
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 17 March 2025
Sec. Water and Wastewater Management
Volume 13 - 2025 | https://doi.org/10.3389/fenvs.2025.1536359
This article is part of the Research TopicMitigating Microbial Contamination of Drinking Water SourcesView all 4 articles
Solar photocatalysis has the potential to reduce chemical and microbial contaminants in water and make it safer for consumption in an effective and sustainable manner. This has been studied and proven well under laboratory-scale conditions. In our previous work, the developed solar photocatalyst, Bi-
Surface water bodies are valuable resources of water supply but highly prone to chemical and microbial contamination (Walker et al., 2019; Singh et al., 2024; Syeed et al., 2023). Chemical contamination is primarily caused by untreated industrial wastewater being discharged into water bodies, introducing pollutants such as dyes, antibiotics, pesticides, and more. Microbial contamination, on the other hand, is mainly the result of both natural processes and anthropogenic activities. Once contaminated, the microbial level can rise rapidly by bacteria multiplication and chemical contamination can lead to several resistances such as antibiotic resistance, algal blooms, etc. (Stevenson et al., 2015). The most common microorganisms that can be present in surface waters are total coliforms, fecal coliforms, and E. coli (E. coli) due to either the organisms found in water or animals having access to that surface water body (Cabral, 2010). Effective and sustainable treatment of surface water can not only utilize that resource but also lead to healthy and safe water consumption throughout the world. For such treatments, chlorination has been proven an effective approach for decades but it has its own limitations; for example, it ends up creating harmful by-products in water which are toxic to consume (Al-Abri et al., 2019). To overcome that, Advanced Oxidation Processes (AOPs) have gained attention in treating water in a more sustainable manner as they can mineralize the contaminants and avoid transferring them into solid and/or air phases (Hübner et al., 2024; Dhamorikar et al., 2024). Among AOPs, photocatalytic oxidation, in particular, is acknowledged to be highly effective in degrading both chemical as well as microbial contaminants in water treatment (Kuspanov et al., 2024; Serik et al., 2024). In order to make this technique even more sustainable, efforts are being put on to drive this technology using solar light (Ren et al., 2021).
Previous field studies (Porley et al., 2020) and assessment of catalyst substrates (Porley and Robertson, 2020) strongly indicate the possibilities of using this technology in a sustainable manner in real-world conditions. In a recent paper, a solar photocatalyst, Bi-
To assess the efficiency of catalyst to degrade chemical organic contaminant and (E. coli) in laboratory scale tests, 4-Chlorophenol was chosen to study microbial degradation (Arora et al., 2025). On achieving efficient results, the study was scaled up, and field trials were conducted in rural India in March’23, where the photocatalyst was tested to degrade total coliform and E. coli in real-world water in the presence of sunlight (Arora et al., 2025). However, field tests have primarily focussed on microbial contaminant degradation to adress the specific need of the area.The results unveiled that the developed catalyst can reduce up to 99% of total coliform and 99.9% of E. coli in 2 h under direct sunlight. However, the treatment process was notably influenced by independent variables, including solar light intensity, bacterial contamination levels, and the reusability of the photocatalyst. After achieving such promising results in field trials, it becomes crucial to understand how environmental uncertainties can affect solar photocatalytic oxidation and how the technology can be improved to achieve even better performances. Advancements in this direction will allow the transfer of technology to a practical scale. For example, the statistics of the treatment time as a function of uncertainties affecting the process variables will allow to better quantify the probability of failure given the size of the reactor and the catalyst or, vice-versa, design the best reactor size and catalyst by imposing the level of risk, etc.
In the present work, two types of uncertainties affecting the reaction process are considered. The first one is the statistical variability of the reaction parameters, namely, the reaction constant and the initial concentration of bacteria. The so-called derived distribution approach is used to study how the statistical change in one or both parameters - considered belonging to a given statistical distribution - impacts the statistical distribution of the treatment time. The second one is the source of variability directly affecting the reaction dynamics via a continuously (randomly) changing reaction constant. The latter case corresponds to environmental noise effects influencing the reaction process such as variable cloudiness and radiation or imperfect and inhomogeneous mixing, which technically transform the reaction kinetics into a stochastic differential equation of the Langevin type (Cox and Miller, 1977; Gardiner et al., 1985; Van Kampen, 1992). Furthermore, the theory of stochastic processes is used to derive mathematically exact solutions for both the probability density functions of the concentration at all times and the treatment time.
