- 1Faculty of Sports Science, Research Academy of Grand Health, Ningbo University, Ningbo, Zhejiang, China
- 2NanJing MaiGaoQiao Community Health Service Center, Nanjing, Jiangsu, China
- 3Nanjing Kuanyue Health Technology Co., Ltd, Nanjing, Jiangsu, China
- 4Ningbo New Fitness Health Technology Co., Ltd, Zhejiang, Ningbo, China
Objective: To evaluate the effects of high-intensity interval training (HIIT) on glycolipid metabolism among type 2 diabetes patients.
Methods: HIIT is consistent with an exercise program (65%-90%VO2max or 75%-95% HRmax; exercise cycle≥2 weeks; frequency ≥ 2 times/week). A meta-analysis was conducted utilizing the random effects model to synthesize the data.
Results: A total of 22 RCT studies with 1034 diabetic patients were included. Compared to moderate-intensity aerobic exercise or conventional controls, HIIT yields noteworthy effects on FBG (MD: -0.55; 95% CI: -0.85- -0.25, Hedges’ g =0.98), 2h-PG (MD: -0.36; 95% CI: -0.57- -0.14, Hedges’ g =1.05), FINS (MD: -0.41; 95% CI: -0.79- -0.03, Hedges’ g =1.07), HbA1c (MD: -0.60; 95% CI: -0.84- -0.36, Hedges’ g =2.69), TC (MD: -0.58; 95% CI: -0.80- -0.36, Hedges’ g =2.36), TG (MD: -0.50; 95% CI: -0.86- -0.14, Hedges’ g =1.50), HDL (MD: 0.62; 95% CI: 0.29–0.95, Hedges’ g =1.19) and LDL (MD: -0.31; 95% CI: -0.56- -0.08, Hedges’ g =0.91), all of the above p<0.01.
Conclusions: HIIT has been shown to improve glucose and lipid metabolism in patients with type 2 diabetes, especially in HbA1c, TC, TG, and HDL. For patients between the ages of 40 and 60 with less than 5 years of disease, exercise programs of moderate to longer duration or moderate to high intensity will produce more favorable results.
1 Introduction
Type 2 diabetes mellitus (T2DM), a highly prevalent chronic metabolic disorder, is the most commonly observed variant of diabetes. It is distinguished by elevated blood glucose levels, relative insufficiency of insulin, and resistance to insulin (1). Abnormal blood glucose levels are commonly accompanied by dyslipidemia or hypertension, resulting in both incapacitation and reduced life expectancy for affected individuals, as well as an elevated susceptibility to sudden cardiac death (2, 3). Studies have shown that diabetes contributes to an annual global mortality of around 3 million individuals, with a consistent increase in the global prevalence of diabetes each year (1). Consensus reports from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) provide updated strategies for the management of type 2 diabetes in adults, proposing person-centered, holistic care that carefully considers the preferences of the person with diabetes to inform the individualization of treatment goals and strategies (3).
Regular aerobic exercise is a routine treatment option for addressing diabetes mellitus, and aerobic training can reduce glycated hemoglobin levels, increase maximal oxygen uptake, and improve insulin sensitivity in patients with T2DM (4). Nevertheless, aerobic exercise takes a long time. American Sports Medicine Association (ACSM) exercise guidelines recommend aerobic exercise for people with diabetes up to 300 min per week (5). Some studies have pointed out that lack of time and low interest in exercise are the primary barriers to physical activity (4, 6). In practical terms, most patients do not fully engage in this self-intervention approach. High-intensity interval training (HIIT) represents a novel exercise regimen comprising multiple high- intensity training and low- intensity training intervals, which significantly reduces the exercise duration to achieve the same effect as aerobic exercise and avoids the appearance of uncomfortable symptoms during the low intensity intervals (6). Reindell suggested that HIIT has the characteristics of a short time, high efficiency, and favorable outcomes. In comparison to other exercise interventions, it can utilize a shorter time to achieve the same training effect (7). Khurshid et al. found that both short-term high-intensity exercise patterns and evenly distributed exercise patterns reduced the risk of cardiovascular disease, with the former being more feasible (8).
