
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Environ. Health, 22 May 2023
Sec. Occupational Safety and Health Interventions
Volume 2 - 2023 | https://doi.org/10.3389/fenvh.2023.1159304
 Lanlan Guo1,2
Lanlan Guo1,2 Zhiqiang Zhou1,2
Zhiqiang Zhou1,2 Ping Dai2
Ping Dai2 Tongyue Zhang2
Tongyue Zhang2 Aerbusili Genjiafu1,2
Aerbusili Genjiafu1,2 Tianzi Jian2,3
Tianzi Jian2,3 Zixin Wen2,4
Zixin Wen2,4 Liwen Zhao1,2
Liwen Zhao1,2 Qilu Li5*
Qilu Li5* Xiangdong Jian1,2*†
Xiangdong Jian1,2*†
Lambda-cyhalothrin is a new-generation pyrethroid II insecticide that is neurotoxic. Most domestic and international reported cases are of oral poisoning, whereas few cases of inhalation and skin absorption poisoning have been reported. Here, we report the case of a 46-year-old man who was poisoned via dermal absorption and inhalation due to the rupture and leakage of the lambda-cyhalothrin pipeline. The patient developed a skin burning sensation, eyeball pain, and upper-limb tremor after exposure. After admission, the patient developed cough, sputum expectoration, chest tightness, and other symptoms. Lung computerized tomography indicated double pneumonia and the patient's electroencephalogram result was abnormal; the patient's condition improved after treatment.
Lambda-cyhalothrin (C23H19CIF3NO3, CAS number: 91465-08-6) has a molecular weight of 449.9 and a relative density (water = 1) of 1.3. According to the World Health Organization classification, it is a Class II (moderately toxic) chemical (1). Inhalation can cause symptoms such as burning sensation, cough, expectoration, and dyspnea. Skin and eye contact causes redness and pain. Poisoning can cause tremors, convulsions, and other neurological symptoms. Long-term repeated exposure can also cause chronic toxicity in the immune, reproductive, skin, and other systems (2–5). In recent years, reports of pyrethroid inhalation have focused on experimental or long-term exposure to pyrethroid pollution in the atmosphere of study (6–9). Reports of poisoning from lambda-cyhalothrin leaks are rare. Here, we report a case of lambda-cyhalothrin intoxication in a lambda-cyhalothrin leak accident who presented with skin burns, upper limbs tremors, cough, phlegm, and tingling eyes. This study was approved by the Ethics Committee of Qilu Hospital of Shandong University, and informed consent was obtained from the patient.
A 46-year-old man was received at our emergency department from a local hospital following pesticide exposure on July 5, 2022. He was agitated by a severe tingling sensation in his eyeballs. After the local doctor irrigated his eyes, he was transferred to our hospital for “inadvertent exposure of the eyes and skin to pesticides for approximately 5 h.” The patient had worked in a pesticide production company for more than 10 years and was responsible for the unprocessed pesticide feeding process (Figure 1). Specifically, he handled approximately 25 kg of unprocessed lambda-cyhalothrin (Figure 2) and other ingredients by introducing them into the feeding port. On the morning of July 5, 2022, the patient closed the discharging mouth after the feeding port was blocked, causing the tank pressure to increase. The circulation line burst, spraying out approximately 2 kg of liquid lambda-cyhalothrin that drenched the eyes and skin of the patient, who was not wearing any protective equipment, apart from a normal dust mask. Before going to the local hospital outpatient that day, he washed his eyes and the whole body because his eyes were stinging and his entire skin was numb. On the way to the hospital the same afternoon, he was fully conscious, although still experiencing the stinging pain in his eyes, numbness in his whole body, and tremor in both upper limbs; he had no nausea, vomiting, dizziness, or headache.

Figure 1. Work site environment. (Part A) Is the tank, which built up pressure and the circulation line ruptured; (Part B) Part is the blower, which caused the feed port to be blocked.

