
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Environ. Sci. , 10 January 2025
Sec. Toxicology, Pollution and the Environment
Volume 12 - 2024 | https://doi.org/10.3389/fenvs.2024.1532169
This article is part of the Research Topic Advances in Soil Pollution Research: Risk Assessment and Ecosystems Management View all 5 articles
Mines are natural reservoirs of various minerals, metals, and metalloids. Several heavy metals (HMs), such as Pb, Cd, Cr, Cu, and Ni, are major anthropogenic pollutants that cause severe environmental pollution. The accumulation of these toxic HMs in soils has raised several concerns for crop growth, food safety, and marketing. Physiological and biochemical processes in plants are severely impacted by HMs, disrupting normal metabolic activities and reducing biomass production. Phytoremediation plays a pivotal role in addressing HM contamination by offering an eco-friendly, economical, and holistic solution. Similarly, arbuscular mycorrhizal fungi (AMF) play a significant role by forming a symbiotic relationship with plant roots. In this association, plants provide root exudates, while AMF enhance plant growth under heavy metal stress by supplying essential nutrients, minerals, and water. These fungi also improve nutrient status, soil quality, and ecosystem stability. The present review and meta-analysis encompass an examination of the global distribution of toxic HMs in mining-affected areas. Furthermore, the study highlights the role of various plant species and microbes, particularly AMF, in mitigating HM stress and its impact on plant growth and nutrition. The meta-analysis also evaluates the efficacy of AMF as a remediation strategy for HM-impacted mine soils.
Human-driven activities such as agriculture, mining, industrial processes, and the extensive use of fertilizers and pesticides have escalated the demand for land resources since the twentieth century (Cheng, 2016). Heavy metal pollution, desertification of land, ecological imbalance of land, soil erosion, land degradation, environmental damage, and decreased soil fertility are all major environmental factors that have severe effects on soil, water, and air (Nosrati and Collins, 2019; Vaverková et al., 2019). Heavy metal (HM) pollution is a global phenomenon. Metal mining and mineral ore processing have a dual effect on the economy and the environment. From one perspective, they provide economic benefits to the country, and simultaneously, they cause environmental pollution. Abundant and active mines are the primary source of toxic HMs. During the rainy season, due to heavy rainfall and strong winds, runoff water washes the toxic waste material into agricultural fields and surrounding water bodies, simultaneously causing air, water, and soil pollution. HM pollution has an irreversible, long-term residual effect and toxicity that poses an immense threat to living beings as well as the environment (Dhaliwal et al., 2020). Once these toxic HMs are released into the surrounding ecosystem, they could migrate to distant areas, accumulate in various biotic and abiotic components of the system, and adversely affect the food chain, human health, and the environment (Peralta-Videa et al., 2009). Lead (Pb), chromium (Cr), mercury (Hg), cadmium (Cd), and arsenic (As) have lethal effects on humans, plants, and animals. Depending on the concentration, a few metals, such as zinc (Zn), copper (Cu), manganese (Mn), and iron (Fe), have another role as essential micronutrients needed for metabolic activity (Schneegurt et al., 2001; Mohan et al., 2007). Heavy metal contamination significantly alters soil characteristics and the surrounding micro-environment. Microorganisms, serving as dynamic bio-indicators, respond to these changes through variations in microbial biomass, respiration rates, and enzyme activity under HM stress conditions (Hinojosa et al., 2005). Long-term or short-term exposure to various toxic HMs causes significant changes in physiological and ecological parameters, including a reduction in basal respiration, microbial biomass, and an increase in metabolic entropy (qCO2) (Crowley, 2008; Zhao et al., 2020). The degree of HM pollution has been evaluated by several indices, like a pollution load index (PLI) and a geo-accumulation index (Igeo) (Duncan et al., 2018; Hamad et al., 2019).
Factors such as plant life cycle, plant biomass, bioaccessibility, and bioavailability of HMs in soil can influence the metal removal process (Ali et al., 2013). Various physical and chemical methods are available for decontamination of toxic HMs and are usually cost-intensive. Given the limitations of conventional cleanup techniques, biological approaches could be considered a potential alternative mitigation option. In some places, bioremediation via phytoremediation of soils contaminated with organic or inorganic pollutants, such as pesticides and hydrocarbons, has become widely accepted. The popularity of bioremediation and phytoremediation for the reclamation of HM-polluted soils is growing even though it has substantial disadvantages due to its economic viability. The term phytoremediation is defined as a green, eco-friendly, low-cost, holistic approach to cleaning toxic contaminants from the environment by a plant-based system (Ali et al., 2013). Numerous phytoremediation projects have been carried out over the past few decades, and as a result, novel phytoremediation techniques, creative concepts, and research have emerged. Several phytoremediation projects have been done in the last few decades, and new phytoremediation strategies, innovative ideas, and research have evolved as a result. More than 500 plant species have been identified as potent HM hyperaccumulators (Ye et al., 2020). A long time span is required for plants to remediate a highly metal-contaminated area. Remediation through plant or phytoremediation is one of the most promising eco-friendly management strategies for reducing toxic contaminants (Burges et al., 2018).
In the past few decades, researchers have worked with various types of plants, their potentiality, and their remediation mechanism strategies for a better understanding of the phytoremediation process. Plants like Cymbopogon citrates (China et al., 2014), Helianthus petiolaris (Saran et al., 2020), Helianthus annuus (Lothe et al., 2016), Bryophyllum laetivirens (Li et al., 2020), Cordyline fruticosa (Herlina et al., 2020), etc., are widely used to remediate heavy metals (Pb, Cd, Cr, Cu, As), and their removal mechanisms have been extensively studied by several researchers in last few years. Vetiver grass (Vetiveria zizanioides) has been widely used for the rehabilitation of mine tailings in several countries like China and Australia. Vetiver is a perennial grass with a huge root system (3–4 m), 1–2 m tall, and non-invasive (Andra et al., 2009). Furthermore, vetiver grass has a strong symbiotic association in the rhizosphere region with a wide range of soil microbes, especially with arbuscular mycorrhizal (AM) fungi, which stipulates phytohormones and essential nutrients for plant development (Bahraminia et al., 2016). The most advantageous properties of mycorrhizal root colonization are an increase in the root surface area to enhance the phytoremediation/phytostabilization potential.
Numerous studies have focused on mining activities and heavy metal contamination, exploring their effects on soil, plants, water resources, ecosystems, and living organisms. Previous studies also examine bioremediation approaches, utilizing plants and microorganisms to mitigate the adverse impacts of HMs effectively. This review aims to provide a comprehensive overview of heavy metal pollution in agricultural soil caused by various mining activities and its associated environmental impacts. Through meta-analysis, the study assesses HM contamination and examines the global distribution of key pollutants, including Cr, Ni, Cd, Pb, and Cu, in mining-affected regions worldwide. This article also sheds light on the role of different plant species and microbes (especially AMF) in mitigating the HM stress condition while supporting plant growth and nutrient uptake. Additionally, through meta-analysis, the study evaluates the efficiency of AMF as a remediation strategy for mine-impacted soils contaminated with HMs such as Cd, Cu, Ni, and Pb.
According to the ancient Shamasastry, 1915, “Mines are a Nation’s treasury.” Mineral resources from mines are abundant in nature. The exploitation of these minerals enhances the world’s economy and development, but at the same time, surface mining, especially open-cast mining, causes severe environmental problems (i.e., loss of surface vegetation, destruction of soil structure, etc.). Mines are the source of various metals and minerals like iron and ferroalloys (Fe, Cr, Co, Mn, Mo, Ni, etc.), non-ferrous metals (Al, Sb, As, Bi, Cd, Cu, Pb, Hg, Li, Zn, etc.), precious metals (Au, Pd, Pt, and Ag), industrial minerals (perlite, sulfur, vermiculite, feldspar, graphite, gypsum, kaolin, etc.) and mineral fuels (uranium, petroleum, cooking coal, natural gas, etc.). China is the largest producer of total minerals, followed by the United States, Russia, Australia, and India (Reichl et al., 2020).
As a fossil fuel, coal is a predominant element in nature. It is mainly composed of carbon with variable amounts of other elements, including hydrogen, oxygen, nitrogen, and sulfur. China is the largest producer of coal. Open-cast mining generates toxic overburden dumps (OB) and coal dust that contain enormous amounts of toxic HMs and are responsible for metal contamination in adjacent agricultural land (Li et al., 2007). In descending order, metals Fe > Mn > Zn > Cu are present in coal mines: the most bioavailable and mobile element is Mn, followed by Zn and Cu, and the least mobile metal is Fe. The reason behind Fe’s lesser mobility is the residual fraction of Fe, which indicates its strong affinity toward minerals, the solid matrix, and strongly bounded clay minerals (Kartal et al., 2006). A pseudo-total concentration of HMs, including Zn (314 mg kg−1), Mn (132 mg kg−1), Pb (82 mg kg−1), Cu (45 mg kg−1), and Co (34 mg kg−1), has been found in reclaimed mine soil (RMS). The bioavailable forms (DTPA-extractable) of Zn, Mn, and Cu are significantly higher in RMS than in control soil. Pb can selectively accumulate in leaves, stem bark, and root bark, whereas Zn and Mn accumulate in leaves, and Cu accumulates in stem wood and root wood. These indicate that the accumulation of metals might be tissue specific (Maiti et al., 2016). The most effective remediation pathway for OB dumps is trees, which can accumulate toxic HMs from OB. Trees that are used for the reclamation of OB dumps should be drought resistant, woody, fast-growing, and able to grow in arid areas and nutrient-deficient areas (Pratas et al., 2005). Various woody plant species, such as A. auriculiformis, M. azedarach, Leucaena leucocephala (Lam.) de Wit, Tectona grandis L. f., Gmelina arborea Roxb., Acacia mangium Wild., Bambusa arundanacea L., Cassia siamea Lam, and Azadirachta indica A. Juss, etc., are used for reclamation of coal OB dumps (Maiti et al., 2016). The Pb concentration in A. auriculiformis, hybrid eucalyptus trees, is significantly higher in root bark than leaf tissue as bark tissue can accumulate lead for longer time while leaves are shed periodically (Sawidis et al., 2011). Through bark exudates, Pb can be removed from plants, which is an important defense mechanism against HM toxicity (Alloway, 2012). In the case of Cu (BCF >1; TFleaf, TFstembark, and TFstem wood <1), two tree species, A. auriculiformis and M. azedarach, might be used for Cu phytostabilization (Sawidis et al., 2011).
Cu mines are a prime source of potentially toxic HMs (Cu, Zn, As, Cd, and Pb) (Cai et al., 2015). South Africa, Chile, and Peru are the largest producers of copper. Due to the chemical weathering process, waste rocks from the Predra Verde mine (Brazil) show potential risks to the environment (Perlatti et al., 2021). In Jiuhuashan, Jiangsu Province, and in eastern China, agricultural soil near abandoned mines contained high levels of copper (816.8 mg kg−1 and 147 mg kg−1, respectively) contamination (Qin et al., 2012; Wu et al., 2011). Acidic drainage compounds (Cu, Zn, and Fe) have been produced from the La Concordia Mine (Argentina) (Nieva et al., 2018). According to China et al. (2014), of environmentally available metal, that is, total metal excluding the silicate matrix-bound metals, Cu (154 mg kg−1) is one of the most abundant heavy metals found in the Mosabani copper mine (India), followed by Ni (136 mg kg−1) and Pb (9.9 mg kg−1). The underground tissues of this plant have an average concentration of 1959 mg kg−1 Cu, which is much higher than shoot (124 mg kg−1 Cu). Therefore, it indicates that HM mobility is limited inside the plant as the translocation factor for Cu (0.06), Ni (0.36), Co (0.68), and Zn (0.24) is less than 1, while Mn is present in higher concentration in the above-ground tissue (TF > 1; Mn 1.37) (Das and Maiti, 2007). The toxicity level of Cu and Ni in plants is 20–100 mg kg-−1 and 10–100 mg kg−1, respectively (Kabata-Pendias, 2011). The bioavailability of copper also depends on soil pH, soil cation exchange capacity (CEC), and total copper content in soil (Bravin et al., 2009; Brun et al., 2001). Copper also plays an essential role in plant growth and development processes such as protein synthesis, CO2 assimilation, ATP synthesis, maintaining homeostasis within chloroplast, photosynthesis, etc. (Hänsch and Mendel, 2009; Yruela, 2013). A high concentration of copper has a toxic effect on seed germination, decreases plant height, causes chlorosis of plant leaves, and reduces plant biomass and grain yield (Adrees et al., 2015).
Ferrochromium is the only natural and economical resource of chromium produced in chromite mines by carbothermic smelting (Beukes et al., 2010). It is a crystalline alloy generally composed of chromium and iron compounds. Globally, South Africa has the most chromite ores, followed by Kazakhstan, India, Albania, and Turkey (ICDA, 2022). The active and abandoned mine wastes are reservoirs of heavy metals that have lethal effects on water, soil, and living beings (Fernández-Caliani et al., 2009). These mine wastes are generally composed of different types of toxic HMs, mainly chromium (Cr) and Nickel (Ni), along with other metals such as Cu, Cd, Pb, Ni, and Mn present in lesser quantities (Bueno et al., 2009). Chromium (Cr) is generally utilized in industrial activities such as the processing and finishing of leather, the production of refractory steel by the stainless steel industry, electroplating cleaning agents, drilling muds, the production of chromic acid and other chemicals, and food preservation (Shanker et al., 2005). Chromium exhibits different levels of toxicity depending on its chemical form, pH, reaction with other elements, and solubility index (Thatoi et al., 2014). Cr (VI) exhibits high toxicity and bioavailability due to its better solubility than Cr (III) (Abyaneh and Fazaelipoor, 2016). A lack of Cr (III) in human and animal diets can cause metabolic deterioration, cardiac problems, and diabetes, but an excess presence in the body has harmful health effects (WHO, 2000). Hexavalent chromium tends to act as a strong oxidizing agent; therefore, Cr (VI) is 10–100 times more toxic than Cr (III) (Zayed et al., 1998). The toxicity level of hexavalent chromium for plants in solution is as low as 0.5 mg kg−1 and 5 mg kg−1 for soil (Turner and Rust, 1971). Highly carcinogenic chromium and asbestos exposure may lead to cancer, mesothelioma, pneumoconiosis, skin irritations, and other respiratory problems such as irritation of the larynx and pharynx, edema, coughing, asthma, etc. (Bloise et al., 2008; Pugnaloni et al., 2013). Cr is mostly accumulated in plant roots rather than in the shoot due to its reduced mobility in root vacuoles. However, Ni accumulation is higher in the shoot than in the root due to greater mobility of Ni through xylem tissue (Pulford et al., 2001; Shanker et al., 2005). The Cr and Ni concentration in the Roro chromite mine waste soil is 3,120 mg kg−1 and 1,620 mg kg−1, respectively, which exceeds the safety level (Cr:75–100 mg kg−1; Ni: 100 mg kg−1) of metals present in soil (IS, 1993). In similar studies in the Almadén mine site in Spain and southern Togo mine sites, Cr and Ni concentrations are 86–35 Cr mg kg−1 and 21.2–126 Ni mg kg−1 and 182–1,029 Cr mg kg−1 and 15–432 Ni mg kg−1, respectively, due to deposition of mine tailings in agricultural soil (Gnandi and Tobschall, 2002; Bueno et al., 2009). In the Daduk mine area of Korea, due to the dispersion of metals from tailings and watercourses, various toxic metals are reported in nearby paddy fields (Lee et al., 2001). In another study in the Co Dinh mine of Vietnam, high levels of potentially toxic elements are also detected in rice fields (5,750 Cr mg kg−1, 375 Co mg kg−1, and 5,590 Ni mg kg−1) (Kien et al., 2010). Based on the dynamic translocation factor (TF dyn>1), Cr and Ni accumulation is higher in plant parts of Oryza sativa growing in contaminated agricultural fields that might have a potential risk of transfer of toxic HMs to livestock or humans (Kien et al., 2010; Kumar and Maiti, 2014).
