
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 13 July 2022
Sec. Clinical Microbiology
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.757844
This article is part of the Research Topic Application of Next Generation Sequencing (NGS) and CRISPR-Cas Systems in The Diagnosis of Infectious Diseases View all 45 articles
 Beatus M. Lyimo1,2†
Beatus M. Lyimo1,2† Zachary R. Popkin-Hall3†
Zachary R. Popkin-Hall3† David J. Giesbrecht4
David J. Giesbrecht4 Celine I. Mandara1
Celine I. Mandara1 Rashid A. Madebe1
Rashid A. Madebe1 Catherine Bakari1
Catherine Bakari1 Dativa Pereus1
Dativa Pereus1 Misago D. Seth1
Misago D. Seth1 Ramadhan M. Ngamba1
Ramadhan M. Ngamba1 Ruth B. Mbwambo1
Ruth B. Mbwambo1 Bronwyn MacInnis5,6
Bronwyn MacInnis5,6 Daniel Mbwambo7
Daniel Mbwambo7 Issa Garimo7
Issa Garimo7 Frank Chacky7
Frank Chacky7 Sijenunu Aaron7
Sijenunu Aaron7 Abdallah Lusasi7
Abdallah Lusasi7 Fabrizio Molteni8
Fabrizio Molteni8 Ritha Njau9
Ritha Njau9 Jane A. Cunningham10
Jane A. Cunningham10 Samwel Lazaro7
Samwel Lazaro7 Ally Mohamed7
Ally Mohamed7 Jonathan J. Juliano3
Jonathan J. Juliano3 Jeffrey A. Bailey4
Jeffrey A. Bailey4 Deus S. Ishengoma1,5,11*
Deus S. Ishengoma1,5,11*Recent developments in molecular biology and genomics have revolutionized biology and medicine mainly in the developed world. The application of next generation sequencing (NGS) and CRISPR-Cas tools is now poised to support endemic countries in the detection, monitoring and control of endemic diseases and future epidemics, as well as with emerging and re-emerging pathogens. Most low and middle income countries (LMICs) with the highest burden of infectious diseases still largely lack the capacity to generate and perform bioinformatic analysis of genomic data. These countries have also not deployed tools based on CRISPR-Cas technologies. For LMICs including Tanzania, it is critical to focus not only on the process of generation and analysis of data generated using such tools, but also on the utilization of the findings for policy and decision making. Here we discuss the promise and challenges of NGS and CRISPR-Cas in the context of malaria as Africa moves towards malaria elimination. These innovative tools are urgently needed to strengthen the current diagnostic and surveillance systems. We discuss ongoing efforts to deploy these tools for malaria detection and molecular surveillance highlighting potential opportunities presented by these innovative technologies as well as challenges in adopting them. Their deployment will also offer an opportunity to broadly build in-country capacity in pathogen genomics and bioinformatics, and to effectively engage with multiple stakeholders as well as policy makers, overcoming current workforce and infrastructure challenges. Overall, these ongoing initiatives will build the malaria molecular surveillance capacity of African researchers and their institutions, and allow them to generate genomics data and perform bioinformatics analysis in-country in order to provide critical information that will be used for real-time policy and decision-making to support malaria elimination on the continent.
Significant progress was made between 2005 and 2015 in malaria control globally but progress has stalled in recent years (World Health Organization, 2020). Changes in malaria burden were attributed to different interventions that target both mosquito vectors and parasites, which have been scaled-up in the past two decades. Recent evidence shows an increase in malaria cases globally from 2017 to 2019 compared to previous years, but the number of deaths remained relatively unchanged from 2017 to 2019. However, in 2020, malaria deaths increased from 2019 levels, with the majority of both cases and deaths involving children and pregnant women from sub-Saharan Africa (SSA) (World Health Organization, 2021). The increase in malaria cases can be partially explained by the emergence and spread of insecticide resistance in Anopheles populations (Kisinza et al., 2017 and antimalarial resistance in parasites (Dondorp et al., 2009; Haldar et al., 2018; Ippolito et al., 2021) both of which threaten the effectiveness of the major malaria interventions, long-lasting insecticide treated bed-nets (LLINs), indoor residual spraying (IRS) and antimalarial drugs (Tawe et al., 2018; Apinjoh et al., 2019; Morgan et al., 2020). Increased malaria cases in 2020 were also attributed to the COVID-19 pandemic, which was believed to have disrupted malaria control activities (World Health Organization, 2021). However, other potential factors such as transmission from asymptomatic reservoirs (Andolina et al., 2021, climate change, and other environmental changes caused by human activities are thought to be responsible for the resurgence of malaria (Upatham et al., 1988; Chaumeau et al., 2019; Mitchell et al., 2022).
In 2020, 29 countries in Africa contributed about 96% of all reported malaria deaths globally, despite consistent implementation of the major malaria interventions since 2002 (World Health Organization, 2021). Malaria control in Africa relies mainly on the use of LLINs and effective antimalarial drugs, predominantly artemisinin-based combination therapy (ACTs) for routine case management and sulphadoxine/pyrimethamine (SP) for intermittent preventive therapy in pregnant women (iPTp) (World Health Organization, 2021). Despite the detection of insecticide resistance and occurrence of mutations in PfKelch13 gene (associated with resistance to artemisinins) in Rwanda, Uganda and Eritrea, treatment failure rates remain below 10%, hence both ACT and vector control measures are still considered highly effective (World Health Organization, 2021). However, a robust surveillance and response strategy is urgently needed to detect any early failures in the current interventions and facilitate effective response to ensure malaria elimination by 2030.
At present, malaria case management and routine surveillance rely on traditional diagnostic methods based on microscopy and rapid diagnostic tests (RDTs). These methods are widely deployed by the National Malaria Control Programmes (NMCPs) in most malaria endemic countries in Africa (Tawe et al., 2018; Abbas et al., 2019; Tessema et al., 2019b; Morgan et al., 2020). Despite their short turnaround time, these methods have major limitations (Apinjoh et al., 2019 (Table 1). In areas of low transmission and those closer to malaria elimination, microscopy and RDTs are less sensitive and unable to detect transmissible but very low parasite densities (Nsanzabana, 2019; Andolina et al., 2021). Since most of the widely used RDTs are based on the detection of histidine rich protein 2 (HRP2), the situation is further exacerbated by the emergence and spread of Plasmodium falciparum with histidine rich protein 2 and 3 (pfhrp2 and pfhrp3) gene deletions (Thomson et al., 2019; Feleke et al., 2021). To strengthen the current surveillance system, innovative methods of malaria diagnosis and population surveillance based on advanced molecular techniques are urgently needed to support effective case management (Ishengoma et al., 2019.
Two key technologies that are slated to have a large impact on surveillance and diagnosis of malaria are Next Generation Sequencing (NGS) and clustered regularly interspaced short palindromic repeats (CRISPR). NGS, which is also termed high-throughput or massive parallel sequencing, allows for thousands to billions of DNA or RNA fragments to be sequenced and analyzed in either a targeted or whole genome approach (Deurenberg et al., 2017; Talundzic et al., 2018). Routine malaria surveillance programs could benefit from deployment of NGS technologies, as they provide a scalable, cost-effective means of surveillance in large numbers of samples (Juliano et al., 2010; Van Tyne et al., 2011; Manske et al., 2012; Lerch et al., 2017; Ngondi et al., 2017; Tawe et al., 2018; ; Moser et al., 2021).
CRISPR and CRISPR associated protein (Cas) derived from type II CRISPR bacterial immune systems, provide the basis for a new family of assays that detect nucleic acid targets with high sensitivity and specificity. While CRISPR-Cas is mostly known to enable easy gene editing, it also has enormous diagnostic potential (Jiang et al., 2013. Indeed, CRISPR-Cas systems have been utilized to detect and differentiate arbovirus strains (Gootenberg et al., 2017, to diagnose SARS-CoV-2 infections (Guo et al., 2020, to distinguish pathogenic bacteria (Strich and Chertow, 2019, to diagnose cancers (Yin et al., 2019, and indeed to diagnose malaria (Lee et al., 2020; Cunningham et al., 2021).
This review discusses ongoing efforts to deploy advanced molecular tools for the diagnosis and surveillance of malaria, developing the capacity for pathogen genomics in Africa, and highlights both potential opportunities presented by these innovative technologies as well as challenges facing these efforts.
Our ability to detect and track the spread of malaria is potentially improved by advances in malaria genomics (particularly those based on NGS) and associated bioinformatics tools in combination with epidemiological data (Escalante et al., 2015; Kwiatkowski, 2015; Watson et al., 2021). These tools can facilitate different studies of parasite populations, antimalarial drug resistance, surveillance of hrp2/3 gene deletions, and monitoring the impact of current and future interventions (Figure 1). Studies utilizing these methods are urgently needed and if scaled-up, can be critical for monitoring transmission to achieve proper control and the ultimate goal of malaria elimination.
