- 1Department of Epidemiology, Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
- 2Department of Parasitology, Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
Transmission dynamics is an important indicator for malaria control and elimination. As we move closer to eliminating malaria in Sub-Saharan Africa (sSA), transmission indices with higher resolution (genomic approaches) will complement our current measurements of transmission. Most of the present programmatic knowledge of malaria transmission patterns are derived from assessments of epidemiologic and clinical data, such as case counts, parasitological estimates of parasite prevalence, and Entomological Inoculation Rates (EIR). However, to eliminate malaria from endemic areas, we need to track changes in the parasite population and how they will impact transmission. This is made possible through the evolving field of genomics and genetics, as well as the development of tools for more in-depth studies on the diversity of parasites and the complexity of infections, among other topics. If malaria elimination is to be achieved globally, country-specific elimination activities should be supported by parasite genomic data from regularly collected blood samples for diagnosis, surveillance and possibly from other programmatic interventions. This presents a unique opportunity to track the spread of malaria parasites and shed additional light on intervention efficacy. In this review, various genetic techniques are highlighted along with their significance for an enhanced understanding of transmission patterns in distinct topological settings throughout Sub-Saharan Africa. The importance of these methods and their limitations in malaria surveillance to guide control and elimination strategies, are explored.
Introduction
Despite increased attempts to manage the disease, malaria still has a high mortality and morbidity rate throughout Africa (1). The most recent figures show that the number of malaria cases and deaths increased by 5 and 12%, respectively, in 2020 from 2019 (1). Due to varying transmission intensities among communities, the malaria burden is highly variable throughout Africa (2). Malaria transmission is a dynamic process that is impacted by a variety of interconnected factors, such as interventional pressures, uncontrollable natural environmental conditions, and human-caused disruptions of the environment (3). In addition, a single symptomatic human with a high de novo mutation rate (4) has the potential to continuously generate genetic variation across the millions of symptomatic and asymptomatic cases recorded annually. These mutations make it difficult to ascertain the genetic make-up of the transmitted parasites in subsequent generations. Furthermore, the infections may also be polyclonal, thus making the parasite highly adaptable and challenging to analyze.
For decades, epidemiological and clinical data analysis, such as prevalence rates and case counts (5, 6), the entomological index known as the Entomological Inoculation Rate (EIR) (7), or parasitological parameters have been used to determine the level of malaria transmission (6). To eradicate malaria, however, surveillance systems must be improved (8) and reliable data for surveillance will come from a more precise assessment of transmission patterns (9, 10). New tools will also be required to improve diagnosis, characterize, and understand the dynamics of the parasite reservoir as it evades host immunity and responds to pressures exerted by interventions (10). As the field is evolving, the tools for measuring transmission intensity and dynamics are also improving with higher resolution. These have been made possible through the growing field of genomic epidemiology and tool development, which are both fueled by advances in the computational analysis of the Plasmodium genome (11). In addition to defining prevalence and incidence with higher resolution than the traditional approaches, genomic technologies for tracking parasite dynamics and informing transmission levels also offer novel and potent ways to quantify genetic indices.
Genetic epidemiology is useful for monitoring; the origin and spread of infections, the effectiveness of interventions like antimalarial drugs and vaccines, and the prevalence of the asymptomatic reservoir of infection which drives transmission. Genomic epidemiology has been used to identify relationships between the genetic diversity of the malaria parasite, dynamic changes in transmission intensity, and the effectiveness of malaria control programs (12–15). In Senegal, a validated molecular barcode with 24 Single Nucleotide Polymorphisms (SNPs) was utilized to track distinct parasite types to monitor changes in transmission intensity (16). More specifically, genomic tools can provide information about the parasite population structure at the local level. Population structure can show whether particular genotypes predominate hotspots from the local transmission of specific strains or whether the landscape of malaria transmission is more genetically diverse. This will have significant potential for outcrossing resulting from sustained transmission or importation of multiple genotypes (16–18). Genomic epidemiology can reveal parasite genetic features underlying local and regional patterns of transmission such as Complexity of Infection (COI), diversity (19), parasite population migration and evolution which cannot be deciphered using conventional measures. Furthermore, genomic data can provide information for characterizing connectivity and transmission of different parasite strains (12, 18). Studies on the declining local transmission in the Thailand–Myanmar border region found that the strongest genetic signal associated with transmission decline was loss of parasite genetic diversity within hosts, as measured by a decline in COI (20). Thus, suggesting that this measure could be a proxy for local transmission intensity (21).
When combined with epidemiological and Geographic Information Systems (GIS) data, genetic information may be utilized to identify transmission hotspots and customize interventions. As a result, comprehensive data on the dynamics of the parasite population in a particular area will become available, allowing for more precisely targeted interventions (17, 22). Other molecular epidemiological parameters used to define the infection dynamics of P. falciparum include the rate at which different genotypes are acquired over time (molecular force of infection, molFOI). In Papua New Guinea, molFOI was used to describe recrudescent Plasmodium vivax infection as opposed to vector-acquired infection and the length of infection (23, 24). If these new tools are incorporated programmatically, surveillance of Plasmodium parasites can give important information about malaria transmission and can be used to inform decision-making processes within National Malaria Control Programs (NMCPs) (25).
