- 1Department of Pharmacy, The Second Hospital of Dalian Medical University, Dalian, China
- 2Department of Cardiovascular, The Second Affiliated Hospital of Dalian Medical University, Dalian, China
Cardiovascular disease (CVD) is the leading cause of human mortality worldwide. Despite Western medicine having made encouraging results in the clinical management of CVD, the morbidity, mortality, and disability rates of the disease remain high. Modern pharmacology has confirmed that traditional Chinese medicine (TCM), characterized by its multi-component, multi-target, and integrity, plays a positive and important role in the prevention and treatment of various CVDs in China, which has notable advantages in stabilizing disease, improving heart function, and enhancing the quality of life. Importantly, TCM is gradually being accepted by the international community due to its low cost, high safety, versatile bioactivity, and low toxicity. Unfortunately, comprehensive studies on the therapeutic effect of TCM on CVD and its mechanisms are very limited, which may restrict the clinical application of TCM in CVD. Therefore, this review is performed to analyze the pathogenesis of CVD, including inflammatory response, oxidative stress, mitochondrial dysfunction, pyroptosis, ferroptosis, dysbiosis of gut microbiota, etc. Moreover, we summarized the latest progress of TCM (formulas, extracts, and compounds) in curing CVD according to published literature from 2018 to 2023, as well as its mechanisms and clinical evidence. In conclusion, this review is expected to provide useful information and reference for the clinical application of TCM in the prevention and treatment of CVD and further drug development of CVD.
1 Introduction
Cardiovascular disease (CVD) is the diseases of the circulatory system, including disorders of the heart and blood vessels. As a chronic progressive condition, CVD is characterized by high morbidity, mortality, hospitalization, and disability rates, causing a huge economic and health burden worldwide (1, 2). According to the World Health Organization, CVD was the leading cause of the highest number of deaths in 2019 (3), and about 23 million CVD-related deaths in 2030 (4). Meanwhile, CVD remains the predominant cause of human mortality in China (5) and Western countries (6). Recent studies have confirmed that the occurrence and progression of CVD are the results of the interaction of genetic and environmental factors, and common risk factors include age, obesity, tobacco use, alcohol consumption, dyslipidemia, hypertension, diabetes (7–12), etc. Meanwhile, other studies have found that air pollution and circadian syndrome as contributing factors to CVD (13, 14). In addition, numerous studies have demonstrated that oxidative stress, inflammatory response, programmed cell death (such as apoptosis and autophagy, pyroptosis, and ferroptosis), and intestinal flora disorders were associated with the abnormalities of structural and functional in the cardiovascular system (15–17). Currently, surgery and drugs are commonly used in the clinical management of various CVDs, but surgical procedures are both risky and expensive. Besides, the effectiveness of cardiovascular drugs decreases with prolonged use and is accompanied by adverse side effects, which has become a major problem that needs to be urgently addressed in the Western medical treatment of CVD. Therefore, the pathogenesis of CVD needs to be further explored and effective prevention and treatment strategies need to be developed.
Traditional Chinese medicine (TCM) is an accumulation of the Chinese Nation’s clinical experience for thousands of years, characterized by comprehensive resources and low cost, and has been widely used for treating various diseases in clinical practice (18, 19). TCM was an important source of modern drug development for more than 2,000 years. More interestingly, TCM has become increasingly popular in many developed countries (20), such as Australia and the United States, because of its unique advantages including low adverse effects, stable efficacy, and a wide range of targets. Modern medical studies have demonstrated that TCM (including formulas, extracts, and compounds) possessed significant effects on the treatment of CVD, and TCM treatments are well tolerated by patients with CVD (21). Currently, the “compound Dan-Shen dropping pill”, which consists of three TCMs for the treatment of coronary heart disease and angina pectoris, was the first TCM formula in the world to complete a phase III randomized, double-blind, and international multicenter clinical trial approved by the U.S. Food and Drug Administration (NCT00797953) and this drug was widely used in Australia after being approved by the Australian Therapeutic Goods Administration. Meanwhile, the standard of Panax notoginseng extracts has been incorporated into the German Drug Code for the benefit of patients with CVD. Functionally, TCM can exert cardioprotective effects through multiple targets on oxidative stress, inflammation, autophagy, lipid metabolism, cardiomyocyte/vascular endothelial cell function, and gut microbiota (22–24), which compensates for the lack of a single drug model for the treatment of CVD in clinical. Several studies have confirmed that TCM combined with Western drugs can more effectively alleviate clinical symptoms and disease progression in patients with CVD (25, 26). Importantly, with the development of omics technologies such as transcriptome, proteome, metabolome, and bioinformatics, the detailed mechanisms of TCM in the prevention and treatment of CVD have been systematically and comprehensively expanded to multiple levels such as RNA, protein, and metabolites, and also extend to the single-cell microscopic level from the perspective of time and space (27). This suggests that TCM provides new perspectives and strategies to combat various CVDs in modern society.
Currently, there are few reviews on TCM for the prevention and treatment of various CVDs. In this review, the current pathogenesis of CVD was comprehensively overviewed. Moreover, the current research on TCM (including TCM formulas, extracts, and compounds) protection against CVD was summarized and discussed based on the published literature from 2018-2023 through global and local databases including PubMed, Web of Science, and China National Knowledge Infrastructure, as well as its mechanisms and clinical efficacy, which may provide a reference for the clinical application of TCM in the treatment of CVD and a theoretical basis for the development of new drugs to combat CVD.
2 The pathogenesis of CVDs
The development and progression of CVD were associated with genetic mutations, obesity, environmental factors, and poor lifestyle (28, 29). Increasing evidence has demonstrated that the possible pathogenesis of CVD includes inflammation, oxidative stress, mitochondrial dysfunction, cell death (e.g., apoptosis, ferroptosis, and pyroptosis), and gut microbiota imbalance, which would lead to cardiomyocyte injury, inflammatory response, and vascular lesions (15, 30, 31), etc.
2.1 Inflammation
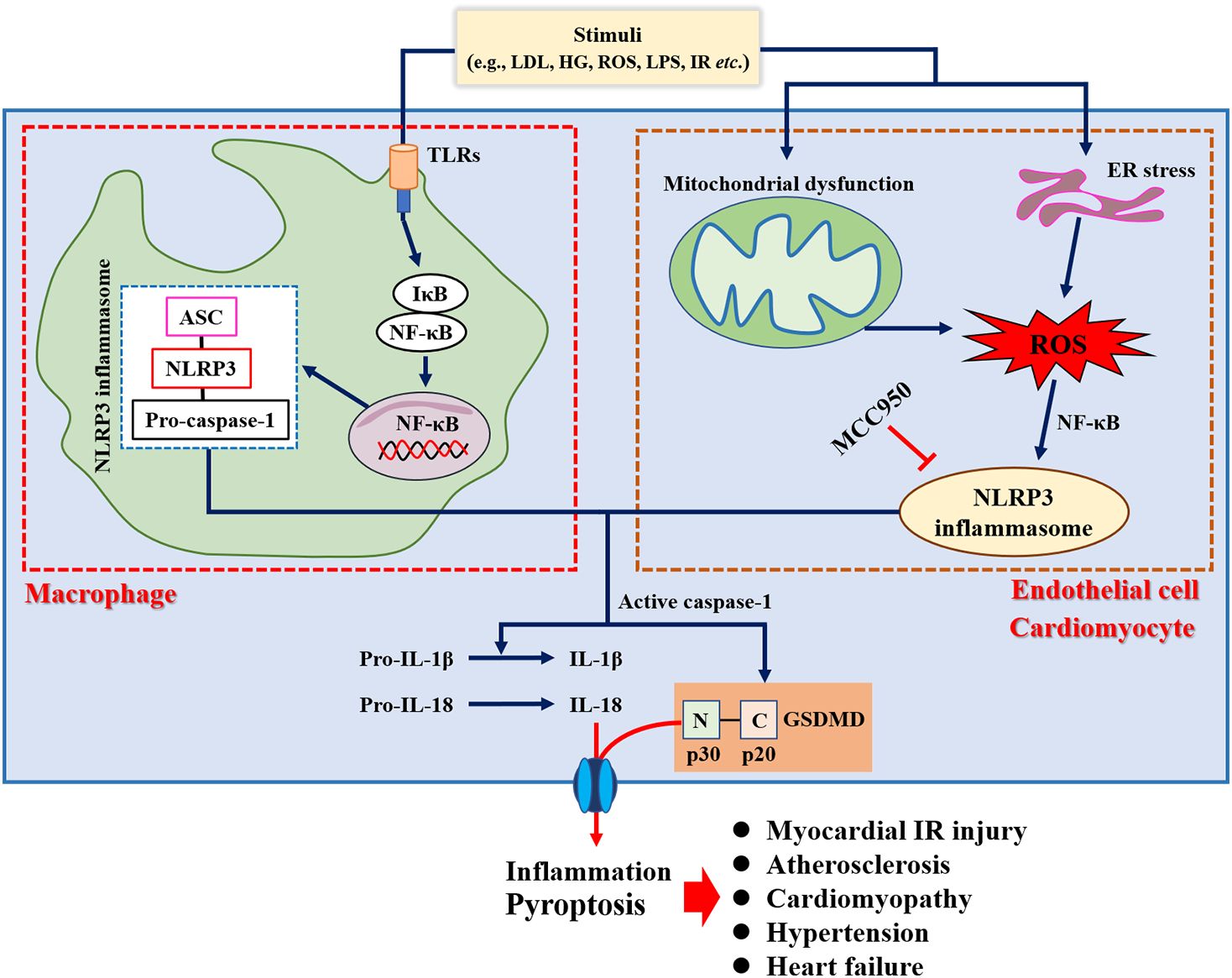
Inflammation plays an important role in the pathogenesis of various CVDs (32), and anti-inflammatory therapies have proven beneficial in several recent clinical trials (33, 34). Increased incidence of cardiovascular events has also been shown in patients with chronic inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, psoriasis, inflammatory myopathies, and inflammatory bowel disease (35). Evidence suggested that the upregulation of circulating C reactive protein resulted in a greater risk of incident acute myocardial infarction (36) or cerebrovascular events (37). Previous studies have shown that atherosclerosis is a low-grade and aseptic inflammatory disease (38). For example, Mai et al. (39) demonstrated that nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome was a key driver of atherosclerosis. Meanwhile, the inflammatory response was considered to be a trigger for the developmental process of atrial fibrillation (40). Over-activation of NLRP3 inflammasome was directly associated with hospitalization rates in patients with cardiac insufficiency and dilated cardiomyopathy, accompanied by cellular scorching of cardiomyocytes (41). In addition, it has also been demonstrated that inhibition of the inflammatory response or NLRP3 gene deletion improved cardiac remodeling and reduced proinflammatory cytokines secretion and fibrotic processes (42, 43), as well as attenuated angiotensin II (Ang II)-induced hypertension (44). Taken together, inflammation was involved in the pathogenesis of several CVDs (Figure 1), which also provides new strategies for the prevention and management of CVD.
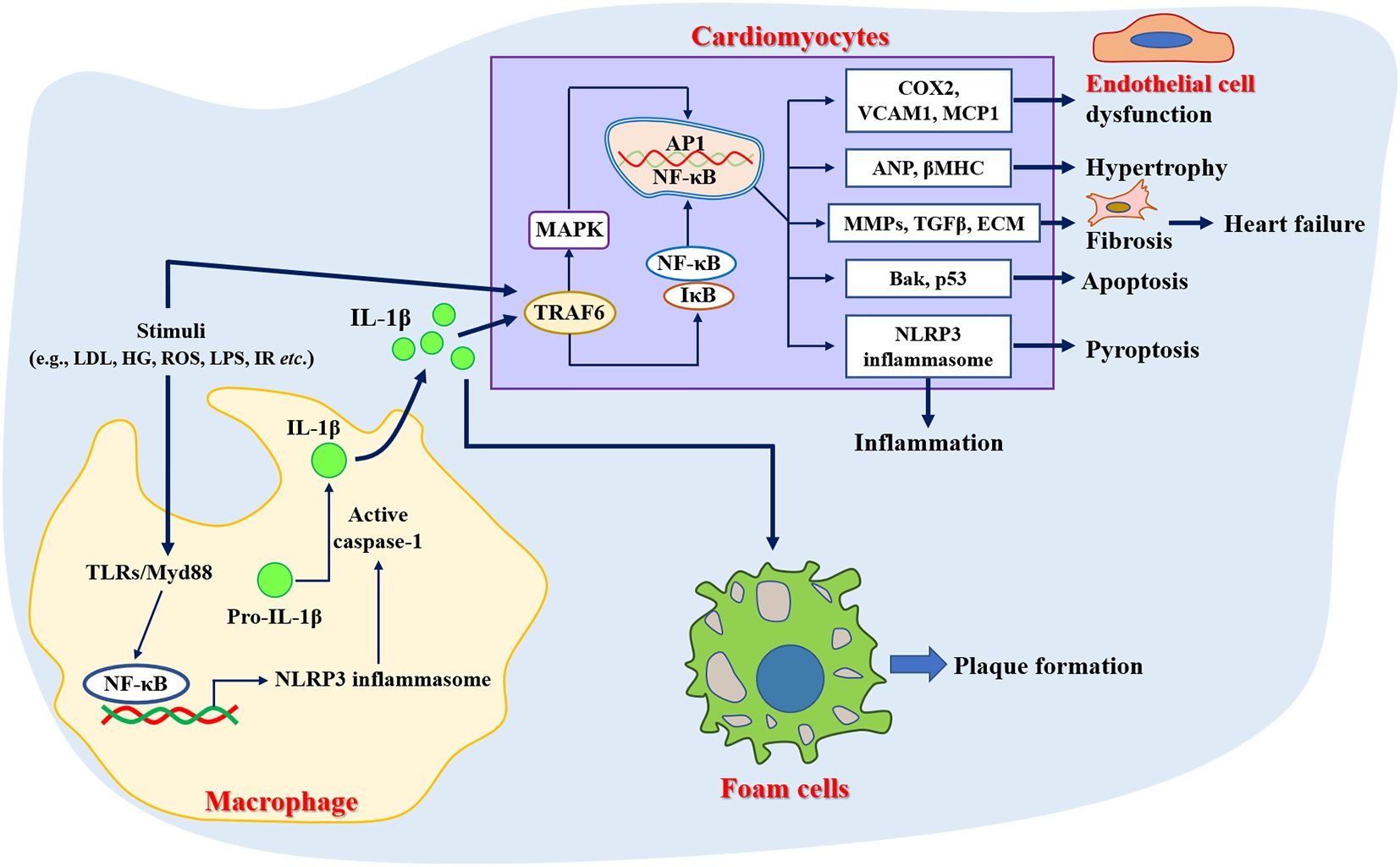
Figure 1. Role of inflammation in the pathogenesis of cardiovascular diseases. ANP, Atrial natriuretic peptide; Bak, Bcl-2 antagonist/killer; COX2, Cyclooxygenase 2; ECM, Extracellular matrix; HG, High glucose; LDL, Low-density lipoprotein; LPS, Lipopolysaccharide; MCP1, Monocyte chemotactic protein 1; NLRP3, Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3; ROS, Reactive oxygen species; TGFβ, Transforming growth factor beta; TLRs, Toll-like receptors; TRAF6, Tumor necrosis factor receptor-associated factor 6; VCAM1, Vascular cell adhesion molecule 1; βMHC, Beta-myosin heavy chain.
2.2 Oxidative stress
Oxidative stress is a pathological state of reactive oxygen species (ROS) accumulation caused by excessive production of oxygen free radicals or impaired intracellular antioxidant defense systems (45). Normal physiological state of ROS levels contributes to the maintenance of cardiovascular homeostasis (46), while excessive and/or sustained increases in ROS production play an important role in the pathological statute of CVD (Figure 2), such as atherosclerosis, hypertension, myocardial ischemia-reperfusion injury, arrhythmia, heart failure, and acute myocardial infarction (47). Of note, oxidative stress has emerged as a new target for the prevention and treatment of CVD (48). It has also been found that common CVD risk factors contribute to a sustained increase in ROS production in the vascular wall (49). Functionally, oxidative stress not only promotes lipid peroxidation, protein and enzyme denaturation, DNA damage, and severe functional impairment of vascular endothelial cells and cardiomyocytes, but also participates in the pathogenesis of hypertension, myocardial ischemia-reperfusion injury, atherosclerosis, and other CVDs by regulating inflammation and stimulating vascular smooth muscle cell proliferation (50). In addition, endogenous antioxidant enzymes (e.g., superoxide dismutase, glutathione peroxidase, catalase, glutathione S-transferase, and peroxidase) and exogenous antioxidants may act by scavenging free radicals and exerting anti-CVD activities. For example, overexpression of glutathione peroxidase 4 (GPX4) inhibited atherosclerosis progression in apolipoprotein E-deficient (ApoE-/-) mice (51). Giam et al. (52) showed that the antioxidant NAC attenuated cardiac injury and prevented cardiac fibrosis which improved cardiac function in mice with heart failure.
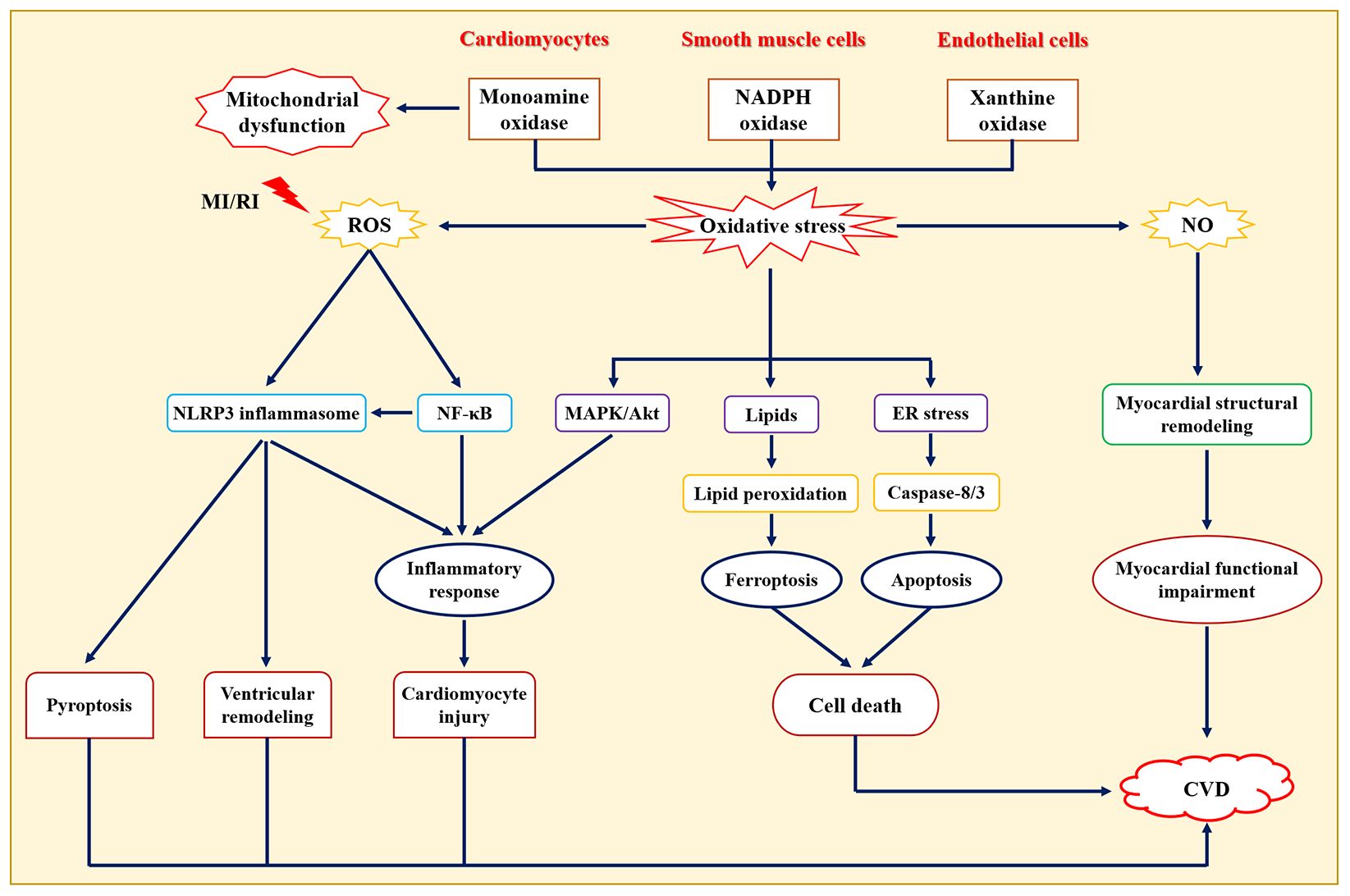
Figure 2. Role of oxidative stress in the pathogenesis of cardiovascular diseases. NO: one of the members of reactive nitrogen, damages cardiomyocytes through direct cytotoxicity or generates ONOO− with O2− to cause cardiomyocyte damage. CVD, Cardiovascular diseases; ER, Endoplasmic reticulum; MAPK, Mitogen-activated protein kinase; MI/RI, Myocardial ischemia/reperfusion injury; NF-κB, Nuclear transcription factor-κB; NLRP3, Nucleotide-binding oligomerization domain-like receptor protein 3.
2.3 Mitochondrial dysfunction
Mitochondria, a key site of cellular metabolism for ATP production, provides enough energy for the contraction and diastole of human cardiomyocytes, but mitochondrial dysfunction accelerates the occurrence and progression of CVD (Figure 3). For example, mitochondrial dysfunction in macrophages contributes to inducing inflammation and inhibiting repair after myocardial infarction, but mitochondrial-targeted ROS scavenging alleviates these phenomena and reduces death after myocardial infarction in mice (53). Currently, mitochondrial dysfunction, mitochondrial DNA and nuclear DNA gene mutation, and the presence of mutant proteins associated with mitochondria are considered to be non-negligible causes of CVD pathogenesis (54). For instance, four mitochondrial DNA mutation genes (e.g., MT-RNR1, MT-TL1, MT-TL2, and MT-CYB) have been reported to be connected with atherosclerosis progression (55). Functionally, mutations in the mitochondrial genome and nuclear genome may disrupt mitochondrial homeostasis, leading to excessive ROS production and reducing oxidative phosphorylation capacity, which are risk factors for CVD (56). For example, specific targeted antioxidant treatments that reduced ROS production and enhanced ROS scavenging have been shown to alleviate impaired mitochondrial-induced oxidative stress (57). Jacinto et al. (58) showed that the overproduction of mitochondrial ROS promoted atherosclerosis progression by triggering DNA fragmentation and cell apoptosis. Moreover, mitophagy plays an important regulatory role in maintaining cellular homeostasis, whereas mitophagy damage predisposes to cause abnormal function of cardiovascular-derived cells (59). Notably, several intervention strategies ameliorate CVD by improving four important characteristics of mitochondria, such as scavenging mitochondrial ROS (60), mitochondrial DNA editing or mitochondrial replacement therapy (61), increased oxidative phosphorylation (62), and enhanced mitophagy (63). Therefore, maintaining normal mitochondrial function has the potential to be used as an effective therapeutic strategy for CVDs.
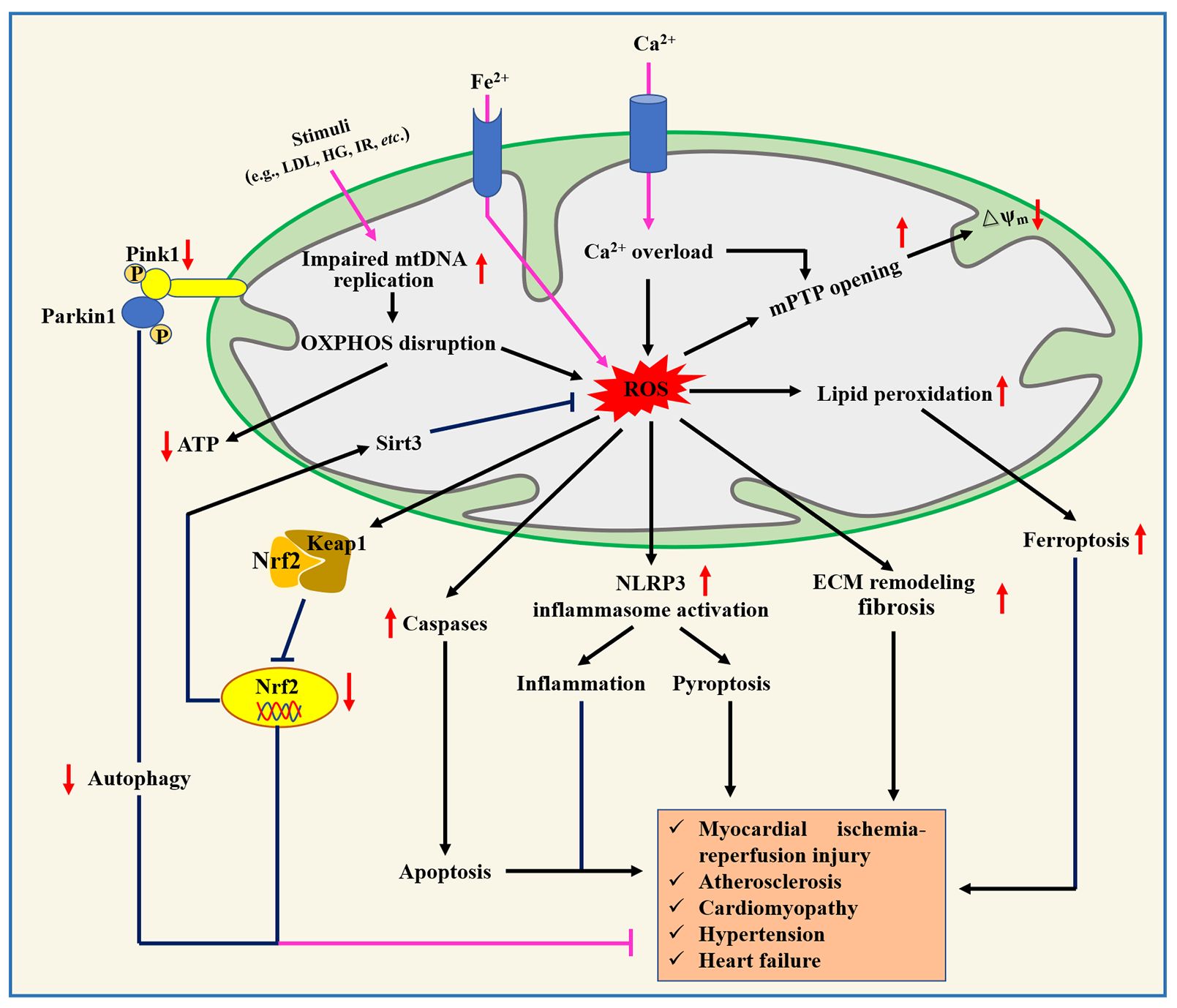
Figure 3. Role of mitochondrial dysfunction in the pathogenesis of cardiovascular diseases. ECM, Extracellular matrix; HG, High glucose; IR, ischemia/reperfusion; Keap1, Kelch-like ECH-associated protein 1; LDL, Low-density lipoprotein; mPTP, Mitochondrial permeability transition pore; Nrf2, Nuclear factor erythroid 2-related factor 2.
2.4 Pyroptosis
Pyroptosis, a form of programmed cell death, is closely related to the inflammatory response, mediated by the Gasdermin protein, and dependent on caspase activity (64). Pyroptosis is typically characterized by the swelling and rupture of cell membranes, the release of pro-inflammatory factors, and cell contents from the plasma membrane to the extracellular environment (65), which aggravates inflammatory response. Recent studies have shown that pyroptosis was involved in the development and progression of several CVDs (Figure 4), including atherosclerosis, diabetic cardiomyopathy, myocardial infarction, myocardial ischemia-reperfusion injury, myocarditis (66), etc. Mechanistically, NLRP3 inflammasome activated caspase-1 and triggered an inflammatory cascade, which plays an important role in pyroptosis (67). For example, NLRP3 inhibitor MCC950 has the potential to prevent NLRP3-related diseases, such as cardiac hypertrophy (68), hypertension (69), atherosclerosis (70), and myocardial injury (71). Jin et al. (72) showed that caspase-1 inhibitor VX765 ameliorated mitochondrial damage induced by the NLRP3 inflammasome activation and inhibition of vascular inflammation in both low-density lipoprotein receptor-deficient (Ldlr-/-) and ApoE-/- mice. These results suggested that inhibition of pyroptosis may provide a new avenue for the treatment and management of CVDs.
