- 1Department of Endocrinology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Department of Endocrinology, Xiangyang No. 1 People’s Hospital, Hubei University of Medicine, Xiangyang, Hubei, China
- 3Department of Endocrinology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, China
- 4Center for Clinical Evidence-Based and Translational Medicine, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, China
- 5Department of Rheumatology and Immunology, Xiangyang No. 1 People’s Hospital, Hubei University of Medicine, Xiangyang, Hubei, China
Context/Objectives: Hydroxychoroquine has hypoglycemic effects and may reduce the risk of diabetes mellitus (DM). We determined the association between hydroxychoroquine use and the incidence of DM in a population-based cohort of pations with Rheumatic disease
Methods: A prospective cohort study among 502392 Potentially eligible participants in the context of UK Biobank, recruitment to the database began between 2006 and 2010. Patients diagnosed with diabetes and fasting glucose greater than or equal to 7 mmol/L at baseline (n=619) were excluded and patients diagnosed with either RA or SLE at baseline (n=6793) were followed up until 2022. Diagnosis was recorded using the International Classification of Diseases, tenth edition (ICD-10) code. The mean follow-up was 13.78 years and the primary outcome was newly recorded type 2 diabetes mellitus (T2DM), with the time of onset of diabetes as the follow-up endpoint date.
Results: During a median follow-up period of 13.78 (12.93, 14.49) years, diabetes developed in 537 participants, with an incidence of 7.9%. New diabetes cases not taking hydroxychloroquine and taking hydroxychloroquine was 504 (8.03%) and 33 (6.36%), respectively. In univariate models, the hazard ratio for diabetes was 0.89 (95% confidence interval, 0.81-0.98, P=0.014) for hydroxychloroquine users compared with those not taking hydroxychloroquine. After adjusting for age, sex, race, education level, and BMI the hazard ratio for incident diabetes among hydroxychloroquine users was 0.88 (95% confidence interval, 0.80-0.97, P=0.008). In complete multivariate model hazard ratio for hydroxychloroquine was 0.87 (95% confidence interval, 0.79- 0.96, P=0.005).
Conclusion: Hydroxychloroquine was associated with decreased risk of DM among rheumatoid arthritis patients, our data taken together with correlational studies, warrant further investigation of the potential preventive effect of hydroxychloroquine against T2DM.
Introduction
Rheumatic disease or rheumatism is a type of autoimmune inflammation that involves multiple organs and tissues of the entire body (1, 2), and it is a significant cause of disability and decline in the daily quality of life (3–5). The mortality rate for patients with rheumatic disease is 1.5 times higher than that for the general population (6), with the principal representative diseases being systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), at prevalence rates of 0.03% and 0.28% (7), respectively. Epidemiologic studies also describe an increased risk of diabetes in individuals with rheumatism, which may be related to multiple factors such as disease-related inflammation, premature menopause, forced inactivity, and drugs used to treat the disease (such as glucocorticoids and immunosuppressants) (5, 8). Considering that diabetes is also a recognized risk factor for cardiovascular death, reducing the risk of developing diabetes in those with rheumatism is crucial (9, 10).
Hydroxychloroquine (HCQ) is a classic traditional synthetic anti-rheumatic drug (2, 11). HCQ has become a background drug for the treatment of SLE (12, 13). ACR guidelines conditionally recommend the use of HCQ in the initial treatment of patients with mild to moderate RA (14). Cases of reduced blood sugar levels after hydroxychloroquine treatment were first reported in 1984. In addition, studies have found that hydroxychloroquine can reduce cardiovascular risk by controlling blood sugar levels when used to treat rheumatoid arthritis and systemic lupus erythematosus (15). Furthermore, HCQ is a known ion channel inhibitor (16), but this property has not been linked to its effect on blood sugar.
The relationship between hydroxychloroquine and diabetes mellitus (DM) has been a cause of increasing concern (17, 18). Hydroxychloroquine, as inferred from chloroquine studies, improves glucose homeostasis and reduces diabetic incidence (19, 20). Unfortunately, studies that have focused on the efficacy of hydroxychloroquine on blood glucose and diabetes risk in patients with rheumatism have proven inadequate, and there are only a few clinical studies in the extant literature. Therefore, it is particularly important to conduct larger population studies with long follow-up times. Based on these considerations, we analyzed the UK Biobank to explore whether hydroxychloroquine use was associated with a lower risk of diabetes in patients with rheumatism.