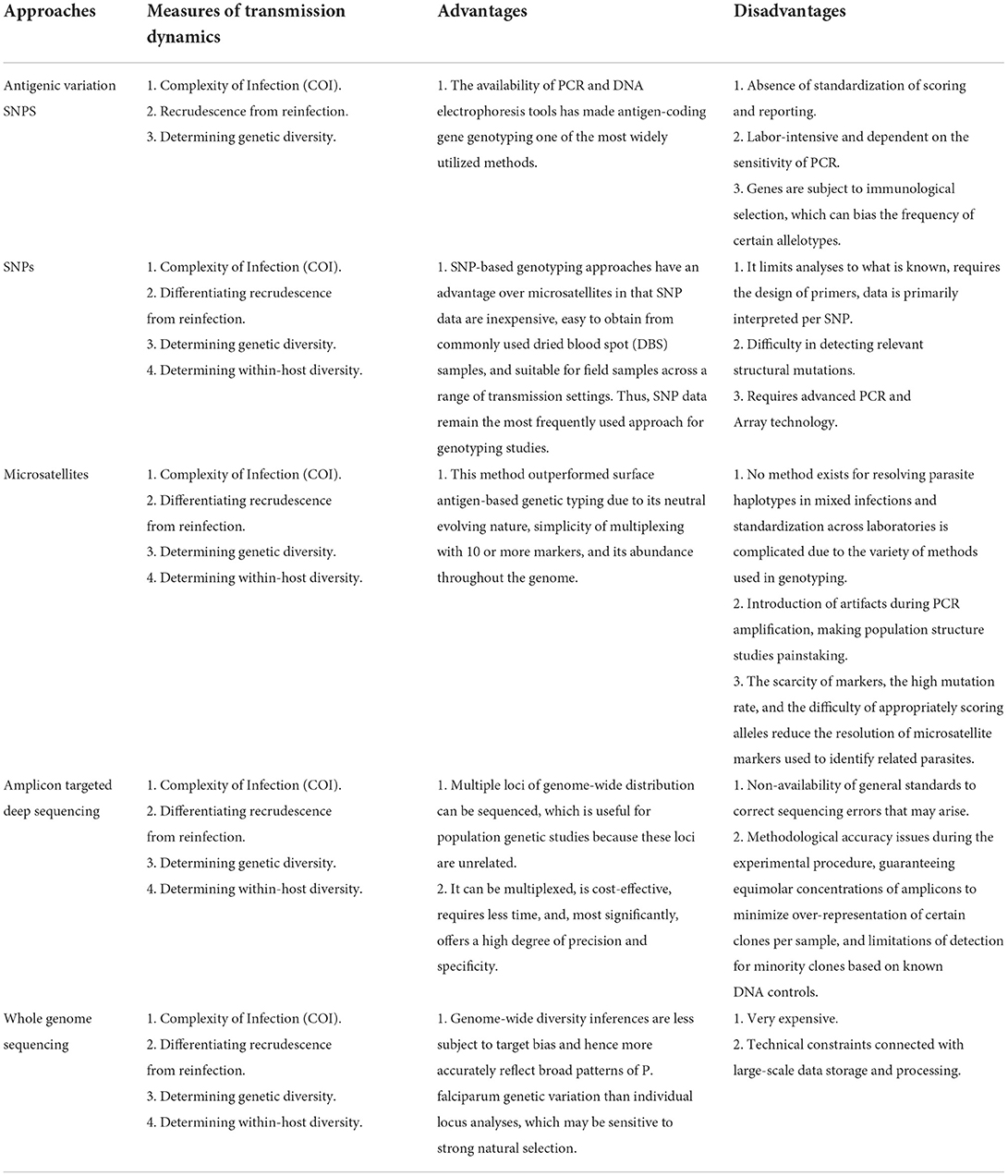
Parasite genotyping to investigate transmission dynamics during malaria surveillance can be done in several ways (Table 1). For example, high-resolution melting assays and polymerase chain reaction for SNP genotyping (PCR). Even though genotyping of vaccine candidate antigens, such as Circumsporozoite Protein (csp), Apical Membrane Antigen 1 (ama1), and Merozoite Surface Proteins 1 and 2 (msp1&2), have been shown to have the potential to pinpoint the major routes and reservoirs of infection, immune selection limits the viability of this method (26–29). These genes have also been used to assess the causes and dynamics of malaria outbreaks, to monitor imported malaria and its role in local transmission, and to predict the frequency of P. vivax relapses (25). However, there are still restrictions on how these genotyping approaches may be used to distinguish between reinfection and recrudescence in order to correctly define the Plasmodium population and to detect low parasitaemia in an asymptomatic human host (26). Some of these methods still have limitations when it comes to their capacity to provide high-throughput data for whole-genome information. For instance, novel structural mutations important to downstream applications cannot be found by PCR genotyping (30).
Microsatellite genotyping, which entails amplification and sequencing, or gel-based characterization of tandem repeats and outputting length polymorphism is another widely used approach. Microsatellites were used to identify the intercontinental spread of P. falciparum antimalarial drug resistance alleles from Asia to Africa (31) and more recently, across the eastern greater Mekong sub-region (32, 33). Whole genome sequencing (WGS) employing next-generation sequencing (NGS) technology was developed in response to the need for improved resolution and more coverage of genomes following the sequencing of the Plasmodium genome. WGS is crucial for discovering novel genetic variations relevant for surveillance and validating improvements in genomic methodology. Higher sequence reads per instrument run at lower costs are produced by NGS technologies (27, 30). Despite the significant decrease in sequencing costs over the past two decades, large-scale WGS of parasites for surveillance purposes is not practical, particularly in Sub-Saharan Africa (sSA). The research community has been actively evaluating the potential role of targeted sequencing and genotyping approaches to recover the most informative genomic regions for malaria genomic epidemiology (25, 34, 35).
The choice of genotyping technique varies for different endemic settings depending on the priority and cost involved in these approaches. The selection of an appropriate approach for certain endemic settings continues to be a challenge, especially for nations in sSA with limited access to new technologies. For countrywide molecular surveillance in sSA to be successful, a combination of techniques and approaches derived from the technological advancement and adaptation to the evolving epidemiology of malaria will be required. This paper reviews current approaches and tools for determining P. falciparum transmission dynamics and offers suggestions for how the various approaches could be applied in resource-limited contexts (29). The importance of these techniques and their limitations in incorporating them into malaria surveillance to guide control and elimination strategies, are discussed.
The research articles were chosen by a search conducted in PubMed and Google Scholar using relevant key terms (malaria, transmission dynamics and the genomic approaches). A review of several papers that met our inclusion criteria uncovered other papers containing relevant information. In all, 94 papers on tool development, assessment of the tools and practical uses of the genomic approaches were included in this review.
Genomic approaches and tools for measuring malaria transmission dynamics
Genotyping of antigen-coding gene
Plasmodium parasites express a large number of different antigens on the surface of their cells. These variations are critical for virulence and host immune evasion (29, 36, 37). They are suitable markers for the identification of genetically distinct P. falciparum parasite sub-populations (29). Earlier studies in genotyping Plasmodium infection used length polymorphisms in antigen-coding genes like msp1, msp2, and glutamate-rich protein (glurp) to determine COI (a measure of the effectiveness of intervention programmes), genetic diversity and to differentiate between recrudescence and reinfection during drug efficacy trials (38–40). Genotyping of antigen-coding genes has become one of the most extensively used approaches due to the availability of PCR and DNA electrophoresis equipment (35). For example, Huang et al. in 2018 (41) and Papa Mze et al. in 2022 (42) demonstrated, by msp1&2 genotyping, a reduction in COI and genetic diversity in the Grande Comore Island, as a result of a drastic drop in transmission caused by past interventions.
Although this approach has shown utility, it has limitations such as the absence of standardization of scoring and reporting formats that enable the comparison of results among laboratories in various endemic sites. In addition, the msp/glurp genotyping process is labor-intensive and is dependent on the sensitivity of PCR, which may fail to amplify low-abundance variants or produce artifacts (43). Moreover, sensitivity is low when agarose gels are used, and interpretation is subjective, particularly in high-transmission areas of Africa, where polyclonal infections result in many bands. Additionally, the msp/glurp genes are subject to immunological selection, which can bias the frequency of certain allelotypes (35, 44). This may affect the precision with which population structure and transmission patterns derived from these loci are estimated (35).