The current findings on the effects of HIIT on glucose and lipid metabolism in type 2 diabetic populations are inconsistent. Liu et al. showed that HIIT was superior to moderate-intensity continuous training (MICT) in reducing blood glucose and lipid indexes; however, no significant difference was observed in lowering 2-hour postprandial glucose levels or improving HbA1c levels (9). Han’s study similarly noted that HIIT is ineffective in terms of improvement in FPG, HbA1c, and lipid metabolism (10). In addition, some studies still have limitations and lack analysis in terms of the biological characteristics of the patients and the subgroup characteristics of the exercise protocol. Liu’s review explored the effects of HIIT on glycemia, cardiorespiratory fitness, and body composition, but no subgroup analyses were performed (9). Chen et al.’s review was published in Chinese, and the study population was mainly Chinese (6). Ivan et al. included studies in English and Spanish before 2017, with a small number of documents, after which some new evidence appeared (11); Yang reviewed the effects of low-intensity HIIT on glucose metabolism and cardiorespiratory endurance in diabetic patients, who showed significant improvements in glycemic control, insulin resistance, and lipids. However, subgroup analyses were not performed due to a smaller number of included studies (12). Jung ME et al. reported that despite patients’ knowledge that exercise is effective, a lack of scientific guidance resulted in decreased adherence to exercise among diabetic patients (13). To enhance patient engagement in self-care practices that can be tailored to individual preferences and traits, further investigation into the various factors that influence exercise regimens is necessary for optimization.
Therefore, we conducted a meta-analysis and systematic review to verify the effects of HIIT on glycemic and lipid markers considering its duration, intensity and aspects related to the disease and sociodemographic factors in the analysis. The results enrich its proposal and can contribute to decision making for prescribing training to patients with type 2 diabetes.
2 Materials and methods
2.1 Registration
The protocol has been registered in the PROSPERO registry (CRD42023401649; http://www.crd.york.ac.uk/PROSPERO) and conducted in accordance with the systematic review checklist (PRISMA 2020).
2.2 Literature search strategy
Literature searches were performed in the PubMed, Scopus, Web of Science, Cochrane Library, and China National Knowledge Infrastructure(CNKI) databases (details of the search strategies are reported in Table 1).
The search dates were all from the creation of the database to April 1, 2023 The scientific databases were searched according to three criteria: study population (“diabetes mellitus”, “type 2 diabetes mellitus”), medical interventions (“high-intensity interval exercise”, “sprint interval training”, “HIIT”) and outcomes (“blood glucose”, “glucose tolerance disorder”, “lipids”). All search strategies were conducted in the relevant databases using English and Chinese. Two researchers independently completed the initial screening of the articles and the statistics of the basic information in the included literature and the changes in the effect indicators before and after the intervention, and the third researcher negotiated the resolution of disputes when they existed.
2.3 Inclusion and exclusion criteria
The eligibility criteria were established depending on the PICOS (population, intervention, comparison, outcome, study design) items.
(P) The study included patients with type 2 diabetes who met the World Health Organization’s diagnostic criteria (fasting blood glucose≥7.0 mmol/L or OGTT 2-hour glucose≥11.1 mmol/L or HbA1C≥6.5%), and who had an age≥18 years; there were no restrictions on the gender or race of the study participants. Patients with clinically manifest cardiovascular diseases, acute complications, and pregnant or lactating women were excluded.
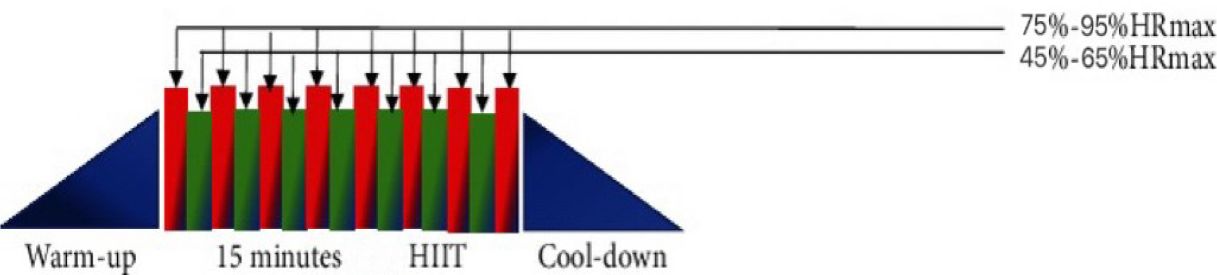
(I) The intervention group participated in only the HIIT exercise program. HIIT exercise protocol consists of three phases: a warm-up phase, an alternating exercise phase, and an exercise recovery phase (13). The alternating phase alternates high-intensity exercise with low-intensity exercise (the exercise protocol of HIIT is shown in Figure 1). During the high-intensity exercise phase, the heart rate should be 75%-95% of the HRmax for 60 s, and during the low-intensity interval phase, the heart rate should be 45%-65% of the HRmax for 60 s. A total of 6–8 sets were completed; HIIT is a form of exercise that can be practiced in a variety of ways (exercise cycle ≥ 2 weeks; frequency ≥ 2 times/week). Exercise types include running, cycling, resistance bands, and unassisted exercise. Studies on joint interventions by combining strength training, diet, medicine, health education, and other means were excluded.
