability of rice bran oil obtained from Ethiopian small-scale rice-milling plants for human consumption. There were no previous efforts to utilize rice-milling byproducts for human consumption in Ethiopia except one work by the same author Bultum et al. (2020). The rice bran samples used in this research were stabilized to inactivate endogenous lipase activity that will become active after rice milling, rendering the fat inedible. Then, crude oil was extracted and evaluated for essential quality attributes and found to have acceptable properties. The crude rice bran oil investigated has a specific gravity of 0.909 g/ml at 26°C and a viscosity of 28.1 mpa.s at 30°C, which are in the acceptable ranges as suggested in different standards and literatures. Moreover, it contains 3.40% free fatty acid as oleic acid, 185.13 mg NOH/g saponification value, 4.02% unsaponifiable content, and 106.09 g I2/100 g iodine value. Furthermore, it contains fatty acid profiles and appreciable antioxidant activity that provides several health benefits.
This work is the first to report the quality attributes of rice bran oil from small-scale rice-milling plants in Ethiopia. The Ethiopian rice-milling sector should integrate with byproduct utilization pipelines in order to get full benefit from the product. The authors believe that the output of this work could be the input for the government and the rice-processing plants to emphasis on rice-milling byproduct utilization to recover all possible benefits from resources. It could also be a starting point for fellow researchers who are interested in further studying the health effects and other aspects of rice bran oil.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
LB, conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, and supervision. SE, conceptualization, methodology, and writing—review and editing. LT, conceptualization and writing—review and editing.
This work was supported by the Technology Development Project of the Ministry of Science and Technology (MoST), Federal Democratic Republic of Ethiopia.
The authors thank Addis Ababa Institute of Technology (AAiT) and the Science faculty of Addis Ababa University and Bahir Dar Institute of Technology (BiT) for providing laboratory facilities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ali, A., and Devarajan, S. (2017). Nutritional and health benefits of rice bran oil. Berlin, Germany: Springer, 135–158. doi:10.1007/978-3-319-59011-0
AOAC, (2000). Official methods of analysis of the association of official agricultural Chemists. Am. J. Public Health Nations Health 46, 916–916. doi:10.2105/ajph.46.7.916-a
Arab, F., Alemzadeh, I., and Maghsoudi, V. (2011). Determination of antioxidant component and activity of rice bran extract. Sci. Iran. 18, 1402–1406. doi:10.1016/j.scient.2011.09.014
Bodie, A. R., Micciche, A. C., Atungulu, G. G., Rothrock, M. J., and Ricke, S. C. (2019). Current trends of rice milling byproducts for agricultural applications and alternative food production systems. Front. Sustain. Food Syst. 3, 1–13. doi:10.3389/fsufs.2019.00047
Bultum, L. E., Emire, S. A., and Wolde, Y. T. (2020). Influence of full fat rice bran from Ethiopian rice milling industries on nutritional qualities, physicochemical and sensory properties of bread and biscuits. Food Meas. 14, 2253–2261. doi:10.1007/s11694-020-00472-7
Cen, R. (2020). Boosting Africa ’ s Rice Sector A research for development strategy 2011 – 2020. Cotonou, Benin: Africa Rice Center.
Childs, N. W., and Lebeau, B. (2023). Rice outlook: May 2022: U.S. 2022/23 rice production projected at 182.7 million cwt. Econ. Res. Serv. Situat. Outlook.
Chiou, T., Ogino, A., Kobayashi, T., and Adachi, S. (2013). Characteristics and antioxidative ability of defatted rice bran extracts obtained using several extractants under subcritical conditions. J. Oleo Sci. 8, 1–8. doi:10.5650/jos.62.1
Chromatography, G. (2006). Modifying AOAC method 996 . 06 for FAME analysis in foods : Faster throughput using hydrogen carrier gas. AvaliableAt: https://gcms.labrulez.com/paper/12806.
Codex Alimentarius International (2011). CODEX STAN 210-1999. Codex standard for named vegetable oils. Adopted 1999. AvaliableAt: http://www.sciepub.com/reference/39559.
