
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 19 February 2025
Sec. Ecosystem Restoration
Volume 13 - 2025 | https://doi.org/10.3389/fenvs.2025.1517852
This article is part of the Research TopicCoastal Wetland Protection and Restoration: Ecosystem Processes, Functions and ServicesView all articles
 Meixiang Gao1,2
Meixiang Gao1,2 Yifei Liu1,2
Yifei Liu1,2 Lihu Xiong3,4
Lihu Xiong3,4 Mengmeng Qi1,2
Mengmeng Qi1,2 Xin Li1,2
Xin Li1,2 Ye Zheng5
Ye Zheng5 Jinwen Liu6
Jinwen Liu6 Zhijing Xie7,8*
Zhijing Xie7,8* Xiujuan Yan6*
Xiujuan Yan6*Drained and diked salt marshes (DDSM) habitats, a typical form of coastal wetland, are undergoing ecological recovery, offering valuable insights into strategies for restoring and protecting biodiversity in reclaimed coastal wetlands. Richness, abundance, and composition of the collembolan community is expected to vary in response to changes in plant and soil in DDSM habitats and agricultural farmlands. However, knowledge on these variations remains limited. Therefore, we aimed to reveal the species richness, abundance, and composition of the collembolan community and the effect exerted by plant and soil variables in DDSM and agricultural farmlands. Soil samples were collected in coastal DDSM (northern enclosure and southern enclosure) and wheat farmland areas in Ningbo City, southeastern China, in April 2023. Species richness, rather than abundance, of the collembolan community, was significantly lower in DDSM habitats than in wheat farmlands. The collembolan community composition differed significantly between these two habitats. Ceratophysella skarzynskii Weiner (1996), Desoria sp12, Isotoma pinnata Fabricius (1781), and Sinella sp. were exclusively in DDSM habitats. Instead, the genera Arrhopalites, Heteraphorura, and Parisotoma preferred wheat farmlands. Plant coverage and height were important variables affecting collembolan community composition in DDSM habitats. DDSM habitats can sustain specific collembolan species, and their soil biodiversity warrants attention, particularly following rigorous reclamation measures. This study provides important information for restoring and protecting biodiversity in reclaimed coastal wetlands.
Several countries have reclaimed extensive coastal tidal flats for agricultural land to meet the increasing demand for food and resource supply (Connor et al., 2001; Eyers and Chmura, 2007; Hong et al., 2010; Janousek et al., 2021; Sato and Kanazaw, 2004). However, such reclamation activities have resulted in severe ecological and environmental issues, including the loss of bird habitats, extinction of local species, reduction in marine biological resources, and extensive pollution in coastal areas (Janousek et al., 2021; Sato and Kanazaw, 2004; Yan et al., 2017). Recently, many countries have strictly controlled land reclamation projects and have strengthened ecological restoration (Cornu and Sadro, 2002; Janousek et al., 2021). Following a clamp down on reclamation efforts, a diverse spectrum of transitional ecosystems, ranging from tidal flats to agricultural lands, can be found in China’s coastal regions. Drained and diked salt marshes (DDSM) are a typical form of coastal wetland. Since 2018, China has issued several documents and implemented multiple measures to strengthen the ecological management of coastal reclamation. Habitat restoration and biodiversity protection of coastal wetlands are receiving increasing attention (Liu et al., 2022).
The restoration of coastal wetlands has focused increasingly on removing dikes and other tidal barriers (Eyers and Chmura, 2007). However, the expertise and methods available for restoring and protecting biodiversity and its function in DDSM habitats reclaimed from tidal flats along the eastern coast of China are limited (Liu et al., 2022). Without further human management, most DDSM habitats are currently undergoing natural succession. This process gradually transforms them into ecosystems capable of sustaining higher biodiversity and providing essential ecosystem functions. In this transitional state, DDSM are undergoing ecological recovery, offering valuable insights into strategies for restoring and protecting biodiversity in reclaimed coastal wetlands. Species richness and community composition in DDSM habitats vary substantially compared to the original tidal flats and targeted agricultural farmlands (Berrenstein et al., 2013; Monfils et al., 2015; Park et al., 2017). Although these marshes are important for protecting and maintaining coastal terrestrial biodiversity (Hazelden and Boorman, 2001; Martínez et al., 2009), a notable mismatch exists between knowledge of community composition and developing restoration strategies for DDSM habitats. Therefore, understanding biodiversity and its underlying drivers in DDSM habitats is imperative to restore and protect biodiversity in coastal reclaimed habitats.
The transformation from tidal flats to DDSM habitats depends the level of ground water, decreases water salinity, alters plant richness, increases vegetation coverage, introduces terrestrial mammals, and accelerates microbial decomposition along with subsidence (Drexler et al., 2019; Janousek et al., 2021; Spencer et al., 2017). Consequently, these changes impact soil aeration, lower soil salinity, alter soil structure (Cornu and Sadro, 2002; Janousek et al., 2021), cause the sequestration of more carbon and nitrogen (Adams et al., 2012), promote thriving soil microbial communities, and affect microbial functions and carbon storage (Fitch et al., 2022). Therefore, the soil fauna adapts to thrive and function in these coastal areas. However, while community composition of plants (Berrenstein et al., 2013), birds (Monfils et al., 2015), soil microbes (Fitch et al., 2022), and benthic animals (Park et al., 2017; Ryu et al., 1997) in these habitats have been studied, the diversity of soil fauna in DDSM habitats remains unclear.
Soil fauna is an important component of the coastal habitats of dunes (Goralczyk, 1998), tundra (Babenko, 2017), forests (Fuangarworn and Lekprayoon, 2010), wetlands (Ge et al., 2014b), and farmlands (Tao et al., 2013; Wang et al., 2015). It plays key roles in ecological processes, such as leaf litter breakdown, soil microstructure formation, and carbon and nitrogen cycling (Cárcamo et al., 2001; Islam et al., 2024). Recently, the soil fauna has gained importance in evaluating biodiversity and soil quality in reclaimed coastal habitats because of its active role and sensitive response to habitat changes (Ge et al., 2014a; Ge et al., 2017). Among various soil fauna, springtail (Hexapoda: Collembola) are among the most abundant and widely distributed arthropods inhabiting coastal wetlands and agricultural landscapes (Li et al., 2018; Lima et al., 2023).They thrive in almost all terrestrial ecosystems and are frequently used as model organisms in ecological research (Potapov et al., 2020). Collembola play a crucial ecological role by feeding on microbes, grazing on decomposing plant material, and interacting with plant roots, thereby influencing the growth and distribution of prokaryotes, fungi, and plants (Potapov et al., 2023). Through these interactions, they contribute significantly to nutrient cycling by processing and stabilizing organic matter. Moreover, springtails are highly sensitive to environmental changes, making them valuable bioindicators (Li et al., 2023). Diversity, functional characteristics, coverage, and presence of plants can change the food resources and habitat of collembolan communities (Krab et al., 2019; Zhang et al., 2021). Collembolan density and diversity significantly increase with plant species variety, plant functional group richness, and coverage (Krab et al., 2019; Li et al., 2018). Moreover, variations in soil organic matter, water content, and pH are possible factors affecting species richness and composition of collembolan communities in wetlands and farmlands (Dou et al., 2019; Sterzynska and Ehrnsberger, 2000). There is a widespread opinion that wetlands support a limited number of soil fauna, including Collembola, owing to waterlogging, anaerobic conditions, low temperatures, and a relative lack of microorganisms (Sławska, 2000). Additionally, construction of embankments, dikes, and activities linked to pond aquaculture and livestock in DDSM habitats have resulted in greater heterogeneity in plant community structure and soil environment compared with its original tidal flat. Generally, they manifest as higher abundance of grassland, freshwater, salt-tolerant, or exotic species, lower soil moisture, and soil salinity (Santoro et al., 2023). Instead, plant composition and diversity vary less, while the soil parameters vary more in targeted agricultural farmlands due to monoculture and frequent human management. Therefore, richness, abundance, and composition of the collembolan community is expected to vary in response to changes in plant and soil in DDSM habitats and agricultural farmlands. However, information on these variations is limited.
This study aimed to determine species richness, abundance, and composition of soil collembolan communities as well as the underlying environmental factors in DDSM and farmland habitats in reclaimed coastal areas. We investigated collembolan communities and associated plant and soil variables within DDSM habitats and adjacent wheat farmlands in Southeastern China. Based on the outcomes of earlier studies on the variability of soil collembolan communities in reclaimed wetlands and farmlands (Santoro et al., 2023; Zhang et al., 2021), we posited the following hypotheses: 1) species richness and abundance of collembolan communities are lower in DDSM habitats than in wheat farmlands; 2) species composition of collembolan communities differs between DDSM habitats and wheat farmlands; and 3) plant variables play a greater role in shaping the composition of collembolan communities in DDSM habitats, whereas soil variables are more influential in wheat farmlands. The results of this study are expected to expand our knowledge of soil biodiversity in DDSM habitats and agricultural farmlands, and provide a broader perspective on the recovery trajectories of reclaimed marshes.