Figure 2. Unprocessed lambda-cyhalothrin. This bag of raw materials weighs approximately 25 kg and was the main cause of poisoning; it is mixed with other ingredients in a jar.
Physical examination at admission revealed a temperature of 36.3°C, pulse of 73 beats/min, respiratory rate of 18 breaths/min, and blood pressure of 147/93 mmHg. Laboratory test results are shown in Table 1. The patient was conscious, energetic, had bilateral bulbar conjunctival hyperemia, bilateral pupillary size of 3 mm, and a positive pupillary light reflex. There was no neck stiffness. Bilateral chest movements and breath sounds were normal and no wet or dry rales were heard. The heart rate was 73 beats/min, with a uniform rhythm and the absence of pathological murmurs in any of the valve areas. The abdomen was flat without tenderness or rebound pain, and the liver and spleen were not palpable. There was no deformity of the ribs, spine, or limbs. Physiological reflexes were present, but pathological reflexes were absent. Flaky skin burns were observed above the cubital fossa on the left upper arm. He had a history of coronary heart disease for one year and denied any other illness. Diagnosis at admission was lambda-cyhalothrin poisoning and skin burn.
After admission, the patient was given ECG monitoring and was administered flucloxacillin (1 g, intravenous infusion, every six hours) to fight infections; betamethasone sodium phosphate (100 mg, intravenous infusion, once a day) to treat inflammation; polyene phosphatidylcholine (232.5 mg, intravenous infusion, once a day) to protect the liver; alanyl glutamine (20 g, intravenous infusion, once a day), fatty milk amino acid (17), and glucose (11%) injection to support nutrition; torasemide (20 mg, intravenous injection, twice a day) to promote diuresis; and other comprehensive treatments. The patient complained of unbearable pain in both eyes and pain and swelling in the left forearm. Dezocine (5 mg, intramuscular injection) and continuous micropump for midazolam were administered for analgesia. The HA330 perfusion device was used to remove the poison in the blood; perfusion was performed twice on the first day after admission and once a day on the second and third days. On July 6, 2022, the patient complained of increased cough, sputum, and occasional chest tightness. We scheduled the patient for chest computerized tomography (CT) the following day. Chest CT scans showed increased texture in both lungs with multiple patchy high-density shadows with blurred edges in the lower lobe (Figure 3), cable strip high-density foci, punctate calcified foci, and coronary artery calcification. The brain and abdomen CT scans revealed no abnormalities. Diagnosis was aspiration pneumonia, and intravenous flucloxacillin sodium was administered every 6 h. Chest CT scans on July 10, 2022, indicated double pneumonia that showed improvement compared to the July 7, 2022, scans, although with persistence of coronary artery calcification. Methylprednisolone (40 mg, intravenous infusion, once daily) was administered for hormone adjustment. Pulmonary function test result indicated mild obstructive ventilatory dysfunction. On July 12, 2022, the electroencephalogram result was mildly abnormal, with a low-amplitude slow wave between the low voltage and significant P4. On July 14, 2022, the patient had a fever with the highest temperature of 38.9 °C. Diclofenac sodium (2 ml, intramuscular injection) was administered to reduce fever. Procalcitonin level was 0.147 ng/ml. Catheter tip culture and drug sensitivity tests showed positivity for Enterobacter cloacae, and the antibiotic was adjusted to etimicin (300 mg, intravenous infusion, once a day). On July 18, 2022, the patient was discharged from the hospital 14 days after hospitalization. Telephone follow-up for the next 1 month showed that the patient lived normally, with no respiratory or nervous system abnormalities nor complaints of discomfort. On July 25, 2022, the patient visited the hospital for reexamination, and chest CTs (Figure 4) indicated double pneumonia, which had improved from July 10, 2022, and coronary artery calcification. The EEG results were normal.
