
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 21 May 2024
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1374496
This article is part of the Research TopicContinuous Glucose Monitoring: Beyond Diabetes ManagementView all 10 articles
A correction has been applied to this article in:
Corrigendum: Accuracy of a novel real-time continuous glucose monitoring system: a prospective self-controlled study in thirty hospitalized patients with type 2 diabetes
Aims: The present study aimed to investigate the accuracy of the Glunovo® real-time continuous glucose monitoring system (rtCGMS).
Methods: We conducted a 14-day interstitial glucose level monitoring using Glunovo® rtCGMS on thirty hospitalized patients with type 2 diabetes. The flash glucose monitoring (FGM) was used as a self-control. Consistency tests, error grid analysis, and calculation of the mean absolute relative difference (MARD) were performed using R software to assess the accuracy of Glunovo® rtCGMS.
Results: Glunovo® exhibited an overall MARD value of 8.89% during hospitalization, compared to 10.42% for FGM. The overall percentages of glucose values within ±10%/10, ± 15%/15, ± 20%/20, ± 30%/30, and ±40%/40 of the venous blood glucose reference value were 63.34%, 81.31%, 90.50%, 97.29%, and 99.36% for Glunovo®, respectively, compared with 61.58%, 79.63%, 88.31%, 96.22% and 99.23% for FGM. The Clarke Error Grid Analysis showed that 99.61% of Glunovo® glucose pairs and 100.00% of FGM glucose pairs within zones A and B.
Conclusion: Our study confirms the superior accuracy of Glunovo® in monitoring blood glucose levels among hospitalized patients with type 2 diabetes.
Diabetes and its complications impose a heavy burden on patients. It is estimated that the global diabetes prevalence among individuals aged 20–79 will increase to 12.2% (783.2 million) (1). Effective management of blood glucose levels is paramount for individuals with diabetes, as abnormal levels can cause irreversible damage to the cardiovascular and nervous systems (2, 3). Traditional self-monitoring of blood glucose (SMBG) often poses challenges due to its painful and inconvenient nature, hindering standardized blood glucose management. Moreover, while HbA1c provides an average of long-term blood glucose levels, it fails to capture short-term fluctuations (4). Continuous glucose monitoring systems (CGMS) have emerged as a solution to address these limitations. CGM measures glucose concentration in the interstitial fluid rather than blood, and its values are determined by the rate of glucose diffusion from plasma to interstitial fluid and the rate at which subcutaneous tissue cells take up glucose (5). Currently, two types of CGMS are available: flash glucose monitoring (FGM) or intermittently scanned CGMS (isCGMS), and real-time CGMS (rtCGMS) (6).
Glunovo® is an rtCGMS consisting of a sensor, transmitter, and a mobile application for data analysis. The sensor, designed for subcutaneous installation, has a 14-day lifespan. It generates electrical signals, which are transmitted to the mobile application for display of blood glucose readings. While previous studies have indicated the stability and repeatability of Glunovo®, there remains a lack of head-to-head research to evaluate its accuracy (7). To address this gap, we conducted a head-to-head study to assess the accuracy of Glunovo®.
Patients with type 2 diabetes who underwent standardized treatment at the Nanjing First Hospital from March 2019 to October 2019 were enrolled in this study.
(1) Age: 18–70 years.
(2) Confirmed diagnosis of type 2 diabetes with a duration of at least 3 months.
(3) No participation in other clinical studies in the past 3 months.
(1) Pregnancy or breastfeeding.
(2) History of adhesive tape allergy.
(3) Acute diabetes complications (e.g., diabetic ketoacidosis and hyperglycemic hyperosmolar coma).
(4) Severe immunosuppressive disorders or systemic neurological diseases.
(1) General clinical data, including name, age, gender, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), hemoglobin A1c (HbA1c), triglyceride (TG), creatinine and duration of diabetes.
(2) Blood glucose values recorded from two groups of CGM devices at three stages: initial (1st or 2nd day), intermediate (7 ± 1 days), and final (14th day), along with paired venous blood glucose measurements.
