
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 12 March 2025
Sec. Clinical Diabetes
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1496851
Diabetic retinopathy (DR) is the predominant vision-threatening complication in individuals with diabetes mellitus. Timely diagnosis and intervention facilitate the prevention of diabetes-associated visual impairment. Classical imaging methods may prevent the timely detection of DR due to shortages of specialized facilities and retinal specialists, particularly in remote areas. In recent years, research on biomarkers related to DR has rapidly developed, playing an important role in risk assessment and early detection of the disease. Some ocular biomarkers from the vitreous body or aqueous humor were invasive, which hampered their application in clinical practice. Meanwhile, biomarkers based on omics were limited by their uneasily accessible use and complicated variables with a relatively low degree of reproducibility. As modern technology progresses, advanced non-ocular biomarkers of DR have established a comprehensive platform for the prompt identification of DR, independent of ophthalmic professionals or devices and accessible to non-ophthalmologists during community screenings. This review focuses on biomarkers derived from non-ocular sample sources, such as nailfold and skin, accessible through non-invasive methods, to reveal if they can be considered as an effective option for the early identification of DR by non-ophthalmologists in community screening initiatives.
Diabetic retinopathy (DR) is a retinal microangiopathy resulting from the chronic effects of diabetes mellitus (DM), which is the predominant sight-threatening complication in patients with DM (1, 2). Recent estimates indicated that the global prevalence of DR among adults with diabetes was 22.3%, with 6.2% of these patients exhibiting vision-threatening diabetic retinopathy (VTDR) (3). In addition to its visual impacts that may result in a lower quality of life, DR has been associated with an elevated risk of systemic vascular problems (4), hence adding a significant burden on individuals and healthcare systems (5). DR can be divided into non-proliferative DR (NPDR) and proliferative DR (PDR). In the initial stage of the disease, it mostly presents as NPDR with mild vascular hyperpermeability. As the disease progresses, NPDR develops from mild to severe with retinal capillary leakage or loss, causing retinal ischemia. Patients with NPDR are typically asymptomatic, making it difficult to detect the disease at an early stage. When the condition further deteriorates, it will progress to the PDR stage with new vessel growth on the optic disc and retina, when the patient may present with a sudden loss of vision due to a vitreous hemorrhage (6). In addition, diabetic macular edema (DME) also plays an important role in the development of DR. DME refers to the retinal thickening in the posterior pole and may occur in either NPDR or PDR. When patients develop DME, their vision gradually declines, severely affecting their quality of life. Consequently, early detection of DR is essential for reasonable risk categorization, early management, optimizing therapeutic effects, and lowering healthcare expenditures.
The prevailing standard of treatment is for an annual dilated retinal examination to facilitate earlier diagnosis (7, 8). The detection of DR mostly employs conventional procedures such as direct or indirect ophthalmoscopy, fundus photography, or optical coherence tomography (OCT). Still, these techniques are protracted and manual and require specialized equipment and retinal experts, leading to resource deficiencies, especially in screening capacity and retinal specialist availability, which may hinder the early identification of DR.
Recently, multiple unique biomarkers from various sample sources connected with DR were discovered, which was essential for early identifying the occurrence and development of DR (Figure 1). Some ocular biomarkers from the vitreous body or aqueous humor (9) are invasive and thus undesirable for both patients and clinicians. In recent years, the rapid advancement of translational medicine has given rise to the investigation of biomarkers from non-ocular sample sources, including nailfold, skin, blood (10–13), saliva (14), feces (15), and urine (16), for the clinical diagnosis and prognosis of DR. These biomarkers are both safe and readily accessible for sample collection, particularly in difficult groups. Non-coding RNAs (17) and genomic (18) and lipidomic (19) biomarkers have also been intensively investigated to predict the risk of DR development, but most of them are limited by their uneasily accessible use and complicated variables with a relatively low degree of reproducibility, preventing their use in clinical practice, particularly in community hospitals or remote areas where ophthalmic investigations are not readily available. Given the foregoing, there is a need for easy, rapid, reliable, and affordable non-ocular biomarkers in community-based DR screening that does not require ophthalmic specialists or ophthalmic technology and may be performed by non-ophthalmologists.
To our knowledge, there is a paucity of studies that have comprehensively assessed non-ocular diagnostic biomarkers of DR and their application in clinical practice. Optimal biomarkers are characterized by specificity and can be readily assessed by non-invasive techniques (20). Therefore, this review concentrates on non-ocular biomarkers, easily obtained through non-invasive methods, to explore their potential as an effective option for non-ophthalmologists to identify DR early in community screening initiatives.
