
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 24 January 2025
Sec. Neuroendocrine Science
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1531723
The protein tyrosine phosphatase receptors N and N2 are encoded by the Ptprn and Ptprn2 genes expressed in neuroendocrine cells of the hypothalamus, pituitary gland, and diffuse neuroendocrine system, including the pancreas, lung, and intestine. Unlike other members of the protein tyrosine phosphatase receptor family, PTPRN and PTPRN2 lack protein tyrosine phosphatase activity due to mutation of two residues in their intracellular catalytic domains. However, during evolution these proteins acquired new cellular roles beyond tyrosine dephosphorylation in the centralized and diffuse neuroendocrine systems. Here we discuss the current understanding and lack of information about the actions of these proteins, focusing on neuroendocrine cells of the hypothalamus, pituitary, and pancreas.
Protein tyrosine phosphatase receptors (PTPRs), a family of proteins encoded by 21 genes in humans, are signaling molecules composed of an extracellular domain, a transmembrane domain, and an intracellular domain. The diverse extracellular domain shares homology with cell adhesion molecules and encompasses a wide range of physiological functions, the transmembrane domain makes them a type of membrane receptor protein and the intracellular domain contains either one or two highly conserved intracellular phosphatase domain (1, 2). Protein tyrosine phosphatase receptor type N (PTPRN, also known as IA-2 and ICA512) and PTPRN2 (also known as IA-2β and phogrin) are atypical members of this protein family. PTPRN consists of 979 amino acids encoded by the Ptprn gene, located on human chromosome 2q35, while PTPRN2 consists of 986 amino acids, and its Ptprn2 gene is located on human chromosome 7q36 (3).
Comparison of the amino acid sequences of human, mouse, and rat PTPRN revealed that the intracellular domain is over 97% conserved in these species, while the extracellular domain is 80–90% conserved. The intracellular domain of PTPRN2 is 92% conserved in human, mouse and rat, whereas the extracellular domain shows only 50–60% identity (3). PTPRN and PTPRN2 share 74% identity within the intracellular, but only 27% in the extracellular domains (4–6). A splice variant of human Ptprn lacking exon 13 encoding the transmembrane region (7, 8), as well as three splice transcripts of human Ptprn2 (9), have been detected in the pancreas. The extracellular domain of PTPRN and PTPRN2 proteins (hereinafter referred to as PTPRNs) show partial homology with the extracellular domain of regulated endocrine specific protein 18, which is another protein marker of neuroendocrine cells (10–12), indicating a potential physiological significance for their function (13).
Western blot analysis and physiological responses suggest that PTPRN is expressed in hippocampal tissues in mice (14, 15). PTPRN expression has also been detected in autonomic nerve fibers and ganglia (16, 17). However, others have argued that PTPRN immunoreactivity is below detectable level in the hippocampus, cerebral cortex, cerebellum, striatum, and thalamus (16). In situ hybridization, western blot, and immunohistochemical analyses revealed PTPRN2 expression in the cerebral cortex, hippocampus, thalamus, choroid plexus, Purkinje cells, granular layer of the cerebellum, and medulla oblongata (18, 19). Immunostaining and immunohistochemical evidence also support PTPRN2 expression in hippocampal interneurons and suggest the existence of molecularly distinct populations of secretory vesicles in different types of inhibitory neurons (20).
The hypothalamus is a central neuroendocrine hub that expresses PTPRN and PTPRN2 proteins and mRNAs (16, 18, 21–23). Within the hypothalamus, Ptprn and Ptprn2 were detected in cells of the arcuate and periventricular nuclei (22) as well as in gonadotroph-releasing hormone (GnRH) neurons (24, 25), and Ptprn+Ptprn2 deletion affected suprachiasmatic function (17). A high level of PTPRN immunoreactivity was detected in the amygdala, which is also considered a neuroendocrine region of the brain. The highest levels of PTPRN immunoreactivity were observed in the infundibular tract, which contains the axons of hypothalamic vasopressin and oxytocin secreting neurons that terminate in the posterior pituitary (16).
Ptprn was originally cloned from the bovine pituitary (26). The rodent pituitary also expresses Ptprn and Ptprn2 (18, 19, 22, 23, 27) as well as immortalized pituitary cells (28). Single cell RNA sequencing experiments with freshly dispersed rat pituitary cells revealed that these genes are expressed in hormone-producing corticotrophs, melanotrophs, gonadotrophs, thyrotrophs, somatotrophs, and lactotrophs, but not in folliculostellate cells and pituicytes (29, 30). Because pituicytes are the resident cells of the posterior pituitary, the finding that PTPRN is detected in the posterior pituitary (16) suggests that nerve endings of hypothalamic vasopressin and oxytocin neurons express this protein.
In addition to neuroendocrine brain and pituitary gland, neuroendocrine cells can be found as single cells or small groups of cells scattered throughout the parenchymal surface epithelium of various tissues, including the pancreas, lung, and intestine. PTPRN is present in alpha, beta, and delta cell of the pancreas, chromaffin cells of the adrenal medulla, and thyroid C cells, also known as parafollicular cells, which are calcitonin-secreted neuroendocrine cells (16, 19, 21, 31). Immunohistochemical analysis also indicated PTPRN expression in rat gastrointestinal neuroendocrine cells (19, 32). Ptprn is also expressed in human lung cancer cell lines with a neuroendocrine phenotype (33).
Unlike other PTPR members, which have a tandem phosphatase domain, PTPRNs have a single phosphatase domain (34). Furthermore, the structure of the catalytic domain is altered in PTPRN and PTPRN2, suggesting that both proteins are pseudophosphatases. According to the bioinformatic definition, a pseudophosphatase is a member of phosphatase family that contains a mutation that predicts impairment or loss of its catalytic activity, regardless of whether this protein is enzymatically active or not (35). However, neither PTPRN nor PTPRN2 exhibit tyrosine phosphatase activity (36, 37). Furthermore, the enzymatic portion of PTPRNs can heterodimerize with another PTPRs and cause a 20% decrease in enzyme activity (38). However, the biological roles of PTPRNs have been investigated for several decades and have provided strong evidence that these proteins have acquired novel cellular roles beyond tyrosine dephosphorylation. In general, the sequence similarity between PTPRN and PTPRN2 suggests some levels of redundancy. Consequently, the effects on neuroendocrine cells are enhanced or observed only in double knockout (DKO) mice (39).