The main steps of the low-cost methodology adopting Bi-
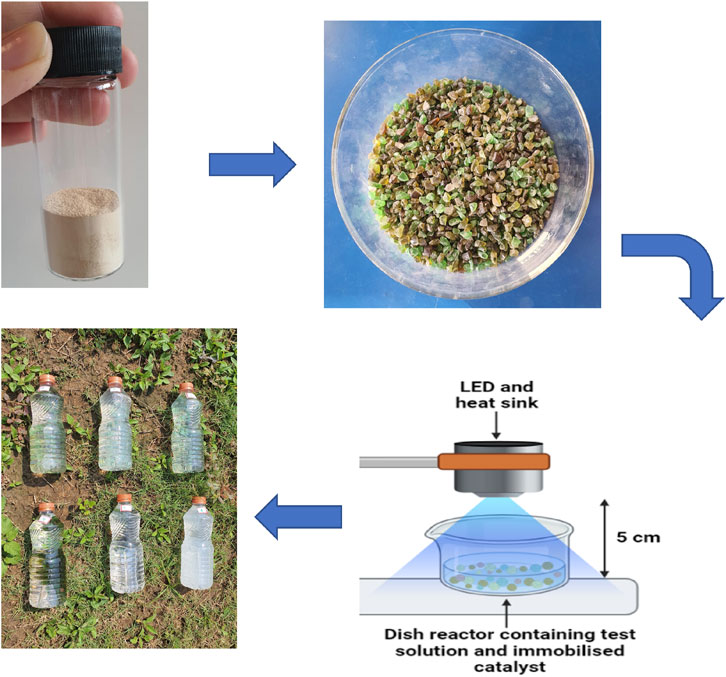
Figure 1. Illustration of the developed low-cost Bi-
For both laboratory experiments and field tests, it is well known that photocatalytic oxidation for microbial contaminants follows first-order kinetics (Lebedev et al., 2018; Tran et al., 2023; Masoom et al., 2024). Accordingly, the corresponding equation describing the temporal evolution of the concentration for the microbial contaminant,
where
with
According to Equations 1 and 2, the reaction and the treatment time depend on the concentration of one reactant only. However, in practical situations the reaction is affected by many factors such as light intensity, catalyst pore size, solubility, catalyst efficiency, band gap, light distance, type of contaminant, etc. (Kim et al., 2024). The field studies also uncovered similar observations for instance, the rate and reaction efficiency significantly depended on environmental factors such as solar intensity and rotation frequency due to mass transport effects (Arora et al., 2025). Therefore, besides considering statistical changes in the initial concentration, it is important to take into account the sources of variability in the reaction constant to predict the photocatalyst’s and overall treatment’s efficiency. Two cases are studied in detail, namely,: i) uncertainty in the initial concentration for a given rate constant
The uncertainty in the initial microbial concentration in the water (colony-forming unit (CFU) per mL) with which the treatment begins is given by the pollution process due to environmental or anthropic origin. As such, the concentration may have temporal and spatial variability (e.g., location of the water source). Without describing the current reason for such variability in the field measurements, in this work, simple probability density functions to describe the uncertainty in the initial concentration are accounted for.
The uncertainty in the reaction rate may depend on the environmental variables governing the treatment process, such as solar intensity, photocatalyst usage, and lifespan (i.e., the time after which regeneration is required). The latter effect, however, would affect the process at much larger timescales and is therefore not considered in this work. In a previous publication, Arora et al. (2025) showed that the treatment efficiency depends on the light wavelength used during the process, as well. Similarly to the previous case, an analysis based on the probability distribution functions is performed to study the effects induced by variability in the rate constant. Additionally, a stochastic approach considering a continuously changing constant rate is involved (see Section 2.4).