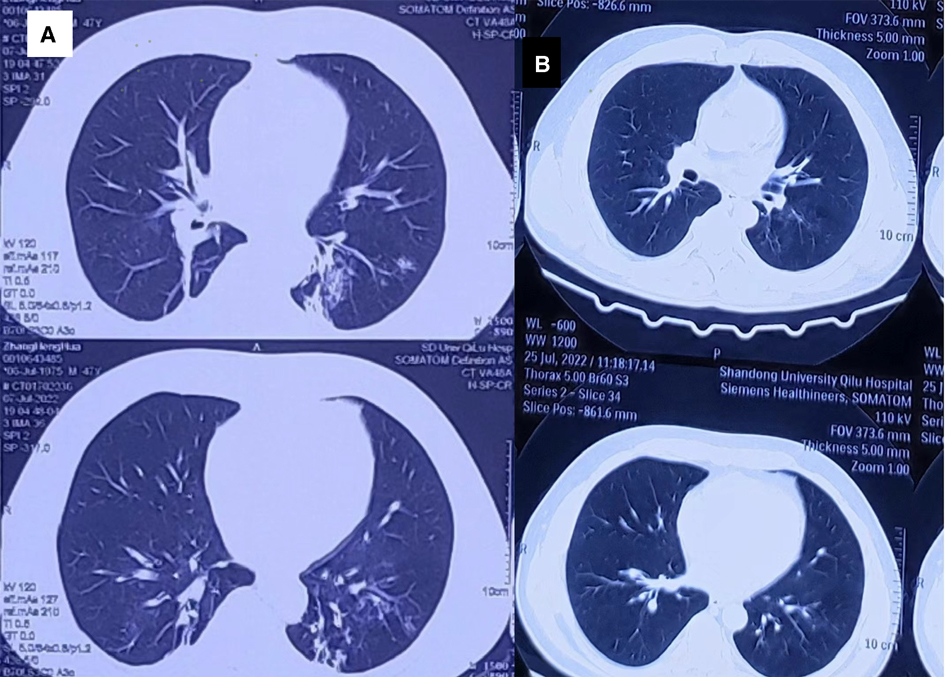
Figure 3. Computed tomography (CT) changes of the patient's lungs. (A) Shows increased texture in both lungs and multiple patchy high-density shadows with blurred edges in the lower lobe. Cable strip high-density foci, punctate calcification foci, and coronary artery calcification are also observed in both lungs, which were most likely caused by inhalation of lambda-cyhalothrin. (B) Shows computed tomography findings of the lungs on July 25, 2022; the pneumonia improved after treatment compared to the presentation in (A).
Lambda-cyhalothrin is a class of synthetic type II pyrethroid insecticides, which is the safest class of existing insecticides because of its broad-spectrum effectiveness, high insecticide efficiency, low toxicity to mammals, and low environmental diffusion (10). In recent decades, as the utilization rate of organophosphorus pesticides has decreased, the use of pyrethroids has increased (11). In 2015, pyrethroids accounted for approximately 38% of the global insecticide market (12). Pyrethroids are highly lipophilic, with higher oral and inhalation absorption rates and lower skin absorption (13); however, transcutaneous absorption has also been reported (14, 15).
Due to lipophilicity, pyrethroids easily cross the blood–brain barrier and are neurotoxic, mainly involving the extrapyramidal system, cerebellar system, spinal cord, and peripheral nerves (16). The toxicity of pyrethroids in insects is 2,250 times higher than that in mammals (17), which is due to the higher nerve sensitivity of insects, lower skin absorption of mammals, and more efficient liver metabolism in mammals (18). Depending on whether cyano is present, pyrethroids can be divided into type I (without cyano) and type II (with cyano), both of which may cause neurological symptoms (19), including sympathetic activation, tremors, and epileptiform seizures (20). Cyhalothrin causes benign damage to the central nervous system, with a good prognosis and few residual neurological deficits (21). In this report, the patient developed involuntary tremor in both upper limbs on the day of poisoning, and the electroencephalogram showed mild abnormalities on the eighth day of poisoning, which returned to normal on the 14th day and was considered transient nerve damage.