2.5 Ferroptosis
Ferroptosis is a new type of cellular iron-dependent programmed cell death, and the process mainly involves the accumulation of lipid peroxidation products and lethal ROS (73). Increasing evidence has demonstrated that ferroptosis was morphologically, biochemically, and genetically distinct from cell apoptosis, necrosis, and autophagy (74), which was mainly characterized by impaired cell membrane integrity, mitochondrial atrophy, normal nuclei, and a significant decrease in the levels of GPX4, glutamate-cystine antiporter system components (SLC3A2 and SLC7A11), and coenzyme II. Available studies have shown that ferroptosis was closely associated with the development of various CVDs including cardiomyopathy, myocardial ischemia-reperfusion injury, heart failure, myocardial infarction, vascular injury, and atherosclerosis (75). For example, Wang et al. (76) reported that increased levels of lipid peroxidation and reduced SLC7A11 levels were observed in the development of diabetic cardiomyopathy. Bai et al. (77) found that ferrostatin-1 (Fer-1, ferroptosis inhibitor) alleviated atherosclerotic lesions by reducing iron accumulation and lipid peroxidation, and enhancing the expression of GPX4 and SLC7A11 in a high-fat diet (HFD)-fed ApoE-/- mice. Another study showed that the inactivation of the Nrf2/GPX4 pathway could aggravate doxorubicin-induced cardiomyopathy by promoting cardiomyocyte ferroptosis (78). Importantly, three types of iron chelators (e.g., deferiprone, deferoxamine, deferasirox) have been used in clinical practice for the treatment of iron overload cardiomyopathy (79). Although many preclinical studies suggest that pharmacological regulation of ferroptosis and genetic inhibition of iron uptake are promising treatment strategies for CVD (Figure 5), the underlying mechanism and regulatory networks need to be fully investigated during the pathological process of CVD, which will provide new ideas and strategies for the prevention and treatment of CVD.
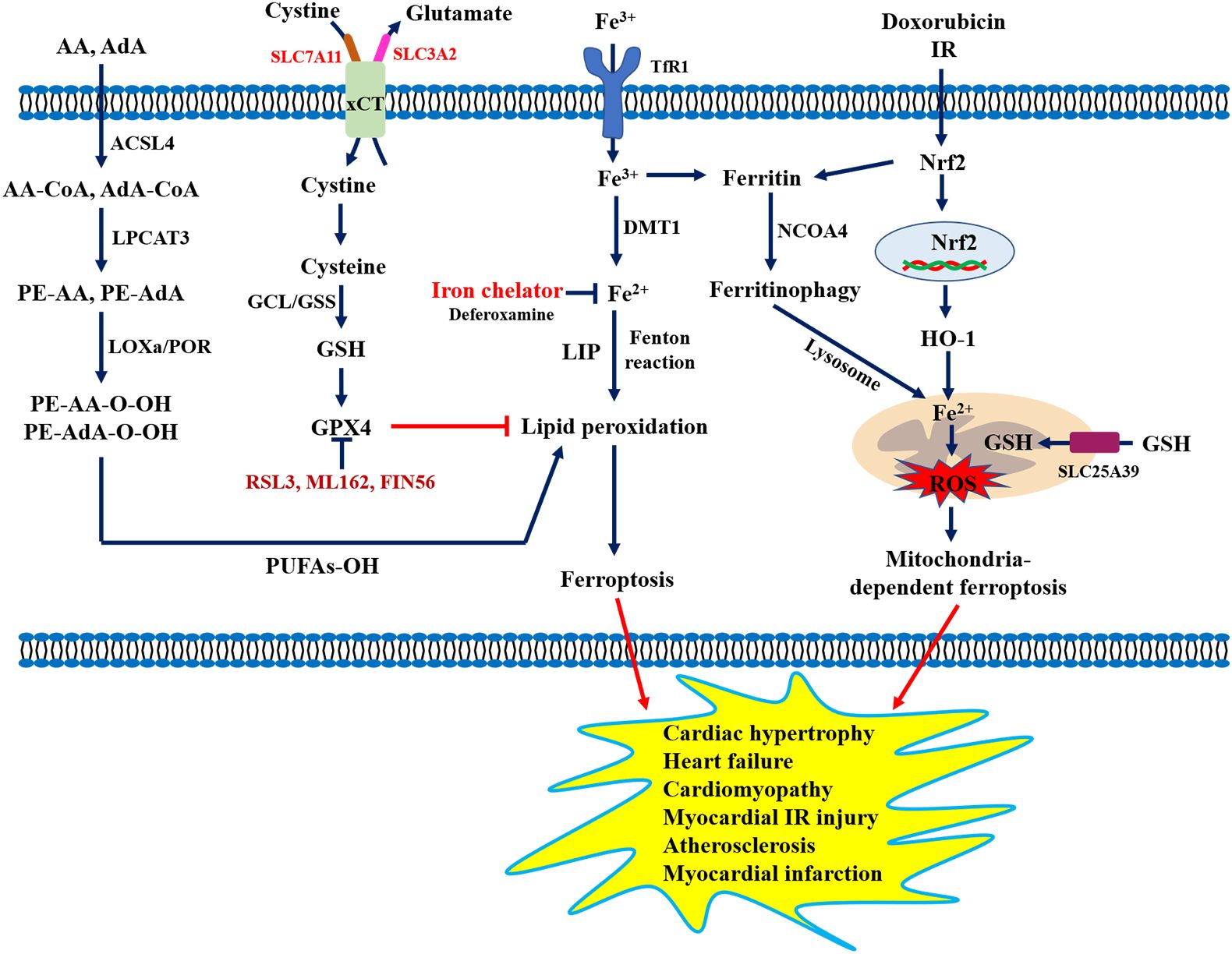
Figure 5. Role of ferroptosis in the pathogenesis of cardiovascular diseases. AA, Arachidonic acid; ACSL4, Long-chain fatty acyl-CoA synthase 4; AdA, Adrenal acid; DMT1, Divalent metal transporter 1; FfR1, Transferrin receptor 1; GCL, Glutamate-cysteine ligase; GPX4, Glutathione peroxidase 4; GSH, Glutathione; GSS, Glutathione synthase; HO-1, Heme oxygenase 1; LPCAT3, Lysolecithin acyltransferase 3; LOXs, Lipoxygenases; NCOA4, Nuclear receptor coactivator 4; POR, Cytochrome P450 oxidoreductase; PUFAs, Polyunsaturated fatty acids; SLC7A11, Solute carrier family 7 member 11; xCT, System Xc-.
2.6 Gut microbiota and metabolomics
Gut microbiota refers to the large number of commensal microorganisms living in the human intestinal tract, which mainly consists of Firmicutes, Bacteroidetes, Proteobacteria, Fusobacteria, and Actinobacteria at the phylum level, but its balance is easily disturbed by food intake, lifestyle, and environment (80). Functionally, the gut microbiota can form the intestinal epithelial barrier, regulate intestinal immunity, and prevent the invasion of pathogenic bacteria and metabolic abnormalities (81), which are essential for human health. Numerous studies have demonstrated that dysbiosis of intestinal bacteria and its metabolites, such as Trimethylamine oxide (TMAO), lipopolysaccharides (LPS), short-chain fatty acids (SCFAs), and bile acids, were closely associated with the development of CVD (82), and targeting the gut microbiota was expected to be a potential new target for the treatment of CVD (Figure 6). For example, Jie et al. (83) reported that patients with atherosclerotic cardiovascular disease (ACVD) possessed an increased relative abundance of Enterobacteriaceae and Streptococcus spp., which contributed to aggravating ACVD as well as other diseases. In another survey, high levels of Prevotella, Hungatella, and Succinclasticum and low levels of Lachnospiraceae family and Faecalibacterium were observed in patients with heart failure (84). Meanwhile, elevated plasma levels of TMAO were positively associated with stroke (85), hypertension (86), and atherosclerosis (87), as well as increased cardiovascular events (88), suggesting that reducing intake of dietary TMAO precursors was an effective strategy to decrease the risk of CVD. The above studies suggest that gut microbiota serves as a “microbial organ” that affects cardiovascular health and the “gut-heart” axis is a potential avenue in the prevention and treatment of CVD.
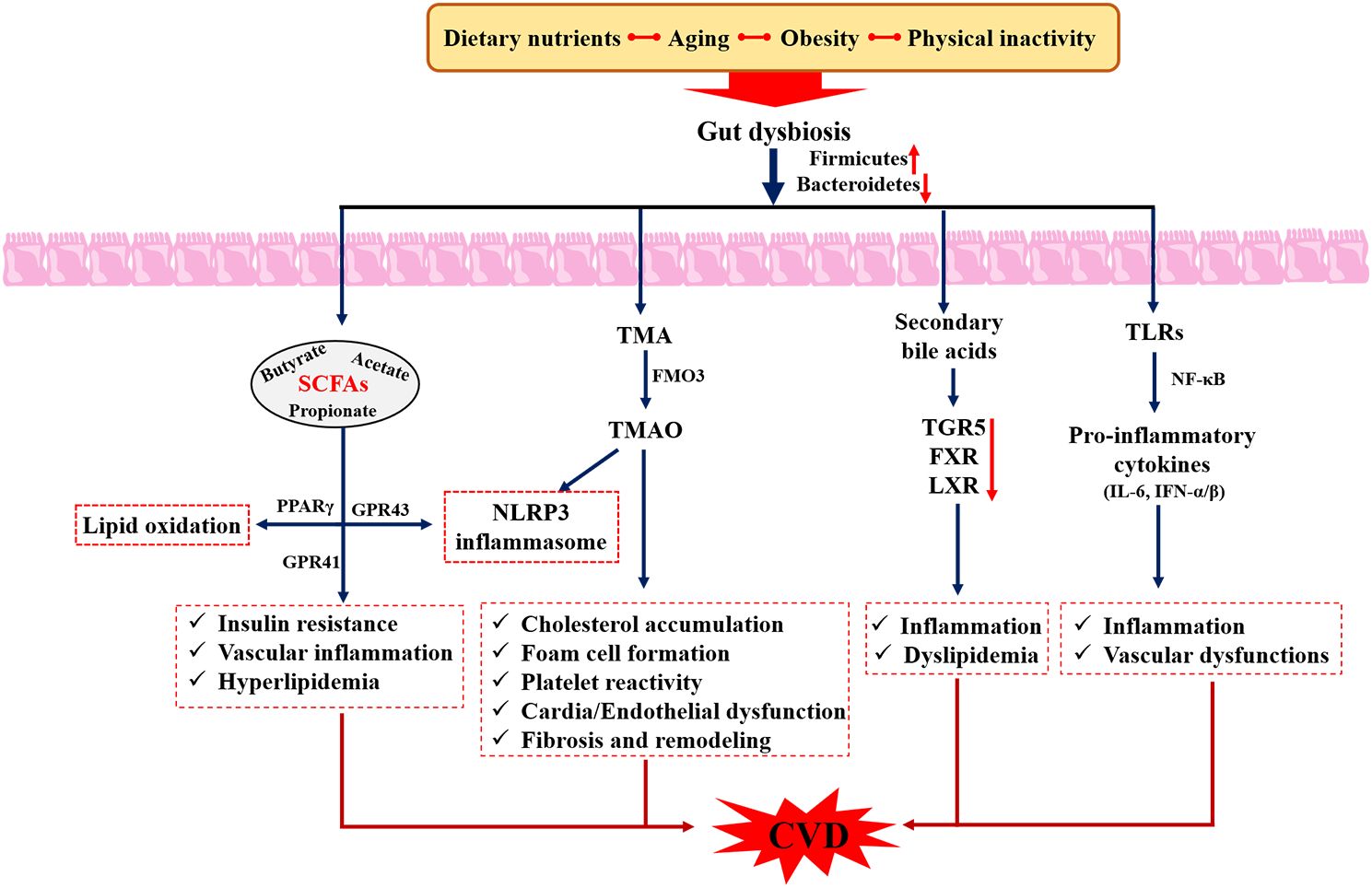
Figure 6. Role of gut microbiota in the pathogenesis of cardiovascular diseases. SCFAs, Short chain fatty acids; LPS, Lipopolysaccharides; TGR5, Takeda G-protein-coupled receptor 5; FXR, farnesoid X receptor; TMAO, trimethylamine-N-oxide; TMA, trimethylamine.
2.7 Others
Except for the pathogenesis mentioned above, researchers believe that CVD is associated with endoplasmic reticulum stress (ERS) (89), autophagy deficiency (90), diabetes (91), metabolic syndrome (92), etc. Moreover, searching for biomarkers used to determine the occurrence and progression of CVDs and revealing their mechanisms are of great clinical significance for the early diagnosis and treatment of CVD. Meanwhile, the exploration of assessment tools for the early identification of people at high risk of CVD is an important guarantee to reduce cardiovascular mortality. However, the drugs developed to address this pathogenesis can only alleviate the symptoms of CVD, but cannot inhibit or reverse CVD progression. Therefore, elucidating the pathogenesis of CVD remains a key clinical problem that needs to be addressed. Of note, understanding the pathogenesis of CVD may provide effective biomarkers and pathways for subsequent therapeutic and new drug development.
3 TCM in the treatment of CVD
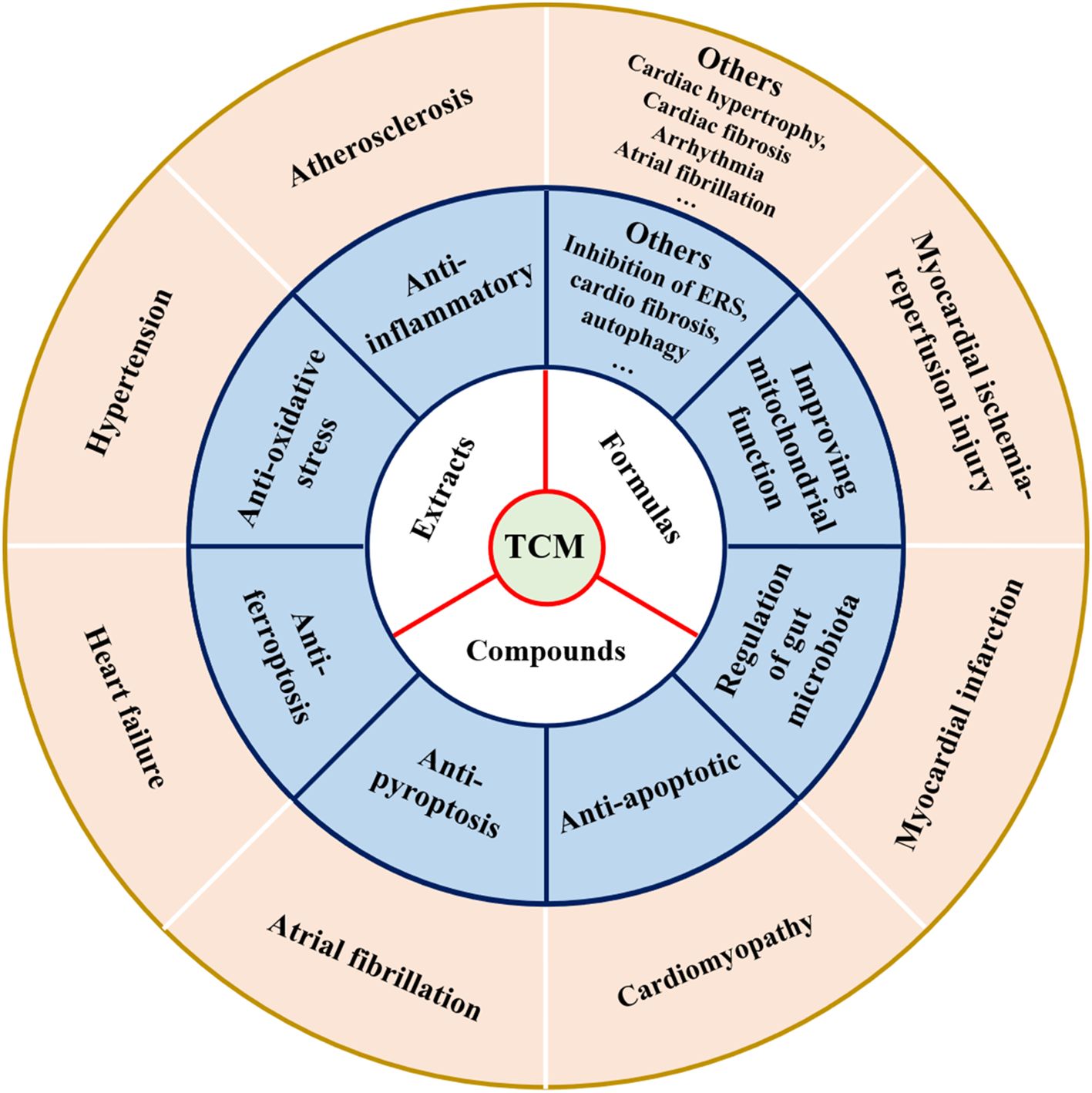
With in-depth research on the pathogenesis of CVD, TCM has shown unique therapeutic advantages in CVD by virtue of its multi-component, multi-target, and integrity (93). More and more studies have demonstrated that TCM (including formulas, extracts, and compounds) exhibited a protective effect on cardiovascular (21), and mechanisms of action of TCM in preventing CVD are shown in Figure 7 and Tables 1–3. Meanwhile, the majority of Chinese patients with CVD have been treated with TCM during the diagnosis and treatment process (94). Herein, we summarized the research progress of TCM in the treatment of various CVDs to provide a reference for the research on the complex mechanism of TCM in combating CVD.
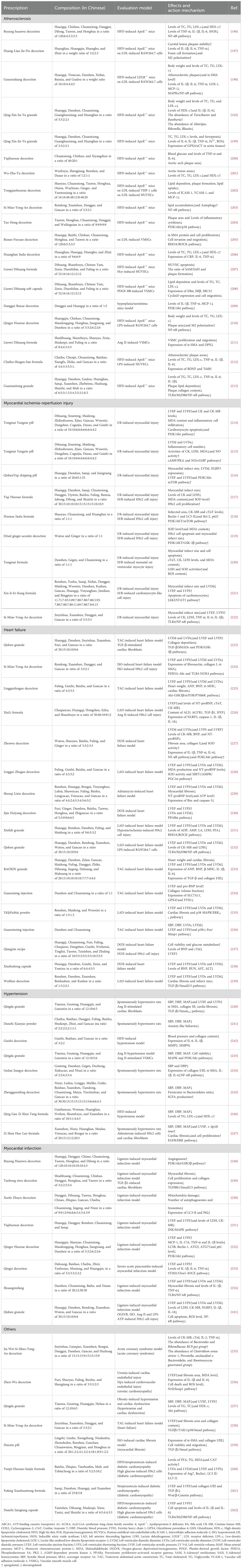
Table 1. Summary of traditional Chinese medicine formulas in the prevention and treatment of various cardiovascular diseases from 2018-2023.
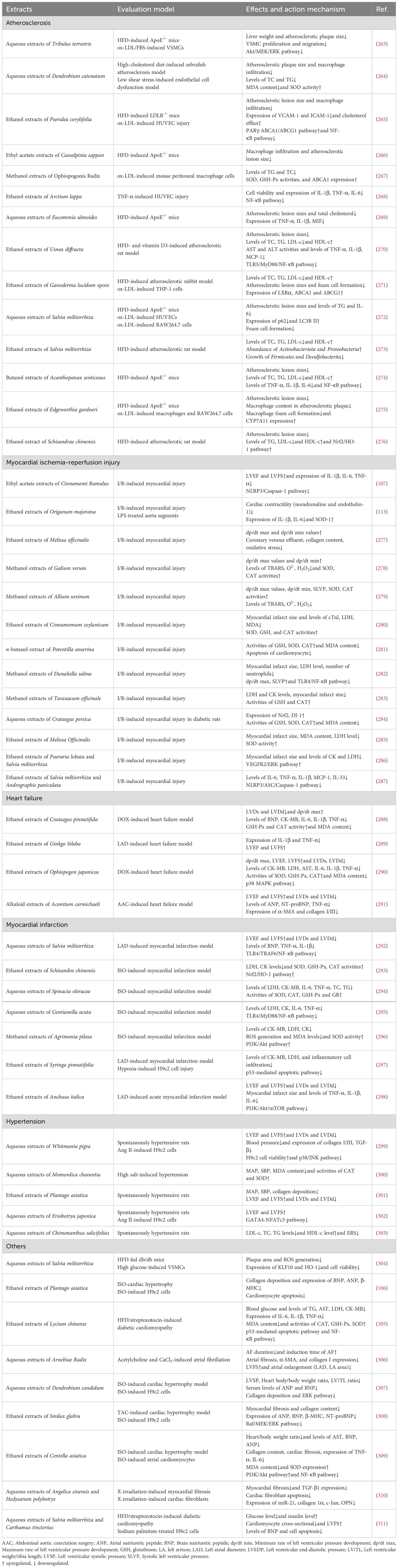
Table 2. Summary of traditional Chinese medicine extracts in the prevention and treatment of various cardiovascular diseases from 2018-2023.
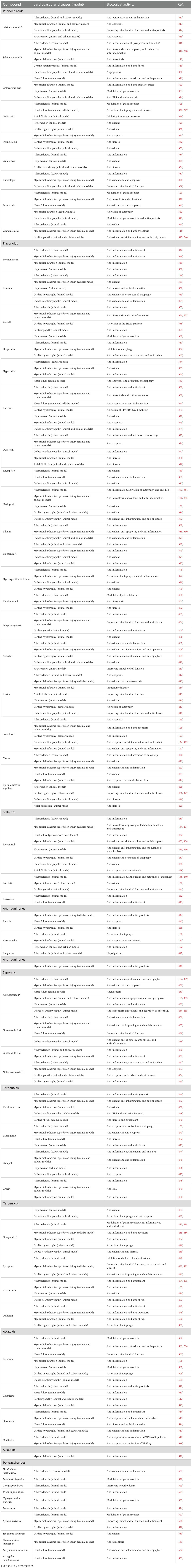
Table 3. Summary of traditional Chinese medicine compounds in the prevention and treatment of various cardiovascular diseases from 2018-2023.
3.1 TCM formulas for CVD
Chinese herbal compounding (fu fang or prescription in Chinese) is the main form of TCM for the prevention and treatment of various diseases, which is the simultaneous application of multiple herbs to regulate the body as a whole for therapeutic purposes in clinical practice. A meta-analysis showed that the efficacy of Bushen Huoxue decoction in treating coronary heart disease was superior to conventional Western medicine (95). Bi and his colleagues (96) confirmed that Qingre Huatan formulae for the phlegm-heat-stasis syndrome pattern of coronary heart disease was safe and can effectively improve vascular endothelial function. In a randomized, multicenter, double-blind, non-inferiority trial, the results showed that treatment with the Songling Xuemaikang capsule had a well-tolerated and improved total hypertension symptom score and total cholesterol in patients with essential hypertension (97). In addition, TCM prescriptions have been shown to improve sleep disorders in patients with CVD (98). Mechanistically, the Qing-Xue-Xiao-Zhi formula can alleviate the development of atherosclerosis by blocking the TLR4/MyD88/NF-κB pathway to promote lipid efflux, reducing atherosclerotic plaques in the aorta and aortic root and serum TMAO levels, and inhibiting macrophage-mediated inflammation (99). Wu et al. (100) observed that the QiShenYiQi dripping pill can inhibit myocardial ischemia-induced ferroptosis in cardiomyocytes by reducing mitochondrial ROS levels and restoring mitochondrial function (e.g., biogenesis and dynamic homeostasis). Chen et al. (101) demonstrated that Qishen granule administration exhibited cardioprotective effects by inactivation of NF-κB/NLRP3/GSDMD pathway in myocardial infarction, as evidenced by improving cardiac function, reducing inflammatory cell infiltration and collagen deposition, as well as inhibiting NLRP3 inflammasome activation and pyroptosis. Qing-Xin-Jie-Yu granule treatment contributed to the alleviation of atherosclerosis development by regulating gut microbiota composition (that is, the relative abundance of Turicibacter and Roseburia was enhanced), increasing bile acids production, and reducing metaflammation induced by HFD (102). Zhou et al. (103) showed by a comprehensive network analysis that Shenfu injection can be used to treat coronavirus disease 2019 (COVID-19) combined with heart failure. Except for the above-mentioned TCM prescriptions, there are still numerous studies reported on the use of some classical TCM formulas for the prevention and treatment of CVD according to ancient works and the modern clinical. Herein, we summarized the pharmacological effects and molecular mechanisms of TCM prescriptions on CVD based on published studies from 2018 to 2023 and listed in Table 1.
3.2 TCM extracts for CVD
Increasing evidence has proved that single TCM extracts also possessed a protective effect against CVD except for TCM preparations mentioned above (Table 2). For example, a network pharmacology study showed that Schisandra extracts have the potential for therapeutic effects on atherosclerosis by regulating immune inflammation and oxidative stress (104). Recently, the key mechanisms of TCM extracts in CVD may be associated with immunomodulation, antioxidant, anti-cell death, anti-inflammatory, and gut microbiota regulation. For example, Quince extract exhibited hypolipidemic, antioxidant, anti-inflammatory, anti-thrombotic, and vascular endothelium protective effects on HFD-induced atherosclerosis (105). Plantago asiatica L. seeds extracts prevented isoproterenol-induced cardiac hypertrophy by restoration of autophagy and inhibition of cardiomyocyte apoptosis (106). The ethyl acetate extracts of Cinnamomi Ramulus protect rats from myocardial ischemia-reperfusion injury by suppression of NLRP3 inflammasome activation and pyroptosis (107). In doxorubicin-induced chronic heart failure, the combination of aqueous extracts of Aconiti Lateralis Radix Praeparata and Zingiberis Rhizoma has a better therapeutic effect than their single aqueous extracts, which may be associated with improving left ventricular function and promoting mitochondrial energy metabolism through activation of the PPARα/PGC-1α/Sirt3 pathway (108). Treatment with bay leaf extracts exhibited an anti-inflammatory effect in the rat model of myocardial infarction (109), reflected by reducing the levels of C-reactive protein and myeloperoxidase. Another study showed that aqueous extracts of Ligustrum robustum attenuated atherosclerosis development by modulating gut microbiota composition and metabolism, as evidenced by increased relative abundance of genus Bifidobacterium, and reduced serum TMAO and bile acid, as well as decreased cholesterol absorption (110). In addition, single TCM extracts used for the treatment of CVD have been shown to regulate mitochondrial homeostasis and maintain normal autophagy function, as well as have anti-ERS and anti-contractile effects. For instance, Vilella et al. (111) reported that green tea extracts ameliorated cardiomyopathy progression by improving mitochondrial function. In streptozotocin-induced diabetic atherosclerosis, Ginkgo biloba leaf extracts reduced plaque lipid deposition and serum inflammatory cytokines secretion via inhibiting ERS and mTOR-mediated autophagy (112). Granado et al. (113) proved that Marjoram extracts prevented inflammatory response, apoptosis, and oxidative stress of cardiomyocytes induced by coronary ischemia-reperfusion, as well as possessed anti-contractile effects in aorta segments. Taken together, the cardioprotective effects of single TCM extracts on various CVDs were confirmed, but its underlying mechanisms and safety need to be further explored before clinical practice.
3.3 Compounds isolated from TCM for CVD
With the development of pharmaceutical chemistry and pharmacology, many scholars have conducted studies on the bioactive components of TCM in recent years. It has been found that a large number of effective compounds extracted from TCM, such as phenolic acids, flavonoids, stilbenes, anthraquinones, saponins, terpenoids, alkaloids, polysaccharides, etc., all of which possessed therapeutic effects on various CVDs (Table 3).
3.3.1 Phenolic acids
Phenolic acids are a subclass of plant phenolics that can be isolated and extracted from many traditional Chinese herbs such as Angelica sinensis, Salvia miltiorrhiza, Cinnamomi ramulus, Lonicera japonica, Radix Paeoniae Rubra, Ligusticum wallichii, etc. Modern pharmacological studies have confirmed that phenolic acids have a variety of biological activities, including antioxidant, anti-inflammation, anti-coagulant, and hypolipidemic (114). Of note, numerous studies have demonstrated that phenolic acids have been shown to have a therapeutic effect on CVD (115, 116). Vanillic acid, a phenolic compound extracted from Angelica sinensis, could alleviate hypoxia/reoxygenation-induced H9c2 cardiomyocyte injury by inhibiting cell apoptosis and oxidative stress (117). Cinnamic acid is an active phenolic acid extracted from Cinnamomi ramulus that has a cardioprotective effect against myocardial ischemia-reperfusion injury by inhibiting NLRP3 inflammasome-mediated inflammation and cardiomyocyte pyroptosis (118). Shen et al. (119) showed that Salvianolic acid B can effectively inhibit ferroptosis and mitochondrial oxidative stress by activation of the Nrf2 pathway, thereby attenuating myocardial infarction. Another study reported that ferulic acid ameliorated atherosclerotic injury by modulating gut microbiota and lipid metabolism (120), as evidenced by reducing the relative abundance of Erysipelotrichaceae and Firmicutes and increasing the relative abundance of Ruminococcaceae, as well as downregulating serum levels of total cholesterol, triglyceride, and low-density lipoprotein cholesterol and atherogenic index in HFD-fed ApoE-/- mice. In addition, we summarized many phenolic acids such as caffeic acid, protocatechuic acid, chlorogenic acid, gallic acid, benzoic acid, and erucic acid for the treatment and prevention of CVD, which are listed in Table 3.