Nailfold capillaroscopy (NFC) is an innovative non-invasive method for detecting systemic disease-related microvascular morphological and functional abnormalities (21). Some studies have established the relationships between nailfold capillary changes and the existence and severity of DR. Changes such as tortuosity (curvature of the capillary limb without crossover), avascular regions (absence of two or more adjacent capillaries in the most distal row), branching capillary (numerous small buds emerging from the distal loop), diminished capillary density, and microhemorrhages were markedly elevated in individuals suffering from DR compared to those DM without DR (22–25). Furthermore, Shikama et al. (26) revealed that individuals with T2DM had an elevated risk of DR correlated with an increased number of crossing capillaries. When data on crossing capillaries were added to a model containing known risk and inhibiting factors for DR (age, sex, diabetes duration, glycated hemoglobin, systolic blood pressure, body mass index, and use of certain medications), the Nagelkerke R2 increased from 0.29 to 0.35. As the value of Nagelkerke R2 ranges from 0 to 1, the closer it is to 1, the better the model fits the data, suggesting that crossing capillaries might serve as a novel biomarker related to the residual risk of DR.
Subgroup studies of DR patients indicated that alterations in nailfold capillaries were substantially correlated with the degree of DR and may run parallel to retinal changes. Several cross-sectional studies found that abnormalities in nailfold capillaries, including diminished capillary density, tortuosity, a larger number of mega capillaries, and dilated apical capillaries, were observed in patients with PDR, significantly greater than in patients with NPDR and those without DR (24, 25, 27). These findings implied that NFC can detect the microvascular changes in the nailfold capillaries accurately, which were predominantly proliferative in the early stages and regressive in the advanced stages (23). Furthermore, previous studies identified that patients with DR who have a prolonged illness duration over 20 years or poor glycemic control (HbA1c > 11%) had significantly higher frequencies of mega capillaries, enhanced tortuosity, and neovascularization in type 2 diabetes mellitus (T2DM) (22, 24), which was also observed in research on juveniles with type 1 diabetes mellitus (T1DM) (28). It indicated that variations in nailfold capillaries may play a role in prognosis alone.
Upon diagnostic test estimation for DR detection, according to the data from a prospective study, Uyar et al. (22) used semiquantitative capillary assessments to evaluate the presence of tortuosity. The receiver operating characteristic (ROC) curve analysis of the diagnostic test estimation showed that the area under the curve (AUC) value for tortuosity was 0.615 (95% CI 0.540 to 0.689). For significance, in the multivariate logistic regression analysis, tortuosity was significantly associated with DR (OR 2.16; P = 0.036). In general, an AUC value ranges from 0 to 1. The closer to 1 indicates a better diagnostic performance, while a value of 0.5 means the test is no better than random chance. Here, an AUC of 0.615 for tortuosity suggests that it has limited diagnostic accuracy for detecting DR, despite the significance in the regression analysis. Although previous research offered new perspectives on the relationships between NFC changes and DR, it should be noted that prior studies were qualitative assessments, which were subjective and lacked the high-resolution quantification ability of the quantitative approach, giving rise to a lack of reliability in their outcomes.
Unlike previous research, Okabe et al. (29) evaluated nailfold capillary alterations quantitatively with NFC, Kekkan-Bijin SC-10, showing an AUC value for nailfold capillary length of 0.83 (95% CI 0.71 to 0.90, P < 0.001). Furthermore, when adding nailfold capillary length into conventional systemic risk factors, it also substantially enhances the discriminating capability for DR, yielding an AUC of 0.89 (95% CI 0.79 to 0.95, P < 0.001). Another study applied a video-based technique for nailfold capillary evaluation, revealing that vasodilation of the nailfold capillaries serves as a reliable indication of DR, reaching an AUC of 0.75 (95% CI 0.634 to 0.875, P < 0.001) (27). Similarly, in the study of Rohit et al. (23), the aforementioned characteristics were recorded by a semiquantitative NFC score in the center 3 mm of each image. They found that NFC score was an important predictor of DR with an AUC of 0.745 (95% CI 0.648 to 0.827, P < 0.001) for correctly predicting DR. Surprisingly, a high diagnostic accuracy of NFC (72%) in retinopathy was observed even in patients with controlled HbA1c levels (<7%) (23). Subsequently, for practical use in a clinical environment, Goydin et al. (30) created a computer program calculating the results of DR predictions according to NFC changes, showing that NFC changes (capillary network density, velocity of arterial and venous blood flow) have a high diagnostic information value for detecting both NPDR and PDR (92.2% vs. 94.4%). Automatic assessment of NFC changes eliminated the effects of grader subjectivity, making it possible for NFC changes to serve as essential non-invasive markers for DR identification.
Overall, the quantitative evaluation of NFC has offered a variety of metrics for the direct assessment of peripheral microvascular structure and has the potential to serve as a promising supplementary method for DR identification due to its high sensitivity and specificity, especially when added to conventional systemic risk factors. To some degree, NFC also seems to have a role in the prognosis and identification of patients at elevated potential for DR. In addition, through a monitor, patients can confirm their microvascular damage visually, which may enhance diabetes self-management and compliance with DR screening.