The study area was situated in Ningbo City, Zhejiang Province, China, spanning 120°55′–122°16′E and 28°51′–30°33′N. Ningbo is in the central part of China’s coastline; its topography is characterized by higher elevations in the southwestern region and lower elevations in the northeastern sector. Urban zones have altitudes ranging from 4 to 5.8 m, whereas suburban ones from 3.6 to 4 m. The region has a subtropical monsoon climate characterized by mild and humid conditions during the four seasons. The city’s mean annual temperature is 17.5°C, with the highest recorded temperature in July reaching 29.1°C and the lowest in January decreasing to 5.8°C. The average annual precipitation is 1,530 mm, of which 60% falls between May and September (Gao et al., 2023). The total land area of Ningbo is 9,816 km2. By the end of 2023, the city’s permanent population was 9.697 million, of which 79.9% was urban (http://www.ningbo.gov.cn/col/col1229099787/index.html, 03 September 2024).
The Zhejiang Province had reclaimed a total of 108,760 ha of coastal land by 2010, with Ningbo City’s reclamation area and wetlands being the most extensive reclaimed (37.8%) and wetland (47.97%) areas in the Province (Yang et al., 2018). The study was performed in the Dasong Enclosure and adjacent farmlands in Zhanqi Town, Yinzhou District of Ningbo City (121.80°–121.89°N, 29.68°–29.7°E). Formerly, this area was an open tidal flat. It was enclosed by seawalls (dikes) in 2012 and featured a typical DDSM habitat. Subsequently, it became an enclosed area named the Dasong Enclosure. The latter was used for agriculture, allowing a few farmers to breed fish, shrimp, shellfish, ducks, and sheep. However, fish breeding and poultry rearing were prohibited in the Dasong Enclosure in 2019, allowing plants that prefer moist habitats, such as Phragmites australis Trinius, Spartina alterniflora Loisel, and Arundo donax Linnaeus, to grow and dominate the Dasong Enclosure (unpublished data from Lihu Xiong). Based on the results of a field investigation in September 2022, in contrast to the outside tidal flats, the Dasong Enclosure presented a reduction in soil moisture, a decline in salinity, and a notable increase in hydrophytic vegetation (unpublished data from Lihu Xiong). In contrast to adjacent crop fields with rice and wheat rotation models, the Dasong Enclosure exhibited a higher plant richness, composition, and coverage, as well as greater bird and mammal diversity (unpublished data from Lihu Xiong). However, the soil animal community remains largely unexplored in the DDSM habitats within the Dasong Enclosure and its adjacent farmlands.
DDSM and wheat farmland sites selected for the present study (Supplementary Table S1) were established to investigate the biodiversity of the soil collembolan community within the northern region of the Dasong Enclosure (NE, 121.80°–121.89°N, 29.69°–29.79°E) and the southern region of the Dasong Enclosure (SE, 121.84°–121.86°N, 29.68°–29.71°E). The NE and SE habitats were originally separated by a river. After the establishment of the Dasong Enclosure, the river channel was modified. As a result, the NE and SE habitats are now separated by a 10-m-wide partially man-made river, and connected only by a 5-m-wide and 15-m-long stone bridge (Supplementary Figure S1).
Wheat farmland (WF) sites were situated in the agricultural region of Zhanqi Town, which have been reclaimed for agricultural land use for more than 40 years. The WF sites were distant approximately 3.2 km from the Dasong Enclosure area. Ten plots, each measuring 10 × 10 m, were established with a minimum separation of 50 m from one another in the NE, SE, and WF habitats (Supplementary Table S1; Supplementary Figure S1). Soil samples of Collembola were collected from these 30 plots.
Within each plot, three soil samples were collected at a depth of 0–10 cm using an auger with a 7-cm inner diameter. Three samples were randomly collected at 3-m intervals. Subsequently, soil samples from the 0–10 cm were collected to measure soil moisture within a 2-m diameter circle around the center of each plot. Soil moisture was quantified using a gravimetric method. The height (cm) of 10 plants was randomly measured within a 2-m circle around each soil sample in each plot. Plant coverage, ranging from 0 to 1, was measured for each plot. Field collection was conducted on 16 April 2023. Ninety soil samples were collected: 3 soil samples/plot × 10 plots/habitat × 3 habitats.
Collembola were extracted from each soil sample by 10 days using dry Tullgren funnels, preserved in 95% ethanol, and stored at −20°C for further identification. Collembola were identified to the species level according to relevant publications (Christiansen and Bellinger, 1998; Potapov, 2021; Xie et al., 2019; Yin et al., 1998) using a set of stereomicroscopes (Olympus SZX16, Tokyo, Japan; equipment sourced from China; and Nikon Eclipse 80i; Shanghai, China).
All data were analyzed using R 4.4.1 (Team, 2024). The full list of species and their corresponding abundances in the NE, SE, and WF habitats are presented in Supplementary Table S2. Species richness (number of species) and abundance (number of individual species) were used to describe the quantitative characteristics of each collembolan community. Consistent with the quantitative classification criteria outlined in the literature (Wei et al., 2022), a dominant species was defined as presenting an abundance of 10% or more within the community. Common species represented 1%–10% of overall abundance. In contrast, species classified as rare amounted to less than 1% of the community.
To evaluate the diversity of the collembolan community in each habitat, Shannon and Simpson indices were calculated by the “diversity” function from the vegan package (Team, 2024). The Shannon index (Shannon, 1948) measures both richness and evenness. Its usual values vary around 1.5–3.5, with values greater than 3.5 indicating that habitat is highly important for biodiversity (Magurran, 2004). The Simpson index (Simpson, 1949) measures evenness, and varies from 0 to 1, with values closer to 1 indicating better evenness among species.
An extrapolated species diversity analysis method was employed to determine whether the field survey of each collembolan community was sufficient (Chao et al., 2014). This method reasonably extrapolates the observed species richness, that is, the number of species, based on existing trends, thereby obtaining corresponding theoretical values. The values were calculated using the “iNEXT” function from the iNEXT package (Hsieh et al., 2024). This study used observed abundances to calculate species richness for rarefaction and extrapolated samples. Rarefaction/extrapolated curves were plottted (Colwell et al., 2012).
To verify Hypothesis 1, a non-parametric statistical test, the Kruskal–Wallis test, was employed to assess variations in the richness, abundance, Shannon, and Simpson indices of collembolan communities, as well as the abundance of individual species across NE, SE, and WF habitats. This approach was necessitated by the noncompliance of these variables with the assumptions of normality and homogeneity of variance. Post-hoc multiple comparisons utilizing pairwise Wilcoxon rank-sum tests were conducted to delineate specific habitats exhibiting significant differences. The Holm method was used to adjust the p-values to control for the error rates of multiple comparisons. The “shapiro.test” function from the stats package was used to assess the normality of the variables, while the “leveneTest” function from the car package was employed to evaluate the homogeneity of variance (Fox and Weisberg, 2019). The “kruskal.test” function from the stat package was employed to conduct the Kruskal–Wallis test, while the “pairwise.wilcox.test” function from the stat package was used to conduct pairwise Wilcoxon rank-sum tests (Team, 2024).
To test Hypothesis 2, the composition of soil collembolan communities in NE, SE, and WF habitats was analyzed using non-metric multi-dimensional scaling (NMDS) based on the Bray–Curtis dissimilarity index. NMDS is a sorting method that simplifies multi-dimensional space objects into lower-dimensional spaces while retaining the original relationships between objects. In cases with many samples and species, the NMDS model can more accurately reflect the numerical sorting information of the distance matrix and is therefore considered the most robust non-restrictive sorting method (Minchin, 1987). This visualization was achieved through the “metaMDS” function from the vegan package (Oksanen et al., 2024). Only the species present in a minimum of three sampling plots were considered (Xie et al., 2021). This exclusion was based on the observation that species found in only one or two plots exhibited consistently low abundance.
To test Hypothesis 3, environmental factors, including soil moisture, plant height, and plant coverage, were used (Supplementary Table S1). Spearman’s correlation was used to evaluate the relationships between species richness, abundance of collembolan communities, and environmental factors. Spearman correlation was conducted using the “cor” function from the corrplot package (Wei and Simko, 2021). General linear regression was used to evaluate the importance of environmental factors affecting species richness and abundance of collembolan communities in each habitat using the “lm” function from the stats package (Team, 2024). Distance-based redundancy analysis (DB-RDA) (Legendre and Legendre, 2012) was used to identify environmental factors related to collembolan community composition in each habitat. Moreover, hierarchical partitioning (HP) (Lai et al., 2022) was introduced to DB-RDA to describe the relative contributions of individual environmental factors by the “rdacca.hp” function from the rdacca.hp package (Lai et al., 2022). To eliminate the influence of extreme values and reduce the weight of high-abundance species, community data in each habitat were standardized using Hellinger transformation by the “decostand” function from the vegan package (Oksanen et al., 2024). The variation explained by environmental variables was assessed using adjusted R2 values. The permutation test of hierarchical partitioning for canonical analysis with 999 permutations was performed to evaluate significance using the “permu.hp” function in the rdacca.hp package (Lai et al., 2022).
Overall, 43 collembolan species were identified among 2,405 individuals. Species richness was 16, 21, and 26 in NE, SE, and WF habitats, respectively (Supplementary Table S2). Species richness was significantly higher in the WF habitat than in the NE habitat (Figure 1A). The abundance of collembolan communities in NE, SE, and WF habitats was 471, 1,126, and 808, respectively. No significant differences in abundance of collembolan communities existed among the three habitats (Figure 1B).