The Glunovo® device featured a 14-day real-time glucose oxidase electrochemical sensor with a flexible sensor probe. Glucose and oxygen from tissue fluid permeate the probe, triggering an electrochemical reaction that generates an electrical signal. This signal, emitted every 3 minutes, was processed by a transmitter (7 mm thick, with a lifespan of 3 years), an applicator for the transmitter applied by a simple click, and software for processing and sharing data. The applicator, designed for ease of use, included a button to position the sensor and retract the insertion needle upon pressing.
The processed signals from the transmitter were converted into blood glucose readings, transmitted via Bluetooth to a mobile application. The application provided real-time display of blood glucose readings, reflected glucose fluctuation trends through trend curves, and enabled exportation of historical data. The analysis software could analyze exported data from the application and conduct statistical analyses for a deeper understanding of the titration of anti-diabetic drugs. All sensors were clinically implanted using an automatic abdominal sensor applicator, with each participant receiving two sensors for improved performance. Paired sensors values were calculated using pairwise average absolute difference and matched to corresponding venous blood glucose levels. In case of sensor failure, the replacement sensor would match the venous blood glucose value.
All participants underwent a 14-day adaptation period using the CGMS. Following the sensor’s recommendations, the device calibrated twice daily using SMBG measurements every 24 hours. After the 14-day adaptation period, paired continuous glucose values and venous blood glucose values were collected for each participant, with a minimum of 24 readings collected within different time periods over 7 hours. The collection of paired continuous glucose and venous blood glucose readings was randomized, assigning each participant a random collection period divided into three stages: initial, middle, and final. FGM was performed as a matched control during this period.
Real-time blood glucose values measured by Glunovo® were compared with venous blood glucose values measured by hospital nurses using the EKF Fast Blood Glucose Analyzer (Biosen-C-Line, EKF Diagnostics, Cardiff, UK). The measurement range of Glunovo® was approximately 2.2–22.2 mmol/L; values outside this range were not included in the analysis. The study was conducted in accordance with the Helsinki Declaration of 1964 and its subsequent amendments and received ethical approval from the Ethics Committee of Nanjing First Hospital (Approval Number: ChiCTR2100045233).
For continuous variables, Shapiro-Wilk test was used to assess normality. Normally distributed data were presented as mean ± SD, and non-normally distributed data as median (interquartile range). Categorical variables were presented as count (percentage). Mean absolute relative difference (MARD) was determined as the average relative difference between the CGMS and venous blood glucose pairs and expressed as a percentage. CGM performance evaluation followed statistical recommendations from Clarke and Kovatchev (8). The numbers of glucose pairs in various risk zones of error grid analyses were determined with the R package “ega,” which is designed for Clarke or Parkes error grid analysis (https://cran.r-project.org/web/packages/ega/ega.pdf). A p-value less than 0.05 was considered statistically significant. All statistical calculations were performed using R software (version 4.3.1).
A total of 31 patients were enrolled, with one participant dropping out midway, resulting in the final collection of data from 30 patients. The patients’ characteristics were presented in Table 1, including 18 females and 12 males, with a median age of 56.00 years and an average BMI of 24.55 kg/m2. The median duration of diabetes was 9.00 years, with average SBP and DBP of 123.60 mmHg and 75.07 mmHg, respectively. Blood indicators, including HbA1c, TG, and creatinine, were 7.81%, 1.41 mmol/L, and 64.31 μmol/L, respectively. A total of 2,327 pairs of matched glucose data were available for evaluation. Venous blood glucose levels were categorized as <3.9 mmol/L (6 pairs), 3.9–10.0 mmol/L (1,422 pairs), and ≥10.0 mmol/L (899 pairs).