Materials and methods
Study design and participants
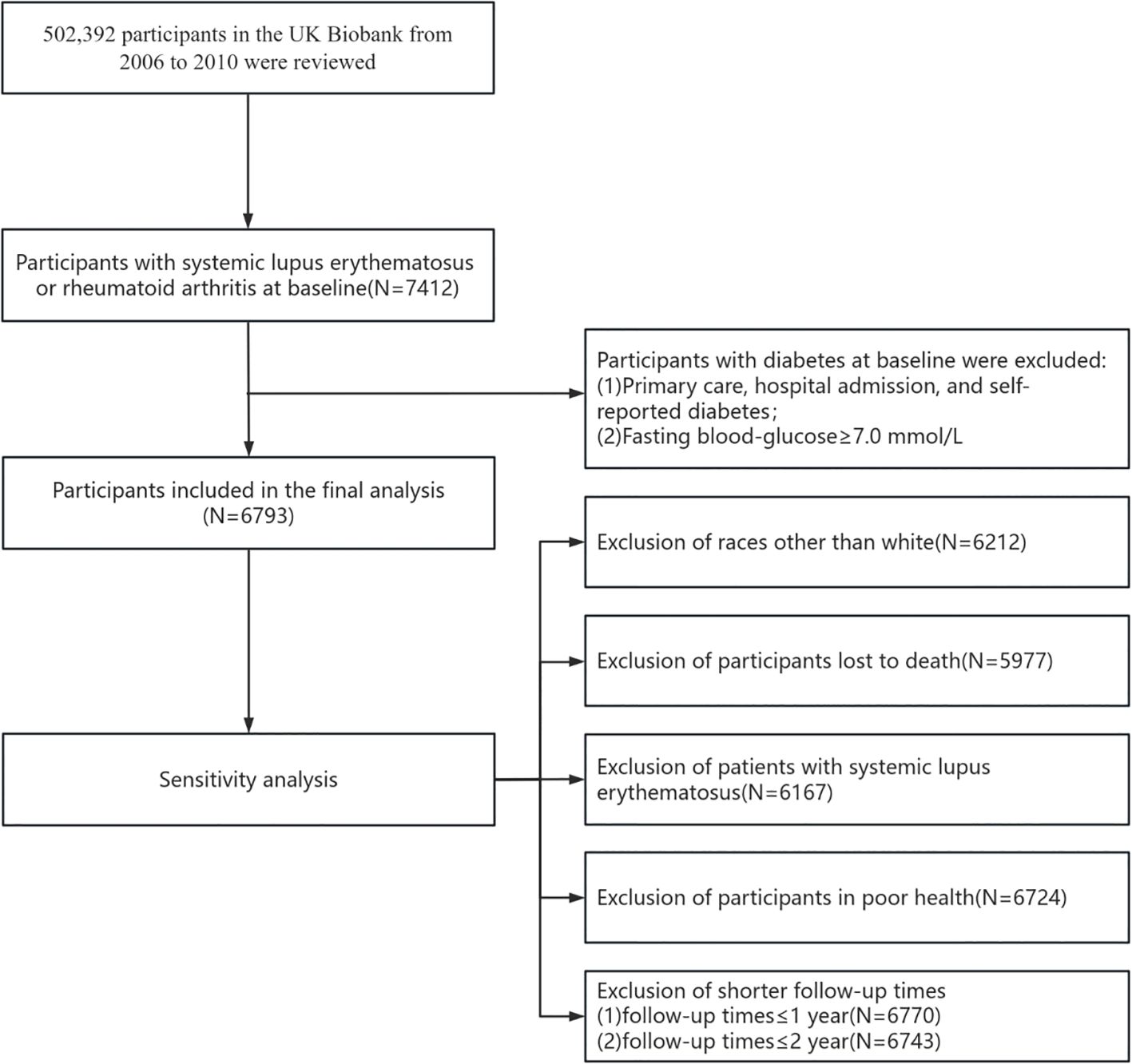
The UK Biobank is a large population-based, prospective cohort study that comprises more than one-half million participants aged 40-69 years recruited in the UK between 2006 and 2010. Each participant underwent a touch-screen questionnaire, oral interviews, and body measurements and provided a biological sample. The UK Biobank has received ethical approval from the North West Multi-centre Research Ethics Committee (MREC), and all participants have provided informed written consent. Participants with RA or SLE prior to the baseline survey were included in our study (N=7412), and participants with a baseline diagnosis of diabetes or a fasting blood glucose (FBG) of ≥7 mmol/L were excluded (N=619), resulting in 6793 participants (Figure 1).
Exposure and outcomes assessment
Hydroxychloroquine was administered orally by trained nurses. This variable included weekly and monthly medication data for routine treatment, excluding short-term medication.
Participant health status was collected using records that contained primary healthcare data, hospital admission data, and self-reported medical status at evaluation centers. Diagnoses were recorded using the International Classification of Diseases, 10th edition (ICD-10), and patients with RA (M05, M06) and SLE (M32) were identified. The primary outcome of our study was the risk of developing T2DM (E11). Participants were accepted for follow-up until diagnosis of T2DM, death, loss to follow-up, or at the date of available data (September 30, 2021), whichever was earlier.
Covariates
Possible confounding factors included sociodemographic levels, lifestyle, a family history of diabetes, and baseline FBG. Information on age, sex, Ethnicity, education level, physical activity, smoking, alcohol consumption, dietary habits, and a family history of diabetes was collected by baseline questionnaires in the UK Biobank. In this study, ethnicity was classified as White, Asian or Asian British, Black or Black British and other ethnic group. Education level was categorized as college or university degree, A/AS level or equivalent, O levels/GCSEs or equivalent and others. Smoking and alcohol consumption were both categorized as never, previous and current. Regarding diet, participants were asked about their intake of sugary beverages, intake of vegetables and fruits, and frequency of their consumption of fish, red meat, and processed meat. In the study, diet frequency was categorized as less than once a week, once per week and more than once per week. Body measurements were performed by trained nurses who applied a uniform method to collect participant height and weight, and body mass index (BMI) was calculated as BMI (kg/m2) = weight (kg)/height (m)2. We measured baseline FBG using hexokinase and a Beckman Coulter AU5800 (Beckman, USA).
Statistical analysis
For descriptive analyses of baseline characteristics, categorical variables are expressed as frequencies (percentages) and continuous variables as means ± standard deviations, or medians (interquartile ranges). Baseline characteristics were compared with or without hydroxychloroquine using Chi-squared tests, one-way analysis of variance (parametric), or the Kruskal-wallis test (nonparametric) followed by Tukey’s test, and P<0.05 indicated a statistical significance.