Globally, the production of crude steel has expanded drastically since 2000 to meet increasing demand. Iron ores are the backbone of the world economy (World Steel Association, 2021). Australia, Brazil, and China are the top three countries for the production of iron (around 69%) (Holmes et al., 2022). To maintain the growing demand, iron ore industries have continuously increased mining activity. As a result, huge concentrations of Cd, Mn, As, Ni, Pb, Zn, and Cr have been found in the agricultural soil near iron mines (Hosseini et al., 2018). Toxic tailing wastes from iron mining are dumped into a tailings pond located at the Noamundi–Jodda belt, India. As a result, during monsoon season, the toxic fine particles washed off by heavy wind and rain are deposited into nearby water bodies and soil, thereby causing air, water, and soil pollution. Although Fe is essential for the synthesis of chlorophyll, chloroplast structure and its functions, excessive concentrations of iron might enter into the food chain and show toxic effects on plant, animal, and human health (Maiti et al., 2005). According to Dhatrak et al. (2017), the health status of mine workers and a nearby population around an open-cast iron mine showed noise-induced hearing loss (NIHL) and anemia as major health effects. Iron mining plays an important role in economic development and simultaneously causes air pollution by blasting, drilling, unloading, and loading minerals and overburdens by wind at mineral handling plants, workshops, etc. These air pollutants affect the flora and fauna of the local environment (Tripathi et al., 2014; Chaturvedi and Patra, 2016). Some native plant species, such as Cassia sophera Eupatorium odoratum, Techtona grandis, Alstonia scholaris, Cassia tora, etc., can be found in Fe tailings. Techtona grandis can accumulate a higher concentration of HMs than Alstonia scholaris, but these HMs have not shown any detrimental effect on native plants. Rather, higher Fe content promotes lavish growth, as stated by Maiti et al. (2005).
Worldwide, Kazakhstan is the largest producer of uranium, followed by Australia, Namibia, Uzbekistan, and Canada. Jaduguda, India’s first, oldest, and most productive underground uranium (U) mine, consists of uraninite and other associated accessory minerals such as copper, nickel, arsenic, cobalt molybdenum, and magnetite, etc. (Sethy et al., 2014). Uranium occurs naturally in the earth’s crust with a mean concentration of approximately 3 mg kg−1 (Gupta and Singh, 2003). Hexavalent U is the most soluble form and is present as the uranyl cation (UO2)+2 in 80%–90% of the soil. It prevails in solutions predominantly as a stable ion (UO2)+2 and as soluble carbonate complexes, that is, UO2CO3, UO2(CO3)2−2, UO2(CO3)3–4, (UO2)2CO3(OH)−3 and (UO2)3(CO3)6–6. In the absence of dissolved inorganic ligands (fluoride, carbonate, sulfate, and phosphate) and a pH range within 4–7.5, the hydrolysis ion UO2OH+ in water and soil forms complexes with these inorganic ligands. As a result, these complexes increase the total solubility of U (Shahandeh and Hossner, 2002). Uranium is a radioactive element that undergoes a continuous decaying process, emits alpha (α), beta (β), and gamma (γ) rays, and produces various isotopes. This transformation stops when the stable product lead (206Pb) is formed (Sarangi, 2003). The radiation that is emitted from these naturally occurring isotopes is very low and does not penetrate due to its high density, which acts as a shield against its own radiation (Wang et al., 2009). According to WHO (2012), a mean concentration of U in ambient air has been reported to be approximately 0.02 ng m−3 in Tokyo, Japan, and 0.076 ng m−3 in New York City, United States of America. Uranium enters the kidney through water or food, and the uranyl ion forms bicarbonate and citrate complexes in blood plasma and affects the proximal tubules of the kidney, causing tubular degeneration, liver damage, genetic malfunction, cancer, and necrosis (Miller et al., 2004; Sethy et al., 2011). The Environmental Protection Agency (EPA) of the United States has categorized U as a carcinogenic element, and in drinking water, the maximum contaminant level (MCL) of U is 30 µg L−1 (EPA, 1999). The proposed interim maximum acceptable level (IMAC) of U in Canada is 20 µg L−1, whereas WHO strictly recommended the permissible level to be 2 µg L−1 (Shin et al., 2002). In humans, the ingestion and intake dose is very low (2 µSv.Y−1), which is far below the WHO permissible level (100 uSv.Y−1). The mean metal pollution index (MPI) value indicates the overall pollution level in ground and surface water to be below the maximum threshold value of 100 (Mohan et al., 1996). Several experiments have been conducted for the accumulation of U in native plant species to determine the mechanism of U uptake absorption by plants and for biological exploration of U from soil (Petrova, 2006). Uranium accumulation varies depending upon plant species as well as genotypes, lines within species, and cultivars; moreover, U is accumulated higher in the root portion than in the shoot. Therefore, only a small portion is translocated to the shoot. Less than 1 mg kg−1 of U is found to be toxic in the soil (Sheppard et al., 1992; Stojanović et al., 2010). According to (Pentyala and Eapen, 2020), Vetiveria zizanioides L. Nash showed good ability for phytoextraction (84%–95% of recovery) of U from hydroponic solution at a concentration below 200 ppm under controlled experimental conditions. U is generally restricted in the root portion of vetiver, but at concentrations above 1,000 ppm, it is translocated from the root to the shoot.
The main aim of the meta-analysis was to compare a selected number of peer-reviewed articles and to determine the potential risk of toxic metals on soil health using global datasets. We searched literature published in the Web of Science database between 2012 and 2022 and selected research according to our objectives. The keywords were “Mine,” “pollution,” “Copper,” “Cadmium,” “Nickel,” “Chromium,” “Lead,” and “World.” From >2,500 published reports, we excluded studies based on data originality. We screened the remaining articles from different origins depending on the title and abstract. From the vast range of published articles, the research papers were selected based on the manuscripts reporting metal toxicity due to mining activity across different types of mines, and proper analytical methods were followed. Finally, based on the inclusion criteria, 55, 41, 98, 52, and 51 research papers were considered for Cr, Ni, Pb, Cd, and Cu, respectively. The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) flowchart is depicted in Supplementary Figure S3.
From the literature survey, we considered parameters like standard error, sample size, and the difference between the tested and control means. The effect size or outcomes were calculated by the mean difference between the maximum concentration of metals (Cr, Ni, Pb, Cd, and Cu) and the permissible limit of metals in mine areas. The maximum permissible limit of metals in soil (Cr, Ni, Pb, and Cu) was that suggested by World Health Organization, 1996 (Cu: 36 mg kg−1, Ni: 35 mg kg−1, Cr: 100 mg kg−1, and Pb: 85 mg kg−1). The result was expressed on mean difference as a continuous factor for statistical analysis at the 95% confidence level (CI) between the group of individual studies and the permissible limit of metals (Cr, Ni, Pb, Cd, and Cu) in the mine areas. Then, from the random effect model (RE), forest plots were designed to summarize all the study information of individual research work, and this plot simultaneously provides a visual representation of heterogeneities. The vertical line in the middle of the forest plot, commonly known as the zero-effect line, shows that there was no difference between the study group mean and the permissible limit. This mean difference is zero at this point.
From Figures 1, 2 it can be observed from the RE model that the overall mean value for Cr was 0.16 (CIs: 0.14–0.17) and for Ni, it was 0.19 (CIs: 0.16–0.22), statistically significant with p < 0.05 and inconsistency indexes (I2) of 98.58% and 96.05%, respectively, which represented substantial heterogeneity [43,49,63,68, 84–87]. Similarly, for Cd, Pb, and Cu (Figures 3–5) from the RE model, the overall mean values were 0.01 (CIs: 0.01–0.01), 0.06 (CIs: 0.06–0.07), and 0.08 (CIs: 0.07–0.09), respectively, which are statistical significance at p < 0.05. The overall inconsistency indexes (I2) of Cd, Pb, and Cu were 47.34%, 99.24%, and 98.02%, respectively, indicating substantial heterogeneity. The positive value indicated that the total concentrations of Cr, Ni, Cd, Pb, and Cu in mine areas were higher than the permissible level recommended by WHO. In a meta-analysis of the summary means, most metals (Cr, Ni, Pb, and Cu) present in mine areas were found to be significant at the p < 0.05 level, as the confidence intervals did not overlap with the zero-effect line except for Cd. Various factors like runoff water and aerial deposition from mines lead to the contamination of nearby agricultural lands, water bodies, etc. As a result, the presence of higher concentrations of metals in different mine areas may influence the potential risk of metal toxicity and its relative risk to the ecosystem (Qu et al., 2012; Chen et al., 2017; Liu et al., 2019; Sun et al., 2018).
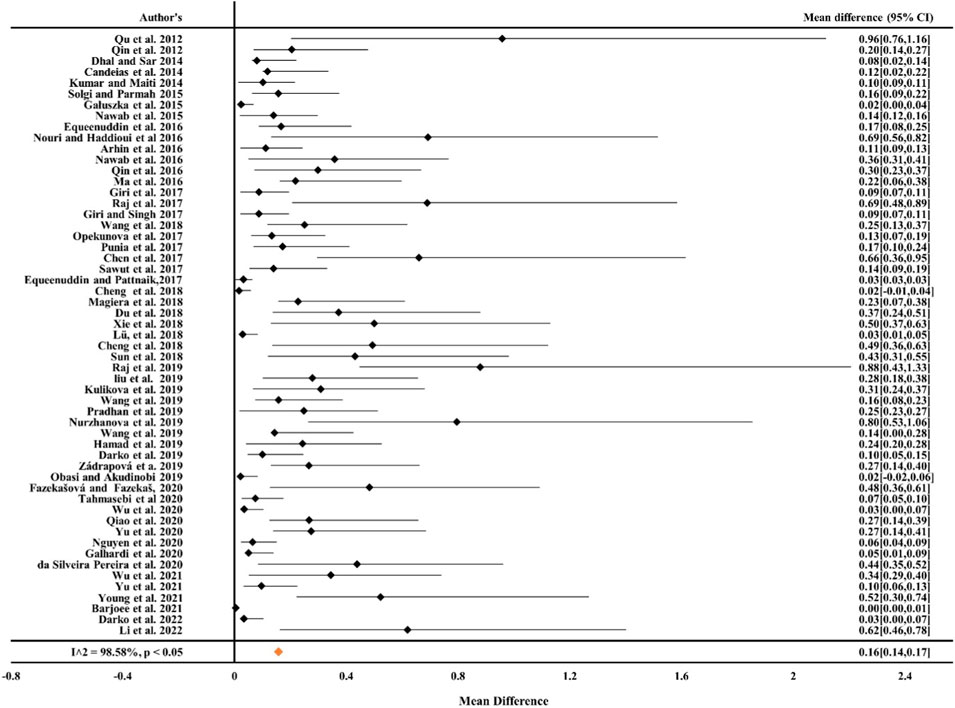
Figure 1. Forest plot indicating the mean difference of individual observations regarding Cr contamination in mine areas.
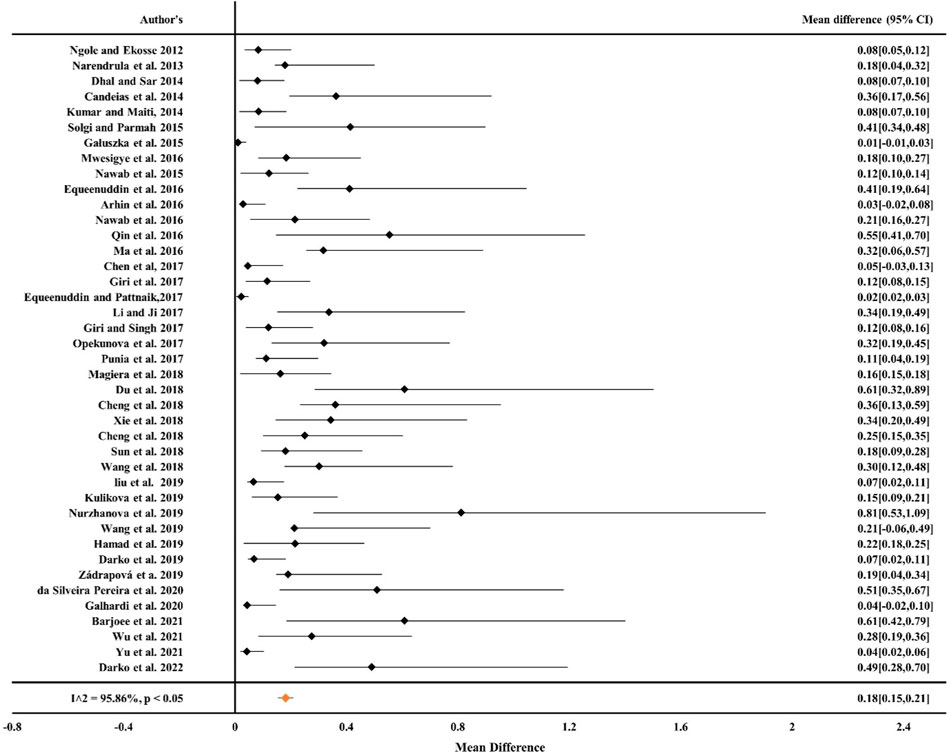
Figure 2. Forest plot indicating the mean difference of individual observations regarding Ni contamination in mine areas.
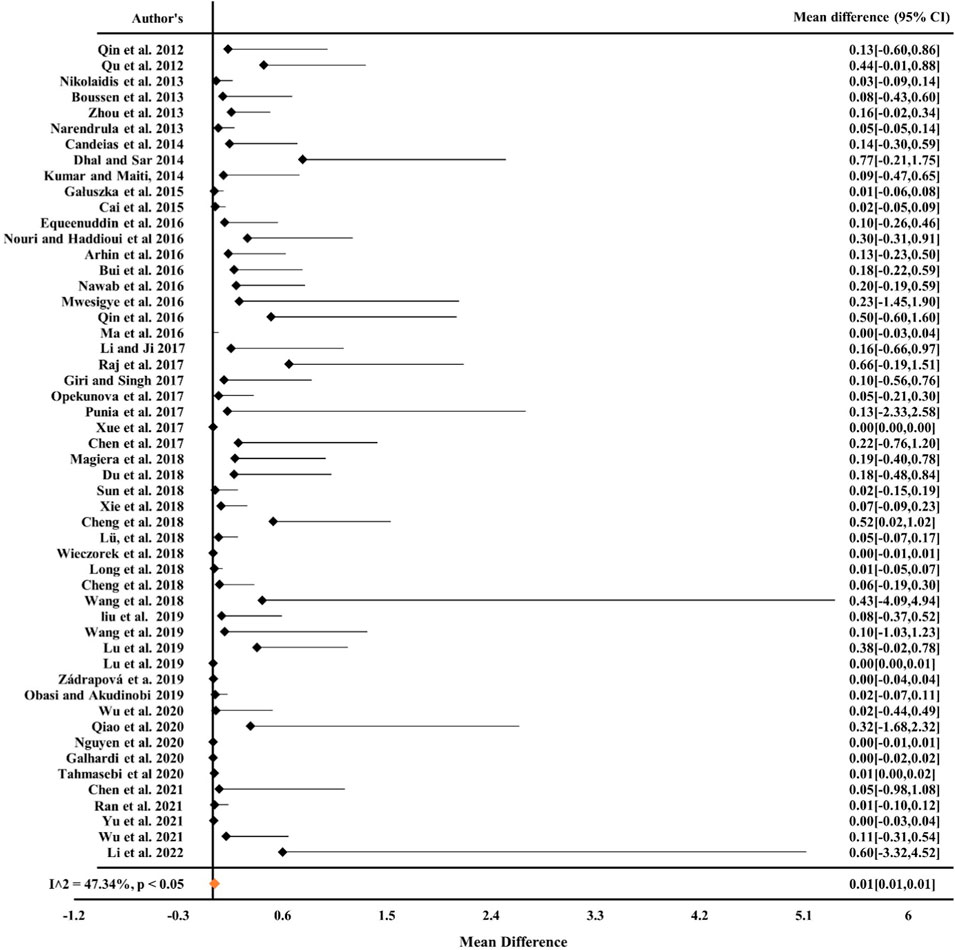
Figure 3. Forest plot indicating the mean difference of individual observations regarding Cd contamination in mine areas.