Additional sources of uncertainty in the final treatment time (Equation 2) are related to the variability of the target concentration,
The derived distribution approach (Kottegoda and Rosso, 2008) allows calculating the probability distribution function (pdf) of a variable,
where the presence of the absolute value on the left-hand side term allows for neglecting the sign of the integral when
By comparing the two integrand functions and rearranging the terms it follows that
where the term
The effect of concurrent variation of both the initial concentration
where
Random temporal changes in the rate constant may affect the actual treatment time. By considering the possibility that
subject to the initial condition that
or, equivalently,
where
Equations 8 and 9 represent the evolution equation of a stochastic process continuous in time and space (i.e., concentration) and affected by multiplicative white Gaussian noise (i.e., the noise term
where
where
Equation 10 is commonly referred to as the Ornstein-Uhlenbeck stochastic process (Uhlenbeck and Ornstein, 1930; Van Kampen, 1992). An example of numerically generated trajectories for this process (Equation 10) is given in Figure 2, from which clearly appears the statistical nature of the concentration and the time to reach the minimum concentration even when starting from the same initial concentration

Figure 2. Sample realizations of stochastic trajectories driving the process from the initial concentration
Solving Equation 10 cannot be done with usual methods given the stochastic nature of the process and therefore the loose mathematical meaning of the solution
where
The exact solution for
From
After some algebra one obtains
Numerical simulations for testing the derived distribution approach are performed via a standard MonteCarlo technique and the MatLab software (MatLab 2023b). In particular, random generation of either initial concentration
As far as the stochastic model is concerned, the effects of changing the parameters of the distribution of
In this study, three probability distributions are used to study the statistics of the water treatment method: uniform (Supplementary Equation S1), lognormal (Supplementary Equation S4), and normal (Supplementary Equation S7) distribution. Except for the uniform one, which is mainly used as a didactical case here, these statistical distributions are chosen because of their broad applicability in describing environmental process uncertainties (Kottegoda and Rosso, 2008). All the three distributions are scaled between minimum and maximum values based on experimental and field measurements (Arora et al., 2025), so that the condition