3.3.2 Flavonoids
Flavonoids are secondary metabolites widely found in TCM and have various pharmacological activities that are beneficial to human health (121), such as antioxidant, anti-apoptosis, anti-inflammation, antitumor, etc. Of note, many studies have found that flavonoid compounds can play an effective protective role in the treatment of CVD (122). Functionally, scutellarin, a flavonoid compound extracted from Erigeron breviscapus, possessed protective effects against cardiac hypertrophy (123), diabetic cardiomyopathy (124), atherosclerosis (125), myocardial ischemia-reperfusion injury (126), and myocardial infarction (127) via inhibition of inflammation, oxidative stress, and apoptosis. Baicalein extracted from Scutellaria baicalensis inhibited Ang II/oxidized low-density lipoprotein-induced inflammation via inactivation of the AMPK/NF-κB pathway, thus showing anti-atherosclerotic activity (128). Wogonin, one of the main flavonoid compounds of Scutellaria radix, ameliorated isoproterenol-induced myocardial infarction via suppression of inflammation and oxidative stress (129). Naringenin was the main flavonoid that existed in various citrus fruits, bergamots, and tomatoes. Naringenin treatment inhibited myocardial ischemia-reperfusion-induced inflammation, lipid peroxidation, and ferroptosis by activating the Nrf2/GPX4 pathway (130). Naringenin suppressed blood pressure, cholesterol triglycerides, LDL, serum malondialdehyde (MDA), and nitric oxide, as well as increased serum superoxide dismutase and glutathione via blocking the STAT3 pathway in obesity-associated hypertension (131). Abukhalil et al. (132) reported that galangin, a natural flavonoid found in lesser galangal and honey, exerted a protective effect on diabetic cardiomyopathy by reduction of oxidative stress, inflammation, and hyperglycemia. Last but not least, pinocembrin belongs to this series of flavonoids and exerts an antioxidant effect on heart failure by activating the Nrf2/HO-1 pathway, evidenced by reducing ROS level in heart tissue and serum MDA level and improving cardiac function (133). Taken together, flavonoids possess a range of biological activities that prevent the development and progression of CVD, and their potential mechanisms are summarized in Table 3.
3.3.3 Stilbenes
Stilbenes are compounds with a stilbene parent structure connected by a vinyl group between two benzene rings and have a typical conjugated structure. Stilbenes are widely found in TCM, including Polygonum cuspidatum and Polygonum multiflorum, and have beneficial effects on human health. Resveratrol, a main compound extracted from Polygonum cuspidatum, can prevent myocardial ischemia-reperfusion injury by inhibition of oxidative stress and ferroptosis (134). Maayah et al. (135) found that resveratrol treatment inhibited cardiac NLRP3 inflammasome activation and reduced inflammatory responses, and thus alleviated doxorubicin-induced cardiomyopathy. Another study showed that resveratrol protects against atherosclerosis by reducing TMAO levels and enhancing hepatic bile acid biosynthesis through the remodeling of intestinal flora (136). Polydatin, an active component in Polygonum cuspidatum, can ameliorate acute myocardial infarction-induced cardiac damage by inhibition of oxidative stress and cell apoptosis via activation of the Nrf2/HO-1 pathway (137). Zhang and colleagues (138) confirmed that polydatin can inhibit inflammation and pyroptosis by blocking the NLRP3/caspase-1 pathway and triggering mTOR-mediated autophagy, thereby exerting an anti-atherosclerosis effect. 2,3,4’,5-tetrahydroxystilbene 2-O-β-D-glucoside (TSG) is extracted and purified from Polygonum multiflorum, which can prevent the development and progression of atherosclerosis by reducing lipid accumulation and inflammation in ApoE-/- mice fed with HFD (139). These results suggested that stilbenes exhibited therapeutic effects on CVD via different mechanisms (Table 3).
3.3.4 Anthraquinones
Anthraquinones are compounds with unsaturated cyclic diketone structures and are widely found in some Chinese herbal medicines (140). Accumulating studies have shown that anthraquinones have various biological activities, including antitumor, antioxidant, and anti-inflammation (141), etc. Emodin (1,3,8-trihydroxy-6-methylanthraquinone), a natural anthraquinone derivative, can be extracted and purified from natural plants such as Rhei radix et rhizoma, Polygoni Cuspidat, Polygoni multiflori, which protects against various CVDs (142). Previous studies have demonstrated that emodin exhibited a therapeutic effect on atherosclerosis via inhibition of inflammatory response (143), suppression of PPAR-γ-mediated lipid metabolism (144) and endothelial cell apoptosis (145), reducing oxidative stress (146). Other studies found that emodin can prevent cardiac hypertrophy (147), restrict vasodilation by activation of K+-ATP channels (148), and inhibition of myocardial fibrosis (149). Aloe-emodin is an active ingredient in Rheum palmatum and Aloe vera, which prevents the progression of various CVDs. For example, Tang et al. (150) reported that aloe-emodin exerted an anti-atherosclerosis effect by reducing atherosclerotic plaque in the aorta and lipid accumulation and promoting endothelial autophagy. Yu et al. (151) showed that aloe-emodin inhibited the development of cardiac fibrosis and hypertrophy in rats with chronic myocardial infarction by suppressing cardiac apoptosis and oxidative stress via the inactivation of the TGF-β/Smad pathway. Another study found that aloe-emodin exhibited specific therapeutic value in hypertension-related CVD by inhibiting NLRP3 inflammasome activation (152). Moreover, other anthraquinone compounds have protective effects against CVD, which is summarized in Table 3.
3.3.5 Saponins
Saponins are a class of glycosides with triterpenoids or steranes, which are widely found in natural plants and have been reported to have many pharmacological activities, including antitumor, anti-inflammation, anti-oxidative stress, etc. Importantly, previous studies have shown that saponins were shown to be effective in treating CVD (Table 3) (153), such as atherosclerosis, myocardial infarction, myocardial ischemia-reperfusion injury, heart failure, cardiomyopathy, and hypertension. Astragaloside IV (AS-IV) is the main active ingredient purified from Astragalus membranaceus and serves as an effective therapeutic agent for the treatment of CVD (154). For example, AS-IV could markedly reduce myocardial infarction-induced myocardial fibrosis, cardiac hypertrophy, and macrophage pyroptosis by inhibition of the ROS/caspase-1/GSDMD pathway (155). Yin et al. (156) showed that AS-IV protects against myocardial ischemia-reperfusion injury by suppressing cardiomyocyte apoptosis and serum cardiac troponin levels via blocking CaSR/ERK1/2 and the related apoptotic pathways. Another study found that AS-IV treatment suppressed inflammation, plaque area, and serum lipids in HFD-induced atherosclerosis by blocking the MAPK/NF-κB pathway (157). Other studies proved that AS-IV can attenuate the progression of myocardial fibrosis (158), heart failure (159), and cardiac hypertrophy (160) by inhibiting Nrf2-mediated oxidative stress. Ginsenosides (mainly including the ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Re, Rg3, and Rh2 and compound K) serve as the main active constituents of Panax ginseng and exert protection against CVD by suppression of oxidative stress, cholesterol accumulation, inflammation, and insulin resistance (161).
3.3.6 Terpenoids
Terpenoids are a large group of organic compounds present in TCM and can be effectively used for treating various diseases. Importantly, the preventive and therapeutic effects of terpenoids on CVD have received increasing attention (Table 3), which was associated with their remarkable biological activities, such as anti-inflammation, antioxidant, and anti-apoptosis. Tanshinone IIA, a fat-soluble component of Salvia miltiorrhiza, could protect against heart failure by inhibition of cardiomyocyte apoptosis via activating the AMPK/mTOR-mediated autophagy pathway (162). Paeoniflorin, a bioactive component extracted from Paeonia lactiflora, can ameliorate ox-LDL-induced atherosclerosis by inhibiting apoptosis and adhesion molecule expression via autophagy enhancement in human umbilical vein endothelial cells (163). Andrographolide, a bioactive labdane diterpenoid extracted from Andrographis paniculate, exhibited anti-oxidative stress capacity against adverse cardiac remodeling after myocardial infarction by activating the Nrf2/HO-1 pathway (164). Artemisinin, a sesquiterpene lactone compound with peroxisome bridging group structure purified from Artemisia annua, prevented myocardial ischemia-reperfusion injury by inhibition of cardiac autophagy and NLRP3 inflammasome activation (165). Taken together, terpenoids may serve as an effective therapeutic agent for the treatment of various CVDs by different mechanisms.
3.3.7 Alkaloids
Alkaloids are a class of nitrogen-containing basic organic compounds and widely found in TCM. Of note, alkaloids exert protective effects against CVDs by suppression of inflammation, oxidative stress, and cardiomyocyte apoptosis (Table 3). Berberine, a natural isoquinoline alkaloid isolated from Rhizoma coptidis, possessed profound pharmacological activities for the treatment of various CVDs (166), including atherosclerosis, cardiac hypertrophy, heart failure, myocardial infarction, and arrhythmia. Similarly, palmatine was a potential candidate drug for the treatment of cardiac hypertrophy by activating the Nrf2/ARE pathway (167). Matrine, a quinolizidine alkaloid derived from Sophora flavescens, could attenuate diabetic cardiomyopathy by reducing inflammatory cytokines levels and oxidative stress (168). Cyclovirobuxine D, a steroidal alkaloid extracted from Buxus microphylla, exerted a cytoprotective effect against HFD diet- and streptozotocin-induced rat diabetic cardiomyopathy by activating Nrf2-mediated antioxidant responses (169). Cordycepin is an active ingredient in Cordyceps sinensis that can prevent myocardial ischemia-reperfusion injury by activating the AMPK/mTOR-mediated autophagy (170). Colchicine, a botanical alkaloid derived from Colchicum autumnale, exerted unique anti-inflammatory effects in the therapy of various CVDs (171), including atherosclerosis, heart failure, atrial fibrillation, and myocardial infarction.
3.3.8 Polysaccharides
Polysaccharides widely exist in natural plants, which are a kind of complex structure of natural polymer compounds (172). Currently, natural polysaccharides are attracting considerable attention worldwide due to their versatile biological activities and few side effects. Of note, numerous studies have shown that bioactive polysaccharides exhibit profound efficiency in controlling the risk factors of CVD (173), such as inflammatory response, oxidative stress, hypertension, and hyperlipidemia. Polysaccharides derived from Gelidium crinale reduced oxidative stress and inflammation in oxidized low-density lipoprotein-induced atherosclerosis (174). Huang et al. (175) found that the administration of polysaccharides from Eriobotrya japonica effectively reduced oxidative damage and inflammation induced by myocardial ischemia-reperfusion injury. Astragalus polysaccharides could ameliorate diabetic cardiomyopathy progression by improving cardiac function and inhibiting cardiomyocyte apoptosis via the inactivation of the ERS pathway (176). Lycium barbarum polysaccharides could reduce the levels of inflammatory cytokines (e.g., IL-6 and TNF-α) and plasma lipid peroxidation in a pressure overload-induced heart failure rat model (177). In addition, polysaccharides extracted from TCM, such as Polygonatum sibiricum, Opuntia dilleniid, Plantago asiatica, Angelica sinensis, and Ganoderma lucidum, also have therapeutic effects on various CVDs (Table 3).
3.3.9 Others
In addition to the above-mentioned compounds isolated from TCM for the prevention of CVD, other active ingredients in TCM have been reported to have therapeutic effects on various CVDs. Schisandrin B, bioactive dibenzocyclooctadiene derivatives found in Schisandra chinensis, could alleviate diabetic cardiomyopathy by reducing cardiac inflammation and damage via blocking MyD88-dependent inflammation (178). Schisandrin B prevented hypoxia/reoxygenation-induced cardiomyocyte injury by inhibiting inflammation and oxidative stress, which was associated with the activation of the AMPK/Nrf2 pathway (179). Morronisid, an iridoid glycoside extracted from Cornus officinalis, promoted angiogenesis and improved cardiac function in rats with acute myocardial infarction (180). Sulforaphane is a natural glucosinolate found in Raphanus sativus, which inhibited cardiac cell ferroptosis by activating the AMPK/Nrf2 pathway (76). Schisandrol A, a bioactive lignan extracted from Schisandra chinensis, could inhibit cardiomyocyte apoptosis induced by myocardial ischemia-reperfusion via increasing 14-3-3θ expression (181). Collectively, natural compounds from TCM exert anti-CVD effects, which may be developed as an effective therapeutic agent for the treatment of CVD in clinical.
4 Clinical study of the TCM for the prevention and treatment of CVD
Accumulating evidence has reported that TCM has a wide range of pharmacological effects in various CVDs and its beneficial efficacy has been proved in vitro cell models or animal experiments. Importantly, several clinical studies are underway to explore the safety and efficacy of TCM decoction and injections for the treatment of various CVDs. For example, several studies provided a reliable evaluation of the efficacy and safety of Xuefu Zhuyu granules (182) and Xuefu Zhuyu granules (183) in the treatment of patients with coronary heart disease. Other randomized controlled trials similarly analyzed the efficacy and safety of Zhuling decoction (184) and Buyang Huanwu decoction (185) in the treatment of heart failure. A multicenter, randomized, double-blind, placebo-controlled clinical trial found that Qing-Xin-Jie-Yu granule reduced inflammation and cardiovascular endpoint in patients with coronary heart disease (186). A phase I clinical trial by Hu et al. (187) showed that Danhong injection promoted endothelial progenitor cell mobilization by increasing the expression of Akt, eNOS, and MMP-9 in patients with coronary heart disease. Lai et al. (97) found that treatment with TCM formula (Songling Xuemaikang capsule) improved blood pressure in patients with mild hypertension and was well tolerated. Another study confirmed that astragalus injection was a safe and effective therapeutic agent in the clinical management of heart failure (188). In addition, several clinical trials have shown that the combination of TCM and standard drugs for CVD treatment was advantageous to simple conventional Western medicine in relieving clinical symptoms (25, 189). Chao et al. (190) reported that TCM formula combined with Western medicine reduced blood lipid levels and inflammatory factors in patients with coronary heart disease. Zhang et al. (191) showed that modified Xiaojianzhong decoction combined with conventional Western medicine alleviated the progression of chronic heart failure by improving heart function and maintaining gastrointestinal hormones. Another study found that treatment with Jianpi Huazhi pill combined with Western medicine (anti-heart failure) led to decreasing the levels of inflammatory cytokines and improving the composition of the gut microbiota (192). Meanwhile, several clinical studies are completed or ongoing to evaluate the safety and efficacy of TCM combined with Western medicine for the treatment of CVD according to Chinese Clinical Trial Registry (Table 4). Many researchers have proved that treatment with TCM based on the standard drug not only prevented CVD progression and improved quality of life but also reduced the incidence of adverse cardiovascular events in patients (193–195). More interestingly, TCM may be an effective alternative method to Western medicine in modern American healthcare, but some barriers prevent its integration into Western health systems, such as the fact that TCM is not accredited by the American Board of Medical Specialties, available TCM therapies may impose an undesired burden for patients, and TCM therapies are individualized. However, no cardiovascular drug or combination of drugs has shown significant efficacy in all patients with CVD, and standard Western medicine can lead to adverse side effects. From an economic point of view, TCM therapies are cheaper than Western medicine and have a better prognosis for patients with CVD. Based on the current situation, TCM may be an attractive alternative for patients with CVD.
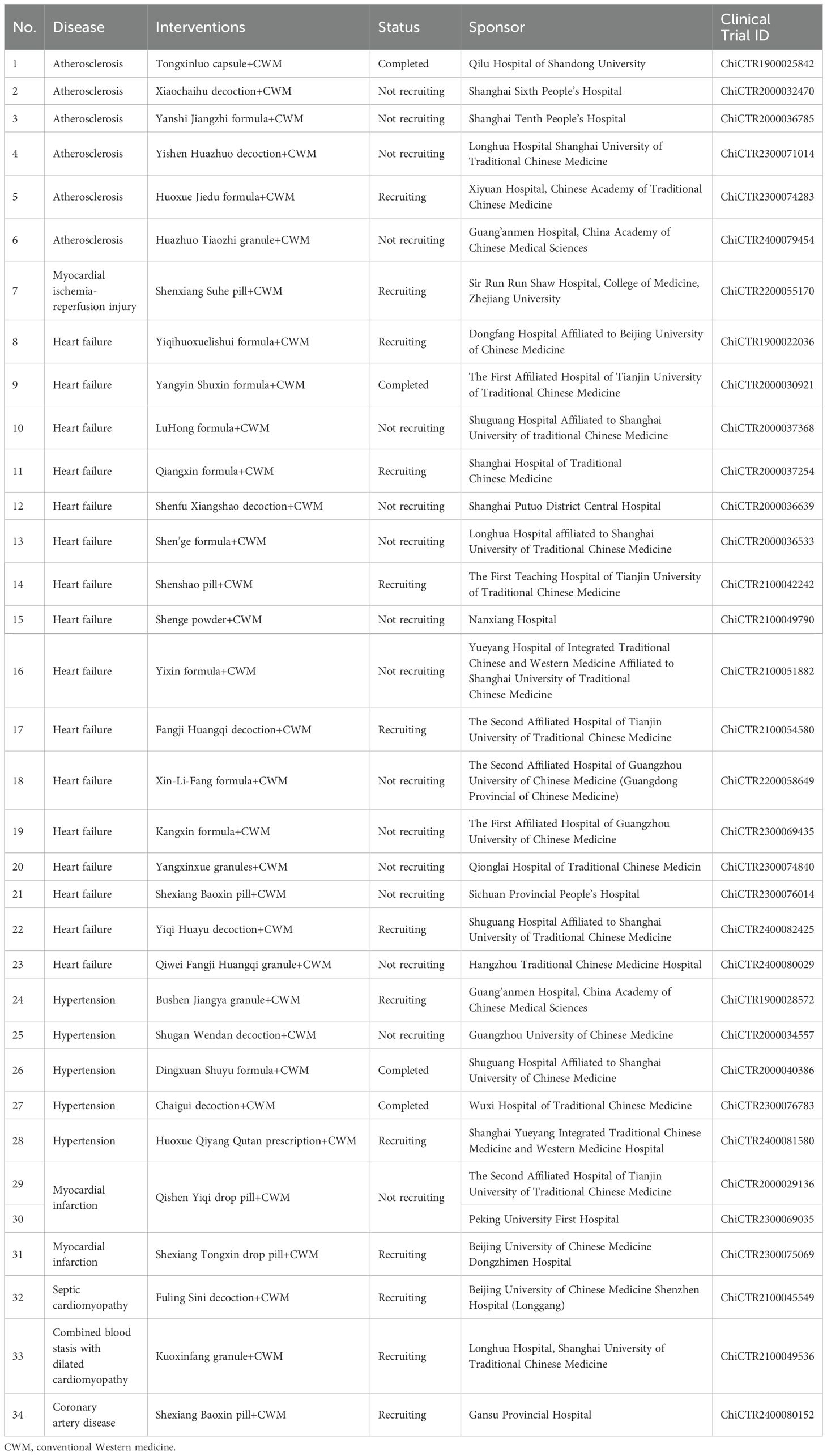
Table 4. The ongoing clinical trials of traditional Chinese medicine combined with Western medicine for cardiovascular diseases therapy from 2018-2023.
5 Conclusion and prospects
As the leading cause of death after malignant tumors, CVD is difficult to treat clinically and imposes a huge economic and health burden on people worldwide. The morbidity and mortality of CVD are continuously increasing, and the treatment is ineffective because of its complex pathogenesis. In recent years, TCM has been particularly prominent in the treatment of 95 certain diseases, including CVD, offering a new perspective in the modern era for the prevention and treatment of diseases such as COVID-19. In this review, we found that TCM (formulas, extracts, and compounds) can combat CVD through multiple mechanisms, including anti-inflammatory, antioxidant, improving mitochondrial dysfunction, anti-cell death (such as autophagy, apoptosis, ferroptosis, pyroptosis), and regulating gut microbiota. Meanwhile, clinical trials have proven the efficacy and safety of TCM in alleviating the symptoms of CVD. However, there are still some challenges that must be overcome in TCM for CVD treatment. (1) With the rapid advancement of science, there is a need to utilize network pharmacology approaches and multi-omics technologies, such as nutrigenomics, metabolomics, proteomics, gut microbial macrogenomics and immunomics, to reveal the physiological functions and mechanism explanations of TCM in combating CVD; (2) The metabolic, toxicity, and pharmacokinetic profiles of TCM fight against patients with CVD in clinical trials need to be further validated; (3) The construction of TCM resources for common quality standards to ensure active ingredient in TCM; (4) Research on active ingredients of TCM is limited by defects includes unstable chemical structure, low bioavailability and easy oxidation, and liposome embedding or nanoparticle formulation can be considered; (5) Development of CVD models with human disease characteristics for exploring the pharmacological activity of TCM, such as primate animal models that can avoid species barriers leading to ineffectiveness; (6) Designing TCM delivery systems to improve its stability, bioavailability, and efficacy in the gastrointestinal tract.
In conclusion, TCM possesses good anti-CVD effects and is an indispensable active drug for the treatment of CVD. Based on the latest evidence, this review summarized the pathogenesis of CVD and systematically analyzed and discussed the mechanisms of TCM in preventing CVD, as well as its clinical trials. This review aims to provide a scientific and effective comprehensive reference for TCM in CVD therapy and to better utilize and develop the treasures of TCM.
Author contributions
JD: Conceptualization, Investigation, Writing – original draft. LQ: Investigation, Writing – original draft. YL: Writing – review & editing. ML: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Provincial Doctoral Research Initiation Fund (NO: 2022-BS-249) and the Natural Science Foundation of Liaoning Province (No.2022-MS-325).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Petersen KS, Kris-Etherton PM. Diet quality assessment and the relationship between diet quality and cardiovascular disease risk. Nutrients. (2021) 13:4305. doi: 10.3390/nu13124305
2. Liu S, Li Y, Zeng X, Wang H, Yin P, Wang L, et al. Burden of cardiovascular diseases in China, 1990-2016: findings from the 2016 global burden of disease study. JAMA Cardiol. (2019) 4:342–52. doi: 10.1001/jamacardio.2019.0295
3. Mamani-Ortiz Y, San Sebastián M, Armaza AX, Luizaga JM, Illanes DE, Ferrel M, et al. Prevalence and determinants of cardiovascular disease risk factors using the WHO STEPS approach in Cochabamba, Bolivia. BMC Public Health. (2019) 19:786. doi: 10.1186/s12889-019-7064-y
4. Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. (2015) 372:1333–41. doi: 10.1056/NEJMoa1406656
5. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. (2016) 387:251–72. doi: 10.1016/S0140-6736(15)00551-6
6. Siasos G, Bletsa E, Stampouloglou PK, Oikonomou E, Tsigkou V, Paschou SA, et al. MicroRNAs in cardiovascular disease. Hellenic J Cardiol. (2020) 61:165–73. doi: 10.1016/j.hjc.2020.03.003
7. Ciumărnean L, Milaciu MV, Negrean V, Orășan OH, Vesa SC, Sălăgean O, et al. Cardiovascular risk factors and physical activity for the prevention of cardiovascular diseases in the elderly. Int J Environ Res Public Health. (2021) 19:207. doi: 10.3390/ijerph19010207
8. Rosenthal T, Touyz RM, Oparil S. Migrating populations and health: risk factors for cardiovascular disease and metabolic syndrome. Curr Hypertens Rep. (2022) 24:325–40. doi: 10.1007/s11906-022-01194-5
9. Gyldenkerne C, Mortensen MB, Kahlert J, Thrane PG, Warnakula Olesen KK, Sørensen HT, et al. 10-year cardiovascular risk in patients with newly diagnosed type 2 diabetes mellitus. J Am Coll Cardiol. (2023) 82:1583–94. doi: 10.1016/j.jacc.2023.08.015
10. Millwood IY, Im PK, Bennett D, Hariri P, Yang L, Du H, et al. Alcohol intake and cause-specific mortality: conventional and genetic evidence in a prospective cohort study of 512 000 adults in China. Lancet Public Health. (2023) 8:e956–67. doi: 10.1016/S2468-2667(23)00217-7
11. Ouyang L, Su X, Li W, Tang L, Zhang M, Zhu Y, et al. ALKBH1-demethylated DNA N6-methyladenine modification triggers vascular calcification via osteogenic reprogramming in chronic kidney disease. J Clin Invest. (2021) 131::e146985. doi: 10.1172/JCI146985
12. An J, Ouyang L, Yu C, Carr SM, Ramprasath T, Liu Z, et al. Nicotine exacerbates atherosclerosis and plaque instability via NLRP3 inflammasome activation in vascular smooth muscle cells. Theranostics. (2023) 13:2825–42. doi: 10.7150/thno.81388
13. Hu X, Nie Z, Ou Y, Lin L, Qian Z, Vaughn MG, et al. Long-term exposure to ambient air pollution, circadian syndrome and cardiovascular disease: A nationwide study in China. Sci Total Environ. (2023) 868:161696. doi: 10.1016/j.scitotenv.2023.161696
14. Huang H, Li Z, Ruan Y, Feng W, Chen J, Li X, et al. Circadian rhythm disorder: a potential inducer of vascular calcification? J Physiol Biochem. (2020) 76:513–24. doi: 10.1007/s13105-020-00767-9
15. Papaconstantinou J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells. (2019) 8:1383. doi: 10.3390/cells8111383
16. Chen F, Yin S, Feng Z, Liu C, Lv J, Chen Y, et al. Knockdown of circ_NEK6 decreased 131I resistance of differentiated thyroid carcinoma via regulating miR-370-3p/MYH9 axis. Technol Cancer Res Treat. (2021) 20:15330338211004950. doi: 10.1177/15330338211004950
17. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. (2020) 127:553–70. doi: 10.1161/CIRCRESAHA.120.316242
18. Song XY, Li YD, Shi YP, Jin L, Chen J. Quality control of traditional Chinese medicines: a review. Chin J Nat Med. (2013) 11:596–607. doi: 10.3724/SP.J.1009.2013.00596
19. Wang X, Wang ZY, Zheng JH, Li S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. (2021) 19:1–11. doi: 10.1016/S1875-5364(21)60001-8
20. Luo Y, Wang CZ, Hesse-Fong J, Lin JG, Yuan CS. Application of Chinese medicine in acute and critical medical conditions. Am J Chin Med. (2019) 47:1223–35. doi: 10.1142/S0192415X19500629
21. Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. (2017) 69:2952–66. doi: 10.1016/j.jacc.2017.04.041
22. Li X, Li L, Lei W, Chua HZ, Li Z, Huang X, et al. Traditional Chinese medicine as a therapeutic option for cardiac fibrosis: Pharmacology and mechanisms. BioMed Pharmacother. (2021) 142:111979. doi: 10.1016/j.biopha.2021.111979
23. Jia Q, Wang L, Zhang X, Ding Y, Li H, Yang Y, et al. Prevention and treatment of chronic heart failure through traditional Chinese medicine: Role of the gut microbiota. Pharmacol Res. (2020) 151:104552. doi: 10.1016/j.phrs.2019.104552
