- 1Department of Urology, Kunming Children’s Hospital, Kunming, Yunnan, China
- 2Department of Orthopedics, Kunming Children’s Hospital, Kunming, Yunnan, China
- 3Yunnan Province Clinical Research Center for Children’s Health and Disease, Kunming Children’s Solid Tumor Diagnosis and Treatment Center, Kunming, Yunnan, China
- 4Yunnan Key Laboratory of Children’s Major Disease Research, Yunnan Clinical Medical Center for Pediatric Diseases, Kunming Children’s Hospital, Kunming, Yunnan, China
Zinc is an essential trace element in the human body, playing a crucial role in cellular metabolism.Dysregulation of zinc homeostasis can lead to abnormal cellular metabolism, contributing to diseases and closely related to tumor development. Adequate zinc intake can maintain zinc homeostasis in the body and support normal cellular metabolism. This review discusses the metabolic processes of zinc in the human body and its close relationship with tumorigenesis. It briefly describes zinc absorption, transport, storage, and release, as well as its important role in gene expression, signal transduction, oxidative stress, immune response, and apoptosis. It focuses on the abnormal cellular metabolism caused by excessive or insufficient zinc, the relationship between zinc homeostasis disruption and metabolic syndrome, and the mechanisms involved in tumor development. It analyzes how changes in the expression and activity of zinc transporters may lead to disrupted zinc homeostasis in tumor tissues. It points out that zinc deficiency is associated with various cancers, including prostate cancer, hepatocellular carcinoma, pancreatic cancer, lung cancer, ovarian cancer, esophageal squamous cell carcinoma, and breast cancer. The summary emphasizes that zinc metalloproteins could serve as potential targets for cancer therapy, and regulating the expression and activity of zinc transport proteins may offer new methods and strategies for clinical cancer treatment.
1 Introduction
Zinc is a vital trace element for the human body, playing a key role in protein composition within cells and participating in metabolic processes. Zinc is involved in the conformation and function of nuclear transcription factors, facilitating protein synthesis, and it also acts as a component of superoxide dismutase (SOD), providing strong antioxidant activity. Additionally, zinc plays a role in processes such as apoptosis and immune response (1). Dysregulation of zinc homeostasis can lead to disturbances in cellular metabolic functions and human diseases, with the relationship between zinc metabolism disorders and metabolic syndrome and tumors needing further investigation. However, there is currently no definitive conclusion regarding the correlation between zinc metabolism and metabolic syndrome, as existing studies show contradictory results, and the specific mechanisms supporting their relationship remain unclear. More research is needed to explore this issue, as numerous studies have proposed a link between zinc homeostasis disruption and cancer (2). This article briefly summarizes zinc absorption, transport, and its involvement in metabolic processes within the human body. It summarizes diseases related to abnormal zinc metabolism and focuses on the relationship between zinc homeostasis disruption, metabolic syndrome, and tumors. It discusses issues and controversies regarding zinc in cancer treatment, offering insights for cancer diagnosis and therapy.
2 Zinc absorption, transport, and its functions
The main source of zinc in the human body is from dietary intake, and it can also be transported from reserves in the liver, muscles, and other tissues to other parts of the body. In the stomach, zinc forms complexes with proteins in food under the action of gastric acid. It is mainly absorbed in the upper part of the small intestine through zinc transporters on the apical membrane of intestinal epithelial cells, particularly the ZIP4 transporter. After absorption, zinc is transported into the plasma through zinc ion channels and the ZnT1 transport protein located on the basolateral membrane of intestinal epithelial cells, and subsequently delivered to body tissues (3). Zinc participates in vital cellular activities (4). It is primarily found in the liver, pancreas, muscles, bones, and prostate, playing significant roles in human health.
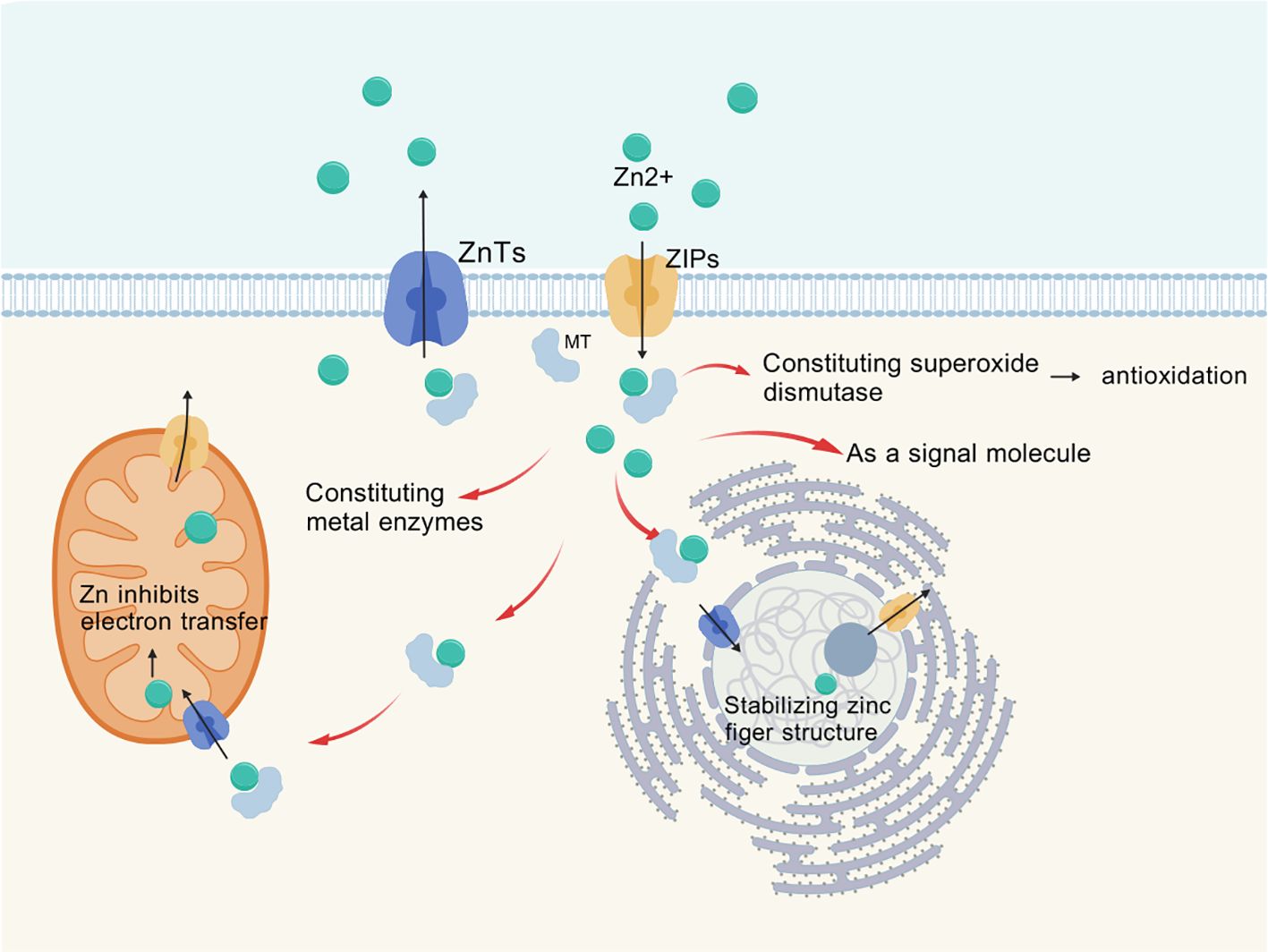
Zinc is transported across membranes via transporters ZnT or ZIP (as shown in Figure 1), with ZIP family proteins facilitating the influx of zinc ions from the extracellular space or from intracellular vesicles and organelles into the cytoplasm (5). Currently, at least 14 types of ZIP proteins and 10 types of ZnT proteins have been identified in the human body, which exhibit tissue-specific differential expression (6). These zinc transporters exhibit structural homology as well as differences, and their varying tissue distribution and functions have been a popular research topic (7). Zinc binds to metallothioneins (MT) in the cytoplasm, which consist of four subtypes; MT-1 and MT-2 are present in all cells of the body, regulating intracellular levels and flux of zinc and copper while detoxifying heavy metals. MTs are also involved in nuclear transcription and play a role in immune function through their chelation of metals (8).