Despite these favorable findings, current studies still had the following limitations that require further consideration. First, the existing research mainly consists of observational studies, making it difficult to analyze the temporal relationship between NFC alterations and DR. Additionally, due to the relatively small sample size of these studies, it can be difficult to apply these present results to all geographic regions and ethnic groups. As a result, larger cohort studies with diverse demographic populations are required to elucidate the precise and temporal relationships between NFC changes and DR. Simultaneously, alterations in NFC could indicate multiple diseases, potentially resulting in an erroneous diagnosis. Future NFC research is required to elucidate the link between various comorbidities and DM.
Previous research in vitro has verified that advanced glycation end-products (AGEs) play a crucial part in DR pathogenesis by inducing pericyte apoptosis (31), increasing proinflammatory mediators (32), hindering retinal microvascular endothelial cell function (33), and adding VEGF secretion (34), thereby facilitating the development of DR. Consequently, AGEs have the potential to function as a biomarker for DR.
Given the low turnover rate, AGEs frequently accumulate in skin tissues over time. The direct measurement of AGEs in skin biopsy specimens can be a marker of future DR development in the DCCT/EDIC study (35). While the direct evaluation of AGE quantification from certain targeted tissues has enhanced our understanding of the correlation between AGE accumulation and the degree of DR, the fact that this method is invasive and time-consuming still impedes its clinical application.
With the unique fluorescence pattern of AGEs, skin autofluorescence (SAF), a non-invasive biomarker for AGE accumulation in epidermal tissues, has recently been developed (36). In recent years, AGEs quantified via SAF have been employed for medical diagnosis (37, 38), particularly in individuals with diabetes (39). Studies to date have suggested that SAF is a non-invasive surrogate biomarker for diabetic microvascular complications (40, 41), as it provides a non-invasive and cost-effective diagnostic method with a high level of reproducibility (37).
Most earlier studies on the correlation between DR prevalence and SAF have focused on T1DM patients. It has been established that SAF correlates with the prevalence of DR in adults (42, 43), as well as in adolescents with T1DM (44). Skin AGE accumulation was also predictive of future DR progression (35). The high area under the ROC curve for DR (AUC: 0.89, 95% CI 0.85 to 0.94) observed by Januszewski et al. (51) suggested that SAF may function as a non-invasive biomarker for DR in T1DM.
Consistent with previous research on T1DM patients, in T2DM patients, SAF was also correlated with the severity of DR (45, 46). After adjusting for variables such as age, smoking, and diabetic nephropathy, which were known to increase SAF measures (42), SAF remained linked with the severity of DR in T2DM patients (47). Also, a current meta-analysis of six studies revealed that SAF was connected to DR and that for every 0.1 unit rise in SAF level, there was a 5% increased risk of DR (OR = 1.05, 95% CI 1.03 to 1.08) (41). Differently, it is unclear whether there is a linear correlation between the severity of the DR in T2DM patients and the degree of AGEs. Takayanagi et al. (48) evaluated the potential role of AGEs during the progression of DR in T2DM patients and discovered that among groups categorized by quartiles of AGE scores, only the top quartile exhibited a substantially elevated rate of PDR. This finding suggests that significantly raised levels of AGEs may contribute to the development of PDR, highlighting the clinical value of the SAF as a non-invasive and dependable biomarker for individuals at risk of VTDR.
Most importantly, accumulating evidence in recent years suggested that SAF was a superior diagnostic biomarker to HbA1c for DR and PDR. Concretely, an ROC analysis revealed that the predictive capacities of SAF and HbA1c for DR were 0.79 and 0.55, while for PDR, the predictive capacities were 0.81 and 0.61, respectively (45). Furthermore, Ling et al. (46) found that sensitivity was considerably greater than that of HbA1c when AGEs >72.3. AGEs demonstrated a considerably greater efficacy in early diagnosis than HbA1c in the context of VTDR. These might be because of the “metabolic memory” effect, where the accumulation of AGEs shows the long-lasting consequences of hyperglycemia, while HbA1c reflects the short-term effects of glycemic management. Given that microvascular issues can arise even in prediabetes, SAF could therefore be more useful than HbA1c for assessing retinal damage (35).
As for the accuracy of SAF for DR detection, the SAF test as a diagnostic tool for DR has demonstrated adequate accuracy for clinical application. However, the ideal cutoff value of SAF for distinguishing patients remains controversial. In a study including 138 T2DM patients, Hirano et al. (47) revealed that the cutoff point between mild NPDR and no DR was 2.25 with an AUC of 0.78, while the cutoff line between severe NPDR and PDR was 2.32 (AUC: 0.75). Another study of 1,471 T2DM patients found that the best cutoff point for SAF to identify any DR was 72.3 (AUC: 0.56) and VTDR was 77.1 (AUC: 0.73) (46). A recent meta-analysis encompassed four studies to assess the efficacy of SAF as an instrument for DR screening. The OR value for detecting DR with SAF was 5.11 (49). These discrepancies may be due to different sample sizes, subject variability, and lifestyle variances. Notably, larger samples and further studies are required to determine suitable SAF reference values across populations.