24. Pan L, Zhang XF, Wei WS, Zhang J, Li ZZ. The cardiovascular protective effect and mechanism of calycosin and its derivatives. Chin J Nat Med. (2020) 18:907–15. doi: 10.1016/S1875-5364(20)60034-6
25. Liu J, Dong Y, Hu X. Efficacy of Yangxin recipe in combination with conventional Western medicine in treatment of angina pectoris of coronary heart disease. Clin Appl Thromb Hemost. (2022) 28:10760296221076152. doi: 10.1177/10760296221076152
26. Lu Y, Wang F, Ni H, Sun Y, Shi H. Observation of curative effect of trimetazidine combined with metoprolol in elderly patients with coronary heart disease complicated with heart failure and the effect of myocardial remodeling by integrated traditional Chinese and Western medicine. BioMed Res Int. (2022) 2022:6098799. doi: 10.1155/2022/6098799
27. Guo R, Luo X, Liu J, Liu L, Wang X, Lu H. Omics strategies decipher therapeutic discoveries of traditional Chinese medicine against different diseases at multiple layers molecular-level. Pharmacol Res. (2020) 152:104627. doi: 10.1016/j.phrs.2020.104627
28. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. (2018) 15:230–40. doi: 10.1038/nrcardio.2017.154
29. Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the uk biobank study. JAMA Cardiol. (2018) 3:693–702. doi: 10.1001/jamacardio.2018.1717
30. Lin X, Ouyang S, Zhi C, Li P, Tan X, Ma W, et al. Focus on ferroptosis, pyroptosis, apoptosis and autophagy of vascular endothelial cells to the strategic targets for the treatment of atherosclerosis. Arch Biochem Biophys. (2022) 715:109098. doi: 10.1016/j.abb.2021.109098
31. Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. (2018) 214:153–7. doi: 10.1016/j.lfs.2018.10.063
32. Akhmerov A, Parimon T. Extracellular vesicles, inflammation, and cardiovascular disease. Cells. (2022) 11:2229. doi: 10.3390/cells11142229
33. Fan Y, Liu J, Miao J, Zhang X, Yan Y, Bai L, et al. Anti-inflammatory activity of the Tongmai Yangxin pill in the treatment of coronary heart disease is associated with estrogen receptor and NF-κB signaling pathway. J Ethnopharmacol. (2021) 276:114106. doi: 10.1016/j.jep.2021.114106
34. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. (2019) 139:1289–99. doi: 10.1161/CIRCULATIONAHA.118.038010
35. Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. (2011) 27:174–82. doi: 10.1016/j.cjca.2010.12.040
36. Holzknecht M, Tiller C, Reindl M, Lechner I, Troger F, Hosp M, et al. C-reactive protein velocity predicts microvascular pathology after acute ST-elevation myocardial infarction. Int J Cardiol. (2021) 338:30–6. doi: 10.1016/j.ijcard.2021.06.023
37. Ayas NT, Hirsch Allen AJ, Fox N, Peres B, Mehrtash M, Humphries KH, et al. C-reactive protein levels and the risk of incident cardiovascular and cerebrovascular events in patients with obstructive sleep apnea. Lung. (2019) 197:459–64. doi: 10.1007/s00408-019-00237-0
38. Henein MY, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. (2022) 23:12906. doi: 10.3390/ijms232112906
39. Mai W, Liao Y. Targeting IL-1β in the treatment of atherosclerosis. Front Immunol. (2020) 11:589654. doi: 10.3389/fimmu.2020.589654
40. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. (2015) 12:230–43. doi: 10.1038/nrcardio.2015.2
41. Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X, et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. (2020) 34:101523. doi: 10.1016/j.redox.2020.101523
42. Willeford A, Suetomi T, Nickle A, Hoffman HM, Miyamoto S, Heller Brown J. CaMKIIδ-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight. (2018) 3:e97054. doi: 10.1172/jci.insight.97054
43. Nguyen MN, Kiriazis H, Gao XM, Du XJ. Cardiac fibrosis and arrhythmogenesis. Compr Physiol. (2017) 7:1009–49. 10.1002/cphy.c160046
44. Li X, Zhang Z, Luo M, Cheng Z, Wang R, Liu Q, et al. NLRP3 inflammasome contributes to endothelial dysfunction in angiotensin II-induced hypertension in mice. Microvasc Res. (2022) 143:104384. doi: 10.1016/j.mvr.2022.104384
45. Lorenzon Dos Santos J, Quadros AS, Weschenfelder C, Garofallo SB, Marcadenti A. Oxidative stress biomarkers, nut-related antioxidants, and cardiovascular disease. Nutrients. (2020) 12:682. doi: 10.3390/nu12030682
46. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. (2002) 82:47–95. doi: 10.1152/physrev.00018.2001
47. Xiang D, Liu Y, Zhou S, Zhou E, Wang Y. Protective effects of estrogen on cardiovascular disease mediated by oxidative stress. Oxid Med Cell Longev. (2021) 2021:5523516. doi: 10.1155/2021/5523516
48. Chang X, Zhang T, Zhang W, Zhao Z, Sun J. Natural drugs as a treatment strategy for cardiovascular disease through the regulation of oxidative stress. Oxid Med Cell Longev. (2020) 2020:5430407. doi: 10.1155/2020/5430407
49. Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. (2017) 120:713–35. doi: 10.1161/CIRCRESAHA.116.309326
50. Pignatelli P, Menichelli D, Pastori D, Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. (2018) 76:713–22. doi: 10.5603/KP.a2018.0071
51. Guo Z, Ran Q, Roberts LJ 2nd, Zhou L, Richardson A, Sharan C, et al. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free Radic Biol Med. (2008) 44:343–52. doi: 10.1016/j.freeradbiomed.2007.09.009
52. Giam B, Chu PY, Kuruppu S, Smith AI, Horlock D, Kiriazis H, et al. N-acetylcysteine attenuates the development of cardiac fibrosis and remodeling in a mouse model of heart failure. Physiol Rep. (2016) 4:e12757. doi: 10.14814/phy2.12757
53. Cai S, Zhao M, Zhou B, Yoshii A, Bugg D, Villet O, et al. Mitochondrial dysfunction in macrophages promotes inflammation and suppresses repair after myocardial infarction. J Clin Invest. (2023) 133:e159498. doi: 10.1172/JCI159498
54. Chistiakov DA, Shkurat TP, Melnichenko AA, Grechko AV, Orekhov AN. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med. (2018) 50:121–7. doi: 10.1080/07853890.2017.1417631
55. SoBenin IA, Sazonova MA, Postnov AY, Bobryshev YV, Orekhov AN. Changes of mitochondria in atherosclerosis: possible determinant in the pathogenesis of the disease. Atherosclerosis. (2013) 227:283–8. doi: 10.1016/j.atherosclerosis.2013.01.006
56. Manolis AS, Manolis AA, Manolis TA, Apostolaki NE, Apostolopoulos EJ, Melita H, et al. Mitochondrial dysfunction in cardiovascular disease: Current status of translational research/clinical and therapeutic implications. Med Res Rev. (2021) 41:275–313. doi: 10.1002/med.21732
57. Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. (2019) 51:1–13. doi: 10.1038/s12276-019-0355-7
58. Jacinto TA, Meireles GS, Dias AT, Aires R, Porto ML, Gava AL, et al. Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol Res. (2018) 51:33. doi: 10.1186/s40659-018-0182-7
59. Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, et al. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res. (2019) 124:1360–71. doi: 10.1161/CIRCRESAHA.118.314607
60. Lee TL, Lee MH, Chen YC, Lee YC, Lai TC, Lin HY, et al. Vitamin D attenuates ischemia/reperfusion-induced cardiac injury by reducing mitochondrial fission and mitophagy. Front Pharmacol. (2020) 11:604700. doi: 10.3389/fphar.2020.604700
61. Zekonyte U, Bacman SR, Moraes CT. DNA-editing enzymes as potential treatments for heteroplasmic mtDNA diseases. J Intern Med. (2020) 287:685–97. doi: 10.1111/joim.v287.6
62. Bagul PK, Katare PB, Bugga P, Dinda AK, Banerjee SK. SIRT-3 modulation by resveratrol improves mitochondrial oxidative phosphorylation in diabetic heart through deacetylation of TFAM. Cells. (2018) 7:235. doi: 10.3390/cells7120235
63. Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, et al. Alternative mitophagy protects the heart against obesity-associated cardiomyopathy. Circ Res. (2021) 129:1105–21. doi: 10.1161/CIRCRESAHA.121.319377
64. Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. (2017) 547:99–103. doi: 10.1038/nature22393
65. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. (2017) 42:245–54. doi: 10.1016/j.tibs.2016.10.004
66. Zeng C, Wang R, Tan H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int J Biol Sci. (2019) 15:1345–57. doi: 10.7150/ijbs.33568
67. Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. (2021) 18:2114–27. doi: 10.1038/s41423-021-00740-6
68. Wang M, Zhao M, Yu J, Xu Y, Zhang J, Liu J, et al. MCC950, a selective NLRP3 inhibitor, attenuates adverse cardiac remodeling following heart failure through improving the cardiometabolic dysfunction in obese mice. Front Cardiovasc Med. (2022) 9:727474. doi: 10.3389/fcvm.2022.727474
69. Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res. (2019) 115:776–87. doi: 10.1093/cvr/cvy252
70. Sharma A, Choi JSY, Stefanovic N, Al-Sharea A, Simpson DS, Mukhamedova N, et al. Specific NLRP3 inhibition protects against diabetes-associated atherosclerosis. Diabetes. (2021) 70:772–87. doi: 10.2337/db20-0357
71. Zhang L, Jiang YH, Fan C, Zhang Q, Jiang YH, Li Y, et al. MCC950 attenuates doxorubicin-induced myocardial injury in vivo and in vitro by inhibiting NLRP3-mediated pyroptosis. BioMed Pharmacother. (2021) 143:112133. doi: 10.1016/j.biopha.2021.112133
72. Jin Y, Liu Y, Xu L, Xu J, Xiong Y, Peng Y, et al. Novel role for caspase 1 inhibitor VX765 in suppressing NLRP3 inflammasome assembly and atherosclerosis via promoting mitophagy and efferocytosis. Cell Death Dis. (2022) 13:512. doi: 10.1038/s41419-022-04966-8
73. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. (2021) 31:107–25. doi: 10.1038/s41422-020-00441-1
74. Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther. (2021) 6:49. doi: 10.1038/s41392-020-00428-9
75. Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. (2021) 11:3052–9. doi: 10.7150/thno.54113
76. Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. (2022) 12:708–22. doi: 10.1016/j.apsb.2021.10.005
77. Bai T, Li M, Liu Y, Qiao Z, Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. (2020) 160:92–102. doi: 10.1016/j.freeradbiomed.2020.07.026
78. Wang Y, Yan S, Liu X, Deng F, Wang P, Yang L, et al. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ. (2022) 29:1982–95. doi: 10.1038/s41418-022-00990-5
79. Pennell DJ, Udelson JE, Arai AE, Bozkurt B, Cohen AR, Galanello R, et al. Cardiovascular function and treatment in β-thalassemia major: a consensus statement from the American Heart Association. Circulation. (2013) 128:281–308. doi: 10.1161/CIR.0b013e31829b2be6
80. Khan S, Moore RJ, Stanley D, Chousalkar KK. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl Environ Microbiol. (2020) 86:e00600–20. doi: 10.1128/AEM.00600-20
81. Qian B, Zhang K, Li Y, Sun K. Update on gut microbiota in cardiovascular diseases. Front Cell Infect Microbiol. (2022) 12:1059349. doi: 10.3389/fcimb.2022.1059349
82. Sanchez-Rodriguez E, Egea-Zorrilla A, Plaza-Díaz J, Aragón-Vela J, Muñoz-Quezada S, Tercedor-Sánchez L, et al. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients. (2020) 12:605. doi: 10.3390/nu12030605
83. Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. (2017) 8:845. doi: 10.1038/s41467-017-00900-1
84. Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol. (2018) 71:1184–6. doi: 10.1016/j.jacc.2017.12.057
85. Sun T, Zhang Y, Yin J, Peng X, Zhou L, Huang S, et al. Association of gut microbiota-dependent metabolite trimethylamine N-oxide with first ischemic stroke. J Atheroscler Thromb. (2021) 28:320–8. doi: 10.5551/jat.55962
86. Jiang S, Shui Y, Cui Y, Tang C, Wang X, Qiu X, et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. (2021) 46:102115. doi: 10.1016/j.redox.2021.102115
87. Xiao L, Huang L, Zhou X, Zhao D, Wang Y, Min H, et al. Experimental periodontitis deteriorated atherosclerosis associated with trimethylamine N-oxide metabolism in mice. Front Cell Infect Microbiol. (2021) 11:820535. doi: 10.3389/fcimb.2021.820535
88. Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, et al. Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol. (2018) 38:2225–35. doi: 10.1161/ATVBAHA.118.311023
89. Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol. (2021) 18:499–521. doi: 10.1038/s41569-021-00511-w
90. Zhao F, Satyanarayana G, Zhang Z, Zhao J, Ma XL, Wang Y. Endothelial autophagy in coronary microvascular dysfunction and cardiovascular disease. Cells. (2022) 11:2081. doi: 10.3390/cells11132081
91. Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep. (2019) 21:21. doi: 10.1007/s11886-019-1107-y
92. Silveira Rossi JL, Barbalho SM, Reverete de Araujo R, Bechara MD, Sloan KP, Sloan LA. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab Res Rev. (2022) 38:e3502. doi: 10.1002/dmrr.3502
93. Cheng X, Hu J, Liu X, Tibenda JJ, Wang X, Zhao Q. Therapeutic targets by traditional Chinese medicine for ischemia-reperfusion injury induced apoptosis on cardiovascular and cerebrovascular diseases. Front Pharmacol. (2022) 13:934256. doi: 10.3389/fphar.2022.934256
94. Gao L, Cao M, Li JQ, Qin XM, Fang J. Traditional Chinese medicine network pharmacology in cardiovascular precision medicine. Curr Pharm Des. (2021) 27:2925–33. doi: 10.2174/1381612826666201112142408
95. Liu LC, Mao QY, Liu C, Hu J, Duan L, Wang J. The effectiveness and safety of Bushen Huoxue decoction on treating coronary heart disease: a meta-analysis. Evid Based Complement Alternat Med. (2021) 2021:5541228. doi: 10.1155/2021/5541228
96. Bi YF, Wang XL, Zhang X, Hou YZ, Zhao ZQ, Ren XY, et al. Protocol to study the effects of traditional Chinese medicine on patients with coronary heart disease showing phlegm-heat-stasis symptom pattern. J Tradit Chin Med. (2021) 41:826–32. doi: 10.19852/j.cnki.jtcm.2021.05.016
97. Lai X, Dong Z, Wu S, Zhou X, Zhang G, Xiong S, et al. Efficacy and safety of Chinese herbal medicine compared with losartan for mild essential hypertension: a randomized, multicenter, double-blind, noninferiority trial. Circ Cardiovasc Qual Outcomes. (2022) 15:e007923. doi: 10.1161/CIRCOUTCOMES.121.007923
98. Song JX, Zhao YS, Zhen YQ, Yang XY, Chen Q, An JR, et al. Banxia-Houpu decoction diminishes iron toxicity damage in heart induced by chronic intermittent hypoxia. Pharm Biol. (2022) 60:609–20. doi: 10.1080/13880209.2022.2043392
99. Li Y, Zhang L, Ren P, Yang Y, Li S, Qin X, et al. Qing-Xue-Xiao-Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-κB pathway regulation. Phytomedicine. (2021) 93:153812. doi: 10.1016/j.phymed.2021.153812
100. Wu L, Fan Z, Gu L, Liu J, Cui Z, Yu B, et al. QiShenYiQi dripping pill alleviates myocardial ischemia-induced ferroptosis via improving mitochondrial dynamical homeostasis and biogenesis. J Ethnopharmacol. (2023) 308:116282. doi: 10.1016/j.jep.2023.116282
101. Chen X, Li Y, Li J, Liu T, Jiang Q, Hong Y, et al. Qishen granule (QSG) exerts cardioprotective effects by inhibiting NLRP3 inflammasome and pyroptosis in myocardial infarction rats. J Ethnopharmacol. (2022) 285:114841. doi: 10.1016/j.jep.2021.114841
102. Wang A, Guan B, Shao C, Zhao L, Li Q, Hao H, et al. Qing-Xin-Jie-Yu Granule alleviates atherosclerosis by reshaping gut microbiota and metabolic homeostasis of ApoE-/- mice. Phytomedicine. (2022) 103:154220. doi: 10.1016/j.phymed.2022.154220
103. Zhou W, Chen Z, Fang Z, Xu D. Network analysis for elucidating the mechanisms of Shenfu injection in preventing and treating COVID-19 combined with heart failure. Comput Biol Med. (2022) 148:105845. doi: 10.1016/j.compbiomed.2022.105845
104. Duan H, Khan GJ, Shang LJ, Peng H, Hu WC, Zhang JY, et al. Computational pharmacology and bioinformatics to explore the potential mechanism of Schisandra against atherosclerosis. Food Chem Toxicol. (2021) 150:112058. doi: 10.1016/j.fct.2021.112058
105. Abulizi A, Simayi J, Nuermaimaiti M, Han M, Hailati S, Talihati Z, et al. Quince extract resists atherosclerosis in rats by down-regulating the EGFR/PI3K/Akt/GSK-3β pathway. BioMed Pharmacother. (2023) 160:114330. doi: 10.1016/j.biopha.2023.114330
106. Fan W, Zhang B, Wu C, Wu H, Wu J, Wu S, et al. Plantago asiatica L. seeds extract protects against cardiomyocyte injury in isoproterenol- induced cardiac hypertrophy by inhibiting excessive autophagy and apoptosis in mice. Phytomedicine. (2021) 91:153681. doi: 10.1016/j.phymed.2021.153681
107. Peng L, Lei Z, Rao Z, Yang R, Zheng L, Fan Y, et al. Cardioprotective activity of ethyl acetate extract of Cinnamomi Ramulus against myocardial ischemia/reperfusion injury in rats via inhibiting NLRP3 inflammasome activation and pyroptosis. Phytomedicine. (2021) 93:153798. doi: 10.1016/j.phymed.2021.153798
108. Wen J, Zou W, Wang R, Liu H, Yang Y, Li H, et al. Cardioprotective effects of Aconiti Lateralis Radix Praeparata combined with Zingiberis Rhizoma on doxorubicin-induced chronic heart failure in rats and potential mechanisms. J Ethnopharmacol. (2019) 238:111880. doi: 10.1016/j.jep.2019.111880
109. Hasan R, Lindarto D, Siregar GA, Mukhtar Z. The effect of bay leaf extract Syzygium polyanthum (Wight) Walp. on C-reactive protein (CRP) and myeloperoxidase (MPO) level in the heart of rat model of myocardial infarction. Med Glas (Zenica). (2020) 17:41–5. doi: 10.17392/1068-20
110. Liu S, He F, Zheng T, Wan S, Chen J, Yang F, et al. Ligustrum robustum alleviates atherosclerosis by decreasing serum TMAO, modulating gut microbiota, and decreasing bile acid and cholesterol absorption in mice. Mol Nutr Food Res. (2021) 65:e2100014. doi: 10.1002/mnfr.202100014
111. Vilella R, Sgarbi G, Naponelli V, Savi M, Bocchi L, Liuzzi F, et al. Effects of standardized green tea extract and its main component, EGCG, on mitochondrial function and contractile performance of healthy rat cardiomyocytes. Nutrients. (2020) 12:2949. doi: 10.3390/nu12102949
112. Tian J, Popal MS, Liu Y, Gao R, Lyu S, Chen K, et al. Ginkgo biloba leaf extract attenuates atherosclerosis in streptozotocin-induced diabetic ApoE-/- mice by inhibiting endoplasmic reticulum stress via restoration of autophagy through the mTOR signaling pathway. Oxid Med Cell Longev. (2019) 2019:8134678. doi: 10.1155/2019/8134678
113. Granado M, González-Hedström D, Amor S, Fajardo-Vidal A, Villalva M, de la Fuente-Fernández M, et al. Marjoram extract prevents ischemia reperfusion-induced myocardial damage and exerts anti-contractile effects in aorta segments of male wistar rats. J Ethnopharmacol. (2022) 282:114660. doi: 10.1016/j.jep.2021.114660
114. Tinikul R, Chenprakhon P, Maenpuen S, Chaiyen P. Biotransformation of plant-derived phenolic acids. Biotechnol J. (2018) 13:e1700632. doi: 10.1002/biot.201700632
115. Reboredo-Rodríguez P, Varela-López A, Forbes-Hernández TY, Gasparrini M, Afrin S, Cianciosi D, et al. Phenolic compounds isolated from olive oil as nutraceutical tools for the prevention and management of cancer and cardiovascular diseases. Int J Mol Sci. (2018) 19:2305. doi: 10.3390/ijms19082305
116. Panda V, Laddha A, Nandave M, Srinath S. Dietary phenolic acids of Macrotyloma uniflorum (Horse Gram) protect the rat heart against isoproterenol-induced myocardial infarction. Phytother Res. (2016) 30:1146–55. doi: 10.1002/ptr.v30.7
117. Yao X, Jiao S, Qin M, Hu W, Yi B, Liu D. Vanillic acid alleviates acute myocardial hypoxia/reoxygenation injury by inhibiting oxidative stress. Oxid Med Cell Longev. (2020) 2020:8348035. doi: 10.1155/2020/8348035
118. Luan F, Rao Z, Peng L, Lei Z, Zeng J, Peng X, et al. Cinnamic acid preserves against myocardial ischemia/reperfusion injury via suppression of NLRP3/Caspase-1/GSDMD signaling pathway. Phytomedicine. (2022) 100:154047. doi: 10.1016/j.phymed.2022.154047
119. Shen Y, Shen X, Wang S, Zhang Y, Wang Y, Ding Y, et al. Protective effects of Salvianolic acid B on rat ferroptosis in myocardial infarction through upregulating the Nrf2 signaling pathway. Int Immunopharmacol. (2022) 112:109257. doi: 10.1016/j.intimp.2022.109257
120. Gu Y, Zhang Y, Li M, Huang Z, Jiang J, Chen Y, et al. Ferulic acid ameliorates atherosclerotic injury by modulating gut microbiota and lipid metabolism. Front Pharmacol. (2021) 12:621339. doi: 10.3389/fphar.2021.621339
121. Luo Y, Jian Y, Liu Y, Jiang S, Muhammad D, Wang W. Flavanols from nature: a phytochemistry and biological activity review. Molecules. (2022) 27:719. doi: 10.3390/molecules27030719
122. Parmenter BH, Croft KD, Hodgson JM, Dalgaard F, Bondonno CP, Lewis JR, et al. An overview and update on the epidemiology of flavonoid intake and cardiovascular disease risk. Food Funct. (2020) 11:6777–806. doi: 10.1039/D0FO01118E
123. Shi X, Hu Y, Jiang Y, Wu J, Zhang C, Zhang J, et al. Scutellarein protects against cardiac hypertrophy via suppressing TRAF2/NF-κB signaling pathway. Mol Biol Rep. (2022) 49:2085–95. doi: 10.1007/s11033-021-07026-0
124. Xu L, Chen R, Zhang X, Zhu Y, Ma X, Sun G, et al. Scutellarin protects against diabetic cardiomyopathy via inhibiting oxidative stress and inflammatory response in mice. Ann Palliat Med. (2021) 10:2481–93. doi: 10.21037/apm-19-516
125. Fu Y, Sun S, Sun H, Peng J, Ma X, Bao L, et al. Scutellarin exerts protective effects against atherosclerosis in rats by regulating the Hippo-FOXO3A and PI3K/AKT signaling pathways. J Cell Physiol. (2019) 234:18131–45. doi: 10.1002/jcp.v234.10
126. Xu LJ, Chen RC, Ma XY, Zhu Y, Sun GB, Sun XB. Scutellarin protects against myocardial ischemia-reperfusion injury by suppressing NLRP3 inflammasome activation. Phytomedicine. (2020) 68:153169. doi: 10.1016/j.phymed.2020.153169
127. Huang H, Geng Q, Yao H, Shen Z, Wu Z, Miao X, et al. Protective effect of scutellarin on myocardial infarction induced by isoprenaline in rats. Iran J Basic Med Sci. (2018) 21:267–76. doi: 10.22038/ijbms.2018.26110.6415
128. Zhang X, Qin Y, Ruan W, Wan X, Lv C, He L, et al. Targeting inflammation-associated AMPK//Mfn-2/MAPKs signaling pathways by baicalein exerts anti-atherosclerotic action. Phytother Res. (2021) 35:4442–55. doi: 10.1002/ptr.v35.8
129. Bei W, Jing L, Chen N. Cardio protective role of wogonin loaded nanoparticle against isoproterenol induced myocardial infarction by moderating oxidative stress and inflammation. Colloids Surf B Biointerfaces. (2020) 185:110635. doi: 10.1016/j.colsurfb.2019.110635
130. Xu S, Wu B, Zhong B, Lin L, Ding Y, Jin X, et al. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) /System xc-/ glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered. (2021) 12:10924–34. doi: 10.1080/21655979.2021.1995994
131. Liu H, Zhao H, Che J, Yao W. Naringenin protects against hypertension by regulating lipid disorder and oxidative stress in a rat model. Kidney Blood Press Res. (2022) 47:423–32. doi: 10.1159/000524172
132. Abukhalil MH, Althunibat OY, Aladaileh SH, Al-Amarat W, Obeidat HM, Al-Khawalde AAA, et al. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. BioMed Pharmacother. (2021) 138:111410. doi: 10.1016/j.biopha.2021.111410
133. Chen X, Wan W, Guo Y, Ye T, Fo Y, Sun Y, et al. Pinocembrin ameliorates post-infarct heart failure through activation of Nrf2/HO-1 signaling pathway. Mol Med. (2021) 27:100. doi: 10.1186/s10020-021-00363-7
134. Li T, Tan Y, Ouyang S, He J, Liu L. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene. (2022) 808:145968. doi: 10.1016/j.gene.2021.145968
135. Maayah ZH, Alam AS, Takahara S, Soni S, Ferdaoussi M, Matsumura N, et al. Resveratrol reduces cardiac NLRP3-inflammasome activation and systemic inflammation to lessen doxorubicin-induced cardiotoxicity in juvenile mice. FEBS Lett. (2021) 595:1681–95. doi: 10.1002/1873-3468.14091
136. Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio. (2016) 7:e02210–15. doi: 10.1128/mBio.02210-15
137. Chen G, Liu G, Cao D, Jin M, Guo D, Yuan X. Polydatin protects against acute myocardial infarction-induced cardiac damage by activation of Nrf2/HO-1 signaling. J Nat Med. (2019) 73:85–92. doi: 10.1007/s11418-018-1241-7
138. Zhang X, Wang Z, Li X, Chen J, Yu Z, Li X, et al. Polydatin protects against atherosclerosis by activating autophagy and inhibiting pyroptosis mediated by the NLRP3 inflammasome. J Ethnopharmacol. (2023) 309:116304. doi: 10.1016/j.jep.2023.116304
139. Li F, Zhang T, He Y, Gu W, Yang X, Zhao R, et al. Inflammation inhibition and gut microbiota regulation by TSG to combat atherosclerosis in ApoE-/- mice. J Ethnopharmacol. (2020) 247:112232. doi: 10.1016/j.jep.2019.112232
140. Wang D, Wang XH, Yu X, Cao F, Cai X, Chen P, et al. Pharmacokinetics of anthraquinones from medicinal plants. Front Pharmacol. (2021) 12:638993. doi: 10.3389/fphar.2021.638993
141. Cui Y, Chen LJ, Huang T, Ying JQ, Li J. The pharmacology, toxicology and therapeutic potential of anthraquinone derivative emodin. Chin J Nat Med. (2020) 18:425–35. doi: 10.1016/S1875-5364(20)30050-9
142. Wang X, Yang S, Li Y, Jin X, Lu J, Wu M. Role of emodin in atherosclerosis and other cardiovascular diseases: Pharmacological effects, mechanisms, and potential therapeutic target as a phytochemical. BioMed Pharmacother. (2023) 161:114539. doi: 10.1016/j.biopha.2023.114539
143. Gao Q, Wang F, Guo S, Li J, Zhu B, Cheng J, et al. Sonodynamic effect of an anti-inflammatory agent–emodin on macrophages. Ultrasound Med Biol. (2011) 37:1478–85. doi: 10.1016/j.ultrasmedbio.2011.05.846