Codex Alimentarius Commission (2005). Joint FAO/WHO food standards programme Codex committee on fats and oils. Rome, Italy: WHO.
Conti, B., Leonardi, M., Pistelli, L., Profeti, R., Ouerghemmi, I., and Benelli, G. (2013). Larvicidal and repellent activity of essential oils from wild and cultivated ruta chalepensis L. (Rutaceae) against Aedes albopictus skuse (Diptera: Culicidae), an arbovirus vector. Parasitol. Res. 112, 991–999. doi:10.1007/s00436-012-3221-2
De Almeida, D. T., Nunes, I. L., Conde, P. L., Rosa, R. P. S., Rogério, W. F., and Machado, E. R. (2013). A quality assessment of crude palm oil marketed in Bahia, Brazil. Grasas Aceites 64, 387–394. doi:10.3989/gya.118412
Farhoosh, R, T.-K., and Sharif, A. (2013). Assaying antioxidant characteristics of sesame seed, rice bran, and bene hull oils and their unsaponifiable matters by using DPPH radical-scavenging model system. J. Agr. Sci. Tech 15, 241–252. 20.1001.1.16807073.2013.15.2.16.5
Firestone, D., and Yurawecz, M. P. (2002). AOAC official methods of analysis. Available at: http://www2.kobe-u.ac.jp/∼toyoda/beef/BEEF/Ref3/AOAC2.pdf.
Friedman, M. (2013). Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. J. Agric. Food Chem 61, 10626–41. doi:10.1021/jf403635v
Ghosh, M. (2007). Review on recent trends in rice bran oil processing. J. Amer. Oil Chem. Soc. 84, 315–324. doi:10.1007/s11746-007-1047-3
Gul, K., Yousuf, B., Singh, A. K., Singh, P., and Abas, A. (2015). Rice bran: Nutritional values and its emerging potential for development of functional food—a review. Bioact. Carbohydrates Diet. Fibre 6, 24–30. doi:10.1016/j.bcdf.2015.06.002
He, D., and Liu, L. (2019). Analytical aspects of rice bran oil. Amsterdam, Netherlands: Elsevier. doi:10.1016/B978-0-12-812828-2.00007-X
Heinonen, I. M., Lehtonen, P. J., and Hopia, A. I. (1998). Antioxidant activity of berry and fruit wines and liquors. J. Agric. Food Chem. 46, 25–31. doi:10.1021/jf970489o
Hemavathy, J., and Prabhakar, J. V. (1987). Lipid composition of rice (Oryza sativa L.) bran. J. Am. Oil Chem. Soc. 64, 1016–1019. doi:10.1007/bf02542441
Kirby, A. J., and Schmidt, R. J. (1997). The antioxidant activity of Chinese herbs for eczema and of placebo herbs - I. J. Ethnopharmacol. 56, 103–108. doi:10.1016/S0378-8741(97)01510-9
Lakkakula, N. R., Lima, M., and Walker, T. (2004). Rice bran stabilization and rice bran oil extraction using ohmic heating. Bioresour. Technol. 92, 157–161. doi:10.1016/j.biortech.2003.08.010
Louisiana, R., and Unive, S. (2000). Lipase and lipoxygenase activity , functionality , and nutrient losses in rice bran during storage lipase and lipoxygenase activity , functionality , and nutrient losses in rice bran during storage. Baton Rouge, LA, USA: Louisiana State University.
Luque de Castro, M. D., and Priego-Capote, F. (2010). Soxhlet extraction: Past and present panacea. J. Chromatogr. A 1217, 2383–2389. doi:10.1016/j.chroma.2009.11.027
Mccaskill, D., and Zhang, F. (1999). Use of rice bran oil in foods : Developing nutraceuticals for the new millenium. Food Technol. 53, 50–53.