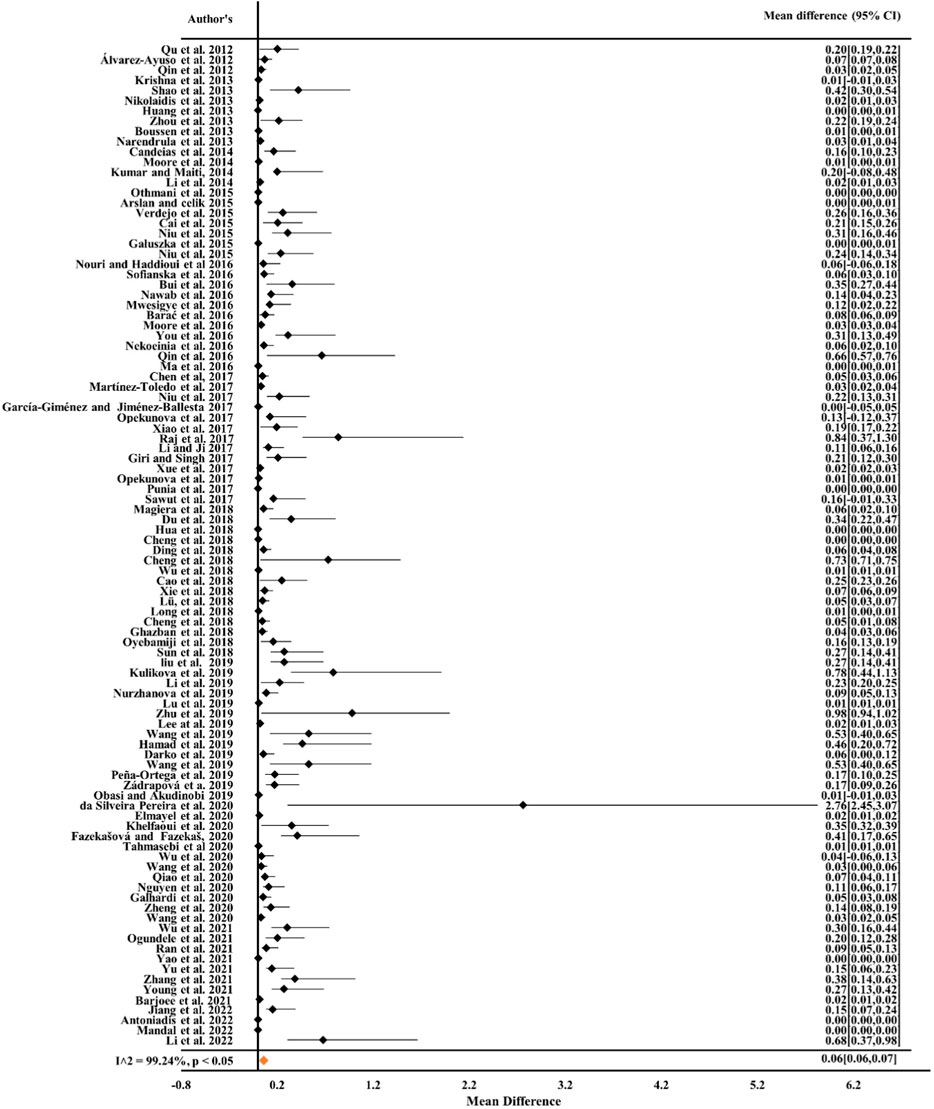
Figure 4. Forest plot indicating the mean difference of individual observations regarding Pb contamination in mine areas.
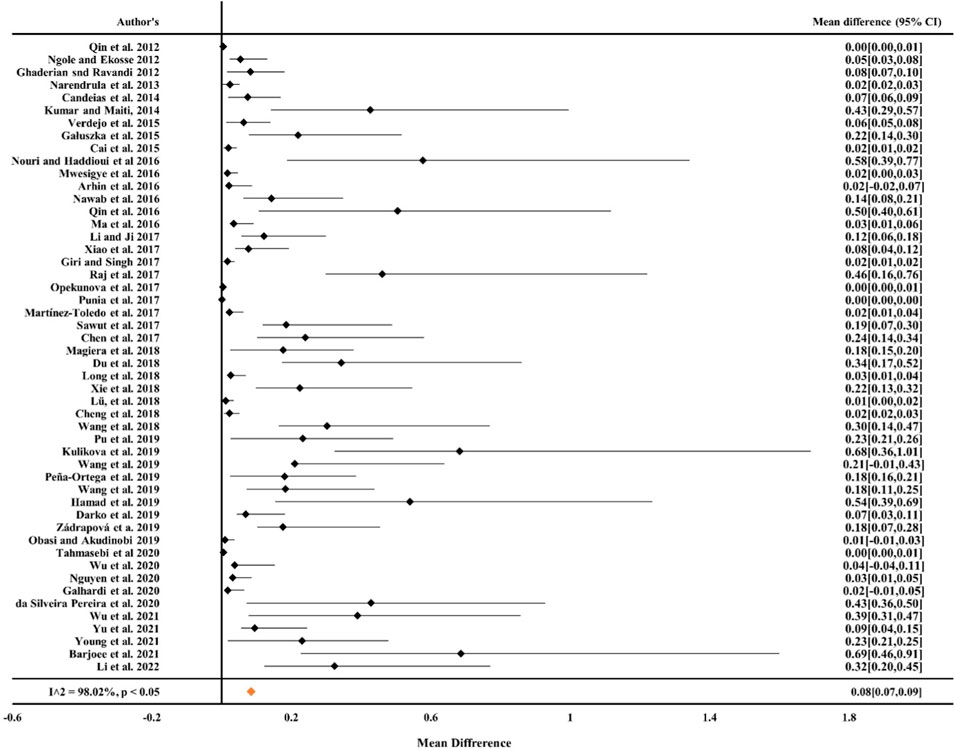
Figure 5. Forest plot indicating the mean difference of individual observations regarding Cu contamination in mine areas.
Worldwide, the immense development in industrial sectors, especially mining, metal, energy supply, agriculture, chemical production, and transport, causes significant pollution of the ecosystem. Globally, heavy metal contamination is a problem for the environment as well as for living beings (Sun et al., 2012; Cachada et al., 2018). As we know, remediation of heavy metals is more complicated than remediation of other organic pollutants. Various traditional, mechanical, and chemical techniques, including electrochemical treatments, thermal methods, incineration, excavation, vitrification, chemical oxidation, and solvent extraction, are widely utilized to remove or destroy these toxic HMs in soil. However, these methods are often costly, time-intensive, and labor-demanding. Moreover, they can lead to soil degradation and generate secondary waste materials, posing additional environmental management challenges (Khan et al., 2018). Bio-remediation techniques have gained attention due to their cost-effectiveness, viability, no generation of secondary waste, and eco-friendly (Akande et al., 2018; ALAM et al., 2018). These techniques include plants and various microbes (bacteria, fungi, mycorrhiza, etc.) that are utilized to decontaminate the hazardous compounds from soil. For soil purification, applications of plants alone or in association with microorganisms help to stabilize, mineralize, transfer, and remove toxic metals (Wang et al., 2018).
The term “phytoremediation” is derived from Greek (“phyton”) and Latin (“remedium”), which means “plant” and “to correct,” respectively (Cunningham et al., 1996). Phytoremediation is a bioremediation process in which plants (alone or in association with microbes) are used as purifying agents to remove, stabilize, or destroy the toxic metals from air, water, and soil in an eco-friendly manner (Wani et al., 2012). Supplementary Table S1 shows different mechanisms of phytoremediation, and Supplementary Figure S2 shows images of such mechanisms. Generally, plants can extract essential nutrients (Fe, Zn, Ni, Mn, and Cu) as well as non-essential metals (Cr, Cd, As, Pb, and Hg) that are not required in their physiological process and can store an enormous amount of the toxic metals (hyper-accumulator) in their parts from contaminated soil and water (Tangahu et al., 2011). Several studies have been done regarding different mechanisms of phytoremediation strategies, as shown in Supplementary Table S2. The advantages of phytoremediation are as follows: 1) inexpensive technology (60%–80% lesser than traditional process); 2) minimize soil deterioration; 3) solar-driven remediation process; 4) no generation of secondary hazardous compounds; 5) suitable and broad-spectrum treatment; 6) sustainable and environment-friendly technique. One limitation is that plants require time for their growth and development (Morikawa and Erkin, 2003). Techniques like phytoextraction and phytostabilization are commonly used for remediating HM-polluted sites. Several plant species have demonstrated the ability to absorb, bioaccumulate, immobilize, and degrade heavy metals (HMs) from contaminated sites. Some examples include C. citrates, H. petiolaris, V. zizanioides L, Pennisetum purpureum cv. Mott, Conocarpus lancifolius, and Cordyline fruticose, etc. (Andra et al., 2009; China et al., 2014; Herlina et al., 2020; Rasheed et al., 2020; Kowitwiwat and Sampanpanish, 2020; Saran et al., 2020). These species are key in cleaning contaminated soils either by extracting HMs into their tissues or stabilizing them in the soil. Vegetation helps limit pollutant transport, reduce wind dispersion, and prevent water erosion (Perronnet et al., 2000). Unlike conventional methods that disturb soil physical properties, phyto-strategies maintain and enhance soil quality. Successful phytoremediation implementation requires considerations of biomass production, heavy metal concentration in plant material, and the time needed for soil remediation (Robinson et al., 1998). Phytodegradation involves the uptake of toxic compounds by plants, where plant enzymes break down these substances into less harmful forms (Sun et al., 2012; Hamdi et al., 2012). Plant species like Arabidopsis thaliana and Azolla filiculoides are used for phytodegradation of pollutants such as 2,4-DNT and bisphenol A in the United States and Iran (Yoon et al., 2008; Zazouli et al., 2014). Phytovolatilization occurs when plants transform the contaminant into volatile compounds and emit them into the atmosphere through transpiration or radial diffusion from their leaves, stems, and roots (Limmer and Burken, 2016; Peter et al., 2017). Rhizodegradation is the breakdown of contaminants facilitated by rhizospheric microorganisms, where root-released enzymes and exudates help decompose pollutants into non-toxic forms (Jia et al., 2016; Gkorezis et al., 2016). Plants like Pteris vittata (Sakakibara et al., 2010) and Salicornia bigelovii (Shrestha et al., 2006) are involved in phytovolatilization of arsenic and selenium in Japan and the United States, respectively. The efficacy of phytoremediation depends on selecting appropriate plant species and various environmental factors. Overall, phytoremediation is a complex process involving multiple plant mechanisms. Understanding these processes can enhance plant adaptation to metal stress and improve efficiency, providing sustainable solutions for heavy metal contamination and ecosystem restoration.
Naturally, plants interact constantly with many microorganisms in their rhizospheric region. Beneficial microorganisms, especially arbuscular mycorrhizal fungi (AMF), have a symbiotic association with plant roots where AMF increase plant nutrient uptake ability, improve biomass accumulation, amplify photosynthesis capacity, and provide protection against heavy metal toxicity. Successively, the plant provides exudates of amino acids, carbon, and photosynthetic products to the AMF for growth and development (Mitra et al., 2020).
Around 80% of terrestrial plants and 90% of agricultural plants have mycorrhizal associations in their roots, where fungal hyphae enter the cortical cells of plant roots, forming vesicles, hyphae, and arbuscles (Smith and Read, 2010). Supplementary Figure S1 denotes the schematic diagram of the heavy metal detoxification mechanism through AMF. AMF help immobilize heavy metals by binding them at the cortical region, preventing translocation to the upper ground part (shoot, stem, leaves), and preventing damage to leaves. Plants are categorized based on TF value into hyper-accumulators (TF > 1) and non-hyperaccumulators (TF < 1). The translocation factor (TF) is higher in non-mycorrhizal-associated plants than in mycorrhizal-associated plants (Arshad et al., 2008). Endomycorrhizal fungi AMF belong to the phylum Glomeromycota. They are considered an eco-friendly, sustainable strategy to enhance plant growth, increase shoot biomass, improve soil health and water uptake capacity, provide protection to the plant against biotic and abiotic stress, and detoxify heavy metal-induced stress (Mishra et al., 2019). Glomeromycota are obligate symbiotic organisms, so they require around 20% of carbon from host plant cells for their survival. Simultaneously, they provide water and nutrients (P, N) through their arbuscles and intracellular and extracellular hyphae to the host plant (Parniske, 2008). AMF combat heavy metal stress by immobilization, precipitation, chelation, and sequestration in the rhizosphere and vacuoles and activate the plant anti-oxidant defense system (Mitra et al., 2020). Another AMF defense mechanism is to secrete a hydrophobic unique glycoprotein called glomalin, which is composed of carbon (39%–59%), phosphorus (0.03%–0.1%), nitrogen (3%–5%), hydrogen (4%–6%), oxygen (33%–49%), and a trace amount of iron (Schindler et al., 2007; Zhang et al., 2017). This protein is basically an N-linked glycoprotein produced from the spores and hyphae of AMF, which helps in soil aggregation, cellular function, toxic heavy metal stress, carbon storage, etc. (Emran et al., 2012; Wu et al., 2015). Easily extractable glomalin-related soil protein (EE-GRSP) and total glomalin-related soil protein (T-GRSP) are both readily quantified from the soil with the help of a citrate buffer (Wright and Upadhyaya, 1996).
For plant growth and nutrition, AMF increase the surface area with the help of extracellular and intracellular hyphae for better absorption of nutrients, water, and the ions that are generally present in an immobilized form in soil. AMF also improve the plant's ability to acquire nutrients from the depleted zone of the rhizosphere (Smith and Read, 2010; Smith and Smith, 2011). As stated by Nakmee et al. (2016), native species of AMF Glomus aggregatum, Acaulospora scrobiculata, and F. mosseae provide positive effect on plant nutrient uptake (total N, P, K) and enhance plant biomass, leaf number, and plant height of sorghum. It was observed that those wheat plants inoculated with AMF culture (F. mosseae and R. intraradices) contain a 1.13–2.76 times higher concentration of Zn than non-inoculated wheat plants (Coccina et al., 2019). Various abiotic stresses include salinity, heavy metals, drought, flooding, extreme temperature, etc. AMF communities independently withstand these unfavorable stress conditions for their host plants, provide sufficient water in drought stress, supply nutrients (phosphorus), and balance osmotic pressure in flooding stress conditions (Zhu et al., 2017; Caradonia et al., 2019). Under drought conditions, tomato plants containing AMF (R. intraradices) colonized on their roots provide sufficient water-based nutrients to the tomato plant for better growth during water-stress situations. Inoculation with G. etunicatum enhances total chlorophyll content, root-shoot height-weight, increased N, P, K, Ca, Zn, Cu concentration, flavonoid content, soluble sugar, proline, glycine betaine, polyamine, POD, and CAT activity in Pistaciavera L under stress conditions (Abbaspour et al., 2012). Studies revealed that G. etunicatum F. mosseae and R. irregularis increased the growth and grain yield of Triticum aestivum L., regulate nutrient uptake capacity, and decreased Na+ and Cl-concentration at times of salinity stress (Daei et al., 2009). According to Hashem et al. (2016), oxidative stress generates a high concentration of malonaldehyde and hydrogen peroxide in Solanum lycopersicum L. AMF strains (Glomus mosseae, Glomus intraradices, and Glomus etunicatum) help to decrease the concentration of these elements and boost the plant’s defense system against Cd stress. AMF also provide protection against biotic stress. Various pathogens, such as root-rot fungi, pathogenic bacteria, nematodes, and other harmful microorganisms, can cause various diseases. However, the presence of AMF significantly reduce pathogen-induced damage and infection by enhancing nutrient availability, stimulating root growth, and improving root morphology. AMF secrete beneficial enzymes in the rhizosphere, strengthening plant defenses and enabling plants to better withstand biotic stress (Vos et al., 2012; Spagnoletti et al., 2020). Fusarium wilt causes damage to Cicer arietinum L, but treatment with an AMF strain (Glomus hoi) provides protection against wilt disease and increases the nitrogen and phosphate content in treated plants as compared with non-treated plants (Singh et al., 2010). Similarly, Glomus sp. synthesizes antimicrobial compounds that help to arrest the mycelia growth of Fusarium oxysporum on L. esculentum plants and increase the chlorophyll, N, P, and K content of the plants. Furthermore, in Capsicum annum, Glomus sp. reduces the activity of the pathogen Pythium aphanidermatum and provides better yield of the plant (Kumari and Prabina, 2019; Kumari and Srimeena, 2019).