Values for either
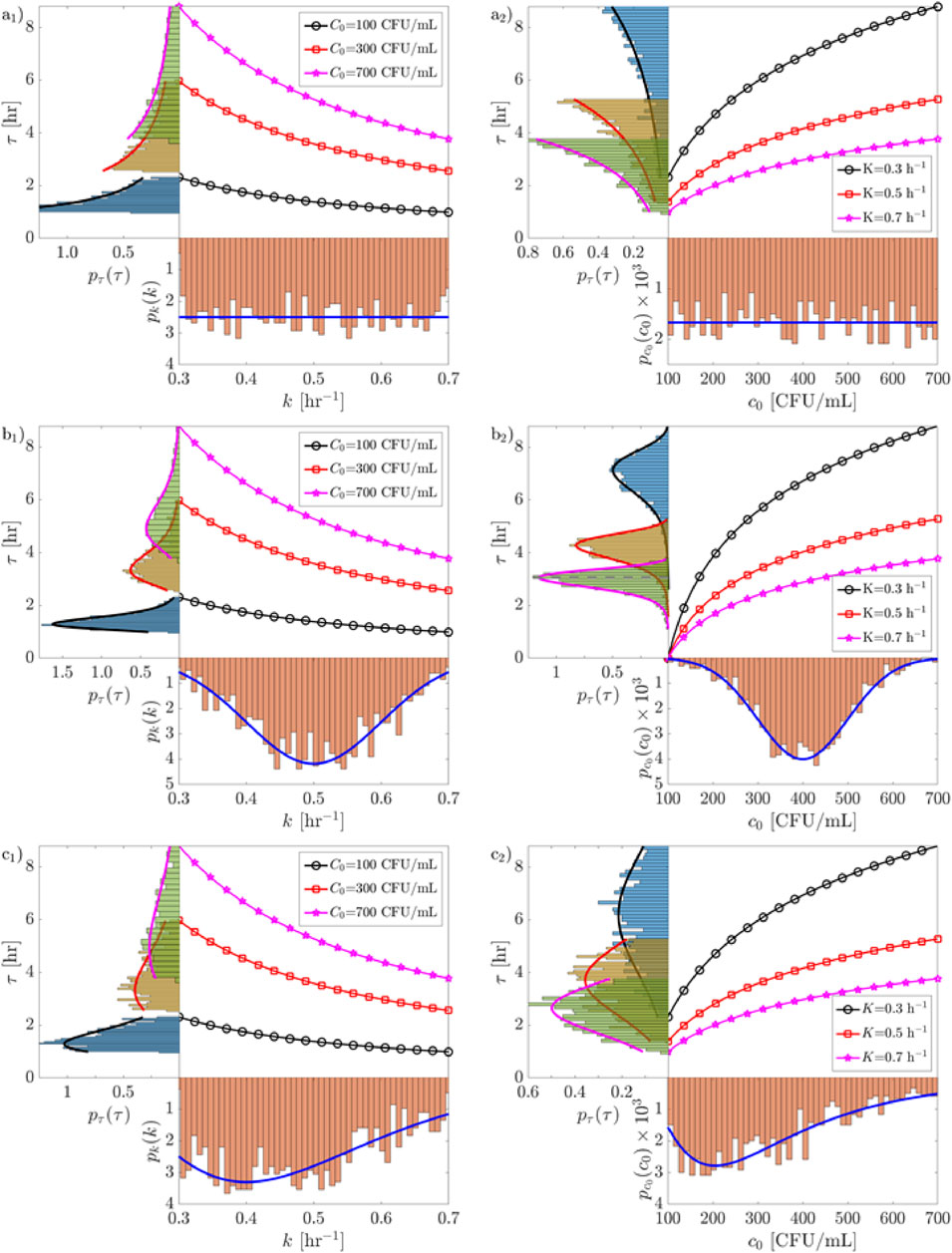
Figure 3. The probability distribution function,
Consider first the case of the uniform distribution (Figure 3A): from a mathematical point of view, one sees that the reaction dynamics (exponential) transforms a uniform distribution into exponential ones (see Supplementary Table S1). Moreover, the effect of the reaction is that of introducing a skewness in the distribution of the treatment times,
In order to move from the laboratory scale to the pilot scale, understanding the impact of all uncertainties involved in the process is critical. These uncertainties play a crucial role in optimizing the efficiency of water treatment using photocatalytic oxidation. In this regard, the proposed approach (i.e., the derived distribution, Equation 5) can be used to assess how uncertainties in
The role of the initial concentration deserves some more attention, though. When the initial concentration of pollutants increases, the treatment time generally increases as well. This is because a higher concentration of contaminants requires more time for the photocatalyst to degrade them effectively. However, at higher concentrations, saturation effects or inhibition due to excess pollutant concentration might also reduce the overall efficiency of the process, thereby altering the expected degradation rate (Hanafi and Sapawe, 2020; Mangrulkar et al., 2012).
The variability in rate constant primarily depends on two factors: i) the life span of the photocatalyst and, ii) solar intensity during the treatment. Uncertainties arising from environmental noises (such as a change in solar intensities during treatment) or the photocatalyst’s life could lead to variation in the reaction rate constant,
As the final point of our analysis, an example of the resulting derived distribution of the treatment time in the case where both
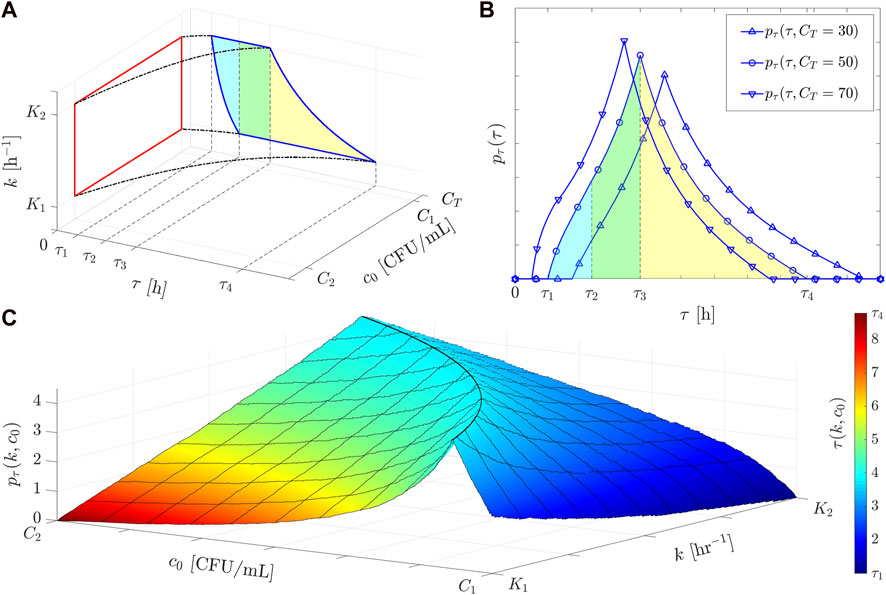
Figure 4. The bivariate derived distribution approach (Equation 6) and its application to calculate
with
To summarize, the proposed study aims to highlight how treatment times are influenced by variations in initial concentration,