It is difficult to interpret population structure using data produced from these loci because it is unclear whether the observed patterns reflect population history or natural selection. Capillary gel separation has been demonstrated to be three times faster, with superior resolution (2.4 x), and separation efficiency (5.4 x) than a typical gel DNA separation (45). Gupta and colleagues (46) demonstrated that capillary electrophoresis provides more accurate outcomes for anti-malarial treatment trials in determining whether recurrent parasitaemia following therapy indicates recrudescence (treatment failure) or a new infection. Although this technique is an update from gel electrophoresis to discriminate P. falciparum infections, it requires an expensive equipment and is not as readily available as gel electrophoresis (46). Current WHO recommendations suggest that all studies in sSA should employ msp1 and msp2 along with a panel of two to three informative microsatellite markers (such as Poly-α, Pfpk2 and TA1) and use the match-counting method for analysis (47).
Microsatellite genotyping approach
Microsatellites are highly polymorphic tandem repeats of one to six base pairs (bp). They occur often in the P. falciparum genome, mostly as [TA]n, [T]n, and [TAA]n repeats (35, 48). Microsatellite markers are extremely abundant in P. falciparum, appearing around every 2–3 kb throughout the genome (48, 49). They are considered “selectively neutral” loci, thus, powerful markers for population genetic studies. Microsatellites can be utilized to fingerprint parasites and resolve infection relatedness at high spatial resolutions in the context of malaria elimination, particularly in settings with low prevalence and high levels of monoclonality (20, 35, 50, 51). Additionally, by combining P. falciparum drug resistance markers with flanking microsatellite loci, one may examine the genetic diversity and evolution of selective signatures surrounding drug resistance genes (35, 52).
This approach surpassed genetic typing approaches based on surface antigens and revealed numerous essential information about the spread of epidemics, the extent of recombination, and the degree of population differentiation (49). The use of multilocus approaches surpasses approaches using surface antigens because it is extremely widespread in P. falciparum, occurring every 2–3 kb throughout the genome. Thus, microsatellites can be used to better distinguish between COI in high and low transmission settings (49) and between local and imported cases. Microsatellites provide a metric that better identifies and quantifies the rate of importation and risk of local spread of malaria infections by comparing the genetic relatedness (53). The neutral evolving nature of the markers, ease of multiplexing with 10 or more markers in one PCR run, and abundance throughout the genome are some other advantages (49, 54–56). For example, this approach was able to determine the genetic diversity in a low transmission setting of the Kingdom of Eswatini (57) and differentiate between the COI of local and imported cases in Southern Africa (58).
As with msp/glurp typing, microsatellite typing is dependent on reliable DNA fragment amplification by PCR and can introduce artifacts in amplification and diversity estimates, thus making population structure studies painstaking. Furthermore, no method exists for resolving parasite haplotypes in mixed infections and standardization across laboratories is complicated because of the variety of methods used in genotyping microsatellites. In addition, the scarcity of markers, the high mutation rate, and the difficulty of appropriately scoring alleles (fragment sizing) reduce the resolution of microsatellite markers used to identify related parasites (54, 59).
The challenges in defining fragment sizes can be addressed using either capillary electrophoresis or next-generation sequencing (NGS) albeit at extra cost. Unlike fragment size estimation in gel electrophoresis, which is subjective, capillary electrophoresis or NGS method is based on fluorescently labeled oligonucleotides or nucleotides, separated in fine capillaries, and detected by laser. They provide more separation efficiency and higher resolution (60). Due to their enormous variability, parasite population substructure may be undetected in sites with high transmission, as panels of ten to fourteen markers may yield a false estimation of relatedness (54). The generation of P. falciparum genome sequences is becoming more popular as the cost decreases, allowing for the design of more effective SNP-based panels tailored to study questions and settings. However, these genomes are now available for mining novel microsatellite loci to enrich existing loci to address specific research questions such as the origin and spread of drug resistance, making it relevant for low resource settings like sSA in the long-term (26, 35).
Single nucleotide polymorphism genotyping
Single nucleotide polymorphisms (SNPs) are regarded as one of the most common forms of genetic variation and for this reason a powerful tool for the identification of disease-causing organisms, their genetic diversity, phylogenetic analysis and outbreak surveillance. The SNP barcode generates a unique haplotype signature for each infection, distinguishing single-clone infections from those with parasite genome mixtures (35, 61). Some studies have used this technique to develop genotyping assays which incorporate SNP barcoding to evaluate parasite genotypes derived from communities, malaria patients, or laboratory strains (35, 62). SNP barcoding is also sensitive for detecting and genotyping sub-microscopic parasitaemia in locations with low malaria transmission, even in the absence of positive Rapid Diagnostic Tests (RDT) (35, 63). Additionally, the approach has the potential to identify epidemic sources (35, 64).
SNP genotyping approaches can be used on qPCR, High resolution Melting genotyping and the Sequenom Mass Array assays. These approaches have an advantage over microsatellites in that SNP data are inexpensive, easy to obtain from commonly used dried blood spot (DBS) samples, and suitable for field samples across a range of transmission settings. Thus, SNP data remain the most frequently used approach for genotyping studies (38, 65). A study on Kenyan and Gambian parasite isolates that used at least 80 SNP genotyping on the Sequenom MassARRAY iPLEX platform revealed no clear spatially confined parasite subpopulations, but rather a diffused spatio-temporal pattern to parasite genotypes (66). Another SNP approach is the TaqMan array card (TAC), a 384-well microfluidic real-time PCR system that compartmentalizes each sample into 48 separate PCRs simultaneously and has been used for the detection of multiple tuberculosis (TB) drug resistance markers, syndromic pathogen detection and has yielded 100% accuracy in detecting known antimalarial drug resistance markers compared to sequencing (67).
Some disadvantages of SNP genotyping is that it limits analyses to what is known, requires the design of primers, data is primarily interpreted per SNP and few laboratories in sSA have been able to utilize these assays due to the absence and cost of advanced PCR or array technologies (64, 68). Moreover, PCR genotyping is ineffective in detecting structural mutations that are relevant for downstream applications (26) thus, there is a need for NGS platforms to provide higher resolution and coverage of genomes.
Targeted amplicon deep sequencing
Targeted amplicon deep sequencing (TADS) is the sequencing of specific regions of the genome using next-generation technologies. This approach increases the sample load, the processing speed and at the same time, lowers the costs of molecular analysis (69). TADS of short amplicons have the potential to overcome some of the limitations associated with long polymorphic genotyping markers, most notably the effect of a marker's fragment length on the detectability of minority clones. In transmission studies, TADS is used to differentiate indigenous infections from imported infections, especially in areas marked for pre-elimination.