Studies published between 2005 and 2022 were searched in the Web of Science database and selected based on their reporting quality. The keywords were “Arbuscular mycorrhizal fungi,” “Mine,” “Soil,” “World,” “Cadmium,” “Nickel,” “Lead,” “Copper,” and “Chromium.” After assessing more than 250 peer-reviewed articles, articles were excluded based on the following reasons: a) Lack of analytical techniques (not mentioning the QA/QC), b) remediation through other microbes, and c) graphical representation of data. A total of 24 studies comprising nine, twelve, seven, and five studies for Cd, Pb, Cu, and Ni, respectively, were included in the meta-analysis, which assessed the efficacy of AMF in remediating metal-contaminated mine soils (Table 1). Studies reporting remediation of Cr with AMF were not found during the systemic review. The PRISMA flowchart is displayed in Supplementary Figure S4.
From the RE models shown in Figures 6A–D, the overall mean values for Pb, Cd, Ni, and Cu were 1.34 (CIs: 1.19–1.49), 1.08 (CIs: 0.86–1.31), 0.79 (CIs: 0.53–1.05), and 1.46 (CIs: 1.02–1.90), respectively. The data showed statistical significance at p < 0.05. The inconsistency indexes (I2) of Pb, Cd, Ni, and Cu were 92.94%, 96.39%, 99.30%, and 98.89%, respectively, indicating substantial heterogeneity. The positive effect sizes for all the metals indicated that the AMF can reduce the metal accumulation capacity in plants. Studies from United States (Punamiya et al., 2010), China (Zhan et al., 2019), Mexico (González-Villalobos et al., 2021), etc., indicate that the Pb accumulation in plants was increased by AMF (Diversispora spurcum, G. mosseae, Rhizophagus irregularis) except for Wu et al. (2010), Solís-Domínguez et al. (2011), and Bahraminia et al. (2016), for which the CI values overlapped the zero-effect line and were determined to be non-significant (Figure 6A). Case studies from Spain (Arriagada et al., 2007), Brazil (de Andrade et al., 2008), China (Zhong et al., 2012), and Canada (Hassan et al., 2013) showed that the accumulation of Cd in plant systems increased in the presence of AMF inoculation of Glomus deserticola, G. intraradices, and R. irregularis. In other studies from China (Wu et al., 2010; Hu et al., 2013; Liu et al., 2014; He et al., 2020), the Cd accumulation in the plant decreased (Glomus constrictum, Glomus caledonium, and G. intraradices) (Figure 6B). For Ni and Cu, the accumulation decreases in the presence of AMF (Glomus tenue, Glomus margarita) (Orłowska et al., 2011; Lam and Lai, 2018; Manyiwa and Ultra Jr, 2022), and accumulation increases with the help of G. mosseae, G. etunicatum (Chen et al., 2005; Lins et al., 2006) (Figures 6C,D). Plant roots are symbiotically associated with AMF, which increase plant nutrient uptake ability, increases biomass accumulation, increases photosynthesis capacity, and modulates metal toxicity. Through the formation of extracellular and intracellular hyphae, AMF increase soil surface area for better absorption of soil nutrients (N and P) and toxic metals (Cr, Ni, Cd, Pb, and Cu), improves root growth and root morphology, and secretes various proteins like glomalin (Amir et al., 2013; Lam and Lai, 2018; Zhan et al., 2019; Manyiwa and Ultra Jr, 2022). In this paradigm, the presence or absence of AMF in plant systems could impact the accumulation capacity of toxic metals, thus increasing or decreasing ecosystem risk.

Figure 6. The forest plot indicates the mean difference of individual observations regarding the metal accumulation levels in plants in the presence of mycorrhizal infection in different countries. (A) Pb, (B) Cd, (C) Ni, and (D) Cu.
Global heavy metal pollution is of great concern to environmentalists. Numerous research papers have explored the toxic effects of HMs on plants, animals, humans, and other living organisms. These studies highlight the detrimental impact of HM contamination on the ecosystem, emphasizing the need for effective remediation strategies. Plants play an efficient role in the remediation of poisonous HMs. However, the effectiveness of phytoremediation is often limited by slow plant growth and lower efficiency in removing HMs. To address these challenges, the use of plant-associated microbes, especially arbuscular mycorrhizal fungi (AMF), can significantly enhance the removal efficiency of HMs from contaminated soils. These microbes can also improve plant health, nutrient uptake, and stress tolerance, thereby boosting the overall phytoremediation process. The success of this bioremediation technology depends on the proper selection and screening of plant species and AMF cultures to optimize their effectiveness in mitigating HMs from contaminated environments. Future research should focus on optimizing AMF-based remediation strategies, particularly in metal-polluted soils, to enhance ecological sustainability and agricultural productivity. Several areas of research could potentially improve the remediation of metal-contaminated soils in the future. Some of these include:
• Developing more efficient and cost-effective methods for removing or treating metal contaminants.
• Improving our understanding of the behavior and mobility of metal contaminants in the environment, which could lead to more targeted and effective remediation methods.
• Developing new technologies for detecting and measuring metal contaminants in soil, which could enable more accurate assessments of contamination levels and the effectiveness of remediation efforts.
• Investigating alternative materials and methods for immobilizing contaminants, such as natural or synthetic zeolites, to overcome the limitations of traditional stabilization/solidification methods.
• Investigating the use of new microorganisms, enzymes, or new biotechnology approaches for bioremediation and making it more efficient.
• Investigating the use of hybrid approaches, such as combining phytoremediation with bioremediation or chemical treatment, to increase the efficiency of remediation.
• Investigating the use of machine learning and AI tools to optimize the effectiveness of remediation methods and better predict the behavior of contaminants in different soil types.
• Conducting more long-term studies to assess the effectiveness of different remediation methods and to identify any potential negative effects on the environment or human health.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
SB: Data curation, Formal analysis, Methodology, Software, and Writing–original draft. JM: Software and Writing–review and editing. DS: Conceptualization, Resources, and Writing–review and editing. RD: Conceptualization, Resources, and Writing–review and editing. PB: Conceptualization, Supervision, and Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors are thankful to the Indian Statistical Institute for providing financial assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1532169/full#supplementary-material
Abbaspour, H., Saeidi-Sar, S., Afshari, H., and Abdel-Wahhab, M. A. (2012). Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant. Physiol. 169, 704–709. doi:10.1016/j.jplph.2012.01.014
Abyaneh, A. S., and Fazaelipoor, M. H. (2016). Evaluation of rhamnolipid (RL) as a biosurfactant for the removal of chromium from aqueous solutions by precipitate flotation. J. Environ. Manage. 165, 184–187. doi:10.1016/j.jenvman.2015.09.034
Adrees, M., Ali, S., Rizwan, M., Ibrahim, M., Abbas, F., Farid, M., et al. (2015). The effect of excess copper on growth and physiology of important food crops: a review. Environ. Sci. Pollut. Res. 22, 8148–8162. doi:10.1007/s11356-015-4496-5
Akande, F., Ogunkunle, C., and Ajayi, S. (2018). Contamination from petroleum products: impact on soil seed banks around an oil storage facility in Ibadan, South-West Nigeria. Pollut 4, 515–525. doi:10.22059/poll.2018.249913.375
Alam, A. K. M. R., Hossain, A. B. M., Hoque, S., and Chowdhury, D. A. (2018). Heavy metals in wetland soil of greater dhaka district, Bangladesh. Pollut 4, 129–141. doi:10.22059/poll.2017.234867.284
Ali, H., Khan, E., and Sajad, M. A. (2013). Phytoremediation of heavy metals—concepts and applications. Chemosphere 91, 869–881. doi:10.1016/j.chemosphere.2013.01.075
Alloway, B. J. (2012) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability, 22. Springer Science and Business Media, 238.
Álvarez-Ayuso, E., Otones, V., Murciego, A., García-Sánchez, A., and Santa, R. I. (2012). Antimony, arsenic and lead distribution in soils and plants of an agricultural area impacted by former mining activities. Sci. Total. Environ. 439, 35–43. doi:10.1016/j.scitotenv.2012.09.023
Amir, H., Lagrange, A., Hassaïne, N., and Cavaloc, Y. (2013). Arbuscular mycorrhizal fungi from New Caledonian ultramafic soils improve tolerance to nickel of endemic plant species. Mycorrhiza 23, 585–595. doi:10.1007/s00572-013-0499-6
Andra, S. S., Datta, R., Sarkar, D., Makris, K. C., Mullens, C. P., Sahi, S. V., et al. (2009). Induction of lead-binding phytochelatins in vetiver grass [Vetiveria zizanioides (L.)]. J. Environ. Qual. 38, 868–877. doi:10.2134/jeq2008.0316
Antoniadis, V., Thalassinos, G., Levizou, E., Wang, J., Wang, S. L., Shaheen, S. M., et al. (2022). Hazardous enrichment of toxic elements in soils and olives in the urban zone of Lavrio, Greece, a legacy, millennia-old silver/lead mining area and related health risk assessment. J. Hazard. Mat. 434, 128906. doi:10.1016/j.jhazmat.2022.128906
Arhin, E., Boansi, A. O., and Zango, M. S. (2016). Trace elements distributions at Datoko-Shega artisanal mining site, northern Ghana. Environ. Geochem. Health. 38, 203–218. doi:10.1007/s10653-015-9705-0
Arriagada, C. A., Herrera, M. A., and Ocampo, J. A. (2007). Beneficial effect of saprobe and arbuscular mycorrhizal fungi on growth of Eucalyptus globulus co-cultured with Glycine max in soil contaminated with heavy metals. J. Environ. Manage. 84, 93–99. doi:10.1016/j.jenvman.2006.05.005
Arshad, M., Silvestre, J., Pinelli, E., Kallerhoff, J., Kaemmerer, M., Tarigo, A., et al. (2008). A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere 71, 2187–2192. doi:10.1016/j.chemosphere.2008.02.013
Arslan, Ş., and Çelik, M. (2015). Assessment of the pollutants in soils and surface waters around Gümüşköy silver mine (Kütahya, Turkey). Bull. Environ. Contam. Toxicol. 95, 499–506. doi:10.1007/s00128-015-1613-6
Attinti, R., Barrett, K. R., Datta, R., and Sarkar, D. (2017). Ethylenediaminedisuccinic acid (EDDS) enhances phytoextraction of lead by vetiver grass from contaminated residential soils in a panel study in the field. Environ. Pollut. 225, 524–533. doi:10.1016/j.envpol.2017.01.088
Bahraminia, M., Zarei, M., Ronaghi, A., and Ghasemi-Fasaei, R. (2016). Effectiveness of arbuscular mycorrhizal fungi in phytoremediation of lead-contaminated soil by vetiver grass. Int. J. Phytoremediation 18, 730–737. doi:10.1080/15226514.2015.1131242
Barać, N., Škrivanj, S., Mutić, J., Manojlović, D., Bukumirić, Z., Živojinović, D., et al. (2016). Heavy metals fractionation in agricultural soils of Pb/Zn mining region and their transfer to selected vegetables. Water. Air. Soil. Pollut. 227, 1–13. doi:10.1007/s11270-016-3177-4
Barjoee, S. S., Abadi, S. Z. M., Elmi, M. R., Varaoon, V. T., and Nikbakht, M. (2021). Evaluation of trace elements pollution in deposited dust on residential areas and agricultural lands around Pb/Zn mineral areas using modified pollution indices. J. Environ. Health. Sci. Eng. 19, 753–769. doi:10.1007/s40201-021-00643-8
Beukes, J. P., Dawson, N. F., and van Zyl, P. G. (2010). Theoretical and practical aspects of Cr (VI) in the South African ferrochrome industry. J. South. Afr. Inst. Min. Metall. 110, 743–750.
Bloise, A., Fornero, E., Belluso, E., Barrese, E., and Rinaudo, C. (2008). Synthesis and characterization of tremolite asbestos fibres. Eur. J. Mineral. 20, 1027–1033. doi:10.1127/0935-1221/2009/0021-1838
Boussen, S., Soubrand, M., Bril, H., Ouerfelli, K., and Abdeljaouad, S. (2013). Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 192, 227–236. doi:10.1016/j.geoderma.2012.08.029
Bravin, M. N., Marti, A. L., Clairotte, M., and Hinsinger, P. (2009). Rhizosphere alkalisation — a major driver of copper bioavailability over a broad pH range in an acidic, copper-contaminated soil. Soil. 318, 257–268. doi:10.1007/s11104-008-9835-6
Brun, L. A., Maillet, J., Hinsinger, P., and Pepin, M. (2001). Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ. Pollut. 111, 293–302. doi:10.1016/s0269-7491(00)00067-1
Bueno, P. C., Bellido, E., Rubí, J. A. M., and Ballesta, R. J. (2009). Concentration and spatial variability of mercury and other heavy metals in surface soil samples of periurban waste mine tailing along a transect in the Almadén mining district (Spain). Environ. Geol. 56, 815–824. doi:10.1007/s00254-007-1182-z
Bui, A. T., Nguyen, H. T., Nguyen, M. N., Tran, T. H. T., Vu, T. V., Nguyen, C. H., et al. (2016). Accumulation and potential health risks of cadmium, lead and arsenic in vegetables grown near mining sites in Northern Vietnam. Environ. Monit. Assess. 188, 525–536. doi:10.1007/s10661-016-5535-5
Burges, A., Alkorta, I., Epelde, L., and Garbisu, C. (2018). From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediation 20, 384–397. doi:10.1080/15226514.2017.1365340
Cachada, A., Rocha-Santos, T., and Duarte, A. C. (2018). Soil and pollution: an introduction to the main issues. Soil. Pollut. 1, 1–28. doi:10.1016/B978-0-12-849873-6.00001-7
Cai, L. M., Xu, Z. C., Qi, J. Y., Feng, Z. Z., and Xiang, T. S. (2015). Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 127, 127–135. doi:10.1016/j.chemosphere.2015.01.027
Candeias, C., Melo, R., Ávila, P. F., da Silva, E. F., Salgueiro, A. R., and Teixeira, J. P. (2014). Heavy metal pollution in mine–soil–plant system in S. Francisco de Assis–Panasqueira mine (Portugal). Appl. Geochem. 44, 12–26. doi:10.1016/j.apgeochem.2013.07.009
Cao, C., Wang, L., Li, H., Wei, B., and Yang, L. (2018). Temporal variation and ecological risk assessment of metals in soil nearby a Pb–Zn mine in Southern China. Int. J. Environ. Res. Public. Health. 15, 940. doi:10.3390/ijerph15050940
Caradonia, F., Francia, E., Morcia, C., Ghizzoni, R., Moulin, L., Terzi, V., et al. (2019). Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria avoid processing tomato leaf damage during chilling stress. Agronomy 9, 299. doi:10.3390/agronomy9060299
Chaturvedi, N., and Patra, H. K. (2016). Iron ore mining, waste generation, environmental problems and their mitigation through phytoremediation technology. Int. J. Sci. Res. Meth. 5, 397–402.
Chen, M., Chen, X., Xing, Y., Liu, Y., Zhang, S., Zhang, D., et al. (2021). Arsenic and cadmium in soils from a typical mining city in Huainan, China: spatial distribution, ecological risk assessment and health risk assessment. Bull. Environ. Contam. Toxicol. 107, 1080–1086. doi:10.1007/s00128-021-03278-5
Chen, M., Lu, W., Hou, Z., Zhang, Y., Jiang, X., and Wu, J. (2017). Heavy metal pollution in soil associated with a large-scale cyanidation gold mining region in southeast of Jilin, China. Environ. Sci. Pollut. Res. 24, 3084–3096. doi:10.1007/s11356-016-7968-3
Chen, X., Wu, C., Tang, J., and Hu, S. (2005). Arbuscular mycorrhizae enhance metal lead uptake and growth of host plants under a sand culture experiment. Chemosphere 60, 665–671. doi:10.1016/j.chemosphere.2005.01.029
Cheng, S., Liu, G., Zhou, C., and Sun, R. (2018). Chemical speciation and risk assessment of cadmium in soils around a typical coal mining area of China. Ecotoxicol. Environ. Saf. 160, 67–74. doi:10.1016/j.ecoenv.2018.05.022
Cheng, X., Danek, T., Drozdova, J., Huang, Q., Qi, W., Zou, L., et al. (2018). Soil heavy metal pollution and risk assessment associated with the Zn-Pb mining region in Yunnan, Southwest China. Environ. Monit. Assess. 190, 194–210. doi:10.1007/s10661-018-6574-x
Cheng, X., Drozdova, J., Danek, T., Huang, Q., Qi, W., Yang, S., et al. (2018). Pollution assessment of trace elements in agricultural soils around copper mining area. Sustainability 10, 4533. doi:10.3390/su10124533
Cheng, Z. (2016). The spatial correlation and interaction between manufacturing agglomeration and environmental pollution. Ecol. Indic. 61, 1024–1032. doi:10.1016/j.ecolind.2015.10.060
China, S. P., Das, M., and Maiti, S. K. (2014). Phytostabilization of Mosaboni Copper mine tailings: a green step towards waste management. Appl. Ecol. Environ. Res. 12, 25–32.