Examples of probability density functions
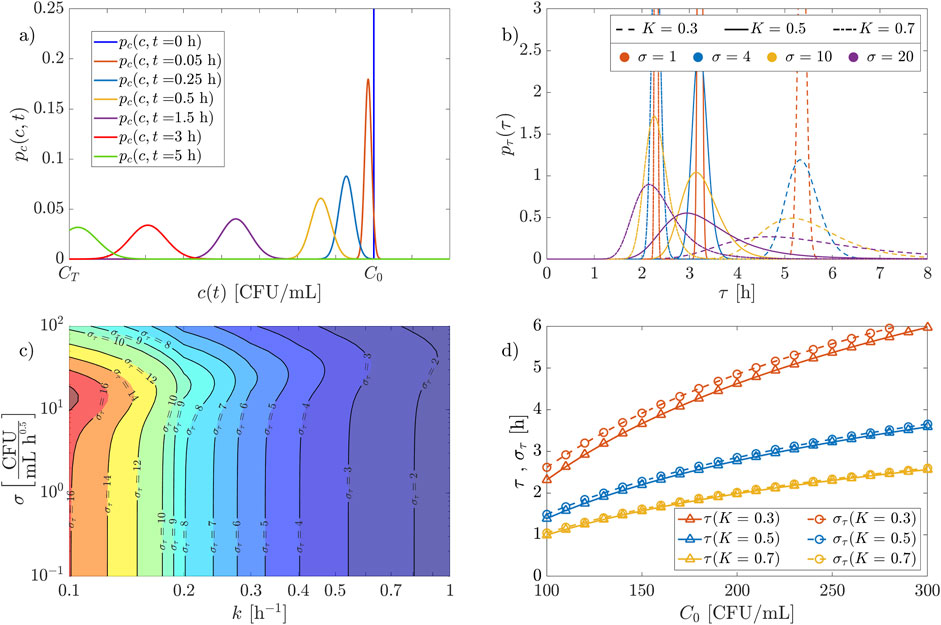
Figure 5. Probability distribution functions of concentration and treatment time for the Ornstein-Uhlenbeck process under analysis. (A) pdf of the concentration at different times (
Although the solution for
As the last aspect of our analysis, the level of dynamic environmental noise that may have affected the experimental data is assessed. From the experimental data, Arora et al. (2025) evaluated the rate constant of the reaction using a simple regression line to fit the experimental data on a logarithmic plot and obtain the mean rate constant,
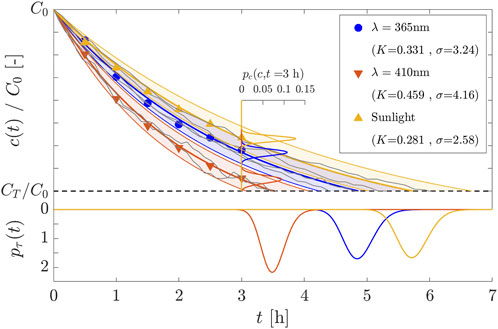
Figure 6. Experimental measurements (points), time evolution and associated pdf.s of the solar photocatalyst reaction using different light sources, i.e., wavelengths (blue for
In the present analysis, the process noise (rate constant variability) is considered to be originated by (random) variations in solar radiation and/or cloud cover, including the presence of precipitation. Due to the chemical nature, it is taken for granted that fluctuations in water temperature and pH affect the overall reaction dynamics. Furthermore, additional factors may impact the reaction dynamics, particularly for pilot-scale treatments. In this case, the size, shape, and orientation of the tank to sunlight can induce variations in concentration, potentially dampened by mechanical mixing. Determining the single contribution of each factor to the overall dynamic randomness is quite challenging, yet beyond the scope of this work. Nevertheless, the process noise
Following the field trials of the low-cost Bi-
The two proposed approaches can be very useful in estimating treatment times during the process, enhancing the practicality of this technology in real-world applications. Additionally, they provide insights into the operational challenges posed by varying conditions and helps to optimize the design of solar photocatalytic systems. An extension of this study could involve examining the stochastic nature of water arrival, both in terms of volume and timing, and using this information to optimize the development of large-scale photocatalytic water treatment systems. This would be particularly valuable for designing photocatalytic reservoirs that can handle fluctuating water inputs and optimize treatment times for large-scale applications.