Zinc homeostasis within cells is maintained by the ZIP (zinc transporter) and ZnT (zinc transport protein) families, as well as metallothioneins (1, 9). These proteins are all regulated during their functions and interact with other metabolic and signaling pathways. Once zinc ions enter the cells, they are transported to various organelles, with a significant presence in mitochondrial metalloproteins. Many zinc metalloproteins are secreted or reside in organelles such as the endoplasmic reticulum, Golgi apparatus, and secretory vesicles (1). Zinc ion signaling regulates cellular proliferation, differentiation, ion transport, and secretory functions (10).
Zinc exists in two forms within human cells (11). One form of zinc is bound to proteins, serving as an essential component of many proteins in the human body (12). It can chelate with negatively charged parts of molecules, such as cysteine and histidine, providing structural bridges to maintain the three-dimensional conformation of polypeptides, known as zinc metalloproteins or zinc metallozymes. Currently, over 3,000 types of such metalloproteins have been identified (1). This includes oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases, which are involved in processes such as oxidative stress, apoptosis, and immune responses within cells (12). The other form is mobile zinc bound to non-protein ligands, the nature of which is still unknown (13). This type of zinc ion forms a loose association with non-protein ligands, known as free zinc ions, which serve as a pool for exchangeable zinc (11).
Zinc plays a crucial role in metabolic processes such as gene expression, signal transduction, oxidative stress, immune response, and apoptosis. It is involved in the conformation and function of nuclear transcription factors, such as zinc fingers and nuclear receptors, both of which are stabilized by four coordinated zinc ions. By stabilizing zinc finger structures, zinc executes important functions in cells, playing significant roles in DNA replication and repair, transcription and translation, cellular proliferation and maturation, as well as apoptosis regulation (14). Zinc, as a component of superoxide dismutase (SOD), exhibits strong antioxidant activity. SOD exists in three isoenzymatic forms in the body: copper-zinc superoxide dismutase, found in the cytoplasm; manganese superoxide dismutase (Mn SOD), located in the mitochondria; and extracellular superoxide dismutase (EC-SOD), which is present in extracellular spaces and fluids (15). These three forms of SOD work together to protect cells from the toxic effects of excessive reactive oxygen species. Zinc also acts as a cofactor in the formation of active thymosin (Zn FTS) released by thymocytes (9). Zn FTS regulates the differentiation of mature T cells in the thymus and the function of mature T cells in peripheral blood, having less impact on B cell development compared to T cells (16). It promotes the host defense functions of the immune system.
3 Disorders related to zinc homeostasis imbalance
Both excess and deficiency of zinc can lead to dysregulation of zinc homeostasis, and ultimately resulting in human diseases. Excessive zinc intake releases soluble zinc salts in acidic gastric fluid, which can directly irritate the gastrointestinal mucosa and lead to ulcer formation (17). This condition manifests as symptoms such as nausea, vomiting, decreased appetite, abdominal cramps, and headaches (18). Excessive zinc, once absorbed into the bloodstream, inhibits normal metabolic processes involving zinc (19). For example, high levels of zinc in the blood can suppress the antioxidant pathways in red blood cells, leading to oxidative damage to the cell membranes, which increases the risk of hemolysis, coagulopathy, and even triggers disseminated intravascular coagulation (DIC) (20). Systemic hypoxemia can then lead to liver dysfunction, pancreatitis, coagulation-related disorders, acute renal failure associated with tubular damage, and neurological abnormalities (19).
Mild micronutrient deficiencies in the human body can lead to chronic and subtle metabolic disturbances, resulting in DNA or mitochondrial damage, which may accelerate aging and contribute to cancer and degenerative diseases (21). Zinc deficiency is associated with diabetes, cirrhosis, inflammatory bowel disease, malabsorption syndrome, and sickle cell anemia (22–28). Zinc deficiency can lead to or exacerbate issues such as immune deficiencies, gastrointestinal problems, endocrine disorders, neurological dysfunction, cancer, aging, and degenerative diseases (19). Zinc deficiency affects all metabolic processes involving zinc (Figure 2), as evidenced by several aspects: first, certain intracellular transcription factors, hormone receptors, and many enzymes require zinc to maintain structural integrity. Zinc stabilizes the tertiary folding of smaller proteins, thereby contributing to the maintenance of their functional activity (29). Zinc also plays a structural role in ribosomes, cell membranes, and nucleic acids (30). Zinc deficiency can lead to protein structural abnormalities and impaired enzyme activity. Secondly, like calcium or nitric oxide (NO), zinc acts as an intracellular and intercellular messenger, activating intracellular signaling pathways and altering gene expression patterns. Free or exchangeable zinc (loosely bound zinc) can serve as a second messenger to control various functions, including gastric acid secretion, hormone release, and cardiac electrophysiology (31). Zinc, as a signaling molecule, functions similarly to other neurotransmitters and possesses neuromodulatory capabilities (32). In the immune system, zinc plays a role in intracellular, extracellular, and intercellular signaling. A classic sign of zinc deficiency in humans is impaired innate and cell-mediated immune functions, characterized by thymic atrophy, lymphopenia, reduced leukocyte function, and recurrent infections (33). Zinc deficiency leads to abnormalities in cellular signaling and neuromodulatory functions. Thirdly, redox reactions and antioxidant activities become dysregulated. Zinc can directly protect cell membranes from oxidative damage (34). Zinc can also indirectly reduce potential free radical formation and lipid peroxidation, as well as oxidative damage to proteins and DNA (34). Zinc deficiency is associated with increased oxidative stress factors and inflammatory biomarkers. Zinc supplementation can reduce biomarkers of oxidative stress, such as thiobarbituric acid reactive substances (TBARS) and malondialdehyde (MDA) levels (35). Most reactive oxygen species (ROS) are produced by NADPH oxidase (NOX), and zinc can scavenge ROS, exerting antioxidant effects. Zinc levels influence the activity and levels of copper-zinc superoxide dismutase (36). Zinc deficiency leads to abnormal thiol redox status in cell membranes, resulting in increased permeability and fragility of red blood cell membranes, as well as inactivation of calcium channel proteins in the membranes (30). The activities of antioxidant enzymes such as Cu/Zn superoxide dismutase, catalase, and peroxidase are affected, leading to weakened antioxidant defenses and accelerating the process of mitochondrial oxidative aging (37). Fourth, the processes of cell growth, development, proliferation, and apoptosis become dysregulated. Zinc deficiency affects the structure of key enzymes and transcription factors involved in DNA repair and replication, including DNA polymerases, DNA-dependent RNA polymerases, and reverse transcriptases (38). Abnormal formation of zinc finger proteins, such as transcription factors, transcriptional repressors, steroid receptors, thyroid receptors, vitamin D receptors, and retinoic acid receptors occurs (39). These enzyme and protein abnormalities contribute to disruptions in cellular growth, development, and proliferation. Zinc deficiency also promotes apoptosis, primarily in rapidly growing tissues such as intestinal crypt cells, the thymus, and other embryonic and fetal tissues (40). Fifth, the metabolism of carbohydrates, lipids, and proteins, as well as cellular respiration, becomes abnormal. Within mitochondria, zinc inhibits mitochondrial respiration, terminal oxidation, and ATP production by altering the function of mitochondrial enzymes and the cytochrome electron transport chain (41). Zinc-containing enzymes and proteins participate in the metabolism of nucleic acids, proteins, carbohydrates, and lipids by interacting with hormone receptors, transcription factors, and enzyme systems (19). When zinc is deficient, the functions of the aforementioned metabolic enzymes are hindered. Additionally, zinc deficiency affects the regulatory roles of zinc-dependent metalloproteins or their antioxidant membrane-stabilizing effects, leading to diminished protection against metal toxicity and other harmful substances (42).