(C) The comparators included moderate-intensity continuous training (MICT, 46%-63% VO2max or 64%-75% HRmax; a duration of usually more than 20 min; types such as running, cycling, walking, body mechanics, and Tai Ji), combined aerobic and resistance training, routine care groups, and static stretching. Studies with control groups practicing low- or moderate-intensity HIIT were excluded.
(O) The outcomes included any of the following indicators. Primary outcomes: fasting blood glucose, glycosylated hemoglobin, fasting insulin, and 2h-PG; secondary outcomes: total cholesterol, triglyceride, high-density lipoprotein, and low-density lipoprotein. Studies that did not contain relevant outcome indicators were excluded.
(S) The study design included randomized controlled trials. Case reports, abstracts, reviews, lectures, commentaries, and data that could not be extracted were excluded.
2.4 Evaluation of bias and quality assessment
We used the Cochrane Quality Assessment Tool, as the included articles were randomized controlled trials. The risk of bias and methodological quality of the included studies were assessed by two evaluators using Review Manager 5.3 in terms of selective bias (randomized sampling, grouping), implementation bias (whether the experiment was blinded to the subjects and experimenters), measurement bias (whether the experiment operator was blinded to the endpoints), follow-up bias (completeness of the results), selective reporting bias and other biases were evaluated. The outcomes were expressed as low- risk, unclear, and high- risk. If a dispute arose between the two evaluators during the quality evaluation, a third evaluator was invited to participate to reach harmonization.
2.5 Data extraction
After screening the literature, two researchers independently extracted the following data from the eligible literature: the external characteristics of the literature (title, authors, year of publication, nationality of the authors); basic information about the subjects (age, gender, country, sample size, duration of the disease); the experimental design and exercise intervention protocol (training period and frequency, duration, intervention mode, intensity); and the outcome indicators related to the study.
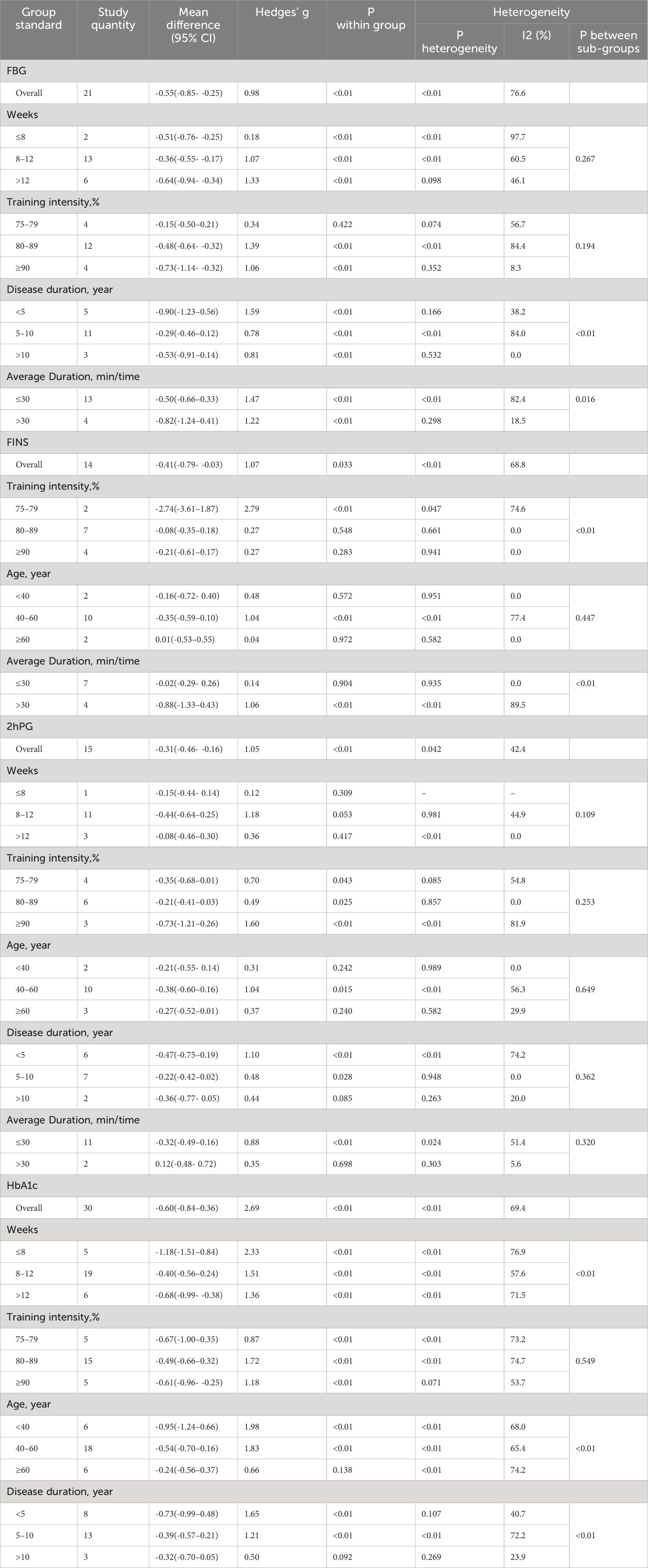