To gain a better understanding of malaria epidemiology and infection dynamics, individual parasite clones are tracked throughout time to determine their acquisition, elimination, or persistence in a human host. Tracking of genotypes over time can be done by taking repeated blood samples at specific time intervals and through longitudinal cohort studies and randomized-controlled trials. TADS-based genotyping of longitudinal samples from Papua New Guinea allowed for the tracking of clone density over time. This was to investigate clone competition or the dynamics of resistance-associated genotypes, and provide an additional parameter for investigating malaria infection dynamics (49). TADS-based genotyping can also be used to determine the incidence of new clones per host and serves as a surrogate measure for the exposure of an individual and the transmission intensity in a population (24). This is important in antimalarial clinical trials, where existing clones must be identified separately from new clones in post-treatment samples from patients with recurrent parasitaemia (70, 71). Previously published studies employed two distinct techniques for genotyping P. falciparum and P. vivax using TADS:
Sequencing of conventional length polymorphic genotyping markers, such as P. falciparum msp1 and msp2 (70, 72). Alternatively, non-repetitive areas containing a high number of single nucleotide polymorphisms (SNPs) are sequenced, such as the P. falciparum circumsporozoite protein (csp) or the P. vivax msp1. The advantage of this approach is that a single sequence read connects all SNPs inside an amplicon, allowing for immediate haplotype identification.
Multiple loci of genome-wide distribution were sequenced, with each locus containing a single SNP (70, 73). This strategy is well suited for population genetic studies, as these loci are unrelated. The disadvantage is that each infecting clone's haplotype must be reconstructed, which is difficult or impossible in data with a high number of co-infecting clones per host (38, 70). Other challenges with TADS include methodological accuracy issues during the experimental procedure, guaranteeing equimolar concentrations of amplicons to minimize over-representation of certain clones per sample, and limitations of detection for minority clones based on known DNA controls.
Molecular inversion probes
Targeted deep sequencing is highly sensitive and offers the opportunity to multiplex in circumstances whereby epidemiological studies involve hundreds of samples, thus decreasing the cost. One such multiplexing approach is the use of Molecular Inversion Probes (MIPs), initially referred to as “padlock probes” (74). This is an enrichment process that allows for multiplexing at different stages. Interest in MIPs has increased because; 1) It is cost-effective, 2) It has high multiplexing potential, 3) It is less time-consuming as only a small number of processing steps are required to achieve targeted region capture, 4) It has low DNA requirements (50–250 ng) as compared to other target enrichment methods, 5) It has high accuracy and specificity, 6) It allows enrichment of target regions at a scale that is matched by NGS platforms (75). MIP-TADS demonstrated that pfhrp2/3-deleted P. falciparum is a common cause of false-negative Rapid Diagnostic Test (RDT) results in two locations in Ethiopia. This genomic method revealed evidence of a recent, significant selection for pfhrp2 deletion in the sites studied, which is cause for concern (76). The downside of this approach is, like other fragment genotyping approaches, genotyping of samples containing multi-clone infections remains an unresolved challenge when multiple genome-wide loci are targeted.
Whole genome sequencing
Whole genome sequencing is the process of determining the entire DNA sequences in the genome of an organism. It is a highly sensitive tool that can be used to gather information about potential drug resistance, identify homologous relapses with improved accuracy, and analyze population structure and gene flow, especially as the cost of this technology declines rapidly. The advent of NGS is making WGS a standard today in the field of life sciences, as PCR genotyping and targeted sequencing provide less information compared to the whole genome (14).
NGS involves large-scale DNA sequencing without the use of gels and reversible terminators and was developed with an emphasis on speed and efficiency in response to advancing technology. Millions of reads can be generated in hours using platforms such as Illumina/Solexa, ABI/SOLiD, 454/Roche, and Helicos, which are at the fore-front of delivering unmatched opportunities for high-throughput genomic research (77). Thus, adapting WGS approaches is pertinent to study the malaria parasite and the epidemiology of the disease, as different regions are at different phases in their malaria elimination agenda (26).
Whole Genome Sequencing has provided deeper insight into the movement, demographics, and evolution of resistant parasites (78) and the opportunity to develop new control methods, including new drugs and vaccines, improved diagnostics and effective vector control techniques (79). A genome-wide analysis of polymorphism in P. falciparum from 24 malaria-endemic settings in 15 African countries revealed major clustering into western, central, and eastern ancestries, in addition to a highly divergent Ethiopian population (80). Whole Genome Sequencing has been used to investigate parasite diversity, with interpretations based on genome-wide diversity across various loci (24). Miotto and colleagues in 2015, through a large multicenter genome-wide association study of P. falciparum resistance to artemisinin, across 15 locations in Southeast Asia identified at least 20 mutations in kelch13 associated with a slow parasite clearance rate after treatment with artemisinin derivatives. This data also revealed that the fd (ferredoxin), arps10 (apicoplast ribosomal protein S10), mdr2 (multidrug resistance protein 2) and crt (chloroquine resistance transporter) polymorphisms are markers of a genetic background on which kelch13 mutations are especially prone to emerge, and that they correlate with the current geographical boundaries and population frequencies of artemisinin resistance (81).
Unlike individual locus analyses, which may be susceptible to strong natural selection, inferences from genome-wide diversity are less subject to target bias and so more correctly reflect broad patterns of P. falciparum genetic variation (35). However, the cost is a significant impediment to implementing WGS and it remains impractical in many situations due to technical constraints connected with large-scale data storage and processing. These studies cannot usually report diversity statistics due to multiple infections in their samples (82).
Recommendations for application of genomics techniques in resource-limited contexts
The incorporation of genomic data into malaria surveillance is a recent development, facilitated considerably by the continuous rise in accessibility to genomic technologies. However, sSA continues to lag behind other regions in the translation of findings to direct control and elimination efforts for a number of reasons. Given that one of the recommendations of the Malaria Eradication Research Agenda (malERA) expert panel is to improve the characterization of the parasite reservoir and develop new tools to assess transmission (10), some of the aforementioned tools should be made available for routine surveillance where useful. To close the gap between sSA and other regions and fully utilize the potential of genomic tools for measuring transmission, several challenges will have to be resolved. These include but not limited to funding, slow reagent supply chain, insufficient local infrastructure, challenges with integrating into routine surveillance systems, lack of technical expertise and staff retention after Bioinformatics training (69).
The restricted infrastructure is the first problem that needs to be solved. Lack of assistance provided by local governments in sSA and, to a lesser extent, donor fatigue internationally are major contributors to this problem. As we work toward malaria elimination and eradication, governments, researchers, NMCPs, and other important stakeholders must work together to use genomics to track malaria parasites. The NMCPs should be heavily involved in the development and execution of genomic investigations to make sure that they address issues of relevance and priority in the local environment. But before that can happen, researchers need to educate all stakeholders on the added value of genomic data and how it may be integrated into routine program activities including surveillance. The COVID-19 experience exposed everyone to some fundamental genomics, which may be leveraged to improve stakeholder participation in malaria genomics as a whole and transmission monitoring specifically. As a result, the time is right to achieve this. This will guarantee that the NMCPs include genomics in their strategic plan, and genomes data will be widely used to support malaria elimination initiatives. Governments will also be eager to assist this worthwhile course using the COVID-19 experience as a model. To close the human resource capacity gap, more genomics and bioinformatics training should be promoted throughout Africa. Technical experts should be kept on staff and paid fairly. They can also be engaged remotely to analyze data. Bulk purchasing and negotiating directly with manufacturers as a sub-region can improve the procurement of reagents and supplies, as demonstrated by the Pathogen Genomics Initiative (PGI) of Africa CDC during COVID-19.