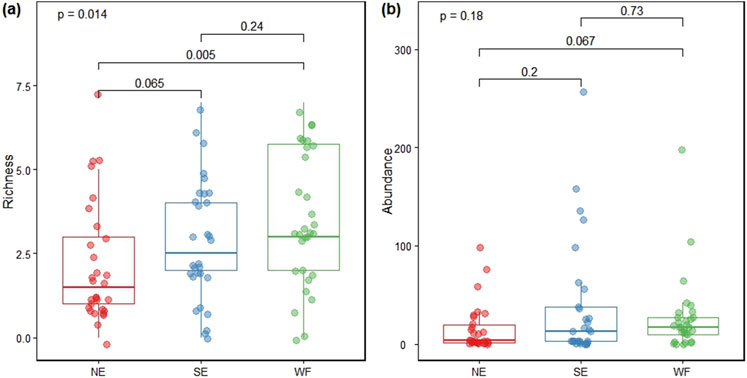
Figure 1. Boxplots summarizing richness (A) and abundance (B) of collembolan communities in NE (northern enclosure), SE (southern enclosure), and WF (wheat farmland) habitats.
Based on the current field survey, species rarefaction curves in all habitats tended to level off gradually. When the number of Collembola collected in NE, SE, and WF habitats reached 942, 2252, and 1,616 individuals, respectively, the extrapolation curves suggested that the corresponding richness was expected to be 18.06, 23.52, and 27.47. These values would have represented an increase of 12.88%, 10.71%, and 5.65%, respectively, in species richness (Figure 2).
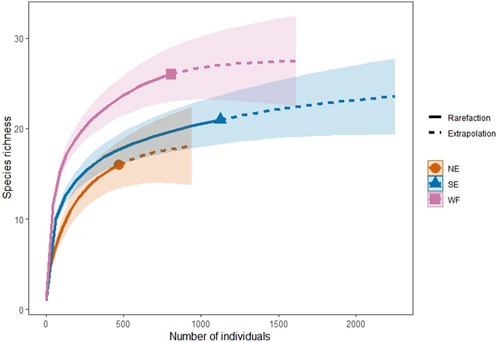
Figure 2. Species diversity extrapolated results showing species richness of collembolan communities in northern enclosure (NE), southern enclosure (SE), and wheat farmland (WF) habitats. “Rarefaction” refers to the rarefaction curves; whereas “Extrapolation” refers to the extrapolation curves. Shaded areas represent 95% confidence intervals.
The Shannon indices of the collembolan community in NE, SE, and WF habitats were 1.147, 1.755, and 2.141, respectively, with the latter two being significantly higher (p < 0.05) than the first one (Figure 3A). The Simpson indices of the collembolan community in NE, SE, and WF habitats were 0.516, 0.724, and 0.808, respectively, with the latter two being again significantly higher (p < 0.05) than the first one (Figure 3B).
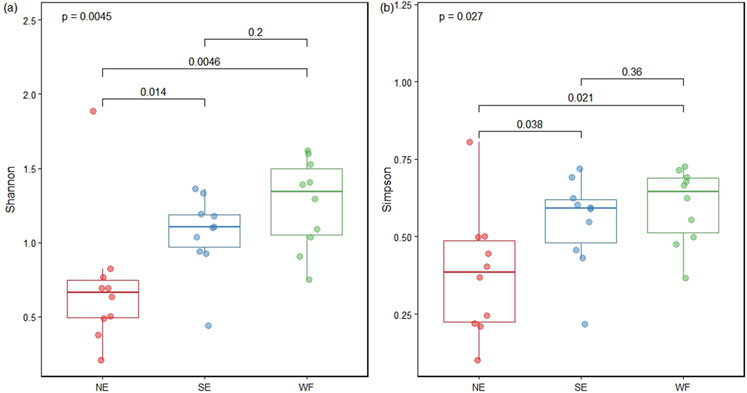
Figure 3. Shannon (A) and Simpson (B) indices of collembolan communities in the northern enclosure (NE), southern enclosure (SE), and wheat farmland (WF) habitats.
Bionychiurus changbaiensis Sun and Wu (2012) and F. ozaena sp1 Yosii (1977) were prevalent in the WF habitat but rare in the NE and SE habitats (Figure 4; Supplementary Table S2). The abundance of B. changbaiensis was notably higher in the WF habitat than in NE (p < 0.001) and SE (p < 0.001) habitats (Figure 5A). The abundance of F. ozaena sp1 was significantly higher in the WF habitat than in the NE (p < 0.05) habitat (Figure 5D). Sinella curviseta Brook (1882) and Ceratophysella sp2 were dominant in the SE habitat but rare in NE and WF habitats (Figure 4; Supplementary Table S2). S. curviseta was significantly more abundance in the SE habitat than in NE (p < 0.001) and WF (p < 0.001) habitats (Figure 5E). Instead, no significant differences in the abundances of Ceratophysella sp2 were observed among the three habitats (Figure 5B). Sinella sp was uniquely prevalent in the NE habitat, where its abundance was significantly higher than that in both SE (p < 0.05) and WF (p < 0.05) habitats (Figure 5F). Instead, Desoria choi Lee (1977) was dominant across all habitats, and its abundance in the WF habitat differed significantly from that in NE (p < 0.001) and SE (p < 0.001) habitats (Figure 5C).
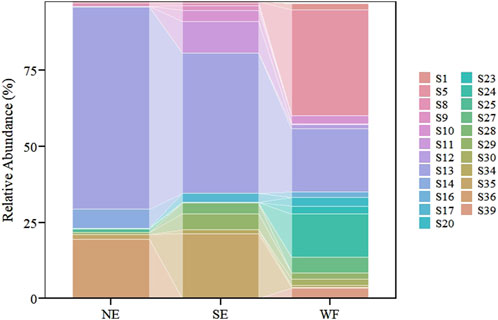
Figure 4. Species richness stack map of collembolan communities in northern enclosure (NE), southern enclosure (SE), and wheat farmland (WF) habitats. Only species common or dominant in at least one habitat are shown. S1, Arrhopalites sp1; S5, Bionychiurus changbaiensis; S8, Bourletiella sp3; S9, Ceratophysella skarzynskii; S10, Ceratophysella sp1; S11, Ceratophysella sp2; S12, Coreanura sp1; S13, Desoria choi; S14, Desoria sp12; S16, Desoria sp7; S17, Desoria sp8; S20, Entomobrya sibirica Stach (1963); S23, Folsomia octoculata Handschin (1925); S24, Folsomia ozaena sp1; S25, Folsomides sp1; S27, Heteraphorura seolagensis Lee (1974); S28, Isotoma pinnata; S29, Isotoma sp1; S30, Parisotoma ekmani; S34, Rambutsinella sp1; S35, Sinella curviseta; S36, Sinella sp; S39, Sinella sp5.
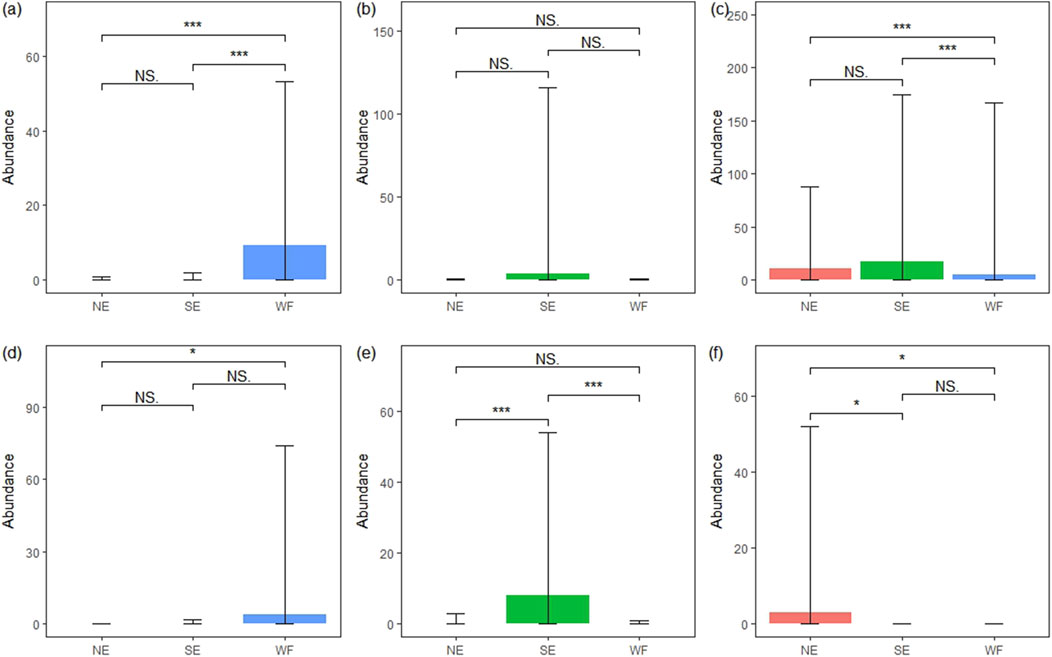
Figure 5. Abundance of six main species in northern enclosure (NE), southern enclosure (SE), and wheat farmland (WF) habitats. Abundance of each dominant species in at least one of the three habitats: (A) S5, Bionychiurus changbaiensis; (B) S11, Ceratophysella sp2; (C) S13, Desoria choi; (D) S24, Folsomia ozaena sp1; (E) S35, Sinella curviseta; (F) S36, Sinella sp.