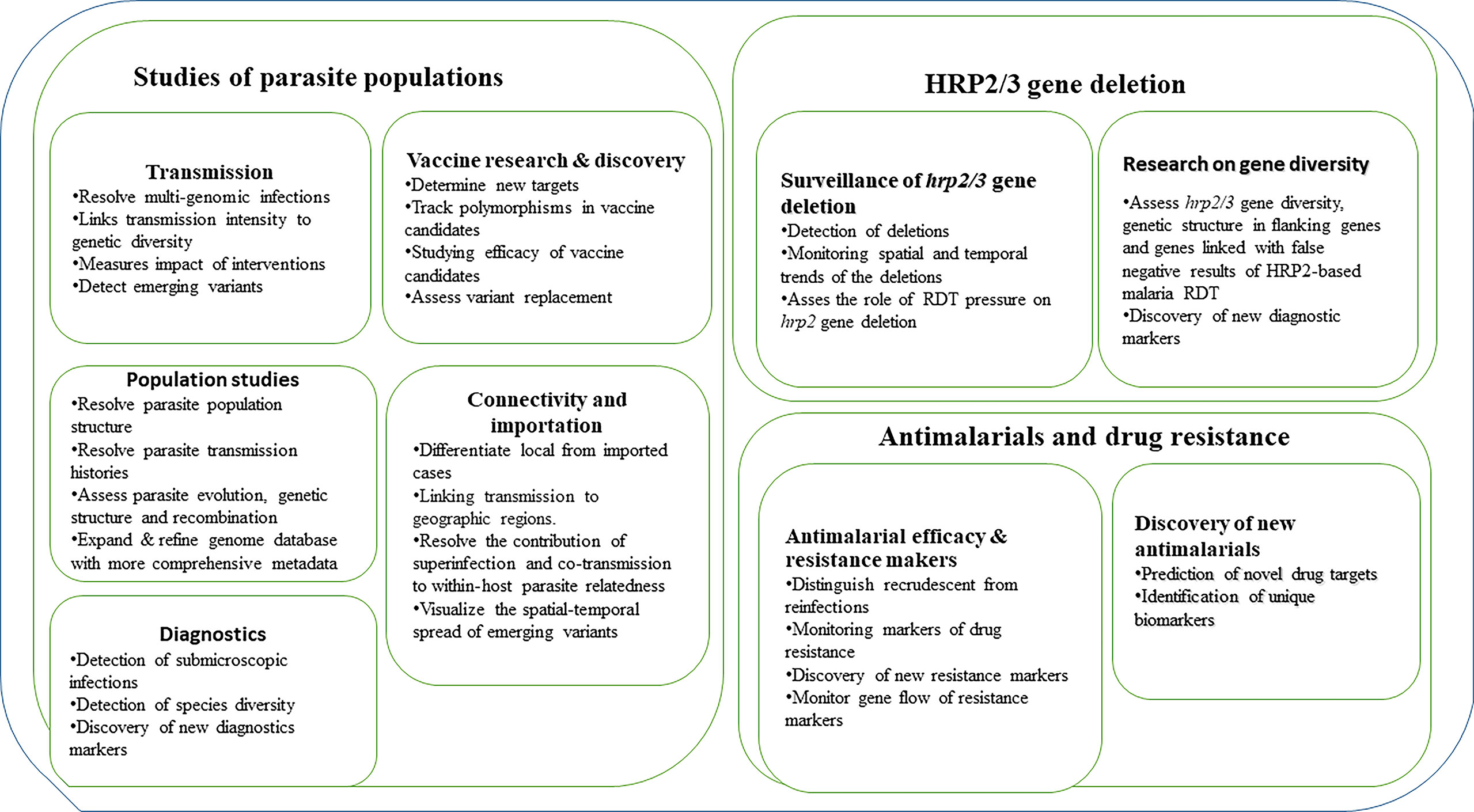
Figure 1 Current and potential application of NGS in studies of parasite populations, drug resistance and surveillance of hrp2/3 gene deletions.
Historically, transmission intensity and dynamics of malaria burden were measured using tools like entomological inoculation rates (EIR), case incidence and prevalence. These tools have helped to generate maps of mortality rates, prevalence and incidence as well as the overall global burden of malaria (Weiss et al., 2019; Neafsey et al., 2021), but recently have become less sensitive especially in areas of very low transmission. Due to their inherent limitations, they are unable to detect genetic signatures and parasite population structure indicative of modified transmission in response to interventions. For example, higher incidence could indicate a decline in the effectiveness of malaria interventions, even though the increase might actually be due to increased transmission rates from a source which was not addressed in the initial interventions (Mohd Abd Razak et al., 2016). Furthermore, decreased transmission limits outcrossing, influencing high inbreeding rates within the population and triggering population fragmentation. As such, conventional surveillance methods may result in misinterpreting the underlying processes shaping malaria epidemiology. Advances in NGS technologies supported by bioinformatic tools and increased availability of epidemiological data have made it possible to generate high quality and sensitive data which may help detect changes in transmission intensity, identify multi-genomic infections, evaluate intervention effectiveness, and identify potential deficiencies in malaria control programmes in both high and low transmission areas (Watson et al., 2021). This improved understanding can be achieved by determining the number of parasite strains, genetic diversity (e.g. within-host diversity index (Fws) as well as relatedness and genetic structure of parasites in an infection.
Polymorphic genes such as merozoite surface protein (msp1), merozoite surface protein 2 (msp2) and glutamate rich protein (glurp) have been used extensively to study parasite genetic diversity and population structure, mostly to detect complexity/multiplicity of infection (COI/MOI), wherein multiple distinct genotypes co-occur in a single host (Lalremruata et al., 2017). Studies using these methods have demonstrated associations between MOI and such factors as host age, clinical severity, and transmission intensity (Mayengue et al., 2009; Lalremruata et al., 2017). However, these PCR-based genotyping methods have limited inter-laboratory reproducibility and also lack sensitivity, hence may underestimate allelic diversity in co-infected hosts (Assefa et al., 2014). Highly sensitive NGS tools, by contrast, can illuminate not only MOI but also parasite transmission history, genetic structure, recombination, and evolutionary history.
The use of these tools to study evolutionary history can improve our understanding of the parasite-host interactions involved in the emergence of new parasites in the region (Su et al., 2020, as well as evolutionary responses to selective pressures caused by intensified malaria elimination interventions, such as antimalarial drugs (Neafsey and Volkman, 2017). It is even possible to estimate recombination events that may lead to evasion of control and detection efforts (Miles et al., 2016). In addition, NGS data facilitates population genetic and evolutionary metric calculations, such as linkage disequilibrium (LD), effective population size (Ne), site frequency spectrum (SFS), identity by descent (IBD), identity by state (IBS), haplotype diversity (H), principal component analysis (PCA), FWS, and FST. The extent of parasite genomic data now available provides opportunities to expand and refine the global genome database and public resources, which in turn will greatly expand our understanding of parasite biology and accelerate the discovery of new interventions.
Traditionally, case incidence was used in combination with human migration data to link parasite populations across geographical and transmission gradients, which would inform intervention strategies. However, this method lacks the precision of NGS-based methods.
Genetic relatedness inferred from genomic data provides much higher geographic resolution of transmission (Tessema et al., 2019a) and can be quantified between 0 (completely unrelated parasites) and 1 (identical clones). Genetic relatedness between parasite samples is positively correlated with reduced transmission, and therefore can be used as a proxy measure of transmission level (Daniels et al., 2015; Cerqueira et al., 2017). Using advanced NGS tools, it is also possible to infer malaria transmission networks over a given timescale in a population (Ajibola et al., 2021). Importation of malaria parasites from higher to lower transmission areas, particularly those in the elimination phase, remains a major challenge to malaria elimination, and is difficult to detect using traditional methods. However, NGS methods are able to do so, as was demonstrated in Zanzibar (Morgan et al., 2020). Strengthening malaria molecular surveillance (MMS) using advanced genomic tools will reveal the number of secondary cases arising from each imported infection, allowing NMCPs to determine what interventions are required to prevent re-establishment of transmission. The data derived from effective MMS systems will also help to resolve the contribution of superinfection and co-transmission (Nair et al., 2014; Nkhoma et al., 2018).
Antimalarial resistance is a major obstacle to the effective control and elimination of malaria. Malaria parasites have evolved resistance to all widely deployed antimalarial drugs, leading to withdrawal of some of the drugs such as chloroquine and SP (Ishengoma et al., 2019). Antimalarial drug resistance has spread globally, although it remains rare and localized in some areas with lower transmission and therefore lower selective pressure (Haldar et al., 2018). We can clearly use these methods to retrospectively understand how and why resistance emerged and spread. However, their prospective use by NMCPs through enhancing genomic surveillance infrastructure can identify signatures of emerging drug resistance both when resistance mutations are known and unknown (Neafsey et al., 2021.
Antimalarial efficacy monitoring is usually done in therapeutic efficacy studies (TES) which enroll infected patients and genotype those with recurrent infection after treatment to distinguish between recrudescence (drug failure) and reinfection (World Health Organization, 2009). Traditional methods rely on the polymorphic markers mentioned above (World Health Organization, 2007) or microsatellites (Greenhouse et al., 2006; Malvy et al., 2018). While these methods are widespread, they are limited by their low throughput, poor resolution, and technical challenges regarding analysis, scoring and interpretation of results. Specifically, these methods under-estimate MOI or misclassify infections as either recrudescent or reinfection (Shaukat et al., 2012). However, new NGS methods using amplicon sequencing (Rao et al., 2016 or SNP barcodes are being optimized and may potentially replace the current methods, which would improve estimates of antimalarial efficacy especially in low transmission areas with limited diversity and polymorphisms in parasite populations.
NGS provides a robust, high throughput tool for identifying and tracking molecular markers of drug resistance (Ishengoma et al., 2019). Currently available markers can be used for spatial and temporal monitoring of resistance to different antimalarials, including both current drugs and those which were used in the past (Rao et al., 2016; Levitt et al., 2017; Nag et al., 2017; Talundzic et al., 2018). This monitoring is especially critical in the context of partial artemisinin resistance, which has already been reported in South-East Asia (SEA) (Leang et al., 2015; Takala-Harrison and Laufer, 2015). Artemisinin resistant parasites may be introduced from SEA (as drug resistance genes are known to spread between populations) (Roper et al., 2004; Dwivedi et al., 2016) or could potentially emerge independently in SSA, underscoring the necessity of this continuous surveillance.