Coccina, A., Cavagnaro, T. R., Pellegrino, E., Ercoli, L., McLaughlin, M. J., and Watts-Williams, S. J. (2019). The mycorrhizal pathway of zinc uptake contributes to zinc accumulation in barley and wheat grain. BMC Plant. Biol. 19, 133–147. doi:10.1186/s12870-019-1741-y
Crowley, D. (2008). Impacts of metals and metalloids on soil microbial diversity and ecosystem function. Rev. la Cienc. del suelo Nutr. vegetal. 8, 6–11. doi:10.4067/s0718-27912008000400003
Cunningham, S. D., Anderson, T. A., Schwab, A. P., and Hsu, F. C. (1996). Phytoremediation of soils contaminated with organic pollutants. Adv. Agron. 56, 55–114. doi:10.1016/s0065-2113(08)60179-0
Daei, G., Ardekani, M. R., Rejali, F., Teimuri, S., and Miransari, M. (2009). Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J. Plant. Physiol. 166, 617–625. doi:10.1016/j.jplph.2008.09.013
Darko, G., Adjei, S., Nkansah, M. A., Borquaye, L. S., Boakye, K. O., and Dodd, M. (2022). Accumulation and bioaccessibility of toxic metals in root tubers and soils from gold mining and farming communities in the Ashanti region of Ghana. Int. J. Environ. Health. Res. 32, 426–436. doi:10.1080/09603123.2020.1772203
Darko, G., Boakye, K. O., Nkansah, M. A., Gyamfi, O., Ansah, E., Yevugah, L. L., et al. (2019). Human health risk and bioaccessibility of toxic metals in topsoils from Gbani mining community in Ghana. J. Health. Pollut. 9, 190602. doi:10.5696/2156-9614-9.22.190602
Das, M., and Maiti, S. K. (2007). Metal accumulation in A. baccifera growing naturally on abandoned copper tailings pond. Environ. Monit. Assess. 127, 119–125. doi:10.1007/s10661-006-9265-y
da Silveira Pereira, W. V., Teixeira, R. A., de Souza, E. S., de Moraes, A. L. F., Campos, W. E. O., do Amarante, C. B., et al. (2020). Chemical fractionation and bioaccessibility of potentially toxic elements in area of artisanal gold mining in the Amazon. J. Environ. Manage. 267, 110644. doi:10.1016/j.jenvman.2020.110644
de Andrade, S. A. L., da Silveira, A. P. D., Jorge, R. A., and de Abreu, M. F. (2008). Cadmium accumulation in sunflower plants influenced by arbuscular mycorrhiza. Int. J. Phytoremediation 10, 1–13. doi:10.1080/15226510701827002
Dhal, P. K., and Sar, P. (2014). Microbial communities in uranium mine tailings and mine water sediment from Jaduguda U mine, India: a culture independent analysis. J. Environ. Sci. Health 49, 694–709. doi:10.1080/10934529.2014.865458
Dhaliwal, S. S., Singh, J., Taneja, P. K., and Mandal, A. (2020). Remediation techniques for removal of heavy metals from the soil contaminated through different sources: a review. Environ. Sci. Pollut. Res. 27, 1319–1333. doi:10.1007/s11356-019-06967-1
Dhatrak, S. V., Subroto, S. N., Sishodiya, P. L., Dhumne, U. L., Ingole, S. V., and Gupta, S. R. (2017). Health Status Evaluation of mine workers and nearby population around iron ore mines in tribal district of Jharkhand, India. Am. J. Prev. Med. 1, 20–26. doi:10.5455/ajpmph.280307
Ding, T., Ma, D., Lu, J., and Zhang, R. (2018). Magnetite as an indicator of mixed sources for W–Mo–Pb–Zn mineralization in the Huangshaping polymetallic deposit, southern Hunan Province, China. Geol. Rev. 95, 65–78. doi:10.1016/j.oregeorev.2018.02.019
Du, F., Yang, Z., Liu, P., and Wang, L. (2018). Accumulation, translocation, and assessment of heavy metals in the soil-rice systems near a mine-impacted region. Environ. Sci. Pollut. Res. 25, 32221–32230. doi:10.1007/s11356-018-3184-7
Duncan, A. E., de Vries, N., and Nyarko, K. B. (2018). Assessment of heavy metal pollution in the sediments of the River Pra and its tributaries. Water. Air. Soil. Pollut. 229, 1–10. doi:10.1007/s11270-018-3899-6
Eapen, S., Suseelan, K. N., Tivarekar, S., Kotwal, S. A., and Mitra, R. (2003). Potential for rhizofiltration of uranium using hairy root cultures of Brassica juncea and Chenopodium amaranticolor. Environ. Res. 91, 127–133. doi:10.1016/s0013-9351(02)00018-x
Elmayel, I., Esbrí, J. M., García-Ordiales, E., Elouaer, Z., Garcia-Noguero, E. M., Bouzid, J., et al. (2020). Biogeochemical assessment of the impact of Zn mining activity in the area of the Jebal Trozza mine, Central Tunisia. Environ. Geochem. Health. 42, 3529–3542. doi:10.1007/s10653-020-00595-2
Emran, M., Gispert, M., and Pardini, G. (2012). Patterns of soil organic carbon, glomalin and structural stability in abandoned Mediterranean terraced lands. Eur. J. Soil. Sci. 63, 637–649. doi:10.1111/j.1365-2389.2012.01493.x
Equeenuddin, S. M., and Pattnaik, B. K. (2017). Assessment of heavy metal contamination in sediment at Sukinda ultramafic complex using HAADF-STEM analysis. Chemosphere 185, 309–320. doi:10.1016/j.chemosphere.2017.06.131
Equeenuddin, S. M., Tripathy, S., Sahoo, P. K., and Ranjan, A. (2016). Geochemical characteristics and mode of occurrence of trace elements in coal at West Bokaro coalfield. Int. J. Coal. Sci. Technol. 3, 399–406. doi:10.1007/s40789-016-0146-x
Farahat, E. A., and Galal, T. M. (2018). Trace metal accumulation by Ranunculus sceleratus: implications for phytostabilization. Environ. Sci. Pollut. Res. Int. 25, 4214–4222. doi:10.1007/s11356-017-0808-2
Fazekašová, D., and Fazekaš, J. (2020). Soil quality and heavy metal pollution assessment of iron ore mines in Nizna Slana (Slovakia). Sustainability 12, 2549. doi:10.3390/su12062549
Fernández-Caliani, J. C., Barba-Brioso, C., González, I., and Galán, E. (2009). Heavy metal pollution in soils around the abandoned mine sites of the iberian pyrite belt (southwest Spain). Air. Soil. Pollut. 200, 211–226. doi:10.1007/s11270-008-9905-7
Galhardi, J. A., de Mello, J. W., and Wilkinson, K. J. (2020). Bioaccumulation of potentially toxic elements from the soils surrounding a legacy uranium mine in Brazil. Chemosphere 261, 127679. doi:10.1016/j.chemosphere.2020.127679
Gałuszka, A., Migaszewski, Z. M., Dołęgowska, S., Michalik, A., and Duczmal-Czernikiewicz, A. (2015). Geochemical background of potentially toxic trace elements in soils of the historic copper mining area: a case study from Miedzianka Mt., Holy Cross Mountains, south-central Poland. Environ. Earth. Sci. 74, 4589–4605. doi:10.1007/s12665-015-4395-6
García-Giménez, R., and Jiménez-Ballesta, R. (2017). Mine tailings influencing soil contamination by potentially toxic elements. Environ. Earth. Sci. 76, 51–12. doi:10.1007/s12665-016-6376-9
Ghaderian, S. M., and Ravandi, A. A. G. (2012). Accumulation of copper and other heavy metals by plants growing on Sarcheshmeh copper mining area, Iran. J. Geochem. Explor. 123, 25–32. doi:10.1016/j.gexplo.2012.06.022
Ghazban, F., Parizanganeh, A., Zamani, A., and Baniardalan, S. (2018). Evaluation of heavy metal contamination of surface soils in Zarshouran Gold District, Northwestern Iran. Int. J. Environ. Res. 12, 843–860. doi:10.1007/s41742-018-0139-2
Giri, S., and Singh, A. K. (2017). Human health risk assessment due to dietary intake of heavy metals through rice in the mining areas of Singhbhum Copper Belt, India. Environ. Sci. Pollut. Res. 24, 14945–14956. doi:10.1007/s11356-017-9039-9
Giri, S., Singh, A. K., and Mahato, M. K. (2017). Metal contamination of agricultural soils in the copper mining areas of Singhbhum shear zone in India. J. Earth. Syst. Sci. 126, 49. doi:10.1007/s12040-017-0833-z
Gkorezis, P., Daghio, M., Franzetti, A., Hamme, J. D. V., Sillen, W., and Vangronsveld, J. (2016). The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: an environmental perspective. Front. Microbiol. 7, 1836–1863. doi:10.3389/fmicb.2016.01836
Gnandi, K., and Tobschall, H. (2002). Heavy metals distribution of soils around mining sites of cadmium-rich marine sedimentary phosphorites of Kpogame and Hahotoe (southern Togo). Environ. Geol. 41, 593–600. doi:10.1007/s002540100425
González-Villalobos, M. A., Martinez-Trinidad, T., Alarcón, A., and Plascencia-Escalante, F. O. (2021). Growth and lead uptake by Parkinsonia aculeata L. inoculated with Rhizophagus intraradices. Int. J. Phytoremediation 23, 272–278. doi:10.1080/15226514.2020.1812506
Guo, P., Wang, T., Liu, Y., Xia, Y., Wang, G., Shen, Z., et al. (2016). Phytostabilization potential of evening primrose (Oenothera glazioviana) for copper-contaminated sites. Environ. Sci. Pollut. Res. Int. 21, 631–640. doi:10.1007/s11356-013-1899-z
Gupta, C., and Singh, H. (2003). Uranium resource processing: secondary resources. Springer Science and Business Media.
Hamad, R., Balzter, H., and Kolo, K. (2019). Assessment of heavy metal release into the soil after mine clearing in Halgurd-Sakran National Park, Kurdistan, Iraq. Environ. Sci. Pollut. Res. 26, 1517–1536. doi:10.1007/s11356-018-3597-3
Hamdi, H., Benzarti, S., Aoyama, I., and Jedidi, N. (2012). Rehabilitation of degraded soils containing aged PAHs based on phytoremediation with alfalfa (Medicago sativa L.). Int. Biodeterior. Biodegr. 67, 40–47. doi:10.1016/j.ibiod.2011.10.009
Hänsch, R., and Mendel, R. R. (2009). Physiological functions of mineral micronutrients (cu, Zn, Mn, Fe, Ni, Mo, B, cl). Curr. Opin. Plant. Biol. 12, 259–266. doi:10.1016/j.pbi.2009.05.006
Hashem, A., Abd Allah, E. F., Alqarawi, A. A., al Huqail, A. A., Egamberdieva, D., and Wirth, S. (2016). Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi. J. Biol. Sci. 23, 272–281. doi:10.1016/j.sjbs.2015.11.002
Hassan, S. E., Hijri, M., and St-Arnaud, M. (2013). Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. N. Biotechnol. 30, 780–787. doi:10.1016/j.nbt.2013.07.002
He, Y., Yang, R., Lei, G., Li, B., Jiang, M., Yan, K., et al. (2020). Arbuscular mycorrhizal fungi reduce cadmium leaching from polluted soils under simulated heavy rainfall. Environ. Pollut. 263, 114406. doi:10.1016/j.envpol.2020.114406
Herlina, L., Widianarko, B., and Sunoko, H. R. (2020). Phytoremediation potential of Cordyline fruticosa for lead contaminated. Soil. J. Pendidik. IPA. Indones. 9, 42–49. doi:10.15294/jpii.v9i1.23422
Hinojosa, M. B., Carreira, J. A., García-Ruíz, R., and Dick, R. P. (2005). Microbial response to heavy metal–polluted soils: community analysis from phospholipid-linked fatty acids and ester-linked fatty acids extracts. J. Environ. Qual. 34, 1789–1800. doi:10.2134/jeq2004.0470
Holmes, R. J., Lu, Y., and Lu, L. (2022) “Introduction: overview of the global iron ore industry,” in Iron ore, 1–56.
Hosseini, S. M., Rezazadeh, M., Salimi, A., and Ghorbanli, M. (2018). Distribution of heavy metals and arsenic in soils and indigenous plants near an iron ore mine in northwest Iran. Acta. Ecol. Sin. 38, 363–367. doi:10.1016/j.chnaes.2018.02.004
Hu, J., Wu, S., Wu, F., Leung, H. M., Lin, X., and Wong, M. H. (2013). Arbuscular mycorrhizal fungi enhance both absorption and stabilization of Cd by Alfred stonecrop (Sedum alfredii Hance) and perennial ryegrass (Lolium perenne L.) in a Cd-contaminated acidic soil. Chemosphere 93, 1359–1365. doi:10.1016/j.chemosphere.2013.07.089
Hua, L., Yang, X., Liu, Y., Tan, X., and Yang, Y. (2018). Spatial distributions, pollution assessment, and qualified source apportionment of soil heavy metals in a typical mineral mining city in China. Sustainability 10, 3115. doi:10.3390/su10093115
Huang, X., Zhu, Y., and Ji, H. (2013). Distribution, speciation, and risk assessment of selected metals in the gold and iron mine soils of the catchment area of Miyun Reservoir, Beijing, China. Environ. Monit. Assess. 185, 8525–8545. doi:10.1007/s10661-013-3193-4
IS (1993). Drinking water specifications (1st revision). Bureau Indian Stand. (IS 10500). Available at: www.bis.org.in/bis/html/10500.html.
Jia, H., Wang, H., Lu, H., Jiang, S., Dai, M., Liu, J., et al. (2016). Rhizodegradation potential and tolerance of Avicennia marina (Forsk.) Vierh in phenanthrene and pyrene contaminated sediments. Mar. Pollut. Bull. 110, 112–118. doi:10.1016/j.marpolbul.2016.06.075
Jiang, Y., Wen, H., Zhang, Q., Yuan, L., and Liu, L. (2022). Source apportionment and health risk assessment of potentially toxic elements in soil from mining areas in northwestern China. Environ. Geochem. Health. 44, 1551–1566. doi:10.1007/s10653-021-00907-0
Kabata-Pendias, A. (2011). Trace elements in soils and plants. Boca Raton London New York: fourth. CRC Press Taylor and Francis Group.
Kartal, Ş., Aydın, Z., and Tokalıoğlu, Ş. (2006). Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. J. Hazard. Mat. 132, 80–89. doi:10.1016/j.jhazmat.2005.11.091
Khan, N. T., Jameel, N., and Khan, M. J. (2018). A brief overview of contaminated soil remediation methods. Biotechnol. Ind. J. 14, 171.