The data analyzed in this study is subject to the following licenses/restrictions: The manuscript with the dataset is currently under review. See the reference in the submitted manuscript. Requests to access these datasets should be directed to YS5hcm9yYS00QHNtcy5lZC5hYy51aw==.
AA: Conceptualization, Investigation, Writing–original draft, Writing–review and editing. GC: Formal analysis, Writing–original draft, Writing–review and editing. EC: Conceptualization, Writing–original draft, Writing–review and editing. NR: Conceptualization, Writing–original draft, Writing–review and editing. PP: Formal analysis, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. AA thanks E4 DTP Project Reference 2581304 Natural Environmental Research Council for the studentship and the Overseas Research and Conference Fund for the travel funding. The Platform of Hydraulic Constructions (PL-LCH) EPFL, and the G-Research are also acknowledged for supporting the research visits.
AA thanks the University of Edinburgh and École Polytechnique Fédérale de Lausanne, EPFL for a smooth collaboration for this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1536359/full#supplementary-material
Al-Abri, M., Al-Ghafri, B., Bora, T., Dobretsov, S., Dutta, J., Castelletto, S., et al. (2019). Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. NPJ Clean. Water 2 (2), 2. doi:10.1038/s41545-018-0024-8
Alimard, P., Gong, C., Itskou, I., and Kafizas, A. (2024). Achieving high photocatalytic nox removal activity using a bi/biobr/tio2 composite photocatalyst. Chemosphere 368, 143728. doi:10.1016/j.chemosphere.2024.143728
Arora, A., Porley, V. E., Meikap, B. C., Sen, R., Debnath, A., Chatzisymeon, E., et al. (2025). Field trials of low-cost Bi-TiO2-P25 solar photocatalyst for water treatment. Adv. Sustain. Syst. doi:10.1002/adsu.202400823
Cabral, J. P. (2010). Water microbiology. bacterial pathogens and water. Int. J. Environ. Res. public health 7, 3657–3703. doi:10.3390/ijerph7103657
Calvani, G., and Perona, P. (2023). Splitting probabilities and mean first-passage times across multiple thresholds of jump-and-drift transition paths. Phys. Rev. E 108, 044105. doi:10.1103/physreve.108.044105
Cox, D. R., and Miller, H. D. (1977). The theory of stochastic processes, 134. Boca Raton, FL: CRC Press.
Daly, E., and Porporato, A. (2006). Probabilistic dynamics of some jump-diffusion systems. Phys. Rev. E—Statistical, Nonlinear, Soft Matter Phys. 73, 026108. doi:10.1103/physreve.73.026108
Dhamorikar, R. S., Lade, V. G., Kewalramani, P. V., and Bindwal, A. B. (2024). Review on integrated advanced oxidation processes for water and wastewater treatment. J. Industrial Eng. Chem. 138, 104–122. doi:10.1016/j.jiec.2024.04.037
Hanafi, M. F., and Sapawe, N. (2020). Effect of initial concentration on the photocatalytic degradation of remazol brilliant blue dye using nickel catalyst. Mater. Today Proc. 31, 318–320. doi:10.1016/j.matpr.2020.06.066
Hübner, U., Spahr, S., Lutze, H., Wieland, A., Rüting, S., Gernjak, W., et al. (2024). Advanced oxidation processes for water and wastewater treatment–Guidance for systematic future research. Heliyon 10, e30402. doi:10.1016/j.heliyon.2024.e30402
Kim, C.-M., Jaffari, Z. H., Abbas, A., Chowdhury, M. F., and Cho, K. H. (2024). Machine learning analysis to interpret the effect of the photocatalytic reaction rate constant (k) of semiconductor-based photocatalysts on dye removal. J. Hazard. Mater. 465, 132995. doi:10.1016/j.jhazmat.2023.132995
Kottegoda, N. T., and Rosso, R. (2008). Applied statistics for civil and environmental engineers. Blackwell Malden, MA.