The subgroups were the intensity of exercise (75%-79% HRmax, 80%-89% HRmax and >=90%, HRmax), exercise period (≤8 weeks, 9–12 weeks, and >12 weeks), exercise duration (≤30 min/time and >30 min/time), age (<40 years, 40–59 years, and ≥60 years), and disease duration (<5 years, 5–10 years, and >10 years). In cases of disagreement between the two persons, a third person summarized them and determined their subgroups through a group discussion. The outcome indicators need to be extracted separately for the mean and standard deviation of the pre-test and the mean and standard deviation of the post-test for the intervention and control groups in the study. The mean standard deviation of the difference between the pre-test and post-test was calculated separately.
For some studies (14–24), as they had multiple control groups, we extracted the data across multiple groups and included them in the meta-analysis (Table 2). For FPG, we evaluated 14 studies, of which 21 compared HIIT and control groups. For 2h-PG, 9 studies were evaluated, with 15 comparisons between the HIIT and control groups. For FINS, 8 studies were evaluated, with 14 comparisons between the HIIT and comparison groups. For HbA1c, 18 studies were evaluated, with 30 comparisons between the HIIT and control groups. For TC, 13 studies were evaluated, with 23 comparisons between the HIIT and control groups. For TG, 13 studies were evaluated, and 23 comparisons were made between the HIIT and comparison groups. For HDL, 15 studies were evaluated, with 24 comparisons between the HIIT and control groups. For LDL, 15 studies were evaluated, with 24 comparisons between the HIIT and comparison groups.
2.6 Sensitivity analysis and publication bias
To explore the heterogeneity, we conducted a sensitivity analysis, applying a literature-by-exclusion approach. Publication bias was assessed with a visual inspection of a funnel plot. When significant bias was detected, we performed a trim-and-fill analysis.
2.7 Statistical analysis
Stata 16 software was used for meta-analysis. The data included in the study were continuous and were expressed as the mean difference (MD) and 95% confidence interval (CI). The presence of heterogeneity among the studies was tested using the I2 test. Subgroup analyses were performed to analyze the characteristics of the studies and sources of heterogeneity across various classifications.
To reflect the practical value of the effect sizes for clinical purposes, the effect size was calculated according to Hedge s’ g. It is also widely used in meta-analysis. Hedges suggested that g values of 0.2, 0.5, and 0.8 represent small, medium, and large effect sizes, respectively (34).