Neural voltage-gated sodium channels (VGSCs) are important targets for neurotoxicity of pyrethroids in mammals, with voltage-gated calcium and chloride channels as secondary action sites (22). Sensory organs and nerve endings are most sensitive to the effects of pyrethroids, although their toxic effects are not limited to one region of the nervous system. In a previous study, all pyrethroids showed similar effects on sodium channel gating, but there were significant differences in neurotoxicity between cyanide and non-cyanide compounds (23). Pyrethroids II prolonged sodium channel inactivation, resulting in continued depolarization of the nerve membrane and decreased action potential amplitude (11). Permethrin acts on VGSCs, Nav1.6, Nav1.3, and Nav1.8 (19), disrupting neural function by altering the rapid dynamic transition of VGSCs between different states. Pyrethroids also affect other voltage-gated and ligand-gated ion channels. Prolonged occupational exposure may cause skin paresthesia, including stinging, itching, and burning sensations on the skin. This is because pyrethroids prolong VGSCs, peripheral nerve membrane depolarization, and synaptic transmission and increase sensory neuron firing (24).
There is no specific antidote for pyrethroid poisoning, with symptomatic and supportive treatment as the standard practice (25, 26). For oral ingestion, the stomach is washed with water or activated carbon. Regarding paresthesia due to skin contact, the skin is washed with water or soapy water and vitamin E can be applied to relieve it (27). The usual symptoms of poisoning are dizziness, headache, and gastrointestinal symptoms (28), as well as seizures, myocardial damage, and increased saliva secretion caused by nerve excitation (29). Severe cases present with respiratory failure, pulmonary edema, and coma. Administration of atropine can improve salivation or pulmonary edema, but caution is required due to the risk of atropine poisoning (28). In addition, diazepam can be used to control convulsions and epilepsy (29). Most patients have good prognoses, and death cases are rare (27, 30).
The accident was caused by an accidental rupture of a circulating pipe containing the pesticide, which was subsequently absorbed through the respiratory tract and skin. Percutaneous absorption is less, skin irritation symptoms are obvious. The patient had no history of respiratory disease before poisoning nor any symptoms such as fever, cough, or sputum production. He remained conscious after the accident, did not vomit, and did not lavage his stomach. In addition, he had a clear history of occupational exposure, which suggested that the pneumonia was caused by the inhalation of permethrin. The upper respiratory symptoms were mild, and pneumonia was obvious, possibly because the solubility of lambda-cyhalothrin was low, and the irritation to the upper respiratory tract was mild. Lambda-cyhalothrin could easily enter the lower respiratory tract and lung tissue and cause pneumonia. The patient's workplace was equipped with a ventilation system, but on the day of the accident, the patient was not wearing regular protective clothing and goggles. It is recommended to pay attention to personal protection and standardize operation procedures to avoid recurrence.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Shandong University Qilu Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LG and XJ obtained research funding and investigated the description of the incident. LG conceived the study and drafted the manuscript. PD, ZZ, TZ, AG, TJ and ZW supervised data collection. LG, QL, and XJ take responsibility for the paper as a whole. All authors contributed to the article and approved the submitted version.
We would like to thank the staff of Qilu Hospital of Shandong University for their support in sample and clinical data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization. The WHO recommended classification of pesticides by hazard and guidelines to classification 2019 edition. Geneva: World Health Organization (2020). Available at: http://nyfzx.com/pdf/dx/FAO%20WHO%20Guidelines%20on%20Highly%20Hazardous%20Pesticides%202016.pdf (September 17, 2022).