It is important to understand the origin and flow of resistance genes in order to anticipate the diffusion of antimalarial resistance and interrupt its spread (Dwivedi et al., 2016). Current methods for doing this are expensive and less robust. For example, Sanger sequencing is considered to be useful in identification of major alleles but has low accuracy in identifying alleles with frequency less than 50% (minor alleles) (Rohlin et al., 2009). NGS based methods are promising as they are high throughput, less labor intensive, have high sensitivity in identifying all variants, even those with minor allele frequencies, and facilitate the discovery of novel markers (Tessema et al, 2019a).
Malaria rapid diagnostic tests (mRDTs) based on lateral flow detection of P. falciparum Histidine-Rich Protein 2 (PfHRP2) have supported prompt and effective identification of P. falciparum malaria infections, particularly in resource poor regions. However, recent studies in various countries have reported some cases where pfHRP2 based mRDTs failed to detect malaria parasites in patients with positive results by either microscopy or PCR (Gamboa et al., 2010; Thomson et al., 2019; Bakari et al., 2020). While other factors may be involved (Murray et al., 2008, deletion of the pfhrp2 gene (as well as the related pfhrp3 that cross-reacts in RDTs) has also been reported to contribute to false-negative test results (Luchavez et al., 2011; Cheng et al., 2014; Thomson et al., 2019), which has significantly compromised the performance of PfHRP2-based RDTs. Thus, WHO currently recommends for all malaria endemic countries to undertake surveys to identify the prevalence and trends of the deletion in space and time (World Health Organization, 2019).
NGS data enables both the detection of emergence and monitoring of pfhrp2/3 gene deletion frequencies in all settings (low and high transmission) in relation to the pressure caused by the use of HRP2-based RDTs. With intensified surveillance, it will therefore be possible to identify areas which may need to change diagnostic strategies for effective malaria control. The deletion is currently detected based on initial clinical evidence (e.g. a microscopy-positive result for P.falciparum but negative RDT) and confirmed with molecular approaches such as PCR and antigen detection assays (Cheng et al., 2014). However, some samples can be RDT-negative but have intact pfhrp2/3 genes (Nsobya et al., 2021). Indeed, a study from the Democratic Republic of Congo that performed WGS on samples with suspected deletions showed that the genes were actually intact (Parr et al., 2021). However, recent data from the Horn of Africa, which utilized complementary molecular, immunological and sequencing assays, demonstrates that there have been multiple independent deletions in pfhrp3, which have been accompanied by strong selection for a single pfhrp2 deletion due to pressure from RDTs (Feleke et al., 2021). As such, the MalariaGEN network has highlighted the utility of widely deployed NGS as a critical tool to detect the true pfhrp2/3 gene deletion prevalence (MalariaGEN et al., 2021.
Gene diversity can potentially explain the variability in the sensitivity of PfHRP2-based RDTs (Baker et al., 2005; ; Wurtz et al., 2013; Li et al., 2015; Gendrot et al., 2019). Indeed, studies conducted in different geographical settings have shown that gene diversity in the pfhrp2 gene plays a role in the sensitivity of RDTs especially among infections with low parasitaemia (≥ 200 asexual parasites/µl) (Baker et al., 2010). Given that countries such as Eritrea (Berhane et al., 2018 and Ethiopia (Golassa et al., 2020 have reported abnormally high prevalence of these deletions, NGS methods will likely be necessary for further characterization and monitoring of the diversity in these genes.
Timely and accurate diagnosis is a key element of effective malaria management. Traditional microscopy and RDT-based diagnostic methods involve detection of malaria parasites or products e.g. antigens in the blood of infected patients (Kasetsirikul et al., 2016). In other laboratories, malaria diagnosis involves molecular methods such as PCR (Table 1). Both microscopy and RDTs have been deployed as diagnostic tools in many malaria endemic areas, despite low sensitivity due to their high detection limits (≥ 50 parasites/μL) and limited ability to detect non-falciparum species. These deficiencies cause under-reporting of infections, specifically in areas with low transmission and for individuals with asymptomatic malaria or low density infections (Slater et al., 2019). By contrast, PCR has high sensitivity and specificity which make it capable of detecting low parasite density of less than 5 parasites/μl. This increased sensitivity will improve our ability to diagnose very low density infections, and detect asymptomatic individuals who can unknowingly contribute to disease transmission. Furthermore, the NGS-enabled ability to scan hundreds of Plasmodium genomes to better understand parasite biology and for novel diagnostic markers facilitates the development of tools that are species-specific, highly sensitive and agreeable to PCR or other nucleic acid amplification techniques (Lucchi et al., 2013.
In addition to the shortcomings outlined above, currently widespread robust malaria diagnostics require substantial financial investment and equipment, along with extensive personnel training. CRISPR-based diagnostics are an exciting development, as they have similar sensitivity and specificity to PCR assays, but require fewer resources, and are fast, cost-effective, and easy to use (Kostyusheva et al., 2021). The simplicity of the CRISPR-Cas based detection system allows the rapid development of diagnostic methods of a wide array of infectious diseases (Jiang et al., 2013; Gootenberg et al., 2017; Kostyusheva et al. 2021). Four CRISPR-based diagnostic methods have particular potential to replace PCR-based methods in low-resource settings: DETECTR, SHERLOCK, CARMEN, and CRISPR-Chip (Kaminski et al., 2021; Kostyusheva et al., 2021). These methods work reliably in isothermal conditions (negating the need for an expensive thermocycler) and results can be determined by lateral flow strips at the point of care, or by fluorescence read by a fluorimeter, or in some cases, with the naked eye. The use of lateral flow strips or visual inspection of fluorescence avoids the use of more technically challenging gel electrophoresis or quantification using a qPCR machine (Kaminski et al., 2021; Kostyusheva et al., 2021). CRISPR-based methods that have the potential to be developed for malaria diagnosis are described in Table 2.
The deployment of CRISPR-based diagnostic methods for P. falciparum could revolutionize the field of malaria surveillance in Africa. The ability of this method to detect as little as one copy of target DNA per reaction will play a major role in the detection of both symptomatic and asymptomatic infections and any associated resistance mutations (Kwiatkowski, 2015. CRISPR has already been used to detect malaria strain variants in mixed infections, an important challenge for malaria control and elimination efforts and a modality that could be extended to many areas of epidemiology (Kwiatkowski, 2015. One-pot systems that are suitable for field deployment due to their minimal technological and logistical prerequisites, high diagnostic reliability, and rapid turnaround time have been developed for SARS-CoV-2 and show additional promise for the expansion of these tools in malaria-endemic countries (Nguyen et al., 2021). The development of isothermal reactions performed with lyophilized reagents and with straightforward visualization at the point-of-care are particularly revolutionary as a means to quickly, cheaply, and accurately diagnose malaria infections in low-resource and/or remote locations (Cunningham et al., 2021). While these tools are still in early development, their accuracy, ease-of-use, cost-effectiveness, and rapidity give them great potential to complement currently available tools.
In Africa, few studies have been undertaken to determine the diversity and other features of parasite populations using NGS methods. Previous studies utilized PCR genotyping of polymorphic genes (msp1, msp2 and glurp) to determine parasite diversity with little focus on population structures and other population genetics metrics (Carlsson et al., 2011; Yavo et al., 2016; Metoh et al., 2020; Amoah et al., 2021). Recently, some regional studies have been conducted in Africa using genomic tools and have provided initial data on parasite population structure (Manske et al., 2012; Amambua-Ngwa et al., 2019; Abera et al., 2021; Moser et al., 2021). However, other areas, particularly those with low transmission, remain largely unexplored (Morgan et al., 2020). In addition, the metrics that are routinely used in these studies [e.g. FWS, FST (the fixation index), COI/MOI, IBD, inbreeding coefficient (F), and coefficient of uniqueness (COU)] have not been fully evaluated to be reliably informative for monitoring intervention-modulated changes in malaria transmission (Abera et al., 2021; Onyango et al., 2021). Therefore, further intensive study and metric development is required. The integration of traditional epidemiological data with NGS data will likely improve malaria surveillance in service of elimination strategies. While CRISPR-based diagnostics could revolutionize the field of malaria surveillance in malaria endemic countries in Africa, they have not yet been deployed or tailored to the specific needs of NMCPs.
Meanwhile, there are ongoing collaborative projects between African researchers, NMCPs, and partners in the USA and Europe to establish local molecular, genetic, and genomic laboratory and analytic capacity to support MMS. These projects are located in several countries in SSA including Senegal, Mozambique, Tanzania, Mali, The Gambia, Ghana, Gabon, Madagascar, Cameroon, Ivory Coast, Burkina Faso, Ethiopia and Uganda (Amambua-Ngwa et al., 2019; MalariaGEN et al., 2021). In Tanzania, previous efforts were made with the support of different funding agencies and partners and set-up the stage for the current initiatives (Amambua-Ngwa et al., 2019; MalariaGEN et al., 2021). Our current collaboration (Molecular Surveillance of Malaria in Tanzania, MSMT) is funded by the Bill and Melinda Gates Foundation and focuses on six key aims to attain the bold and ambitious goal of establishing local capacity for in-country generation and analysis of genomics data to support policy and decision making by the NMCP and its stakeholders. To that end, Tanzanian stakeholders identified three scientific aims for MSMT: 1) unraveling parasite population genetics, 2) country-wide drug resistance monitoring, and 3) country-wide surveys of pfhrp2/3 gene deletion. In addition, the project will address three supportive aims with a focus on training for capacity building, career development, and science leadership for sustainability of MMS. The project will also focus on developing a reliable model for effective engagement with NMCP and other stakeholders, and developing use cases for MMS in Tanzania. Lastly, the project will develop and implement a framework and platforms for storage, management, analysis, and sharing of the genomic data and associated metadata.To successfully adopt and transfer NGS and bioinformatics tools to Africa and build local capacity, a robust strategy of capacity building is critical. The MSMT team has developed a “counterpart model” of capacity building. This model involves pairing Tanzanian researchers with US-based expert (counterparts) collaborators with the role ensuring successful technology transfer.