Currently, there is no definitive conclusion regarding the relationship between zinc metabolism-related diseases and metabolic syndrome. Metabolic syndrome is a cluster of conditions associated with obesity, hypertension, hyperglycemia, hyperlipidemia, and hyperuricemia (43). Some animal studies have suggested that zinc nanoparticles improve obesity-induced cardiovascular diseases by reducing blood pressure, oxidative stress, cardiac iron accumulation, insulin resistance, and inflammatory markers (44). Althanoon Zeina et al. also suggested that zinc supplementation in patients with metabolic syndrome is associated with improvements in systolic blood pressure, body mass index, and metabolic parameters, recommending the correction of zinc deficiency in these patients (45). These studies suggest that there may be a connection between zinc and factors associated with metabolic syndrome. However, current research remains controversial regarding whether there is a link between zinc metabolism and metabolic syndrome (46). Research shows that adequate dietary zinc consumption is linked to a decrease in the risk of MetS (47–49). A study in China has also indicated that zinc levels in children are indeed associated with components of metabolic syndrome (50). A meta-analysis of observational studies by Ding J et al. indicated that dietary zinc intake is negatively correlated with metabolic syndrome (46). Wu Y et al. found that higher blood zinc concentrations are associated with adverse changes in metabolic risk factors related to metabolic syndrome, particularly concerning BMI and LDL-c, and this relationship exhibits gender differences, mainly affecting women (51). In Aydogdu A’s study, serum zinc levels were significantly elevated in children with metabolic syndrome (52). Due to limited evidence, more well-designed prospective cohort studies are needed to clarify the relationship between serum zinc levels and metabolic syndrome. The specific mechanisms linking zinc to metabolic syndrome remain unclear; a case-control study suggested that higher serum zinc levels might be related to the number of metabolic factors, independent of BMI and insulin resistance (48). Research on this issue is limited, necessitating further studies to clarify the role of zinc status in the mechanisms associated with metabolic syndrome and to determine the optimal range of blood zinc levels in the body (51). In contrast, a cross-sectional study conducted in Iran on the relationship between serum zinc levels and metabolic syndrome in children and adolescents suggested that there is no association between serum zinc levels and metabolic syndrome in children (53). A case-control study by Ennes Dourado Ferro F et al. also indicated that there is no relationship between zinc nutritional status and biochemical markers of metabolic syndrome (54). In summary, current research on the relationship between zinc metabolism and metabolic syndrome is contradictory, and the specific mechanisms in studies supporting their correlation remain unclear, necessitating further investigation into this issue.
4 Zinc metabolism abnormalities and tumor
Approximately two-thirds of tumors in the human body are related to addictive behaviors, diet, lack of exercise, excessive sun exposure, or infections. Zinc can protect cells from damage caused by inflammation and oxidative stress, leading to the hypothesis that zinc has anti-cancer properties (55). As mentioned earlier, zinc stabilizes the structure of proteins, DNA, RNA, and ribosomes within cells, and regulates gene expression through zinc finger transcription factors, thereby altering the expression of different components in the DNA damage response (DDR) (56). Zinc deficiency is involved in various aspects of cancer cell generation and growth. Changes in zinc ion concentration can directly and specifically affect the activity of YY1 (YY1 is an intrinsically disordered transcription factor, a protein regulator of gene expression that has been shown to be related to the progression of many cancers), leading to altered gene expression patterns and potentially resulting in tumor transformation or progression (57). Zinc deficiency (ZD) is present in various