We exploited Cox proportional hazards models to analyze the association between hydroxychloroquine use and diabetes risk in participants with RA, SLE and Rheumatic Disease (RA or SLE), respectively. Schoenfeld residuals validated the assumption of equal proportional risk (P>0.05). The time variable was follow-up time from baseline (2006-2010) to diabetes onset or follow-up cut-off date (2021). Results are reported as hazard ratio (HR) and 95% confidence interval (CI). In model 2, we further adjusted for smoking, alcohol consumption, physical activity, and diet (including intake of sugar or sugar-sweetened beverages, vegetables, fruits, processed meats, red meat, and oily fish). In model 3, we further adjusted for family history of diabetes and FBG.
We subsequently performed several additional analyses to assess the robustness of the results. We first examined by stratified analysis whether the association between hydroxychloroquine and diabetes risk varied by type of rheumatic immune disease (RA or SLE), age (≤60 years vs. >60 years), BMI, sex, or FBG. The association between hydroxychloroquine and diabetes risk was also investigated by a series of sensitivity analyses. We initially excluded participants with SLE, included only RA patients for analysis, and performed subgroup analyses for RA patients. Second, the association between hydroxychloroquine and diabetes risk was only evaluated in individuals who were white, thus excluding other races. Third, we excluded participants who developed diabetes within 1 or 2 years of follow-up. Fourth, eliminating participants who self-reported poor health reduced the impact of poor health on lifestyle behavior. Fifth, we excluded participants who died before the endpoint event. We performed all statistical analyses using SAS 9.4 software.
Results
Population characteristics
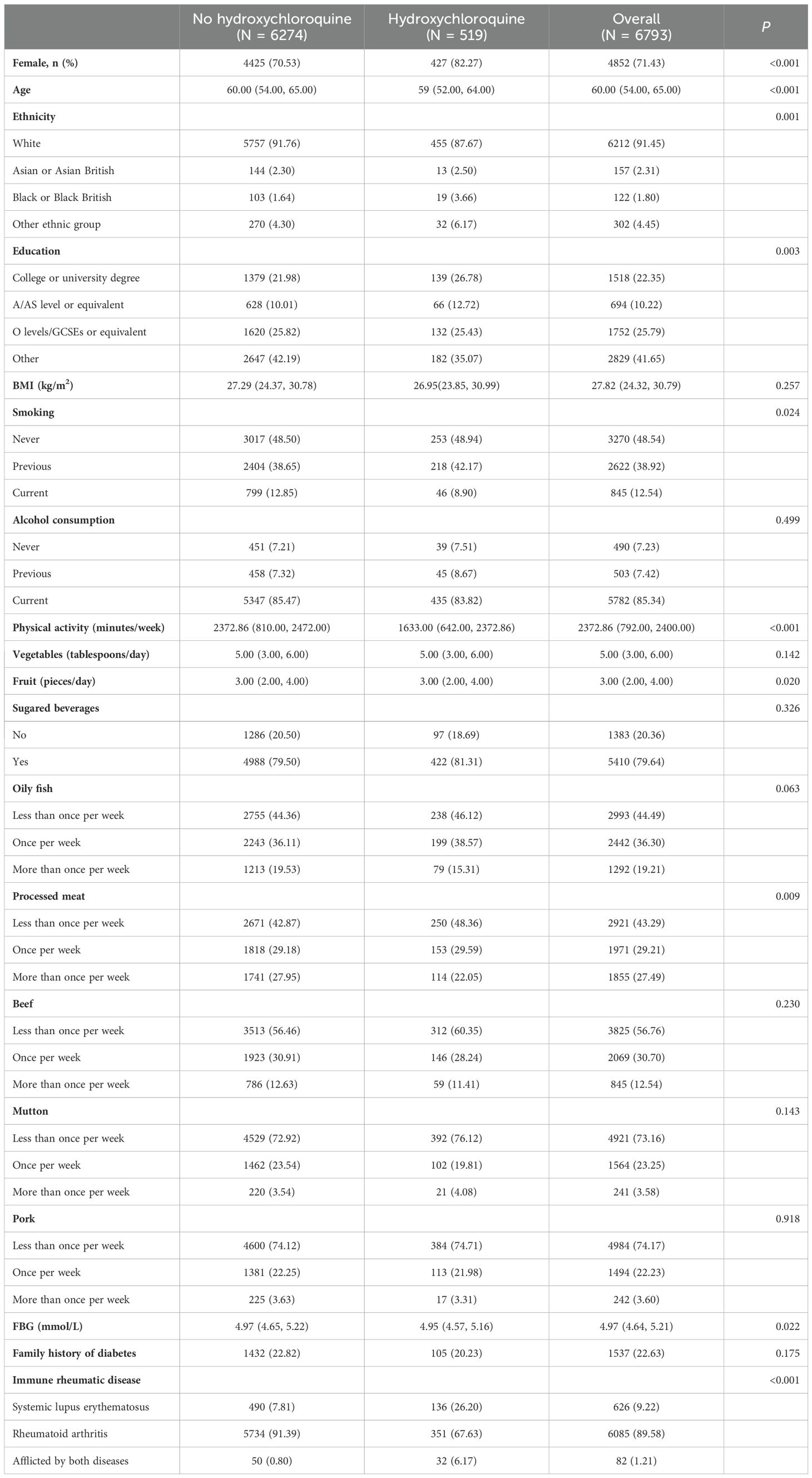
Baseline characteristics of the study population are shown in Table 1: 6793 participants with SLE or RA were included in the study for analysis, with a median age of 60.00 (54.00, 65.00) years, of whom 4852 (71.43%) were female and 6085 (89.58%) had RA. Compared with those who did not take hydroxychloroquine, participants who took hydroxychloroquine were more likely to be female, younger, more educated, nonwhite, former smokers and drinkers; with less physical activity, greater fruit intake, processed meat intake, and lower baseline FBG (P<0.05).