Considering the widespread agreement that SAF is a potential biomarker for the onset and development of DR, multiple confounding variables should be evaluated before they are used in clinical trials. Firstly, in individuals with T2DM, renal function could affect the correlation between SAF and retinopathy, as renal insufficiency alone can elevate SAF levels even in non-diabetic individuals (50, 51). Consequently, in reality, SAF may assist in identifying certain participants for DR screening; however, individuals with renal insufficiency and diabetes should be examined regardless of their SAF levels, as they exhibit a three-fold greater prevalence of retinopathies (52), not associated with SAF (53). Furthermore, SAF can be impacted by skin pigmentation, resulting in diminished measurement accuracy (54). Fortunately, the AGE sensor may make up for this deficiency (55). Subsequently, Kim et al. (56) tested a novel SAF measuring system transmitted through the first dorsal interossei muscles, which provided better screening performance, especially in Asian T2DM subjects. When measuring SAF, it is essential to consider other confounding factors, such as the heightened generation or intake of AGEs from dietary sources or smoking, which could exacerbate AGE buildup, alongside the diminished elimination of AGEs (57–59).
Generally, the SAF measurement of AGEs as a diagnostic biomarker for DR is accurate enough for clinical use; however, it cannot replace the fundus test due to its limitations. In order to address its variability and ensure its appropriate use, more patient evaluations are required. Additionally, we also need more research to establish reference cutoffs and account for all the variables that could influence the test.
In general, NFC changes and SAF are sensitive, cost-effective biomarkers that enable the early detection of DR Table 1. Quantitative evaluation of NFC, such as the nailfold capillary length and arterial blood flow velocity, showed distinct advantages in differentiating the different stages of DR. Furthermore, adding these biomarkers to risk factors can enhance the discrimination of DR. Moreover, in both T1DM and T2DM patients, SAF has been shown to be correlated with the prevalence and severity of DR. Furthermore, SAF may be a superior diagnostic biomarker to HbA1c for DR and PDR, as it reflects long-term hyperglycemia effects. As both NFC changes and SAF are sensitive and cost-effective biomarkers for early DR detection, more longitudinal studies and basic research are needed to clarify their relationships with DR, validate them in independent cohorts, and establish appropriate reference values.
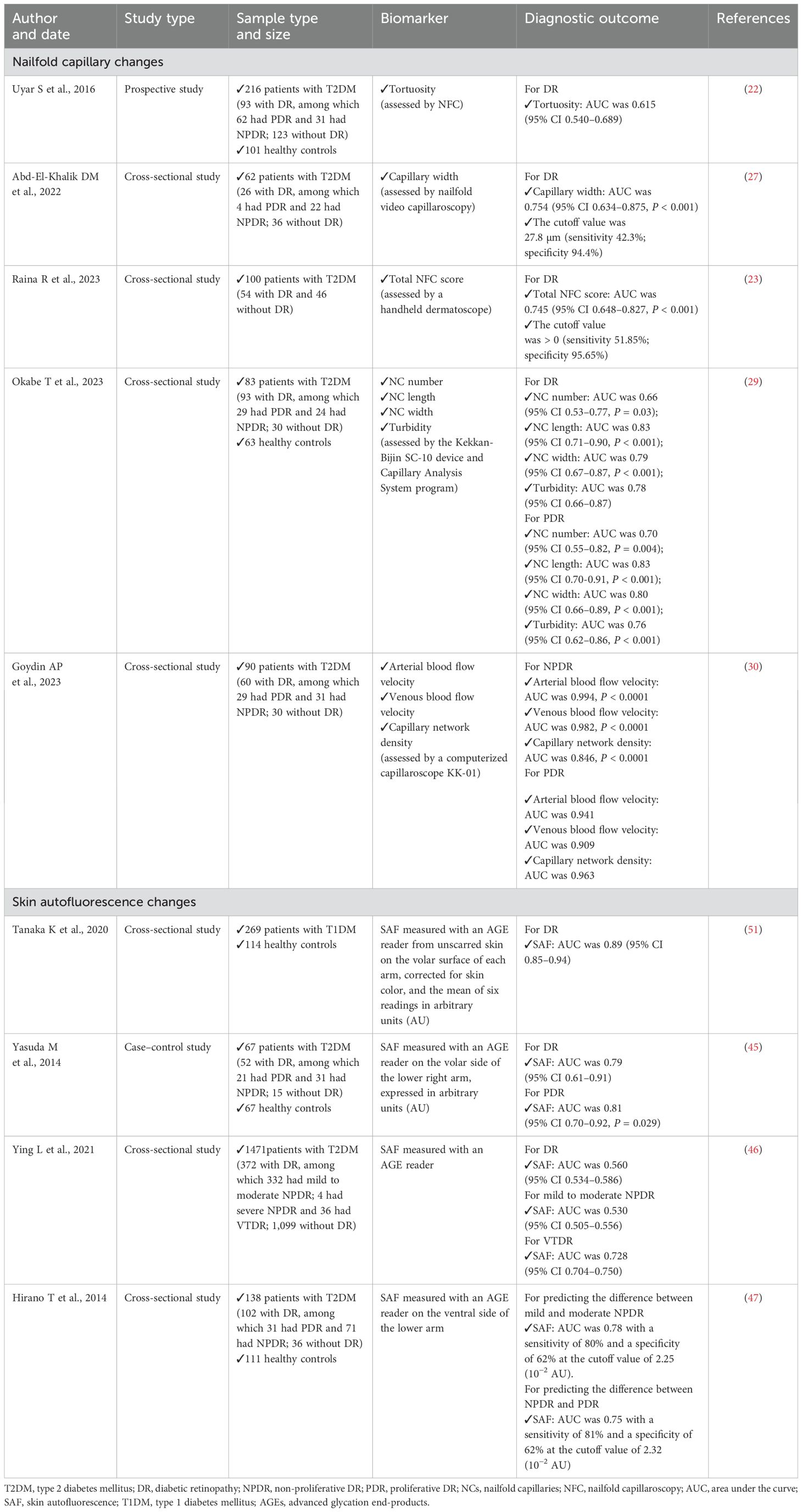
Table 1. Summary of key articles about non-ocular diagnostic biomarkers of DR by non-invasive methods.
This review provided researchers with vast and absolute knowledge about the current schemes and drawbacks in non-ocular and non-invasive diagnostic biomarkers of DR. Although these non-invasive diagnostic biomarkers have only moderate accuracy, we still consider them to be of diagnostic value and sufficient accuracy for their use by non-ophthalmologists in primary screenings for DR, particularly in the absence of fundus investigations. Moreover, combinatorial biomarkers merit consideration since they have substantially greater sensitivity compared to individual biomarkers. Consequently, extensive investigations and validations are necessary to determine whether specific non-ocular biomarkers or their combinations exhibit the greatest predictive efficacy for use as a screening tool in routine clinical practice for non-ophthalmologists.