MARD values were shown in Table 2. Overall, the MARD for Glunovo® was 8.89%, and for FGM, it was 10.42%. The data were further categorized into rate of change in venous blood glucose groups defined by intervals: <-0.11, (-0.11, -0.06], (-0.06, 0], (0, 0.06], (0.06, 0.11], >0.11 mmol/L/min. The Glunovo® exhibited MARD values of 10.09%, 7.44%, 7.93%, 9.41%, 12.70%, and 17.11% for these respective intervals, whereas the FGM demonstrated MARD values of 10.73%, 9.81%, 10.12%, 10.19%, 11.25%, and 21.30%. For venous blood glucose categorizations: < 3.90, [3.90, 10.00), ≥ 10.00 mmol/L, Glunovo® exhibited MARD values of 8.65%, 8.09%, and 10.58%, respectively, while FGM demonstrates MARD values of 15.21%, 9.60%, and 8.57%. In the initial, middle, and final stages of data collection, MARD values were 8.65%, 8.09%, and 10.58% for Glunovo®, while 15.21%, 9.60%, and 8.57% for FGM.
Agreement analyses were presented in Table 3. The overall percentages of glucose values within ±10%/10 mmol/L, ± 15%/15 mmol/L, ± 20%/20 mmol/L, ± 30%/30 mmol/L, and ±40%/40 mmol/L of the venous blood glucose reference value were 63.34%, 81.31%, 90.50%, 97.29%, and 99.36% for Glunovo®, respectively, compared with 61.58%, 79.63%, 88.31%, 96.22% and 99.23% for FGM.
As shown in Figure 1, Clarke Error Grid Analysis demonstrated acceptable clinical accuracy. For Glunovo®, 99.61% of glucose values fell within zones A (93.64%, n = 2,179) and B (5.97%, n = 139). In comparison, for FGM, 100.0% of glucose values were within zones A (90.29%, n = 2,101) and B (9.71%, n = 226). As shown in Figure 2, Parkes Error Grid Analysis demonstrated acceptable clinical accuracy. For Glunovo®, 100.0% of glucose values fell within zones A (92.52%, n = 2,153) and B (7.48%, n = 174). In comparison, for FGM, 100.0% of glucose values were within zones A (90.29%, n = 2,101) and B (9.71%, n = 226).
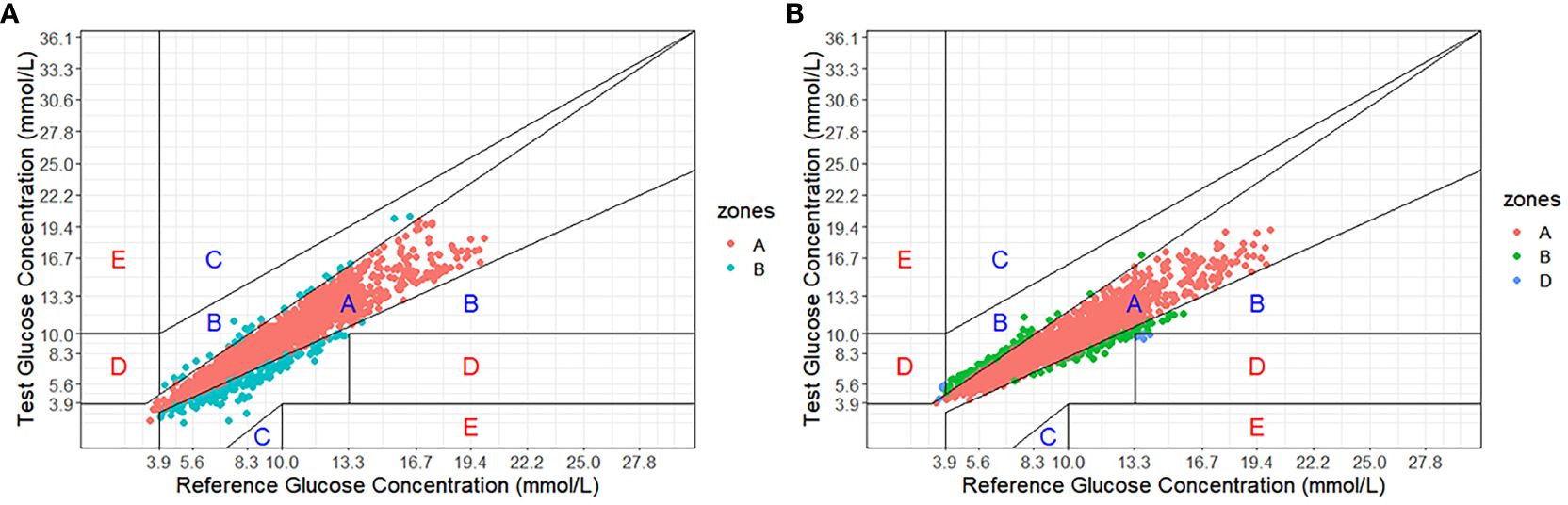
Figure 1. Clarke error grid analysis. (A) flash glucose monitoring; (B) Glunovo®. The percentage of measuring points falling in A + B zones was 100.00% for flash glucose monitoring and 99.61% for Glunovo®. Zone A, clinically accurate; Zone B, benign errors; Zone C, overcorrection errors; Zone D, failure to treat errors; and Zone E, erroneous treatment errors.