Depending on financing support and the stage of a country (malaria elimination or control), a genomic strategy to monitor malaria transmission must be chosen. A reasonably inexpensive, reliable, simple but low through-put method for analyzing the SNPs to monitor malaria transmission at the control stage is the Taqman based real-time PCR assay and high-resolution melting genotyping (83, 84). In reference labs in malaria-endemic areas, the technique can be simply applied with the right staff and facilities.
In general, NGS techniques (WGS, TADS) will provide additional information, particularly when used to analyze the population dynamics and structure of parasites at a lower scale (15, 85). Although whole genome sequencing provides comprehensive information on the genetics of the parasite, it is better suitable for pre-elimination situations. During the pre-elimination era, WGS will give insights into the local and/or regional parasite populations to guide the selection of the right markers to be used in the elimination phase. On the other hand, TADS would be more economical and would offer the most pertinent data at both the control and elimination settings. Nonetheless, to analyze sequencing data, especially NGS, complex infrastructure with sufficient computer capacity and highly skilled employees are needed.
The creation of national and sub-regional reference labs could reduce the amount of equipment and qualified personnel needed. Malaria genomics for monitoring transmission dynamics in sSA should learn from the very effective PGI COVID-19 approach for a successful implementation. It will take coordinated efforts from various stakeholders at the national, sub-regional, and international levels to create the necessary framework for the establishment and maintenance of these reference laboratories, as well as cost-effective planning to ensure the efficient use of available resources. The Pathogen Diversity Network Africa (PDNA) collaboration model and the Malaria Genomic Epidemiology (MalariaGEN) consortium sample collecting, storage, or data exchange approaches will be helpful in this situation (86, 87).
In conclusion, we believe that the time is right to incorporate genomics into surveillance of malaria transmission in sSA. The successful application of malaria genomics for the monitoring of transmission across Africa will be ensured by effective planning and implementation. This includes the development and sharing of standardized operational procedures (SOP), consolidated procurements across sSA, training a cadre of bioinformaticians and genomics experts, and a strong stakeholder engagement. Using lessons learned from the COVID-19 sequencing projects across Africa, we can start with TAD and move on to WGS as costs continue to further lower and WGS can be operationalized into routine program activities.
Author contributions
AG suggested the topic and contributed to the writing and review of the manuscript. BM mainly wrote the first draft of the manuscript and worked on the review. NA-B contributed to the writing of the first draft and was involved in the review process. All authors contributed to the article and approved the submitted version.
Funding
AG was funded by the Bill and Melinda Gates Foundation as a Calestous Juma Science Leadership Fellow. Investment ID INV-037564.
Acknowledgments
The authors acknowledge Henrietta Sasu, Michaella Ahorbo, Lawrencia Appiah-Danquah, Vanesa Tima, and Albright Essuman for the initial literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. World Malaria Report 2021. World Health Organization. (2021). Available online at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed April 27, 2022).
2. Fontenille D, Simard F. Unravelling complexities in human malaria transmission dynamics in Africa through a comprehensive knowledge of vector populations. Comp Immunol Microbiol Infect Dis. (2004) 27:357–75. doi: 10.1016/j.cimid.2004.03.005
3. Kar NP, Kumar A, Singh OP, Carlton JM, Nanda N. A review of malaria transmission dynamics in forest ecosystems. Parasites and Vectors. (2014) 7:265. doi: 10.1186/1756-3305-7-265
4. Hamilton WL, Claessens A, Otto TD, Kekre M, Fairhurst RM, Rayner JC, et al. Extreme mutation bias and high AT content in plasmodium falciparum. Nucleic Acids Res. (2017) 45:1889–901. doi: 10.1093/nar/gkw1259
5. Chanda E, Coleman M, Kleinschmidt I, Hemingway J, Hamainza B, Masaninga F, et al. Impact assessment of malaria vector control using routine surveillance data in Zambia: implications for monitoring and evaluation. Malar J. (2012) 11:1437. doi: 10.1186/1475-2875-11-437
6. Vitor-Silva S, Siqueira AM, Sampaio VDS, Guinovart C, Reyes-Lecca RC, Melo GC de, et al. Declining malaria transmission in rural Amazon: changing epidemiology and challenges to achieve elimination. Malar J. (2016) 15:266. doi: 10.1186/s12936-016-1326-2
7. Shaukat AM, Breman JG, McKenzie FE. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar J. (2010) 8:1222. doi: 10.1186/1475-2875-9-122
8. World Health Organization. Global Technical Strategy for Malaria 2016–2030. World Health Organization. 2016 1–35. Available online at: https://apps.who.int/iris/bitstream/handle/10665/186671/9789243564999_spa.pdf?sequence=1 (accessed April 27, 2022).