tumors and affects their occurrence and progression. For instance, zinc levels in the serum or plasma of breast cancer patients are significantly reduced (58–61). The serum zinc levels are significantly reduced in patients with acute leukemia (62). Similarly, serum zinc levels are also significantly lower in bladder cancer patients, as well as in those with esophageal squamous cell carcinoma (ESCC), malignant prostate cancer, and ovarian cancer (63). This decrease in serum zinc levels may be due to the increased uptake by tumor cells and enhanced enzyme activity, leading to a higher demand for zinc in cancerous tissues (64). Conversely, some studies have found that higher toenail zinc levels in men are associated with an increased risk of prostate cancer (65).
4.1 Zinc metabolic abnormalities and tumor-related mechanisms
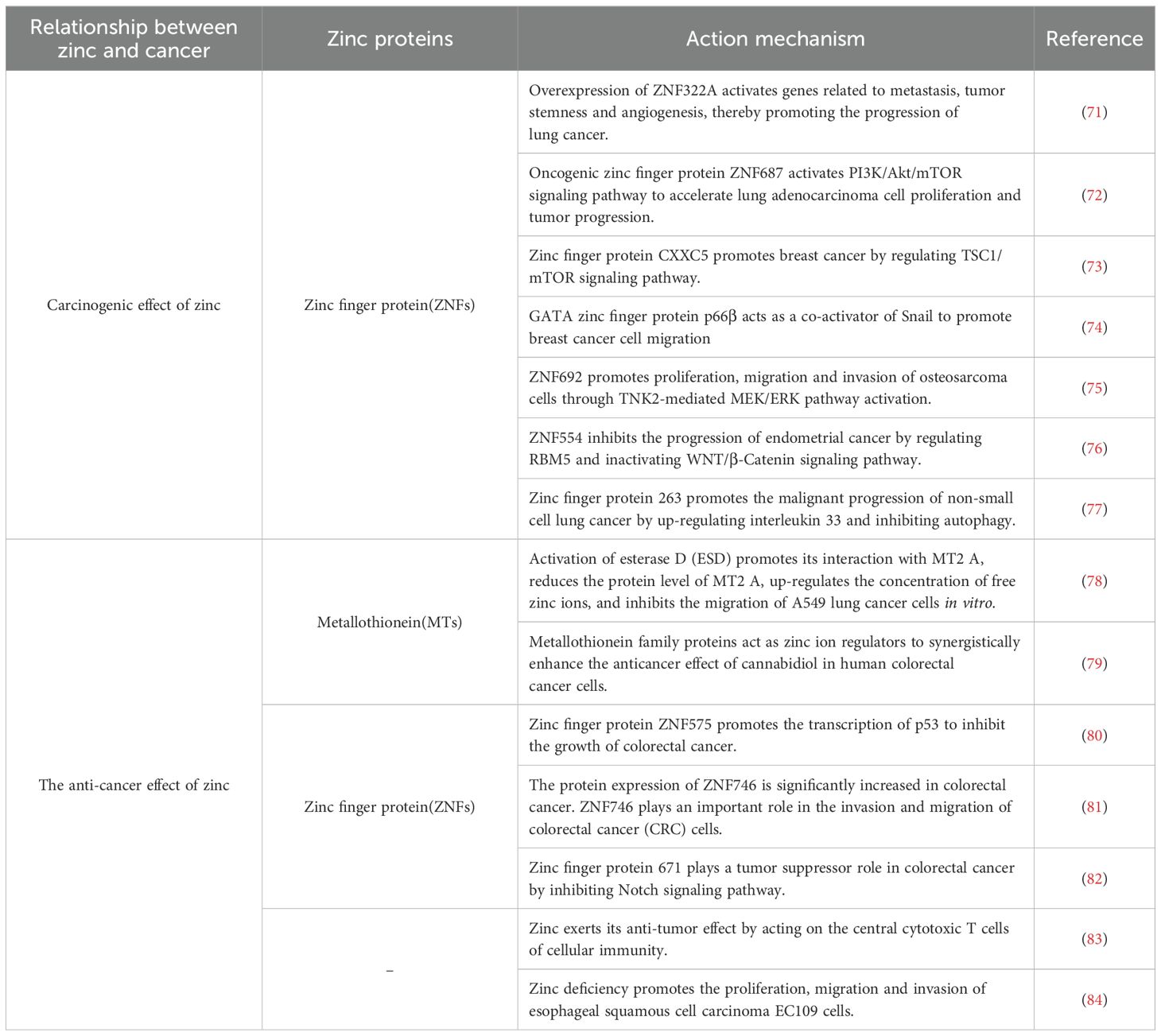
Disruption of zinc homeostasis is associated with tumor development, and the role of zinc varies across different types of cancer (66). Zinc indirectly affects tumor cells by influencing gene expression and cell survival, and directly impacts tumor cells by regulating the activation, function, and/or survival of immune cells (67). Under physiological conditions, Th2 and Th1 cells collaboratively engage in anti-tumor immunity, with cytokines like IL-4, IL-5, and IL-6 promoting B lymphocyte antibody synthesis and contributing to cancer prevention, and zinc is essential for activating this series of responses (67). Zinc deficiency disrupts processes such as oxidative stress, DNA damage, DNA repair, cell cycle, apoptosis, metabolic changes, microRNA expression, and inflammatory factors, thereby promoting cancer development (36). Zinc deficiency reduces the number of T and B cells in the thymus and bone marrow, increasing the body’s susceptibility to infections and weakening its defenses, which results in a higher incidence of tumors. Another potential mechanism by which zinc inhibits tumor growth is closely related to its suppression of the activity of the nuclear transcription factor NF-κB (68, 69). NF-κB in its active form induces the expression of approximately 200 genes, which are related to angiogenesis, metastasis, and cell proliferation. Zinc influences gene expression at the nuclear level by stabilizing structures and regulating various transcription factors, including NF-κB (69). The NF-κB transcription factor can enhance inflammatory responses, particularly by boosting the production of pro-inflammatory cytokines by macrophages. Zinc ions negatively regulate NF-κB activity through proteins like A20 with zinc finger structures and by reversibly inhibiting phosphodiesterase (PDE), thus suppressing inflammatory responses and cancer development. The tumor-suppressing effect of zinc is also related to its antioxidant properties. Established cancer cells generate large amounts of reactive oxygen species (ROS), and the clearance of these ROS relies on the activity of antioxidant enzymes. However, zinc can protect healthy cells from the cytotoxic and genotoxic effects of hydrogen peroxide, but in tumor tissues, it can exacerbate the toxicity of H2O2, leading to oxidative stress dysregulation in cancer cells (70). The mechanism of zinc and cancer is shown in Table 1.
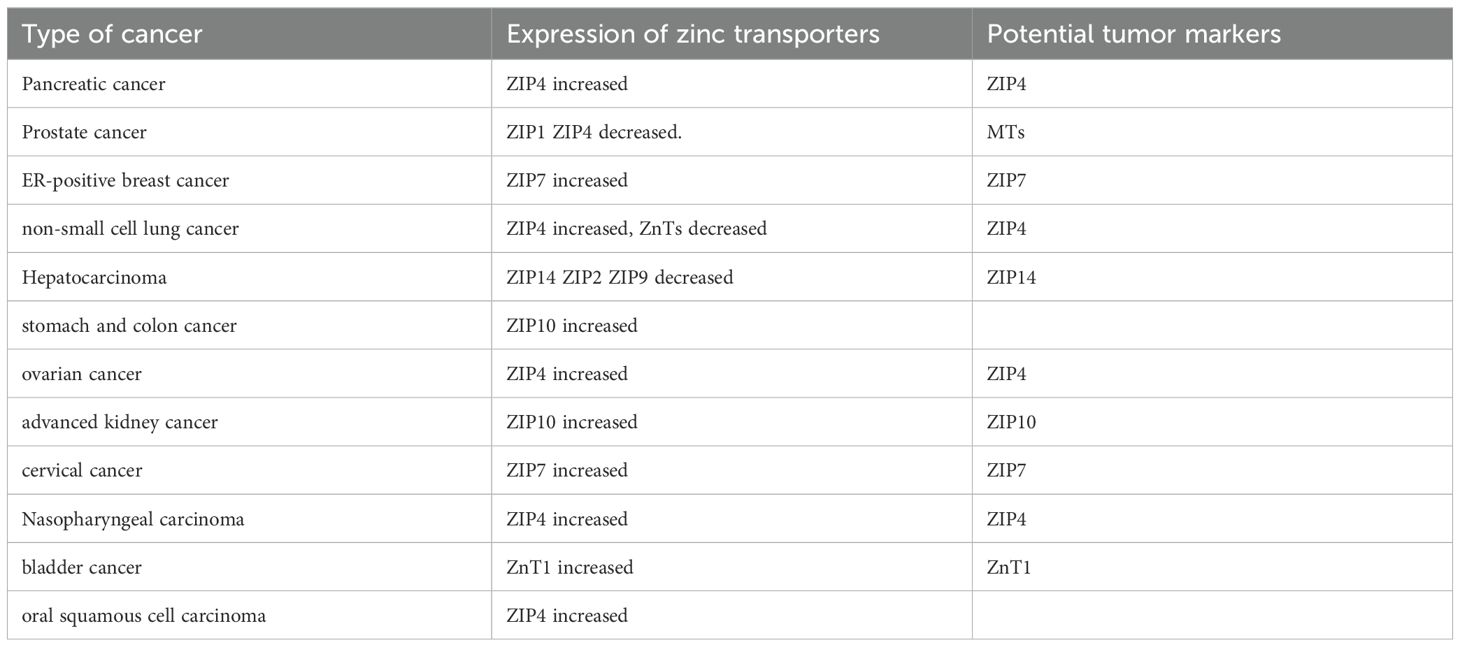
Altered expression levels of zinc transporters are one of the reasons for zinc homeostasis disruption in tumor tissues (85). For example, current studies show that zinc levels are high in prostate tissue cells, and this elevated intracellular zinc level facilitates the production and secretion of citrate in prostatic fluid, as well as aids normal cells in exerting cytotoxic effects to eliminate harmful cells. In contrast, prostate tumor cells exhibit reduced zinc levels, which may be due to low expression of the ZIP1 transporter protein, leading to low intracellular zinc and consequently promoting tumor cell proliferation (86, 87). In ER-positive breast cancer, elevated expression levels of ZIP7 increase zinc levels in ER-positive breast cancer cells. ZIP7 is activated by serine phosphorylation and is involved in the pathways that promote the progression of ER-positive breast cancer (88).