Mesfin, A. H., and Zemedu, L. (2018). Choices of varieties and demand for improved rice seed in fogera district of Ethiopia. Rice Sci. 25, 350–356. doi:10.1016/j.rsci.2018.10.005
Nariya, P. B., Bhalodia, N. R., Shukla, V. J., Acharya, R., and Nariya, M. B. (2013). In vitro evaluation of antioxidant activity of Cordia dichotoma (Forst f.) bark. Ayu 34, 124–128. doi:10.4103/0974-8520.115451
Orthoefer, F. T. (1996). Rice bran oil detection. Bailey’s Ind. Oil Fat. Prod.. 6th Edn. , 393–409. doi:10.1002/047167849X.bio015.pub2
Patterson, H. B. W. (2011). “Quality and control,” in Hydrogenation of fats and oils (Amsterdam, Netherlands: Elsevier), 329–350.
Punia, S., Kumar, M., Siroha, A. K., and Purewal, S. S. (2021). Rice bran oil: Emerging trends in extraction, health benefit, and its industrial application. Rice Sci. 28, 217–232. doi:10.1016/j.rsci.2021.04.002
Raghuram, T. C., and Rukmini, C. (1995). Nutritional significance of rice bran oil. Indian J. Med. Res. 102, 241–244. Available at: http://europepmc.org/abstract/MED/8675245.
Ramezanzadeh, F. M., Rao, R. M., Prinyawiwatkul, W., Marshall, W. E., and Windhauser, M. (2000). Effects of microwave heat , packaging , and storage temperature on fatty acid and proximate compositions in rice bran. J. Agric. Food Chem. 48, 464–467. doi:10.1021/jf9909609
Res, J. R., Nayik, G. A., Majid, I., Gull, A., and Muzaffar, K. (2015). Rice bran oil , the future edible oil of India. A mini Rev. 3, 4–6. doi:10.4172/2375-4338.1000151
Sahu, S., Ghosh, M., and Bhattacharyya, D. K. (2018). Isolation of the unsaponifiable matter (squalene, phytosterols, tocopherols, γ-oryzanol and fatty alcohols) from a fatty acid distillate of rice bran oil. Grasas Aceites 69, 262–268. doi:10.3989/gya.1112172
Srigley, C. T. (2017). Current analytical techniques for food lipids. Available at: http://digitalcommons.unl.edu/usfda.
Sugano, M., Koba, K., and Tsuji, E. (2017). Health benefits of rice bran oil. Available at: http://europepmc.org/abstract/MED/10625933.
Tao, J., Rao, R., and Liuzzo, J. (1993). Microwave heating for rice bran stabilization. J. Microw. Power Electromagn. Energy 28, 156–164. doi:10.1080/08327823.1993.11688217
Wilson, T. A., Nicolosi, R. J., Woolfrey, B., and Kritchevsky, D. (2007). Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters. J. Nutr. Biochem. 18, 105–112. doi:10.1016/j.jnutbio.2006.03.006
World Data Atlas (2020). Ethiopia—Rice, paddy production quantity. Available at: https://knoema.com/atlas/Ethiopia/topics/Agriculture/Crops-Production-Quantitytonnes/Rice-paddy-production (Accessed March 29, 2021).
Keywords: rice bran, rice bran stabilization, rice bran oil, oil characterization, antioxidant activity assay
Citation: Bultum LE, Emire SA and Tufa LT (2022) Physicochemical characterization of microwave-stabilized rice bran oil from Ethiopian small-scale rice-processing plants. Front. Food. Sci. Technol. 2:1011445. doi: 10.3389/frfst.2022.1011445
Received: 04 August 2022; Accepted: 27 September 2022;
Published: 19 October 2022.
Edited by:
Tanima Bhattacharya, Hubei University, ChinaReviewed by:
Mohammad Hojjati, Khuzestan University of Agricultural Sciences and Natural Resources, IranCopyright © 2022 Bultum, Emire and Tufa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lemessa Etana Bultum, bGVtZTMzODhAZ21haWwuY29t; Lemma Teshome Tufa, bGVtbWF0MjAwM0B5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.