Kuspanov, Z., Serik, A., Matsko, N., Bissenova, M., Issadykov, A., Yeleuov, M., et al. (2024). Efficient photocatalytic degradation of methylene blue via synergistic dual co-catalyst on srtio3@ al under visible light: experimental and dft study. J. Taiwan Inst. Chem. Eng. 165, 105806. doi:10.1016/j.jtice.2024.105806
Lebedev, A., Anariba, F., Tan, J. C., Li, X., and Wu, P. (2018). A review of physiochemical and photocatalytic properties of metal oxides against Escherichia coli. J. Photochem. Photobiol. A Chem. 360, 306–315. doi:10.1016/j.jphotochem.2018.04.013
Mangrulkar, P. A., Kamble, S. P., Joshi, M. M., Meshram, J. S., Labhsetwar, N. K., and Rayalu, S. S. (2012). Photocatalytic degradation of phenolics by n-doped mesoporous titania under solar radiation. Int. J. Photoenergy 2012, 1–10. doi:10.1155/2012/780562
Masoom, W., Iqbal, S., Rasool, M., Musaddiq, S., and Javaid, A. (2024). “Light driven microbial disinfection of water; mechanisms, kinetic models and factors influencing it,” in Graphene-based photocatalysts: from fundamentals to applications (Springer), 413–439.
Porley, V., Chatzisymeon, E., Meikap, B. C., Ghosal, S., and Robertson, N. (2020). Field testing of low-cost titania-based photocatalysts for enhanced solar disinfection (SODIS) in rural India. Environ. Sci. Water Res. and Technol. 6, 809–816. doi:10.1039/c9ew01023h
Porley, V., and Robertson, N. (2020). Substrate and support materials for photocatalysis. Nanostructured photocatalysts. Elsevier, 129–171.
Ren, G., Han, H., Wang, Y., Liu, S., Zhao, J., Meng, X., et al. (2021). Recent advances of photocatalytic application in water treatment: a review. Nanomaterials 11, 1804. doi:10.3390/nano11071804
Serik, A., Kuspanov, Z., Bissenova, M., Idrissov, N., Yeleuov, M., Umirzakov, A., et al. (2024). Effective photocatalytic degradation of sulfamethoxazole using pan/srtio3 nanofibers. J. Water Process Eng. 66, 106052. doi:10.1016/j.jwpe.2024.106052
Singh, P. K., Kumar, U., Kumar, I., Dwivedi, A., Singh, P., Mishra, S., et al. (2024). Critical review on toxic contaminants in surface water ecosystem: sources, monitoring, and its impact on human health. Environ. Sci. Pollut. Res. 31, 56428–56462. doi:10.1007/s11356-024-34932-0
Stevenson, A., Burkhardt, J., Cockell, C. S., Cray, J. A., Dijksterhuis, J., Fox-Powell, M., et al. (2015). Multiplication of microbes below 0.690 water activity: implications for terrestrial and extraterrestrial life. Environ. Microbiol. 17, 257–277. doi:10.1111/1462-2920.12598
Syeed, M. M., Hossain, M. S., Karim, M. R., Uddin, M. F., Hasan, M., and Khan, R. H. (2023). Surface water quality profiling using the water quality index, pollution index and statistical methods: a critical review. Environ. Sustain. Indic. 18, 100247. doi:10.1016/j.indic.2023.100247
Tran, H. D., Nguyen, D. Q., Do, P. T., and Tran, U. N. (2023). Kinetics of photocatalytic degradation of organic compounds: a mini-review and new approach. RSC Adv. 13, 16915–16925. doi:10.1039/d3ra01970e
Uhlenbeck, G. E., and Ornstein, L. S. (1930). On the theory of the Brownian motion. Phys. Rev. 36, 823–841. doi:10.1103/physrev.36.823
Walker, D., Baumgartner, D., Gerba, C., and Fitzsimmons, K. (2019). Surface water pollution. Elsevier.
Keywords: derived distribution simulations, solar photocatalysis, sustainable water treatment, photocatalytic oxidation, stochastic process
Citation: Arora A, Calvani G, Chatzisymeon E, Robertson N and Perona P (2025) Uncertainty analysis of field trials of low-cost Bi-
Received: 28 November 2024; Accepted: 21 February 2025;
Published: 17 March 2025.
Edited by:
Ilunga Kamika, University of South Africa, South AfricaReviewed by:
Marco Carnevale Miino, University of Insubria, ItalyCopyright © 2025 Arora, Calvani, Chatzisymeon, Robertson and Perona. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayushi Arora, YS5hcm9yYS00QHNtcy5lZC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.