Relationship between Hydroxychloroquine and diabetes mellitus
During a median follow-up period of 13.78 (12.93, 14.49) years, diabetes developed in 537 participants, with an incidence of 7.9%. The numbers of individuals not taking hydroxychloroquine vs. taking hydroxychloroquine were 504 (8.03%) and 33 (6.36%), respectively.
Table 2 shows the association between hydroxychloroquine use and diabetes risk. Participants with RA taking hydroxychloroquine had reduced risk of diabetes compared with those not taking hydroxychloroquine (HR=0.82 [95%CI: 0.73-0.92], P< 0.001). However, in the SLE population, hydroxychloroquine use is not significantly associated with the risk of diabetes mellitus (HR=1.07 [95%CI: 0.86-1.31], P= 0.553). In participants with rheumatic immune, using univariate models, the hazard ratio for diabetes was 0.89 (95% CI: 0.81–0.98) for hydroxychloroquine users when compared with those not taking hydroxychloroquine (P=0.014). The hazard ratio for DM after adjusting for age, sex, race, educational level, and BMI (model 1) was 0.88 (95% CI: 0.80-0.97) for hydroxychloroquine (P=0.008), and the hazard ratio for hydroxychloroquine was 0.87 (95% CI: 0.79–0.96) in model 3 (P=0.005) (see Supplementary Table 1 for details of the variable adjustments).
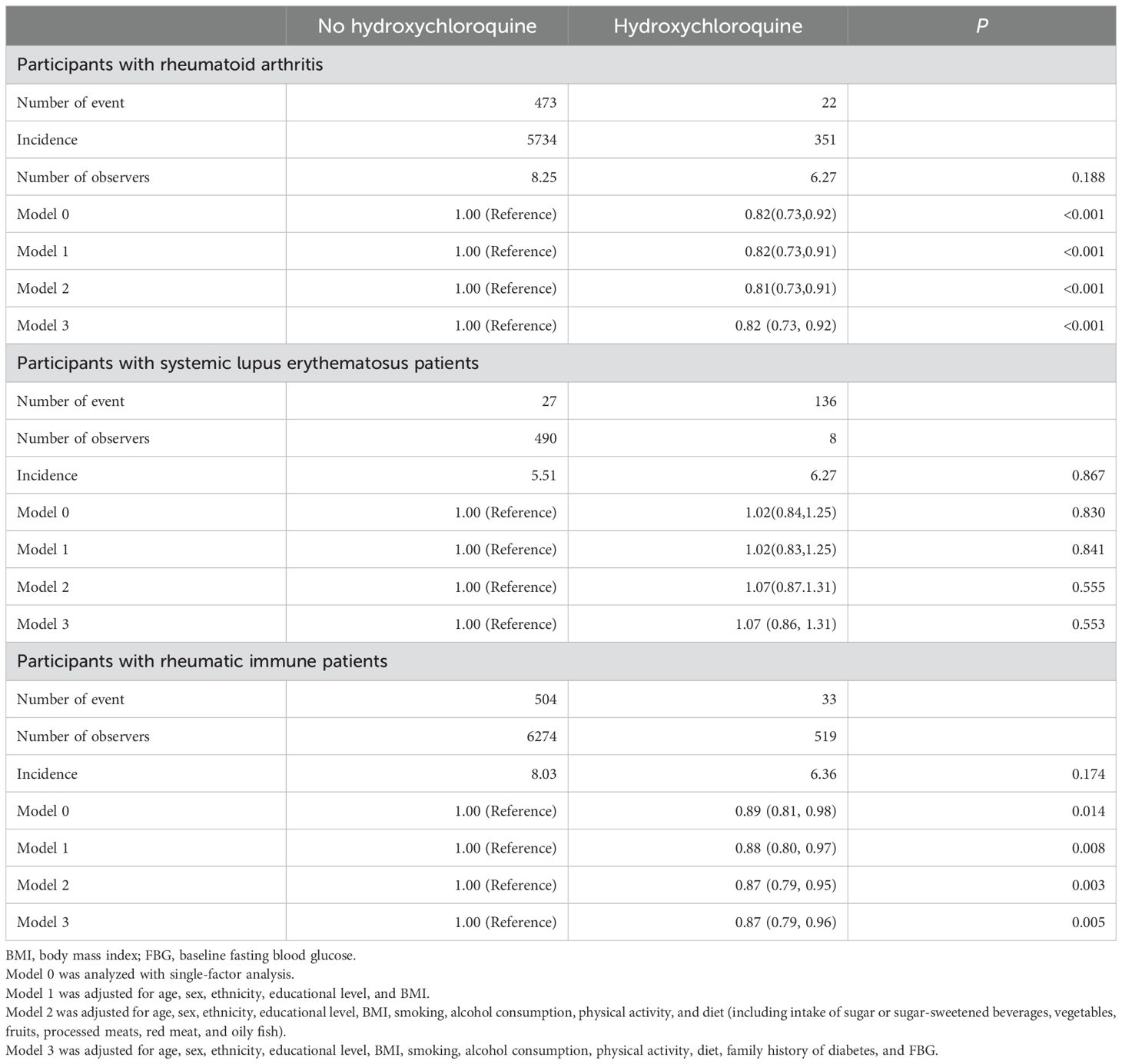
Table 2. Association between hydroxychloroquine use and diabetes incidence in rheumatic immune patients.