To ensure key local stakeholders are effectively engaged, the MMS projects should bring on board all local stakeholders and ensure they are fully engaged in the project. In Tanzania, the MSMT project established an advisory committee which is under the Chief Medical Officer of the Tanzanian Ministry of Health with the role of providing oversight and guiding the implementation and sustainability of the project. The project is run by the joint implementation committee, which includes the study team, NIMR, NMCP and the Ministry in charge of the local governments (which owns and runs all health facilities in Tanzania). The technical implementation committee involves NIMR researchers, NMCP and collaborating partners who are advised by a committee of experts with experience and track records in MMS and capacity building particularly in Africa.
Thus far, a genomics laboratory has been established at NIMR in Dar es Salaam and will soon be equipped with state-of-the-art equipment to ensure genomics data are generated in-country and analysed by local experts from the second year of the project in 2022. The Tanzanian researchers have sequenced over 8,500 field collected samples in laboratories in the US as part of their training, and future analysis will take place in the NIMR laboratory with further training and coaching from the US-based collaborators. It is anticipated that our training and capacity building model and the laboratory capacity to be built in Tanzania will pave a way to a sustainable MMS for supporting not only malaria but other pathogens as part of the broader pathogen genomics initiative.
African researchers seeking to answer biological questions using NGS face many challenges, ranging from funding to procurement to lack of trained personnel (Table 3). The long-standing imbalance between collaborators in the global North and South has resulted in unsustainable patterns of research practice that must be broken if African researchers are to be free to initiate and execute NGS-dependent research studies. For the wide application of NGS technology in malaria surveillance, African countries need substantial investment to establish genomic capacity (Haanshuus et al., 2019 including human capacity, ancillary equipment for library preparation, quality control and sequencing (Escalante et al., 2015.
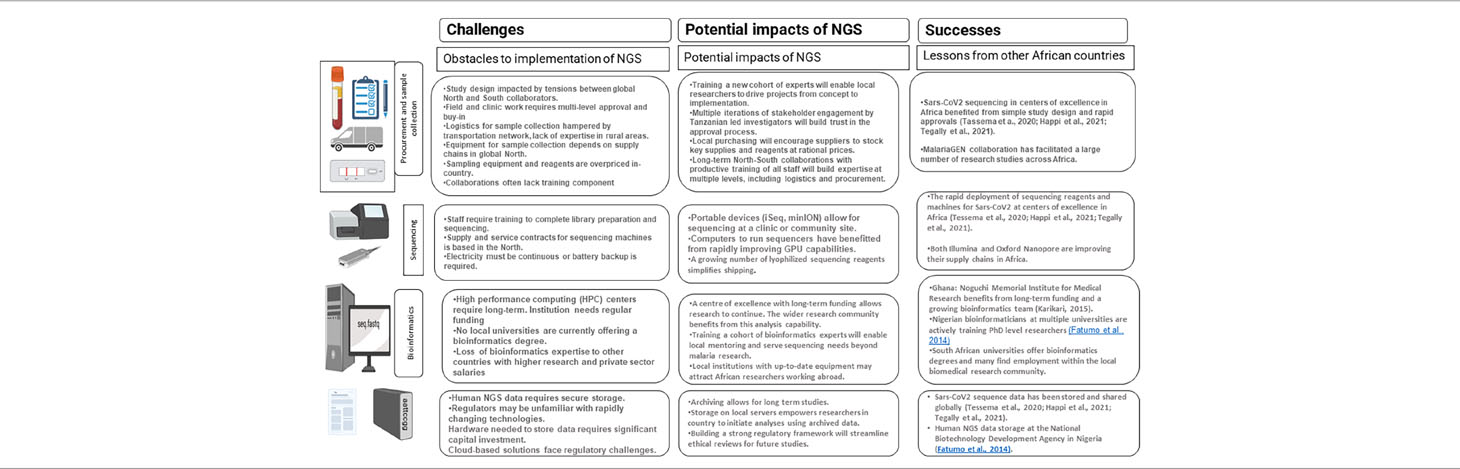
Table 3 Challenges and opportunities for NGS application in a resource constrained country such as Tanzania.
Historical imbalances are visible beginning with the funding and design of research programs. Too often, minimal intellectual input is sought from African researchers during this phase, and donors must critique their funding calls to address this imbalance. For large-scale studies where samples are collected from many districts within a country, buy-in from a large number of authorities is needed. The need to meet funding deadlines for an overseas collaborator may create tension during this phase. African procurement services and scientific suppliers to furnish basic sample collection necessities are often limited or unavailable, creating further imbalance between collaborators. To overcome this challenge, long-term partnerships that emphasize training are needed. Funding for NGS projects must be balanced, and local supply chains must be developed to support sample collection and sequencing without reliance on procurement abroad. One of the best recent examples of the rapid implementation of NGS for infectious disease research was seen in the centres of excellence for SARS-CoV-2 sequencing (Tessema et al., 2020; Happi et al., 2021; Tegally et al., 2021). To monitor the emergence of novel viral strains, several African laboratories rapidly pivoted to sequencing, taking advantage of publicly shared protocols, using kits and consumables tailored to this problem, and laboratories in Botswana and South Africa were able to quickly detect and raise awareness of the omicron variant of concern (Viana et al., 2022).
The sequencing phase of an NGS study faces many related challenges. Until sequencing becomes routine, skilled staff are difficult to find and retain. Sequencing machines, consumables and quality control reagents are challenging to procure, and are priced above the budgets of many African laboratories. Both Illumina and Oxford Nanopore Technologies have taken steps to address procurement challenges by supplying flow cells and consumables directly to several African agencies, but gaps in procurement continue to be filled by collaborating Northern institutions. Equipment and flow cells were rapidly distributed to several laboratories early in the spread of SARS-CoV-2. Robust supply chains and investment from the major suppliers of sequencing technology are needed to expand similar supply chains.
NGS studies produce large amounts of data that is analyzed with an ever-evolving suite of bioinformatics tools. Adequate data storage is a significant cost and requires in-house expertise. Few African institutions are training bioinformaticians, and those that are trained may leave for better paying jobs in the North-centered biotechnology industry. Building high performance computing clusters will require sustained investment, as will building up a cohort of bioinformaticians to work on African infectious disease sequencing projects. There is also a need to develop local expertise for equipment installation and maintenance to prevent long delays and additional costs accrued from overseas outsourcing (Apinjoh et al., 2019). Examples of institutions that have successfully built bioinformatics systems include the Noguchi institute in Ghana (Karikari, 2015, Redeemer’s University in Nigeria (Fatumo et al., 2014, and several South African universities.
Human and pathogen sequence data is sensitive, requiring adequate long-term storage, and a regulatory framework to protect patients’ information. Relatively low-cost storage exists in cloud storage systems, but privacy concerns must be addressed for this to be allowed by regulators. Hardware for long-term storage must be budgeted for in research proposals, and efficient storage formats are needed.
Skilled Workforce
Despite their potential, the initial investments in infrastructure for NGS are high, the methods require highly skilled personnel to perform sample processing, bioinformaticians, molecular epidemiologists for data analysis and interpretation, field epidemiologists, disease specialists for interpretation of the generated data, and public health specialists for adoption of the findings into policy (Escalante et al., 2015; Ishengoma et al., 2019). However, there are limited initiatives to train, retain, mentor and maintain highly skilled experts in Africa. The ongoing capacity building initiatives such as those under the African Academy of Sciences (https://www.aasciences.africa/), The human heredity and Health (H3Africa Initiative, https://h3africa.org/) and others need to be strongly supported by African countries for sustainability.
The application of advanced NGS tools and CRISPR-Cas technologies to malaria diagnosis and surveillance has great potential for malaria elimination in Africa. These tools will improve our understanding of malaria transmission dynamics, including our ability to track the spread of parasites and their associated clinically relevant mutations. With the wide scale distribution of genomics facilities and expertise, as well as state-of-the-art CRISPR-based diagnostics that substantially reduce equipment and training needs, our understanding of many aspects of malaria biology will dramatically expand. In addition to wider reaching and more accurate mobile and point-of-care diagnostics, African capacity for malaria control and ultimately elimination will result from this technological expansion. Furthermore, the deployment of these technologies and associated spread of expertise is not limited to malaria, but can also facilitate the better understanding and control of other infectious diseases as part of the ongoing initiative of building the capacity for pathogen genomics in Africa.
Nevertheless, significant challenges remain, which need to be addressed to facilitate smooth implementation of these tools necessary for malaria surveillance and elimination. Simulation studies which consider surveillance tools as an intervention strategy can also be performed to quantitatively evaluate deployment of these tools. Available simulation models such as openmalaria can be used to optimize these tools in terms of cost-effectiveness and public health. These challenges range from technological hurdles to a lack of funding investment and sufficiently skilled workforce in the areas where malaria interventions are most desperately needed. However, should these hurdles be overcome, the potential public health implications of deploying NGS and CRISPR-Cas tools in Tanzania and elsewhere are invaluable.