HG: Writing – original draft, Writing – review & editing. JL: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-037A) and 2022 Tianjin Binhai New Area Health and Health Commission Science and Technology Project (2022BWKY009).
The authors would like to sincerely thank Dr. Manhong Xu, who helped with the design of the picture and table.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care: The international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. (2018) 125:1608–22. doi: 10.1016/j.ophtha.2018.04.007
2. Tan GS, Cheung N, Simó R, Cheung GCM, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. (2017) 5:143–55. doi: 10.1016/S2213-8587(16)30052-3
3. Teo ZL, Tham Y-C, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology. (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
4. Xu X-H, Sun B, Zhong S, Wei D-D, Hong Z, Dong A-Q. Diabetic retinopathy predicts cardiovascular mortality in diabetes: A meta-analysis. BMC Cardiovasc Disord. (2020) 20:478. doi: 10.1186/s12872-020-01763-z
5. Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20-79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. (2017) 5:423–30. doi: 10.1016/S2213-8587(17)30097-9
6. Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. (2003) 110:1677–82. doi: 10.1016/S0161-6420(03)00475-5
7. Screening guidelines for diabetic retinopathy. American college of physicians, American diabetes association, and american academy of ophthalmology. Ann Internal Med. (1992) 116:683–5. doi: 10.7326/0003-4819-116-8-683
8. Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes Care. (2004) 27 Suppl 1:S84–87. doi: 10.2337/diacare.27.2007.s84
9. Al-Dwairi R, El-Elimat T, Aleshawi A, Al Sharie AH, Abu Mousa BM, Al Beiruti S, et al. Vitreous levels of vascular endothelial growth factor and platelet-derived growth factor in patients with proliferative diabetic retinopathy: A clinical correlation. Biomolecules. (2023) 13:1630. doi: 10.3390/biom13111630
10. Wang JR, Chen Z, Yang K, Yang HJ, Tao WY, Li YP, et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol Metab Syndr. (2020) 12:55. doi: 10.1186/s13098-020-00562-y
11. Zeng J, Chen M, Feng Q, Wan H, Wang J, Yang F, et al. The platelet-to-lymphocyte ratio predicts diabetic retinopathy in type 2 diabetes mellitus. Diabetes Metab Syndrome Obesity: Targets Ther. (2022) 15:3617–26. doi: 10.2147/DMSO.S378284
12. Yang L, Yu W, Pan W, Chen S, Ye X, Gu X, et al. A clinical epidemiological analysis of prognostic nutritional index associated with diabetic retinopathy. Diabetes Metab Syndr Obes. (2021) 14:839–46. doi: 10.2147/DMSO.S295757
13. Xu Y-X, Pu S-D, Zhang Y-T, Tong X-W, Sun X-T, Shan Y-Y, et al. Insulin resistance is associated with the presence and severity of retinopathy in patients with type 2 diabetes. Clin Experiment Ophthalmol. (2024) 52:63–77. doi: 10.1111/ceo.14344
14. Khairul-Anwar I, Wan-Nazatul-Shima S, Siti-Lailatul-Akmar Z, Siti-Azrin AH, Zunaina E. Evaluation of TNF-α and IL-6 in saliva among diabetic retinopathy patients in east coast Malaysia. Trop Med Int Health: TM IH. (2022) 27:310–6. doi: 10.1111/tmi.13724
15. Bai J, Wan Z, Zhang Y, Wang T, Xue Y, Peng Q. Composition and diversity of gut microbiota in diabetic retinopathy. Front Microbiol. (2022) 13:926926. doi: 10.3389/fmicb.2022.926926