Subgroup analyses
Subgroup analyses showed consistent results as stratified by age, with relative risks of diabetes for participants aged 60 years or older/younger of 0.87 (95% CI: 0.77–0.99, P=0.034) and 0.85 (95% CI: 0.74–0.98, P=0.027) for hydroxychloroquine users compared with non-users. Hydroxychloroquine significantly reduced the risk of diabetes among male participants with low baseline FBG (<6.1 mmol/L), non-overweight obesity, longer duration of rheumatic immune disease (≥10 years), and RA (P<0.05) (Figure 2). The association of hydroxychloroquine with diabetes was not significantly different in all subgroups (P for interaction >0.05).
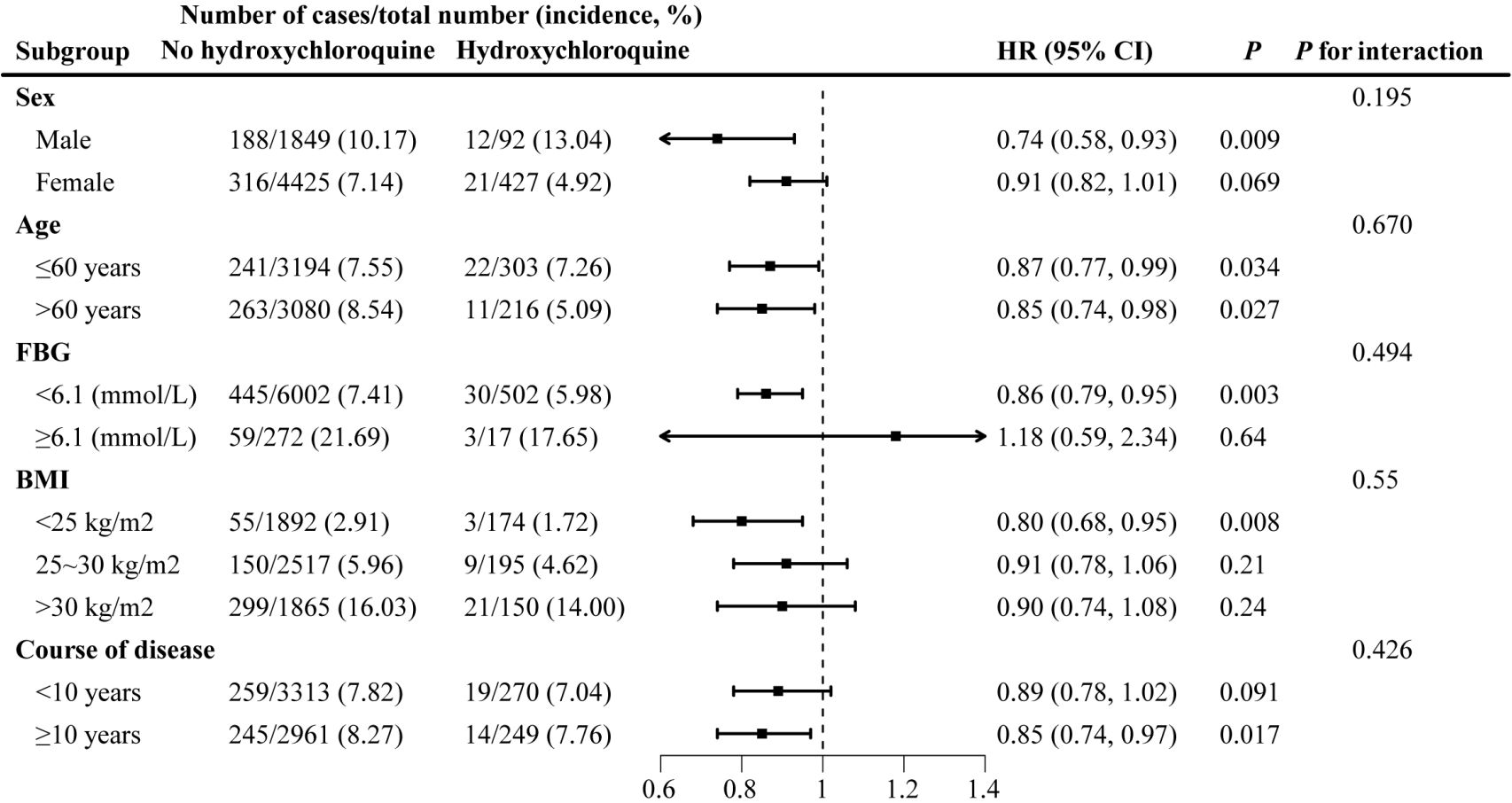
Figure 2. Subgroup analysis: stratified analysis by sex, age, baseline FBG, BMI and course of disease.
Sensitivity analysis
In sensitivity analyses that excluded SLE, race other than white, poor health within one or two years of follow-up, lost to follow-up due to death, and hydroxychloroquine use were associated with a similar reduction in diabetes risk (P=0.002) (Table 3). We observed no association between hydroxychloroquine use and diabetes onset among obese participants with a baseline FBG ≥6.1 mmol/L (P>0.05).