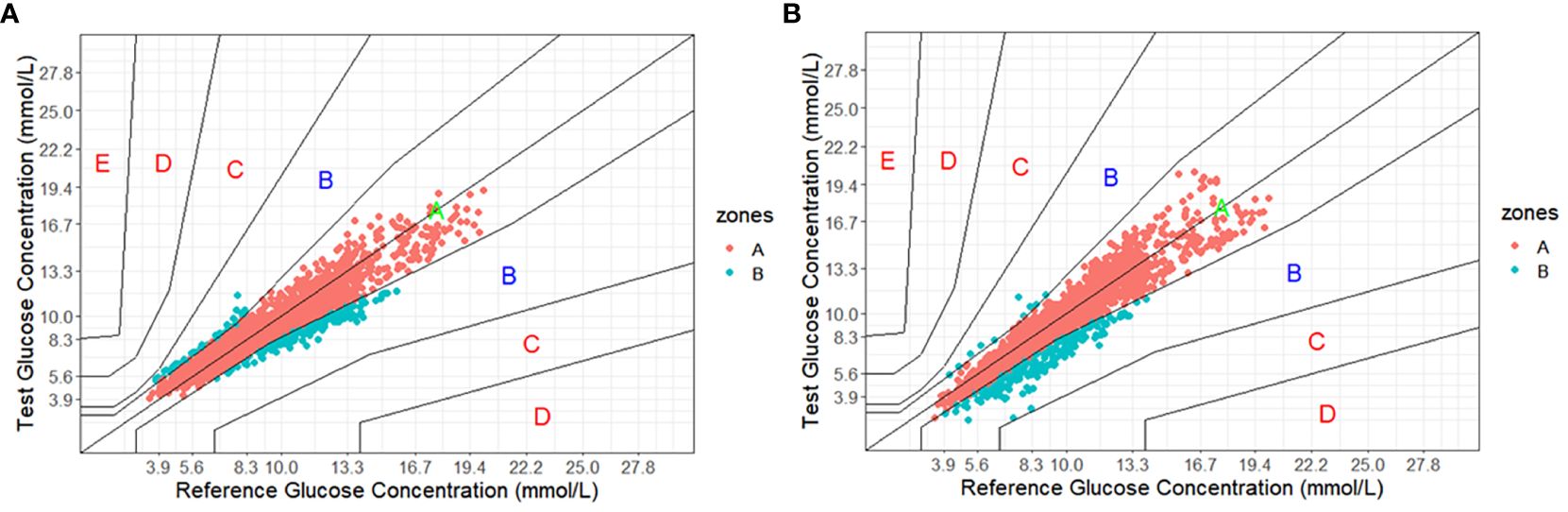
Figure 2. Parkes error grid analysis. (A) flash glucose monitoring; (B) Glunovo®. The percentage of measuring points falling in A + B zones was 100.00% for flash glucose monitoring and 100.00% for Glunovo®. Zone A, clinically accurate; Zone B, benign errors; Zone C, overcorrection errors; Zone D, failure to treat errors; and Zone E, erroneous treatment errors.