DI- Formulated the original idea, supervised and edited the manuscript; BL, ZPH, DG, CB, DP, MS, JJ, JB, and DI - wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported, in whole, by the Bill & Melinda Gates Foundation [grant number 02202]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. JJ also received funding from NIH K24AI134990.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer DN declared a shared affiliation with the author BM to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, N., Saba, T., Rehman, A., Mehmood, Z., Javaid, N., Tahir, M., et al. (2019). Plasmodium Species Aware Based Quantification of Malaria Parasitemia in Light Microscopy Thin Blood Smear. Microsc. Res. Tech. 82, 1198–1214. doi: 10.1002/jemt.23269
Abera, D., Kibet, C. K., Degefa, T., Amenga-Etego, L., Bargul, J. L., Golassa, L. (2021). Genomic Analysis Reveals Independent Evolution of Plasmodium Falciparum Populations in Ethiopia. Malar. J. 20, 129. doi: 10.1186/s12936-021-03660-y
Ackerman, C. M., Myhrvold, C., Thakku, S. G., Freije, C. A., Metsky, H. C., Yang, D. K., et al. (2020). Massively Multiplexed Nucleic Acid Detection With Cas13. Nature 582, 277–282. doi: 10.1038/s41586-020-2279-8
Ajibola, O., Diop, M. F., Ghansah, A., Amenga-Etego, L., Golassa, L., Apinjoh, T., et al. (2021). In Silico Characterisation of Putative Plasmodium Falciparum Vaccine Candidates in African Malaria Populations. Sci. Rep. 11, 16215. doi: 10.1038/s41598-021-95442-4
Amambua-Ngwa, A., Amenga-Etego, L., Kamau, E., Amato, R., Ghansah, A., Golassa, L., et al. (2019). Major Subpopulations of Plasmodium Falciparum in Sub-Saharan Africa. Science 365, 813–816. doi: 10.1126/science.aav5427
Amoah, L. E., Abukari, Z., Dawson-Amoah, M. E., Dieng, C. C., Lo, E., Afrane, Y. A. (2021). Population Structure and Diversity of Plasmodium Falciparum in Children With Asymptomatic Malaria Living in Different Ecological Zones of Ghana. BMC Infect. Dis. 21, 439. doi: 10.1186/s12879-021-06120-9
Andolina, C., Rek, J. C., Briggs, J., Okoth, J., Musiime, A., Ramjith, J., et al. (2021). Sources of Persistent Malaria Transmission in a Setting With Effective Malaria Control in Eastern Uganda: A Longitudinal, Observational Cohort Study. Lancet Infect. Dis. 21, 1568–1578. doi: 10.1016/S1473-3099(21)00072-4
Apinjoh, T. O., Ouattara, A., Titanji, V. P. K., Djimde, A., Amambua-Ngwa, A. (2019). Genetic Diversity and Drug Resistance Surveillance of Plasmodium Falciparum for Malaria Elimination: Is There an Ideal Tool for Resource-Limited Sub-Saharan Africa? Malar. J. 18, 217. doi: 10.1186/s12936-019-2844-5
Arizti-Sanz, J., Freije, C. A., Stanton, A. C., Petros, B. A., Boehm, C. K., Siddiqui, S., et al. (2020). Streamlined Inactivation, Amplification, and Cas13-Based Detection of SARS-CoV-2. Nat. Commun. 11, 5921. doi: 10.1038/s41467-020-19097-x
Assefa, S. A., Preston, M. D., Campino, S., Ocholla, H., Sutherland, C. J., Clark, T. G. (2014). estMOI: Estimating Multiplicity of Infection Using Parasite Deep Sequencing Data. Bioinformatics 30, 1292–1294. doi: 10.1093/bioinformatics/btu005
Bakari, C., Jones, S., Subramaniam, G., Mandara, C. I., Chiduo, M. G., Rumisha, S., et al. (2020). Community-Based Surveys for Plasmodium Falciparum Pfhrp2 and Pfhrp3 Gene Deletions in Selected Regions of Mainland Tanzania. Malar. J. 19, 391. doi: 10.1186/s12936-020-03459-3
Baker, J., Ho, M.-F., Pelecanos, A., Gatton, M., Chen, N., Abdullah, S., et al. (2010). Global Sequence Variation in the Histidine-Rich Proteins 2 and 3 of Plasmodium Falciparum: Implications for the Performance of Malaria Rapid Diagnostic Tests. Malar. J. 9, 129. doi: 10.1186/1475-2875-9-129
Baker, J., McCarthy, J., Gatton, M., Kyle, D. E., Belizario, V., Luchavez, J., et al. (2005). Genetic Diversity of Plasmodium Falciparum Histidine-Rich Protein 2 (PfHRP2) and its Effect on the Performance of PfHRP2-Based Rapid Diagnostic Tests. J. Infect. Dis. 192, 870–877. doi: 10.1086/432010
Berhane, A., Anderson, K., Mihreteab, S., Gresty, K., Rogier, E., Mohamed, S., et al. (2018). Major Threat to Malaria Control Programs by Plasmodium Falciparum Lacking Histidine-Rich Protein 2, Eritrea. Emerging Infect. Dis. 24, 462–470. doi: 10.3201/eid2403.171723
Boonma, P., Christensen, P. R., Suwanarusk, R., Price, R. N., Russell, B., Lek-Uthai, U. (2007). Comparison of Three Molecular Methods for the Detection and Speciation of Plasmodium Vivax and Plasmodium Falciparum. Malar. J. 6, 124. doi: 10.1186/1475-2875-6-124
Carlsson, A. M., Ngasala, B. E., Dahlström, S., Membi, C., Veiga, I. M., Rombo, L., et al. (2011). Plasmodium Falciparum Population Dynamics During the Early Phase of Anti-Malarial Drug Treatment in Tanzanian Children With Acute Uncomplicated Malaria. Malar. J. 10, 380. doi: 10.1186/1475-2875-10-380
Cerqueira, G. C., Cheeseman, I. H., Schaffner, S. F., Nair, S., McDew-White, M., Phyo, A. P., et al. (2017). Longitudinal Genomic Surveillance of Plasmodium Falciparum Malaria Parasites Reveals Complex Genomic Architecture of Emerging Artemisinin Resistance. Genome Biol. 18, 78. doi: 10.1186/s13059-017-1204-4
Chaumeau, V., Kajeechiwa, L., Fustec, B., Landier, J., Naw Nyo, S., Nay Hsel, S., et al. (2019). Contribution of Asymptomatic Plasmodium Infections to the Transmission of Malaria in Kayin State, Myanmar. J. Infect. Dis. 219, 1499–1509. doi: 10.1093/infdis/jiy686
Cheng, Q., Gatton, M. L., Barnwell, J., Chiodini, P., McCarthy, J., Bell, D., et al. (2014). Plasmodium Falciparum Parasites Lacking Histidine-Rich Protein 2 and 3: A Review and Recommendations for Accurate Reporting. Malar. J. 13, 283. doi: 10.1186/1475-2875-13-283
Cordray, M. S., Richards-Kortum, R. R. (2012). Emerging Nucleic Acid-Based Tests for Point-of-Care Detection of Malaria. Am. J. Trop. Med. Hyg. 87, 223–230. doi: 10.4269/ajtmh.2012.11-0685
Cunningham, C. H., Hennelly, C. M., Lin, J. T., Ubalee, R., Boyce, R. M., Mulogo, E. M., et al. (2021). A Novel CRISPR-Based Malaria Diagnostic Capable of Plasmodium Detection, Species Differentiation, and Drug-Resistance Genotyping. EBioMedicine 68, 103415. doi: 10.1016/j.ebiom.2021.103415
Daniels, R. F., Schaffner, S. F., Wenger, E. A., Proctor, J. L., Chang, H.-H., Wong, W., et al. (2015). Modeling Malaria Genomics Reveals Transmission Decline and Rebound in Senegal. Proc. Natl. Acad. Sci. U.S.A. 112, 7067–7072. doi: 10.1073/pnas.1505691112
Deurenberg, R. H., Bathoorn, E., Chlebowicz, M. A., Couto, N., Ferdous, M., García-Cobos, S., et al. (2017). Application of Next Generation Sequencing in Clinical Microbiology and Infection Prevention. J. Biotechnol. 243, 16–24. doi: 10.1016/j.jbiotec.2016.12.022
Dondorp, A. M., Nosten, F., Yi, P., Das, D., Phyo, A. P., Tarning, J., et al. (2009). Artemisinin Resistance in Plasmodium Falciparum Malaria. N. Engl. J. Med. 361, 455–467. doi: 10.1056/NEJMoa0808859
Dwivedi, A., Khim, N., Reynes, C., Ravel, P., Ma, L., Tichit, M., et al. (2016). Plasmodium Falciparum Parasite Population Structure and Gene Flow Associated to Anti-Malarial Drugs Resistance in Cambodia. Malar. J. 15, 319. doi: 10.1186/s12936-016-1370-y
Escalante, A. A., Ferreira, M. U., Vinetz, J. M., Volkman, S. K., Cui, L., Gamboa, D., et al. (2015). Malaria Molecular Epidemiology: Lessons From the International Centers of Excellence for Malaria Research Network. Am. J. Trop. Med. Hyg. 93, 79–86. doi: 10.4269/ajtmh.15-0005
Fatumo, S. A., Adoga, M. P., Ojo, O. O., Oluwagbemi, O., Adeoye, T., Ewejobi, I., et al. (2014). Computational Biology and Bioinformatics in Nigeria. PloS Comput. Biol. 10, e1003516. doi: 10.1371/journal.pcbi.1003516
Feleke, S. M., Reichert, E. N., Mohammed, H., Brhane, B. G., Mekete, K., Mamo, H., et al. (2021). Plasmodium Falciparum is Evolving to Escape Malaria Rapid Diagnostic Tests in Ethiopia. Nat. Microbiol. 6, 1289–1299. doi: 10.1038/s41564-021-00962-4