Discussion
We herein analyzed the association between hydroxychloroquine and diabetes in a UK population sample of 500,000 and found that hydroxychloroquine use reduced the risk of diabetes by a 13% (HR=0.87 [95%CI: 0.79-0.96], P=0.005), particularly in RA patients. We also conducted a large number of sensitivity analyses, with consistent results. The advantages of the present study were our large population cohort, adequate subgroup analyses, and sensitivity analyses.
Previous investigators have demonstrated that hydroxychloroquine reduced the risk of diabetes in rheumatic diseases, and this was consistent with our findings. For example, the results of a prospective, multicenter observational study (21) showed that patients with RA who took hydroxychloroquine reflected a hazard ratio of 0.62 (95% CI, 0.42–0.92) with respect to developing diabetes compared with non-users. Ozen and colleagues conducted another retrospective cohort study that encompassed 13,669 patients with RA and depicted an adjusted hazard ratio of 0.67 (95% CI, 0.77–0.80) (22). Patients with RA who received hydroxychloroquine thus generated a significantly reduced risk of diabetes compared with those who did not. Many studies have consistently revealed that hydroxychloroquine use reduced the risk of type 2 diabetes in RA patients (20, 22–26), but there are few extant studies on hydroxychloroquine in SLE patients. Salmasi et al (27) ascertained that the use of antimalarial drugs can effectively reduce the risk of type 2 diabetes in SLE patients by continuously following 1498 SLE patients for 4.62 years. Their findings differed from our study in which we did not uncover an association between hydroxychloroquine and type 2 diabetes risk in SLE patients, but this difference might be explained by the limitations of the present study. First, we had a very small number of SLE subjects who used hydroxychloroquine in the current study, with a correspondingly small number of diabetes endpoint events: only 136 SLE patients used hydroxychloroquine, of whom only eight developed DM, implying that this study may not have reflected enough power for us to discern a modest association between the two conditions.
In addition, our data lacked specific information on patients’ actual use of hydroxychloroquine. Previous studies have shown a dose-dependent protective effect of hydroxychloroquine on diabetes in SLE patients. For example, Chen et al. (7) ascertained that after 10 years of follow-up of newly diagnosed SLE patients, hydroxychloroquine was dose-dependently associated with a reduced risk of diabetes. Patients with cumulative hydroxychloroquine doses of ≥129 g exhibited the lowest hazard ratio for diabetes (HR 0.26 [95% CI: 0.18-0.37], P<0.001); however, patients with cumulative hydroxychloroquine doses <129 g did not avoid diabetes. Unfortunately, the UK Biobank database does not contain data on hydroxychloroquine doses used by patients, and the negative results in this study may have been due to our low drug doses. One cohort study showed that patients who persisted in using hydroxychloroquine were 39% less likely to develop T2DM compared with patients who halted treatment (27). Nam et al. in a case-control study found that the hydroxychloroquine adjusted odds ratio of 0.76 with a cumulative exposure time >270 days/year was associated with a significantly reduced risk of diabetes (25). While the specific pattern of diabetes risk reduction with hydroxychloroquine remains unclear, our study is consistent with many previous studies that depicted hydroxychloroquine as reducing the development of type 2 diabetes in rheumatic patients.
Given the aforementioned limitations, an association between hydroxychloroquine and diabetes in SLE patients cannot be eliminated, although we uncovered no significant association in the present study. Our findings rather highlight the need for further research to determine the intriguing question of whether hydroxychloroquine protects SLE patients from a lower incidence of type 2 diabetes. Randomized controlled trials with larger sample sizes are therefore needed to verify the action of hydroxychloroquine on blood glucose metabolism among patients with rheumatism. The study was also limited by confounding factors that did not account for other drugs or different treatment strategies administered by physicians. A retrospective cohort study of 12880 patients (24) demonstrated that individuals with RA or psoriasis who used hydroxychloroquine had a lower risk of diabetes than those who used other antirheumatic drugs, with a hazard ratio of 0.54 (95% CI, 0.36–0.80). An individual drug analysis of 5530 RA cohort studies revealed that hydroxychloroquine reduced the type 2 diabetes hazard ratio of 0.52 (95% CI, 0.42–0.65) (26). These findings suggested that the protective effect of hydroxychloroquine on diabetes was independent of the effect of disease treatment. The protective effect of hydroxychloroquine on blood glucose metabolism is not only observed in autoimmune diseases but also in individuals without systemic inflammation but taking hydroxychloroquine to improve insulin sensitivity. A growing body of evidence therefore supports a protective association between hydroxychloroquine and type 2 diabetes (10, 28–30), but its functional underlying mechanisms in the prevention of diabetes are not well understood.
Our study did not address the mechanism of action by which hydroxychloroquine affects blood sugar, and this warrants further investigation. The primary defects in type 2 diabetics are the dysfunction of beta-islet cells and impaired insulin secretion in response to glucose stimulation (31), which involve a series of ion-channel activities (32). Given that HCQ treatment reduces insulin requirements (15, 18, 28, 33), we hypothesize that its mechanism of action on glucose metabolism may be related to reduced insulin degradation through changes in lysosomal enzyme activity and endosomal pH (15). Professor Yang’s team (34) reported a “new switch” role of the KCNH6 channel in insulin secretion (35), the team also found that KCNH2 potassium channel enhances endogenous incretin secretion (36), and whether the characterization of hydroxychloroquine ion channel inhibitor (16) plays an important role in regulating blood glucose hormone secretion is worthy of further study. Based on these studies, we hypothesize that hydroxychloroquine regulates blood glucose levels by inhibiting potassium channels to promote insulin and/or incretin secretion.