4.2 Zinc and tumor therapeutic targets
The mechanisms by which zinc homeostasis dysregulation leads to tumors have given rise to different anticancer targets for various cancers. Zinc transport proteins may serve as potential targets for cancer therapy, as modulating their function or zinc levels could offer new strategies for cancer treatment (89). Zinc transport proteins serve as targets for cancer therapy. Messenger RNA analysis in pancreatic cancer cells shows overexpression of ZIP4, while other ZIP variants are downregulated. ZIP4 promotes cell proliferation and tumor progression, and interfering with the RNA involved in the generation of ZIP4 can inhibit tumor cell proliferation and invasion; however, more research is needed to further explore the related mechanisms (90). Excess zinc accumulates in breast tumor cells, and this excess zinc has a toxic effect on them. Breast tumor cells increase the expression levels of ZnT2, which transports the excess zinc into vesicles, thereby reducing its toxic effects. Inhibiting the activity of ZnT2 in breast tumor cells can release excess zinc from these vesicles, resulting in cytotoxic effects on malignant breast cancer cells (91). In esophageal squamous cell carcinoma (ESCC), ZIP6 promotes cancer cell proliferation, invasion, and metastasis by increasing intracellular zinc levels, thereby activating the PI3K/AKT and MAPK/Erk pathways. Targeting ZIP6 may represent a potential strategy for treating the aggressiveness of ESCC (92). In various tumor cells, the expression levels of zinc transporters differ (see Table 2), and regulating the expression or activity of these transporters could also serve as a strategy for cancer treatment. The increased expression of MT has been linked to the proliferation rate of tumor cells (93), indicating that MT could be a potential target for future cancer suppression research (93). Zinc finger proteins (ZNFs) regulate the expression of various target genes, influencing tumor occurrence, progression, and patient prognosis. ZNFs are also expected to serve as new biological markers or therapeutic targets for malignant tumors (94).
4.3 The controversy of zinc supplementation in tumor treatment
Zinc supplementation is beneficial for the treatment of many tumors. Studies have found that zinc supplementation can induce cytotoxicity in pancreatic cancer cells and reduce their invasiveness (95). Zinc oxide nanoparticles can also promote apoptosis in liver and ovarian cancer cells by inducing autophagy (96, 97). Metal chelators can form stable complexes with metals, reducing the consumption of metal ions. The antibiotic chloroquine is a metal chelator that can chelate zinc, increasing intracellular zinc levels in tumor cells and enhancing anti-cancer effects (98). Metal chelating compounds (such as disulfiram, chloroquine, and dithiocarbamate derivatives) serve as coordination complexes targeting metals like copper, zinc, and gold in the ubiquitin-proteasome pathway, potentially acting as anti-cancer drugs (99). However, zinc chelation may also produce side effects. Some studies suggest that ferroptosis is a cell death mechanism that can be targeted for cancer treatment, but zinc chelation may inhibit this mechanism (100). This presents a major controversy in the use of metal chelates for cancer treatment, and further research is needed to confirm the impact of these side effects. The use of metal chelators should consider the specific characteristics of the cancer being treated. The benefits and risks of targeted therapies involving zinc in different types of tumors still require further investigation.
The application of zinc supplementation in cancer treatment is becoming increasingly common, but there remains significant controversy regarding its therapeutic use and effects for some tumors. Current studies have many shortcomings, and the benefits and risks of zinc supplementation for cancer treatment require further investigation. A study examining whether a combination of antioxidant vitamins and minerals can reduce the risk of skin cancer (SC) randomly assigned 7,876 French women and 5,141 French men to receive either a daily antioxidant capsule (containing 20 mg of zinc) or a matched placebo. With a median follow-up time of 7.5 years, the results indicated that zinc-containing antioxidant supplements had differential effects on SC incidence, increasing the risk in women but not in men (101). Taking prostate cancer, which has been extensively studied, as an example, there is considerable controversy regarding the therapeutic and preventive roles of zinc in this context. Numerous experimental studies have confirmed that the application of zinc derivatives and supplements can inhibit the proliferation, migration, and invasion of prostate cancer cells. However, some studies suggest that the efficacy of zinc supplementation in any form appears to be limited (87), primarily because malignant tumor cells with ZIP1 deficiencies cannot uptake and accumulate zinc from increased plasma zinc concentrations. Additionally, zinc supplementation formulations, especially those containing cadmium and lead, may have potential contaminants, and the bioavailability of different zinc compounds (such as sulfates, gluconates, and less commonly used citrates) varies (102). Some research has suggested that direct intratumoral injection of zinc can inhibit the growth of prostate cancer cells in xenograft mice (103), but the practicality of this intratumoral administration method in humans remains to be debated. Another controversial aspect of zinc’s use in prostate cancer treatment is that zinc levels in metastatic and late-stage hormone-independent prostate cancer have not been established, and effective treatments for advanced malignant prostate tumors and metastases are lacking. A large prospective cohort study found that low-dose zinc supplementation (1 to 24 mg/d) after diagnosis was associated with a reduced risk of lethal prostate cancer in men with non-metastatic prostate cancer (104). Conversely, another 30-year follow-up study indicated that daily supplementation of more than 75 mg of zinc or supplementation for more than 15 years could significantly increase the risk of lethal and aggressive prostate cancer (102). Therefore, the potential risks and benefits of low-dose zinc supplementation after diagnosis, as well as the duration of supplementation, require further investigation regarding prostate cancer survival.
In summary, the trace element zinc is involved in the formation of intracellular proteins and plays a crucial role in cellular processes such as gene expression, signal transduction, oxidative stress, immune response, and apoptosis. Both excess and deficiency of zinc can lead to metabolic abnormalities in cells, resulting in disease; therefore, it is essential to ensure an appropriate zinc intake to maintain zinc homeostasis and normal cellular metabolism. Current research on the relationship between zinc metabolism and metabolic syndrome is contradictory, and the specific mechanisms supporting their correlation remain unclear, necessitating further studies to explore this issue. Cancer is a major disease impacting human health, and zinc homeostasis is closely related to cancer; zinc deficiency can disrupt cellular immune responses and oxidative stress, thereby promoting cancer development. Zinc supplementation has been shown to be beneficial for various cancers, including pancreatic, colorectal, liver, ovarian, and cervical cancers. The role of zinc in cancer varies by cancer type, allowing for the selection of different therapeutic targets based on specific mechanisms of action. The expression levels of zinc transport proteins vary across different cancer cells; thus, regulating the expression or activity of these transport proteins is also a therapeutic approach for cancer treatment. Many studies currently focus on zinc transport proteins as targets for cancer therapy, but further exploration is needed. Other proteins involved in zinc metabolism, such as metallothioneins and zinc finger structures, may also serve as potential research directions for future tumor suppressive targets, providing new approaches for clinical cancer treatment.