Loss of tyrosine phosphatase activity of PTPRNs due to mutation of two residues in the catalytic domain does not exclude the possibility that these proteins act as enzymes for other substrates. To date, no replacement substrate for PTPRN has been identified, but PTPRN2 has been reported to be able to dephosphorylate specific inositol phospholipids, including PI(3)P, PI(4,5)P2, but not PI(3,4,5)P3. When the transmembrane form of PTPRN2 was overexpressed in mammalian cells, it reduced plasma membrane PI(4,5)P2 levels in a dose-dependent manner (40). Other have reported that PTPRN2 and phospholipase C beta enzymatically reduce plasma membrane PI(4,5)P2 levels in metastatic breast cancer cells. They also found that the expression of these genes was increased in these cells, which coincided with human metastatic relapse. The authors further suggested that depletion of PI(4,5)P2 by these enzymes releases the PI(4,5)P2‐binding protein cofilin into the cytoplasm where it increases cellular migration and metastatic capacity (41). However, we observed no significant changes in InsP3-dependent calcium oscillations in gonadotrophs from DKO mice, which argues against a physiologically significant reduction in phospholipase C activity (23).
Several lines of research with pancreatic β-cells have shown that the secretory pathway is affected by deletion of Ptprn and/or Ptprn2. These genes appear to be required to accumulate normal levels of insulin-containing vesicles and prevent their degradation (42). Global knockout of Ptprn led to impaired glucose-mediated insulin secretion (43), whereas overexpression of Ptprn in an insulinoma cell line led to increased insulin secretion (42). Ptprn2 knockout mice also show impaired glucose tolerance and reduced glucose-induced insulin secretion but was not sufficient to prevent the development of diabetes (27). However, the role of PTPRNs in exocytosis appears to be specific to β-cells. Renin release from dense core vesicles of neuroendocrine juxtaglomerular granular cells is not directly inhibited by DKO, but reflects reduced catecholamine release from sympathetic nerve endings (17). In female but not in male mice, it was originally suggested that DKO inhibits the accumulation and secretion of the pituitary gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone, leading to infertility (27). Subsequent studies have shown that this is not the case for these hormones, that the exocytotic pathway of other anterior pituitary cells is also intact, and that the levels of hormones secretes by melanotrophs from the intermediate pituitary lobe are higher in DKO animals (see below).
Immunocytochemistry performed on cultured cells suggests that PTPRN is colocalized with neurosecretory granules and it is not a resident plasma membrane protein (16). PTPRN2 has also been reported to be enriched in the membranes of β-cell secretory granules (44). Proteomics analysis also revealed the presence of PTPRN and PTPRN2, as well as peptidyl-glycine-α-amidating monooxygenase (PAM), a neuropeptide processing enzyme (45), in insulin secretory granules (46). PTPRN has also been identified in the secretory granules of chromaffin cells (47). Expression of a fusion construct between PTPRN2-enhanced green fluorescent protein in β-cells and pheochromocytoma PC12 cells revealed the presence of this chimera in dense-core secretory granules (48, 49). PTPRN has also been reported to tether insulin secretory granules to actin microfilaments via its association with the adapter protein syntrophin beta 2 (50, 51). The same group also reported that the F-actin modifier villin-1 regulates insulin granule dynamic and exocytosis downstream of PTPRN (52). However, PTPRNs were not detected in pituitary corticotroph dense core vesicles (53) and TT endocrine cells (54), unlike PAM, the typical enzyme for this organelle. This may provide a rationale for the lack of effects of DKO on pituitary corticotroph secretion. Furthermore, Sntb2 encoding syntrophin beta 2, and Vil1 encoding Villin-1, are unlikely to play this role in pituitary hormone release because these genes are not expressed or are below detection by single cell RNA sequencing in hormone-producing cells (30).
Solimena’s laboratory proposed the association of PTPRN with insulin secretory granules not only to explain the initiation of the exocytotic process, but also to have a post-exocytotic functions. First, they reported that exocytosis of secretory granules leads to insertion of PTPRN in the plasma membrane, which promotes calcium-dependent cleavage of its cytoplasmic domain by mu-calpain. It has been suggested that this cleavage results in the generation of a cytosolic fragment of PTPRN that is targeted to the nucleus, causing upregulation of insulin gene expression. Therefore, this new pathway links regulated exocytosis to the control of gene expression and suggests that calcium acts as a dual signal: it triggers exocytosis and activates the retrograde pathway (55). Second, the same group proposed signal transducer and activator of transcription 5 (STAT5) as the binding domain for the cytosolic fragment of PTPRN and described the synergy of glucose and growth hormone signaling (56). Third, the C-terminal fragment of PTPRN has been proposed to promote β-cell proliferation by linking signaling by STAT3 and STAT5 (57). Consistently with these findings, effects of DKO on gene expression and/or cell proliferation have also been observed in neuroendocrine cells of the hypothalamus and pituitary gland (see below). Furthermore, knockout of Ptprn has been reported to reduce, whereas overexpression of PTPRN increases proliferation and migration of glioma cells (58). PTPRN2 has also been suggested to play a role in other types of cancers (59).
Early work with the pituitary gland suggested that DKO directly affects gonadotroph functions, particularly in females but not in males. In parallel with the β-cell secretion model, it has been suggested that PTPRNs are required for LH secretion, and DKO causes a lack of LH surge and ovulation. Therefore, PTPRNs in female gonadotrophs have been described as crucial for the structure and function of the dense core secretory vesicles, implying sexual dimorphism in exocytotic LH release (27). However, proteomics analysis of dense core secretory vesicles was not performed in pituitary cells to elucidate the presence/absence of PTPRNs in dense core secretory vesicles in female/male gonadotrophs. In addition, the authors did not examine the status of hypothalamic neurosecretory neurons that control pituitary gonadotroph functions.