2. Wang X, Martínez MA, Dai M, Chen D, Ares I, Romero A, et al. Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ Res (2016) 149:86–104. doi: 10.1016/j.envres.2016.05.003
3. Marettova E, Maretta M, Legáth J. Effect of pyrethroids on female genital system. Review. Anim Reprod Sci. (2017) 184:132–8. doi: 10.1016/j.anireprosci.2017.07.007
4. Yoshinaga J, Imai K, Shiraishi H, Nozawa S, Yoshiike M, Mieno MN, et al. Pyrethroid insecticide exposure and reproductive hormone levels in healthy Japanese male subjects. Andrology. (2014) 2:416–20. doi: 10.1111/j.2047-2927.2014.00202.x
5. Meeker JD, Barr DB, Hauser R. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod Toxicol. (2009) 27:155–60. doi: 10.1016/j.reprotox.2008.12.012
6. Yoshida T, Mimura M, Sakon N. Estimating household exposure to pyrethroids and the relative contribution of inhalation pathway in a sample of Japanese children. Environ Sci Pollut Res Int. (2021) 28:19310–24. doi: 10.1007/s11356-020-12060-9
7. Guida Y, Pozo K, Carvalho GO, Capella R, Targino AC, Torres JPM, et al. Occurrence of pyrethroids in the atmosphere of urban areas of southeastern Brazil: inhalation exposure and health risk assessment. Environ Pollut. (2021) 290:118020. doi: 10.1016/j.envpol.2021.118020
8. Pauluhn J. Upper respiratory tract nociceptor stimulation and stress response following acute and repeated cyfluthrin inhalation in normal and pregnant rats: physiological rat-specific adaptions can easily be misunderstood as adversities. Toxicol Lett. (2018) 282:8–24. doi: 10.1016/j.toxlet.2017.10.003
9. Saka WA, Akhigbe RE, Azeez OM, Babatunde TR. Effects of pyrethroid insecticide exposure on haematological and haemostatic profiles in rats. Pak J Biol Sci. (2011) 14:1024–7. doi: 10.3923/pjbs.2011.1024.1027
10. Costa C, Rapisarda V, Catania S, Di Nola C, Ledda C, Fenga C. Cytokine patterns in greenhouse workers occupationally exposed to α-cypermethrin: an observational study. Environ Toxicol Pharmacol. (2013) 36:796–800. doi: 10.1016/j.etap.2013.07.004
11. Bao W, Liu B, Simonsen DW, Lehmler HJ. Association between exposure to pyrethroid insecticides and risk of all-cause and cause-specific mortality in the general US adult population. JAMA Intern Med. (2020) 180:367–74. doi: 10.1001/jamainternmed.2019.6019
12. Andersen HR, David A, Freire C, Fernández MF, D’Cruz SC, Reina-Pérez I, et al. Pyrethroids and developmental neurotoxicity—a critical review of epidemiological studies and supporting mechanistic evidence. Environ Res. (2022) 214:113935. doi: 10.1016/j.envres.2022.113935
13. Côté J, Bouchard M. Dose reconstruction in workers exposed to two major pyrethroid pesticides and determination of biological reference values using a toxicokinetic model. J Expo Sci Environ Epidemiol (2018) 28:599–614. doi: 10.1038/s41370-017-0004-y
14. Hughes MF, Edwards BC. In vitro dermal absorption of pyrethroid pesticides in human and rat skin. Toxicol Appl Pharmacol. (2010) 246:29–37. doi: 10.1016/j.taap.2010.04.003
15. Hughes MF, Edwards BC. In vivo dermal absorption of pyrethroid pesticides in the rat. J Toxicol Environ Health A. (2016) 79:83–91. doi: 10.1080/15287394.2015.1109571
16. Saillenfait AM, Ndiaye D, Sabaté JP. Pyrethroids: exposure and health effects–an update. Int J Hyg Environ Health. (2015) 218:281–92. doi: 10.1016/j.ijheh.2015.01.002