Gamboa, D., Ho, M.-F., Bendezu, J., Torres, K., Chiodini, P. L., Barnwell, J. W., et al. (2010). A Large Proportion of P. Falciparum Isolates in the Amazon Region of Peru Lack Pfhrp2 and Pfhrp3: Implications for Malaria Rapid Diagnostic Tests. PloS One 5, e8091. doi: 10.1371/journal.pone.0008091
Gendrot, M., Fawaz, R., Dormoi, J., Madamet, M., Pradines, B. (2019). Genetic Diversity and Deletion of Plasmodium Falciparum Histidine-Rich Protein 2 and 3: A Threat to Diagnosis of P. Falciparum Malaria. Clin. Microbiol. Infect. 25, 580–585. doi: 10.1016/j.cmi.2018.09.009
Goh, B., Ching, K., Soares Magalhães, R. J., Ciocchetta, S., Edstein, M. D., Maciel-de-Freitas, R., et al. (2021). The Application of Spectroscopy Techniques for Diagnosis of Malaria Parasites and Arboviruses and Surveillance of Mosquito Vectors: A Systematic Review and Critical Appraisal of Evidence. PloS Negl. Trop. Dis. 15, e0009218. doi: 10.1371/journal.pntd.0009218
Golassa, L., Messele, A., Amambua-Ngwa, A., Swedberg, G. (2020). High Prevalence and Extended Deletions in Plasmodium Falciparum Hrp2/3 Genomic Loci in Ethiopia. PloS One 15, e0241807. doi: 10.1371/journal.pone.0241807
Gootenberg, J. S., Abudayyeh, O. O., Kellner, M. J., Joung, J., Collins, J. J., Zhang, F. (2018). Multiplexed and Portable Nucleic Acid Detection Platform With Cas13, Cas12a, and Csm6. Science 360, 439–444. doi: 10.1126/science.aaq0179
Gootenberg, J. S., Abudayyeh, O. O., Lee, J. W., Essletzbichler, P., Dy, A. J., Joung, J., et al. (2017). Nucleic Acid Detection With CRISPR-Cas13a/C2c2. Science 356, 438–442. doi: 10.1126/science.aam9321
Greenhouse, B., Myrick, A., Dokomajilar, C., Woo, J. M., Carlson, E. J., Rosenthal, P. J., et al. (2006). Validation of Microsatellite Markers for Use in Genotyping Polyclonal Plasmodium Falciparum Infections. Am. J. Trop. Med. Hyg. 75, 836–842. doi: 10.4269/ajtmh.2006.75.836
Guo, L., Sun, X., Wang, X., Liang, C., Jiang, H., Gao, Q., et al. (2020). SARS-CoV-2 Detection With CRISPR Diagnostics. Cell Discovery 6, 34. doi: 10.1038/s41421-020-0174-y
Haanshuus, C. G., Mørch, K., Blomberg, B., Strøm, G. E. A., Langeland, N., Hanevik, K., et al. (2019). Assessment of Malaria Real-Time PCR Methods and Application With Focus on Low-Level Parasitaemia. PloS One 14, e0218982. doi: 10.1371/journal.pone.0218982
Haldar, K., Bhattacharjee, S., Safeukui, I. (2018). Drug Resistance in Plasmodium. Nat. Rev. Microbiol. 16, 156–170. doi: 10.1038/nrmicro.2017.161
Han, E.-T. (2013). Loop-Mediated Isothermal Amplification Test for the Molecular Diagnosis of Malaria. Expert Rev. Mol. Diagn. 13, 205–218. doi: 10.1586/erm.12.144
Happi, A. N., Ugwu, C. A., Happi, C. T. (2021). Tracking the Emergence of New SARS-CoV-2 Variants in South Africa. Nat. Med. 27, 372–373. doi: 10.1038/s41591-021-01265-1
Hopkins, H., González, I. J., Polley, S. D., Angutoko, P., Ategeka, J., Asiimwe, C., et al. (2013). Highly Sensitive Detection of Malaria Parasitemia in a Malaria-Endemic Setting: Performance of a New Loop-Mediated Isothermal Amplification Kit in a Remote Clinic in Uganda. J. Infect. Dis. 208, 645–652. doi: 10.1093/infdis/jit184
Imwong, M., Hanchana, S., Malleret, B., Rénia, L., Day, N. P. J., Dondorp, A., et al. (2014). High-Throughput Ultrasensitive Molecular Techniques for Quantifying Low-Density Malaria Parasitemias. J. Clin. Microbiol. 52, 3303–3309. doi: 10.1128/JCM.01057-14
Ippolito, M. M., Moser, K. A., Kabuya, J.-B. B., Cunningham, C., Juliano, J. J. (2021). Antimalarial Drug Resistance and Implications for the WHO Global Technical Strategy. Curr. Epidemiol. Rep. 8, 46–62. doi: 10.1007/s40471-021-00266-5
Ishengoma, D. S., Saidi, Q., Sibley, C. H., Roper, C., Alifrangis, M. (2019). Deployment and Utilization of Next-Generation Sequencing of Plasmodium Falciparum to Guide Anti-Malarial Drug Policy Decisions in Sub-Saharan Africa: Opportunities and Challenges. Malar. J. 18, 267. doi: 10.1186/s12936-019-2853-4
Jiang, W., Bikard, D., Cox, D., Zhang, F., Marraffini, L. A. (2013). RNA-Guided Editing of Bacterial Genomes Using CRISPR-Cas Systems. Nat. Biotechnol. 31, 233–239. doi: 10.1038/nbt.2508
Joung, J., Ladha, A., Saito, M., Kim, N.-G., Woolley, A. E., Segel, M., et al. (2020). Detection of SARS-CoV-2 With SHERLOCK One-Pot Testing. N. Engl. J. Med. 383, 1492–1494. doi: 10.1056/NEJMc2026172
Juliano, J. J., Ariey, F., Sem, R., Tangpukdee, N., Krudsood, S., Olson, C., et al. (2009). Misclassification of Drug Failure in Plasmodium Falciparum Clinical Trials in Southeast Asia. J. Infect. Dis. 200, 624–628. doi: 10.1086/600892
Juliano, J. J., Porter, K., Mwapasa, V., Sem, R., Rogers, W. O., Ariey, F., et al. (2010). Exposing Malaria in-Host Diversity and Estimating Population Diversity by Capture-Recapture Using Massively Parallel Pyrosequencing. Proc. Natl. Acad. Sci. U.S.A. 107, 20138–20143. doi: 10.1073/pnas.1007068107
Kamau, E., Alemayehu, S., Feghali, K. C., Saunders, D., Ockenhouse, C. F. (2013). Multiplex qPCR for Detection and Absolute Quantification of Malaria. PloS One 8, e71539. doi: 10.1371/journal.pone.0071539
Kaminski, M. M., Abudayyeh, O. O., Gootenberg, J. S., Zhang, F., Collins, J. J. (2021). CRISPR-Based Diagnostics. Nat. Biomed. Eng. 5, 643–656. doi: 10.1038/s41551-021-00760-7
Karikari, T. K. (2015). Bioinformatics in Africa: The Rise of Ghana? PloS Comput. Biol. 11, e1004308. doi: 10.1371/journal.pcbi.1004308
Kasetsirikul, S., Buranapong, J., Srituravanich, W., Kaewthamasorn, M., Pimpin, A. (2016). The Development of Malaria Diagnostic Techniques: A Review of the Approaches With Focus on Dielectrophoretic and Magnetophoretic Methods. Malar. J. 15, 358. doi: 10.1186/s12936-016-1400-9
Kellner, M. J., Koob, J. G., Gootenberg, J. S., Abudayyeh, O. O., Zhang, F. (2019). SHERLOCK: Nucleic Acid Detection With CRISPR Nucleases. Nat. Protoc. 14, 2986–3012. doi: 10.1038/s41596-019-0210-2
Kisinza, W. N., Nkya, T. E., Kabula, B., Overgaard, H. J., Massue, D. J., Mageni, Z., et al. (2017). Multiple Insecticide Resistance in Anopheles Gambiae From Tanzania: A Major Concern for Malaria Vector Control. Malar. J. 16, 439. doi: 10.1186/s12936-017-2087-2
Kostyusheva, A., Brezgin, S., Babin, Y., Vasilyeva, I., Glebe, D., Kostyushev, D., et al (2021). CRISPR-Cas Systems for Diagnosing Infectious Diseases. Methods. 203, 431–446. doi: 10.1016/j.ymeth.2021.04.007
Kwiatkowski, D. (2015). Malaria Genomics: Tracking a Diverse and Evolving Parasite Population. Int. Health 7, 82–84. doi: 10.1093/inthealth/ihv007
Lalremruata, A., Jeyaraj, S., Engleitner, T., Joanny, F., Lang, A., Bélard, S., et al. (2017). Species and Genotype Diversity of Plasmodium in Malaria Patients From Gabon Analysed by Next Generation Sequencing. Malar. J. 16, 398. doi: 10.1186/s12936-017-2044-0
Leang, R., Taylor, W. R. J., Bouth, D. M., Song, L., Tarning, J., Char, M. C., et al. (2015). Evidence of Plasmodium Falciparum Malaria Multidrug Resistance to Artemisinin and Piperaquine in Western Cambodia: Dihydroartemisinin-Piperaquine Open-Label Multicenter Clinical Assessment. Antimicrob. Agents Chemother. 59, 4719–4726. doi: 10.1128/AAC.00835-15
Lee, R. A., Puig, H. D., Nguyen, P. Q., Angenent-Mari, N. M., Donghia, N. M., McGee, J. P., et al. (2020). Ultrasensitive CRISPR-Based Diagnostic for Field-Applicable Detection of Plasmodium Species in Symptomatic and Asymptomatic Malaria. Proc. Natl. Acad. Sci. U.S.A. 117, 25722–25731. doi: 10.1073/pnas.2010196117
Lerch, A., Koepfli, C., Hofmann, N. E., Messerli, C., Wilcox, S., Kattenberg, J. H., et al. (2017). Development of Amplicon Deep Sequencing Markers and Data Analysis Pipeline for Genotyping Multi-Clonal Malaria Infections. BMC Genomics 18, 864. doi: 10.1186/s12864-017-4260-y