Pituitary gonadotroph gene expression and hormone secretion are controlled by GnRH-secreting neurons (60), whose function is critically dependent on connections with kisspeptinergic neurons (61). GnRH is released in a pulsatile manner in females and males, causing oscillatory release of LH, a secretory pattern required for gonadal spermatogenesis/oogenesis and steroidogenesis (62). Pulsatile GnRH/LH release is driven by “GnRH pulse generator”, a neuronal assembly in the arcuate nucleus of the hypothalamus (63), which consist of kisspeptin-secreting neurons that control the distal processing of GnRH neurons and their secretion at the median eminence (64). The pulsatile release of GnRH/LH is sufficient for male fertility, but female fertility also depends on the sustained release of GnRH called the surge, which is necessary for ovulation (65). A distinct population of kisspeptin neurons is located in the rostral periventricular region of the third ventricle (RP3V) and stimulates the cell bodies of GnRH neurons to release GnRH, which causes the LH surge necessary for ovulation (65). Both GnRH and kisspeptin neurons also express Ptprn+Ptprn2 (24, 25), so DKO may affect their functions.
In a recent study (23), we showed that the density of kisspeptin staining was significantly reduced in both the arcuate nucleus and the RP3V region of DKO mice (Figures 1A, B). Moreover, the expression of Gnrh1 and Kiss1 was decreased in the hypothalamic tissue of DKO animals (Figure 1C). Expression of the pituitary gonadotroph-specific genes Lhb, Fshb, and Gnrhr was also significantly reduced in females and males (23). These changes were accompanied by significantly reduced pituitary LH accumulation and released in both females and males, arguing against sexual dimorphism at the pituitary level (Figure 1D). Significant changes in ovarian steroidogenesis and gene expression were also observed in DKO females, leading to the delay in puberty and female reproductive organ development (23). Others have also reported a delay in onset of puberty in Ptprn2-only knockout females (22). Finally, DKO females were in constant diestrus, indicating a lack of ovulation, in contrast to control females that had a 4–5-day estrous cycle. However, no changes were observed in testicular steroidogenesis and spermatogenesis, and seminal vesicles development in DKO males (23). The interpretation of these findings is summarized in the scheme shown in Figure 1E.
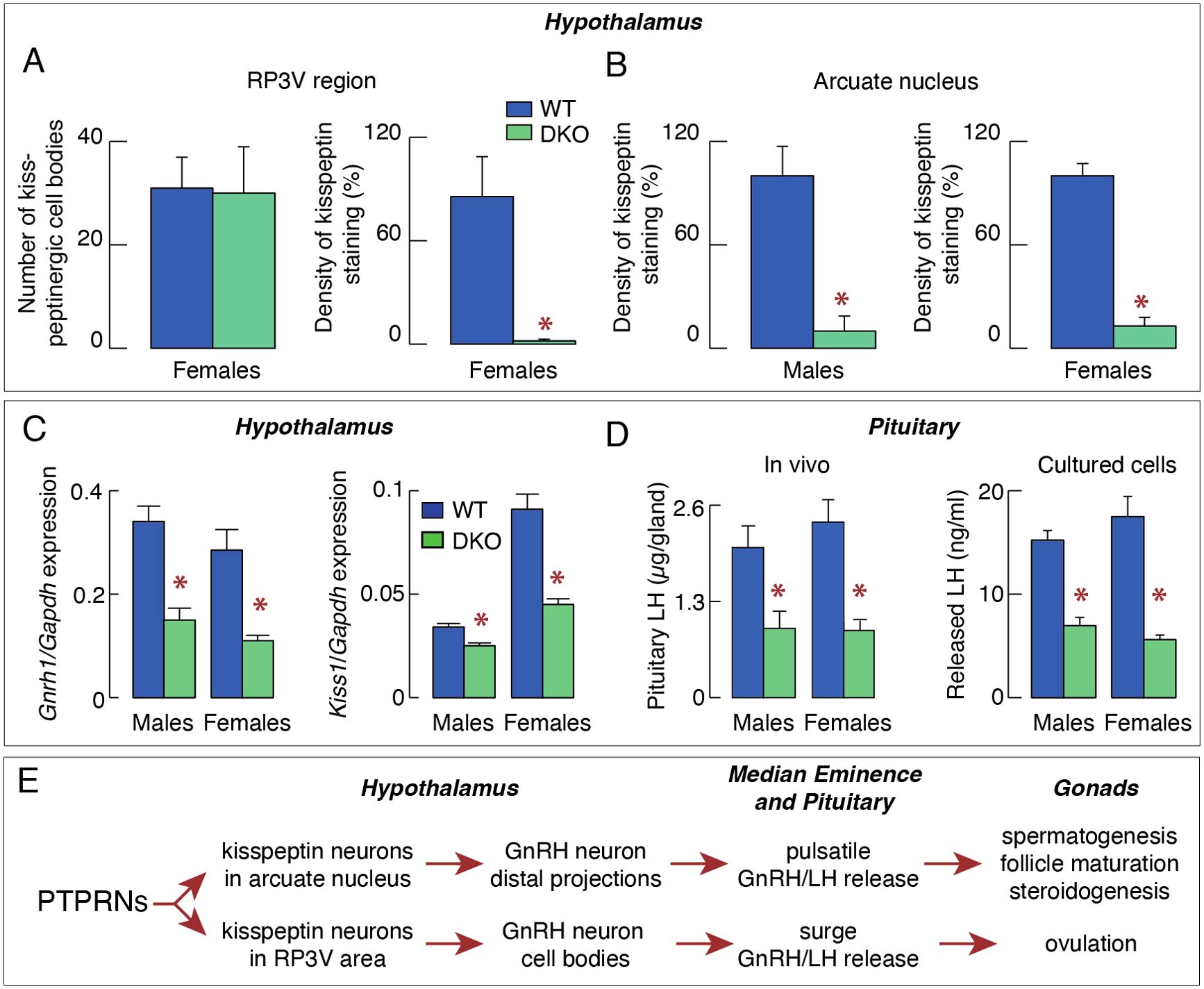
Figure 1. PTPRNs contribute to the control of reproduction by stimulating kisspeptin-GnRH secreting neurons. (A) Quantification of kisspeptinergic cell bodies (left) and fiber densities (right) in the RP3V region of WT and DKO females. (B) Quantification of kisspeptinergic fiber densities in the arcuate nucleus of WT and DKO males and females. (C) Inhibition of expression of Gnrh1 (left) and Kiss1 (right) in DKO animals. (D) Inhibition of pituitary and serum luteinizing hormone (LH) in DKO mice. (E) Schematic representation of the proposed stimulatory effect of PTPRN on the hypothalamic-pituitary-gonadal axis by increasing the synthesis and release of kisspeptin, which influences the pulsatile and surge release of GnRH/LH, the former being responsible for spermatogenesis, follicle maturation and steroidogenesis, and later for ovulation. Asterisks indicate significant differences between pairs, P < 0.01 Asterisks.