Khelfaoui, M., Medjram, M. S., Kabir, A., Zouied, D., Mehri, K., Chikha, O., et al. (2020). Chemical and mineralogical characterization of weathering products in mine wastes, soil, and sediment from the abandoned Pb/Zn mine in Skikda, Algeria. Environ. Earth. Sci. 79, 293–308. doi:10.1007/s12665-020-09043-x
Kien, C. N., Noi, N. V., Son, L. T., Ngoc, H. M., Tanaka, S., Nishina, T., et al. (2010). Heavy metal contamination of agricultural soils around a chromite mine in Vietnam. Sci. Plant. Nutr. 56, 344–356. doi:10.1111/j.1747-0765.2010.00451.x
Kowitwiwat, A., and Sampanpanish, P. (2020). Phytostabilization of arsenic and manganese in mine tailings using Pennisetum purpureum cv. Mott supplemented with cow manure and acacia wood-derived biochar. Heliyon 6, e04552. doi:10.1016/j.heliyon.2020.e04552
Krishna, A. K., Mohan, K. R., Murthy, N. N., Periasamy, V., Bipinkumar, G., Manohar, K., et al. (2013). Assessment of heavy metal contamination in soils around chromite mining areas, Nuggihalli, Karnataka, India. Environ. Earth. Sci. 70, 699–708. doi:10.1007/s12665-012-2153-6
Kulikova, T., Hiller, E., Jurkovič, Ľ., Filová, L., Šottník, P., and Lacina, P. (2019). Total mercury, chromium, nickel and other trace chemical element contents in soils at an old cinnabar mine site (Merník, Slovakia): anthropogenic versus natural sources of soil contamination. Environ. Monit. Assess. 191, 263–281. doi:10.1007/s10661-019-7391-6
Kumar, A., and Maiti, S. K. (2014). Translocation and bioaccumulation of metals in Oryza sativa and Zea mays growing in chromite-asbestos contaminated agricultural fields, Jharkhand, India. Bull. Environ. Contam. Toxicol. 93, 434–441. doi:10.1007/s00128-014-1339-x
Kumar, A., Maiti, S. K., Prasad, M. N. V., and Singh, R. S. (2017). Grasses and legumes facilitate phytoremediation of metalliferous soils in the vicinity of an abandoned chromite–asbestos mine. J. Soils. Sediments. 17, 1358–1368. doi:10.1007/s11368-015-1323-z
Kumari, S. M. P., and Prabina, B. J. (2019). Protection of tomato, Lycopersicon esculentum from wilt pathogen, Fusarium oxysporum f. sp. lycopersici by arbuscular mycorrhizal fungi, Glomus sp. Int. J. Curr. Microbiol. Appl. Sci. 8, 1368–1378. doi:10.20546/ijcmas.2019.804.159
Kumari, S. M. P., and Srimeena, N. (2019). Arbuscular mycorrhizal fungi (AMF) induced defense factors against the Damping-off disease pathogen, Pythium aphanidermatum in chilli (Capsicum annum). Int. J. Curr. Microbiol. Appl. Sci. 8, 2243–2248. doi:10.20546/ijcmas.2019.806.267
Lam, C. M., and Lai, H. Y. (2018). Effect of inoculation with arbuscular mycorrhizal fungi and blanching on the bioaccessibility of heavy metals in water spinach (Ipomoea aquatica Forsk.). Ecotoxicol. Environ. Saf. 162, 563–570. doi:10.1016/j.ecoenv.2018.07.047
Lee, C. G., Chon, H. T., and Jung, M. C. (2001). Heavy metal contamination in the vicinity of the Daduk Au–Ag–Pb–Zn mine in Korea. Appl. Geochem. 16, 1377–1386. doi:10.1016/s0883-2927(01)00038-5
Lee, M., and Yang, M. (2010). Rhizofiltration using sunflower (Helianthus annuus L.) and bean (Phaseolus vulgaris L. var. vulgaris) to remediate uranium contaminated groundwater. J. Hazard. Mat. 173, 589–596. doi:10.1016/j.jhazmat.2009.08.127
Lee, S. W., Cho, H. G., and Kim, S. O. (2019). Comparisons of human risk assessment models for heavy metal contamination within abandoned metal mine areas in Korea. Environ. Geochem. Health. 41, 481–505. doi:10.1007/s10653-018-0108-x
Li, D., Liu, G., Li, X., Li, R., Wang, J., and Zhao, Y. (2022). Heavy metal (loid) s pollution of agricultural soils and health risk assessment of consuming soybean and wheat in a typical non-ferrous metal mine area in Northeast China. Sustainability 14, 2953. doi:10.3390/su14052953
Li, F., Yang, F., Chen, Y., Jin, H., Leng, Y., and Wang, J. (2020). Chemical reagent-assisted phytoextraction of heavy metals by Bryophyllum laetivirens from garden soil made of sludge. Chemosphere 253, 126574. doi:10.1016/j.chemosphere.2020.126574
Li, H., and Ji, H. (2017). Chemical speciation, vertical profile and human health risk assessment of heavy metals in soils from coal-mine brownfield, Beijing, China. J. Geochem. Explor. 183, 22–32. doi:10.1016/j.gexplo.2017.09.012
Li, M. S., Luo, Y. P., and Su, Z. Y. (2007). Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ. Pollut. 147, 168–175. doi:10.1016/j.envpol.2006.08.006
Li, Q., Ji, H., Qin, F., Tang, L., Guo, X., and Feng, J. (2014). Sources and the distribution of heavy metals in the particle size of soil polluted by gold mining upstream of Miyun Reservoir, Beijing: implications for assessing the potential risks. Environ. Monit. Assess. 186, 6605–6626. doi:10.1007/s10661-014-3877-4
Li, Z., Deblon, J., Zu, Y., Colinet, G., Li, B., and He, Y. (2019). Geochemical baseline values determination and evaluation of heavy metal contamination in soils of Lanping Mining Valley (Yunnan Province, China). Int. J. Environ. Res. Public. Health. 16, 4686. doi:10.3390/ijerph16234686
Limmer, M., and Burken, J. (2016). Phytovolatilization of organic contaminants. Environ. Sci. Technol. 50, 6632–6643. doi:10.1021/acs.est.5b04113
Lins, C. E. L., Cavalcante, U. M. T., Sampaio, E. V. S. B., Messias, A. S., and Maia, L. C. (2006). Growth of mycorrhized seedlings of Leucaena leucocephala (Lam.) de Wit. in a copper contaminated soil. Appl. Soil. Ecol. 31, 181–185. doi:10.1016/j.apsoil.2005.06.004
Liu, L., Gong, Z., Zhang, Y., and Li, P. (2014). Growth, cadmium uptake and accumulation of maize (Zea mays L.) under the effects of arbuscular mycorrhizal fungi. Ecotoxicol. 23, 1979–1986. doi:10.1007/s10646-014-1331-6
Liu, X., Bai, Z., Shi, H., Zhou, W., and Liu, X. (2019). Heavy metal pollution of soils from coal mines in China. Nat. Hazards. 99, 1163–1177. doi:10.1007/s11069-019-03771-5
Long, J., Tan, D., Deng, S., and Lei, M. (2018). Pollution and ecological risk assessment of antimony and other heavy metals in soils from the world's largest antimony mine area, China. Hum. Ecol. Risk. Assess. Int. J. 24, 679–690. doi:10.1080/10807039.2017.1396531
Lothe, A. G., Hansda, A., and Kumar, V. (2016). Phytoremediation of copper-contaminated soil using Helianthus annuus, Brassica nigra, and Lycopersicon esculentum Mill.: a pot scale study. Environ. Qual. Manage. 25, 63–70. doi:10.1002/tqem.21463
Lü, J., Jiao, W. B., Qiu, H. Y., Chen, B., Huang, X. X., and Kang, B. (2018). Origin and spatial distribution of heavy metals and carcinogenic risk assessment in mining areas at You'xi County southeast China. Geoderma 310, 99–106. doi:10.1016/j.geoderma.2017.09.016
Lu, N., Li, G., Hav, J. C., Wang, H. Y., Wei, Y., and Sun, Y. Y. (2019). Investigation of lead and cadmium contamination in mine soil and metal accumulation in selected plants growing in a gold mining area. Appl. Ecol. Environ. Res. 17, 10587–10597. doi:10.15666/aeer/1705_1058710597
Lu, Q., Xu, Z., Xu, X., Liu, L., Liang, L., Chen, Z., et al. (2019). Cadmium contamination in a soil-rice system and the associated health risk: an addressing concern caused by barium mining. Ecotoxicol. Environ. Saf. 183, 109590. doi:10.1016/j.ecoenv.2019.109590
Ma, Y., Dickinson, N. M., and Wong, M. H. (2006). Beneficial effects of earthworms and arbuscular mycorrhizal fungi on establishment of leguminous trees on Pb/Zn mine tailings. Soil. Biol. biochem. 38, 1403–1412. doi:10.1016/j.soilbio.2005.10.016
Ma, Z., Chen, K., Li, Z., Bi, J., and Huang, L. (2016). Heavy metals in soils and road dusts in the mining areas of Western Suzhou, China: a preliminary identification of contaminated sites. J. Soils. Sediments. 16, 204–214. doi:10.1007/s11368-015-1208-1
Magiera, T., Zawadzki, J., Szuszkiewicz, M., Fabijańczyk, P., Steinnes, E., Fabian, K., et al. (2018). Impact of an iron mine and a nickel smelter at the Norwegian/Russian border close to the Barents Sea on surface soil magnetic susceptibility and content of potentially toxic elements. Chemosphere 195, 48–62. doi:10.1016/j.chemosphere.2017.12.060
Maiti, S. K., Kumar, A., and Ahirwal, J. (2016). Bioaccumulation of metals in timber and edible fruit trees growing on reclaimed coal mine overburden dumps. Int. J. Min. Reclam. Environ. 30, 231–244. doi:10.1080/17480930.2015.1038864
Maiti, S. K., Nandhini, S., and Das, M. (2005). Accumulation of metals by naturally growing herbaceous and tree species in iron ore tailings. Int. J. Environ. Stud. 62, 593–603. doi:10.1080/00207230500241652
Mandal, J., Bakare, W. A., Rahman, M. M., Rahman, M. A., Siddique, A. B., Oku, E., et al. (2022). Varietal differences influence arsenic and lead contamination of rice grown in mining impacted agricultural fields of Zamfara State, Nigeria. Chemosphere 305, 135339. doi:10.1016/j.chemosphere.2022.135339
Manyiwa, T., and Ultra Jr, V. U. (2022). Soil amendments and arbuscular mycorrhiza influenced the growth and heavy metal accumulation of Colosphospermum mopane (Kirk Ex Benth.) in heavy metal contaminated soil. Soil. Sediment. Contam. Int. J. 31, 81–96. doi:10.1080/15320383.2021.1910623
Martínez-Toledo, Á., Montes-Rocha, A., González-Mille, D. J., Espinosa-Reyes, G., Torres-Dosal, A., Mejia-Saavedra, J. J., et al. (2017). Evaluation of enzyme activities in long-term polluted soils with mine tailing deposits of San Luis Potosí, México. México. J. Soils. Sediments. 17, 364–375. doi:10.1007/s11368-016-1529-8
Meyer, E., Londoño, D. M. M., de Armas, R. D., Giachini, A. J., Rossi, M. J., Stoffel, S. C. G., et al. (2017). Arbuscular mycorrhizal fungi in the growth and extraction of trace elements by Chrysopogon zizanioides (vetiver) in a substrate containing coal mine wastes. Int. J. Phytoremediation 19, 113–120. doi:10.1080/15226514.2016.1207596
Miller, J. R., Hudson-Edwards, K. A., Lechler, P. J., Preston, D., and Macklin, M. G. (2004). Heavy metal contamination of water, soil and produce within riverine communities of the Rıo Pilcomayo basin, Bolivia. Sci. Total. Environ. 320, 189–209. doi:10.1016/j.scitotenv.2003.08.011
Mishra, A., Bhattacharya, A., and Mishra, N. (2019). Mycorrhizal symbiosis: an effective tool for metal bioremediation. New Future Dev. Microb. Biotechnol. Bioeng., 113–128. doi:10.1016/b978-0-12-818258-1.00007-8
Mitra, D., Uniyal, N., Panneerselvam, P., Senapati, A., and Ganeshamurthy, A. N. (2020). Role of mycorrhiza and its associated bacteria on plant growth promotion and nutrient management in sustainable agriculture. Int. J. Life. Sci. Appl. Sci. 1.
Mohammadi, H., Amani-Ghadim, A. R., Matin, A. A., and Ghorbanpour, M. (2020). Fe nanoparticles improve physiological and antioxidative attributes of sunflower (Helianthus annuus) plants grown in soil spiked with hexavalent chromium. Biotech 10, 19. doi:10.1007/s13205-019-2002-3
Mohan, D., Pittman Jr, C. U., Bricka, M., Smith, F., Yancey, B., Mohammad, J., et al. (2007). Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid. Interface. Sci. 310, 57–73. doi:10.1016/j.jcis.2007.01.020
Mohan, S. V., Nithila, P., and Reddy, S. J. (1996). Estimation of heavy metals in drinking water and development of heavy metal pollution index. J. Environ. Sci. Health 31, 283–289. doi:10.1080/10934529609376357
Moore, F., Dehghani, S., and Keshavarzi, B. (2014). Characterization of soil contamination in Miduk mining district, SW Iran. Soil. Sediment. Contam. Int. J. 23, 614–627. doi:10.1080/15320383.2014.856856
Moore, F., Sheykhi, V., Salari, M., and Bagheri, A. (2016). Soil quality assessment using GIS-based chemometric approach and pollution indices: nakhlak mining district, Central Iran. Environ. Monit. Assess. 188, 214–216. doi:10.1007/s10661-016-5152-3
Morikawa, H., and Erkin, Ö. C. (2003). Basic processes in phytoremediation and some applications to air pollution control. Chemosphere 52, 1553–1558. doi:10.1016/s0045-6535(03)00495-8
Mwesigye, A. R., Young, S. D., Bailey, E. H., and Tumwebaze, S. B. (2016). Population exposure to trace elements in the Kilembe copper mine area, Western Uganda: a pilot study. Sci. Total. Environ. 573, 366–375. doi:10.1016/j.scitotenv.2016.08.125
Nakmee, P. S., Techapinyawat, S., and Ngamprasit, S. (2016). Comparative potentials of native arbuscular mycorrhizal fungi to improve nutrient uptake and biomass of Sorghum bicolor Linn. Agric. Nat. Resour. 50, 173–178. doi:10.1016/j.anres.2016.06.004
Narendrula, R., Nkongolo, K. K., Beckett, P., and Spiers, G. (2013). Total and bioavailable metals in two contrasting mining regions (Sudbury in Canada and Lubumbashi in DR-Congo): relation to genetic variation in plant populations. Chem. Ecol. 29, 111–127. doi:10.1080/02757540.2012.696617
Nawab, J., Khan, S., Shah, M. T., Khan, K., Huang, Q., and Ali, R. (2015). Quantification of heavy metals in mining affected soil and their bioaccumulation in native plant species. Int. J. Phytoremediation 17, 801–813. doi:10.1080/15226514.2014.981246
Nawab, J., Li, G., Khan, S., Sher, H., Aamir, M., Shamshad, I., et al. (2016). Health risk assessment from contaminated foodstuffs: a field study in chromite mining-affected areas northern Pakistan. Environ. Sci. Pollut. Res. 23, 12227–12236. doi:10.1007/s11356-016-6379-9
Nekoeinia, M., Mohajer, R., Salehi, M. H., and Moradlou, O. (2016). Multivariate statistical approach to identify metal contamination sources in agricultural soils around Pb–Zn mining area, Isfahan province, Iran. Environ. Earth. Sci. 75, 760–770. doi:10.1007/s12665-016-5597-2
Ngole, V. M., and Ekosse, G. I. E. (2012). Copper, nickel and zinc contamination in soils within the precincts of mining and landfilling environments. Int. J. Environ. Sci. Technol. 9, 485–494. doi:10.1007/s13762-012-0055-5
Nguyen, T. H., Hoang, H. N. T., Bien, N. Q., Tuyen, L. H., and Kim, K. W. (2020). Contamination of heavy metals in paddy soil in the vicinity of Nui Phao multi-metal mine, North Vietnam. Environ. Geochem. Health. 42, 4141–4158. doi:10.1007/s10653-020-00611-5
Nieva, N. E., Borgnino, L., and García, M. G. (2018). Long term metal release and acid generation in abandoned mine wastes containing metal-sulphides. Environ. Pollut. 242, 264–276. doi:10.1016/j.envpol.2018.06.067
Nikolaidis, C., Orfanidis, M., Hauri, D., Mylonas, S., and Constantinidis, T. (2013). Public health risk assessment associated with heavy metal and arsenic exposure near an abandoned mine (Kirki, Greece). Int. J. Environ. Health. Res. 23, 507–519. doi:10.1080/09603123.2013.769202
Niu, S., Gao, L., and Zhao, J. (2015). Distribution and risk assessment of heavy metals in the xinzhuangzi reclamation soil from the Huainan coal mining area, China. China. Hum. Ecol. risk. Assess. Int. J. 21, 900–912. doi:10.1080/10807039.2014.943572
Niu, S., Gao, L., and Zhao, J. (2015). Risk analysis of metals in soil from a restored coal mining area. Bull. Environ. Contam. Toxicol. 95, 183–187. doi:10.1007/s00128-015-1576-7
Niu, S., Gao, L., and Zhao, J. (2017). Heavy metals in the soils and plants from a typical restored coal-mining area of Huainan coalfield, China. Environ. Monit. Assess. 189, 484–496. doi:10.1007/s10661-017-6207-9
Nosrati, K., and Collins, A. L. (2019). A soil quality index for evaluation of degradation under land use and soil erosion categories in a small mountainous catchment, Iran. J. Mt. Sci. 16, 2577–2590. doi:10.1007/s11629-019-5567-8
Nouri, M., and Haddioui, A. (2016). Human and animal health risk assessment of metal contamination in soil and plants from Ait Ammar abandoned iron mine, Morocco. Environ. Monit. Assess. 188, 6–12. doi:10.1007/s10661-015-5012-6
Nurzhanova, A., Pidlisnyuk, V., Abit, K., Nurzhanov, C., Kenessov, B., Stefanovska, T., et al. (2019). Comparative assessment of using Miscanthus × giganteus for remediation of soils contaminated by heavy metals: a case of military and mining sites. Environ. Sci. Pollut. Res. 26, 13320–13333. doi:10.1007/s11356-019-04707-z
Obasi, P. N., and Akudinobi, B. E. B. (2019). Pollution status of arable soils and stream sediments in mining areas of Abakaliki, Lower Benue Trough, Nigeria. Int. J. Environ. Sci. Technol. 16, 7869–7884. doi:10.1007/s13762-019-02337-z
Ogundele, L. T., Oluwajana, O. A., Ogunyel, A. C., and Inuyomi, S. O. (2021). Heavy metals, radionuclides activity and mineralogy of soil samples from an artisanal gold mining site in Ile-Ife, Nigeria: implications on human and environmental health. Environ. Earth. Sci. 80, 1–15. doi:10.1007/s12665-021-09494-w
Opekunova, M. G., Somov, V. V., and Papyan, E. E. (2017). Soil contamination in the impact zone of mining enterprises in the Bashkir Transural region. Eur. Soil. Sci. 50, 732–745. doi:10.1134/s1064229317060084
Organization, W. H. (2000). Air quality guidelines for Europe. Regional Office for Europe: World Health Organization.