Collectively, our data support a significant association between hydroxychloroquine treatment and a reduced risk of type 2 diabetes in patients with rheumatism. Studies have shown that hydroxychloroquine is a potentially effective drug for the prevention of type 2 diabetes, at least in patients with RA. In the future, high-quality randomized controlled trials are needed to verify the hypoglycemic effect of hydroxychloroquine, and basic studies are also needed to explore its hypoglycemic mechanism of action.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
C-XL: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. M-LF: Data curation, Formal analysis, Writing – original draft. B-WP: Validation, Visualization, Writing – review & editing. X-JZ: Conceptualization, Visualization, Writing – review & editing. H-ZZ: Validation, Visualization, Writing – review & editing. J-JZ: Data curation, Formal analysis, Writing – review & editing. J-KY: Conceptualization, Project administration, Visualization, Writing – review & editing. S-yX: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Natural Science Foundation of Hubei Provincial Department of Education(B2023104); Innovative Research Program of Xiangyang No.1 People’s Hospital (XYY2023MS06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1381321/full#supplementary-material
References
1. Huang JY, Leong PY, Ker A, Chen HH, Wei JC. The long-term persistence of tumor necrosis factor inhibitors in patients with moderate to severe immune-mediated rheumatic diseases: A nation-wide, population-based real-world study. Int J Rheum Dis. (2022) 25:1295–305. doi: 10.1111/1756-185X.14423
2. Xu WD, Huang Q, Huang AF. Emerging role of ezh2 in rheumatic diseases: A comprehensive review. Int J Rheum Dis. (2022) 25:1230–8. doi: 10.1111/1756-185X.14416
3. Sitia S, Atzeni F, Sarzi-Puttini P, Di Bello V, Tomasoni L, Delfino L, et al. Cardiovascular involvement in systemic autoimmune diseases. Autoimmun Rev. (2009) 8:281–6. doi: 10.1016/j.autrev.2008.08.004
4. Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the uk. Lancet. (2023) 401:1878–90. doi: 10.1016/S0140-6736(23)00457-9
5. Su YJ, Leong PY, Wang YH, Wei JC. Sjogren syndrome is a hidden contributor of macrovascular and microvascular complications in patients with type 2 diabetes. Int J Rheum Dis. (2022) 25:1176–85. doi: 10.1111/1756-185X.14400
6. Sokka BA T, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. (2008) 26:S35–61.
7. Chen YM, Lin CH, Lan TH, Chen HH. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: A population-based cohort study. Rheumatol (Oxford). (2015) 54:1244–9. doi: 10.1093/rheumatology/keu451
8. Li C, Wang XR, Ji HJ, Zhang XY, Li XF. Cardiovascular disease in rheumatoid arthritis: medications and risk factors in China. Clin Rheumatol. (2017) 36:1023–9. doi: 10.1007/s10067-017-3596-7
9. Udupa A, Leverenz D, Balevic SJ, Sadun RE, Tarrant TK, Rogers JL. Hydroxychloroquine and covid-19: A rheumatologist’s take on the lessons learned. Curr Allergy Asthma Rep. (2021) 21:5. doi: 10.1007/s11882-020-00983-9
10. Infante M, Ricordi C, Fabbri A. Antihyperglycemic properties of hydroxychloroquine in patients with diabetes: risks and benefits at the time of covid-19 pandemic. J Diabetes. (2020) 12:659–67. doi: 10.1111/1753-0407.13053
11. Liang S, Wei Z, Fang H, Guan T. Meta-analysis of effectiveness and safety of glucocorticoid combined with hydroxychloroquine in the treatment of systemic lupus erythematosus rash. Int J Rheum Dis. (2023) 26:1686–96. doi: 10.1111/1756-185X.14791
12. Fanouriakis A, Kostopoulou M, Andersen J, Aringer M, Arnaud L, Bae SC, et al. Eular recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. (2024) 83:15–29. doi: 10.1136/ard-2023-224762
13. Mok CC, Hamijoyo L, Kasitanon N, Chen DY, Chen S, Yamaoka K, et al. The asia-pacific league of associations for rheumatology consensus statements on the management of systemic lupus erythematosus. Lancet Rheumatol. (2021) 3:e517–e31. doi: 10.1016/s2665-9913(21)00009-6
14. Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. (2021) 73:1108–23. doi: 10.1002/art.41752
15. Fierro JJ, Velasquez-Berrio M, Ospina A, Henning S, de Leeuw K, Cadavid JA. The effects of hydroxychloroquine and its promising use in refractory obstetric antiphospholipid syndrome. Rheumatol Int. (2024) 44:223–34. doi: 10.1007/s00296-023-05457-5
16. Capel RA, Herring N, Kalla M, Yavari A, Mirams GR, Douglas G, et al. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current if: novel electrophysiological insights and therapeutic potential. Heart Rhythm. (2015) 12:2186–94. doi: 10.1016/j.hrthm.2015.05.027
17. Chakrabarti K, McCune WJ. Advances in the clinical use of hydroxychloroquine levels. Curr Opin Rheumatol. (2022) 34:151–7. doi: 10.1097/BOR.0000000000000872