A marker gene for the hypothalamic-pituitary-adrenal axis is Pomc, which is expressed in both the hypothalamus and the pituitary gland. In the hypothalamus, Pomc is expressed in the arcuate nucleus (66) and in the pituitary Pomc is expressed in corticotrophs and melanotrophs (67). DKO did not affect Pomc expression in the hypothalamus (Figure 2A), but dramatically increased expression in the pituitary (Figure 2B), although both tissues express PTPRNs. Pomc regulatory sequences in the pituitary and hypothalamic tissues differ (68), indicating that the tissue-specific PTPRNs actions are transcriptionally related. Single knockouts of Ptprn and Ptprn2 also increased Pomc expression, but of smaller amplitudes compared to DKO (69).
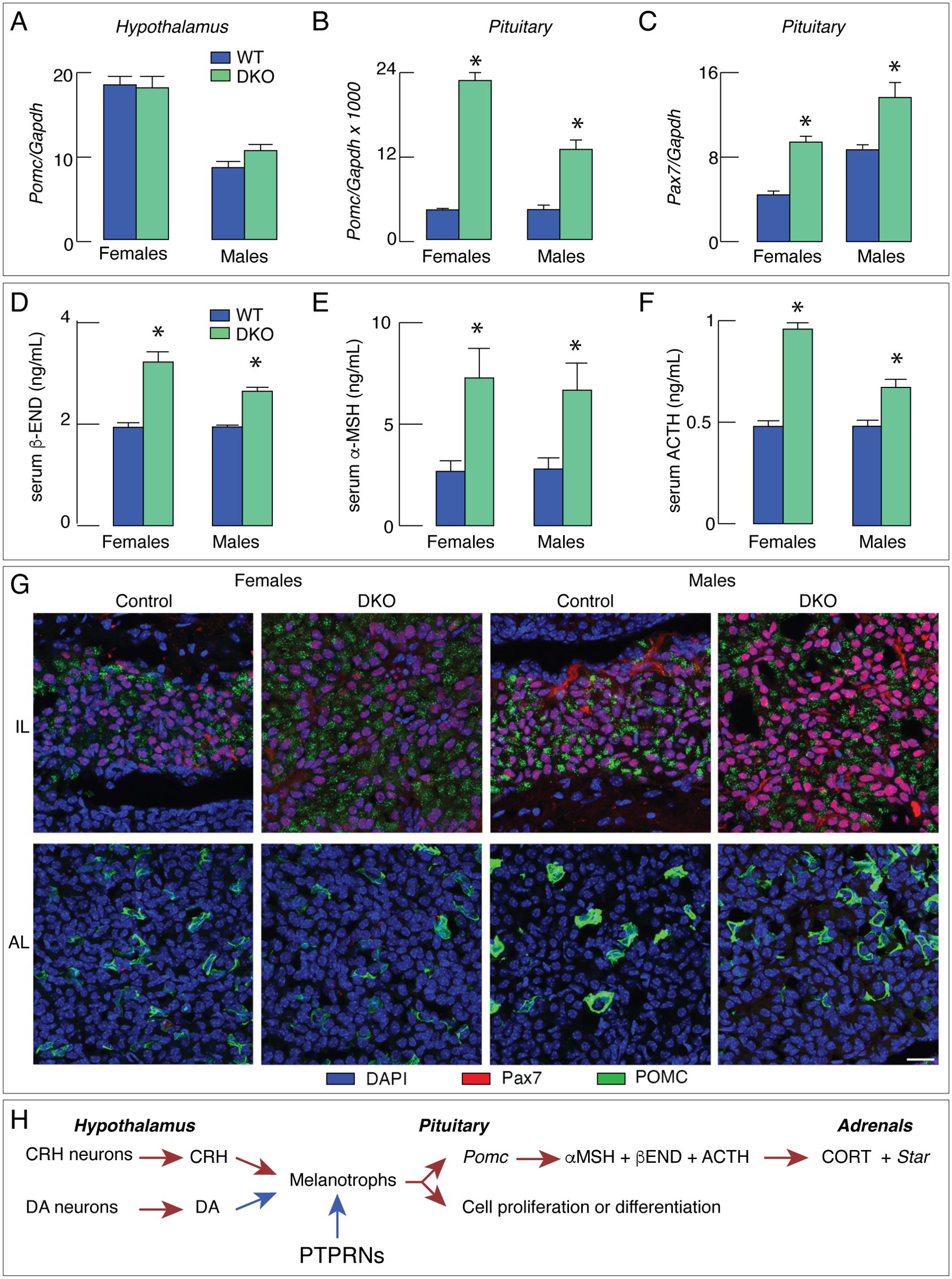
Figure 2. DKO increases pituitary melanotroph gene expression and hormone secretion, reflecting an increase in pituitary melanotroph population. (A–F) DKO does not affect Pomc expression in hypothalamus (A) but stimulates expression of this gene in pituitary (B) as well as Pax7 expression in pituitary (C). (D–F) Increase in Pomc expression was accompanied by elevation in serum hormone concentration: beta-endorphin (β-END; D), alpha-melanocyte stimulating hormone (α−MSH; E) and adrenocorticotropic hormone (ACTH; F). (G) Representative images of PAX7 (a maker protein of melanotrophs) and POMC immunostaining in intermediate lobe (IL) and anterior lobe (AL) of control and DKO female and male mice. The PAX7-immunopositive cells (red) were detected in IL, while POMC immunoreactivity (green) was present in both AL and IL of control and DKO animals. Note that IL of DKO mice of both sexes was larger than those from controls. The scale bar of 20 µm applies to all panels. (H) Schematic representation of the proposed inhibitory effect of PTPRNs on the hypothalamic-pituitary-gonadal axis by reducing Pomc expression and slowing melanotroph proliferation/differentiation. Red arrows - stimulation; blue arrows – inhibition. Asterisks indicate significant differences between pairs, P < 0.01.