144. Fu X, Xu AG, Yao MY, Guo L, Zhao LS. Emodin enhances cholesterol efflux by activating peroxisome proliferator-activated receptor-γ in oxidized low density lipoprotein-loaded THP1 macrophages. Clin Exp Pharmacol Physiol. (2014) 41:679–84. doi: 10.1111/cep.2014.41.issue-9
145. Zhang X, Qin Q, Dai H, Cai S, Zhou C, Guan J. Emodin protects H9c2 cells from hypoxia-induced injury by up-regulating miR-138 expression. Braz J Med Biol Res. (2019) 52:e7994. doi: 10.1590/1414-431x20187994
146. Pang X, Liu J, Li Y, Zhao J, Zhang X. Emodin inhibits homocysteine-induced C-reactive protein generation in vascular smooth muscle cells by regulating PPARγ expression and ROS-ERK1/2/p38 signal pathway. PloS One. (2015) 10:e0131295. doi: 10.1371/journal.pone.0131295
147. Evans LW, Bender A, Burnett L, Godoy L, Shen Y, Staten D, et al. Emodin and emodin-rich rhubarb inhibits histone deacetylase (HDAC) activity and cardiac myocyte hypertrophy. J Nutr Biochem. (2020) 79:108339. doi: 10.1016/j.jnutbio.2019.108339
148. Zhou GH, Zhang F, Wang XN, Kwon OJ, Kang DG, Lee HS, et al. Emodin accentuates atrial natriuretic peptide secretion in cardiac atria. Eur J Pharmacol. (2014) 735:44–51. doi: 10.1016/j.ejphar.2014.04.014
149. Carver W, Fix E, Fix C, Fan D, Chakrabarti M, Azhar M. Effects of emodin, a plant-derived anthraquinone, on TGF-β1-induced cardiac fibroblast activation and function. J Cell Physiol. (2021) 236:7440–9. doi: 10.1002/jcp.v236.11
150. Tang X, Zhang Y, Liu X, Li X, Zhao H, Cui H, et al. Aloe-emodin derivative produces anti-atherosclerosis effect by reinforcing AMBRA1-mediated endothelial autophagy. Eur J Pharmacol. (2022) 916:174641. doi: 10.1016/j.ejphar.2021.174641
151. Yu J, Zhao X, Yan X, Li W, Liu Y, Wang J, et al. Aloe-emodin ameliorated MI-induced cardiac remodeling in mice via inhibiting TGF-β/SMAD signaling via up-regulating SMAD7. Phytomedicine. (2023) 114:154793. doi: 10.1016/j.phymed.2023.154793
152. Zhang Y, Song Z, Huang S, Zhu L, Liu T, Shu H, et al. Aloe emodin relieves Ang II-induced endothelial junction dysfunction via promoting ubiquitination mediated NLRP3 inflammasome inactivation. J Leukoc Biol. (2020) 108:1735–46. doi: 10.1002/JLB.3MA0520-582R
153. Liu J, Wang Y, Qiu L, Yu Y, Wang C. Saponins of Panax notoginseng: chemistry, cellular targets and therapeutic opportunities in cardiovascular diseases. Expert Opin Investig Drugs. (2014) 23:523–39. doi: 10.1517/13543784.2014.892582
154. Tan YQ, Chen HW, Li J. Astragaloside IV: an effective drug for the treatment of cardiovascular diseases. Drug Des Devel Ther. (2020) 14:3731–46. doi: 10.2147/DDDT.S272355
155. Zhang X, Qu H, Yang T, Liu Q, Zhou H. Astragaloside IV attenuate MI-induced myocardial fibrosis and cardiac remodeling by inhibiting ROS/caspase-1/GSDMD signaling pathway. Cell Cycle. (2022) 21:2309–22. doi: 10.1080/15384101.2022.2093598
156. Yin B, Hou XW, Lu ML. Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol Sin. (2019) 40:599–607. doi: 10.1038/s41401-018-0082-y
157. Zhang Y, Du M, Wang J, Liu P. Astragaloside IV relieves atherosclerosis and hepatic steatosis via MAPK/NF-κB signaling pathway in LDLR-/- mice. Front Pharmacol. (2022) 13:828161. doi: 10.3389/fphar.2022.828161
158. Luo LF, Guan P, Qin LY, Wang JX, Wang N, Ji ES. Astragaloside IV inhibits adriamycin-induced cardiac ferroptosis by enhancing Nrf2 signaling. Mol Cell Biochem. (2021) 476:2603–11. doi: 10.1007/s11010-021-04112-6
159. Sui YB, Zhang KK, Ren YK, Liu L, Liu Y. The role of Nrf2 in astragaloside IV-mediated antioxidative protection on heart failure. Pharm Biol. (2020) 58:1192–8. doi: 10.1080/13880209.2020.1849319
160. Nie P, Meng F, Zhang J, Wei X, Shen C. Astragaloside IV exerts a myocardial protective effect against cardiac hypertrophy in rats, partially via activating the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. (2019) 2019:4625912. doi: 10.1155/2019/4625912
161. Fan W, Huang Y, Zheng H, Li S, Li Z, Yuan L, et al. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. BioMed Pharmacother. (2020) 132:110915. doi: 10.1016/j.biopha.2020.110915
162. Zhang X, Wang Q, Wang X, Chen X, Shao M, Zhang Q, et al. Tanshinone IIA protects against heart failure post-myocardial infarction via AMPKs/mTOR-dependent autophagy pathway. BioMed Pharmacother. (2019) 112:108599. doi: 10.1016/j.biopha.2019.108599
163. Wang Y, Che J, Zhao H, Tang J, Shi G. Paeoniflorin attenuates oxidized low-density lipoprotein-induced apoptosis and adhesion molecule expression by autophagy enhancement in human umbilical vein endothelial cells. J Cell Biochem. (2019) 120:9291–9. doi: 10.1002/jcb.v120.6
164. Xie S, Deng W, Chen J, Wu QQ, Li H, Wang J, et al. Andrographolide protects against adverse cardiac remodeling after myocardial infarction through enhancing Nrf2 signaling pathway. Int J Biol Sci. (2020) 16:12–26. doi: 10.7150/ijbs.37269
165. Wang F, Gao Q, Yang J, Wang C, Cao J, Sun J, et al. Artemisinin suppresses myocardial ischemia-reperfusion injury via NLRP3 inflammasome mechanism. Mol Cell Biochem. (2020) 474:171–80. doi: 10.1007/s11010-020-03842-3
166. Feng X, Sureda A, Jafari S, Memariani Z, Tewari D, Annunziata G, et al. Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics. (2019) 9:1923–51. doi: 10.7150/thno.30787
167. Cheng D, Liu P, Wang Z. Palmatine attenuates the doxorubicin-induced inflammatory response, oxidative damage and cardiomyocyte apoptosis. Int Immunopharmacol. (2022) 106:108583. doi: 10.1016/j.intimp.2022.108583
168. Hou H, Zhang Q, Dong H, Ge Z. Matrine improves diabetic cardiomyopathy through TGF-β-induced protein kinase RNA-like endoplasmic reticulum kinase signaling pathway. J Cell Biochem. (2019) 120:13573–82. doi: 10.1002/jcb.v120.8
169. Jiang Z, Fu L, Xu Y, Hu X, Yang H, Zhang Y, et al. Cyclovirobuxine D protects against diabetic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Sci Rep. (2020) 10:6427. doi: 10.1038/s41598-020-63498-3
170. Xu H, Cheng J, He F. Cordycepin alleviates myocardial ischemia/reperfusion injury by enhancing autophagy via AMPK-mTOR pathway. J Physiol Biochem. (2022) 78:401–13. doi: 10.1007/s13105-021-00816-x
171. Deftereos SG, Beerkens FJ, Shah B, Giannopoulos G, Vrachatis DA, Giotaki SG, et al. Colchicine in cardiovascular disease: in-depth review. Circulation. (2022) 145:61–78. doi: 10.1161/CIRCULATIONAHA.121.056171
172. Yin M, Zhang Y, Li H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front Immunol. (2019) 10:145. doi: 10.3389/fimmu.2019.00145
173. Dong X, Zhou M, Li Y, Li Y, Ji H, Hu Q. Cardiovascular protective effects of plant polysaccharides: a review. Front Pharmacol. (2021) 12:783641. doi: 10.3389/fphar.2021.783641
174. Zheng H, Pei Y, Zhou C, Hong P, Qian ZJ. Amelioration of atherosclerosis in ox-LDL induced HUVEC by sulfated polysaccharides from Gelidium crinale with antihypertensive activity. Int J Biol Macromol. (2023) 228:671–80. doi: 10.1016/j.ijbiomac.2022.12.245
175. Huang X, Hou R, Pan W, Wu D, Zhao W, Li Q. A functional polysaccharide from Eriobotrya japonica relieves myocardial ischemia injury via anti-oxidative and anti-inflammatory effects. Food Funct. (2022) 13:113–20. doi: 10.1039/D1FO03208A
176. Sun S, Yang S, An N, Wang G, Xu Q, Liu J, et al. Astragalus polysaccharides inhibits cardiomyocyte apoptosis during diabetic cardiomyopathy via the endoplasmic reticulum stress pathway. J Ethnopharmacol. (2019) 238:111857. doi: 10.1016/j.jep.2019.111857
177. Pop C, Berce C, Ghibu S, Scurtu I, Sorițău O, Login C, et al. Effects of Lycium barbarum L. polysaccharides on inflammation and oxidative stress markers in a pressure overload-induced heart failure rat model. Molecules. (2020) 25:466. doi: 10.3390/molecules25030466
178. Luo W, Lin K, Hua J, Han J, Zhang Q, Chen L, et al. Schisandrin B attenuates diabetic cardiomyopathy by targeting MyD88 and inhibiting MyD88-dependent inflammation. Adv Sci (Weinh). (2022) 9:e2202590. doi: 10.1002/advs.202202590
179. Zhao B, Li GP, Peng JJ, Ren LH, Lei LC, Ye HM, et al. Schizandrin B attenuates hypoxia/reoxygenation injury in H9c2 cells by activating the AMPK/Nrf2 signaling pathway. Exp Ther Med. (2021) 21:220. doi: 10.3892/etm.2021.9651
180. Liu T, Sun F, Cui J, Zheng S, Li Z, Guo D, et al. Morroniside enhances angiogenesis and improves cardiac function following acute myocardial infarction in rats. Eur J Pharmacol. (2020) 872:172954. doi: 10.1016/j.ejphar.2020.172954
181. Gong S, Liu J, Wan S, Yang W, Zhang Y, Yu B, et al. Schisandrol A attenuates myocardial ischemia/reperfusion-induced myocardial apoptosis through upregulation of 14-3-3θ. Oxid Med Cell Longev. (2021) 2021:5541753. doi: 10.1155/2021/5541753
182. Liu D, Zeng Y, Liang P, Jiang Y, An S, Ren P. Efficacy and safety of Xuefu Zhuyu Granules combined with western medicine in the treatment of angina pectoris of coronary heart disease: A study protocol of a randomized, double-blind, placebo-controlled clinical trial. Med (Baltimore). (2022) 101:e31235. doi: 10.1097/MD.0000000000031235
183. Li Y, Tao T, Song D, He T, Liu X. Effects of Xuefu Zhuyu granules on patients with stable coronary heart disease: a double-blind, randomized, and placebo-controlled study. Oxid Med Cell Longev. (2021) 2021:8877296. doi: 10.1155/2021/8877296
184. Yunhu C, Lihua F, Tao Z, Xueqian L. Effectiveness of Zhuling decoction on diuretic resistance in patients with heart failure: a randomized, controlled trial. J Tradit Chin Med. (2022) 42:439–45. doi: 10.19852/j.cnki.jtcm.20220311.003
185. Zhu M, Wei J, Li Y, Wang Y, Ren J, Li B, et al. Efficacy and mechanism of buyang huanwu decoction in patients with ischemic heart failure: a randomized, double-blind, placebo-controlled trial combined with proteomic analysis. Front Pharmacol. (2022) 13:831208. doi: 10.3389/fphar.2022.831208
186. Li J, Gao Z, Zhang L, Li S, Yang Q, Shang Q, et al. Qing-Xin-Jie-Yu Granule for patients with stable coronary artery disease (QUEST Trial): A multicenter, double-blinded, randomized trial. Complement Ther Med. (2019) 47:102209. doi: 10.1016/j.ctim.2019.102209
187. Hu Z, Wang H, Fan G, Zhang H, Wang X, Mao J, et al. Danhong injection mobilizes endothelial progenitor cells to repair vascular endothelium injury via upregulating the expression of Akt, eNOS and MMP-9. Phytomedicine. (2019) 61:152850. doi: 10.1016/j.phymed.2019.152850
188. Fu S, Zhang J, Menniti-Ippolito F, Gao X, Galeotti F, Massari M, et al. Huangqi injection (a traditional Chinese patent medicine) for chronic heart failure: a systematic review. PloS One. (2011) 6:e19604. doi: 10.1371/journal.pone.0019604
189. Xu Y, Hu H, Li Y, Cen R, Yao C, Ma W, et al. Effects of huoxin formula on the arterial functions of patients with coronary heart disease. Pharm Biol. (2019) 57:13–20. doi: 10.1080/13880209.2018.1561726
190. Chao W, Qiong WU, Ping LI, Zhigang W, Xusheng L, Yuanyuan LI, et al. Effect of Traditional Chinese Medicine combined with Western Medicine on blood lipid levels and inflammatory factors in patients with angina pectoris in coronary heart disease identified as intermingled phlegm and blood stasis syndrome: a network Meta-analysis. J Tradit Chin Med. (2023) 43:640–9. doi: 10.19852/j.cnki.jtcm.20230506.001
191. Zhang H, Yin KQ, Liu XL, Zhang M, Ren HF, Zhou HW, et al. Clinical efficacy of modified Xiaojianzhong decoction in patients with chronic heart failure and constipation. J Shaanxi Univ Chin Med. (2019) 42:117–119+138. doi: 10.13424/j.cnki.jsctcm.2019.06.031
192. Peng JX. Clinical study on the regulation effect of Jianpi Huazhi pill on intestinal flora in patients with acute exacerbation of chronic heart failure. Asia-Pacific Traditional Med. (2020) 16:140–2. doi: 10.11954/ytctyy.202006044
193. Shen Z, Chen T, Deng B, Fan M, Hua J, Zhang M, et al. Effects on Suxiao Jiuxin Pills in the treatment of patients with acute coronary syndrome undergoing early percutaneous coronary intervention: A multicenter randomized double-blind placebo-controlled trial. J Altern Complement Med. (2020) 26:1055–63. doi: 10.1089/acm.2020.0014
194. Liang B, Zou FH, Fu L, Liao HL. Chinese herbal medicine Dingji Fumai decoction for ventricular premature contraction: a real-world trial. BioMed Res Int. (2020) 2020:5358467. doi: 10.1155/2020/5358467
195. Chen Y, Xiao X, Xu X, Zhang Z, Deng Y. Traditional Chinese Medicine in the prevention and treatment of stable angina pectoris in patients with coronary heart disease based on the theory of "phlegm and blood stasis" under guidance of evidence-based medicine: a prospective cohort study. J Tradit Chin Med. (2021) 41:150–6. doi: 10.19852/j.cnki.jtcm.2021.01.017
196. Liu B, Song Z, Yu J, Li P, Tang Y, Ge J. The atherosclerosis-ameliorating effects and molecular mechanisms of BuYangHuanWu decoction. BioMed Pharmacother. (2020) 123:109664. doi: 10.1016/j.biopha.2019.109664
197. Cai Y, Wen J, Ma S, Mai Z, Zhan Q, Wang Y, et al. Huang-Lian-Jie-Du decoction attenuates atherosclerosis and increases plaque stability in high-fat diet-induced ApoE-/- mice by inhibiting m1 macrophage polarization and promoting M2 macrophage polarization. Front Physiol. (2021) 12:666449. doi: 10.3389/fphys.2021.666449
198. Zhang Y, Ding J, Wang Y, Feng X, Du M, Liu P. Guanxinkang decoction attenuates the inflammation in atherosclerosis by regulating efferocytosis and MAPKs signaling pathway in LDLR-/- mice and RAW264.7 cells. Front Pharmacol. (2021) 12:731769. doi: 10.3389/fphar.2021.731769
199. Zhang J, Wang X, Guan B, Wang X, An X, Wang T, et al. Qing-Xin-Jie-Yu Granule inhibits ferroptosis and stabilizes atherosclerotic plaques by regulating the GPX4/xCT signaling pathway. J Ethnopharmacol. (2023) 301:115852. doi: 10.1016/j.jep.2022.115852
200. Zhou QB, Chen Y, Zhang Y, Li DD, Wang HQ, Jia ZJ, et al. Hypermethylation effects of Yiqihuoxue decoction in diabetic atherosclerosis using genome-wide DNA methylation analyses. J Inflammation Res. (2022) 15:163–76. doi: 10.2147/JIR.S335374
201. Li C, Chi C, Li W, Li Z, Wang X, Wang M, et al. An integrated approach for identifying the efficacy and potential mechanisms of TCM against atherosclerosis-Wu-Zhu-Yu decoction as a case study. J Ethnopharmacol. (2022) 296:115436. doi: 10.1016/j.jep.2022.115436
202. Ha E, Kim M, Chun J, Seo CS, Ahn Y, Jung J. Tongqiaohuoxue hinders development and progression of atherosclerosis: a possible role in Alzheimer's disease. Biol (Basel). (2020) 9:363. doi: 10.3390/biology9110363
203. Zhu ZB, Song K, Huang WJ, Li H, Yang H, Bai YQ, et al. Si-Miao-Yong-An (SMYA) decoction may protect the renal function through regulating the autophagy-mediated degradation of ubiquitinated protein in an atherosclerosis model. Front Pharmacol. (2020) 11:837. doi: 10.3389/fphar.2020.00837
204. Li S, Liu P, Feng X, Du M, Zhang Y, Wang Y, et al. Mechanism of Tao Hong Decoction in the treatment of atherosclerosis based on network pharmacology and experimental validation. Front Cardiovasc Med. (2023) 10:1111475. doi: 10.3389/fcvm.2023.1111475
205. Guo HY, Lu ZY, Zhao B, Jiang WW, Xiong YH, Wang K. Effects of Bunao-Fuyuan decoction serum on proliferation and migration of vascular smooth muscle cells in atherosclerotic. Chin J Nat Med. (2021) 19:36–45. doi: 10.1016/S1875-5364(21)60004-3
206. Liang J, Huang Y, Mai Z, Zhan Q, Lin H, Xie Y, et al. Integrating network pharmacology and experimental validation to decipher the mechanism of action of Huanglian Jiedu decoction in treating atherosclerosis. Drug Des Devel Ther. (2021) 15:1779–95. doi: 10.2147/DDDT.S304911
207. Chen Q, Zhang Y, Meng Q, Wang S, Yu X, Cai D, et al. Liuwei Dihuang prevents postmenopausal atherosclerosis and endothelial cell apoptosis via inhibiting DNMT1-medicated ERα methylation. J Ethnopharmacol. (2020) 252:112531. doi: 10.1016/j.jep.2019.112531
208. Meng Q, Yu X, Chen Q, Wu X, Kong X, Wang S, et al. Liuwei Dihuang soft capsules inhibits the phenotypic conversion of VSMC to prevent the menopausal atherosclerosis by up-regulating the expression of myocardin. J Ethnopharmacol. (2020) 246:112207. doi: 10.1016/j.jep.2019.112207
209. Xu H, Zhang T, He L, Yuan M, Yuan X, Wang S. Exploring the mechanism of Danggui Buxue Decoction in regulating atherosclerotic disease network based on integrated pharmacological methods. Biosci Rep. (2021) 41:BSR20211429. doi: 10.1042/BSR20211429
210. Jin Z, Luo Y, Zhao H, Cui J, He W, Li J, et al. Qingre Huoxue Decoction regulates macrophage polarisation to attenuate atherosclerosis through the inhibition of NF-κB signalling-mediated inflammation. J Ethnopharmacol. (2023) 301:115787. doi: 10.1016/j.jep.2022.115787
211. Zhang Y, Qian X, Sun X, Lin C, Jing Y, Yao Y, et al. Liuwei Dihuang, a traditional Chinese medicinal formula, inhibits proliferation and migration of vascular smooth muscle cells via modulation of estrogen receptors. Int J Mol Med. (2018) 42:31–40. doi: 10.3892/ijmm.2018.3622
212. Li L, Yu AL, Wang ZL, Chen K, Zheng W, Zhou JJ, et al. Chaihu-Shugan-San and absorbed meranzin hydrate induce anti-atherosclerosis and behavioral improvements in high-fat diet ApoE-/- mice via anti-inflammatory and BDNF-TrkB pathway. BioMed Pharmacother. (2019) 115:108893. doi: 10.1016/j.biopha.2019.108893
213. Yang M, Jiao H, Li Y, Zhang L, Zhang J, Zhong X, et al. Guanmaitong granule attenuates atherosclerosis by inhibiting inflammatory immune response in ApoE-/- mice fed high-fat diet. Drug Des Devel Ther. (2022) 16:3145–68. doi: 10.2147/DDDT.S372143
214. Chen R, Chen T, Wang T, Dai X, Meng K, Zhang S, et al. Tongmai Yangxin pill reduces myocardial no-reflow by regulating apoptosis and activating PI3K/Akt/eNOS pathway. J Ethnopharmacol. (2020) 261:113069. doi: 10.1016/j.jep.2020.113069
215. Chen R, Chen T, Wang T, Dai X, Zhang S, Jiang D, et al. Tongmai Yangxin pill reduces myocardial No-reflow via endothelium-dependent NO-cGMP signaling by activation of the cAMP/PKA pathway. J Ethnopharmacol. (2021) 267:113462. doi: 10.1016/j.jep.2020.113462
216. Li M, Wang Y, Qi Z, Yuan Z, Lv S, Zheng Y, et al. QishenYiqi dripping pill protects against myocardial ischemia/reperfusion injury via suppressing excessive autophagy and NLRP3 inflammasome based on network pharmacology and experimental pharmacology. Front Pharmacol. (2022) 13:981206. doi: 10.3389/fphar.2022.981206
217. Chen M, Zhong G, Liu M, He H, Zhou J, Chen J, et al. Integrating network analysis and experimental validation to reveal the mitophagy-associated mechanism of Yiqi Huoxue (YQHX) prescription in the treatment of myocardial ischemia/reperfusion injury. Pharmacol Res. (2023) 189:106682. doi: 10.1016/j.phrs.2023.106682
218. Long L, Yu Z, Chen S, Wu J, Liu Y, Peng J, et al. Pretreatment of Huoxue Jiedu formula ameliorates myocardial ischaemia/reperfusion injury by decreasing autophagy via activation of the PI3K/AKT/mTOR pathway. Front Pharmacol. (2021) 12:608790. doi: 10.3389/fphar.2021.608790
219. Xie F, Wu YY, Duan GJ, Wang B, Gao F, Wei PF, et al. Anti-myocardial ischemia reperfusion injury mechanism of dried ginger-aconite decoction based on network pharmacology. Front Pharmacol. (2021) 12:609702. doi: 10.3389/fphar.2021.609702
220. Zhao Y, Guo R, Li L, Li S, Fan G, Zhao X, et al. Tongmai formula improves cardiac function via regulating mitochondrial quality control in the myocardium with ischemia/reperfusion injury. BioMed Pharmacother. (2020) 132:110897. doi: 10.1016/j.biopha.2020.110897
221. Zhou K, Chen H, Wang XY, Xu YM, Liao YF, Qin YY, et al. Targeted pharmacokinetics and bioinformatics screening strategy reveals JAK2 as the main target for Xin-Ji-Er-Kang in treatment of MIR injury. BioMed Pharmacother. (2022) 155:113792. doi: 10.1016/j.biopha.2022.113792
222. Cui Y, Zhang F, Xu W, Li Z, Zou J, Gao P, et al. Effects of Si-Miao-Yong-An decoction on myocardial I/R rats by regulating gut microbiota to inhibit LPS-induced TLR4/NF-κB signaling pathway. BMC Complement Med Ther. (2023) 23:180. doi: 10.1186/s12906-023-04013-9
223. Zeng Z, Wang Q, Yang X, Ren Y, Jiao S, Zhu Q, et al. Qishen granule attenuates cardiac fibrosis by regulating TGF-β /Smad3 and GSK-3β pathway. Phytomedicine. (2019) 62:152949. doi: 10.1016/j.phymed.2019.152949
224. Liao M, Xie Q, Zhao Y, Yang C, Lin C, Wang G, et al. Main active components of Si-Miao-Yong-An decoction (SMYAD) attenuate autophagy and apoptosis via the PDE5A-AKT and TLR4-NOX4 pathways in isoproterenol (ISO)-induced heart failure models. Pharmacol Res. (2022) 176:106077. doi: 10.1016/j.phrs.2022.106077
225. Chen Y, Li L, Hu C, Zhao X, Zhang P, Chang Y, et al. Lingguizhugan decoction dynamically regulates MAPKs and AKT signaling pathways to retrogress the pathological progression of cardiac hypertrophy to heart failure. Phytomedicine. (2022) 98:153951. doi: 10.1016/j.phymed.2022.153951
226. Wei XH, Liu WJ, Jiang W, Lan TH, Pan H, Ma MY, et al. XinLi formula, a traditional Chinese decoction, alleviates chronic heart failure via regulating the interaction of AGTR1 and AQP1. Phytomedicine. (2023) 113:154722. doi: 10.1016/j.phymed.2023.154722
227. Hu Y, Qu H, Zhou H. Integrating network pharmacology and an experimental model to investigate the effect of Zhenwu Decoction on doxorubicin-induced heart failure. Comb Chem High Throughput Screen. (2023) 26:2502–16. doi: 10.2174/1386207326666230413091715
228. Yu S, Qian H, Tian D, Yang M, Li D, Xu H, et al. Linggui Zhugan Decoction activates the SIRT1-AMPK-PGC1α signaling pathway to improve mitochondrial and oxidative damage in rats with chronic heart failure caused by myocardial infarction. Front Pharmacol. (2023) 14:1074837. doi: 10.3389/fphar.2023.1074837
229. Zhuang J, Zhu J, Dou Y, Chen X, Chen H, Liu X, et al. Shenqi Lixin Decoction improves cardiac function in rats with adriamycin-induced heart failure through modulation of PGC-1α and mitochondrial apoptosis pathway. Ann Transl Med. (2021) 9:1592. doi: 10.21037/atm-21-5350
230. Zhang W, Yu M, Zhang C, Yu Q, Xu S, Yan Q, et al. Active ingredient paeonol of jijiu huiyang decoction alleviates isoproterenol-induced chronic heart failure via the GSK3A/PPARα pathway. Oxid Med Cell Longev. (2023) 2023:3271057. doi: 10.1155/2023/3271057
231. Su YN, Lu PP, Yan SY, Guo XT, Ma J, Guo CX, et al. Xinfuli granule alleviates metabolic remodeling through inhibition of endoplasmic reticulum stress and mitochondrial injury in heart failure. J Ethnopharmacol. (2023) 303:115782. doi: 10.1016/j.jep.2022.115782
232. Li Y, Li X, Chen X, Sun X, Liu X, Wang G, et al. Qishen granule (QSG) inhibits monocytes released from the spleen and protect myocardial function via the TLR4-MyD88-NF-κB p65 pathway in heart failure mice. Front Pharmacol. (2022) 13:850187. doi: 10.3389/fphar.2022.850187
233. Qiu X, Ma J, Shi Y, Zhang D, Li D, Dong Z, et al. BAOXIN granules protected mouse model with elevated afterload from cardiac hypertrophy by suppressing both inflammatory reaction and collagen deposition. Front Physiol. (2019) 10:820. doi: 10.3389/fphys.2019.00820
234. Wang C, Zhou J, Wang S, Liu Y, Long K, Sun T, et al. Guanxining injection alleviates fibrosis in heart failure mice and regulates SLC7A11/GPX4 axis. J Ethnopharmacol. (2023) 310:116367. doi: 10.1016/j.jep.2023.116367
235. Nie Y, Zhang Y, Li Z, Wan M, Li D. Injection of YiQiFuMai powder protects against heart failure via inhibiting p38 and ERK1/2 MAPKs activation. Pharm Biol. (2022) 60:570–8. doi: 10.1080/13880209.2022.2038207
236. Fan S, Xiao G, Ni J, Zhao Y, Du H, Liang Y, et al. Guanxinning injection ameliorates cardiac remodeling in HF mouse and 3D heart spheroid models via p38/FOS/MMP1-mediated inhibition of myocardial hypertrophy and fibrosis. BioMed Pharmacother. (2023) 162:114642. doi: 10.1016/j.biopha.2023.114642
237. Yuan C, Wu Z, Jin C, Cao W, Dong Y, Chen J, et al. Qiangxin recipe improves doxorubicin-induced chronic heart failure by enhancing KLF5-mediated glucose metabolism. Phytomedicine. (2023) 112:154697. doi: 10.1016/j.phymed.2023.154697
238. Tan C, Zeng J, Wu G, Zheng L, Huang M, Huang X. Xinshuitong Capsule extract attenuates doxorubicin-induced myocardial edema via regulation of cardiac aquaporins in the chronic heart failure rats. BioMed Pharmacother. (2021) 144:112261. doi: 10.1016/j.biopha.2021.112261
239. Qiu H, Huang ZY, Cao H, Zhang Z, Ma J, Li XQ, et al. Deciphering mechanism of the herbal formula WuShen in the treatment of postinfarction heart failure. Phytomedicine. (2022) 95:153878. doi: 10.1016/j.phymed.2021.153878
240. Chen X, Long L, Cheng Y, Chu J, Shen Z, Liu L, et al. Qingda granule attenuates cardiac fibrosis via suppression of the TGF-β1/Smad2/3 signaling pathway in vitro and in vivo. BioMed Pharmacother. (2021) 137:111318. doi: 10.1016/j.biopha.2021.111318
241. Chen S, Hu J, Lu DC, Liu HY, Wei SS. Metabolomic characteristics of spontaneously hypertensive rats under chronic stress and the treatment effect of Danzhi Xiaoyao Powder, a traditional Chinese medicine formula. J Integr Med. (2022) 20:73–82. doi: 10.1016/j.joim.2021.11.007