The abundances of Arrhopalites sp1 (Supplementary Figure S2A), Ceratophysella sp1 (Supplementary Figure S2E), Parisotoma ekmani Fjellberg (1977) (Supplementary Figure S2G), and Sinella sp5 (Supplementary Figure S2H) were notably higher in the WF habitat than in NE and SE habitats (p < 0.05, p < 0.01, p < 0.001, respectively). The abundance of Ceratophysella skarzynskii Weiner (1996) (Supplementary Figure S2B, p < 0.05) and Isotoma pinnata Fabricius (1781) (Supplementary Figure S2F, p < 0.001) in the SE habitat significantly exceeded that in NE and WF habitats, respectively. The abundance of Ceratophysella sp1 in the SE (p < 0.05) and WF (p < 0.01) habitats was notably higher than that in the NE habitat (Supplementary Figure S2C). Additionally, the abundance of Desoria sp12 was significantly higher in the NE habitat than in SE (p < 0.001) and WF (p < 0.001) habitats (Supplementary Figure S2D). No significant differences in abundance were observed among the three habitats for the other species.
According to NMDS results, the distribution of collembolan species in NE and SE habitats was relatively dispersed; whereas in the WF habitat, it was more concentrated. Collembolan communities in the three habitats partially overlapped near the middle, indicating a small overall similarity in species composition. The collembolan communities in NE and SE habitats showed a relatively high degree of overlap, suggesting that species composition in these habitats was more similar than that in the WF habitat. The second axis separated the collembolan community in the WF habitat from that in the other two habitats (Figure 6).
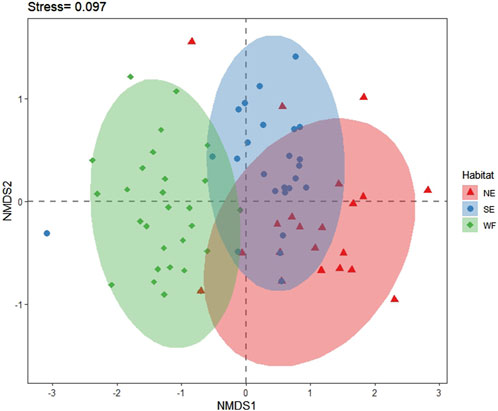
Figure 6. Non-metric multidimensional scaling (NMDS) ordination based on the Bray–Curtis dissimilarity index of collembolan community composition in northern enclosure (NE), southern enclosure (SE), and wheat farmland (WF) habitats. NMDS was conducted to assess the dissimilarity of species composition between each habitat (stress = 0.097). Each point in the figure represents a plot, and closure proximity indicates greater similarity in species composition.
Species richness correlated significantly with soil moisture (p < 0.05) and plant coverage (p < 0.01). Abundance in the collembolan community correlated significantly with soil moisture (p < 0.01) in the NE habitat according to Spearman’s correlation results (Figure 7A). Instead, species richness and abundance were not significantly correlated with environmental factors in SE and WF habitats (Figures 7B,C).

Figure 7. Spearman correlation of species richness, abundance of the collembolan community, and environmental factors in NE (A), SE (B), and WF (C) habitats. Rich, species richness; Abun, abundance; SM, soil moisture (%); Height, plant height (cm); Coverage, plant coverage.
Based on the outcomes of general linear regression analysis, only species richness showed a significant increase with enhanced plant coverage in the NE habitat (R2 = 0.157, p < 0.05) (Figure 8A). Conversely, no other significant associations were identified between species richness, abundance, and the environmental factors examined in each habitat (Figures 8B–D).
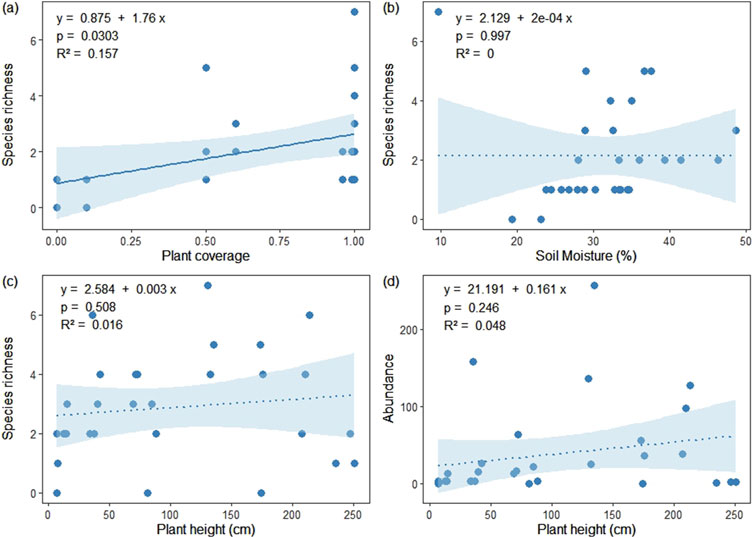
Figure 8. Linear relationships between species richness and abundance of the collembolan community and certain environmental factors in NE (A, B) and SE (C, D) habitats. The solid line represents a significant (p < 0.05) result; dotted lines represent non-significant (p > 0.05) results.
The three environmental factors collectively explained 11.6% of total variance in collembolan community composition in the NE habitat (adjusted R2 = 0.116). Plant coverage contributed significantly to collembolan community composition (adjusted R2 = 0.794, p < 0.01). Plant coverage and soil moisture exerted a high synergistic effect on collembolan community composition (adjusted R2 = 0.233) owing to larger collinearity between the two factors. However, these three environmental factors could not explain the relatively large residual (88.4%) (Figure 9A).
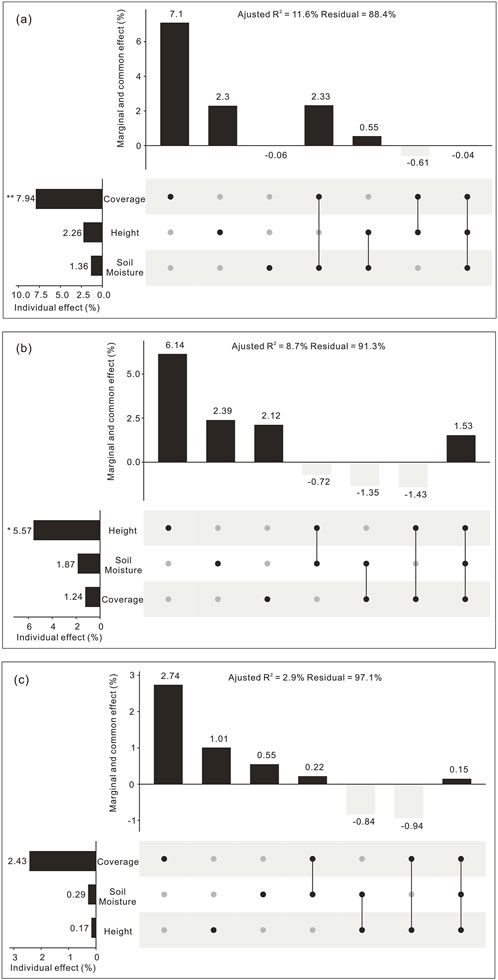
Figure 9. UpSet matrix layout of variation partitioning based on DB-RDA and hierarchical partitioning results showing the relative importance of three environmental factors on collembolan community composition in the NE (A), SE (B), and WF (C) habitats. Each row corresponds to an environmental factor in the dot-matrix plot on the right. For each column, the isolated black dot represents the marginal effect of each environmental factor, lines connecting multiple dots represent the common effect of the corresponding environmental factors. The percentage of variation explained by each component (from variation partitioning) is shown in the top column diagram. The column diagram on the left shows the individual effect of each environmental factor (from hierarchical partitioning); its value is equal to its marginal effect in addition to its average shared common effect with other environmental factors. Coverage, plant coverage; Height, plant height (cm); Soil moisture (%).
Environmental factors collectively explained 8.7% of total variance in collembolan community composition in the SE habitat (adjusted R2 = 0.087). Plant height contributed significantly to collembolan community composition (adjusted R2 = 0.056, p < 0.05). Plant height, soil moisture, and plant coverage showed a high synergistic effect on collembolan community composition (adjusted R2 = 0.015). Nevertheless, a substantial proportion of the residue (91.3%) was unaccounted for by plant and soil variables (Figure 9B).
Environmental factors collectively explained 2.9% of total variance in collembolan community composition in the WF habitat (adjusted R2 = 0.029), although none of them contributed significantly. Overall, 97.1% of variance could not be explained by these environmental factors (Figure 9C).
The first part of Hypothesis 1 was confirmed: the collembolan community’s species richness was lower in the DDSM habitat than in wheat farmland. In the NE habitat, species richness was significantly lower than in wheat farmland. Contrary to Hypothesis 1, the average abundance of collembolan communities in the DDSM habitat was almost equivalent to that in wheat farmland. Similarly, Yahya et al. (2020) found that collembolan abundance was lower in wetlands than in wheat-rice farmland, but the difference was not significant.