Pituitary expression of Pomc is stimulated by hypothalamic corticotropin-releasing hormone (68), which is a ligand for corticotropin-releasing hormone receptor 1 expressed in corticotrophs and melanotrophs (67). However, DKO did not affect Crh expression (69). Tbx19 is a common developmental transcription factor gene for corticotrophs and melanotrophs, whereas Pax7 is expressed only in melanotroph (67) and ts expression in the pituitary was significantly elevated in DKO females and males (Figure 2C). TBX19 controls terminal differentiation of both lineages and, in cooperation with PITX1, activates Pomc transcription (70). PAX7 controls melanotroph differentiation (71) and facilitates TBX19-controlled Pomc transcription via chromatin remodeling (72).
DKO-increased Pomc expression in the pituitary gland was associated with increased hormone secretion in vivo and in vitro. Serum beta-endorphin was significantly elevated in both DKO females and males (Figure 2D), as were serum concentrations of alpha-melanocyte stimulating hormone (Figure 2E) and adrenocorticotropic hormone (Figure 2F). In cultured pituitary cells, both hormone release and cellular content of these hormones were significantly elevated, further indicating that Ptprn and Ptprn2 regulate their synthesis ad release. DKO also increased serum corticosterone concentration, adrenal mass, and gene expression of the steroidogenic enzyme Star in adrenal tissue in both sexes (69).
Elevated expression of Pax7 in the DKO pituitary is consistent with the hypothesis that Pomc expression and hormone synthesis and release are elevated in melanotrophs. The hypothesis was additionally confirmed by the finding that the expression of melanotroph-specific genes Pcsk2, Esm1, Doc2g, and Oacyl was also increased in DKO pituitaries. In contrast, there was no increase in the expression of the corticotrophs-specific genes Chrna1, Clrn1, Trdn, and Hspb3 (69). Finally, immunohistochemical analysis using a POMC/adrenocorticotropic hormone-specific antibody to identify corticotrophs and melanotrophs and PAX7-specific antibody to label melanotrophs, showed that the intermediate lobe (home to melanotrophs) was enlarged, reflecting an increase in the population size of DKO melanotrophs. In contrast, we failed to detect an increase in the number of corticotrophs in the anterior lobe (Figure 2G). Therefore, both melanotrophs. hyperplasia and increased Pomc expression per cell in DKO mice are responsible for the significant increase in POMC-derived hormones. The scheme shown in Figure 2H illustrates the interpretation of these findings.
A review of the PTPRN literature suggests an important conclusion; all neuroendocrine cells express PTPRN genes, but their knockout disrupts the function of only some of these cell types, suggesting a cell type-specific role for these pseudophosphatases. In the diffuse neuroendocrine system, the cells themselves control their own secretion, gene expression, and proliferation in response to stimulation. Thus, the role of PTPRNs in their function is more readily elucidated. In pancreatic β-cells, PTPRNs have been suggested to be an integral part of a system for monitoring β-cell-stimulated secretory activity and for adjusting insulin expression and initiating cell proliferation. There has also been significant progress in characterizing the molecular mechanism of action of PTPRNs in the exocytotic pathway, insulin gene expression, and cell proliferation. A growing number of reports also indicate that PTPRNs are involved in the tumorigenesis, but their mechanism of action has not been proven. The role of PTPRNs in other diffuse neuroendocrine cells has not been studied.
In the centralized neuroendocrine system organized as the hypothalamic-pituitary-target organ axes, there is a complex relationship as the participating cells synchronize their activity through feedforward and feedback mechanisms. It is therefore more difficult to identify the cell type directly affected by DKO, as demonstrated in work with the hypothalamic-pituitary-gonadal axis in female and male mice. Although PTPRNs are expressed in kisspeptinergic and GnRH neurons, as well as in gonadotrophs, only kisspeptinergic neurons in both regions of the hypothalamus have been identified as PTPRNs-responsive cell types, suggesting that stimulation of Kiss1 expression increases hormone gene expression and secretion downstream of the axis (Figure 1E). In contrast, in the hypothalamic-pituitary-adrenal axis, melanotrophs have been identified as directly responding cells. Moreover, PTPRNs appear to inhibit both Pomc expression and melanotroph proliferation/differentiation, in parallel with the action of dopamine (Figure 2G). Further studies are needed to characterize the role of PTPRNs in the function of other hypothalamic and pituitary cells.
It is known that transcription factors can act as activators and repressors in different cells, but it is currently unknown whether PTPRNs act as transcription factors or upstream elements in the control of gene transcription. Further studies are needed to elucidate the molecular mechanism of this process. The cellular specificity of the PTPRNs actions is consistent with the specificity of promoter activation and repression for different genes, as suggested by the failure of DKO to increase Pomc expression in the hypothalamus but facilitate Pomc expression in the pituitary. The role of PTPRNs in normal and carcinoma cell proliferation also requires further studies.
SSS: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. SJS: Writing – review & editing. SC: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, award number: Z01 HD000195-31; grant recipient SSS.