16. Mujeeb S, Rodrigues GR, Nayak RR, Kamath AR, Kamath SJ, Kamath G. Urine protein: Urine creatinine ratio correlation with diabetic retinopathy. Indian J Ophthalmol. (2021) 69:3359–63. doi: 10.4103/ijo.IJO_1269_21
17. Shaker OG, Abdelaleem OO, Mahmoud RH, Abdelghaffar NK, Ahmed TI, Said OM, et al. Diagnostic and prognostic role of serum miR-20b, miR-17-3p, HOTAIR, and MALAT1 in diabetic retinopathy. IUBMB Life. (2019) 71:310–20. doi: 10.1002/iub.1970
18. Liu J, Li J, Tang Y, Zhou K, Zhao X, Zhang J, et al. Transcriptome analysis combined with mendelian randomization screening for biomarkers causally associated with diabetic retinopathy. Front Endocrinol. (2024) 15:1410066. doi: 10.3389/fendo.2024.1410066
19. He M, Hou G, Liu M, Peng Z, Guo H, Wang Y, et al. Lipidomic studies revealing serological markers associated with the occurrence of retinopathy in type 2 diabetes. J Transl Med. (2024) 22:448. doi: 10.1186/s12967-024-05274-9
20. Ting DSW, Tan K-A, Phua V, Tan GSW, Wong CW, Wong TY. Biomarkers of diabetic retinopathy. Curr Diabetes Rep. (2016) 16:125. doi: 10.1007/s11892-016-0812-9
21. Sirufo MM, Bassino EM, De Pietro F, Ginaldi L, De Martinis M. Nailfold capillaroscopy: Clinical practice in non-rheumatic conditions. Microvasc Res. (2021) 134:104122. doi: 10.1016/j.mvr.2020.104122
22. Uyar S, Balkarlı A, Erol MK, Yeşil B, Tokuç A, Durmaz D, et al. Assessment of the relationship between diabetic retinopathy and nailfold capillaries in type 2 diabetics with a noninvasive method: Nailfold videocapillaroscopy. J Diabetes Res. (2016) 2016:7592402. doi: 10.1155/2016/7592402
23. Raina R, Chhabra N, Barnwal S, Vasisht S, Kansal NK, Kant R. Predictability of nailfold capillaroscopic score in diagnosing retinopathy in patients with type 2 diabetes mellitus and its utility as a non-invasive tool for differentiating from those not having retinopathy: A pilot observational cross-sectional analytical study. Indian J Dermatol. (2023) 68:354. doi: 10.4103/ijd.ijd_289_23
24. Mahajan M, Kaur T, Singh K, Mahajan BB. Evaluation of nail fold capillaroscopy changes in patients with diabetic retinopathy and healthy controls, and its correlation with disease duration, HbA1c levels and severity of diabetic retinopathy: an observational study. Indian J Dermatol Venereol Leprol. (2024) 90(6):782–88. doi: 10.25259/IJDVL_232_2023
25. Mohanty G, Padhan P, Chilakamarthy S, Das MK, Bhuyan D. Can nailfold capillaroscopy be a screening tool for diabetic retinopathy - a hospital based cross-sectional study in orissa, India. J Evidence Based Med Healthcare. (2021) 8:1479–83. doi: 10.18410/jebmh/2021/280
26. Shikama M, Sonoda N, Morimoto A, Suga S, Tajima T, Kozawa J, et al. Association of crossing capillaries in the finger nailfold with diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Invest. (2021) 12:1007–14. doi: 10.1111/jdi.13444
27. Abd-El-Khalik DM, Hafez EA, Hassan HE, Mahmoud AE, Ashour DM, Morshedy NA. Nail folds capillaries abnormalities associated with type 2 diabetes mellitus progression and correlation with diabetic retinopathy. Clin Med Insights Endocrinol Diabetes. (2022) 15:11795514221122828. doi: 10.1177/11795514221122828
28. Kaminska-Winciorek G, Deja G, Polańska J, Jarosz-Chobot P. Diabetic microangiopathy in capillaroscopic examination of juveniles with diabetes type 1. Postepy Higieny I Medycyny Doswiadczalnej (Online). (2012) 66:51–9.