Overall, species richness and abundance exhibited different responses in the two habitats. The results of this study partially align with the findings from a study on reclaimed coastal areas along the west coast of the Yellow Sea in China, which detected higher taxonomic richness and abundance of soil macrofauna in wheat farmlands and lower values in unutilized wetlands (Ge et al., 2014b). The expected outcome of species richness could be due to frequent waterlogging, anaerobic conditions, and a relative lack of microorganisms in the DDSM habitat of the study area (Sławska, 2000). For example, the present study area was found to harbor significantly fewer soil microorganisms in the halophyte plants Suaeda salsa Linnaeus, S. alterniflora Loisel, and Phragmites communis Trinius growing in coastal wetlands than in adjacent rice fields (Li and Shen, 2011). Additionally, specific plot conditions in DDSM habitats with low plant richness and coverage could contribute to lower species richness. Plots NE9, NE10, SE4, and SE8 showed lower plant coverage, especially the NE9 and NE10 plots, which were located in the drainage engineering area and frequently subject to disturbances from workers, vehicles, and construction activities (Supplementary Figure S3). As vegetation cover and land use affect the taxonomic richness of soil fauna in coastal wetlands and farmland (Ge et al., 2014b), we speculated that poor conditions with low vegetation cover or bare land in certain areas of DDSM habitats lowered species richness of the collembolan community. Furthermore, species richness of the collembolan community was significantly lower in the NE habitat than in the SE habitat. This coincided with lower elevation of the NE habitat (0.04 ± 0.9 m) compared with the SE habitat (1.08 ± 1.71 m) (unpublished data from Lihu Xiong), suggesting that the soil drainage process is likely slower in the NE habitat. Consequently, variations in elevation may have led to more extensive and frequent waterlogging in the NE habitat than in the SE habitat (Sławska, 2000). Elevation and drainage processes within DDSM habitats can influence soil salinity, waterlogging conditions, and microorganisms (Li and Shen, 2011). Indeed, soil salinity (Owojori et al., 2009), waterlogging environments (Sławska, 2000), and soil microbial biomass (Filser et al., 2002) have been shown to affect abundance, survival, and juvenile production of collembolan communities. Consequently, we propose that changes in salinity, waterlogging environments, and microbial communities, may indirectly affect species richness across DDSM habitats.
The significantly higher abundance of dominant species in the DDSM habitat may be attributed to unexpected results (Wiwatwitaya and Takeda, 2005). D. choi was dominant in NE and SE habitats, representing 13.01% and 21.58% of total abundance among collembolan communities in this study (combined collected Collembola), respectively. The collembolan community tends to be dominated by a handful of species across various habitats, thereby contributing to abundance and composition of the community (Sousaa et al., 2004; Wiwatwitaya and Takeda, 2005).
Species composition of the collembolan community differed between the DDSM habitat and wheat farmland, confirming Hypothesis 2. Several collembolan species, inhabit wetlands, and many of them are found exclusively in these habitats (Sławska, 2000). A similar phenomenon was observed in this study. C. skarzynskii, Desoria sp12, I. pinnata, and Sinella sp were only present in the DDSM habitat, in which they were also dominant. Accordingly, they might be specialized for such areas. Microtubercules and hair are key adaptations enabling Collembola to thrive in moist environments (Marx et al., 2012). Both C. skarzynskii and I. pinnata exhibited these features, which may explain their dominance and exclusivity in DDSM habitats. Arrhopalites sp1, Heteraphorura seolagensis, P. ekmani, and Sinella sp5 were exclusively distributed in wheat farmland and were significantly dominated in this habitat in the present study. Previous studies have reported that Collembola from the genera Ceratophysella, Isotomurus, Ballistura, Arrhopalites, and Sminthurides were stenotypic species in mire habitats (Sławska, 2000); whereas Isotogastrura mucrospatulata Palacios-Vargas, Lima and Zeppelini (2015) was endemic in tidal flats (Lima et al., 2023). Similarly, we found that certain Collembola from the genera Ceratophysella, Desoria, Sinella, and Isotoma were preferentially distributed in DDSM habitats. We also observed that certain species from the genera Arrhopalites, Heteraphorura, and Parisotoma were particularly well-adapted to the cultivated environment of wheat farmland. Therefore, we suggest that these species, which dominate and are exclusive to DDSM and wheat farmland, could serve as potential indicators for restoring and protecting soil biodiversity in reclaimed coastal wetlands.
Some Collembola were found in both DDSM and wheat farmland habitats, but showed habitat preferences. S. curviseta, Ceratophysella sp1, B. changbaiensis, and F. ozaena sp1 existed in both habitats; however, the first two were significantly dominant in DDSM, whereas the last two were significantly dominant in wheat farmland. D. choi was dominant in both the DDSM habitat and wheat farmland, but was significantly more abundant in the former. As Desoria mulyeongariensis prefers wetland habitats in Korea (Lim and Park, 2011), we speculated that D. choi might also prefer and thrive in moist habitats in coastal marshes. Therefore, although distributed in both habitats, certain collembolan species from the genera Sinella, Ceratophysella, and Desoria preferred DDSM habitats, whereas the genera Bionychiurus and Folsomia favored farmland habitats. A similar phenomenon was observed by Chang et al. (2013), who found that species from the genus Folsomia were preferentially distributed in rice and soybean farmlands, with marsh soils and located in temperate zones. This suggests that habitat preferences by collembolan species may be driven by specific environmental factors, such as soil moisture, organic content, and the availability of food resources (Potapov et al., 2020). These factors could play a crucial role in shaping the distribution of these species, with certain genera thriving in the DDSM habitats compared to the more disturbed conditions of farmland. Further research is needed to examine the mechanisms guiding habitat preferences.
The Shannon and Simpson indices of collembolan communities were significantly higher in wheat farmland than in the DDSM habitat, indicating a clear separation between the two areas regarding species diversity and evenness. Additionally, a significant difference in the Shannon and Simpson indices was observed between the NE and SE habitats. The higher species richness and the greater dominance of certain species in the SE habitat, compared to the NE habitat, may have contributed to this difference (Shannon, 1948; Simpson, 1949). Therefore, the biodiversity of the collembolan community across DDSM habitats warrants further attention. Whereas, the composition of the collembolan community was similar in the two reclaimed DDSM habitats, according to the outcomes of NMDS, its composition in wheat farmland differed from that of the DDSM habitat. Xie et al. (2021) found that certain collembolan species contributed to the structure and pattern of different habitats. In the present study, Arrhopalites sp1, Desoria sp7, Folsomia inoculata sp1, Folsomia octoculata, H. seolagensis, P. ekmani, and Sinella sp5 only existed in wheat farmland; therefore, these seven species contributed to the significant separation between wheat farmland and DDSM habitat.
Our findings partially support Hypothesis 3. Plant parameters significantly affect the composition of collembolan communities in the DDSM habitat; however, contrary to our expectations, soil does not significantly influence the collembolan community in wheat farmland.
In DDSM habitats, plant height and coverage were important environmental factors affecting the composition of the collembolan community. Collembola are detritivores or fungivores, whose abundance can be quite high in some wetlands (Batzer and Wu, 2020), and are affected by plant variables. Plant species richness, biomass, coverage, and vegetation type contribute to collembolan community composition because diverse plant communities provide a wide range of food resources (Maceda-Veiga et al., 2016; Zhang et al., 2023). Collembola are particularly abundant in wetlands with vegetation and tend to accumulate in areas where plant growth and leaf litter accumulate (Giordano et al., 2014). These microhabitats offer a wealth of food sources and shelter from predators and environmental pressure in wetlands (Giordano et al., 2014; Maceda-Veiga et al., 2016). Similarly, the significant influence of plant height and coverage on collembolan communities observed in this study may be due to the greater availability of food resources and shelter in the DDSM habitat. Although soil moisture exhibited a significant positive correlation with species richness and abundance of the collembolan community (Figure 7), its individual influence was not pronounced based on the DB-RDA results. This study revealed considerable collinearity between soil moisture and factors, such as plant coverage and height. Consequently, the observed positive correlations among soil moisture, species richness, and abundance may be attributed to the substantial contributions of plant coverage and plant height (Figure 8). We did not detect a significant contribution of soil moisture to collembolan community composition in the DDSM habitat; however, other studies did. The lengths of the antenna and body of Collembola were negatively associated with soil water content, which could be explained by there being more opportunities for these organisms to migrate on the surface of a coastal mudflat when it was submerged in water (Li et al., 2023). These findings suggest that the relationship between the collembolan community and soil moisture is case-specific.
Collembola respond to plant and soil variables in farmlands (Chang et al., 2013; Dou et al., 2019; Xie et al., 2021); however, we did not detect significant relationships between collembolan communities and these variables. Studies have reported positive correlations between plant variables and collembolan communities in wheat farmlands. For example, wheat roots (Becker et al., 2001) and crop identity (Chauvat et al., 2014) are key determinants of collembolan community composition in wheat fields. However, other studies have reported different findings; wheat litter (Sereda et al., 2015) and crop species (Bokova et al., 2023) do not significantly influence the collembolan community in wheat farmlands. Additionally, some studies have reported the significant influence of soil moisture on the collembolan community in wheat fields (Asif et al., 2016; Salmon et al., 2021). Suitable soil moisture may increase the accessibility of microorganisms to food, thereby affecting the collembolan community (Meyer et al., 2021). However, some studies have only detected minor or insignificant roles of soil moisture on collembolan composition (Shaki and Ahmed, 2015) and suggested that Collembola tended to be abundant in wetter soil and soil with sufficient amounts of nutrients (Shaki and Ahmed, 2015; Steinberger et al., 1984). In this study, insufficient soil nutrients in the reclaimed farmland may have contributed to the non-significant effect of soil moisture. Therefore, further studies combining soil moisture and nutrients are needed.