We are grateful to Drs. Abner L. Notkins, Tao Cai, and Gilberto N Carmona for providing Ptprn and Ptprn2 knockout mice after their laboratory was closed and for helpful discussion of preliminary data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DKO, double knockout; GnRH, gonadotroph-releasing hormone; LH, luteinizing hormone; RP3V, the rostral periventricular region of the third ventricle, PTPR, protein tyrosine phosphatase receptors; PTPRN, PTPR type N; PTPRN2, PTPR type N2; PTPRNs, PTPRN+PTPRN2
1. Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. (2006) 7:833–46. doi: 10.1038/nrm2039
2. Stoker AW. Protein tyrosine phosphatases and signalling. J Endocrinol. (2005) 185:19–33. doi: 10.1677/joe.1.06069
3. Notkins AL, Lan MS, Leslie RD. IA-2 and IA-2beta: the immune response in IDDM. Diabetes Metab Rev. (1998) 14:85–93. doi: 10.1002/(SICI)1099-0895(199803)14:1<85::AID-DMR205>3.0.CO;2-I
4. Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. (1994) 13:505–14. doi: 10.1089/dna.1994.13.505
5. Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, Maclaren NK, et al. Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci U S A. (1996) 93:2307–11. doi: 10.1073/pnas.93.6.2307
6. Xie J, Zhang B, Lan MS, Notkins AL. Genomic structure and promoter sequence of the insulin-dependent diabetes mellitus autoantigen, IA-2 (PTPRN). Genomics. (1998) 54:338–43. doi: 10.1006/geno.1998.5583
7. Park YS, Kawasaki E, Kelemen K, Yu L, Schiller MR, Rewers M, et al. Humoral autoreactivity to an alternatively spliced variant of ICA512/IA-2 in Type I diabetes. Diabetologia. (2000) 43:1293–301. doi: 10.1007/s001250051525
8. Diez J, Park Y, Zeller M, Brown D, Garza D, Ricordi C, et al. Differential splicing of the IA-2 mRNA in pancreas and lymphoid organs as a permissive genetic mechanism for autoimmunity against the IA-2 type 1 diabetes autoantigen. Diabetes. (2001) 50:895–900. doi: 10.2337/diabetes.50.4.895
9. Torii S. Expression and function of IA-2 family proteins, unique neuroendocrine-specific protein-tyrosine phosphatases. Endocr J. (2009) 56:639–48. doi: 10.1507/endocrj.K09E-157
10. Bloomquist BT, Darlington DN, Mueller GP, Mains RE, Eipper BA. Regulated endocrine-specific protein-18: a short-lived novel glucocorticoid-regulated endocrine protein. Endocrinology. (1994) 135:2714–22. doi: 10.1210/endo.135.6.7988462
11. Zhang G, Hirai H, Cai T, Miura J, Yu P, Huang H, et al. RESP18, a homolog of the luminal domain IA-2, is found in dense core vesicles in pancreatic islet cells and is induced by high glucose. J Endocrinol. (2007) 195:313–21. doi: 10.1677/JOE-07-0252
12. Atari E, Perry MC, Jose PA, Kumarasamy S. Regulated endocrine-Specific protein-18, an emerging endocrine protein in physiology: A literature review. Endocrinology. (2019) 160:2093–100. doi: 10.1210/en.2019-00397
13. Sosa L, Torkko JM, Primo ME, Llovera RE, Toledo PL, Rios AS, et al. Biochemical, biophysical, and functional properties of ICA512/IA-2 RESP18 homology domain. Biochim Biophys Acta. (2016) 1864:511–22. doi: 10.1016/j.bbapap.2016.01.013
14. Wang Y, Yang H, Li N, Wang L, Guo C, Ma W, et al. A novel ubiquitin ligaseaAdaptor PTPRN suppresses seizure susceptibility through endocytosis of Na(V)1.2 sodium channels. Adv Sci (Weinh). (2024) 11:e2400560. doi: 10.1002/advs.202400560
15. Carmona GN, Nishimura T, Schindler CW, Panlilio LV, Notkins AL. The dense core vesicle protein IA-2, but not IA-2beta, is required for active avoidance learning. Neuroscience. (2014) 269:35–42. doi: 10.1016/j.neuroscience.2014.03.023
16. Solimena M, Dirkx R Jr., Hermel JM, Pleasic-Williams S, Shapiro JA, Caron L, et al. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. (1996) 15:2102–14. doi: 10.1002/j.1460-2075.1996.tb00564.x
17. Kim SM, Theilig F, Qin Y, Cai T, Mizel D, Faulhaber-Walter R, et al. Dense-core vesicle proteins IA-2 and IA-2{beta} affect renin synthesis and secretion through the {beta}-adrenergic pathway. Am J Physiol Renal Physiol. (2009) 296:F382–389. doi: 10.1152/ajprenal.90543.2008
18. Shimizu S, Saito N, Kubosaki A, SungWook S, Takeyama N, Sakamoto T, et al. Developmental expression and localization of IA-2 mRNA in mouse neuroendocrine tissues. Biochem Biophys Res Commun. (2001) 288:165–71. doi: 10.1006/bbrc.2001.5754
19. Takeyama N, Ano Y, Wu G, Kubota N, Saeki K, Sakudo A, et al. Localization of insulinoma associated protein 2, IA-2 in mouse neuroendocrine tissues using two novel monoclonal antibodies. Life Sci. (2009) 84:678–87. doi: 10.1016/j.lfs.2009.02.012
20. Ramirez-Franco JJ, Munoz-Cuevas FJ, Lujan R, Jurado S. Excitatory and inhibitory neurons in the hippocampus exhibit molecularly distinct large dense core vesicles. Front Cell Neurosci. (2016) 10:202. doi: 10.3389/fncel.2016.00202
21. Dirkx R Jr., Hermel JM, Rabin DU, Solimena M. ICA 512, a receptor tyrosine phosphatase-like protein, is concentrated in neurosecretory granule membranes. Adv Pharmacol. (1998) 42:243–6. doi: 10.1016/s1054-3589(08)60738-3
22. Kang T, Ye J, Qin P, Li H, Yao Z, Liu Y, et al. Knockdown of Ptprn-2 delays the onset of puberty in female rats. Theriogenology. (2021) 176:137–48. doi: 10.1016/j.theriogenology.2021.09.029
23. Sokanovic SJ, Constantin S, Lamarca Dams A, Mochimaru Y, Smiljanic K, Bjelobaba I, et al. Common and female-specific roles of protein tyrosine phosphatase receptors N and N2 in mice reproduction. Sci Rep. (2023) 13:355. doi: 10.1038/s41598-023-27497-4
24. Burger LL, Vanacker C, Phumsatitpong C, Wagenmaker ER, Wang L, Olson DP, et al. Identification of genes enriched in gnRH neurons by translating ribosome affinity purification and RNAseq in mice. Endocrinology. (2018) 159:1922–40. doi: 10.1210/en.2018-00001
25. Stephens SBZ, Kauffman AS. Estrogen regulation of the molecular phenotype and active translatome of AVPV kisspeptin neurons. Endocrinology. (2021) 162. doi: 10.1210/endocr/bqab080
26. Hermel JM, Dirkx R Jr., Solimena M. Post-translational modifications of ICA512, a receptor tyrosine phosphatase-like protein of secretory granules. Eur J Neurosci. (1999) 11:2609–20. doi: 10.1046/j.1460-9568.1999.00677.x
27. Kubosaki A, Nakamura S, Clark A, Morris JF, Notkins AL. Disruption of the transmembrane dense core vesicle proteins IA-2 and IA-2beta causes female infertility. Endocrinology. (2006) 147:811–5. doi: 10.1210/en.2005-0638
28. Lee MS, Dirkx R Jr., Solimena M, Dannies PS. Stabilization of the receptor protein tyrosine phosphatase-like protein ICA512 in GH4C1 cells upon treatment with estradiol, insulin, and epidermal growth factor. Endocrinology. (1998) 139:2727–33. doi: 10.1210/endo.139.6.6039
29. Fletcher PA, Smiljanic K, Maso Previde R, Iben JR, Li T, Rokic MB, et al. Cell type- and sex-Dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol (Lausanne). (2019) 10:623. doi: 10.3389/fendo.2019.00623
30. Fletcher PA, Smiljanic K, Previde RM, Constantin S, Sherman AS, Coon SL, et al. The astroglial and stem cell functions of adult rat folliculostellate cells. Glia. (2023) 71:205–28. doi: 10.1002/glia.24267
31. Bergman J, Botling J, Fagerberg L, Hallstrom BM, Djureinovic D, Uhlen M, et al. The human adrenal gland proteome defined by transcriptomics and antibody-based profiling. Endocrinology. (2017) 158:239–51. doi: 10.1210/en.2016-1758
32. Gomi H, Kubota-Murata C, Yasui T, Tsukise A, Torii S. Immunohistochemical analysis of IA-2 family of protein tyrosine phosphatases in rat gastrointestinal endocrine cells. J Histochem Cytochem. (2013) 61:156–68. doi: 10.1369/0022155412466872
33. Xie H, Notkins AL, Lan MS. IA-2, a transmembrane protein tyrosine phosphatase, is expressed in human lung cancer cell lines with neuroendocrine phenotype. Cancer Res. (1996) 56:2742–4.