29. Okabe T, Kunikata H, Yasuda M, Kodama S, Maeda Y, Nakano J, et al. Relationship between nailfold capillaroscopy parameters and the severity of diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol. (2024) 262(3):759–68. doi: 10.1007/s00417-023-06220-z
30. Goydin AP, Shutova SV, Fabrikantov OL. Evaluation of the diagnostic capabilities of nailfold capillaroscopy in diabetic retinopathy. Vestn Oftalmol. (2023) 139:16–26. doi: 10.17116/oftalma202313901116
31. Kim J, Kim KM, Kim C-S, Sohn E, Lee YM, Jo K, et al. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free Radic Biol Med. (2012) 53:357–65. doi: 10.1016/j.freeradbiomed.2012.04.030
32. Portillo J-AC, Pfaff A, Vos S, Weng M, Nagaraj RH, Subauste CS. Advanced glycation end products upregulate CD40 in human retinal endothelial and müller cells: Relevance to diabetic retinopathy. Cells. (2024) 13:429. doi: 10.3390/cells13050429
33. Radeva MY, Waschke J. Mind the gap: Mechanisms regulating the endothelial barrier. Acta Physiol (Oxford England). (2018) 222:e12860. doi: 10.1111/apha.12860
34. Zhao B, Smith G, Cai J, Ma A, Boulton M. Vascular endothelial growth factor C promotes survival of retinal vascular endothelial cells via vascular endothelial growth factor receptor-2. Br J Ophthalmol. (2007) 91:538–45. doi: 10.1136/bjo.2006.101543
35. Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, et al. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. (2005) 54:3103–11. doi: 10.2337/diabetes.54.11.3103
36. Mulder DJ, Bieze M, Graaff R, Smit AJ, Hooymans JMM. Skin autofluorescence is elevated in neovascular age-related macular degeneration. Br J Ophthalmol. (2010) 94:622–5. doi: 10.1136/bjo.2009.162990
37. DaMouraSemedo C, Webb M, Waller H, Khunti K, Davies M. Skin autofluorescence, a non-invasive marker of advanced glycation end products: Clinical relevance and limitations. Postgrad Med J. (2017) 93:289–94. doi: 10.1136/postgradmedj-2016-134579
38. Smit AJ, van de Zande SC, Mulder DJ. Skin autofluorescence as tool for cardiovascular and diabetes risk prediction. Curr Opin Nephrol Hypertens. (2022) 31:522–26. doi: 10.1097/MNH.0000000000000835
39. Boersma HE, van der Klauw MM, Smit AJ, Wolffenbuttel BHR. A non-invasive risk score including skin autofluorescence predicts diabetes risk in the general population. Sci Rep. (2022) 12:21794. doi: 10.1038/s41598-022-26313-9
40. Yoshioka K. Skin autofluorescence is a noninvasive surrogate marker for diabetic microvascular complications and carotid intima-media thickness in Japanese patients with type 2 diabetes: a cross-sectional study. Diabetes Therapy. (2018) 9(1):75–85. doi: 10.1007/s13300-017-0339-3
41. Hosseini MS, Razavi Z, Ehsani AH, Firooz A, Afazeli S. Clinical significance of non‑invasive skin autofluorescence measurement in patients with diabetes: A systematic review and meta‑analysis. EClinicalMedicine. (2021) 42:101194. doi: 10.1016/j.eclinm.2021.101194
42. Cleary PA, Braffett BH, Orchard T, Lyons TJ, Maynard J, Cowie C, et al. Clinical and technical factors associated with skin intrinsic fluorescence in subjects with type 1 diabetes from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Technol. (2013) 15:466–74. doi: 10.1089/dia.2012.0316
43. Orchard TJ, Lyons TJ, Cleary PA, Braffett BH, Maynard J, Cowie C, et al. The association of skin intrinsic fluorescence with type 1 diabetes complications in the DCCT/EDIC study. Diabetes Care. (2013) 36:3146–53. doi: 10.2337/dc12-2661
44. Cho YH, Craig ME, Januszewski AS, Benitez-Aguirre P, Hing S, Jenkins AJ, et al. Higher skin autofluorescence in young people with type 1 diabetes and microvascular complications. Diabetic Med: A J Br Diabetic Assoc. (2017) 34:543–50. doi: 10.1111/dme.13280
45. Yasuda M, Shimura M, Kunikata H, Kanazawa H, Yasuda K, Tanaka Y, et al. Relationship of skin autofluorescence to severity of retinopathy in type 2 diabetes. Curr Eye Res. (2015) 40:338–45. doi: 10.3109/02713683.2014.918152