Levitt, B., Obala, A., Langdon, S., Corcoran, D., O’Meara, W. P., Taylor, S. M. (2017). Overlap Extension Barcoding for the Next Generation Sequencing and Genotyping of Plasmodium Falciparum in Individual Patients in Western Kenya. Sci. Rep. 7, 41108. doi: 10.1038/srep41108
Li, P., Xing, H., Zhao, Z., Yang, Z., Cao, Y., Li, W., et al. (2015). Genetic Diversity of Plasmodium Falciparum Histidine-Rich Protein 2 in the China-Myanmar Border Area. Acta Trop. 152, 26–31. doi: 10.1016/j.actatropica.2015.08.003
Lucchi, N. W., Oberstaller, J., Kissinger, J. C., Udhayakumar, V. (2013). Malaria Diagnostics and Surveillance in the Post-Genomic Era. Public Health Genomics 16, 37–43. doi: 10.1159/000345607
Luchavez, J., Baker, J., Alcantara, S., Belizario, V., Cheng, Q., McCarthy, J. S., et al. (2011). Laboratory Demonstration of a Prozone-Like Effect in HRP2-Detecting Malaria Rapid Diagnostic Tests: Implications for Clinical Management. Malar. J. 10, 286. doi: 10.1186/1475-2875-10-286
MalariaGEN, Ahouidi, A., Ali, M., Almagro-Garcia, J., Amambua-Ngwa, A., Amaratunga, C., et al. (2021). An Open Dataset of Plasmodium Falciparum Genome Variation in 7,000 Worldwide Samples. Wellcome Open Res. 6, 42. doi: 10.12688/wellcomeopenres.16168.2
Malvy, D., Torrentino-Madamet, M., L’Ollivier, C., Receveur, M.-C., Jeddi, F., Delhaes, L., et al. (2018). Plasmodium Falciparum Recrudescence Two Years After Treatment of an Uncomplicated Infection Without Return to an Area Where Malaria Is Endemic. Antimicrob. Agents Chemother. 62, e01892-17. doi: 10.1128/AAC.01892-17
Manske, M., Miotto, O., Campino, S., Auburn, S., Almagro-Garcia, J., Maslen, G., et al. (2012). Analysis of Plasmodium Falciparum Diversity in Natural Infections by Deep Sequencing. Nature 487, 375–379. doi: 10.1038/nature11174
Matiya, D. J., Philbert, A. B., Kidima, W., Matowo, J. J. (2019). Dynamics and Monitoring of Insecticide Resistance in Malaria Vectors Across Mainland Tanzania From 1997 to 2017: A Systematic Review. Malar. J. 18, 102. doi: 10.1186/s12936-019-2738-6
Mayengue, P. I., Luty, A. J. F., Rogier, C., Baragatti, M., Kremsner, P. G., Ntoumi, F. (2009). The Multiplicity of Plasmodium Falciparum Infections is Associated With Acquired Immunity to Asexual Blood Stage Antigens. Microbes Infect. 11, 108–114. doi: 10.1016/j.micinf.2008.10.012
Mbanefo, A., Kumar, N. (2020). Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs. Trop. Med. Infect. Dis. 5 (2), 102. doi: 10.3390/tropicalmed5020102
Metoh, T. N., Chen, J.-H., Fon-Gah, P., Zhou, X., Moyou-Somo, R., Zhou, X.-N. (2020). Genetic Diversity of Plasmodium Falciparum and Genetic Profile in Children Affected by Uncomplicated Malaria in Cameroon. Malar. J. 19, 115. doi: 10.1186/s12936-020-03161-4
Miles, A., Iqbal, Z., Vauterin, P., Pearson, R., Campino, S., Theron, M., et al. (2016). Indels, Structural Variation, and Recombination Drive Genomic Diversity in Plasmodium Falciparum. Genome Res. 26, 1288–1299. doi: 10.1101/gr.203711.115
Mitchell, C. L., Janko, M. M., Mwandagalirwa, M. K., Tshefu, A. K., Edwards, J. K., Pence, B. W., et al. (2022). Impact of Extractive Industries on Malaria Prevalence in the Democratic Republic of the Congo: A Population-Based Cross-Sectional Study. Sci. Rep. 12, 1737. doi: 10.1038/s41598-022-05777-9
Mohd Abd Razak, M. R., Sastu, U. R., Norahmad, N. A., Abdul-Karim, A., Muhammad, A., Muniandy, P. K., et al. (2016). Genetic Diversity of Plasmodium Falciparum Populations in Malaria Declining Areas of Sabah, East Malaysia. PloS One 11, e0152415. doi: 10.1371/journal.pone.0152415
Morgan, A. P., Brazeau, N. F., Ngasala, B., Mhamilawa, L. E., Denton, M., Msellem, M., et al. (2020). Falciparum Malaria From Coastal Tanzania and Zanzibar Remains Highly Connected Despite Effective Control Efforts on the Archipelago. Malar. J. 19, 47. doi: 10.1186/s12936-020-3137-8
Moser, K. A., Madebe, R. A., Aydemir, O., Chiduo, M. G., Mandara, C. I., Rumisha, S. F., et al. (2021). Describing the Current Status of Plasmodium Falciparum Population Structure and Drug Resistance Within Mainland Tanzania Using Molecular Inversion Probes. Mol. Ecol. 30, 100–113. doi: 10.1111/mec.15706
Murray, C. K., Gasser, R. A., Magill, A. J., Miller, R. S. (2008). Update on Rapid Diagnostic Testing for Malaria. Clin. Microbiol. Rev. 21, 97–110. doi: 10.1128/CMR.00035-07
Nag, S., Dalgaard, M. D., Kofoed, P.-E., Ursing, J., Crespo, M., Andersen, L. O., et al. (2017). High Throughput Resistance Profiling of Plasmodium Falciparum Infections Based on Custom Dual Indexing and Illumina Next Generation Sequencing-Technology. Sci. Rep. 7, 2398. doi: 10.1038/s41598-017-02724-x
Nair, S., Nkhoma, S. C., Serre, D., Zimmerman, P. A., Gorena, K., Daniel, B. J., et al. (2014). Single-Cell Genomics for Dissection of Complex Malaria Infections. Genome Res. 24, 1028–1038. doi: 10.1101/gr.168286.113
Neafsey, D. E., Taylor, A. R., MacInnis, B. L. (2021). Advances and Opportunities in Malaria Population Genomics. Nat. Rev. Genet. 22, 502–517. doi: 10.1038/s41576-021-00349-5
Neafsey, D. E., Volkman, S. K. (2017). Malaria Genomics in the Era of Eradication. Cold Spring Harb. Perspect. Med. 7, a025544. doi: 10.1101/cshperspect.a025544
Ngondi, J. M., Ishengoma, D. S., Doctor, S. M., Thwai, K. L., Keeler, C., Mkude, S., et al. (2017). Surveillance for Sulfadoxine-Pyrimethamine Resistant Malaria Parasites in the Lake and Southern Zones, Tanzania, Using Pooling and Next-Generation Sequencing. Malar. J. 16, 236. doi: 10.1186/s12936-017-1886-9
Nguyen, L. T., Macaluso, N. C., Pizzano, B. L. M., Cash, M. N., Spacek, J., Karasek, J., et al. (2021). A Thermostable Cas12b From Brevibacillus Leverages One-Pot Detection of SARS-CoV-2 Variants of Concern. eBioMedicine, 77,103926. doi: 10.1016/j.ebiom.2022.103926
Nkhoma, S. C., Banda, R. L., Khoswe, S., Dzoole-Mwale, T. J., Ward, S. A. (2018). Intra-Host Dynamics of Co-Infecting Parasite Genotypes in Asymptomatic Malaria Patients. Infect. Genet. Evol. 65, 414–424. doi: 10.1016/j.meegid.2018.08.018
Nsanzabana, C. (2019). Strengthening Surveillance Systems for Malaria Elimination by Integrating Molecular and Genomic Data. Trop. Med. Infect. Dis. 4 (4), 139. doi: 10.3390/tropicalmed4040139
Nsobya, S. L., Walakira, A., Namirembe, E., Kiggundu, M., Nankabirwa, J. I., Ruhamyankaka, E., et al. (2021). Deletions of Pfhrp2 and Pfhrp3 Genes Were Uncommon in Rapid Diagnostic Test-Negative Plasmodium Falciparum Isolates From Uganda. Malar. J. 20, 4. doi: 10.1186/s12936-020-03547-4
Onyango, S. A., Ochwedo, K. O., Machani, M. G., Omondi, C. J., Debrah, I., Ogolla, S. O., et al. (2021). Genetic Diversity and Population Structure of the Human Malaria Parasite Plasmodium Falciparum Surface Protein Pfs47 in Isolates From the Lowlands in Western Kenya. PloS One 16, e0260434. doi: 10.1371/journal.pone.0260434
Pardee, K., Green, A. A., Takahashi, M. K., Braff, D., Lambert, G., Lee, J. W., et al. (2016). Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 165, 1255–1266. doi: 10.1016/j.cell.2016.04.059
Parr, J. B., Kieto, E., Phanzu, F., Mansiangi, P., Mwandagalirwa, K., Mvuama, N., et al. (2021). Analysis of False-Negative Rapid Diagnostic Tests for Symptomatic Malaria in the Democratic Republic of the Congo. Sci. Rep. 11, 6495. doi: 10.1038/s41598-021-85913-z
Pham, N. M., Karlen, W., Beck, H.-P., Delamarche, E. (2018). Malaria and the “Last” Parasite: How can Technology Help? Malar. J. 17, 260. doi: 10.1186/s12936-018-2408-0
Rao, P. N., Uplekar, S., Kayal, S., Mallick, P. K., Bandyopadhyay, N., Kale, S., et al. (2016). A Method for Amplicon Deep Sequencing of Drug Resistance Genes in Plasmodium Falciparum Clinical Isolates From India. J. Clin. Microbiol. 54, 1500–1511. doi: 10.1128/JCM.00235-16