34. Streuli M, Krueger NX, Thai T, Tang M, Saito H. Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. (1990) 9:2399–407. doi: 10.1002/j.1460-2075.1990.tb07415.x
35. Reiterer V, Pawlowski K, Desrochers G, Pause A, Sharpe HJ, Farhan H. The dead phosphatases society: a review of the emerging roles of pseudophosphatases. FEBS J. (2020) 287:4198–220. doi: 10.1111/febs.v287.19
36. Magistrelli G, Toma S, Isacchi A. Substitution of two variant residues in the protein tyrosine phosphatase-like PTP35/IA-2 sequence reconstitutes catalytic activity. Biochem Biophys Res Commun. (1996) 227:581–8. doi: 10.1006/bbrc.1996.1549
37. Fitzgerald LR, Walton KM, Dixon JE, Largent BL. PTP NE-6: a brain-enriched receptor-type protein tyrosine phosphatase with a divergent catalytic domain. J Neurochem. (1997) 68:1820–9. doi: 10.1046/j.1471-4159.1997.68051820.x
38. Gross S, Blanchetot C, Schepens J, Albet S, Lammers R, den Hertog J, et al. Multimerization of the protein-tyrosine phosphatase (PTP)-like insulin-dependent diabetes mellitus autoantigens IA-2 and IA-2beta with receptor PTPs (RPTPs). Inhibition RPTPalpha Enzymatic Activity J Biol Chem. (2002) 277:48139–45. doi:10.1074/jbc.M208228200
39. Kubosaki A, Nakamura S, Notkins AL. Dense core vesicle proteins IA-2 and IA-2beta: metabolic alterations in double knockout mice. Diabetes. (2005) 54:S46–51. doi: 10.2337/diabetes.54.suppl_2.S46
40. Caromile LA, Oganesian A, Coats SA, Seifert RA, Bowen-Pope DF. The neurosecretory vesicle protein phogrin functions as a phosphatidylinositol phosphatase to regulate insulin secretion. J Biol Chem. (2010) 285:10487–96. doi: 10.1074/jbc.M109.066563
41. Sengelaub CA, Navrazhina K, Ross JB, Halberg N, Tavazoie SF. PTPRN2 and PLCbeta1 promote metastatic breast cancer cell migration through PI(4,5)P2-dependent actin remodeling. EMBO J. (2016) 35:62–76. doi: 10.15252/embj.201591973
42. Harashima S, Clarke A, Christie MR, Notkins AL. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc Natl Acad Sci U S A. (2005) 102:8704–9. doi: 10.1073/pnas.0408887102
43. Saeki K, Zhu M, Kubosaki A, Xie J, Lan MS, Notkins AL. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes. (2002) 51:1842–50. doi: 10.2337/diabetes.51.6.1842
44. Wasmeier C, Hutton JC. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J Biol Chem. (1996) 271:18161–70. doi: 10.1074/jbc.271.30.18161
45. Trivellin G, Daly AF, Hernandez-Ramirez LC, Araldi E, Tatsi C, Dale RK, et al. Germline loss-of-function PAM variants are enriched in subjects with pituitary hypersecretion. Front Endocrinol (Lausanne). (2023) 14:1166076. doi: 10.3389/fendo.2023.1166076
46. Brunner Y, Coute Y, Iezzi M, Foti M, Fukuda M, Hochstrasser DF, et al. Proteomics analysis of insulin secretory granules. Mol Cell Proteomics. (2007) 6:1007–17. doi: 10.1074/mcp.M600443-MCP200
47. Wegrzyn JL, Bark SJ, Funkelstein L, Mosier C, Yap A, Kazemi-Esfarjani P, et al. Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J Proteome Res. (2010) 9:5002–24. doi: 10.1021/pr1003104
48. Pouli AE, Emmanouilidou E, Zhao C, Wasmeier C, Hutton JC, Rutter GA. Secretory-granule dynamics visualized in vivo with a phogrin-green fluorescent protein chimaera. Biochem J. (1998) 333:193–9. doi: 10.1042/bj3330193
49. Bright NA, Walters J, Wasmeier C, Hutton JC. Targeting of a phogrin-green fluorescent protein chimaera to insulin secretory granules of pancreatic beta-cells in transgenic mice. Diabetes Metab. (2002) 28:3S29–36.