17. Diez-Sepulveda JC, Uribe-Buritica FL, Angel-Isaza AM, Bustamante-Cristancho LA, Mejia-Herrera F, Watts-Pajaro FA, et al. An 80-year-old woman with Alzheimer disease and accidental poisoning with pyrethroid pesticide successfully treated with intravenous lipid emulsion. Am J Case Rep. (2021) 22:e928420. doi: 10.12659/AJCR.928420
18. Skarbinski J, Mwandama D, Wolkon A, Luka M, Jafali J, Smith A, et al. Impact of indoor residual spraying with lambda-cyhalothrin on malaria parasitemia and anemia prevalence among children less than five years of age in an area of intense, year-round transmission in Malawi. Am J Trop Med Hyg. (2012) 86:997–1004. doi: 10.4269/ajtmh.2012.11-0621
19. Chrustek A, Hołynska-Iwan I, Dziembowska I, Bogusiewicz J, Wróblewski M, Cwynar A, et al. Current research on the safety of pyrethroids used as insecticides. Medicina (Kaunas). (2018) 54:E61. doi: 10.3390/medicina54040061
20. Mallick P, Moreau M, Song G, Efremenko AY, Pendse SN, Creek MR, et al. Development and application of a life-stage physiologically based pharmacokinetic (PBPK) model to the assessment of internal dose of pyrethroids in humans. Toxicol Sci. (2020) 173:86–99. doi: 10.1093/toxsci/kfz211
21. Piner P, Üner N. Neurotoxic effects of lambda-cyhalothrin modulated by piperonyl butoxide in the brain of oreochromis niloticus. Environ Toxicol. (2014) 29:1275–82. doi: 10.1002/tox.21858
22. Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol. (2012) 86:165–81. doi: 10.1007/s00204-011-0726-x
23. Vijverberg HP, van den Bercken J. Annotation. Action of pyrethroid insecticides on the vertebrate nervous system. Neuropathol Appl Neurobiol. (1982) 8:421–40. doi: 10.1111/j.1365-2990.1982.tb00311.x
24. Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacol Toxicol. (1996) 79:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x
25. Pallavidino M, Arango Uribe D, Baskaran S, Saqib A, Elmesserey M, Onsy A, et al. Accidental pyrethroid ingestion in toddler: near-fatal atypical presentation and successful recovery. Front Pediatr. 2020;7:542. doi: 10.3389/fped.2019.00542
27. Bradberry SM, Cage SA, Proudfoot AT, Vale JA. Poisoning due to pyrethroids. Toxicol Rev. (2005) 24(2):93–106. doi: 10.2165/00139709-200524020-00003
28. Akelma H, Kilic ET, Salik F, Bicak EA, Yektas A. Pyrethroid intoxication: a rare case report and literature review. Niger J Clin Pract. (2019) 22(3):442–4. doi: 10.4103/njcp.njcp_241_18
29. Ray DE, Forshaw PJ. Pyrethroid insecticides: poisoning syndromes, synergies, and therapy. J Toxicol Clin Toxicol. (2000) 38(2):95–101. doi: 10.1081/clt-100100922
Keywords: acute poisoning, occupational accident, pneumonia, lambda-cyhalothrin poisoning, neurotoxicity
Citation: Guo L, Zhou Z, Dai P, Zhang T, Genjiafu A, Jian T, Wen Z, Zhao L, Li Q and Jian X (2023) Case report: occupational acute poisoning caused by the accidental release of lambda-cyhalothrin. Front. Environ. Health 2:1159304. doi: 10.3389/fenvh.2023.1159304
Received: 21 March 2023; Accepted: 4 May 2023;
Published: 22 May 2023.
Edited by:
Bente Elisabeth Moen, University of Bergen, NorwayReviewed by:
Andrea Kaifie-Pechmann, University Hospital RWTH Aachen, Germany© 2023 Guo, Zhou, Dai, Zhang, Genjiafu, Jian, Wen, Zhao, Li and Jian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qilu Li bGlxaWx1QHNkdS5lZHUuY24= Xiangdong Jian amlhbnhpYW5nZG9uZ3ZpcEB2aXAuMTYzLmNvbQ==
Abbreviations CT, computerized tomography; VGSCs, voltage-gated sodium channels; EEG, electroencephalogram.
†ORCID Xiangdong Jian orcid.org/0000-0002-2277-6817
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.