242. Chen J, Zhang Y, Wang Y, Jiang P, Zhou G, Li Z, et al. Potential mechanisms of Guizhi decoction against hypertension based on network pharmacology and Dahl salt-sensitive rat model. Chin Med. (2021) 16:34. doi: 10.1186/s13020-021-00446-x
243. Yu N, Shen A, Chu J, Huang Y, Zhang L, Lin S, et al. Qingda granule inhibits angiotensin II induced VSMCs proliferation through MAPK and PI3K/AKT pathways. J Ethnopharmacol. (2020) 258:112767. doi: 10.1016/j.jep.2020.112767
244. Mohammed SAD, Liu H, Baldi S, Chen P, Lu F, Liu S. GJD modulates cardiac/vascular inflammation and decreases blood pressure in hypertensive rats. Mediators Inflammation. (2022) 2022:7345116. doi: 10.1155/2022/7345116
245. Yu X, Zhang X, Jin H, Wu Z, Yan C, Liu Z, et al. Zhengganxifeng decoction affects gut microbiota and reduces blood pressure via renin-angiotensin system. Biol Pharm Bull. (2019) 42:1482–90. doi: 10.1248/bpb.b19-00057
246. Zhu Y, Huang JJ, Zhang XX, Yan Y, Yin XW, Ping G, et al. Qing Gan Zi Shen Tang alleviates adipose tissue dysfunction with up-regulation of SIRT1 in spontaneously hypertensive rat. BioMed Pharmacother. (2018) 105:246–55. doi: 10.1016/j.biopha.2018.05.022
247. Song X, Zhao Y, Wang S, Wang Y, Chen Q, Zhao H, et al. Zi Shen Huo Luo formula enhances the therapeutic effects of angiotensin-converting enzyme inhibitors on hypertensive left ventricular hypertrophy by interfering with aldosterone breakthrough and affecting caveolin-1/mineralocorticoid receptor colocalization and downstream extracellular signal-regulated kinase signaling. Front Pharmacol. (2020) 11:383. doi: 10.3389/fphar.2020.00383
248. Han X, Zhang G, Chen G, Wu Y, Xu T, Xu H, et al. Buyang Huanwu Decoction promotes angiogenesis in myocardial infarction through suppression of PTEN and activation of the PI3K/Akt signalling pathway. J Ethnopharmacol. (2022) 287:114929. doi: 10.1016/j.jep.2021.114929
249. Tan Z, Jiang X, Zhou W, Deng B, Cai M, Deng S, et al. Taohong siwu decoction attenuates myocardial fibrosis by inhibiting fibrosis proliferation and collagen deposition via TGFBR1 signaling pathway. J Ethnopharmacol. (2021) 270:113838. doi: 10.1016/j.jep.2021.113838
250. Yang Y, Su C, Zhang XZ, Li J, Huang SC, Kuang HF, et al. Mechanisms of Xuefu Zhuyu Decoction in the treatment of coronary heart disease based on integrated metabolomics and network pharmacology approach. J Chromatogr B Analyt Technol BioMed Life Sci. (2023) 1223:123712. doi: 10.1016/j.jchromb.2023.123712
251. Li FH, Guo SW, Zhan TW, Mo HR, Chen X, Wang H, et al. Integrating network pharmacology and experimental evidence to decipher the cardioprotective mechanism of Yiqihuoxue decoction in rats after myocardial infarction. J Ethnopharmacol. (2021) 279:114062. doi: 10.1016/j.jep.2021.114062
252. Jin Z, Zhang W, Luo Y, Li X, Qing L, Zuo Q, et al. Protective effect of Qingre Huoxue decoction against myocardial infarction via PI3K/Akt autophagy pathway based on UPLC-MS, network pharmacology, and in vivo evidence. Pharm Biol. (2021) 59:1607–18. doi: 10.1080/13880209.2021.2001542
253. Li L, Li YQ, Sun ZW, Xu CM, Wu J, Liu GL, et al. Qingyi decoction protects against myocardial injuries induced by severe acute pancreatitis. World J Gastroenterol. (2020) 26:1317–28. doi: 10.3748/wjg.v26.i12.1317
254. Sun Y, Wang Z, Hou J, Shi J, Tang Z, Wang C, et al. Shuangxinfang prevents S100A9-induced macrophage/microglial inflammation to improve cardiac function and depression-like behavior in rats after acute myocardial infarction. Front Pharmacol. (2022) 13:832590. doi: 10.3389/fphar.2022.832590
255. Zhao N, Wang Y, Ma Y, Liang X, Zhang X, Gao Y, et al. Jia-Wei-Si-Miao-Yong-An decoction modulates intestinal flora and metabolites in acute coronary syndrome model. Front Cardiovasc Med. (2022) 9:1038273. doi: 10.3389/fcvm.2022.1038273
256. Liu X, Li Y, Ni SH, Sun SN, Deng JP, Ou-Yang XL, et al. Zhen-Wu decoction and lactiflorin, an ingredient predicted by in silico modelling, alleviate uremia induced cardiac endothelial injury via Nrf2 activation. J Ethnopharmacol. (2022) 298:115579. doi: 10.1016/j.jep.2022.115579
257. Gao Q, Ma E, Chen J, Zhao Q, He J, Peng J, et al. Qingda granule prevents obesity-induced hypertension and cardiac dysfunction by inhibiting adverse Akt signaling activation. Heliyon. (2022) 8:e12099. doi: 10.1016/j.heliyon.2022.e12099
258. Su C, Wang Q, Luo H, Jiao W, Tang J, Li L, et al. Si-Miao-Yong-An decoction attenuates cardiac fibrosis via suppressing TGF-β1 pathway and interfering with MMP-TIMPs expression. BioMed Pharmacother. (2020) 127:110132. doi: 10.1016/j.biopha.2020.110132
259. Peng M, Yang M, Lu Y, Lin S, Gao H, Xie L, et al. Huoxin Pill inhibits isoproterenol-induced transdifferentiation and collagen synthesis in cardiac fibroblasts through the TGF-β/Smads pathway. J Ethnopharmacol. (2021) 275:114061. doi: 10.1016/j.jep.2021.114061
260. Zhang X, You LY, Zhang ZY, Jiang DX, Qiu Y, Ruan YP, et al. Integrating pharmacological evaluation and computational identification for deciphering the action mechanism of Yunpi-Huoxue-Sanjie formula alleviates diabetic cardiomyopathy. Front Pharmacol. (2022) 13:957829. doi: 10.3389/fphar.2022.957829
261. Peng M, Liu H, Ji Q, Ma P, Niu Y, Ning S, et al. Fufang Xueshuantong improves diabetic cardiomyopathy by regulating the Wnt/β-Catenin pathway. Int J Endocrinol. (2022) 2022:3919161. doi: 10.1155/2022/3919161
262. Shi H, Zhou P, Ni YQ, Wang SS, Song R, Shen AL, et al. In vivo and in vitro studies of Danzhi Jiangtang capsules against diabetic cardiomyopathy via TLR4/MyD88/NF-κB signaling pathway. Saudi Pharm J. (2021) 29:1432–40. doi: 10.1016/j.jsps.2021.11.004
263. Zhang J, Zhao WR, Shi WT, Tan JJ, Zhang KY, Tang JY, et al. Tribulus terrestris L. extract ameliorates atherosclerosis by inhibition of vascular smooth muscle cell proliferation in ApoE-/- mice and A7r5 cells via suppression of Akt/MEK/ERK signaling. J Ethnopharmacol. (2022) 297:115547. doi: 10.1016/j.jep.2022.115547
264. Han J, Dong J, Zhang R, Zhang X, Chen M, Fan X, et al. Dendrobium catenatum Lindl. water extracts attenuate atherosclerosis. Mediators Inflammation. (2021) 2021:9951946. doi: 10.1155/2021/9951946
265. Liu J, Zhang W, Li Y, Li X, Li Y, Guo F. Flavonoids extract from the seeds of Psoralea corylifolia L. (PFE) alleviates atherosclerosis in high-fat diet-induced LDLR-/- mice. Phytomedicine. (2022) 98:153983. doi: 10.1016/j.phymed.2022.153983
266. Liu Z, Wang H, Li C, Yang J, Suo Q, Zhou Y, et al. Ethyl acetate extract of Caesalpinia sappan L. for the treatment of atherosclerosis in ApoE-/- mice and its mechanism. Mol Omics. (2022) 18:977–90. doi: 10.1039/D2MO00254J
267. Tian Y, Chang S, Xu J, Gong P, Yu B, Qi J. Investigation of the effective components inhibited macrophage foam cell formation in Ophiopogonis Radix. J Ethnopharmacol. (2022) 283:114678. doi: 10.1016/j.jep.2021.114678
268. Lee J, Ha SJ, Park J, Kim YH, Lee NH, Kim YE, et al. Arctium lappa root extract containing L-arginine prevents TNF-α-induced early atherosclerosis in vitro and in vivo. Nutr Res. (2020) 77:85–96. doi: 10.1016/j.nutres.2020.03.003
269. Hashikawa-Hobara N, Hashikawa N, Sugiman N, Hosoo S, Hirata T, Yamaguchi Y, et al. Oral administration of Eucommia ulmoides oliv. leaves extract protects against atherosclerosis by improving macrophage function in ApoE knockout mice. J Food Sci. (2020) 85:4018–24. doi: 10.1111/1750-3841.15461
270. Zhao X, Zhu J, Wang L, Li Y, Zhao T, Chen X, et al. U. diffracta extract mitigates high fat diet and VD3-induced atherosclerosis and biochemical changes in the serum liver and aorta of rats. BioMed Pharmacother. (2019) 120:109446. doi: 10.1016/j.biopha.2019.109446
271. Lai P, Cao X, Xu Q, Liu Y, Li R, Zhang J, et al. Ganoderma lucidum spore ethanol extract attenuates atherosclerosis by regulating lipid metabolism via upregulation of liver X receptor alpha. Pharm Biol. (2020) 58:760–70. doi: 10.1080/13880209.2020.1798471
272. Ko M, Oh GT, Park J, Kwon HJ. Extract of high hydrostatic pressure-treated danshen (Salvia miltiorrhiza) ameliorates atherosclerosis via autophagy induction. BMB Rep. (2020) 53:652–7. doi: 10.5483/BMBRep.2020.53.12.184
273. Ai ZL, Zhang X, Ge W, Zhong YB, Wang HY, Zuo ZY, et al. Salvia miltiorrhiza extract may exert an anti-obesity effect in rats with high-fat diet-induced obesity by modulating gut microbiome and lipid metabolism. World J Gastroenterol. (2022) 28:6131–56. doi: 10.3748/wjg.v28.i43.6131
274. Jia A, Shi Y, Zhang Y, Diao Y, Chang B, Jiang M, et al. Butanol extract of Acanthopanax senticosus (Rupr. et Maxim.) harms alleviates atherosclerosis in apolipoprotein E-deficient mice fed a high-fat diet. Chem Biodivers. (2023) 20:e202200949. doi: 10.1002/cbdv.202200949
275. Tang L, Kuang C, Shan D, Shi M, Li J, Qiu L, et al. The ethanol extract of Edgeworthia gardneri (Wall.) Meisn attenuates macrophage foam cell formation and atherogenesis in ApoE-/- mice. Front Cardiovasc Med. (2022) 9:1023438. doi: 10.3389/fcvm.2022.1023438
276. Chen X, Cao J, Sun Y, Dai Y, Zhu J, Zhang X, et al. Ethanol extract of Schisandrae chinensis fructus ameliorates the extent of experimentally induced atherosclerosis in rats by increasing antioxidant capacity and improving endothelial dysfunction. Pharm Biol. (2018) 56:612–9. doi: 10.1080/13880209.2018.1523933
277. Draginic N, Milosavljevic I, Andjic M, Jeremic J, Nikolic M, Sretenovic J, et al. Short-term administration of lemon balm extract ameliorates myocardial ischemia/reperfusion injury: focus on oxidative stress. Pharm (Basel). (2022) 15:840. doi: 10.3390/ph15070840
278. Bradic J, Jeremic N, Petkovic A, Jeremic J, Zivkovic V, Srejovic I, et al. Cardioprotective effects of Galium verum L. extract against myocardial ischemia-reperfusion injury. Arch Physiol Biochem. (2020) 126:408–15. doi: 10.1080/13813455.2018.1551904
279. Rankovic M, Krivokapic M, Bradic J, Petkovic A, Zivkovic V, Sretenovic J, et al. New insight into the cardioprotective effects of Allium ursinum L. extract against myocardial ischemia-reperfusion injury. Front Physiol. (2021) 12:690696. doi: 10.3389/fphys.2021.690696
280. Sedighi M, Nazari A, Faghihi M, Rafieian-Kopaei M, Karimi A, Moghimian M, et al. Protective effects of Cinnamon bark extract against ischemia-reperfusion injury and arrhythmias in rat. Phytother Res. (2018) 32:1983–91. doi: 10.1002/ptr.v32.10
281. Zhang L, Jian LL, Li JY, Jin X, Li LZ, Zhang YL, et al. Possible involvement of alpha B-crystallin in the cardioprotective effect of n-butanol extract of Potentilla anserina L. @ on myocardial ischemia/reperfusion injury in rat. Phytomedicine. (2019) 55:320–9. doi: 10.1016/j.phymed.2018.10.024
282. Tsai CF, Lin HW, Liao JM, Chen KM, Tsai JW, Chang CS, et al. Dunaliella salina alga protects against myocardial ischemia/reperfusion injury by attenuating TLR4 signaling. Int J Mol Sci. (2023) 24:3871. doi: 10.3390/ijms24043871
283. Sharma M, Pal P, Pottoo F, Kumar S. Mechanistic role of methanolic extract of Taraxacum officinale roots as cardioprotective against ischemia-reperfusion injury-induced myocardial infarction in rats. Appl Biochem Biotechnol. (2023) 195:3384–405. doi: 10.1007/s12010-022-04282-z
284. Asgari M, Salehi I, Ranjbar K, Khosravi M, Zarrinkalam E. Interval training and Crataegus persica ameliorate diabetic nephropathy via miR-126/Nrf-2 mediated inhibition of stress oxidative in rats with diabetes after myocardial ischemia-reperfusion injury. BioMed Pharmacother. (2022) 153:113411. doi: 10.1016/j.biopha.2022.113411
285. Sedighi M, Faghihi M, Rafieian-Kopaei M, Rasoulian B, Nazari A. Cardioprotective effect of ethanolic leaf extract of Melissa officinalis L against regional ischemia-induced arrhythmia and heart injury after five days of reperfusion in rats. Iran J Pharm Res. (2019) 18:1530–42. doi: 10.22037/ijpr.2019.1100761
286. Zhai S, Zhang XF, Lu F, Chen WG, He X, Zhang CF, et al. Chinese medicine GeGen-DanShen extract protects from myocardial ischemic injury through promoting angiogenesis via up-regulation of VEGF/VEGFR2 signaling pathway. J Ethnopharmacol. (2021) 267:113475. doi: 10.1016/j.jep.2020.113475
287. Qu S, Li K, Yang T, Yang Y, Zheng Z, Liu H, et al. Shenlian extract protects against ultrafine particulate matter-aggravated myocardial ischemic injury by inhibiting inflammation response via the activation of NLRP3 inflammasomes. Environ Toxicol. (2021) 36:1349–61. doi: 10.1002/tox.23131
288. Cheng F, Jiang W, Xiong X, Chen J, Xiong Y, Li Y. Ethanol extract of Chinese Hawthorn (Crataegus pinnatifida) fruit reduces inflammation and oxidative stress in rats with doxorubicin-induced chronic heart failure. Med Sci Monit. (2020) 26:e926654. doi: 10.12659/MSM.926654
289. Zhang L, Liu J, Ge Y, Liu M. Ginkgo biloba extract reduces hippocampus inflammatory responses, improves cardiac functions and depressive behaviors in a heart failure mouse model. Neuropsychiatr Dis Treat. (2019) 15:3041–50. doi: 10.2147/NDT.S229296
290. Wu Z, Zhao X, Miyamoto A, Zhao S, Liu C, Zheng W, et al. Effects of steroidal saponins extract from Ophiopogon japonicus root ameliorates doxorubicin-induced chronic heart failure by inhibiting oxidative stress and inflammatory response. Pharm Biol. (2019) 57:176–83. doi: 10.1080/13880209.2019.1577467
291. Xu X, Xie X, Zhang H, Wang P, Li G, Chen J, et al. Water-soluble alkaloids extracted from Aconiti radix lateralis praeparata protect against chronic heart failure in rats via a calcium signaling pathway. BioMed Pharmacother. (2021) 135:111184. doi: 10.1016/j.biopha.2020.111184
292. Wang X, Guo D, Li W, Zhang Q, Jiang Y, Wang Q, et al. Danshen (Salvia miltiorrhiza) restricts MD2/TLR4-MyD88 complex formation and signalling in acute myocardial infarction-induced heart failure. J Cell Mol Med. (2020) 24:10677–92. doi: 10.1111/jcmm.v24.18
293. Shen Z, Geng Q, Huang H, Yao H, Du T, Chen L, et al. Antioxidative and cardioprotective effects of Schisandra chinensis Bee pollen extract on isoprenaline-induced myocardial infarction in rats. Molecules. (2019) 24:1090. doi: 10.3390/molecules24061090
294. Panda V, Bhandare N, Mistry K, S S, Dande P. Cardioprotective potential of Spinacia oleracea (Spinach) against isoproterenol-induced myocardial infarction in rats. Arch Physiol Biochem. (2022) 128:101–10. doi: 10.1080/13813455.2019.1665074
295. Sun JH, Yang HX, Yao TT, Li Y, Ruan L, Xu GR, et al. Gentianella acuta prevents acute myocardial infarction induced by isoproterenol in rats via inhibition of galectin-3/TLR4/MyD88/NF-кB inflammatory signalling. Inflammopharmacology. (2021) 29:205–19. doi: 10.1007/s10787-020-00708-4
296. Zhang M, Chen J, Wang Y, Kang G, Zhang Y, Han X. Network pharmacology-based combined with experimental validation study to explore the underlying mechanism of Agrimonia pilosa Ledeb. extract in treating acute myocardial infarction. Drug Des Devel Ther. (2022) 16:3117–32. doi: 10.2147/DDDT.S370473
297. Feng X, Zhang R, Li J, Cao Y, Zhao F, Du X, et al. Syringa pinnatifolia Hemsl. fraction protects against myocardial ischemic injury by targeting the p53-mediated apoptosis pathway. Phytomedicine. (2019) 52:136–46. doi: 10.1016/j.phymed.2018.09.188
298. Wang S, Zhao Y, Song J, Wang R, Gao L, Zhang L, et al. Total flavonoids from Anchusa italica Retz. improve cardiac function and attenuate cardiac remodeling post myocardial infarction in mice. J Ethnopharmacol. (2020) 257:112887. doi: 10.1016/j.jep.2020.112887
299. Wang CH, Pandey S, Sivalingam K, Shibu MA, Kuo WW, Yu L, et al. Leech extract: A candidate cardioprotective against hypertension-induced cardiac hypertrophy and fibrosis. J Ethnopharmacol. (2021) 264:113346. doi: 10.1016/j.jep.2020.113346
300. Zeng L, Chen M, Ahmad H, Zheng X, Ouyang Y, Yang P, et al. Momordica charantia extract confers protection against hypertension in dahl salt-sensitive rats. Plant Foods Hum Nutr. (2022) 77:373–82. doi: 10.1007/s11130-022-00971-6
301. Tong RC, Qi M, Yang QM, Li PF, Wang DD, Lan JP, et al. Extract of Plantago asiatica L. seeds ameliorates hypertension in spontaneously hypertensive rats by inhibition of angiotensin converting enzyme. Front Pharmacol. (2019) 10:403. doi: 10.3389/fphar.2019.00403
302. Chiang JT, Badrealam KF, Shibu MA, Kuo CH, Huang CY, Chen BC, et al. Eriobotrya japonica ameliorates cardiac hypertrophy in H9c2 cardiomyoblast and in spontaneously hypertensive rats. Environ Toxicol. (2018) 33:1113–22. doi: 10.1002/tox.v33.11
303. Zhang X, Xu P, Lin B, Deng X, Zhu J, Chen X, et al. Chimonanthus salicifolius attenuated vascular remodeling by alleviating endoplasmic reticulum stress in spontaneously hypertensive rats. Food Funct. (2022) 13:6293–305. doi: 10.1039/D1FO04381A
304. Zhou J, Zhang L, Zheng B, Zhang L, Qin Y, Zhang X, et al. Salvia miltiorrhiza bunge exerts anti-oxidative effects through inhibiting KLF10 expression in vascular smooth muscle cells exposed to high glucose. J Ethnopharmacol. (2020) 262:113208. doi: 10.1016/j.jep.2020.113208
305. Wen C, Liu C, Li Y, Xia T, Zhang X, Xue S, et al. Ameliorative potentials of the ethanolic extract from Lycium chinense leaf extract against diabetic cardiomyopathy. Insight into oxido-inflammatory and apoptosis modulation. BioMed Pharmacother. (2022) 154:113583. doi: 10.1016/j.biopha.2022.113583
306. Zhou Q, Chen B, Chen X, Wang Y, Ji J, Kizaibek M, et al. Arnebiae radix prevents atrial fibrillation in rats by ameliorating atrial remodeling and cardiac function. J Ethnopharmacol. (2020) 248:112317. doi: 10.1016/j.jep.2019.112317
307. Cao YY, Li K, Li Y, Tian XT, Ba HX, Wang A, et al. Dendrobium candidum aqueous extract attenuates isoproterenol-induced cardiac hypertrophy through the ERK signalling pathway. Pharm Biol. (2020) 58:176–83. doi: 10.1080/13880209.2020.1723648
308. Fu D, Zhou J, Xu S, Tu J, Cai Y, Liu J, et al. Smilax glabra Roxb. flavonoids protect against pathological cardiac hypertrophy by inhibiting the Raf/MEK/ERK pathway: In vivo and in vitro studies. J Ethnopharmacol. (2022) 292:115213. doi: 10.1016/j.jep.2022.115213
309. Ding B, Niu W, Wang S, Zhang F, Wang H, Chen X, et al. Centella asiatica (L.) Urb. attenuates cardiac hypertrophy and improves heart function through multi-level mechanisms revealed by systems pharmacology. J Ethnopharmacol. (2022) 291:115106. doi: 10.1016/j.jep.2022.115106
310. Ma C, Fu Z, Guo H, Wei H, Zhao X, Li Y. The effects of Radix Angelica Sinensis and Radix Hedysari ultrafiltration extract on X-irradiation-induced myocardial fibrosis in rats. BioMed Pharmacother. (2019) 112:108596. doi: 10.1016/j.biopha.2019.01.057
311. Li J, Xu M, Xing B, Liu Y, Zhang Q, Guo J, et al. Combination of Salviae miltiorrhizae Radix et Rhizoma and Carthami Flos improves cardiac function of diabetic cardiomyopathy mice by regulating the unfolded protein response signaling pathway. Phytother Res. (2022) 36:3571–83. doi: 10.1002/ptr.v36.9
312. Xie D, Song L, Xiang D, Gao X, Zhao W. Salvianolic acid A alleviates atherosclerosis by inhibiting inflammation through Trc8-mediated 3-hydroxy-3-methylglutaryl-coenzyme A reductase degradation. Phytomedicine. (2023) 112:154694. doi: 10.1016/j.phymed.2023.154694
313. Zhou R, Gao J, Xiang C, Liu Z, Zhang Y, Zhang J, et al. Salvianolic acid A attenuated myocardial infarction-induced apoptosis and inflammation by activating Trx. Naunyn Schmiedebergs Arch Pharmacol. (2020) 393:991–1002. doi: 10.1007/s00210-019-01766-4
314. Gong DF, Sun SC, Wang RR, Dawuti A, Kong DW, Liu RQ, et al. Salvianolic acid A improve mitochondrial respiration and cardiac function via inhibiting apoptosis pathway through CRYAB in diabetic cardiomyopathy. BioMed Pharmacother. (2023) 160:114382. doi: 10.1016/j.biopha.2023.114382
315. Wu Q, Yuan X, Li B, Han R, Zhang H, Xiu R. Salvianolic acid alleviated blood-brain barrier permeability in spontaneously hypertensive rats by inhibiting apoptosis in pericytes via P53 and the Ras/Raf/MEK/ERK pathway. Drug Des Devel Ther. (2020) 14:1523–34. doi: 10.2147/DDDT.S245959
316. Tang Y, Wa Q, Peng L, Zheng Y, Chen J, Chen X, et al. Salvianolic acid B suppresses ER stress-induced NLRP3 inflammasome and pyroptosis via the AMPK/FoxO4 and syndecan-4/rac1 signaling pathways in human endothelial progenitor cells. Oxid Med Cell Longev. (2022) 2022:8332825. doi: 10.1155/2022/8332825
317. Xu X, Mao C, Zhang C, Zhang M, Gong J, Wang X. Salvianolic acid B inhibits ferroptosis and apoptosis during myocardial ischemia/reperfusion injury via decreasing the ubiquitin-proteasome degradation of GPX4 and the ROS-JNK/MAPK pathways. Molecules. (2023) 28:4117. doi: 10.3390/molecules28104117
318. Zhao M, Li F, Jian Y, Wang X, Yang H, Wang J, et al. Salvianolic acid B regulates macrophage polarization in ischemic/reperfused hearts by inhibiting mTORC1-induced glycolysis. Eur J Pharmacol. (2020) 871:172916. doi: 10.1016/j.ejphar.2020.172916
319. Ma D, Mandour AS, Elfadadny A, Hendawy H, Yoshida T, El-Husseiny HM, et al. Changes in cardiac function during the development of uremic cardiomyopathy and the effect of Salvianolic acid B administration in a rat model. Front Vet Sci. (2022) 9:905759. doi: 10.3389/fvets.2022.905759
320. Li CL, Liu B, Wang ZY, Xie F, Qiao W, Cheng J, et al. Salvianolic acid B improves myocardial function in diabetic cardiomyopathy by suppressing IGFBP3. J Mol Cell Cardiol. (2020) 139:98–112. doi: 10.1016/j.yjmcc.2020.01.009
321. Tian L, Su CP, Wang Q, Wu FJ, Bai R, Zhang HM, et al. Chlorogenic acid: A potent molecule that protects cardiomyocytes from TNF-α-induced injury via inhibiting NF-κB and JNK signals. J Cell Mol Med. (2019) 23:4666–78. doi: 10.1111/jcmm.2019.23.issue-7
322. Wang D, Tian L, Lv H, Pang Z, Li D, Yao Z, et al. Chlorogenic acid prevents acute myocardial infarction in rats by reducing inflammatory damage and oxidative stress. BioMed Pharmacother. (2020) 132:110773. doi: 10.1016/j.biopha.2020.110773
323. Zhu Q, Zhu Y, Liu Y, Tao Y, Lin Y, Lai S, et al. Moderation of gut microbiota and bile acid metabolism by chlorogenic acid improves high-fructose-induced salt-sensitive hypertension in mice. Food Funct. (2022) 13:6987–99. doi: 10.1039/D2FO00038E
324. Preetha Rani MR, Salin Raj P, Nair A, Ranjith S, Rajankutty K, Raghu KG. In vitro and in vivo studies reveal the beneficial effects of chlorogenic acid against ER stress mediated ER-phagy and associated apoptosis in the heart of diabetic rat. Chem Biol Interact. (2022) 351:109755. doi: 10.1016/j.cbi.2021.109755
325. Clark M, Centner AM, Ukhanov V, Nagpal R, Salazar G. Gallic acid ameliorates atherosclerosis and vascular senescence and remodels the microbiome in a sex-dependent manner in ApoE-/- mice. J Nutr Biochem. (2022) 110:109132. doi: 10.1016/j.jnutbio.2022.109132
326. Yan X, Zhang YL, Zhang L, Zou LX, Chen C, Liu Y, et al. Gallic acid suppresses cardiac hypertrophic remodeling and heart failure. Mol Nutr Food Res. (2019) 63:e1800807. doi: 10.1002/mnfr.201800807
327. Jin L, Sun S, Ryu Y, Piao ZH, Liu B, Choi SY, et al. Gallic acid improves cardiac dysfunction and fibrosis in pressure overload-induced heart failure. Sci Rep. (2018) 8:9302. doi: 10.1038/s41598-018-27599-4
328. Han D, Zhang QY, Zhang YL, Han X, Guo SB, Teng F, et al. Gallic acid ameliorates angiotensin II-induced atrial fibrillation by inhibiting immunoproteasome-mediated PTEN degradation in mice. Front Cell Dev Biol. (2020) 8:594683. doi: 10.3389/fcell.2020.594683
329. Yan X, Zhang QY, Zhang YL, Han X, Guo SB, Li HH. Gallic acid attenuates angiotensin ii-induced hypertension and vascular dysfunction by inhibiting the degradation of endothelial nitric oxide synthase. Front Pharmacol. (2020) 11:1121. doi: 10.3389/fphar.2020.01121
330. Sundaresan S, John S, Paneerselvam G, Andiapppan R, Christopher G, Selvam GS. Gallic acid attenuates cadmium mediated cardiac hypertrophic remodelling through upregulation of Nrf2 and PECAM-1signalling in rats. Environ Toxicol Pharmacol. (2021) 87:103701. doi: 10.1016/j.etap.2021.103701
331. Dong X, Zeng Y, Liu Y, You L, Yin X, Fu J, et al. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother Res. (2020) 34:270–81. doi: 10.1002/ptr.v34.2
332. Han X, Bai L, Kee HJ, Jeong MH. Syringic acid mitigates isoproterenol-induced cardiac hypertrophy and fibrosis by downregulating Ereg. J Cell Mol Med. (2022) 26:4076–86. doi: 10.1111/jcmm.v26.14
333. Sabahi Z, Khoshnoud MJ, Hosseini S, Khoshraftar F, Rashedinia M. Syringic acid attenuates cardiomyopathy in streptozotocin-induced diabetic rats. Adv Pharmacol Pharm Sci. (2021) 2021:5018092. doi: 10.1155/2021/5018092
334. Sun R, Wu T, Xing S, Wei S, Bielicki JK, Pan X, et al. Caffeic acid protects against atherosclerotic lesions and cognitive decline in ApoE-/- mice. J Pharmacol Sci. (2023) 151:110–8. doi: 10.1016/j.jphs.2022.12.006