Author contributions
GY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. ZW: Data curation, Formal analysis, Writing – original draft. RX: Conceptualization, Investigation, Methodology, Writing – original draft. CZ: Writing – original draft, Writing – review & editing. BY: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by: Scientific Research Foundation of Education Department of Yunnan Province (No. 2020J0228, 2023J0295), Kunming Medical University Joint Project of Department of Science and Technology of Yunnan Province (No. 202301AY070001-108); Kunming City Health Science and Technology Talent “1000” training Project (No. 2020-SW (Reserve)-112), Kunming Health Personnel Training Project Technology Center Construction Project (No. 2020-SW (Tech) -15) and Yunnan Province Clinical Research Center for Children’s Health and Disease. The funding bodies played no role in the study’s design and collection, analysis and interpretation of data, and writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maret W. Zinc and the zinc proteome. Met Ions Life Sci. (2013) 12:479–501. doi: 10.1007/978-94-007-5561-1_14
2. Sugimoto R, Lee L, Tanaka Y, Morita Y, Hijioka M, Hisano T, et al. Zinc deficiency as a general feature of cancer: a review of the literature. Biol Trace Elem Res. (2024) 202:1937–47. doi: 10.1007/s12011-023-03818-6
3. Stiles LI, Ferrao K, Mehta KJ. Role of zinc in health and disease. Clin Exp Med. (2024) 24:38. doi: 10.1007/s10238-024-01302-6
4. Liu H, Li L, Lu R. ZIP transporters-regulated Zn2+ homeostasis: A novel determinant of human diseases. J Cell Physiol. (2024) 239:e31223. doi: 10.1002/jcp.v239.5
5. Wang X, Zhang M, Ma J, Tie Y, Wang S. Biochemical markers of zinc nutrition. Biol Trace Elem Res. (2024) 202(12):5328–38. doi: 10.1007/s12011-024-04091-x
6. Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. (2006) 281:24085–9. doi: 10.1074/jbc.R600011200
7. Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical,and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. (2015) 95:749–84. doi: 10.1152/physrev.00035.2014
8. Solomons NW. Update on zinc biology. Ann Nutr Metab. (2013) 62 Suppl 1:8–17. doi: 10.1159/000348547
10. Levaot N, Hershfinkel M. How cellular Zn2+ signaling drives physiological functions. Cell Calcium. (2018) 75:53–63. doi: 10.1016/j.ceca.2018.08.004
11. Costello LC, Fenselau CC, Franklin RB. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J Inorg Biochem. (2011) 105:589–99. doi: 10.1016/j.jinorgbio.2011.02.002
12. Vallee BL, Galdes A. The metallobiochemistry of zinc enzymes. Adv Enzymol Relat Areas Mol Biol. (1984) 56:283–430. doi: 10.1002/9780470123027.ch5
13. Maret W. Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals. (2011) 24:411–8. doi: 10.1007/s10534-010-9406-1
14. Krishna SS, Majumdar I, Grishin NV. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. (2003) 31:532–50. doi: 10.1093/nar/gkg161
15. Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. (2002) 33:337–49. doi: 10.1016/S0891-5849(02)00905-X
16. Rink L, Kirchner H. Zinc-altered immune function and cytokine production. J Nutr. (2000) 130:1407S–11S. doi: 10.1093/jn/130.5.1407S
17. Meurs KM, Breitschwerdt EB. CVT update: zinc toxicity. In: Bonagura JD, editor. Kirk’s Current Veterinary Therapy XII Small Animal Practice. W.B. Saunders, Philadelphia, PA (1995). p. 238239.
18. Walsh CT, Sandstead HH, Prasad AS, Newberne PM, Fraker PJ. Zinc: health effects and research priorities for the 1990s. Environ Health Perspect. (1994) 102 Suppl 2:5–46. doi: 10.1289/ehp.941025
19. Cummings JE, Kovacic JP. The ubiquitous role of zinc in health and disease. J Vet Emerg Crit Care (San Antonio). (2009) 19:215–40. doi: 10.1111/j.1476-4431.2009.00418.x
20. Khan SA. Zinc toxicosis. In: Cote E, editor. Clinical Veterinary Advisor Dogs and Cats. Mosby, St Louis, MO (2007). p. 1171–3.
21. Ames BN. Supplements and tuning up metabolism. J Nutr. (2004) 134:3164S–8S. doi: 10.1093/jn/134.11.3164S
22. Sun W, Yang J, Wang W, Hou J, Cheng Y, Fu Y, et al. The beneficial effects of Zn on Akt-mediated insulin and cell survival signaling pathways in diabetes. J Trace Elem Med Biol. (2018) 46:117–27. doi: 10.1016/j.jtemb.2017.12.005
23. Barman S, Srinivasan K. Diabetes and zinc dyshomeostasis: Can zinc supplementation mitigate diabetic complications? Crit Rev Food Sci Nutr. (2022) 62:1046–61. doi: 10.1080/10408398.2020.1833178
24. Barman S, Srinivasan K. Zinc supplementation alleviates hyperglycemia and associated metabolic abnormalities in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. (2016) 94:1356–65. doi: 10.1139/cjpp-2016-0084
25. Himoto T, Yoneyama H, Kurokochi K, Inukai M, Masugata H, Goda F, et al. Contribution of zinc deficiency to insulin resistance in patients with primary biliary cirrhosis. Biol Trace Elem Res. (2011) 144:133–42. doi: 10.1007/s12011-011-9049-2
26. Himoto T, Masaki T. Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease. Nutrients. (2018) 10:88. doi: 10.3390/nu10010088
27. Ullah MI, Alameen AAM, Al-Oanzi ZH, Eltayeb LB, Atif M, Munir MU, et al. Biological role of zinc in liver cirrhosis: an updated review. Biomedicines. (2023) 11:1094. doi: 10.3390/biomedicines11041094
28. Barman S, Srinivasan K. Attenuation of oxidative stress and cardioprotective effects of zinc supplementation in experimental diabetic rats. Br J Nutr. (2017) 117:335–50. doi: 10.1017/S0007114517000174
29. Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. (1996) 271:1081–5. doi: 10.1126/science.271.5252.1081
30. O’Dell BL. Role of zinc in plasma membrane function. J Nutr. (2000) 130:1432S–6S. doi: 10.1093/jn/130.5.1432S
31. Hershfinkel M, Silverman WF, Sekler I. The zinc sensing receptor, a link between zinc and cell signaling. Mol Med. (2007) 13:331–6. doi: 10.2119/2006-00038.Hershfinkel
32. Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. (2000) 130:1471S–83S. doi: 10.1093/jn/130.5.1471S
33. Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. (1998) 68:447S–63S. doi: 10.1093/ajcn/68.2.447S
34. Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. (1990) 8:281–91. doi: 10.1016/0891-5849(90)90076-U
35. Zarezadeh M, Faghfouri AH, Aghapour B, Rostamkhani H, Malekahmadi M, Naemi Kermanshahi M, et al. Investigation of the clinical efficacy of Zn supplementation in improvement of oxidative stress parameters: A systematic review and dose-response meta-analysis of controlled clinical trials. Int J Clin Pract. (2021) 75:e14777. doi: 10.1111/ijcp.v75.12
36. Zhang Y, Tian Y, Zhang H, Xu B, Chen H. Potential pathways of zinc deficiency-promoted tumorigenesis. BioMed Pharmacother. (2021) 133:110983. doi: 10.1016/j.biopha.2020.110983
37. Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. (1998) 78:547–81. doi: 10.1152/physrev.1998.78.2.547
38. Frassinetti S, Bronzetti G, Caltavuturo L, Cini M, Croce CD. The role of zinc in life: a review. J Environ Pathol Toxicol Oncol. (2006) 25:597–610. doi: 10.1615/JEnvironPatholToxicolOncol.v25.i3.40
39. Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. (1996) 271:1081–5. doi: 10.1126/science.271.5252.1081
40. Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. (2001) 14:315–30. doi: 10.1023/A:1012993017026
41. Lemire J, Mailloux R, Appanna VD. Zinc toxicity alters mitochondrial metabolism and leads to decreased ATP production in hepatocytes. J Appl Toxicol. (2008) 28:175–82. doi: 10.1002/jat.v28:2
42. Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. (2002) 59:627–47. doi: 10.1007/s00018-002-8454-2
43. Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. (2017) 960:1–17. doi: 10.1007/978-3-319-48382-5
44. Bashandy SAE, El-Seidy AMA, Ibrahim FAA, Abdelrahman SS, Abdelmottaleb Moussa SA, ElBaset MA. Zinc nanoparticles ameliorated obesity-induced cardiovascular disease: role of metabolic syndrome and iron overload. Sci Rep. (2023) 13:16010. doi: 10.1038/s41598-023-42550-y
45. Althanoon Z, Merkhan M. Effects of zinc supplementation on metabolic status in patients with metabolic syndrome. Acta Poloniae Pharm - Drug Res. (2021) 78:521–26. doi: 10.32383/appdr/141348
46. Ding J, Liu Q, Liu Z, Guo H, Liang J, Zhang Y. Association between dietary zinc intake and metabolic syndrome. A Meta-Analysis Observational Stud Front Nutr. (2022) 9:825913. doi: 10.3389/fnut.2022.825913
47. Qu R, Jia Y, Liu J, Jin S, Han T, Na L. Dietary flavonoids, copper intake, and risk of metabolic syndrome in Chinese adults. Nutrients. (2018) 10:991. doi: 10.3390/nu10080991
48. Lu CW, Lee YC, Kuo CS, Chiang CH, Chang HH, Huang KC. Association of serum levels of zinc, copper, and iron with risk of metabolic syndrome. Nutrients. (2021) 13:548. doi: 10.3390/nu13020548
49. Yang S, Chen Q, Wang L. Association of zinc intake, tobacco smoke exposure, with metabolic syndrome: evidence from NHANES 2007-2018. Biol Trace Elem Res. (2024) 202(12):5429–37. doi: 10.1007/s12011-024-04120-9
50. Zhang H, Man Q, Song P, Li S, Liu X, Wang L, et al. Association of whole blood copper, magnesium and zinc levels with metabolic syndrome components in 6-12-year-old rural Chinese children: 2010-2012 China National Nutrition and Health Survey. Nutr Metab (Lond). (2021) 18:67. doi: 10.1186/s12986-021-00593-w
51. Wu Y, Xu G, Bai R, Yu P, He Z, Chen M, et al. Association between circulating zinc levels and risk factors of metabolic syndrome: insights from a bi-directional Mendelian randomization analysis and cross-sectional study. Biol Trace Elem Res. (2023) 202(7):3051–61. doi: 10.1007/s12011-023-03918-3
52. Aydogdu A, Unal Ö, Baltaci SB, Menevse E, Mogulkoc R, Erdem SS, et al. Plasma leptin, nesfatin 1, NPY, and zinc levels in obese and metabolic syndrome children. Eur J Ther. (2023) 29:856–65. doi: 10.58600/eurjther1760
53. Qorbani M, Movasaghi N, Mohammadian Khonsari N, Daneshzad E, Shafiee G, Ashraf H, et al. Association of zinc serum level with metabolic syndrome in Iranian children and adolescents: The CASPIAN-V study. Front Nutr. (2022) 9:932746. doi: 10.3389/fnut.2022.932746
54. Ennes Dourado Ferro F, de Sousa Lima VB, Mello Soares NR, Franciscato Cozzolino SM, do Nascimento Marreiro D. Biomarkers of metabolic syndrome and its relationship with the zinc nutritional status in obese women. Nutr Hosp. (2011) 26:650–4. doi: 10.1590/S0212-16112011000300032
55. Skrajnowska D, Bobrowska-Korczak B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients. (2019) 11:2273. doi: 10.3390/nu11102273
56. Samavarchi Tehrani S, Mahmoodzadeh Hosseini H, Yousefi T, Abolghasemi M, Qujeq D, Maniati M, et al. The crosstalk between trace elements with DNA damage response, repair, and oxidative stress in cancer. J Cell Biochem. (2019) 120:1080–105. doi: 10.1002/jcb.v120.2
57. Figiel M, Górka AK, Górecki A. Zinc ions modulate YY1 activity: relevance in carcinogenesis. Cancers (Basel). (2023) 15:4338. doi: 10.3390/cancers15174338
58. Jouybari L, Kiani F, Akbari A, Sanagoo A, Sayehmiri F, Aaseth J, et al. A meta-analysis of zinc levels in breast cancer. J Trace Elem Med Biol. (2019) 56:90–9. doi: 10.1016/j.jtemb.2019.06.017
59. Tu K, Liu K, Wang Y, Jiang Y, Zhang C. Association of dietary intake of zinc and selenium with breast cancer risk: A case-control study in Chinese women. Nutrients. (2023) 15:3253. doi: 10.3390/nu15143253
60. Laya A. Trace elements homeostasis in biological samples as new candidate biomarkers for early diagnosis and prognosis of female breast cancer and therapeutic response: systematic review. Arch Breast Cancer. (2023) 10:26–37. doi: 10.32768/abc.202310126-37
61. Feng Y, Zeng JW, Ma Q, Zhang S, Tang J, Feng JF. Serum copper and zinc levels in breast cancer: A meta-analysis. J Trace Elem Med Biol. (2020) 62:126629. doi: 10.1016/j.jtemb.2020.126629
62. Kim S, Freeland-Graves JH, Babaei M, Sachdev PK, Beretvas SN. Quantifying the association between acute leukemia and serum zinc, copper, and selenium: a meta-analysis. Leuk Lymphoma. (2019) 60:1548–56. doi: 10.1080/10428194.2018.1540043
63. Mao S, Huang S. Zinc and copper levels in bladder cancer: a systematic review and meta-analysis. Biol Trace Elem Res. (2013) 153:5–10. doi: 10.1007/s12011-013-9682-z
64. Drake EN 2nd, Sky-Peck HH. Discriminant analysis of trace element distribution in normal and Malignant human tissues. Cancer Res. (1989) 49:4210–5.
65. Gutiérrez-González E, Pastor-Barriuso R, Castelló A, Castaño-Vinyals G, Fernández de Larrea-Baz N, Dierssen-Sotos T, et al. Toenail zinc and risk of prostate cancer in the MCC-Spain case-control study. Environ Res. (2024) 245:118065. doi: 10.1016/j.envres.2023.118065
66. Bendellaa M, Lelièvre P, Coll JL, Sancey L, Deniaud A, Busser B. Roles of zinc in cancers: From altered metabolism to therapeutic applications. Int J Cancer. (2024) 154:7–20. doi: 10.1002/ijc.v154.1
67. John E, Laskow TC, Buchser WJ, Pitt BR, Basse PH, Butterfield LH, et al. Zinc in innate and adaptive tumor immunity. J Transl Med. (2010) 8:118. doi: 10.1186/1479-5876-8-118
68. Rink L, Kirchner H. Zinc-altered immune function and cytokine production. J Nutr. (2000) 130:1407S–11S. doi: 10.1093/jn/130.5.1407S
69. Skrajnowska D, Bobrowska-Korczak B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients. (2019) 11:2273. doi: 10.3390/nu11102273
70. Sliwinski T, Czechowska A, Kolodziejczak M, Jajte J, Wisniewska-Jarosinska M, Blasiak J. Zinc salts differentially modulate DNA damage in normal and cancer cells. Cell Biol Int. (2009) 33:542–7. doi: 10.1016/j.cellbi.2009.02.004
71. Huang SH, Hsieh HC, Shieh JM, Su WC, Wang YC. Downregulation of microRNA-326 enhances ZNF322A expression, transcriptional activity and tumorigenic effects in lung cancer. Biofactors. (2024) 50:214–27. doi: 10.1002/biof.v50.1
72. Li M, Liu Z, Hou Z, Wang X, Shi H, Li Y, et al. Oncogenic zinc finger protein ZNF687 accelerates lung adenocarcinoma cell proliferation and tumor progression by activating the PI3K/AKT signaling pathway. Thorac Cancer. (2023) 14:1223–38. doi: 10.1111/1759-7714.14856
73. Wang W, Zhang Z, Zhao M, Wang Y, Ge Y, Shan L. Zinc-finger protein CXXC5 promotes breast carcinogenesis by regulating the TSC1/mTOR signaling pathway. J Biol Chem. (2023) 299:102812. doi: 10.1016/j.jbc.2022.102812