9. THE MAL, ERARCPOTFME. malERA: an updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication. PLOS Med. (2017) 14:e1002455 doi: 10.1371/journal.pmed.1002455
10. THE MAL, ERARCPOTFME. & Measuring T. malERA: an updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLOS Med. (2017) 14:e1002452. doi: 10.1371/journal.pmed.1002452
11. Kwiatkowski D. Malaria genomics: tracking a diverse and evolving parasite population. Int Health. (2017) 7:82–4. doi: 10.1093/inthealth/ihv007
12. Escalante AA, Ferreira MU, Vinetz JM, Volkman SK, Cui L, Gamboa D, et al. Malaria molecular epidemiology: lessons from the international centers of excellence for malaria research network. Am J Trop Med Hyg. (2015) 93(Suppl. 3) 79–36. doi: 10.4269/ajtmh.15-0005
13. Mwingira F, Nkwengulila G, Schoepflin S, Sumari D, Beck HP, Snounou G, et al. Plasmodium falciparum msp1, msp2 and glurp allele frequency and diversity in sub-Saharan Africa. Malar J. (2011) 10:79. doi: 10.1186/1475-2875-10-79
14. Daniels RF, Schaffner SF, Wenger EA, Proctor JL, Chang HH, Wong W, et al. Modeling malaria genomics reveals transmission decline and rebound in Senegal. Proc Natl Acad Sci U S A. (2015) 112:7067–72. doi: 10.1073/pnas.1505691112
15. Wesolowski A, Taylor AR, Chang HH, Verity R, Tessema S, Bailey JA, et al. Mapping malaria by combining parasite genomic and epidemiologic data. BMC Med. (2018) 16:190. doi: 10.1186/s12916-018-1181-9
16. Neafsey DE, Volkman SK. Malaria Genomics in the Era of Eradication. Cold Spring Harb Perspect Med. (2017) 7:25554. doi: 10.1101/cshperspect.a025544
17. Dalmat R, Naughton B, Kwan-Gett TS, Slyker J, Stuckey EM. Use cases for genetic epidemiology in malaria elimination. Malar J. (2019) 18:163. doi: 10.1186/s12936-019-2784-0
18. Volkman SK, Ndiaye D, Diakite M, Koita OA, Nwakanma D, Daniels RF, et al. Application of genomics to field investigations of malaria by the international centers of excellence for malaria research. Acta Trop. (2012) 121:324–32. doi: 10.1016/j.actatropica.2011.12.002
19. Auburn S, Barry AE. Dissecting malaria biology and epidemiology using population genetics and genomics. Int J Parasitol. (2017) 7:77–85. doi: 10.1016/j.ijpara.2016.08.006
20. Nkhoma SC, Nair S, Al-Saai S, Ashley E, McGready R, Phyo AP, et al. Population genetic correlates of declining transmission in a human pathogen. Mol Ecol. (2013) 22:273–85. doi: 10.1111/mec.12099
21. Galinsky K, Valim C, Salmier A, de Thoisy B, Musset L, Legrand E, et al. COIL: A methodology for evaluating malarial complexity of infection using likelihood from single nucleotide polymorphism data. Malar J. (2015) 14:4. doi: 10.1186/1475-2875-14-4
22. Tessema SK, Raman J, Duffy CW, Ishengoma DS, Amambua-Ngwa A, Greenhouse B. Applying next-generation sequencing to track falciparum malaria in sub-Saharan Africa. Malar J. (2019) 18:268. doi: 10.1186/s12936-019-2880-1
23. Hofmann NE, Karl S, Wampfler R, Kiniboro B, Teliki A, Iga J, et al. The complex relationship of exposure to new Plasmodium infections and incidence of clinical malaria in Papua New Guinea. Elife. (2017) 6:23708. doi: 10.7554/eLife.23708
24. Lerch A, Koepfli C, Hofmann NE, Kattenberg JH, Rosanas-Urgell A, Betuela I, et al. Longitudinal tracking and quantification of individual Plasmodium falciparum clones in complex infections OPEN. Sci Rep. (2019) 9:3333. doi: 10.1038/s41598-019-39656-7
25. Noviyanti R, Miotto O, Barry A, Marfurt J, Siegel S, Thuy-Nhien N, et al. Implementing parasite genotyping into national surveillance frameworks: feedback from control programmes and researchers in the Asia-Pacific region. Malaria J. (2020) 19:271. doi: 10.1186/s12936-020-03330-5
26. Akoniyon OP, Adewumi TS, Maharaj L, Oyegoke OO, Roux A, Adeleke MA, et al. Whole genome sequencing contributions and challenges in disease reduction focused on Malaria. Biology (Basel) [Internet]. (2022) 11:587. doi: 10.3390/biology11040587
27. Akter J, Thriemer K, Khan WA, Sullivan DJ, Noedl H, Haque R. Genotyping of Plasmodium falciparum using antigenic polymorphic markers and to study anti-malarial drug resistance markers in malaria endemic areas of Bangladesh. Malar J. (2012) 11:386. doi: 10.1186/1475-2875-11-386
28. Molina-Cruz A, Raytselis N, Withers R, Dwivedi A, Crompton PD, Traore B, et al. A genotyping assay to determine geographic origin and transmission potential of Plasmodium falciparum malaria cases. Commun Biol. (2021) 4:1145. doi: 10.1038/s42003-021-02667-0
29. Usman-Yamman H, Omalu C J I, Abubakar A, Abolarinwa S O, Eke S S, Otuu CA. Genetic diversity of plasmodium falciparum isolates in Minna, North Central Nigeria inferred by PCR genotyping of Merozoite surface protein 1 and 2. Infect Genetics Evolution. (2021) 96:5143. doi: 10.1016/j.meegid.2021.105143
30. Mardis ER. A decade's perspective on DNA sequencing technology. Nature. (2011) 470:198–203. doi: 10.1038/nature09796
31. Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science (1979). (2004) 305:1124. doi: 10.1126/science.1098876
32. Imwong M, Suwannasin K, Kunasol C, Sutawong K, Mayxay M, Rekol H, et al. The spread of artemisinin-resistant Plasmodium falciparum in the greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis. (2017) 17:491–7. doi: 10.1016/S1473-3099(17)30048-8
33. Imwong M, Hien TT, Thuy-Nhien NT, Dondorp AM, White NJ. Spread of a single multidrug resistant malaria parasite lineage (PfPailin) to Vietnam. Lancet Infect Dis. (2017) 17:1022–3. doi: 10.1016/S1473-3099(17)30524-8
34. Jacob CG, Thuy-Nhien N, Mayxay M, Maude RJ, Quang HH, Hongvanthong B, et al. Genetic surveillance in the greater mekong subregion and south asia to support malaria control and elimination. Elife. (2021) 10:e62997. doi: 10.7554/eLife.62997
35. Apinjoh TO, Ouattara A, Titanji VPK, Djimde A. Amambua-Ngwa A. Genetic diversity and drug resistance surveillance of Plasmodium falciparum for malaria elimination: Is there an ideal tool for resource-limited sub-Saharan Africa? Malaria J. (2019) 18:217. doi: 10.1186/s12936-019-2844-5
36. Jemmely NY, Niang M, Preiser PR. Small variant surface antigens and Plasmodium evasion of immunity. Future Microbiol. (2010) 5:663–82. doi: 10.2217/fmb.10.21
37. Frech C, Chen N. Variant surface antigens of malaria parasites: functional and evolutionary insights from comparative gene family classification and analysis. BMC Genomics. (2013) 14:427. doi: 10.1186/1471-2164-14-427
38. Chang HH, Worby CJ, Yeka A, Nankabirwa J, Kamya MR, Staedke SG, et al. THE REAL McCOIL: a method for the concurrent estimation of the complexity of infection and SNP allele frequency for malaria parasites. PLoS Comput Biol. (2017) 13:e1005348. doi: 10.1371/journal.pcbi.1005348
39. Snounou G, Beck HP. The use of PCR genotyping in the assessment of recrudescence or reinfection after antimalarial drug treatment. Parasitology Today. (1998) 14:462–7. doi: 10.1016/S0169-4758(98)01340-4
40. Viriyakosol S, Siripoon N, Petcharapirat C, Petcharapirat P, Jarra W, Thaithong S, et al. Genotyping of Plasmodium falciparum isolates by the polymerase chain reaction and potential uses in epidemiological studies. Bull World Health Organ. (1995) 73:85–95.