Large residuals in ether the DDSM habitat or wheat farmland could not be explained by plant and soil variables. Besides variables measured in this study, other parameters, such as plant genetic diversity, plant roots (Chateil et al., 2013), soil pH or organic matter (Becker et al., 2001), particle size (Salmon et al., 2021), climate [e.g., flooding (Krediet et al., 2023) or warming (Zhang et al., 2023)], topography, and management (e.g., tillage, fertilizer) (Chang et al., 2013) or dikes (Ge et al., 2017) have also been reported as key determinants of collembolan composition in wetlands and wheat farmlands. Therefore, more plant and soil variables and their interactions should be considered in future studies.
Although reclamation generally leads to a loss of marine biodiversity, this study confirmed that reclamation of tidal flats to DDSM habitats could maintain a certain richness and abundance of the soil collembolan community after 11 years. Therefore, ensuring high biodiversity in DDSM habitats and reclaimed farmlands may help maintain local biodiversity, consistent with other studies on reclaimed forests and farmlands (Ge et al., 2014a). Overall, the results of this study provide basic knowledge of the soil faunal biodiversity in DDSM habitats and their targeted farmlands in subtropical areas. Whether DDSM habitats are restored to their original tidal flats or left to succeed to their targeted agricultural farmland, they can maintain a certain unique and general biodiversity of soil fauna, providing a “biodiversity resource.” Future investigations ought to reveal the function of collembolan communities in processes of restoration of biodiversity and the conservation of reclaimed DDSM and agricultural areas. These findings will contribute to the development of restoration strategies for reclaimed coastal areas in southeastern China.
This study aimed to determine the richness, abundance, and composition of the collembolan community and the underlying environmental factors in the DDSM habitat and adjacent farmland. This study showed that species richness, rather than the abundance of collembolan communities, was higher in the DDSM habitat than in wheat farmland. The composition of collembolan communities differed significantly between the DDSM habitat and wheat farmland. Certain species from the genera Ceratophysella, Desoria, Sinella, and Isotoma were preferentially distributed in the DDSM habitat, whereas C. skarzynskii, Desoria sp12, I. pinnata, and Sinella sp were present exclusively in the DDSM habitat. We also observed that certain species from the genera Arrhopalites, Heteraphorura, and Parisotoma were particularly well-adapted to the reclaimed wheat farmland. Plant coverage and height were important factors affecting collembolan community composition in the DDSM habitat; whereas plant coverage, height, and soil moisture were not critical in wheat farmland. Furthermore, a snapshot investigation was performed and certain environmental factors were considered in the present study. Multiple investigations explaining community variations on different temporal scales and more potentially critical environmental variables should be considered to understand soil biodiversity and its underlying processes in DDSM and farmlands. Overall, this study suggests that DDSM habitats have the potential to support certain collembolan species, and soil biodiversity within these habitats warrants attention, especially after stringent reclamation activities have been implemented. Furthermore, these soils could be considered as a “biodiversity resource” during restoration processes.
The datasets presented in this article are not readily available because The data are part of an ongoing study. Requests to access the datasets should be directed to Meixiang Gao, Z2FvbWVpeGlhbmdAbmJ1LmVkdS5jbg==.
The manuscript presents research on animals that do not require ethical approval for their study.
MG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing–original draft, Writing–review and editing. YL: Data curation, Formal Analysis, Investigation, Software, Writing–original draft, Writing–review and editing. LX: Formal Analysis, Investigation, Software, Writing–original draft, Writing–review and editing. MQ: Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. XL: Data curation, Formal Analysis, Software, Writing–original draft, Writing–review and editing. YZ: Formal Analysis, Methodology, Software, Writing–original draft, Writing–review and editing. JL: Formal Analysis, Software, Writing–original draft, Writing–review and editing. ZX: Conceptualization, Data curation, Methodology, Supervision, Writing–original draft, Writing–review and editing. XY: Conceptualization, Data curation, Methodology, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (42271051, 42471054, and 42201449), the Zhejiang Public Welfare Technology Application Research Project (LGN22D010006), the Zhejiang Provincial Natural Science Foundation of China (LQ23D010005), the Ningbo Natural Science Foundation Project (2021J129 and 2023J006), and the Science and technology program of Jilin Province (CXGC2021ZY120).
We thank Jiangshan Lai for his assistance in data analysis. We thank Jiahuan Sun, Shuning Zhang, Jiaqi Zhu, Yanyan Ye, Chen Peng, Zemeng Zhou, Yige Jiang, and Yanting Li for their assistance with field work. We thank Haixia Peng for here assistance with plotting figure. We would like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1517852/full#supplementary-material
Adams, C. A., Andrews, J. E., and Jickells, T. (2012). Nitrous oxide and methane fluxes vs. carbon, nitrogen and phosphorous burial in new intertidal and saltmarsh sediments. Sci. Total Environ. 434, 240–251. doi:10.1016/j.scitotenv.2011.11.058
Asif, M. U., Ahmed, S., Khan, R. R., and Atiq, M. (2016). Relationship of Collembola population wth different abiotic factors in an agricultural ecosystem of Faisalabad, Punjab, Pakistan. Pak. J. Agric. Sci. 53, 201–208. doi:10.21162/pakjas/16.4179
Babenko, A. B. (2017). Springtails (Collembola) of the eastern chukotka peninsula: peculiarities of fauna and assemblages. Zool. Zhurnal 96, 1141–1164. doi:10.7868/S0044513417100038
Batzer, D. P., and Wu, H. (2020). Ecology of terrestrial arthropods in freshwater wetlands. Annu. Rev. Entomology 65, 101–119. doi:10.1146/annurev-ento-011019-024902
Becker, J., Makus, P., and Schrader, S. (2001). Interactions between soil micro- and mesofauna and plants in an ecofarming system. Eur. J. Soil Biol. 37, 245–249. doi:10.1016/s1164-5563(01)01091-3
Berrenstein, H. J., Mans, D. R. A., Bhansing, M., Wakiran, W. S., Atmopawiro, S. L., Chotkan, N. R., et al. (2013). Effects of diking on the biological performance of the black mangrove (Avicennia germinans L.) in an Atlantic mangrove forest. Wetl. Ecol. Manag. 21, 165–172. doi:10.1007/s11273-013-9287-5
Bokova, A. I., Panina, K. S., Dridiger, V. K., Gadzhiumarov, R. G., Kuznetsova, N. A., and Potapov, M. B. (2023). Soil-dwelling springtails as indicators of the efficiency of No-till technologies with different amounts of mineral fertilizers in the crop rotation on chernozem soils. Soil and Tillage Res. 232, 105760. doi:10.1016/j.still.2023.105760
Brook, G. (1882). On a new genus of Collembola (Sinella) allied to Degeeria Nicolet. Zool. J. Linn. Soc. 16, 541–545. doi:10.1111/j.1096-3642.1882.tb02398.x
Cárcamo, H. A., Prescott, C. E., Chanway, C. P., and Abe, T. A. (2001). Do soil fauna increase rates of litter breakdown and nitrogen release in forests of British Columbia, Canada? Can. J. For. Research-Revue Can. De Recherche For. 31, 1195–1204. doi:10.1139/cjfr-31-7-1195
Chang, L., Wu, H., Wu, D., and Sun, X. (2013). Effect of tillage and farming management on Collembola in marsh soils. Appl. Soil Ecol. 64, 112–117. doi:10.1016/j.apsoil.2012.11.007
Chao, A., Gotelli, N. J., Hsieh, T. C., Sander, E. L., Ma, K. H., Colwell, R. K., et al. (2014). Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67. doi:10.1890/13-0133.1
Chateil, C., Goldringer, I., Tarallo, L., Kerbiriou, C., Viol, I. L., Ponge, J.-F., et al. (2013). Crop genetic diversity benefits farmland biodiversity in cultivated fields. Agric. Ecosyst. and Environ. 171, 25–32. doi:10.1016/j.agee.2013.03.004
Chauvat, M., Perez, G., Hedde, M. l., and Lamy, I. (2014). Establishment of bioenergy crops on metal contaminated soils stimulates belowground fauna. Biomass Bioenergy 62, 207–211. doi:10.1016/j.biombioe.2014.01.042
Christiansen, K., and Bellinger, P. (1998). The collembola of north America, north of the rio grande. A taxonomic analysis. 2nd edn, 1520.
Colwell, R. K., Chao, A., Gotelli, N. J., Lin, S., Mao, C. X., Chazdon, R. L., et al. (2012). Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 5, 3–21. doi:10.1093/jpe/rtr044
Connor, R. F., Chmura, G. L., and Beecher, C. B. (2001). Carbon accumulation in Bay of Fundy salt marshes: implications for restoration of reclaimed marshes. Glob. Biogeochem. Cycles 15, 943–954. doi:10.1029/2000gb001346
Cornu, C. E., and Sadro, S. (2002). Physical and functional responses to experimental marsh surface elevation manipulation in Coos Bay’s South Slough. Restor. Ecol. 10, 474–486. doi:10.1046/j.1526-100x.2002.01035.x
Dou, Y., Chang, L., Zhang, B., and Wu, D. (2019). Effect of soybean cultivation on soil Collembola community in marshland of Sanjiang Plain, China. Russ. J. Ecol. 49, 570–576. doi:10.1134/s1067413618660013
Drexler, J. Z., Woo, I., Fuller, C. C., and Nakai, G. (2019). Carbon accumulation and vertical accretion in a restored versus historic salt marsh in southern Puget Sound, Washington, United States. Restor. Ecol. 27, 1117–1127. doi:10.1111/rec.12941
Eyers, S. E., and Chmura, G. L. (2007). Salt marsh vegetation recovery on the bay of fundy. Estuaries Coasts 30, 869–877. doi:10.1007/BF02841340
Filser, J., Mebes, K.-H., Winter, K., Lang, A., and Kampichler, C. (2002). Long-term dynamics and interrelationships of soil Collembola and microorganisms in an arable landscape following land use change. Geoderma 105, 201–221. doi:10.1016/s0016-7061(01)00104-5
Fitch, A. A., Blount, K., Reynolds, L., and Bridgham, S. D. (2022). Partial recovery of microbial function in restored coastal marshes of Oregon, USA. Soil Sci. Soc. Am. J. 86, 831–846. doi:10.1002/saj2.20383
Fjellberg, A. (1977). On the identity of Isotoma ekmani nom. nov. pro I. pallida Agrell, 1939 (nec Nicolet, 1842, Moniez, 1894) (Collembola, Isotomidae). Entomol. scand. 8, 9–11. doi:10.1163/187631277X00026
Fox, J., and Weisberg, S. (2019). An R companion to applied regression. Third edition. Thousand Oaks CA: Sage. Available at: https://socialsciencesmcmasterca/jfox/Books/Companion/.