Our findings demonstrated that the Glunovo® exhibited high accuracy, with an overall MARD of 8.89%. In the initial, middle, and final stages of data collection, Glunovo® consistently exhibited excellent performance. The 2013 CGM Roundtable emphasized that MARD values below 14% are desirable, while values exceeding 18% indicate poor accuracy (9). In comparison, the FGM system exhibited a slightly higher MARD value of 10.42%. A study of 72 diabetic patients evaluated a Dexcom G4 Platinum CGMS with a MARD value of 13% (10). The study on Dexcom G5 Platinum CGMS indicated a MARD value of 9.5% (11). In addition, a separate study of Dexcom G6 Platinum CGMS showed a MARD value of 9.0% (12). The Guardian Connect CGMS had a MARD value of 9.7% (13). Notably, due to limited available data within the hypoglycemic range, the accuracy of the sensors in the low blood glucose range (< 3.9 mmol/L) could not be effectively assessed. Previous studies have indicated that MARD values during hypoglycemia were significantly higher than those within the normal glucose range (14). Therefore, the focus of rtCGM in predicting hypoglycemia should be increased in the future.
The accuracy of Glunovo® was impaired during rapid changes in blood glucose, especially when the blood glucose change rate surpasses 0.11 mmol/L/min. Similarly, in a study of CGM in patients with type 1 diabetes, overall MARD during acute exercise was 29.8% (15). Since CGM does not directly measure glucose concentration in the veins, its values are determined by the rate of glucose diffusion from the plasma to the interstitial fluid and the rate of glucose uptake by cells in subcutaneous tissue (5). The rate of change in glucose concentration in interstitial fluid within tissues is typically slower than that in plasma, often resulting in a delay (16). When blood glucose undergoes rapid fluctuations, this delay was amplified, which could compromise the accuracy of CGM.
The Clarke Error Grid Analysis estimated high clinical performance, with 99.61% of samples in the clinically acceptable error zones A and B. In a multicenter study focusing on the Eversense implantable CGM sensor, the results showed that 99.2% of samples were within the clinically acceptable error zones A (84.3%) and B (14.9%) (17). Moreover, real-time continuous glucose monitoring (rtCGM) has shown promising results in monitoring diabetes for peritoneal dialysis patients, with 99.9% of data points falling within zones A and B (18). The evidence mentioned above strongly supports the implementation of rtCGM, providing patients with viable monitoring options.
Several limitations should be considered. First, as subjects received standardized hospital treatment, results may not apply to home care. Second, the potential impact of confounding factors, such as patient medication profiles and the severity of diabetes, may not have been comprehensively addressed. Third, limited hypoglycemia data may impact the assessment of monitoring effectiveness in low glucose conditions. Future studies should aim for larger sample sizes to detect differences in the low blood glucose range, thereby providing more insights for physicians.
In conclusion, our study highlights the enhanced accuracy of Glunovo® in blood glucose monitoring for hospitalized patients, providing an alternative for diabetes assessment and management. Nevertheless, the reliability of Glunovo® in low blood glucose monitoring requires verification. Further research is warranted to provide insights for the utilization of Glunovo® in the future.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of Nanjing First Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was obtained from the participants or the participants’ legal guardians/next of kin.