18. Infante M, Padilla N, Alejandro R, Caprio M, Della-Morte D, Fabbri A, et al. Diabetes-modifying antirheumatic drugs: the roles of dmards as glucose-lowering agents. Medicina (Kaunas). (2022) 58:571–. doi: 10.3390/medicina58050571
19. Pareek A, Chandurkar N, Thomas N, Viswanathan V, Deshpande A, Gupta OP, et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: A double blind, randomized comparison with pioglitazone. Curr Med Res Opin. (2014) 30:1257–66. doi: 10.1185/03007995.2014.909393
20. Li HZ, Xu XH, Lin N, Lu HD. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann Rheum Dis. (2019) 78:e21. doi: 10.1136/annrheumdis-2018-213157
21. Wasko MCM, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. (2007) 298:187–93. doi: 10.1001/jama.298.2.187
22. Ozen G, Pedro S, Holmqvist ME, Avery M, Wolfe F, Michaud K. Risk of diabetes mellitus associated with disease-modifying antirheumatic drugs and statins in rheumatoid arthritis. Ann Rheum Dis. (2016) 76:848–54. doi: 10.1136/annrheumdis-2016-209954
23. Bili A, Sartorius JA, Kirchner HL, Morris SJ, Ledwich LJ, Antohe JL, et al. Hydroxychloroquine use and decreased risk of diabetes in rheumatoid arthritis patients. J Clin Rheumatol. (2011) 17:115–20. doi: 10.1097/RHU.0b013e318214b6b5
24. Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. (2011) 305:2525–31. doi: 10.1001/jama.2011.878
25. Nam SH, Kim M, Kim YJ, Ahn SM, Hong S, Lee CK, et al. Risk of new-onset diabetes mellitus associated with antirheumatic drugs in patients with rheumatoid arthritis: A nationwide population study. J Clin Med. (2022) 11:2109. doi: 10.3390/jcm11082109
26. Su YJ, Chen HM, Chan TM, Cheng TT, Yu SF, Chen JF, et al. Disease-modifying anti-rheumatic drugs associated with different diabetes risks in patients with rheumatoid arthritis. RMD Open. (2023) 9:e003045. doi: 10.1136/rmdopen-2023-003045
27. Salmasi S, Sayre EC, Antonio Avina-Zubieta J, Esdaile JM, De Vera MA. Adherence to antimalarial therapy and risk of type 2 diabetes mellitus among patients with systemic lupus erythematosus: A population-based study. Arthritis Care Res (Hoboken). (2021) 73:702–6. doi: 10.1002/acr.24147
28. Quatraro A, Consoli G, Magno M, Caretta F, Nardozza A, Ceriello A, et al. Hydroxychloroquine in decompensated, treatment-refractory noninsulin-dependent diabetes mellitus. Ann Internal Med. (1990) 112:678–81. doi: 10.7326/0003-4819-112-9-678
29. Emami J, Pasutto FM, Jamali F. Insulin-sparing effect of hydroxychloroquine in diabetic rats is concentration dependen. Can J Physiol Pharmacol. (1999) 77:118–23. doi: 10.1139/y98-146
30. Toledo FGS, Miller RG, Helbling NL, Zhang Y, DeLany JP. The effects of hydroxychloroquine on insulin sensitivity, insulin clearance and inflammation in insulin-resistant adults: A randomized trial. Diabetes Obes Metab. (2021) 23:1252–61. doi: 10.1111/dom.14333
31. White MG, Shaw JA, Taylor R. Type 2 diabetes: the pathologic basis of reversible beta-cell dysfunction. Diabetes Care. (2016) 39:2080–8. doi: 10.2337/dc16-0619
32. Jacobson DA, Shyng SL. Ion channels of the islets in type 2 diabetes. J Mol Biol. (2020) 432:1326–46. doi: 10.1016/j.jmb.2019.08.014
33. El-Solia A, Al-Otaibi K, Ai-Hwiesh AK. Hydroxychloroquine-induced hypoglycaemia in non-diabetic renal patient on peritoneal dialysis. BMJ Case Rep. (2018) 2018:bcr2017223639. doi: 10.1136/bcr-2017-223639
34. Zhao M-M, Lu J, Li S, Wang H, Cao Xi, Li Qi, et al. Berberine is an insulin secretagogue targeting the kcnh6 potassium channel. Nat Commun. (2021) 12:5616 1–13. doi: 10.1038/s41467-021-25952-2
35. Yang JK, Lu J, Yuan SS, Asan, Cao X, Qiu HY, et al. From hyper- to hypoinsulinemia and diabetes: effect of kcnh6 on insulin secretion. Cell Rep. (2018) 25:3800–10 e6. doi: 10.1016/j.celrep.2018.12.005
Keywords: hydroxychloroquine, diabetes mellitus, UK Biobank, risk, prospective cohort study, rheumatic disease
Citation: Li C-X, Fan M-L, Pang B-W, Zhou X-J, Zhang H-Z, Zeng J-J, Yang J-K and Xu S-y (2024) Association between hydroxychloroquine use and risk of diabetes mellitus in systemic lupus erythematosus and rheumatoid arthritis: a UK Biobank-based study. Front. Endocrinol. 15:1381321. doi: 10.3389/fendo.2024.1381321
Received: 20 February 2024; Accepted: 21 October 2024;
Published: 06 November 2024.
Edited by:
James Cheng-Chung Wei, Chung Shan Medical University Hospital, TaiwanReviewed by:
Yingying Mao, Zhejiang Chinese Medical University, ChinaChien-Hsien Lo, Chung Shan Medical University, Taiwan
Copyright © 2024 Li, Fan, Pang, Zhou, Zhang, Zeng, Yang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Kui Yang, amt5YW5nQGNjbXUuZWR1LmNu; Shao-yong Xu, eW9qaV94dUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Chen-Xia Li
Chen-Xia Li Meng-Lin Fan3,4†
Meng-Lin Fan3,4† Jin-Kui Yang
Jin-Kui Yang Shao-yong Xu
Shao-yong Xu