50. Ort T, Voronov S, Guo J, Zawalich K, Froehner SC, Zawalich W, et al. Dephosphorylation of beta2-syntrophin and Ca2+/mu-calpain-mediated cleavage of ICA512 upon stimulation of insulin secretion. EMBO J. (2001) 20:4013–23. doi: 10.1093/emboj/20.15.4013
51. Schubert S, Knoch KP, Ouwendijk J, Mohammed S, Bodrov Y, Jager M, et al. beta2-Syntrophin is a Cdk5 substrate that restrains the motility of insulin secretory granules. PLoS One. (2010) 5:e12929. doi: 10.1371/journal.pone.0012929
52. Mziaut H, Mulligan B, Hoboth P, Otto O, Ivanova A, Herbig M, et al. The F-actin modifier villin regulates insulin granule dynamics and exocytosis downstream of islet cell autoantigen 512. Mol Metab. (2016) 5:656–68. doi: 10.1016/j.molmet.2016.05.015
53. Gauthier DJ, Sobota JA, Ferraro F, Mains RE, Lazure C. Flow cytometry-assisted purification and proteomic analysis of the corticotropes dense-core secretory granules. Proteomics. (2008) 8:3848–61. doi: 10.1002/pmic.200700969
54. Sasaki K, Satomi Y, Takao T, Minamino N. Snapshot peptidomics of the regulated secretory pathway. Mol Cell Proteomics. (2009) 8:1638–47. doi: 10.1074/mcp.M900044-MCP200
55. Trajkovski M, Mziaut H, Altkruger A, Ouwendijk J, Knoch KP, Muller S, et al. Nuclear translocation of an ICA512 cytosolic fragment couples granule exocytosis and insulin expression in {beta}-cells. J Cell Biol. (2004) 167:1063–74. doi: 10.1083/jcb.200408172
56. Mziaut H, Trajkovski M, Kersting S, Ehninger A, Altkruger A, Lemaitre RP, et al. Synergy of glucose and growth hormone signalling in islet cells through ICA512 and STAT5. Nat Cell Biol. (2006) 8:435–45. doi: 10.1038/ncb1395
57. Mziaut H, Kersting S, Knoch KP, Fan WH, Trajkovski M, Erdmann K, et al. ICA512 signaling enhances pancreatic beta-cell proliferation by regulating cyclins D through STATs. Proc Natl Acad Sci U S A. (2008) 105:674–9. doi: 10.1073/pnas.0710931105
58. Wang D, Tang F, Liu X, Fan Y, Zheng Y, Zhuang H, et al. Expression and tumor-Promoting effect of tyrosine phosphatase receptor type N (PTPRN) in human glioma. Front Oncol. (2021) 11:676287. doi: 10.3389/fonc.2021.676287
59. Sorokin AV, Nair BC, Wei Y, Aziz KE, Evdokimova V, Hung MC, et al. Aberrant expression of proPTPRN2 in cancer cells confers resistance to apoptosis. Cancer Res. (2015) 75:1846–58. doi: 10.1158/0008-5472.CAN-14-2718
60. Constantin S, Bjelobaba I, Stojilkovic SS. Pituitary gonadotroph-specific patterns of gene expression and hormone secretion. Curr Opin Pharmacol. (2022) 66:102274. doi: 10.1016/j.coph.2022.102274
61. Constantin S. Targeting KNDy neurons to control GnRH pulses. Curr Opin Pharmacol. (2022) 67:102316. doi: 10.1016/j.coph.2022.102316
62. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. (2018) 159:3723–36. doi: 10.1210/en.2018-00653
63. Knobil E. Discovery of the hypothalamic gonadotropin-releasing hormone pulse generator and of its physiologic significance. 1992. Am J Obstet Gynecol. (2005) 193:1765–6. doi:10.1016/j.ajog.2005.06.025
64. Han SY, Kane G, Cheong I, Herbison AE. Characterization of gnRH pulse generator activity in male mice using GCaMP fiber photometry. Endocrinology. (2019) 160:557–67. doi: 10.1210/en.2018-01047
65. Goodman RL, Herbison AE, Lehman MN, Navarro VM. Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion. J Neuroendocrinol. (2022), 34:e13094. doi: 10.1111/jne.13094
66. Gali Ramamoorthy T, Begum G, Harno E, White A. Developmental programming of hypothalamic neuronal circuits: impact on energy balance control. Front Neurosci. (2015) 9:126. doi: 10.3389/fnins.2015.00126
67. Stojilkovic SS, Previde RM, Sherman AS, Fletcher PA. Pituitary corticotroph identity and receptor-mediated signaling: a transcriptomics perspective. Curr Opin Endocr Metab Res. (2022) 25. doi: 10.1016/j.coemr.2022.100364
68. Drouin J. 60 YEARS OF POMC: Transcriptional and epigenetic regulation of POMC gene expression. J Mol Endocrinol. (2016) 56:T99–T112. doi: 10.1530/JME-15-0289
69. Constantin S, Sokanovic SJ, Mochimaru Y, Dams AL, Smiljanic K, Previde RM, et al. Protein tyrosine phosphatase receptors N and N2 control pituitary melanotroph development and POMC expression. Endocrinology. (2024), 165. doi: 10.1210/endocr/bqae076
70. Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. (2001) 104:849–59. doi: 10.1016/S0092-8674(01)00282-3
71. Mayran A, Khetchoumian K, Hariri F, Pastinen T, Gauthier Y, Balsalobre A, et al. Pioneer factor Pax7 deploys a stable enhancer repertoire for specification of cell fate. Nat Genet. (2018) 50:259–69. doi: 10.1038/s41588-017-0035-2
Keywords: PTPRN, PTPRN2, GnRH neurons, kisspeptinergic neurons, pancreatic β-cells, gonadotrophs, melanotrophs
Citation: Stojilkovic SS, Sokanovic SJ and Constantin S (2025) What is known and unknown about the role of neuroendocrine genes Ptprn and Ptprn2. Front. Endocrinol. 16:1531723. doi: 10.3389/fendo.2025.1531723
Received: 20 November 2024; Accepted: 08 January 2025;
Published: 24 January 2025.
Edited by:
Nils Lambrecht, United States Department of Veterans Affairs, United StatesReviewed by:
T. John Wu, Uniformed Services University of the Health Sciences, United StatesCopyright © 2025 Stojilkovic, Sokanovic and Constantin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stanko S. Stojilkovic, c3Rvamlsa3NAbWFpbC5uaWguZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.