SG: Data curation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. HZ: Writing – review & editing, Investigation, Software, Validation. JW: Investigation, Software, Validation, Writing – review & editing. HL: Data curation, Investigation, Writing – review & editing. XS: Data curation, Investigation, Writing – review & editing. DD: Project administration, Supervision, Writing – review & editing. JM: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (81870563) and Nanjing Medical Science and Technology Development Fund (ZKX22038).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Malone JI. Diabetic central neuropathy: CNS damage related to hyperglycemia. Diabetes. (2016) 65:355–7. doi: 10.2337/dbi15–0034
3. Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther. (2012) 14 Suppl 1:S51–58. doi: 10.1089/dia.2012.0031
4. Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond hbA1c: role of continuous glucose monitoring. Diabetes Ther. (2019) 10:853–63. doi: 10.1007/s13300–019-0619–1
5. Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. (2009) 11:S–11-S-16. doi: 10.1089/dia.2009.0002
6. Bruttomesso D, Laviola L, Avogaro A, Bonora E, Del Prato S, Frontoni S, et al. The use of real time continuous glucose monitoring or flash glucose monitoring in the management of diabetes: A consensus view of Italian diabetes experts using the Delphi method. Nutr Metab Cardiovasc Dis. (2019) 29:421–31. doi: 10.1016/j.numecd.2019.01.018
7. Meng R, Gu T, Yang F, Liu J, Sun Q, Zhu D. Performance evaluation of the glunovo® Continuous blood glucose monitoring system in chinese participants with diabetes: A multicenter, self-controlled trial. Diabetes Ther. (2021) 12:3153–65. doi: 10.1007/s13300–021-01171–2
8. Clarke W, Kovatchev B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther. (2009) 11 Suppl 1:S45–54. doi: 10.1089/dia.2008.0138
9. Wernerman J, Desaive T, Finfer S, Foubert L, Furnary A, Holzinger U, et al. Continuous glucose control in the ICU: report of a 2013 round table meeting. Crit Care. (2014) 18:226. doi: 10.1186/cc13921
10. Nakamura K, Balo A. The accuracy and efficacy of the dexcom G4 platinum continuous glucose monitoring system. J Diabetes Sci Technol. (2015) 9:1021–6. doi: 10.1177/1932296815577812
11. Link M, Kamecke U, Waldenmaier D, Pleus S, Garcia A, Haug C, et al. Comparative accuracy analysis of a real-time and an intermittent-scanning continuous glucose monitoring system. J Diabetes Sci Technol. (2021) 15:287–93. doi: 10.1177/1932296819895022
12. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. (2018) 20:428–33. doi: 10.1089/dia.2018.0143
13. Yeoh E, Png D, Khoo J, Chee YJ, Sharda P, Low S, et al. A head-to-head comparison between Guardian Connect and FreeStyle Libre systems and an evaluation of user acceptability of sensors in patients with type 1 diabetes. Diabetes Metab Res Rev. (2022) 38:e3560. doi: 10.1002/dmrr.3560
14. Jin Z, Thackray AE, King JA, Deighton K, Davies MJ, Stensel DJ. Analytical performance of the factory-calibrated flash glucose monitoring system freeStyle libre2(TM) in healthy women. Sensors (Basel). (2023) 23:402–4. doi: 10.3390/s23177417
15. Moser O, Eckstein ML, McCarthy O, Deere R, Pitt J, Williams DM, et al. Performance of the Freestyle Libre flash glucose monitoring (flash GM) system in individuals with type 1 diabetes: A secondary outcome analysis of a randomized crossover trial. Diabetes Obes Metab. (2019) 21:2505–12. doi: 10.1111/dom.13835
16. Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. (2003) 52:2790–4. doi: 10.2337/diabetes.52.11.2790
17. Kropff J, Choudhary P, Neupane S, Barnard K, Bain SC, Kapitza C, et al. Accuracy and longevity of an implantable continuous glucose sensor in the PRECISE study: A 180-day, prospective, multicenter, pivotal trial. Diabetes Care. (2017) 40:63–8. doi: 10.2337/dc16–1525
Keywords: Glunovo®, rtCGMS, type 2 diabetes, flash glucose monitoring, venous blood glucose
Citation: Ge S, Zhang H, Wang J, Li H, Su X, Ding D and Ma J (2024) Accuracy of a novel real-time continuous glucose monitoring system: a prospective self-controlled study in thirty hospitalized patients with type 2 diabetes. Front. Endocrinol. 15:1374496. doi: 10.3389/fendo.2024.1374496
Received: 22 January 2024; Accepted: 02 May 2024;
Published: 21 May 2024.
Edited by:
Lingling Xu, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Yaru Zhou, The Third hospital of Hebei University, ChinaCopyright © 2024 Ge, Zhang, Wang, Li, Su, Ding and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Ma, bWFqaWFuaHVhMTk2NTAzQDEyNi5jb20=; Dafa Ding, ZGluZ2RhZmFAbmptdS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.