74. Zou X, Ma L, Zhang Y, Zhang Q, Xu C, Zhang D, et al. GATA zinc finger protein p66β promotes breast cancer cell migration by acting as a co-activator of Snail. Cell Death Dis. (2023) 14:382. doi: 10.1038/s41419-023-05887-w
75. Zheng D, Wei Z, Zhang C, Liu W, Gong C, Wu F, et al. ZNF692 promotes osteosarcoma cell proliferation, migration, and invasion through TNK2-mediated activation of the MEK/ERK pathway. Biol Direct. (2024) 19:28. doi: 10.1186/s13062-024-00472-3
76. Zhu CC, Sun HL, Long TF, Lyu YY, Liu JL, Ni GT. ZNF554 inhibits endometrial cancer progression via regulating RBM5 and inactivating WNT/β-catenin signaling pathway. Curr Med Sci. (2024) 44:406–18. doi: 10.1007/s11596-024-2845-7
77. Xu J, Zhou Y, Wang Q, Liu Y, Tang J. Zinc finger protein 263 upregulates interleukin 33 and suppresses autophagy to accelerate the Malignant progression of non-small cell lung cancer. Clin Transl Oncol. (2024) 26:924–35. doi: 10.1007/s12094-023-03325-z
78. Yao W, Chen X, Cui X, Zhou B, Zhao B, Lin Z, et al. Esterase D interacts with metallothionein 2A and inhibits the migration of A549 lung cancer cells in vitro. J Cell Biochem. (2023) 124:373–81. doi: 10.1002/jcb.v124.3
79. Kwon IS, Hwang YN, Park JH, Na HH, Kwon TH, Park JS, et al. Metallothionein family proteins as regulators of zinc ions synergistically enhance the anticancer effect of cannabidiol in human colorectal cancer cells. Int J Mol Sci. (2023) 24:16621. doi: 10.3390/ijms242316621
80. An N, Peng H, Hou M, Su D, Wang L, Shen X, et al. The zinc figure protein ZNF575 impairs colorectal cancer growth via promoting p53 transcription. Oncol Res. (2023) 31:307–16. doi: 10.32604/or.2023.028564
81. Huang CR, Chu YT, Chang CL, Yip HK, Chen HH. ZNF746 plays cardinal roles on colorectal cancer (CRC) cell invasion and migration and regulates mitochondrial dynamics and morphological changes of CRC cells-Role of combined melatonin and 5-FU regimen. J Cell Biochem. (2024) 125:e30507. doi: 10.1002/jcb.v125.2
82. Wang Y, Chen FR, Wei CC, Sun LL, Liu CY, Yang LB, et al. Zinc finger protein 671 has a cancer-inhibiting function in colorectal carcinoma via the deactivation of Notch signaling. Toxicol Appl Pharmacol. (2023) 458:116326. doi: 10.1016/j.taap.2022.116326
83. Nishida K, Nakagawa N. Zinc suppresses colorectal cancer development through cell-mediated immunity. Yakugaku Zasshi. (2024) 144:475–81. doi: 10.1248/yakushi.23-00154-1
84. Yang P, Li H, Sun M, Guo X, Liao Y, Hu M, et al. Zinc deficiency drives ferroptosis resistance by lactate production in esophageal squamous cell carcinoma. Free Radic Biol Med. (2024) 213:512–22. doi: 10.1016/j.freeradbiomed.2024.01.041
85. Pan Z, Choi S, Ouadid-Ahidouch H, Yang JM, Beattie JH, Korichneva I. Zinc transporters and dysregulated channels in cancers. Front Biosci (Landmark Ed). (2017) 22:623–43. doi: 10.2741/4507
86. Costello LC, Franklin RB. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys. (2016) 611:100–12. doi: 10.1016/j.abb.2016.04.014
87. To PK, Do MH, Cho JH, Jung C. Growth modulatory role of zinc in prostate cancer and application to cancer therapeutics. Int J Mol Sci. (2020) 21:2991. doi: 10.3390/ijms21082991
88. Taylor KM, Hiscox S, Nicholson RI, Hogstrand C, Kille P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci Signal. (2012) 5:ra11. doi: 10.1126/scisignal.2002585
89. Wang J, Zhao H, Xu Z, Cheng X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol Med. (2020) 17:612–25. doi: 10.20892/j.issn.2095-3941.2020.0106
90. Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. (2009) 458:438–44. doi: 10.1038/nature07960
91. Lopez V, Foolad F, Kelleher SL. ZnT2-overexpression represses the cytotoxic effects of zinc hyper-accumulation in Malignant metallothionein-null T47D breast tumor cells. Cancer Lett. (2011) 304:41–51. doi: 10.1016/j.canlet.2011.01.027
92. Cheng X, Wei L, Huang X, Zheng J, Shao M, Feng T, et al. Solute carrier family 39 member 6 gene promotes aggressiveness of esophageal carcinoma cells by increasing intracellular levels of zinc, activating phosphatidylinositol 3-kinase signaling, and up-regulating genes that regulate metastasis. Gastroenterology. (2017) 152:1985–1997.e12. doi: 10.1053/j.gastro.2017.02.006
94. Zhao J, Wen D, Zhang S, Jiang H, Di X. The role of zinc finger proteins in Malignant tumors. FASEB J. (2023) 37:e23157. doi: 10.1096/fj.202300801R
95. Jayaraman AK, Jayaraman S. Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the ZnT-1 zinc transporter gene in pancreatic cancer cells. J Nutr Biochem. (2011) 22:79–88. doi: 10.1016/j.jnutbio.2009.12.001
96. Padmanabhan A, Kaushik M, Niranjan R, Richards JS, Ebright B, Venkatasubbu GD. Zinc Oxide nanoparticles induce oxidative and proteotoxic stress in ovarian cancer cells and trigger apoptosis Independent of p53-mutation status. Appl Surf Sci. (2019) 487:807–818. doi: 10.1016/j.apsusc.2019.05.099
97. Yang R J, Wu R, Mei J, Hu FR, Lei CJ. Zinc oxide nanoparticles promotes liver cancer cell apoptosis through inducing autophagy and promoting p53. Eur Rev Med Pharmacol Sci. (2021) 25:1557–63.
98. Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE. Anticancer activity of the antibiotic clioquinol. Cancer Res. (2005) 65:3389–95. doi: 10.1158/0008-5472.CAN-04-3577
99. Schmitt SM, Frezza M, Dou QP. New applications of old metal-binding drugs in the treatment of human cancer. Front Biosci (Schol Ed). (2012) 4:375–91. doi: 10.2741/s274
100. Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. (2022) 22:381–96. doi: 10.1038/s41568-022-00459-0
101. Hercberg S, Ezzedine K, Guinot C, Preziosi P, Galan P, Bertrais S, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. (2007) 137:2098–105. doi: 10.1093/jn/137.9.2098
102. Zhang Y, Song M, Mucci LA, Giovannucci EL. Zinc supplement use and risk of aggressive prostate cancer: a 30-year follow-up study. Eur J Epidemiol. (2022) 37:1251–60. doi: 10.1007/s10654-022-00922-0
103. Shah MR, Kriedt CL, Lents NH, Hoyer MK, Jamaluddin N, Klein C, et al. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J Exp Clin Cancer Res. (2009) 28:84. doi: 10.1186/1756-9966-28-84
Keywords: zinc, zinc metabolism, metabolic syndrome, cancer therapy, controversy
Citation: Yao G, Wang Z, Xie R, Zhanghuang C and Yan B (2024) Trace element zinc metabolism and its relation to tumors. Front. Endocrinol. 15:1457943. doi: 10.3389/fendo.2024.1457943
Received: 01 July 2024; Accepted: 19 November 2024;
Published: 05 December 2024.
Edited by:
Parmanand Malvi, University of Alabama at Birmingham, United StatesReviewed by:
Ashish Toshniwal, The University of Utah, United StatesMeghna Saxena, University of Minnesota Medical Center, United States
Copyright © 2024 Yao, Wang, Xie, Zhanghuang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Yan, eWJ3Y3lAMTYzLmNvbQ==; Chenghao Zhanghuang, NzM2NTY0MTQ1QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Guiping Yao1†
Guiping Yao1† Chenghao Zhanghuang
Chenghao Zhanghuang