Orłowska, E., Przybyłowicz, W., Orlowski, D., Mongwaketsi, N. P., Turnau, K., and Mesjasz-Przybyłowicz, J. (2013). Mycorrhizal colonization affects the elemental distribution in roots of Ni-hyperaccumulator Berkheya coddii Roessler. Environ. Pollut. 175, 100–109. doi:10.1016/j.envpol.2012.12.028
Orłowska, E., Przybyłowicz, W., Orlowski, D., Turnau, K., and Mesjasz-Przybyłowicz, J. (2011). The effect of mycorrhiza on the growth and elemental composition of Ni-hyperaccumulating plant Berkheya coddii Roessler. Environ. Pollut. 159, 3730–3738. doi:10.1016/j.envpol.2011.07.008
Othmani, M. A., Souissi, F., Durães, N., Abdelkader, M., and Da Silva, E. F. (2015). Assessment of metal pollution in a former mining area in the NW Tunisia: spatial distribution and fraction of Cd, Pb and Zn in soil. Environ. Monit. Assess. 187, 523–541. doi:10.1007/s10661-015-4734-9
Oyebamiji, A., Amanambu, A., Zafar, T., Adewumi, A. J., and Akinyemi, D. S. (2018). Expected impacts of active mining on the distribution of heavy metals in soils around Iludun-Oro and its environs, Southwestern Nigeria. Environ. Sci. 4, 1495046. doi:10.1080/23311843.2018.1495046
Parniske, M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 6, 763–775. doi:10.1038/nrmicro1987
Peña-Ortega, M., Rio-Salas, D., Valencia-Sauceda, J., Mendívil-Quijada, H., Minjarez-Osorio, C., Molina-Freaner, F., et al. (2019). Environmental assessment and historic erosion calculation of abandoned mine tailings from a semi-arid zone of northwestern Mexico: insights from geochemistry and unmanned aerial vehicles. Environ. Sci. Pollut. Res. 26, 26203–26215. doi:10.1007/s11356-019-05849-w
Pentyala, V. B., and Eapen, S. (2020). High efficiency phytoextraction of uranium using Vetiveria zizanioides L. Nash. Int. J. Phytoremediation 221, 1137–1146. doi:10.1080/15226514.2020.1741506
Peralta-Videa, J. R., Lopez, M. L., Narayan, M., Saupe, G., and Gardea-Torresdey, J. (2009). The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int. J. Biochem. Cell. Biol. 41, 1665–1677. doi:10.1016/j.biocel.2009.03.005
Perlatti, F., Martins, E. P., de Oliveira, D. P., Ruiz, F., Asensio, V., Rezende, C. F., et al. (2021). Copper release from waste rocks in an abandoned mine (NE, Brazil) and its impacts on ecosystem environmental quality. Chemosphere 262, 127843. doi:10.1016/j.chemosphere.2020.127843
Perronnet, K., Schwartz, C., Gérard, E., and Morel, J. L. (2000). Availability of cadmium and zinc accumulated in the leaves of Thlaspi caerulescens incorporated into soil. Plant. Soil. 227, 257–263. doi:10.1023/A:1026583317702
Peter, L., Clausen, W., Broholm, M. M., Gosewinkel, U., and Trapp, S. (2017). Test of aerobic TCE degradation by willows (Salix viminalis) and willows inoculated with TCE-cometabolizing strains of Burkholderia cepacia. Environ. Sci. Pollut. Res. 24, 18320–18331. doi:10.1007/s11356-017-9420-8
Petrova, R. (2006) “Accumulation of natural radionuclides in wooden and grass vegetation from abandoned uranium mines. Opportunities for phytoremediation,” in Uranium in the environment. Mining impact and consequences, 507–518.
Pradhan, S. K., Kumar, U., Singh, N. R., and Thatoi, H. (2020). Functional diversity and metabolic profile of microbial community of mine soils with different levels of chromium contamination. Int. J. Environ. Health. Res. 30, 461–473. doi:10.1080/09603123.2019.1601686
Pratas, J., Prasad, M. N. V., Freitas, H., and Conde, L. (2005). Plants growing in abandoned mines of Portugal are useful for biogeochemical exploration of arsenic, antimony, tungsten and mine reclamation. J. Geochem. Explor. 85, 99–107. doi:10.1016/j.gexplo.2004.11.003
Pu, W., Sun, J., Zhang, F., Wen, X., Liu, W., and Huang, C. (2019). Effects of copper mining on heavy metal contamination in a rice agrosystem in the Xiaojiang River Basin, southwest China. Acta. Geochim. 38, 753–773. doi:10.1007/s11631-019-00321-5
Pugnaloni, A., Giantomassi, F., Lucarini, G., Capella, S., Bloise, A., di Primio, R., et al. (2013). Cytotoxicity induced by exposure to natural and synthetic tremolite asbestos: an in vitro pilot study. Acta. Histochem. 115, 100–112. doi:10.1016/j.acthis.2012.04.004
Pulford, I. D., Watson, C., and McGregor, S. D. (2001). Uptake of chromium by trees: prospects for phytoremediation. Environ. Geochem. Health. 23, 307–311. doi:10.1023/a:1012243129773
Punamiya, P., Datta, R., Sarkar, D., Barber, S., Patel, M., and Das, P. (2010). Symbiotic role of Glomus mosseae in phytoextraction of lead in vetiver grass [Chrysopogon zizanioides (L.)]. J. Hazard. Mat. 177, 465–474. doi:10.1016/j.jhazmat.2009.12.056
Punia, A., Siddaiah, N. S., and Singh, S. K. (2017). Source and assessment of metal pollution at Khetri copper mine tailings and neighboring soils, Rajasthan, India. Bull. Environ. Contam. Toxicol. 99, 633–641. doi:10.1007/s00128-017-2175-6
Qiao, D., Wang, G., Li, X., Wang, S., and Zhao, Y. (2020). Pollution, sources and environmental risk assessment of heavy metals in the surface AMD water, sediments and surface soils around unexploited Rona Cu deposit, Tibet, China. Chemosphere 248, 125988. doi:10.1016/j.chemosphere.2020.125988
Qin, C., Luo, C., Chen, Y., and Shen, Z. (2012). Spatial-based assessment of metal contamination in agricultural soils near an abandoned copper mine of Eastern China. Bull. Environ. Contam. Toxicol. 89, 113–118. doi:10.1007/s00128-012-0639-2
Qin, F. X., Wei, C. F., Zhong, S. Q., Huang, X. F., Pang, W. P., and Jiang, X. (2016). Soil heavy metal(loid)s and risk assessment in vicinity of a coal mining area from southwest Guizhou, China. China. J. Cent. South. Univ. 23, 2205–2213. doi:10.1007/s11771-016-3278-7
Qu, C., Sun, K., Wang, S., Huang, L., and Bi, J. (2012). Monte Carlo simulation-based health risk assessment of heavy metal soil pollution: a case study in the Qixia mining area, China. Hum. Ecol. Risk. Assess. Int. J. 18, 733–750. doi:10.1080/10807039.2012.688697
Raj, D., Chowdhury, A., and Maiti, S. K. (2017). Ecological risk assessment of mercury and other heavy metals in soils of coal mining area: a case study from the eastern part of a Jharia coal field, India. Hum. Ecol. Risk. Assess. Int. J. 23, 767–787. doi:10.1080/10807039.2016.1278519
Raj, D., Kumar, A., and Maiti, S. K. (2019). Evaluation of toxic metal (loid) s concentration in soils around an open-cast coal mine (Eastern India). Environ. Earth. Sci. 78, 645–664. doi:10.1007/s12665-019-8657-6
Ramos, D. T., Maranho, L. T., Godoi, A. F. L., Filho, M. A. S. C., and Lacerda, L. G. (2009). Vasconcelos, E.C. Petroleum hydrocarbons rhizodegradation by Sebastiania commersoniana (Baill.) L. B. SM. and downs. Water. Air. Soil. Pollut. 9, 293–302. doi:10.1007/s11267-009-9208-z
Ran, H., Guo, Z., Yi, L., Xiao, X., Zhang, L., Hu, Z., et al. (2021). Pollution characteristics and source identification of soil metal (loid) s at an abandoned arsenic-containing mine, China. J. Hazard. Mat. 413, 125382. doi:10.1016/j.jhazmat.2021.125382
Rasheed, F., Zafar, Z., Waseem, Z. A., Rafay, M., Abdullah, M., Salam, M. M. A., et al. (2020). Phytoaccumulation of Zn, Pb, and Cd in Conocarpus lancifolius irrigated with wastewater: does physiological response influence heavy metal uptake. Int. J. Phytoremediation. 22, 287–294. doi:10.1080/15226514.2019.1658711
Reichl, C., Schatz, M., and Zsak, G. (2020). World mining data 2020. Fed. Ministry Agric. Regions Tour. 35, 265.
Robinson, B. H., Leblanc, M., Petit, D., Brooks, R. R., Kirkman, J. H., and Gregg, P. E. (1998). The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant. Soil 203, 47–56. doi:10.1023/a:1004328816645
Sakakibara, M., Watanabe, A., Inoue, M., Sano, S., and Kaise, T. (2010). Phytoextraction and phytovolatilization of arsenic from as-contaminated soils by Pteris vittata. Proc. Annu. Int. Conf. Soils. Sediments. Water. Energy. 12, 26.
Saran, A., Fernandez, L., Cora, F., Savio, M., Thijs, S., Vangronsveld, J., et al. (2020). Phytostabilization of Pb and Cd polluted soils using Helianthus petiolaris as pioneer aromatic plant species. Int. J. Phytoremediation 22, 459–467. doi:10.1080/15226514.2019.1675140
Sarangi, A. K. (2003). Grade control in Jaduguda uranium mine. Jharkhand. Trans. Min. Geol. Metallurgical Inst. India 99, 2002–2003.
Sawidis, T., Breuste, J., Mitrovic, M., Pavlovic, P., and Tsigaridas, K. (2011). Trees as bioindicator of heavy metal pollution in three European cities. Environ. Pollut. 159, 3560–3570. doi:10.1016/j.envpol.2011.08.008
Sawut, R., Tiyip, T., Abliz, A., Kasim, N., Nurmemet, I., Sawut, M., et al. (2017). Using regression model to identify and evaluate heavy metal pollution sources in an open pit coal mine area, Eastern Junggar, China. Environ. Earth. Sci. 76, 822–835. doi:10.1007/s12665-017-7035-5
Schindler, F. V., Mercer, E. J., and Rice, J. A. (2007). Chemical characteristics of glomalin-related soil protein (GRSP) extracted from soils of varying organic matter content. Soil. Biol. biochem. 39, 320–329. doi:10.1016/j.soilbio.2006.08.017
Schneegurt, M. A., Jain, J. C., Menicucci, J. A., Brown, S. A., Kemner, K. M., Garofalo, D. F., et al. (2001). Biomass byproducts for the remediation of wastewaters contaminated with toxic metals. Environ. Sci. Technol. 35, 3786–3791. doi:10.1021/es010766e
Sethy, N. K., Jha, V. N., Sutar, A. K., Rath, P., Sahoo, S. K., Ravi, P. M., et al. (2014). Assessment of naturally occurring radioactive materials in the surface soil of uranium mining area of Jharkhand, India. J. Geochem. Explor. 142, 29–35. doi:10.1016/j.gexplo.2013.11.009
Sethy, N. K., Tripathi, R. M., Jha, V. N., Sahoo, S. K., Shukla, A. K., and Puranik, V. D. (2011). Assessment of natural uranium in the ground water around Jaduguda uranium mining complex, India. J. Environ. Prot. 2, 1002–1007. doi:10.4236/jep.2011.27115
Shahandeh, H., and Hossner, L. R. (2002). Role of soil properties in phytoaccumulation of uranium. Water. Air. Soil. Pollut. 141, 165–180. doi:10.1023/a:1021346828490
Shanker, A. K., Cervantes, C., Loza-Tavera, H., and Avudainayagam, S. (2005). Chromium toxicity in plants. Environ. Int. 31, 739–753. doi:10.1016/j.envint.2005.02.003
Shao, X., Cheng, H., Duan, X., and Lin, C. (2013). Concentrations and chemical forms of heavy metals in agricultural soil near the world’s largest and oldest tungsten mine located in China. Chem. Speciat. Bioavailab. 25, 125–132. doi:10.3184/095422913x13707909633728
Sheppard, S. C., Evenden, W. G., and Anderson, A. J. (1992). Multiple assays of uranium toxicity in soil. Environ. Toxicol. Water. Qual. 7, 275–294. doi:10.1002/tox.2530070307
Shin, D. C., Kim, Y. S., Moon, J. Y., Park, H. S., Kim, J. Y., and Park, S. K. (2002). International trends in risk management of groundwater radionuclides. J. Environ. Toxicol. 17, 273–284.