335. Oboh G, Ojueromi OO, Ademosun AO, Omayone TP, Oyagbemi AA, Ajibade TO, et al. Effects of caffeine and caffeic acid on selected biochemical parameters in L-NAME-induced hypertensive rats. J Food Biochem. (2021) 45:e13384. doi: 10.1111/jfbc.13384
336. Lee SY, Kuo YH, Du CX, Huang CW, Ku HC. A novel caffeic acid derivative prevents angiotensin II-induced cardiac remodeling. BioMed Pharmacother. (2023) 162:114709. doi: 10.1016/j.biopha.2023.114709
337. Huwait E, Almowallad S, Al-Massabi R, Saddeek S, Gauthaman K, Prola A. Punicalagin targets atherosclerosis: gene expression profiling of THP-1 macrophages treated with punicalagin and molecular docking. Curr Issues Mol Biol. (2022) 44:2153–66. doi: 10.3390/cimb44050145
338. Yu LM, Dong X, Xue XD, Zhang J, Li Z, Wu HJ, et al. Protection of the myocardium against ischemia/reperfusion injury by punicalagin through an SIRT1-NRF-2-HO-1-dependent mechanism. Chem Biol Interact. (2019) 306:152–62. doi: 10.1016/j.cbi.2019.05.003
339. Fu F, Liu C, Shi R, Li M, Zhang M, Du Y, et al. Punicalagin protects against diabetic cardiomyopathy by promoting Opa1-mediated mitochondrial fusion via regulating PTP1B-Stat3 pathway. Antioxid Redox Signal. (2021) 35:618–41. doi: 10.1089/ars.2020.8248
340. Liu X, Qi K, Gong Y, Long X, Zhu S, Lu F, et al. Ferulic acid alleviates myocardial ischemia reperfusion injury via upregulating AMPKα2 expression-mediated ferroptosis depression. J Cardiovasc Pharmacol. (2021) 79:489–500. doi: 10.1097/FJC.0000000000001199
341. Zhang XJ, Cui ZH, Zhao YX, He TT, Wang L, Liang XW. Ferulic acid ameliorates isoproterenol-induced heart failure by decreasing oxidative stress and inhibiting cardiocyte apoptosis via activating Nrf2 signaling pathway in rats. Biol Pharm Bull. (2021) 44:396–403. doi: 10.1248/bpb.b20-00783
342. Li C, Chen L, Song M, Fang Z, Zhang L, Coffie JW, et al. Ferulic acid protects cardiomyocytes from TNF-α/cycloheximide-induced apoptosis by regulating autophagy. Arch Pharm Res. (2020) 43:863–74. doi: 10.1007/s12272-020-01252-z
343. Salin Raj P, Nair A, Preetha Rani MR, Rajankutty K, Ranjith S, Raghu KG. Ferulic acid attenuates high glucose-induced MAM alterations via PACS2/IP3R2/FUNDC1/VDAC1 pathway activating proapoptotic proteins and ameliorates cardiomyopathy in diabetic rats. Int J Cardiol. (2023) 372:101–9. doi: 10.1016/j.ijcard.2022.12.003
344. Chen C. Anti-atherosclerotic activity of para methoxy cinnamic acid in high fat diet induced hyperlipidemia model rats. Appl Biochem Biotechnol. (2022) 194:1911–24. doi: 10.1007/s12010-021-03735-1
345. Nair A, Preetha Rani MR, Salin Raj P, Ranjit S, Rajankutty K, Raghu KG. Cinnamic acid is beneficial to diabetic cardiomyopathy via its cardioprotective, anti-inflammatory, anti-dyslipidemia, and antidiabetic properties. J Biochem Mol Toxicol. (2022) 36:e23215. doi: 10.1002/jbt.v36.12
346. Koczurkiewicz-Adamczyk P, Klaś K, Gunia-Krzyżak A, Piska K, Andrysiak K, Stępniewski J, et al. Cinnamic acid derivatives as cardioprotective agents against oxidative and structural damage induced by doxorubicin. Int J Mol Sci. (2021) 22:6217. doi: 10.3390/ijms22126217
347. Fan M, Li Z, Hu M, Zhao H, Wang T, Jia Y, et al. Formononetin attenuates Aβ(25-35)-induced adhesion molecules in HBMECs via Nrf2 activation. Brain Res Bull. (2022) 183:162–71. doi: 10.1016/j.brainresbull.2022.03.009
348. Wang DS, Yan LY, Yang DZ, Lyu Y, Fang LH, Wang SB, et al. Formononetin ameliorates myocardial ischemia/reperfusion injury in rats by suppressing the ROS-TXNIP-NLRP3 pathway. Biochem Biophys Res Commun. (2020) 525:759–66. doi: 10.1016/j.bbrc.2020.02.147
349. Yang Y, Huang T, Zhang H, Li X, Shi S, Tian X, et al. Formononetin improves cardiac function and depressive behaviours in myocardial infarction with depression by targeting GSK-3β to regulate macrophage/microglial polarization. Phytomedicine. (2023) 109:154602. doi: 10.1016/j.phymed.2022.154602
350. Wu Y, Cai C, Yang L, Xiang Y, Zhao H, Zeng C. Inhibitory effects of formononetin on the monocrotaline−induced pulmonary arterial hypertension in rats. Mol Med Rep. (2020) 21:1192–200. doi: 10.3892/mmr.2020.10911
351. Li J, Chang WT, Qin G, Wojcik KR, Li CQ, Hsu CW, et al. Baicalein preconditioning cardioprotection involves pro-oxidant signaling and activation of pyruvate dehydrogenase. Am J Chin Med. (2022) 50:1255–67. doi: 10.1142/S0192415X22500513
352. Shi R, Zhu D, Wei Z, Fu N, Wang C, Liu L, et al. Baicalein attenuates monocrotaline-induced pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition. Life Sci. (2018) 207:442–50. doi: 10.1016/j.lfs.2018.06.033
353. Liu BY, Li L, Liu GL, Ding W, Chang WG, Xu T, et al. Baicalein attenuates cardiac hypertrophy in mice via suppressing oxidative stress and activating autophagy in cardiomyocytes. Acta Pharmacol Sin. (2021) 42:701–14. doi: 10.1038/s41401-020-0496-1
354. Ma L, Li XP, Ji HS, Liu YF, Li EZ. Baicalein protects rats with diabetic cardiomyopathy against oxidative stress and inflammation injury via phosphatidylinositol 3-kinase (PI3K)/AKT pathway. Med Sci Monit. (2018) 24:5368–75. doi: 10.12659/MSM.911455
355. Zhao J, Wang Z, Yuan Z, Lv S, Su Q. Baicalin ameliorates atherosclerosis by inhibiting NLRP3 inflammasome in apolipoprotein E-deficient mice. Diabetes Vasc Dis Res. (2020) 17:1479164120977441. doi: 10.1177/1479164120977441
356. Fan Z, Cai L, Wang S, Wang J, Chen B. Baicalin prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis. Front Pharmacol. (2021) 12:628988. doi: 10.3389/fphar.2021.628988
357. Xu M, Li X, Song L. Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm Biol. (2020) 58:655–63. doi: 10.1080/13880209.2020.1779318
358. Cai Y, Jiang S, Huang C, Shen A, Zhang X, Yang W, et al. Baicalin inhibits pressure overload-induced cardiac hypertrophy by regulating the SIRT3-dependent signaling pathway. Phytomedicine. (2023) 114:154747. doi: 10.1016/j.phymed.2023.154747
359. Feng P, Yang Y, Liu N, Wang S. Baicalin regulates TLR4/IκBα/NFκB signaling pathway to alleviate inflammation in Doxorubicin related cardiotoxicity. Biochem Biophys Res Commun. (2022) 637:1–8. doi: 10.1016/j.bbrc.2022.10.061
360. Wu D, Ding L, Tang X, Wang W, Chen Y, Zhang T. Baicalin protects against hypertension-associated intestinal barrier impairment in part through enhanced microbial production of short-chain fatty acids. Front Pharmacol. (2019) 10:1271. doi: 10.3389/fphar.2019.01271
361. Koga M, Kanaoka Y, Inada K, Omine S, Kataoka Y, Yamauchi A. Hesperidin blocks varenicline-aggravated atherosclerotic plaque formation in apolipoprotein E knockout mice by downregulating net uptake of oxidized low-density lipoprotein in macrophages. J Pharmacol Sci. (2020) 143:106–11. doi: 10.1016/j.jphs.2020.01.012
362. Li X, Hu X, Wang J, Xu W, Yi C, Ma R, et al. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. Int J Mol Med. (2018) 42:1917–24. doi: 10.3892/ijmm.2018.3794
363. Bhargava P, Verma VK, Malik S, Khan SI, Bhatia J, Arya DS. Hesperidin regresses cardiac hypertrophy by virtue of PPAR-γ agonistic, anti-inflammatory, antiapoptotic, and antioxidant properties. J Biochem Mol Toxicol. (2019) 33:e22283. doi: 10.1002/jbt.2019.33.issue-5
364. Jang SA, Park DW, Sohn EH, Lee SR, Kang SC. Hyperoside suppresses tumor necrosis factor α-mediated vascular inflammatory responses by downregulating mitogen-activated protein kinases and nuclear factor-κB signaling. Chem Biol Interact. (2018) 294:48–55. doi: 10.1016/j.cbi.2018.08.013
365. Shi Y, Qiu X, Dai M, Zhang X, Jin G. Hyperoside attenuates hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Transplant Proc. (2019) 51:2051–9. doi: 10.1016/j.transproceed.2019.04.066
366. Yang Y, Li J, Rao T, Fang Z, Zhang J. The role and mechanism of hyperoside against myocardial infarction in mice by regulating autophagy via NLRP1 inflammation pathway. J Ethnopharmacol. (2021) 276:114187. doi: 10.1016/j.jep.2021.114187
367. Guo X, Zhang Y, Lu C, Qu F, Jiang X. Protective effect of hyperoside on heart failure rats via attenuating myocardial apoptosis and inducing autophagy. Biosci Biotechnol Biochem. (2020) 84:714–24. doi: 10.1080/09168451.2019.1685369
368. Chang X, Zhang T, Liu D, Meng Q, Yan P, Luo D, et al. Puerarin attenuates LPS-induced inflammatory responses and oxidative stress injury in human umbilical vein endothelial cells through mitochondrial quality control. Oxid Med Cell Longev. (2021) 2021:6659240. doi: 10.1155/2021/6659240
369. Ding Y, Li W, Peng S, Zhou G, Chen S, Wei Y, et al. Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting ferroptosis. Biol Pharm Bull. (2023) 46:524–32. doi: 10.1248/bpb.b22-00174
370. He L, Wang T, Chen BW, Lu FM, Xu J. Puerarin inhibits apoptosis and inflammation in myocardial cells via PPARα expression in rats with chronic heart failure. Exp Ther Med. (2019) 18:3347–56. doi: 10.3892/etm.2019.7984
371. Hou N, Huang Y, Cai SA, Yuan WC, Li LR, Liu XW, et al. Puerarin ameliorated pressure overload-induced cardiac hypertrophy in ovariectomized rats through activation of the PPARα/PGC-1 pathway. Acta Pharmacol Sin. (2021) 42:55–67. doi: 10.1038/s41401-020-0401-y
372. Shi W, Yuan R, Chen X, Xin Q, Wang Y, Shang X, et al. Puerarin reduces blood pressure in spontaneously hypertensive rats by targeting eNOS. Am J Chin Med. (2019) 47:19–38. doi: 10.1142/S0192415X19500022
373. Chen F, Chen ZQ, Wang H, Zhu JJ. Puerarin pretreatment inhibits myocardial apoptosis and improves cardiac function in rats after acute myocardial infarction through the PI3K/Akt signaling pathway. Adv Clin Exp Med. (2021) 30:255–61. doi: 10.17219/acem/131754
374. Yin MS, Zhang YC, Xu SH, Liu JJ, Sun XH, Liang C, et al. Puerarin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of inflammation. J Asian Nat Prod Res. (2019) 21:476–93. doi: 10.1080/10286020.2017.1405941
375. Cao H, Jia Q, Yan L, Chen C, Xing S, Shen D. Quercetin suppresses the progression of atherosclerosis by regulating MST1-mediated autophagy in ox-LDL-induced RAW264.7 macrophage foam cells. Int J Mol Sci. (2019) 20:6093. doi: 10.3390/ijms20236093
376. Tang J, Lu L, Liu Y, Ma J, Yang L, Li L, et al. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling. J Cell Biochem. (2019) 120:9747–57. doi: 10.1002/jcb.v120.6
377. Jiang C, Li D, Chen L, Liu Y, Zhao Y, Mei G, et al. Quercetin ameliorated cardiac injury via reducing inflammatory actions and the glycerophospholipid metabolism dysregulation in a diabetic cardiomyopathy mouse model. Food Funct. (2022) 13:7847–56. doi: 10.1039/D2FO00912A
378. Albadrani GM, BinMowyna MN, Bin-Jumah MN, El-Akabawy G, Aldera H, Al-Farga AM. Quercetin prevents myocardial infarction adverse remodeling in rats by attenuating TGF-β1/Smad3 signaling: Different mechanisms of action. Saudi J Biol Sci. (2021) 28:2772–82. doi: 10.1016/j.sjbs.2021.02.007
379. Feng ST, Wang XL, Wang YT, Yuan YH, Li ZP, Chen NH, et al. Efficacy of traditional Chinese medicine combined with selective serotonin reuptake inhibitors on the treatment for Parkinson's disease with depression: a systematic review and meta-analysis. Am J Chin Med. (2021) 49:627–43. doi: 10.1142/S0192415X21500282
380. Feng Z, Wang C, Yue, Jin, Meng Q, Wu J, et al. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm Biol. (2021) 59:1106–16. doi: 10.1080/13880209.2021.1961823
381. Zhang L, Guo Z, Wang Y, Geng J, Han S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev Res. (2019) 80:294–309. doi: 10.1002/ddr.21495
382. Alshehri AS, El-Kott AF, Eleawa SM, El-Gerbed MSA, Khalifa HS, El-Kenawy AE, et al. Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J Physiol Pharmacol. (2021) 72:339–55. doi: 10.26402/jpp.2021.3.04
383. Zhao R, Xiao H, Jin T, Xu F, Li Y, Li H, et al. Naringenin promotes cell autophagy to improve high-fat-diet-induced atherosclerosis in ApoE-/- mice. Braz J Med Biol Res. (2021) 54:e9764. doi: 10.1590/1414-431x20209764
384. Xu X, Lei T, Li W, Ou H. Enhanced cellular cholesterol efflux by naringenin is mediated through inhibiting endoplasmic reticulum stress - ATF6 activity in macrophages. Biochim Biophys Acta Mol Cell Biol Lipids. (2019) 1864:1472–82. doi: 10.1016/j.bbalip.2019.06.005
385. Ma K, Liu W, Liu Q, Hu P, Bai L, Yu M, et al. Naringenin facilitates M2 macrophage polarization after myocardial ischemia-reperfusion by promoting nuclear translocation of transcription factor EB and inhibiting the NLRP3 inflammasome pathway. Environ Toxicol. (2023) 38:1405–19. doi: 10.1002/tox.23774
386. Li Y, He B, Zhang C, He Y, Xia T, Zeng C. Naringenin attenuates isoprenaline-induced cardiac hypertrophy by suppressing oxidative stress through the AMPK/NOX2/MAPK signaling pathway. Nutrients. (2023) 15:1340. doi: 10.3390/nu15061340
387. He Y, Wang S, Sun H, Li Y, Feng J. Naringenin ameliorates myocardial injury in STZ-induced diabetic mice by reducing oxidative stress, inflammation and apoptosis via regulating the Nrf2 and NF-κB signaling pathways. Front Cardiovasc Med. (2022) 9:946766. doi: 10.3389/fcvm.2022.946766
388. Shen W, Anwaier G, Cao Y, Lian G, Chen C, Liu S, et al. Atheroprotective mechanisms of tilianin by inhibiting inflammation through down-regulating NF-κB pathway and foam cells formation. Front Physiol. (2019) 10:825. doi: 10.3389/fphys.2019.00825
389. Tian L, Cao W, Yue R, Yuan Y, Guo X, Qin D, et al. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J Pharmacol Sci. (2019) 139:352–60. doi: 10.1016/j.jphs.2019.02.008
390. Jiang H, Xing J, Fang J, Wang L, Wang Y, Zeng L, et al. Tilianin protects against ischemia/reperfusion-induced myocardial injury through the inhibition of the Ca(2+)/calmodulin-dependent protein kinase ii-dependent apoptotic and inflammatory signaling pathways. BioMed Res Int. (2020) 2020:5939715. doi: 10.1155/2020/5939715
391. Yao J, Li Y, Jin Y, Chen Y, Tian L, He W. Synergistic cardioptotection by tilianin and syringin in diabetic cardiomyopathy involves interaction of TLR4/NF-κB/NLRP3 and PGC1a/SIRT3 pathways. Int Immunopharmacol. (2021) 96:107728. doi: 10.1016/j.intimp.2021.107728
392. Yu XH, Chen JJ, Deng WY, Xu XD, Liu QX, Shi MW, et al. Biochanin A mitigates atherosclerosis by inhibiting lipid accumulation and inflammatory response. Oxid Med Cell Longev. (2020) 2020:8965047. doi: 10.1155/2020/8965047
393. Bai Y, Li Z, Liu W, Gao D, Liu M, Zhang P. Biochanin A attenuates myocardial ischemia/reperfusion injury through the TLR4/NF-κB/NLRP3 signaling pathway. Acta Cir Bras. (2019) 34:e201901104. doi: 10.1590/s0102-865020190110000004
394. Oza MJ, Kulkarni YA. Biochanin A attenuates cardiomyopathy in type 2 diabetic rats by increasing SIRT1 expression and reducing oxidative stress. Chem Biodivers. (2022) 19:e202100591. doi: 10.1002/cbdv.202100591
395. Sangeethadevi G, VS V, Jansy Isabella RAR, Saravanan G, Ponmurugan P, Chandrasekaran P, et al. Attenuation of lipid metabolic abnormalities, proinflammatory cytokines, and matrix metalloproteinase expression by biochanin-A in isoproterenol-induced myocardial infarction in rats. Drug Chem Toxicol. (2022) 45:1951–62. doi: 10.1080/01480545.2021.1894707
396. Feng X, Du M, Li S, Zhang Y, Ding J, Wang J, et al. Hydroxysafflor yellow A regulates lymphangiogenesis and inflammation via the inhibition of PI3K on regulating AKT/mTOR and NF-κB pathway in macrophages to reduce atherosclerosis in ApoE-/- mice. Phytomedicine. (2023) 112:154684. doi: 10.1016/j.phymed.2023.154684
397. Ye J, Lu S, Wang M, Ge W, Liu H, Qi Y, et al. Hydroxysafflor yellow A protects against myocardial ischemia/reperfusion injury via suppressing NLRP3 inflammasome and activating autophagy. Front Pharmacol. (2020) 11:1170. doi: 10.3389/fphar.2020.01170
398. Yao R, Cao Y, Jiang R, Zhang X, Li F, Wang S. Pharmacokinetic characteristics of hydroxysafflor yellow A in normal and diabetic cardiomyopathy mice. BioMed Chromatogr. (2021) 35:e5173. doi: 10.1002/bmc.v35.10
399. Ni B, Zhou D, Jing Y, Liu S. Hydroxysafflor yellow A protects against angiotensin II−induced hypertrophy. Mol Med Rep. (2018) 18:3649–56. doi: 10.3892/mmr.2018.9399
400. Thang SK, Chen PY, Gao WY, Wu MJ, Pan MH, Yen JH. Xanthohumol suppresses NPC1L1 gene expression through downregulation of HNF-4α and inhibits cholesterol uptake in Caco-2 cells. J Agric Food Chem. (2019) 67:11119–28. doi: 10.1021/acs.jafc.9b05221
401. Lin JH, Yang KT, Lee WS, Ting PC, Luo YP, Lin DJ, et al. Xanthohumol protects the rat myocardium against ischemia/reperfusion injury-induced ferroptosis. Oxid Med Cell Longev. (2022) 2022:9523491. doi: 10.1155/2022/9523491
402. Sun TL, Li WQ, Tong XL, Liu XY, Zhou WH. Xanthohumol attenuates isoprenaline-induced cardiac hypertrophy and fibrosis through regulating PTEN/AKT/mTOR pathway. Eur J Pharmacol. (2021) 891:173690. doi: 10.1016/j.ejphar.2020.173690
403. Yang Z, Li T, Wang C, Meng M, Tan S, Chen L. Dihydromyricetin inhibits M1 macrophage polarization in atherosclerosis by modulating miR-9-mediated SIRT1/NF-κB signaling pathway. Mediators Inflammation. (2023) 2023:2547588. doi: 10.1155/2023/2547588
404. Wei L, Sun X, Qi X, Zhang Y, Li Y, Xu Y. Dihydromyricetin ameliorates cardiac ischemia/reperfusion injury through Sirt3 activation. BioMed Res Int. (2019) 2019:6803943. doi: 10.1155/2019/6803943
405. Chen Y, Zheng Y, Chen R, Shen J, Zhang S, Gu Y, et al. Dihydromyricetin attenuates diabetic cardiomyopathy by inhibiting oxidative stress, inflammation and necroptosis via sirtuin 3 activation. Antioxidants (Basel). (2023) 12:200. doi: 10.3390/antiox12010200
406. Chen Y, Luo HQ, Sun LL, Xu MT, Yu J, Liu LL, et al. Dihydromyricetin attenuates myocardial hypertrophy induced by transverse aortic constriction via oxidative stress inhibition and SIRT3 pathway enhancement. Int J Mol Sci. (2018) 19:2592. doi: 10.3390/ijms19092592
407. Wu Y, Song F, Li Y, Li J, Cui Y, Hong Y, et al. Acacetin exerts antioxidant potential against atherosclerosis through Nrf2 pathway in ApoE-/- mice. J Cell Mol Med. (2021) 25:521–34. doi: 10.1111/jcmm.16106
408. Wu C, Chen RL, Wang Y, Wu WY, Li G. Acacetin alleviates myocardial ischaemia/reperfusion injury by inhibiting oxidative stress and apoptosis via the Nrf-2/HO-1 pathway. Pharm Biol. (2022) 60:553–61. doi: 10.1080/13880209.2022.2041675
409. Cui YK, Hong YX, Wu WY, Han WM, Wu Y, Wu C, et al. Acacetin ameliorates cardiac hypertrophy by activating Sirt1/AMPK/PGC-1α pathway. Eur J Pharmacol. (2022) 920:174858. doi: 10.1016/j.ejphar.2022.174858
410. Song F, Mao YJ, Hu Y, Zhao SS, Wang R, Wu WY, et al. Acacetin attenuates diabetes-induced cardiomyopathy by inhibiting oxidative stress and energy metabolism via PPAR-α/AMPK pathway. Eur J Pharmacol. (2022) 922:174916. doi: 10.1016/j.ejphar.2022.174916
411. Li Y, Dang Q, Li Z, Han C, Yang Y, Li M, et al. Restoration of mitochondrial function is essential in the endothelium-dependent vasodilation induced by acacetin in hypertensive rats. Int J Mol Sci. (2022) 23:11350. doi: 10.3390/ijms231911350
412. Huang P, Wang F, Zhang Y, Zhang Y, Qin M, Ji J, et al. Icariin alleviates atherosclerosis by regulating the miR-205-5p/ERBB4/AKT signaling pathway. Int Immunopharmacol. (2023) 114:109611. doi: 10.1016/j.intimp.2022.109611
413. Liu XJ, Lv YF, Cui WZ, Li Y, Liu Y, Xue YT, et al. Icariin inhibits hypoxia/reoxygenation-induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1 signaling pathway. FEBS Open Bio. (2021) 11:2966–76. doi: 10.1002/2211-5463.13276
414. Sai X, Li Z, Deng G, Wang L, Xiaowu W, Nasser MI, et al. Immunomodulatory effects of icariin in a myocardial infarction mouse model. Bioengineered. (2022) 13:12504–15. doi: 10.1080/21655979.2022.2076453
415. Yu LM, Dong X, Xu YL, Zhou ZJ, Huang YT, Zhao JK, et al. Icariin attenuates excessive alcohol consumption-induced susceptibility to atrial fibrillation through SIRT3 signaling. Biochim Biophys Acta Mol Basis Dis. (2022) 1868:166483. doi: 10.1016/j.bbadis.2022.166483
416. Liu QW, Yang ZH, Jiang J, Jiang R. Icariin modulates eNOS activity via effect on post-translational protein-protein interactions to improve erectile function of spontaneously hypertensive rats. Andrology. (2021) 9:342–51. doi: 10.1111/andr.12875
417. Hu L, Wang Z, Li H, Wei J, Tang F, Wang Q, et al. Icariin inhibits isoproterenol-induced cardiomyocyte hypertropic injury through activating autophagy via the AMPK/mTOR signaling pathway. Biochem Biophys Res Commun. (2022) 593:65–72. doi: 10.1016/j.bbrc.2022.01.029
418. Ni T, Lin N, Huang X, Lu W, Sun Z, Zhang J, et al. Icariin ameliorates diabetic cardiomyopathy through Apelin/Sirt3 signalling to improve mitochondrial dysfunction. Front Pharmacol. (2020) 11:256. doi: 10.3389/fphar.2020.00256
419. Su Y, Fan X, Li S, Li Z, Tian M, Li S. Scutellarin improves type 2 diabetic cardiomyopathy by regulating cardiomyocyte autophagy and apoptosis. Dis Markers. (2022) 2022:3058354. doi: 10.1155/2022/3058354
420. Zhang X, Han X, Zhang P, Zhou T, Chen Y, Jin J, et al. Morin attenuates oxidized low-density lipoprotein-mediated injury by inducing autophagy via activating AMPK signalling in HUVECs. Clin Exp Pharmacol Physiol. (2019) 46:1053–60. doi: 10.1111/1440-1681.13160
421. Verma VK, Malik S, Mutneja E, Sahu AK, Bhatia J, Arya DS. Attenuation of ROS-mediated myocardial ischemia-reperfusion injury by morin via regulation of RISK/SAPK pathways. Pharmacol Rep. (2020) 72:877–89. doi: 10.1007/s43440-019-00011-2
422. Salameh A, Dhein S, Mewes M, Sigusch S, Kiefer P, Vollroth M, et al. Anti-oxidative or anti-inflammatory additives reduce ischemia/reperfusions injury in an animal model of cardiopulmonary bypass. Saudi J Biol Sci. (2020) 27:18–29. doi: 10.1016/j.sjbs.2019.04.003
423. Mou Q, Jia Z, Luo M, Liu L, Huang X, Quan J, et al. Epigallocatechin-3-gallate exerts cardioprotective effects related to energy metabolism in pressure overload-induced cardiac dysfunction. Arch Biochem Biophys. (2022) 723:109217. doi: 10.1016/j.abb.2022.109217
424. Wang J, Li P, Qin T, Sun D, Zhao X, Zhang B. Protective effect of epigallocatechin-3-gallate against neuroinflammation and anxiety-like behavior in a rat model of myocardial infarction. Brain Behav. (2020) 10:e01633. doi: 10.1002/brb3.v10.6
425. Mohd Sabri NA, Lee SK, Murugan DD, Ling WC. Epigallocatechin gallate (EGCG) alleviates vascular dysfunction in angiotensin II-infused hypertensive mice by modulating oxidative stress and eNOS. Sci Rep. (2022) 12:17633. doi: 10.1038/s41598-022-21107-5
426. Li G, Pan B, Liu L, Xu X, Zhao W, Mou Q, et al. Epigallocatechin-3-gallate restores mitochondrial homeostasis impairment by inhibiting HDAC1-mediated NRF1 histone deacetylation in cardiac hypertrophy. Mol Cell Biochem. (2023) 479:963–73. doi: 10.1007/s11010-023-04768-2
427. Cui Y, Wang Y, Liu G. Epigallocatechin gallate (EGCG) attenuates myocardial hypertrophy and fibrosis induced by transverse aortic constriction via inhibiting the Akt/mTOR pathway. Pharm Biol. (2021) 59:1305–13. doi: 10.1080/13880209.2021.1972124
428. Gui L, Wang F, Hu X, Liu X, Yang H, Cai Z, et al. Epigallocatechin gallate protects diabetes mellitus rats complicated with cardiomyopathy through TGF-β1/JNK signaling pathway. Curr Pharm Des. (2022) 28:2758–70. doi: 10.2174/1381612828666220902115437
429. Li T, Tong Q, Wang Z, Yang Z, Sun Y, Cai J, et al. Epigallocatechin-3-gallate inhibits atrial fibrosis and reduces the occurrence and maintenance of atrial fibrillation and its possible mechanisms. Cardiovasc Drugs Ther. (2023). doi: 10.1007/s10557-023-07447-y
430. Guo L, Zhang X, Lv N, Wang L, Gan J, Jiang X, et al. Therapeutic role and potential mechanism of resveratrol in atherosclerosis: TLR4/NF-κB/HIF-1α. Mediators Inflammation. (2023) 2023:1097706. doi: 10.1155/2023/1097706
431. Zheng M, Bai Y, Sun X, Fu R, Liu L, Liu M, et al. Resveratrol reestablishes mitochondrial quality control in myocardial ischemia/reperfusion injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α pathway. Molecules. (2022) 27:5545. doi: 10.3390/molecules27175545
432. Gal R, Deres L, Horvath O, Eros K, Sandor B, Urban P, et al. Resveratrol improves heart function by moderating inflammatory processes in patients with systolic heart failure. Antioxidants (Basel). (2020) 9:1108. doi: 10.3390/antiox9111108
433. Jiang J, Gu X, Wang H, Ding S. Resveratrol improves cardiac function and left ventricular fibrosis after myocardial infarction in rats by inhibiting NLRP3 inflammasome activity and the TGF-β1/SMAD2 signaling pathway. PeerJ. (2021) 9:e11501. doi: 10.7717/peerj.11501
434. Liu J, Zhang M, Qin C, Wang Z, Chen J, Wang R, et al. Resveratrol attenuate myocardial injury by inhibiting ferroptosis via inducing KAT5/GPX4 in myocardial infarction. Front Pharmacol. (2022) 13:906073. doi: 10.3389/fphar.2022.906073