41. Huang B, Tuo F, Liang Y, Wu W, Wu G, Huang S, et al. Temporal changes in genetic diversity of msp-1, msp-2, and msp-3 in Plasmodium falciparum isolates from Grande Comore Island after introduction of ACT. Malar J. (2018) 17:83. doi: 10.1186/s12936-018-2227-3
42. Mze NP, Bogreau H, Diedhiou CK, Herdell V, Rahamatou S, Bei AK, et al. Genetic diversity of Plasmodium falciparum in Grande Comore Island. Malar J. (2020) 19:320. doi: 10.21203/rs.3.rs-26560/v1
43. Juliano JJ, Ariey F, Sem R, Tangpukdee N, Krudsood S, Olson C, et al. Misclassification of drug failure in plasmodium falciparum clinical trials in southeast asia. J Infect Dis. (2009) 200:624–8. doi: 10.1086/600892
44. Amambua-Ngwa A, Tetteh KKA, Manske M, Gomez-Escobar N, Stewart LB, Deerhake ME, et al. Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites. PLoS Genet. (2012) 8:e1002992. doi: 10.1371/journal.pgen.1002992
45. Swerdlow H, Gesteland R. Capillary gel electrophoresis for rapid, high resolution DNA sequencing. Nucleic Acids Res. (1990) 8:1415–9. doi: 10.1093/nar/18.6.1415
46. Gupta V, Dorsey G, Hubbard AE, Rosenthal PJ, Greenhouse B. Gel Versus Capillary Electrophoresis Genotyping for Categorizing Treatment Outcomes in Two Anti-Malarial Trials in Uganda. (2010). Available online at: http://www.malariajournal.com/content/9/1/19 (accessed April 27, 2022).
47. WHO. Informal consultation on methodology to distinguish reinfection from recrudescence in high malaria transmission areas. Available online at: https://apps.who.int/iris/rest/bitstreams/1389752/retrieve.2021 (accessed April 27, 2022).
48. Su XZ, Wellems TE. Toward a high-resolution Plasmodium falciparum linkage map: Polymorphic markers from hundreds of simple sequence repeats. Genomics. (1996) 33:386. doi: 10.1006/geno.1996.0218
49. Anderson TJC, Haubold B, Williams JT, Estrada-Franco JG, Richardson L, Mollinedo R, et al. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol. (2000) 17:1467–82. doi: 10.1093/oxfordjournals.molbev.a026247
50. Razak MRMA, Sastu UR, Norahmad NA, Abdu-Karim A, Muhammad A, Muniandy PK, et al. Genetic diversity of plasmodium falciparum populations in malaria declining areas of Sabah, East Malaysia. PLoS ONE. (2016) 11:e0152415. doi: 10.1371/journal.pone.0152415
51. Wei G, Zhang L, Yan H, Zhao Y, Hu J, Pan W. Evaluation of the population structure and genetic diversity of Plasmodium falciparum in southern China. Malar J. (2015) 14:283. doi: 10.1186/s12936-015-0786-0
52. Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. (2002) 418:320–3. doi: 10.1038/nature00813
53. Daniels RF, Schaffner SF, Dieye Y, Dieng G, Hainsworth M, Fall FB, et al. Genetic evidence for imported malaria and local transmission in Richard Toll, Senegal. Malar J. (2020) 19:276. doi: 10.1186/s12936-020-03346-x
54. Fola AA, Kattenberg E, Razook Z, Lautu-Gumal D, Lee S, Mehra S, et al. SNP barcodes provide higher resolution than microsatellite markers to measure Plasmodium vivax population genetics. Malar J. (2020) 19:375. doi: 10.1186/s12936-020-03440-0
55. Madesis P, Ganopoulos I, Tsaftaris A. Microsatellites: evolution and contribution. Methods Mol Biol. (2013) 1006:1–13. doi: 10.1007/978-1-62703-389-3_1
56. Sutton PL, A. call to arms: On refining Plasmodium vivax microsatellite marker panels for comparing global diversity. Malar J. (2013) 12:447. doi: 10.1186/1475-2875-12-447
57. Roh ME, Roh ME, Tessema SK, Murphy M, Nhlabathi N, Mkhonta N, et al. The Journal of Infectious Diseases High Genetic Diversity of Plasmodium falciparum in the Low-Transmission Setting of the Kingdom of Eswatini. J Infect Dis®. (2019) 220:1346–54. doi: 10.1093/infdis/jiz305
58. Tessema S, Wesolowski A, Chen A, Murphy M, Wilheim J, Mupiri AR, et al. Using parasite genetic and human mobility data to infer local and cross- border malaria connectivity in Southern Africa. Elife. (2019) 8:43510. doi: 10.7554/eLife.43510
59. Gunawardena S, Karunaweera ND, Ferreira MU, Phone-Kyaw M, Pollack RJ, Alifrangis M, et al. Geographic structure of Plasmodium vivax: microsatellite analysis of parasite populations from Sri Lanka, Myanmar, and Ethiopia. Am J Tropical Med Hygiene. (2010) 82:235–42. doi: 10.4269/ajtmh.2010.09-0588
60. Liljander A, Wiklund L, Falk N, Kweku M, Mrtensson A, Felger I, et al. Optimization and validation of multi-coloured capillary electrophoresis for genotyping of Plasmodium falciparum merozoite surface proteins (msp1 and 2). Malar J. (2009) 8:78. doi: 10.1186/1475-2875-8-78
61. Daniels R, Volkman SK, Milner DA, Mahesh N, Neafsey DE, Park DJ, et al. A general SNP-based molecular barcode for Plasmodium falciparum identification and tracking. Malar J. (2008) 7:223. doi: 10.1186/1475-2875-7-223
62. Tripathi M, Das A. Genotyping malaria parasites with DNA barcodes. Tropical Med Int Health. (2015) 20:1636–8. doi: 10.1111/tmi.12594
63. Searle KM, Katowa B, Kobayashi T, Siame MNS, Mharakurwa S, Carpi G, et al. Distinct parasite populations infect individuals identified through passive and active case detection in a region of declining malaria transmission in southern Zambia. Malar J. (2017) 16:154. doi: 10.1186/s12936-017-1810-3
64. Obaldia N, Baro NK, Calzada JE, Santamaria AM, Daniels R, Wong W, et al. Clonal outbreak of plasmodium falciparum infection in eastern panama. J Infect Dis®. (2015) 21:1087–96. doi: 10.1093/infdis/jiu575
65. Carlton JM, Volkman SK, Uplekar S, Hupalo DN, Pereira Alves JM, Cui L, et al. Population genetics, evolutionary genomics, and genome-wide studies of malaria: a view across the international centers of excellence for malaria research. Am J Trop Med Hyg. (2015) 93:87–98. doi: 10.4269/ajtmh.15-0049
66. Omedo I, Mogeni P, Bousema T, Rockett K, Amambua-Ngwa A, Oyier I, et al. Micro-epidemiological structuring of plasmodium falciparum parasite populations in regions with varying transmission intensities in Africa. Wellcome Open Res. (2017) 2:10. doi: 10.12688/wellcomeopenres.10784.2
67. Pholwat S, Liu J, Stroup S, Jacob ST, Banura P, Moore CC, et al. The malaria TaqMan array card includes 87 assays for Plasmodium falciparum drug resistance, identification of species, and genotyping in a single reaction. Antimicrob Agents Chemother. (2017) 61:17. doi: 10.1128/AAC.00110-17
68. Campino S, Auburn S, Kivinen K, Zongo I, Ouedraogo JB, Mangano V, et al. Population genetic analysis of plasmodium falciparum parasites using a customized illumina goldengate genotyping assay. PLoS ONE. (2011) 6:e20251. doi: 10.1371/journal.pone.0020251
69. Ghansah A, Kamau E, Amambua-Ngwa A, Ishengoma DS, Maiga-Ascofare O, Amenga-Etego L, et al. Targeted Next Generation Sequencing for malaria research in Africa: Current status and outlook. Malaria J. (2019) 18:324. doi: 10.1186/s12936-019-2944-2
70. Lerch A, Koepfli C, Hofmann NE, Messerli C, Wilcox S, Kattenberg JH, et al. Development of amplicon deep sequencing markers and data analysis pipeline for genotyping multi-clonal malaria infections. BMC Genomics. (2017) 18:864. doi: 10.1186/s12864-017-4260-y
71. WHO. Methods and Techniques for Clinical Trials on Antimalarial Drug Efficacy: Genotyping to Identify Parasite Populations. World Health Organization (2017).