Shrestha, B., Lipe, S., Johnson, K. A., Zhang, T. Q., Retzlaff, W., and Lin, Z. (2006). Soil hydraulic manipulation and organic amendment for the enhancement of selenium volatilization in a soil-pickleweed system. Plant. Soil. 288, 189–196. doi:10.1007/s11104-006-9107-2
Singh, P. K., Singh, M., and Vyas, D. (2010). Biocontrol of fusarium wilt of chickpea using arbuscular mycorrhizal fungi and Rhizobium leguminosorum biovar. Caryologia 63, 349–353. doi:10.1080/00087114.2010.10589745
Smith, S. E., and Smith, F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu., Rev. Plant. Biol. 62, 227–250. doi:10.1146/annurev-arplant-042110-103846
Sofianska, E., and Michailidis, K. (2016). Assessment of heavy metals contamination and potential ecological risk in soils affected by a former Mn mining activity, drama district, northern Greece. Soil. Sediment. Contam. Int. J. 25, 296–312. doi:10.1080/15320383.2016.1133560
Solgi, E., and Parmah, J. (2015). Analysis and assessment of nickel and chromium pollution in soils around Baghejar chromite mine of Sabzevar Ophiolite belt, Northeastern Iran. Trans. Nonferrous Metals Soc. China 25, 2380–2387. doi:10.1016/s1003-6326(15)63853-5
Solís-Domínguez, F. A., Valentín-Vargas, A., Chorover, J., and Maier, R. M. (2011). Effect of arbuscular mycorrhizal fungi on plant biomass and the rhizosphere microbial community structure of mesquite grown in acidic lead/zinc mine tailings. Sci. Total. Environ. 409, 1009–1016. doi:10.1016/j.scitotenv.2010.11.020
Spagnoletti, F. N., Cornero, M., Chiocchio, V., Lavado, R. S., and Roberts, I. N. (2020). Arbuscular mycorrhiza protects soybean plants against Macrophomina phaseolina even under nitrogen fertilization. Eur. J. Plant. Pathol. 156, 839–849. doi:10.1007/s10658-020-01934-w
Stojanović, M. D., Stevanović, D. R., Milojković, J. V., Grubišić, M. S., and Ileš, D. A. (2010). Phytotoxic effect of the uranium on the growing up and development the plant of corn. Water. Air. Soil. Pollut. 209, 401–410. doi:10.1007/s11270-009-0208-4
Sun, B., Zhang, L., Yang, L., Zhang, F., Norse, D., and Zhu, Z. (2012). Agricultural non-point source pollution in China: causes and mitigation measures. AMBIO. 41, 370–379. doi:10.1007/s13280-012-0249-6
Sun, Z., Xie, X., Wang, P., Hu, Y., and Cheng, H. (2018). Heavy metal pollution caused by small-scale metal ore mining activities: a case study from a polymetallic mine in South China. Sci. Total. Environ. 639, 217–227. doi:10.1016/j.scitotenv.2018.05.176
Tahmasebi, P., Taheri, M., and Gharaie, M. H. (2020). Heavy metal pollution associated with mining activity in the Kouh-e Zar region, NE Iran. Bull. Eng. Geol. Environ. 79, 1113–1123. doi:10.1007/s10064-019-01574-3
Tangahu, B. V., Sheikh Abdullah, S. R., Basri, H., Idris, M., Anuar, N., and Mukhlisin, M. (2011). A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 1–31. doi:10.1155/2011/939161
Thatoi, H., Das, S., Mishra, J., Rath, B. P., and Das, N. (2014). Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J. Environ. Manage. 146, 383–399. doi:10.1016/j.jenvman.2014.07.014
Topal, M., Senel, G. U., Obek, E., and Topal, E. I. A. (2015). Removal of tetracycline and the degradation products by Lemna gibba L. exposed to secondary effluents. Environ. Prog. Sustain. Energy. 34, 1311–1325. doi:10.1002/ep.12118
Tripathi, N., Singh, R. S., and Nathanail, C. P. (2014). Mine spoil acts as a sink of carbon dioxide in Indian dry tropical environment. Sci. Total. Environ. 468, 1162–1171. doi:10.1016/j.scitotenv.2013.09.024
Turner, M. A., and Rust, R. H. (1971). Effects of chromium on growth and mineral nutrition of soybeans. Soil. Sci. Soc. Am. J. 35, 755–758. doi:10.2136/sssaj1971.03615995003500050035x
USEPA (1999). Draft guidelines for carcinogen risk assessment (review draft). Washington, D.C: U. S. Environmental Protection Agency, Risk Assess Forum.
Vaverková, M. D., Maxianová, A., Winkler, J., Adamcová, D., and Podlasek, A. (2019). Environmental consequences and the role of illegal waste dumps and their impact on land degradation. Land. use. Policy 89, 104234. doi:10.1016/j.landusepol.2019.104234
Verdejo, J., Ginocchio, R., Sauvé, S., Salgado, E., and Neaman, A. (2015). Thresholds of copper phytotoxicity in field-collected agricultural soils exposed to copper mining activities in Chile. Ecotoxicol. Environ. Saf. 122, 171–177. doi:10.1016/j.ecoenv.2015.07.026
Vos, C. M., Tesfahun, A. N., Panis, B., de Waele, D., and Elsen, A. (2012). Arbuscular mycorrhizal fungi induce systemic resistance in tomato against the sedentary nematode Meloidogyne incognita and the migratory nematode Pratylenchus penetrans. Appl. Soil. Ecol. 61, 1–6. doi:10.1016/j.apsoil.2012.04.007
Wang, D., Xu, Q., Zheng, Q., and Wu, L. (2020). Assessment of the health effects of heavy metals pollution of agricultural soils in the iron ore mining area of the northern Piedmont of mount Wutai, Shanxi province, China. Sustainability 12, 1926. doi:10.3390/su12051926
Wang, G., Cao, F., Shan, B., Meng, M., Wang, W., and Sun, R. (2019). Sources and assessment of mercury and other heavy metal contamination in soils surrounding the Wuda underground coal fire area, Inner Mongolia, China. Bull. Environ. Contam. Toxicol. 103, 828–833. doi:10.1007/s00128-019-02734-7
Wang, L. K., Hung, Y. T., and Shammas, N. K. (2009). Handbook of advanced industrial and hazardous wastes treatment. Boca Raton: CRC Press. doi:10.1201/9781420072228
Wang, M., Zhu, Y., Cheng, L., Andserson, B., Zhao, X., Wang, D., et al. (2018). Review on utilization of biochar for metal-contaminated soil and sediment remediation. J. Environ. Sci. 63, 156–173. doi:10.1016/j.jes.2017.08.004
Wang, Z., Hong, C., Xing, Y., Wang, K., Li, Y., Feng, L., et al. (2018). Spatial distribution and sources of heavy metals in natural pasture soil around copper-molybdenum mine in Northeast China. Ecotoxicol. Environ. Saf. 154, 329–336. doi:10.1016/j.ecoenv.2018.02.048
Wang, Z., Qin, H., and Liu, X. (2019). Health risk assessment of heavy metals in the soil-water-rice system around the Xiazhuang uranium mine, China. Environ. Sci. Pollut. Res. 26, 5904–5912. doi:10.1007/s11356-018-3955-1
Wani, S. H., Sanghera, G. S., Athokpam, H., Nongmaithem, J., Nongthongbam, R., Naorem, B. S., et al. (2012). Phytoremediation: curing soil problems with crops. Afr. J. Agric. Res. 7, 3991–4002. doi:10.5897/ajar12.1061
Wieczorek, J., Baran, A., Urbański, K., Mazurek, R., and Klimowicz-Pawlas, A. (2018). Assessment of the pollution and ecological risk of lead and cadmium in soils. Environ. Geochem. Health. 40, 2325–2342. doi:10.1007/s10653-018-0100-5
World Health Organization (1996). Permissible limits of heavy metals in soil and plants. Geneva, Switzerland.
World steel Association (2021). Available at: https://www.worldsteel.org/steel-by-topic/statistics/steel-statistical-yearbook.html.
Wright, S. F., and Upadhyaya, A. (1996). Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil. Sci. 161, 575–586. doi:10.1097/00010694-199609000-00003
Wu, B., Peng, H., Sheng, M., Luo, H., Wang, X., Zhang, R., et al. (2021). Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 220, 112368. doi:10.1016/j.ecoenv.2021.112368
Wu, F., Liu, Y., Xia, Y., Shen, Z., and Chen, Y. (2011). Copper contamination of soils and vegetables in the vicinity of Jiuhuashan copper mine, China. Environ. Earth. Sci. 64, 761–769. doi:10.1007/s12665-010-0897-4
Wu, F., Wang, X., Tan, K., and Liu, Z. (2020). “Assessment of heavy metal pollution in agricultural soil around a gold mine area in Yitong county,” in Igarss 2020-2020 IEEE Int. Geosci. Remote. Sensing. Symposium, 5034–5037.
Wu, J., Long, J., Liu, L., Li, J., Liao, H., Zhang, M., et al. (2018). Risk assessment and source identification of toxic metals in the agricultural soil around a Pb/Zn mining and smelting area in Southwest China. Int. J. Environ. Res. Public. Health. 15, 1838. doi:10.3390/ijerph15091838
Wu, Q. S., Li, Y., Zou, Y. N., and He, X. H. (2015). Arbuscular mycorrhiza mediates glomalin-related soil protein production and soil enzyme activities in the rhizosphere of trifoliate orange grown under different P levels. Mycorrhiza 25, 121–130. doi:10.1007/s00572-014-0594-3
Wu, S. C., Wong, C. C., Shu, W. S., Khan, A. G., and Wong, M. H. (2010). Mycorrhizo-remediation of lead/zinc mine tailings using vetiver: a field study. Int. J. Phytoremediation 13, 61–74. doi:10.1080/15226511003671353
Xiao, R., Wang, S., Li, R., Wang, J. J., and Zhang, Z. (2017). Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 141, 17–24. doi:10.1016/j.ecoenv.2017.03.002
Xie, W., Peng, C., Wang, H., and Chen, W. (2018). Bioaccessibility and source identification of heavy metals in agricultural soils contaminated by mining activities. Environ. Earth. Sci. 77, 606–612. doi:10.1007/s12665-018-7783-x
Xue, S., Shi, L., Wu, C., Wu, H., Qin, Y., Pan, W., et al. (2017). Cadmium, lead, and arsenic contamination in paddy soils of a mining area and their exposure effects on human HEPG2 and keratinocyte cell-lines. Environ. Res. 156, 23–30. doi:10.1016/j.envres.2017.03.014
Yao, Y., Li, J., He, C., Hu, X., Yin, L., Zhang, Y., et al. (2021). Distribution characteristics and relevance of heavy metals in soils and colloids around a mining area in Nanjing, China. Bull. Environ. Contam. Toxicol. 107, 996–1003. doi:10.1007/s00128-021-03350-0
Ye, L. L., Chen, Y. S., Chen, Y. D., Qian, L. W., Xiong, W. L., Xu, J. H., et al. (2020). Phytomanagement of a chromium-contaminated soil by a high-value plant: phytostabilization of heavy metal contaminated site. BioResour. 15, 3545–3565. doi:10.15376/biores.15.2.3545-3565
Yihui, B. A. N., Zhouying, X. U., Yurong, Y. A. N. G., Zhang, H., Hui, C. H. E. N., and Ming, T. A. N. G. (2017). Effect of dark septate endophytic fungi Gaeumannomyces cylindrosporus on plant growth, photosynthesis and Pb tolerance of maize (Zea mays L.). Pedosphere 27, 283–292. doi:10.1016/s1002-0160(17)60316-3
Yoon, J. M., Oliver, D. J., and Shanks, J. V. (2008). Phytotransformation of 2,4-dinitrotoluene in Arabidopsis thaliana: toxicity, fate, and gene expression studies in vitro. Biotechnol. Program 22, 1524–1531. doi:10.1021/bp0601443
You, M., Huang, Y., Lu, J., and Li, C. (2016). Fractionation characterizations and environmental implications of heavy metal in soil from coal mine in Huainan, China. Environ. Earth. Sci. 75, 78–79. doi:10.1007/s12665-015-4815-7
Young, G., Chen, Y., and Yang, M. (2021). Concentrations, distribution, and risk assessment of heavy metals in the iron tailings of Yeshan National Mine Park in Nanjing, China. Chemosphere 271, 129546. doi:10.1016/j.chemosphere.2021.129546
Yruela, I. (2013). Transition metals in plant photosynthesis. Metallomics. 5, 1090–1109. doi:10.1039/c3mt00086a
Yu, G., Chen, F., Zhang, H., and Wang, Z. (2021). Pollution and health risk assessment of heavy metals in soils of Guizhou, China. Ecosys. Health. Sustain. 7, 1859948. doi:10.1080/20964129.2020.1859948
Yu, P., Sun, Y., Huang, Z., Zhu, F., Sun, Y., and Jiang, L. (2020). The effects of ectomycorrhizal fungi on heavy metals’ transport in Pinus massoniana and bacteria community in rhizosphere soil in mine tailing area. J. Hazard. Mat. 381, 121203. doi:10.1016/j.jhazmat.2019.121203
Zádrapová, D., Titěra, A., Száková, J., Čadková, Z., Cudlín, O., Najmanová, J., et al. (2019). Mobility and bioaccessibility of risk elements in the area affected by the long-term opencast coal mining. J. Environ. Sci. Health 54, 1159–1169. doi:10.1080/10934529.2019.1633854
Zayed, A., Lytle, C. M., Qian, J. H., and Terry, N. (1998). Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206, 293–299. doi:10.1007/s004250050403
Zazouli, M. A., Mahdavi, Y., Bazrafshan, E., and Balarak, D. (2014). Phytodegradation potential of bisphenolA from aqueous solution by Azolla Filiculoides. J. Environ. Health. Sci. Eng. 12, 66. doi:10.1186/2052-336x-12-66
Zhan, F., Li, B., Jiang, M., Li, T., He, Y., Li, Y., et al. (2019). Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int. J. Phytoremediation 21, 849–856. doi:10.1080/15226514.2019.1577353
Zhang, H., Zhang, F., Song, J., Tan, M. L., and Johnson, V. C. (2021). Pollutant source, ecological and human health risks assessment of heavy metals in soils from coal mining areas in Xinjiang, China. Environ. Res. 202, 111702. doi:10.1016/j.envres.2021.111702
Zhang, Z., Wang, Q., Wang, H., Nie, S., and Liang, Z. (2017). Effects of soil salinity on the content, composition, and ion binding capacity of glomalin-related soil protein (GRSP). Sci. Total. Environ. 81, 657–665. doi:10.1016/j.scitotenv.2016.12.176
Zhao, X., Sun, Y., Huang, J., Wang, H., and Tang, D. (2020). Effects of soil heavy metal pollution on microbial activities and community diversity in different land use types in mining areas. Environ. Sci. Pollut. Res. 27, 20215–20226. doi:10.1007/s11356-020-08538-1
Zheng, X. J., Chen, M., Wang, J. F., Li, F. G., Liu, Y., and Liu, Y. C. (2020). Ecological risk assessment of heavy metals in the vicinity of tungsten mining areas, Southern Jiangxi province. Soil. Sediment. Contam. Int. J. 29, 665–679. doi:10.1080/15320383.2020.1763912
Zhong, W., Li, J., Chen, Y., Shu, W., and Liao, B. (2012). A study on the effects of lead, cadmium and phosphorus on the lead and cadmium uptake efficacy of Viola baoshanensis inoculated with arbuscular mycorrhizal fungi. J. Environ. Monit. 14, 2497–2504. doi:10.1039/c2em30333g
Zhou, H., Zeng, M., Zhou, X., Liao, B. H., Liu, J., Lei, M., et al. (2013). Assessment of heavy metal contamination and bioaccumulation in soybean plants from mining and smelting areas of southern Hunan Province, China. Environ. Toxicol. Chem. 32, 2719–2727. doi:10.1002/etc.2389
Zhu, X., Cao, L., and Liang, Y. (2019). Spatial distribution and risk assessment of heavy metals inside and outside a typical lead-zinc mine in southeastern China. Environ. Sci. Pollut. Res. 26, 26265–26275. doi:10.1007/s11356-019-05724-8
Keywords: mines, heavy metals, phytoremediation, arbuscular mycorrhizal fungi, meta-analysis
Citation: Banerjee S, Mandal J, Sarkar D, Datta R and Bhattacharyya P (2025) A review and meta-analysis of the efficacy of arbuscular mycorrhizal fungi in remediating toxic metals in mine-affected soils. Front. Environ. Sci. 12:1532169. doi: 10.3389/fenvs.2024.1532169
Received: 22 November 2024; Accepted: 12 December 2024;
Published: 10 January 2025.
Edited by:
Pavol Midula, Czech University of Life Sciences Prague, CzechiaReviewed by:
Sudipta Tripathi, Ramakrishna Mission Vivekananda Educational and Research Institute, IndiaCopyright © 2025 Banerjee, Mandal, Sarkar, Datta and Bhattacharyya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pradip Bhattacharyya, cHJhZGlwLmJoYXR0YWNoYXJ5eWFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.