435. Grujić-Milanović J, Jaćević V, Miloradović Z, Jovović D, Milosavljević I, Milanović SD, et al. Resveratrol protects cardiac tissue in experimental Malignant hypertension due to antioxidant, anti-inflammatory, and anti-apoptotic properties. Int J Mol Sci. (2021) 22:5006. doi: 10.3390/ijms22095006
436. Tain YL, Lee WC, Wu KLH, Leu S, Chan JYH. Resveratrol prevents the development of hypertension programmed by maternal plus post-weaning high-fructose consumption through modulation of oxidative stress, nutrient-sensing signals, and gut microbiota. Mol Nutr Food Res. (2018) 62:e1800066. doi: 10.1002/mnfr.201800066
437. Guan P, Sun ZM, Wang N, Zhou J, Luo LF, Zhao YS, et al. Resveratrol prevents chronic intermittent hypoxia-induced cardiac hypertrophy by targeting the PI3K/AKT/mTOR pathway. Life Sci. (2019) 233:116748. doi: 10.1016/j.lfs.2019.116748
438. Chen TS, Chuang SY, Shen CY, Ho TJ, Chang RL, Yeh YL, et al. Antioxidant Sirt1/Akt axis expression in resveratrol pretreated adipose-derived stem cells increases regenerative capability in a rat model with cardiomyopathy induced by diabetes mellitus. J Cell Physiol. (2021) 236:4290–302. doi: 10.1002/jcp.v236.6
439. Zhang Y, Zhang S, Liu Z, Zhao X, Yuan Y, Sheng L, et al. Resveratrol prevents atrial fibrillation by inhibiting atrial structural and metabolic remodeling in collagen-induced arthritis rats. Naunyn Schmiedebergs Arch Pharmacol. (2018) 391:1179–90. doi: 10.1007/s00210-018-1554-9
440. Xiong Q, Yan Z, Liang J, Yuan J, Chen X, Zhou L, et al. Polydatin alleviates high-fat diet induced atherosclerosis in apolipoprotein E-deficient mice by autophagic restoration. Phytomedicine. (2021) 81:153301. doi: 10.1016/j.phymed.2020.153301
441. Yu LM, Dong X, Li N, Jiang H, Zhao JK, Xu YL, et al. Polydatin attenuates chronic alcohol consumption-induced cardiomyopathy through a SIRT6-dependent mechanism. Food Funct. (2022) 13:7302–19. doi: 10.1039/D2FO00966H
442. Luo P, Shi W, Wang Y, Ma H, Liu T, Yan D, et al. Raloxifene inhibits IL-6/STAT3 signaling pathway and protects against high-fat-induced atherosclerosis in ApoE-/- mice. Life Sci. (2020) 261:118304. doi: 10.1016/j.lfs.2020.118304
443. Huo S, Shi W, Ma H, Yan D, Luo P, Guo J, et al. Alleviation of inflammation and oxidative stress in pressure overload-induced cardiac remodeling and heart failure via IL-6/STAT3 inhibition by raloxifene. Oxid Med Cell Longev. (2021) 2021:6699054. doi: 10.1155/2021/6699054
444. Ye B, Chen X, Dai S, Han J, Liang X, Lin S, et al. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther. (2019) 13:975–90. doi: 10.2147/DDDT.S195412
445. Liu J, Ning L. Protective role of emodin in rats with post-myocardial infarction heart failure and influence on extracellular signal-regulated kinase pathway. Bioengineered. (2021) 12:10246–53. doi: 10.1080/21655979.2021.1983977
446. Xiao D, Zhang Y, Wang R, Fu Y, Zhou T, Diao H, et al. Emodin alleviates cardiac fibrosis by suppressing activation of cardiac fibroblasts via upregulating metastasis associated protein 3. Acta Pharm Sin B. (2019) 9:724–33. doi: 10.1016/j.apsb.2019.04.003
447. Li X, Hu X, Pan T, Dong L, Ding L, Wang Z, et al. Kanglexin, a new anthraquinone compound, attenuates lipid accumulation by activating the AMPK/SREBP-2/PCSK9/LDLR signalling pathway. BioMed Pharmacother. (2021) 133:110802. doi: 10.1016/j.biopha.2020.110802
448. Bian Y, Li X, Pang P, Hu XL, Yu ST, Liu YN, et al. Kanglexin, a novel anthraquinone compound, protects against myocardial ischemic injury in mice by suppressing NLRP3 and pyroptosis. Acta Pharmacol Sin. (2020) 41:319–26. doi: 10.1038/s41401-019-0307-8
449. Shao X, Liu Z, Liu S, Lin N, Deng Y. Astragaloside IV alleviates atherosclerosis through targeting circ_0000231/miR-135a-5p/CLIC4 axis in AS cell model in vitro. Mol Cell Biochem. (2021) 476:1783–95. doi: 10.1007/s11010-020-04035-8
450. Jiang M, Ni J, Cao Y, Xing X, Wu Q, Fan G. Astragaloside IV attenuates myocardial ischemia-reperfusion injury from oxidative stress by regulating succinate, lysophospholipid metabolism, and ROS scavenging system. Oxid Med Cell Longev. (2019) 2019:9137654. doi: 10.1155/2019/9137654
451. Sui YB, Wang Y, Liu L, Liu F, Zhang YQ. Astragaloside IV alleviates heart failure by promoting angiogenesis through the JAK-STAT3 pathway. Pharm Biol. (2019) 57:48–54. doi: 10.1080/13880209.2019.1569697
452. Cheng S, Zhang X, Feng Q, Chen J, Shen L, Yu P, et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. (2019) 227:82–93. doi: 10.1016/j.lfs.2019.04.040
453. Jing H, Xie R, Bai Y, Duan Y, Sun C, Wang Y, et al. The mechanism actions of astragaloside IV prevents the progression of hypertensive heart disease based on network pharmacology and experimental pharmacology. Front Pharmacol. (2021) 12:755653. doi: 10.3389/fphar.2021.755653
454. Li X, Li Z, Dong X, Wu Y, Li B, Kuang B, et al. Astragaloside IV attenuates myocardial dysfunction in diabetic cardiomyopathy rats through downregulation of CD36-mediated ferroptosis. Phytother Res. (2023) 37:3042–56. doi: 10.1002/ptr.v37.7
455. Zhu Y, Qian X, Li J, Lin X, Luo J, Huang J, et al. Astragaloside-IV protects H9c2 cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artif Cells Nanomed Biotechnol. (2019) 47:4172–81. doi: 10.1080/21691401.2019.1687492
456. Wang ZC, Niu KM, Wu YJ, Du KR, Qi LW, Zhou YB, et al. A dual Keap1 and p47(phox) inhibitor Ginsenoside Rb1 ameliorates high glucose/ox-LDL-induced endothelial cell injury and atherosclerosis. Cell Death Dis. (2022) 13:824. doi: 10.1038/s41419-022-05274-x
457. Jiang L, Yin X, Chen YH, Chen Y, Jiang W, Zheng H, et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics. (2021) 11:1703–20. doi: 10.7150/thno.43895
458. Li C, Zhang X, Li J, Liang L, Zeng J, Wen M, et al. Ginsenoside Rb1 promotes the activation of PPARα pathway via inhibiting FADD to ameliorate heart failure. Eur J Pharmacol. (2023) 947:175676. doi: 10.1016/j.ejphar.2023.175676
459. Zhang C, Han M, Zhang X, Tong H, Sun X, Sun G. Ginsenoside Rb1 protects against diabetic cardiomyopathy by regulating the adipocytokine pathway. J Inflammation Res. (2022) 15:71–83. doi: 10.2147/JIR.S348866
460. Wang S, Yang S, Chen Y, Chen Y, Li R, Han S, et al. Ginsenoside Rb2 alleviated atherosclerosis by inhibiting M1 macrophages polarization induced by microRNA-216a. Front Pharmacol. (2021) 12:764130. doi: 10.3389/fphar.2021.764130
461. Xue Y, Fu W, Liu Y, Yu P, Sun M, Li X, et al. Ginsenoside Rb2 alleviates myocardial ischemia/reperfusion injury in rats through SIRT1 activation. J Food Sci. (2020) 85:4039–49. doi: 10.1111/1750-3841.15505
462. Zhao J, Cui L, Sun J, Xie Z, Zhang L, Ding Z, et al. Notoginsenoside R1 alleviates oxidized low-density lipoprotein-induced apoptosis, inflammatory response, and oxidative stress in HUVECS through modulation of XIST/miR-221-3p/TRAF6 axis. Cell Signal. (2020) 76:109781. doi: 10.1016/j.cellsig.2020.109781
463. Zeng JJ, Shi HQ, Ren FF, Zhao XS, Chen QY, Wang DJ, et al. Notoginsenoside R1 protects against myocardial ischemia/reperfusion injury in mice via suppressing TAK1-JNK/p38 signaling. Acta Pharmacol Sin. (2023) 44:1366–79. doi: 10.1038/s41401-023-01057-y
464. Zhang B, Zhang J, Zhang C, Zhang X, Ye J, Kuang S, et al. Notoginsenoside R1 protects against diabetic cardiomyopathy through activating estrogen receptor α and its downstream signaling. Front Pharmacol. (2018) 9:1227. doi: 10.3389/fphar.2018.01227
465. Xiao J, Zhu T, Yin YZ, Sun B. Notoginsenoside R1, a unique constituent of Panax notoginseng, blinds proinflammatory monocytes to protect against cardiac hypertrophy in ApoE-/- mice. Eur J Pharmacol. (2018) 833:441–50. doi: 10.1016/j.ejphar.2018.07.004
466. Wen J, Chang Y, Huo S, Li W, Huang H, Gao Y, et al. Tanshinone IIA attenuates atherosclerosis via inhibiting NLRP3 inflammasome activation. Aging (Albany NY). (2020) 13:910–32. doi: 10.18632/aging.202202
467. Zhu PC, Shen J, Qian RY, Xu J, Liu C, Hu WM, et al. Effect of tanshinone IIA for myocardial ischemia/reperfusion injury in animal model: preclinical evidence and possible mechanisms. Front Pharmacol. (2023) 14:1165212. doi: 10.3389/fphar.2023.1165212
468. Sun M, Wang W, Min L, Chen C, Li Q, Weng W. Secreted frizzled-related protein 5 (SFRP5) protects ATDC5 cells against LPS-induced inflammation and apoptosis via inhibiting Wnt5a/JNK pathway. J Orthop Surg Res. (2021) 16:129. doi: 10.1186/s13018-021-02260-5
469. Wu S, Lu D, Gajendran B, Hu Q, Zhang J, Wang S, et al. Tanshinone IIA ameliorates experimental diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress in cardiomyocytes via SIRT1. Phytother Res. (2023) 37:3543–58. doi: 10.1002/ptr.v37.8
470. Huang L, Zhu J, Zheng M, Zou R, Zhou Y, Zhu M. Tanshinone IIA protects against subclinical lipopolysaccharide induced cardiac fibrosis in mice through inhibition of NADPH oxidase. Int Immunopharmacol. (2018) 60:59–63. doi: 10.1016/j.intimp.2018.04.036
471. Wu F, Ye B, Wu X, Lin X, Li Y, Wu Y, et al. Paeoniflorin on rat myocardial ischemia reperfusion injury of protection and mechanism research. Pharmacology. (2020) 105:281–8. doi: 10.1159/000503583
472. Liu M, Feng J, Du Q, Ai J, Lv Z. Paeoniflorin attenuates myocardial fibrosis in isoprenaline-induced chronic heart failure rats via inhibiting P38 MAPK pathway. Curr Med Sci. (2020) 40:307–12. doi: 10.1007/s11596-020-2178-0
473. Liu X, Chen K, Zhuang Y, Huang Y, Sui Y, Zhang Y, et al. Paeoniflorin improves pressure overload-induced cardiac remodeling by modulating the MAPK signaling pathway in spontaneously hypertensive rats. BioMed Pharmacother. (2019) 111:695–704. doi: 10.1016/j.biopha.2018.12.090
474. Hu H, Wang C, Jin Y, Meng Q, Liu Q, Liu Z, et al. Catalpol inhibits homocysteine-induced oxidation and inflammation via inhibiting Nox4/NF-κB and GRP78/PERK pathways in human aorta endothelial cells. Inflammation. (2019) 42:64–80. doi: 10.1007/s10753-018-0873-9
475. Ge H, Lin W, Lou Z, Chen R, Shi H, Zhao Q, et al. Catalpol alleviates myocardial ischemia reperfusion injury by activating the Nrf2/HO-1 signaling pathway. Microvasc Res. (2022) 140:104302. doi: 10.1016/j.mvr.2021.104302
476. Xia Y, Lu YW, Hao RJ, Yu GR. Catalpol relieved angiotensin II-induced blood-brain barrier destruction via inhibiting the TLR4 pathway in brain endothelial cells. Pharm Biol. (2022) 60:2210–8. doi: 10.1080/13880209.2022.2142801
477. Zou G, Zhong W, Wu F, Wang X, Liu L. Catalpol attenuates cardiomyocyte apoptosis in diabetic cardiomyopathy via Neat1/miR-140-5p/HDAC4 axis. Biochimie. (2019) 165:90–9. doi: 10.1016/j.biochi.2019.05.005
478. Song R, Han S, Gao H, Jiang H, Li X. Crocin alleviates cognitive impairment associated with atherosclerosis via improving neuroinflammation in LDLR-/- mice fed a high-fat/cholesterol diet. Phytother Res. (2022) 36:1284–96. doi: 10.1002/ptr.v36.3
479. Wang X, Yuan B, Cheng B, Liu Y, Zhang B, Wang X, et al. Crocin alleviates myocardial ischemia/reperfusion-induced endoplasmic reticulum stress via regulation of miR-34a/Sirt1/Nrf2 pathway. Shock. (2019) 51:123–30. doi: 10.1097/SHK.0000000000001116
480. Yuan C, Chen Z, Zhou Q. Crocin inhibits KBTBD7 to prevent excessive inflammation and cardiac dysfunction following myocardial infarction. Mol Med Rep. (2023) 27:20. doi: 10.3892/mmr.2022.12907
481. Chen X, Huang J, Lv Y, Chen Y, Rao J. Crocin exhibits an antihypertensive effect in a rat model of gestational hypertension and activates the Nrf-2/HO-1 signaling pathway. Hypertens Res. (2021) 44:642–50. doi: 10.1038/s41440-020-00609-7
482. Feidantsis K, Mellidis K, Galatou E, Sinakos Z, Lazou A. Treatment with crocin improves cardiac dysfunction by normalizing autophagy and inhibiting apoptosis in STZ-induced diabetic cardiomyopathy. Nutr Metab Cardiovasc Dis. (2018) 28:952–61. doi: 10.1016/j.numecd.2018.06.005
483. Lv Z, Shan X, Tu Q, Wang J, Chen J, Yang Y. Ginkgolide B treatment regulated intestinal flora to improve high-fat diet induced atherosclerosis in ApoE-/- mice. BioMed Pharmacother. (2021) 134:111100. doi: 10.1016/j.biopha.2020.111100
484. Feng Z, Yang X, Zhang L, Ansari IA, Khan MS, Han S, et al. Ginkgolide B ameliorates oxidized low-density lipoprotein-induced endothelial dysfunction via modulating lectin-like ox-LDL-receptor-1 and NADPH oxidase 4 expression and inflammatory cascades. Phytother Res. (2018) 32:2417–27. doi: 10.1002/ptr.v32.12
485. Zhang R, Xu L, Zhang D, Hu B, Luo Q, Han D, et al. Cardioprotection of Ginkgolide B on myocardial ischemia/reperfusion-induced inflammatory injury via regulation of A20-NF-κB pathway. Front Immunol. (2018) 9:2844. doi: 10.3389/fimmu.2018.02844
486. Liu J, Wu P, Xu Z, Zhang J, Liu J, Yang Z. Ginkgolide B inhibits hydrogen peroxide−induced apoptosis and attenuates cytotoxicity via activating the PI3K/Akt/mTOR signaling pathway in H9c2 cells. Mol Med Rep. (2020) 22:310–6. doi: 10.3892/mmr.2020.11099
487. Ge Y, Xu W, Zhang L, Liu M. Ginkgolide B attenuates myocardial infarction-induced depression-like behaviors via repressing IL-1β in central nervous system. Int Immunopharmacol. (2020) 85:106652. doi: 10.1016/j.intimp.2020.106652
488. Jiang Q, Lu M, Li J, Zhu Z. Ginkgolide B protects cardiomyocytes from angiotensin II-induced hypertrophy via regulation of autophagy through SIRT1-FoxO1. Cardiovasc Ther. (2021) 2021:5554569. doi: 10.1155/2021/5554569
489. Jiang YX, Li W, Wang J, Wang GG. Cardiac dysfunction is attenuated by ginkgolide B via reducing oxidative stress and fibrosis in diabetic rats. Iran J Basic Med Sci. (2020) 23:1078–84. doi: 10.22038/ijbms.2020.44210.10358
490. Mannino F, Pallio G, Altavilla D, Squadrito F, Vermiglio G, Bitto A, et al. Atherosclerosis plaque reduction by lycopene is mediated by increased energy expenditure through AMPK and PPARα in ApoE KO mice fed with a high fat diet. Biomolecules. (2022) 12:973. doi: 10.3390/biom12070973
491. Li X, Jia P, Huang Z, Liu S, Miao J, Guo Y, et al. Lycopene protects against myocardial ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Drug Des Devel Ther. (2019) 13:2331–42. doi: 10.2147/DDDT.S194753
492. Fan S, Sun JB, Li R, Song X, Li J. Lycopene protects myocardial ischemia injury through anti-apoptosis and anti-oxidative stress. Eur Rev Med Pharmacol Sci. (2019) 23:3096–104. doi: 10.26355/eurrev_201904_17593
493. Zeng J, Zhao J, Dong B, Cai X, Jiang J, Xue R, et al. Lycopene protects against pressure overload-induced cardiac hypertrophy by attenuating oxidative stress. J Nutr Biochem. (2019) 66:70–8. doi: 10.1016/j.jnutbio.2019.01.002
494. Jiang Y, Du H, Liu X, Fu X, Li X, Cao Q. Artemisinin alleviates atherosclerotic lesion by reducing macrophage inflammation via regulation of AMPK/NF-κB/NLRP3 inflammasomes pathway. J Drug Target. (2020) 28:70–9. doi: 10.1080/1061186X.2019.1616296
495. Wang P, Tian X, Tang J, Duan X, Wang J, Cao H, et al. Artemisinin protects endothelial function and vasodilation from oxidative damage via activation of PI3K/Akt/eNOS pathway. Exp Gerontol. (2021) 147:111270. doi: 10.1016/j.exger.2021.111270
496. Liu X, Wang X, Pan Y, Zhao L, Sun S, Luo A, et al. Artemisinin improves acetylcholine-induced vasodilatation in rats with primary hypertension. Drug Des Devel Ther. (2021) 15:4489–502. doi: 10.2147/DDDT.S330721
497. Kong L, Ji X, Liu Y, Du Y. Effect of artemisinin combined with allicin on improving cardiac function, fibrosis and NF-κB signaling pathway in rats with diabetic cardiomyopathy. Acta Biochim Pol. (2023) 70:401–5. doi: 10.18388/abp.2020_6692
498. Wang L, Zhao X, Ding J, Liu Y, Liu H, Zheng L, et al. Oridonin attenuates the progression of atherosclerosis by inhibiting NLRP3 and activating Nrf2 in apolipoprotein E-deficient mice. Inflammopharmacology. (2023) 31:1993–2005. doi: 10.1007/s10787-023-01161-9
499. Lin J, Lai X, Fan X, Ye B, Zhong L, Zhang Y, et al. Oridonin protects against myocardial ischemia-reperfusion injury by inhibiting GSDMD-mediated pyroptosis. Genes (Basel). (2022) 13:2133. doi: 10.3390/genes13112133
500. Gao RF, Li X, Xiang HY, Yang H, Lv CY, Sun XL, et al. The covalent NLRP3-inflammasome inhibitor Oridonin relieves myocardial infarction induced myocardial fibrosis and cardiac remodeling in mice. Int Immunopharmacol. (2021) 90:107133. doi: 10.1016/j.intimp.2020.107133
501. Xu M, Wan CX, Huang SH, Wang HB, Fan D, Wu HM, et al. Oridonin protects against cardiac hypertrophy by promoting P21-related autophagy. Cell Death Dis. (2019) 10:403. doi: 10.1038/s41419-019-1617-y
502. Ma SR, Tong Q, Lin Y, Pan LB, Fu J, Peng R, et al. Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota. Signal Transduct Target Ther. (2022) 7:207. doi: 10.1038/s41392-022-01027-6
503. Jia X, Shao W, Tian S. Berberine alleviates myocardial ischemia-reperfusion injury by inhibiting inflammatory response and oxidative stress: the key function of miR-26b-5p-mediated PTGS2/MAPK signal transduction. Pharm Biol. (2022) 60:652–63. doi: 10.1080/13880209.2022.2048029
504. Zhu N, Li J, Li Y, Zhang Y, Du Q, Hao P, et al. Berberine protects against simulated ischemia/reperfusion injury-induced H9C2 cardiomyocytes apoptosis in vitro and myocardial ischemia/reperfusion-induced apoptosis in vivo by regulating the mitophagy-mediated HIF-1α/BNIP3 pathway. Front Pharmacol. (2020) 11:367. doi: 10.3389/fphar.2020.00367
505. Abudureyimu M, Yu W, Cao RY, Zhang Y, Liu H, Zheng H. Berberine promotes cardiac function by upregulating PINK1/Parkin-mediated mitophagy in heart failure. Front Physiol. (2020) 11:565751. doi: 10.3389/fphys.2020.565751
506. Tian CX, Li MY, Shuai XX, Jiang F, Dong YL, Gui Y, et al. Berberine plays a cardioprotective role by inhibiting macrophage Wnt5a/β-catenin pathway in the myocardium of mice after myocardial infarction. Phytother Res. (2023) 37:50–61. doi: 10.1002/ptr.v37.1
507. Yang B, Li J, Wang B, Wang G, Li P, Guo H, et al. Hydroxycitrate prevents calcium oxalate crystallization and kidney injury in a nephrolithiasis rat model. Urolithiasis. (2022) 50:47–53. doi: 10.1007/s00240-021-01283-1
508. Chen X, Jiang X, Cheng C, Chen J, Huang S, Xu M, et al. Berberine attenuates cardiac hypertrophy through inhibition of mTOR signaling pathway. Cardiovasc Drugs Ther. (2020) 34:463–73. doi: 10.1007/s10557-020-06977-z
509. Yang B, Wang G, Li Y, Yang T, Guo H, Li P, et al. Hydroxycitric acid prevents hyperoxaluric-induced nephrolithiasis and oxidative stress via activation of the Nrf2/Keap1 signaling pathway. Cell Cycle. (2023) 22:1884–99. doi: 10.1080/15384101.2023.2247251
510. Yang M, Lv H, Liu Q, Zhang L, Zhang R, Huang X, et al. Colchicine alleviates cholesterol crystal-induced endothelial cell pyroptosis through activating AMPK/SIRT1 pathway. Oxid Med Cell Longev. (2020) 2020:9173530. doi: 10.1155/2020/9173530
511. Shen S, Duan J, Hu J, Qi Y, Kang L, Wang K, et al. Colchicine alleviates inflammation and improves diastolic dysfunction in heart failure rats with preserved ejection fraction. Eur J Pharmacol. (2022) 929:175126. doi: 10.1016/j.ejphar.2022.175126
512. Sun X, Duan J, Gong C, Feng Y, Hu J, Gu R, et al. Colchicine ameliorates dilated cardiomyopathy via SIRT2-mediated suppression of NLRP3 inflammasome activation. J Am Heart Assoc. (2022) 11:e025266. doi: 10.1161/JAHA.122.025266
513. Li YW, Chen SX, Yang Y, Zhang ZH, Zhou WB, Huang YN, et al. Colchicine inhibits NETs and alleviates cardiac remodeling after acute myocardial infarction. Cardiovasc Drugs Ther. (2022) 38:31–41. doi: 10.1007/s10557-022-07326-y
514. Geng P, Xu X, Gao Z. Sinomenine suppress the vitamin D3 and high fat induced atherosclerosis in rats via suppress of oxidative stress and inflammation. J Oleo Sci. (2021) 70:1815–28. doi: 10.5650/jos.ess21255
515. Xia B, Li Q, Wu J, Yuan X, Wang F, Lu X, et al. Sinomenine confers protection against myocardial ischemia reperfusion injury by preventing oxidative stress, cellular apoptosis, and inflammation. Front Pharmacol. (2022) 13:922484. doi: 10.3389/fphar.2022.922484
516. Fu YF, Li L, Fang P, Song J, Sun XH, Meng TH, et al. Sinomenine's protective role and mechanism in stress load-induced heart failure. J Pharm Pharmacol. (2020) 72:209–17. doi: 10.1111/jphp.13181
517. Yuan M, Zhao B, Jia H, Zhang C, Zuo X. Sinomenine ameliorates cardiac hypertrophy by activating Nrf2/ARE signaling pathway. Bioengineered. (2021) 12:12778–88. doi: 10.1080/21655979.2021.2000195
518. Xiao M, Xian C, Wang Y, Qi X, Zhang R, Liu Z, et al. Nuciferine attenuates atherosclerosis by regulating the proliferation and migration of VSMCs through the Calm4/MMP12/AKT pathway in ApoE((-/-)) mice fed with High-Fat-Diet. Phytomedicine. (2023) 108:154536. doi: 10.1016/j.phymed.2022.154536
519. Li R, Qin X, Yue L, Liu W, Gao Y, Zhu F, et al. Nuciferine improves cardiac function in mice subjected to myocardial ischemia/reperfusion injury by upregulating PPAR-γ. Heliyon. (2023) 9:e13630. doi: 10.1016/j.heliyon.2023.e13630
520. HarishKumar R, Selvaraj CI. Nuciferine from Nelumbo nucifera Gaertn. attenuates isoproterenol-induced myocardial infarction in Wistar rats. Biotechnol Appl Biochem. (2022) 69:1176–89. doi: 10.1002/bab.v69.3
521. Fan X, Han J, Zhu L, Chen Z, Li J, Gu Y, et al. Protective activities of Dendrobium huoshanense C. Z. Tang et S. J. Cheng polysaccharide against high-cholesterol diet-induced atherosclerosis in zebrafish. Oxid Med Cell Longev. (2020) 2020:8365056. doi: 10.1155/2020/8365056
522. Li QM, Zha XQ, Zhang WN, Liu J, Pan LH, Luo JP. Laminaria japonica polysaccharide prevents high-fat-diet-induced insulin resistance in mice via regulating gut microbiota. Food Funct. (2021) 12:5260–73. doi: 10.1039/D0FO02100H
523. Yin F, Lin P, Yu WQ, Shen N, Li Y, Guo SD. The Cordyceps militaris-derived polysaccharide CM1 alleviates atherosclerosis in LDLR-/- mice by improving hyperlipidemia. Front Mol Biosci. (2021) 8:783807. doi: 10.3389/fmolb.2021.783807
524. Song Z, Li H, Liang J, Xu Y, Zhu L, Ye X, et al. Sulfated polysaccharide from Undaria pinnatifida stabilizes the atherosclerotic plaque via enhancing the dominance of the stabilizing components. Int J Biol Macromol. (2019) 140:621–30. doi: 10.1016/j.ijbiomac.2019.08.173
525. Xiong Q, Zhu L, Zhang F, Li H, Wu J, Liang J, et al. Protective activities of polysaccharides from Cipangopaludina chinensis against high-fat-diet-induced atherosclerosis via regulating gut microbiota in ApoE-deficient mice. Food Funct. (2019) 10:6644–54. doi: 10.1039/C9FO01530B
526. Li W, Yu J, Zhao J, Xiao X, Li W, Zang L, et al. Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE-/- mice by inhibiting inflammation. Phytother Res. (2021) 35:2220–9. doi: 10.1002/ptr.v35.4
527. Zhang Z, Liu H, Yu B, Tao H, Li J, Wu Z, et al. Lycium barbarum polysaccharide attenuates myocardial injury in high-fat diet-fed mice through manipulating the gut microbiome and fecal metabolome. Food Res Int. (2020) 138:109778. doi: 10.1016/j.foodres.2020.109778
528. Pan H, Niu L, Wu Y, Chen L, Zhou X, Zhao Y. Lycium barbarum polysaccharide protects rats and cardiomyocytes against ischemia/reperfusion injury via Nrf2 activation through autophagy inhibition. Mol Med Rep. (2021) 24. doi: 10.3892/mmr.2021.12418
529. Liu Q, Han Q, Lu M, Wang H, Tang F. Lycium barbarum polysaccharide attenuates cardiac hypertrophy, inhibits calpain-1 expression and inhibits NF-κB activation in streptozotocin-induced diabetic rats. Exp Ther Med. (2019) 18:509–16. doi: 10.3892/etm.2019.7612
530. Shi X, Han B, Zhang B, Chu Z, Zhang X, Lu Q, et al. Schisandra chinensis polysaccharides prevent cardiac hypertrophy by dissociating thioredoxin-interacting protein/thioredoxin-1 complex and inhibiting oxidative stress. BioMed Pharmacother. (2021) 139:111688. doi: 10.1016/j.biopha.2021.111688
531. He P, Zhang M, Zhao M, Zhang M, Ma B, Lv H, et al. A novel polysaccharide from Chuanminshen violaceum and its protective effect against myocardial injury. Front Nutr. (2022) 9:961182. doi: 10.3389/fnut.2022.961182
532. Zhu X, Wu W, Chen X, Yang F, Zhang J, Hou J. Protective effects of Polygonatum sibiricum polysaccharide on acute heart failure in rats 1. Acta Cir Bras. (2018) 33:868–78. doi: 10.1590/s0102-865020180100000001
Keywords: cardiovascular disease, traditional Chinese medicine, heart function, therapeutic mechanisms, gut microbiota
Citation: Dai J, Qiu L, Lu Y and Li M (2024) Recent advances of traditional Chinese medicine against cardiovascular disease: overview and potential mechanisms. Front. Endocrinol. 15:1366285. doi: 10.3389/fendo.2024.1366285
Received: 06 January 2024; Accepted: 03 September 2024;
Published: 30 September 2024.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Ping Chung Leung, The Chinese University of Hong Kong, ChinaLiu Ouyang, Georgia State University, United States
Ying Xie, University of California, Berkeley, United States
Copyright © 2024 Dai, Qiu, Lu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miao Li, bGltaWFvZHlleUAxNjMuY29t; Yi Lu, NzUwMTA5NDcxQHFxLmNvbQ==
†These authors have contributed equally to this work
 Junting Dai1†
Junting Dai1† Miao Li
Miao Li