Fuangarworn, M., and Lekprayoon, C. (2010). Adamystis thailandensis sp. nov. (Acari: prostigmata: Adamystidae), a new species of soil mites from Thailand with a key to world species of Adamystidae. Zootaxa 2649, 61–68. doi:10.11646/zootaxa.2649.1.3
Gao, M., Peng, C., Hu, Y., Liu, W., Ye, Y., Zheng, Y., et al. (2023). Composition and vertical distribution of agricultural soil macrofauna community after an extreme high temperature event in the summer of 2022. Ecol. Indic. 153, 110439. doi:10.1016/j.ecolind.2023.110439
Ge, B., Zhang, D., Cui, J., Zhang, H., Zhou, C., and Tang, B. (2014a). Biodiversity variations of soil macrofauna communities in forests in a reclaimed coast with different diked history. Pak. J. Zoology 46, 1053–1059.
Ge, B., Zhang, D., Liu, Q., Jiang, S., Cui, J., Zhou, C., et al. (2017). Influence of dike age on the distribution pattern of pill bug Armadillidium vulgare (Latreille, 1804) (Crustacea: isopoda) in the forests at a reclaimed coast. Pak. J. Zoology 49, 1273–1278. doi:10.17582/journal.pjz/2017.49.4.1273.1278
Ge, B., Zhang, D., Tang, B., and Zhou, C. (2014b). Effect of land cover on biodiversity and composition of a soil macrofauna community in a reclaimed coastal area at Yancheng, China. Turkish J. Zoology 38, 229–233. doi:10.3906/zoo-1302-37
Giordano, R., Weber, E., Darby, B. J., Soto-Adames, F. N., Murray, R. E., and Drizo, A. (2014). Invertebrates associated with a horizontal-flow, subsurface constructed wetland in a northern climate. Environ. Entomol. 43, 283–290. doi:10.1603/en13096
Goralczyk, K. (1998). Nematodes in a coastal dune succession: indicators of soil properties? Appl. Soil Ecol. 9, 465–469. doi:10.1016/s0929-1393(98)00106-1
Hazelden, J., and Boorman, L. A. (2001). Soils and ‘managed retreat’ in south east england. Soil Use Manag. 17, 150–154. doi:10.1079/sum200166
Hong, S.-K., Koh, C.-H., Harris, R. R., Kim, J.-E., Lee, J.-S., and Ihm, B.-S. (2010). Land use in Korean tidal wetlands: impacts and management strategies. Environ. Manag. 45, 1014–1026. doi:10.1007/s00267-006-0164-3
Hsieh, T. C., Ma, K. H., and Chao, A. (2024). iNEXT: iNterpolation and EXTrapolation for species diversity. R. package version 3.0.1. Available at: http://chao.stat.nthu.edu.tw/wordpress/software-download/.
Islam, M. A., Billah, M. M., Idris, M. H., Hussin, W. M. R. W., Bhuiyan, M. K. A., Sukeri, M. S. B. M., et al. (2024). Microbiota and soil fauna mediate litter decomposition and associated carbon and nitrogen dynamics in mangrove blue carbon ecosystems: insights from a coastal lagoon in Malaysia. Hydrobiologia 851, 2469–2486. doi:10.1007/s10750-024-05470-0
Janousek, C. N., Bailey, S. J., and Brophy, L. S. (2021). Early ecosystem development varies with elevation and pre-restoration land use/land cover in a pacific northwest tidal wetland restoration project. Estuaries Coasts 44, 13–29. doi:10.1007/s12237-020-00782-5
Krab, E. J., Monteux, S., Weedon, J. T., and Dorrepaal, E. (2019). Plant expansion drives bacteria and collembola communities under winter climate change in frost-affected tundra. Soil Biol. Biochem. 138, 107569. doi:10.1016/j.soilbio.2019.107569
Krediet, A. F., Ellers, J., and Berg, M. P. (2023). Collembola community contains larger species in frequently flooded soil. Pedobiologia 99-100, 150892. doi:10.1016/j.pedobi.2023.150892
Lai, J., Zou, Y., Zhang, J., and Peres-Neto, P. R. (2022). Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 13, 782–788. doi:10.1111/2041-210x.13800
Lee, B. H. (1977). A study on the Collembola fauna of Korea. The Family Isotomidae (Insecta), with description of five new species. Pac. Insects 17, 155–169.
Lee, B. H. (1974). Étude de la faune Coréenne des Insectes Collemboles III. Description de huit espèces nouvelles de Neanuridae et Onychiuridae. Paris: Bulletin du Muséum National d‗Histoire Naturelle 3 série 220, 573–598.
Li, H., and Shen, Y. (2011). Amount otherness of soil microorganism in three Halophyte plants wetland soils in coastal beach areas. Res. Agric. Mod. 32, 253–256. doi:10.3969/j.issn.1000-0275.2011.02.029
Li, J., Gao, Y., Li, C., Jin, Y., Yang, S., Xia, J., et al. (2023). Effects of species invasion and inundation on the Collembola community in coastal mudflat wetland from the perspective of functional traits. Insects 14, 210. doi:10.3390/insects14020210
Li, Y., Chen, Y., Xu, C., Xu, H., Zou, X., Chen, H. Y. H., et al. (2018). The abundance and community structure of soil arthropods in reclaimed coastal saline soil of managed poplar plantations. Geoderma 327, 130–137. doi:10.1016/j.geoderma.2018.05.004
Lim, M. H., and Park, K. H. (2011). New species of Desoria (collembola: isotomidae) from Korea. Entomological Res. 41, 95–97. doi:10.1111/j.1748-5967.2011.00325.x
Lima, E. C. A., and Zeppelini, D. (2015). First survey of Collembola (Hexapoda: entognatha) fauna in soil of archipelago Fernando de Noronha, Brazil. Fla. Entomol 98, 368–369. doi:10.1653/024.098.0161
Lima, E. C. A. d., Zeppelini, D., Ferreira, A. S., Brito, R. A. d., Oliveira, J. V. L. C. d., Medeiros, E. S. F., et al. (2023). Collembola biocenoses (Arthropoda: Hexapoda) in the archipelago of Fernando de Noronha, Brazil. Eur. J. Soil Biol. 117, 103496. doi:10.1016/j.ejsobi.2023.103496
Liu, Y., Wang, H., Wu, S., and Yue, Q. (2022). A Study of ecological restoration techniques in the reclamation area. J. Ocean Technol. 41, 112–120. doi:10.3969/j.issn.1003-2029.2022.04.012
Maceda-Veiga, A., Basas, H., Lanzaco, G., Sala, M., Sostoa, A. d., and Serra, A. (2016). Impacts of the invader giant reed (Arundo donax) on riparian habitats and ground arthropod communities. Biol. Invasions 18, 731–749. doi:10.1007/s10530-015-1044-7
Martínez, M. L., Pérez-Maqueo, O., Vázquez, G., Castillo-Campos, G., García-Franco, J., Mehltreter, K., et al. (2009). Effects of land use change on biodiversity and ecosystem services in tropical montane cloud forests of Mexico. For. Ecol. Manag. 258, 1856–1863. doi:10.1016/j.foreco.2009.02.023
Marx, M. T., Guhmann, P., and Decker, P. (2012). Adaptations and predispositions of different middle European arthropod taxa (Collembola, Araneae, Chilopoda, Diplopoda) to flooding and drought conditions. Animals 2, 564–590. doi:10.3390/ani2040564
Meyer, S., Kundel, D., Birkhofer, K., Fliessbach, A., and Scheu, S. (2021). Soil microarthropods respond differently to simulated drought in organic and conventional farming systems. Ecol. Evol. 11, 10369–10380. doi:10.1002/ece3.7839
Minchin, P. R. (1987). An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 69, 89–107. doi:10.1007/bf00038690
Monfils, M. J., Brown, P. W., Hayes, D. B., and Soulliere, G. J. (2015). Post-breeding and early migrant bird use and characteristics of diked and undiked coastal wetlands in Michigan, USA. Waterbirds 38, 373–386. doi:10.1675/063.038.0414
Oksanen, J., Simpson, G., Blanchet, F., Kindt, R., Legendre, P., Minchin, P., et al. (2024). Vegan: community ecology package_. R. package version 2.6-6.1. Available at: https://CRANR-projectorg/package=vegan.