72. Juliano JJ, Porter K, Mwapasa V, Sem R, Rogers WO, Ariey F, et al. Exposing malaria in-host diversity and estimating population diversity by capture-recapture using massively parallel pyrosequencing. Proc Natl Acad Sci U S A. (2010) 107:20138–43. doi: 10.1073/pnas.1007068107
73. Friedrich LR, Popovici J, Kim S, Dysoley L, Zimmerman PA, Menard D, et al. Complexity of infection and genetic diversity in cambodian plasmodium vivax. PLoS Negl Trop Dis. (2016) 10:e0004526. doi: 10.1371/journal.pntd.0004526
74. Nilsson M, Malmgren H, Samiotaki M, Kwiatkowski M, Chowdhary BP, Landegren U. Padlock probes: circularizing oligonucleotides for localized DNA detection. Science (1979). (1994) 265:2085-8. doi: 10.1126/science.7522346
75. Aydemir O, Janko M, Hathaway NJ, Verity R, Mwandagalirwa MK, Tshefu AK, et al. Drug-Resistance and population structure of plasmodium falciparum across the democratic Republic of Congo using high-Throughput molecular inversion probes. J Infect Dis. (2018) 218:946–55. doi: 10.1093/infdis/jiy223
76. Feleke SM, Reichert EN, Mohammed H, Brhane BG, Mekete K, Mamo H, et al. Plasmodium falciparum is evolving to escape malaria rapid diagnostic tests in Ethiopia. Nat Microbiol. (2021) 6:1289–99. doi: 10.1038/s41564-021-00962-4
77. Govindan VP, Murthy PK. Next generation sequencing technologies in Malaria. J Next Generation Sequencing Appl. (2016) 03 138. doi: 10.4172/2469-9853.1000138
78. Amato R, Miotto O, Woodrow CJ, Almagro-Garcia J, Sinha I, Campino S, et al. Genomic epidemiology of artemisinin resistant malaria. Elife. (2016) 5:e08714. doi: 10.7554/eLife.08714
79. Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. (2002) 419:498–511.
80. Amambua-Ngwa A, Amenga-Etego L, Kamau E, Amato R, Ghansah A, Golassa L, et al. Major subpopulations of Plasmodium falciparum in sub-Saharan Africa. Science (1979). (2019) 365:813–816. doi: 10.1126/science.aav5427
81. Miotto O, Amato R, Ashley EA, Macinnis B, Almagro-Garcia J, Amaratunga C, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. (2015) 47:226–34. doi: 10.1038/ng.3189
82. Winter DJ, Pacheco MA, Vallejo AF, Schwartz RS, Arevalo-Herrera M, Herrera S, et al. Whole genome sequencing of field isolates reveals extensive genetic diversity in plasmodium vivax from colombia. PLoS Negl Trop Dis. (2015) 9:e0004526. doi: 10.1101/025338
83. Daniels R, Ndiaye D, Wall M, McKinney J, Séne PD, Sabeti PC, et al. Rapid, field-deployable method for genotyping and discovery of single-nucleotide polymorphisms associated with drug resistance in Plasmodium falciparum. Antimicrob Agents Chemother. (2012) 56:2976–86. doi: 10.1128/AAC.05737-11
84. Ndiaye YD, Diédhiou CK, Bei AK, Dieye B, Mbaye A, Mze NP, et al. High resolution melting: a useful field-deployable method to measure dhfr and dhps drug resistance in both highly and lowly endemic Plasmodium populations. Malar J. (2017) 16:153. doi: 10.1186/s12936-017-1811-2
85. Taylor AR, Schaffner SF, Cerqueira GC, Nkhoma SC, Anderson TJC, Sriprawat K, et al. Quantifying connectivity between local Plasmodium falciparum malaria parasite populations using identity by descent. PLoS Genet. (2017) 13:e1007065. doi: 10.1371/journal.pgen.1007065
86. MalariaGEN. Malaria Genomic Epidemiology Network. Available online at: https://www.malariagen.net/about (accessed April 27, 2022).
87. MRC. Centre for Genomics and Global Health Plasmodium Diversity Network Africa. Available online at: https://www.cggh.org/collaborations/plasmodium-diversity-network-africa (accessed April 29, 2022).
Keywords: malaria, transmission, genomics, techniques, surveillance
Citation: Mensah BA, Akyea-Bobi NE and Ghansah A (2022) Genomic approaches for monitoring transmission dynamics of malaria: A case for malaria molecular surveillance in Sub–Saharan Africa. Front. Epidemiol. 2:939291. doi: 10.3389/fepid.2022.939291
Received: 09 May 2022; Accepted: 10 October 2022;
Published: 28 October 2022.
Edited by:
Alyssa E. Barry, Deakin University, AustraliaReviewed by:
Lynette Isabella Oyier, KEMRI Wellcome Trust Research Programme, KenyaEimear Cleary, University of Southampton, United Kingdom
Copyright © 2022 Mensah, Akyea-Bobi and Ghansah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anita Ghansah, YWdoYW5zYWhAbm9ndWNoaS51Zy5lZHUuZ2g=
 Benedicta A. Mensah
Benedicta A. Mensah Nukunu E. Akyea-Bobi
Nukunu E. Akyea-Bobi Anita Ghansah
Anita Ghansah