46. Ying L, Shen Y, Zhang Y, Wang Y, Liu Y, Yin J, et al. Association of advanced glycation end products with diabetic retinopathy in type 2 diabetes mellitus. Diabetes Res Clin Pract. (2021) 177:108880. doi: 10.1016/j.diabres.2021.108880
47. Hirano T, Iesato Y, Toriyama Y, Imai A, Chiba D, Murata T. Correlation between diabetic retinopathy severity and elevated skin autofluorescence as a marker of advanced glycation end-product accumulation in type 2 diabetic patients. J Diabetes Complications. (2014) 28:729–34. doi: 10.1016/j.jdiacomp.2014.03.003
48. Takayanagi Y, Yamanaka M, Fujihara J, Matsuoka Y, Gohto Y, Obana A, et al. Evaluation of relevance between advanced glycation end products and diabetic retinopathy stages using skin autofluorescence. Antioxid (Basel Switzerland). (2020) 9(11):1100. doi: 10.3390/antiox9111100
49. Martínez-García I, Cavero-Redondo I, Álvarez-Bueno C, Pascual-Morena C, Gómez-Guijarro MD, Saz-Lara A. Non-invasive skin autofluorescence as a screening method for diabetic retinopathy. Diabetes Metab Res Rev. (2024) 40:e3721. doi: 10.1002/dmrr.3721
50. Januszewski AS, Xu D, Cho YH, Benitez-Aguirre PZ, O’Neal DN, Craig ME, et al. Skin autofluorescence in people with type 1 diabetes and people without diabetes: An eight-decade cross-sectional study with evidence of accelerated aging and associations with complications. Diabetic Med: A J Br Diabetic Assoc. (2021) 38:e14432. doi: 10.1111/dme.14432
51. Tanaka K, Tani Y, Asai J, Nemoto F, Kusano Y, Suzuki H, et al. Skin autofluorescence is associated with renal function and cardiovascular diseases in pre-dialysis chronic kidney disease patients. Nephrology Dialysis Transplantation: Off Publ Eur Dialysis Transplant Assoc Eur Renal Assoc. (2011) 26:214–20. doi: 10.1093/ndt/gfq369
52. Man REK, Sasongko MB, Wang JJ, MacIsaac R, Wong TY, Sabanayagam C, et al. The association of estimated glomerular filtration rate with diabetic retinopathy and macular edema. Invest Ophth Vis Sci. (2015) 56:4810–6. doi: 10.1167/iovs.15-16987
53. Bentata R, Cougnard-Grégoire A, Delyfer MN, Delcourt C, Blanco L, Pupier E, et al. Skin autofluorescence, renal insufficiency and retinopathy in patients with type 2 diabetes. J Diabetes Complications. (2017) 31:619–23. doi: 10.1016/j.jdiacomp.2016.10.028
54. Koetsier M, Nur E, Chunmao H, Lutgers HL, Links TP, Smit AJ, et al. Skin color independent assessment of aging using skin autofluorescence. Opt Express. (2010) 18:14416–29. doi: 10.1364/OE.18.014416
55. Shirakami T, Yamanaka M, Fujihara J, Matsuoka Y, Gohto Y, Obana A, et al. Advanced glycation end product accumulation in subjects with open-angle glaucoma with and without exfoliation. Antioxid (Basel Switzerland). (2020) 9:755. doi: 10.3390/antiox9080755
56. Kim JJ, Jeong B, Cho Y, Kwon M-H, Lee Y-H, Kang U, et al. The association between skin auto-fluorescence of palmoplantar sites and microvascular complications in asian patients with type 2 diabetes mellitus. Sci Rep. (2018) 8:6309. doi: 10.1038/s41598-018-24707-2
57. Perrone A, Giovino A, Benny J, Martinelli F. Advanced glycation end products (AGEs): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxid Med Cell Longev. (2020) 2020:3818196. doi: 10.1155/2020/3818196
58. Noordzij MJ, Lefrandt JD, Graaff R, Smit AJ. Dermal factors influencing measurement of skin autofluorescence. Diabetes Technol. (2011) 13:165–70. doi: 10.1089/dia.2010.0123
Keywords: diabetic retinopathy, biomarkers, non-ocular, non-invasive, community screening, nailfold capillaroscopy, skin autofluorescence
Citation: Gou H and Liu J (2025) Non-ocular biomarkers for early diagnosis of diabetic retinopathy by non-invasive methods. Front. Endocrinol. 16:1496851. doi: 10.3389/fendo.2025.1496851
Received: 15 September 2024; Accepted: 19 February 2025;
Published: 12 March 2025.
Edited by:
Bert B. Little, University of Louisville, United StatesReviewed by:
Shaminul Shakib, University of Louisville, United StatesCopyright © 2025 Gou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juping Liu, dHlkbGpwQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.