Owojori, O. J., Reinecke, A. J., Voua-Otomo, P., and Reinecke, S. A. (2009). Comparative study of the effects of salinity on life-cycle parameters of four soil-dwelling species (Folsomia candida, Enchytraeus doerjesi, Eisenia fetida and Aporrectodea caliginosa). Pedobiologia 52, 351–360. doi:10.1016/j.pedobi.2008.12.002
Park, H. J., Kang, H. Y., Park, T. H., and Kang, C.-K. (2017). Comparative trophic structures of macrobenthic food web in two macrotidal wetlands with and without a dike on the temperate coast of Korea as revealed by stable isotopes. Mar. Environ. Res. 131, 134–145. doi:10.1016/j.marenvres.2017.09.018
Potapov, A., Bellini, B. C., Chown, S. L., Deharveng, L., Janssens, F., Kováč, Ľ., et al. (2020). Towards a global synthesis of Collembola knowledge – challenges and potential solutions. Soil Org. 92, 161–188. doi:10.25674/so92iss3pp161
Potapov, A. M., Guerra, C. A., Hoogen, J. v.d., Babenko, A., Bellini, B. C., Berg, M. P., et al. (2023). Globally invariant metabolism but densitydiversity mismatch in springtails. Nat. Commun. 14, 674. doi:10.1038/s41467-023-36216-6
Potapov, M. (2021). Synopses on Palaearctic Collembola: Isotomidae. Abhandlungen und Berichte des Naturkundemuseums Görlitz, 73.
Ryu, J., Choi, J.-W., Kang, S., Koh, C., and Huh, S. (1997). Temporal and spatial changes in the species composition and abundance of benthic polychaetes after the construction of Shihwa dike (west coast of Korea). J. Korean Soc. Oceanogr. 2, 101–109. doi:10.1016/j.chemgeo.2008.08.004
Salmon, S., Vittier, T., Barot, S., Ponge, J.-F., Assoula, F. B., Lusley, P., et al. (2021). Responses of Collembola communities to mixtures of wheat varieties: a trait-based approach. Pedobiologia 87-88, 150755. doi:10.1016/j.pedobi.2021.150755
Santoro, V. A., Carol, E., and Kandus, P. (2023). Vegetation changes in coastal wetlands of the outer estuary of the Río de la Plata as a result of anthropic-induced hydrological modifications. Sci. Total Environ. 866, 161325. doi:10.1016/j.scitotenv.2022.161325
Sato, S., and Kanazaw, A. T. (2004). Faunal change of bivalves in Ariake Sea after the construction of the dike for reclamation in Isahaya Bay western Kyushu, Japan. Fossils 76, 90–99. doi:10.14825/kaseki.76.0_90
Sereda, E., Wolters, V., and Birkhofer, K. (2015). Addition of crop residues affects a detritus-based food chain depending on litter type and farming system. Basic Appl. Ecol. 16, 746–754. doi:10.1016/j.baae.2015.07.005
Shaki, M. M., and Ahmed, S. (2015). Seasonal abundance of soil arthropods in relation to meteorological and edaphic factors in the agroecosystems of Faisalabad, Punjab, Pakistan. Int. J. Biometeorology 59, 605–616. doi:10.1007/s00484-014-0874-9
Shannon, C. E. (1948). A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. doi:10.1002/j.1538-7305.1948.tb01338.x
Sławska, M. (2000). Collembola communities in Sphagnum basin bogs and their importance to biodiversity of pine forest. Pedobiologia 44, 413–420. doi:10.1078/s0031-4056(04)70059-1
Sousaa, J. P., Gama, M. M. d., Pinto, C., Keating, A. n., Calhoa, F., Lemos, M., et al. (2004). Effects of land-use on Collembola diversity patterns in a Mediterranean landscape. Pedobiologia 48, 609–622. doi:10.1016/j.pedobi.2004.06.004
Spencer, K. L., Carr, S. J., Diggens, L. M., Tempest, J. A., Morris, M. A., and Harvey, G. L. (2017). The impact of pre-restoration land-use and disturbance on sediment structure, hydrology and the sediment geochemical environment in restored saltmarshes. Sci. Total Environ. 587-588, 47–58. doi:10.1016/j.scitotenv.2016.11.032
Stach, J. (1963). The Apterygotan fauna of Poland in relation to the world-fauna of this group of insects. Tribe: Entomobryini. Kraków: Państwowe Wydawnictwo Naukowe.
Steinberger, Y., Freckman, D., Parker, L., and Whitford, W. (1984). Effects of simulated rainfall and litter quantities on desert soil biota: nematodes and microarthropods. Pedobiologia 26, 267–274. doi:10.1016/s0031-4056(23)05981-4
Sterzynska, M., and Ehrnsberger, R. (2000). The distribution and diversity of Collembola in saltmarsh habitats of the German North Sea – a preliminary study. Pedobiologia 44, 402–412. doi:10.1078/s0031-4056(04)70058-x
Sun, X., and Wu, D. (2012). Two new species of the Genus Sensillonychiurus Pomorski et Sveenkova, 2006 (Collembola: Onychiuridae) from Changbai Mountains, China. Ann. Zool. 62, 563–570. doi:10.3161/000345412X659623
Tao, Y., Gu, W., Chen, J., Tao, J., Xu, Y. J., and Zhang, H. (2013). The influence of land use practices on earthworm communities in saline agriculture soils of the west coast region of China's Bohai Bay. Plant, Soil Environ. 59, 8–13. doi:10.17221/374/2012-pse
Team, R. C. (2024). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://wwwR-projectorg/.
Wang, S., Tan, Y., Fan, H., Ruan, H., and Zheng, A. (2015). Responses of soil microarthropods to inorganic and organic fertilizers in a poplar plantation in a coastal area of eastern China. Appl. Soil Ecol. 89, 69–75. doi:10.1016/j.apsoil.2015.01.004
Wei, Q., Zhou, Y., Xiao, N., Huang, J., Xiao, H., and Chen, H. (2022). Effects of three typical grass cultivation patterns on the community structure of soil mites in rocky desertification control area, Guizhou, China. Environ. Res. Commun. 4, 045008. doi:10.1088/2515-7620/ac6656
Wei, T., and Simko, V. (2021). R package corrplot: visualization of a correlation matrix. Available at: https://github.com/taiyun/corrplot.
Wiwatwitaya, D., and Takeda, H. (2005). Seasonal changes in soil arthropod abundance in the dry evergreen forest of north-east Thailand, with special reference to collembolan communities. Ecol. Res. 20, 59–70. doi:10.1007/s11284-004-0013-x
Xie, Z., Sun, X., Lux, J., Chen, T.-W., Potapov, M., Wu, D., et al. (2021). Drivers of Collembola assemblages along an altitudinal gradient in northeast China. Ecol. Evol. 12, e8559. doi:10.1002/ece3.8559
Xie, Z. J., Potapov, M., and Sun, X. (2019). Two new species of the genus tetracanthella (collembola: isotomidae) from China. Zootaxa 4585, 11–580. doi:10.11646/zootaxa.4585.3.11
Yahya, M., Afzal, M., Majeed, M. Z., Sarwar, I., Shehzad, K., Luqman, M., et al. (2020). Differential impact of land-use, season and soil characteristics on the abundance of edaphic springtails (insecta: collembola) and mites (arachnida: Acari). Pak. J. Zoology 52, 1483–1491. doi:10.17582/journal.pjz/20190817120809
Yan, F., Li, N., Yang, W., Qiao, Y., and An, S. (2017). Effects of reclamation on wetland waterbird populations, behaviors and habitats. Chin. J. Ecol. 36, 2045–2051. doi:10.13292/j.1000-4890.201707.003
Yang, K., Li, J., Xu, L., Yuan, Q., and Liu, Y. (2018). Spatial-temporal characteristics of flat reclamation in Zhejiang Province, China in the past 20 years. Mar. Sci. Bull. 20, 56–70.
Yin, W., Hu, S., Shen, Y., Ning, Y., Sun, X., Wu, J., et al. (1998). Pictorial keys to soil animals of China. Beijing: Science Press.
Yosii, R. (1977). Critical Check List of the Japanese Species of Collembola. Contr. Biol. Lab. Kyoto Univ. 25, 141–170.
Zhang, H., Sun, X., Liu, D., W, H., and Chen, H. (2021). Air warming and drainage influences soil microarthropod communities. Front. Ecol. Evol. 9, 731735. doi:10.3389/fevo.2021.731735
Keywords: coastal reclaimed wetland, coastal reclaimed farmland, drained and diked coastal mashes, wheat farmlands, springtail
Citation: Gao M, Liu Y, Xiong L, Qi M, Li X, Zheng Y, Liu J, Xie Z and Yan X (2025) Variations of collembolan communities in drained and diked salt marsh and adjacent farmland in coastal southeastern China. Front. Environ. Sci. 13:1517852. doi: 10.3389/fenvs.2025.1517852
Received: 28 October 2024; Accepted: 27 January 2025;
Published: 19 February 2025.
Edited by:
Mingxi Zhou, Nanjing Normal University, ChinaReviewed by:
Rentao Liu, Ningxia University, ChinaCopyright © 2025 Gao, Liu, Xiong, Qi, Li, Zheng, Liu, Xie and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijing Xie, eGllemhpamluZ0BuZW51LmVkdS5jbg==; Xiujuan Yan, amlud2VuX3NvaWxAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.