Rohlin, A., Wernersson, J., Engwall, Y., Wiklund, L., Björk, J., Nordling, M. (2009). Parallel Sequencing Used in Detection of Mosaic Mutations: Comparison With Four Diagnostic DNA Screening Techniques. Hum. Mutat. 30, 1012–1020. doi: 10.1002/humu.20980
Roper, C., Pearce, R., Nair, S., Sharp, B., Nosten, F., Anderson, T. (2004). Intercontinental Spread of Pyrimethamine-Resistant Malaria. Science 305 (5687), 1124. doi: 10.1126/science.1098876
Shaukat, A. M., Gilliams, E. A., Kenefic, L. J., Laurens, M. B., Dzinjalamala, F. K., Nyirenda, O. M., et al. (2012). Clinical Manifestations of New Versus Recrudescent Malaria Infections Following Anti-Malarial Drug Treatment. Malar. J. 11, 207. doi: 10.1186/1475-2875-11-207
She, R. C., Rawlins, M. L., Mohl, R., Perkins, S. L., Hill, H. R., Litwin, C. M. (2007). Comparison of Immunofluorescence Antibody Testing and Two Enzyme Immunoassays in the Serologic Diagnosis of Malaria. J. Travel Med. 14, 105–111. doi: 10.1111/j.1708-8305.2006.00087.x
Slater, H. C., Ross, A., Felger, I., Hofmann, N. E., Robinson, L., Cook, J., et al. (2019). The Temporal Dynamics and Infectiousness of Subpatent Plasmodium Falciparum Infections in Relation to Parasite Density. Nat. Commun. 10, 1433. doi: 10.1038/s41467-019-09441-1
Strich, J. R., Chertow, D. S. (2019). CRISPR-Cas Biology and Its Application to Infectious Diseases. J. Clin. Microbiol. 57, e01307-18. doi: 10.1128/JCM.01307-18
Su, X.-Z., Zhang, C., Joy, D. A. (2020). Host-Malaria Parasite Interactions and Impacts on Mutual Evolution. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.587933
Takala-Harrison, S., Laufer, M. K. (2015). Antimalarial Drug Resistance in Africa: Key Lessons for the Future. Ann. N. Y. Acad. Sci. 1342, 62–67. doi: 10.1111/nyas.12766
Talundzic, E., Ravishankar, S., Kelley, J., Patel, D., Plucinski, M., Schmedes, S., et al. (2018). Next-Generation Sequencing and Bioinformatics Protocol for Malaria Drug Resistance Marker Surveillance. Antimicrob. Agents Chemother. 62, e02474-17. doi: 10.1128/AAC.02474-17
Tangpukdee, N., Duangdee, C., Wilairatana, P., Krudsood, S. (2009). Malaria Diagnosis: A Brief Review. Korean J. Parasitol. 47, 93–102. doi: 10.3347/kjp.2009.47.2.93
Tawe, L., Menegon, M., Ramatlho, P., Muthoga, C. W., Mutukwa, N., Vurayai, M., et al. (2018). Molecular Surveillance of Plasmodium Falciparum Drug Resistance Markers in Clinical Samples From Botswana. Am. J. Trop. Med. Hyg. 99, 1499–1503. doi: 10.4269/ajtmh.18-0440
Tegally, H., Wilkinson, E., Lessells, R. J., Giandhari, J., Pillay, S., Msomi, N., et al. (2021). Sixteen Novel Lineages of SARS-CoV-2 in South Africa. Nat. Med. 27, 440–446. doi: 10.1038/s41591-021-01255-3
Tessema, S. K., Inzaule, S. C., Christoffels, A., Kebede, Y., de Oliveira, T., Ouma, A. E. O., et al. (2020). Accelerating Genomics-Based Surveillance for COVID-19 Response in Africa. Lancet Microbe 1, e227–e228. doi: 10.1016/S2666-5247(20)30117-8
Tessema, S. K., Raman, J., Duffy, C. W., Ishengoma, D. S., Amambua-Ngwa, A., Greenhouse, B. (2019b). Applying Next-Generation Sequencing to Track Falciparum Malaria in Sub-Saharan Africa. Malar. J. 18, 268. doi: 10.1186/s12936-019-2880-1
Tessema, S., Wesolowski, A., Chen, A., Murphy, M., Wilheim, J., Mupiri, A.-R., et al. (2019a). Using Parasite Genetic and Human Mobility Data to Infer Local and Cross-Border Malaria Connectivity in Southern Africa. eLife 8, e43510. doi: 10.7554/eLife.43510
Thomson, R., Beshir, K. B., Cunningham, J., Baiden, F., Bharmal, J., Bruxvoort, K. J., et al. (2019). Pfhrp2 and Pfhrp3 Gene Deletions That Affect Malaria Rapid Diagnostic Tests for Plasmodium Falciparum: Analysis of Archived Blood Samples From 3 African Countries. J. Infect. Dis. 220, 1444–1452. doi: 10.1093/infdis/jiz335
Tusting, L. S., Bousema, T., Smith, D. L., Drakeley, C. (2014). Measuring Changes in Plasmodium Falciparum Transmission: Precision, Accuracy and Costs of Metrics. Adv. Parasitol. 84, 151–208. doi: 10.1016/B978-0-12-800099-1.00003-X
Upatham, E. S., Prasittisuk, C., Ratanatham, S., Green, C. A., Rojanasunan, W., Setakana, P., et al. (1988). Bionomics of Anopheles Maculatus Complex and Their Role in Malaria Transmission in Thailand. Southeast Asian J. Trop. Med. Public Health 19, 259–269.
Van Tyne, D., Park, D. J., Schaffner, S. F., Neafsey, D. E., Angelino, E., Cortese, J. F., et al. (2011). Identification and Functional Validation of the Novel Antimalarial Resistance Locus PF10_0355 in Plasmodium Falciparum. PloS Genet. 7, e1001383. doi: 10.1371/journal.pgen.1001383
Viana, R., Moyo, S., Amoako, D. G., Tegally, H., Scheepers, C., Althaus, C. L., et al. (2022). Rapid Epidemic Expansion of the SARS-CoV-2 Omicron Variant in Southern Africa. Nature 603, 679–686. doi: 10.1038/s41586-022-04411-y
Watson, O. J., Okell, L. C., Hellewell, J., Slater, H. C., Unwin, H. J. T., Omedo, I., et al. (2021). Evaluating the Performance of Malaria Genetics for Inferring Changes in Transmission Intensity Using Transmission Modeling. Mol. Biol. Evol. 38, 274–289. doi: 10.1093/molbev/msaa225
Weiss, D. J., Lucas, T. C. D., Nguyen, M., Nandi, A. K., Bisanzio, D., Battle, K. E., et al. (2019). Mapping the Global Prevalence, Incidence, and Mortality of Plasmodium Falciparum 2000-17: A Spatial and Temporal Modelling Study. Lancet. 394 (10195), 322–331. doi: 10.1016/S0140-6736(19)31097-9
World Health Organization (2007) Methods and Techniques for Clinical Trials on Antimalarial Drug Efficacy: Genotyping to Identify Parasite Populations (World Health Organization). Available at: http://www.who.int/malaria/publications/atoz/9789241596305/en/ (Accessed October 8, 2020).
World Health Organization (2009). Methods for Surveillance of Antimalarial Drug Efficacy (Geneva: World Health Organization).
World Health Organization (2019). Response Plan to Pfhrp2 Gene Deletions (World Health Organization).
World Health Organization (2020). Master Protocol for Surveillance of Pfhrp2/3 Deletions and Biobanking to Support Future Research (World Health Organization).
Wurtz, N., Fall, B., Bui, K., Pascual, A., Fall, M., Camara, C., et al. (2013). Pfhrp2 and Pfhrp3 Polymorphisms in Plasmodium Falciparum Isolates From Dakar, Senegal: Impact on Rapid Malaria Diagnostic Tests. Malar. J. 12, 34. doi: 10.1186/1475-2875-12-34
Yavo, W., Konaté, A., Mawili-Mboumba, D. P., Kassi, F. K., Tshibola Mbuyi, M. L., Angora, E. K., et al. (2016). Genetic Polymorphism of Msp1 and Msp2 in Plasmodium Falciparum Isolates From Côte D’Ivoire Versus Gabon. J. Parasitol. Res. 2016, 3074803. doi: 10.1155/2016/3074803
Keywords: next generation sequencing, CRISPR-Cas systems, pathogen genomics, malaria diagnosis, malaria molecular surveillance, Africa, Tanzania
Citation: Lyimo BM, Popkin-Hall ZR, Giesbrecht DJ, Mandara CI, Madebe RA, Bakari C, Pereus D, Seth MD, Ngamba RM, Mbwambo RB, MacInnis B, Mbwambo D, Garimo I, Chacky F, Aaron S, Lusasi A, Molteni F, Njau R, Cunningham JA, Lazaro S, Mohamed A, Juliano JJ, Bailey J A and Ishengoma DS (2022) Potential Opportunities and Challenges of Deploying Next Generation Sequencing and CRISPR-Cas Systems to Support Diagnostics and Surveillance Towards Malaria Control and Elimination in Africa. Front. Cell. Infect. Microbiol. 12:757844. doi: 10.3389/fcimb.2022.757844
Received: 12 August 2021; Accepted: 17 March 2022;
Published: 13 July 2022.
Edited by:
Yanfei Chen, Zhejiang University, ChinaReviewed by:
Changchun Liu, University of Connecticut Health Center, United StatesCopyright © 2022 Lyimo, Popkin-Hall, Giesbrecht, Mandara, Madebe, Bakari, Pereus, Seth, Ngamba, Mbwambo, MacInnis, Mbwambo, Garimo, Chacky, Aaron, Lusasi, Molteni, Njau, Cunningham, Lazaro, Mohamed, Juliano, Bailey and Ishengoma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deus S. Ishengoma, ZGV1c2lzaGVAeWFob28uY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.