3 Results
3.1 Description of studies
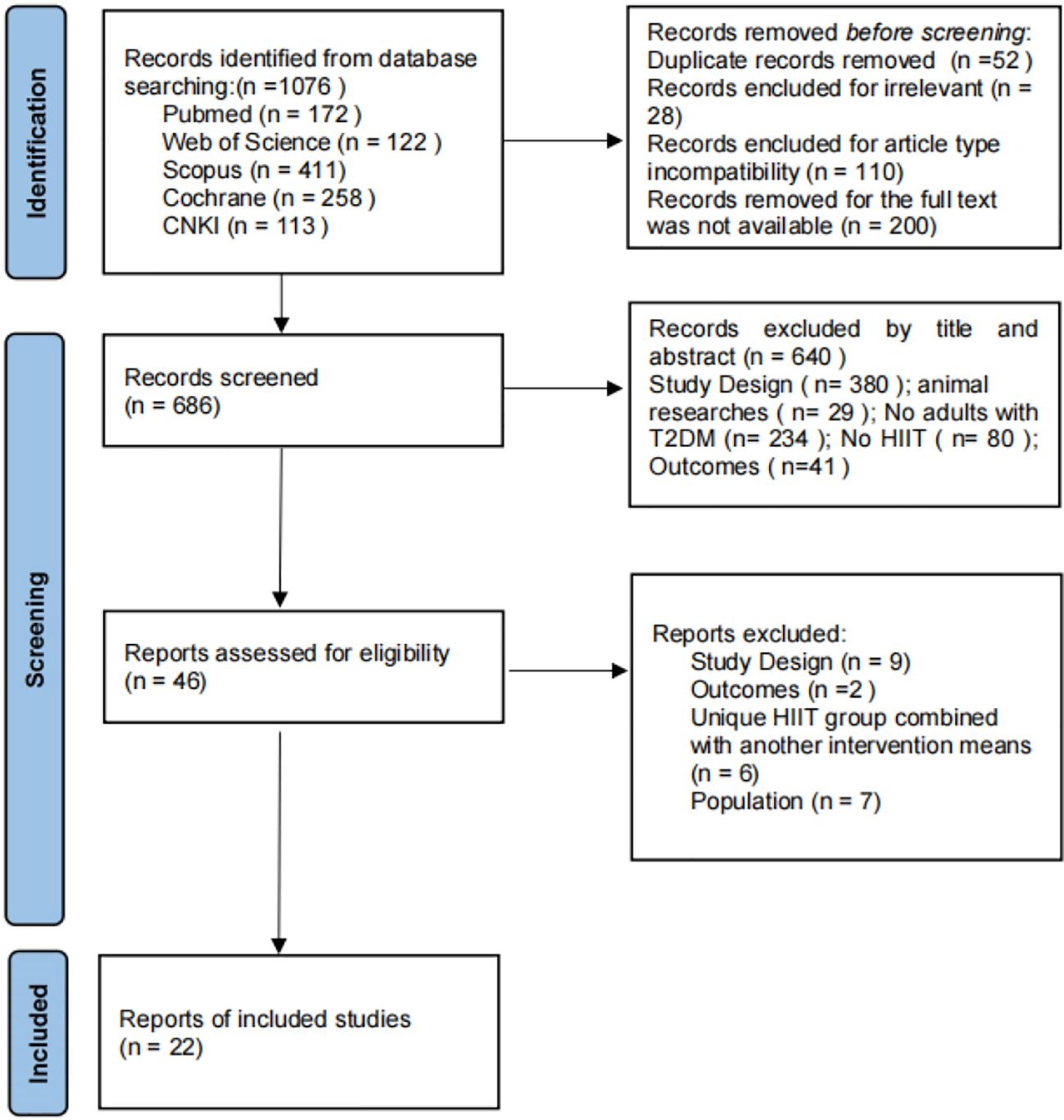
Through the search strategy, a total of 1076 articles were initially retrieved from 5 databases, and 390 articles that were duplicates or for which the full text could not be accessed were deleted. After reading the article titles and abstracts, 640 articles were excluded, and the full text of the remaining 46 articles was read and screened according to the inclusion and exclusion criteria, resulting in the inclusion of 22 articles. The specific flow of the included studies is shown in Figure 2.
This study encompassed RCTs exclusively as part of its research design, with all subjects belonging to the type 2 diabetic population, totaling 1268 patients. The experimental group received interventions involving HIIT exercise, which included running (n=8) (17, 26–28, 33, 35, 36), cycling (n=23) (15, 17, 27, 29–31, 35, 37, 38), unassisted exercise (n=5) (16, 20, 25), resistance bands (n=2) (30, 31), and walking (n=2) (22). Meanwhile, the control group was based on aerobic exercise and usual care. The exercise intensity was categorized as moderate in 17 studies, low in 5 studies, and high in 6 studies. The primary outcome indicators were addressed in 32 studies. Details of the basic characteristics of the included literature are shown in Table 2.
3.2 Quality evaluation
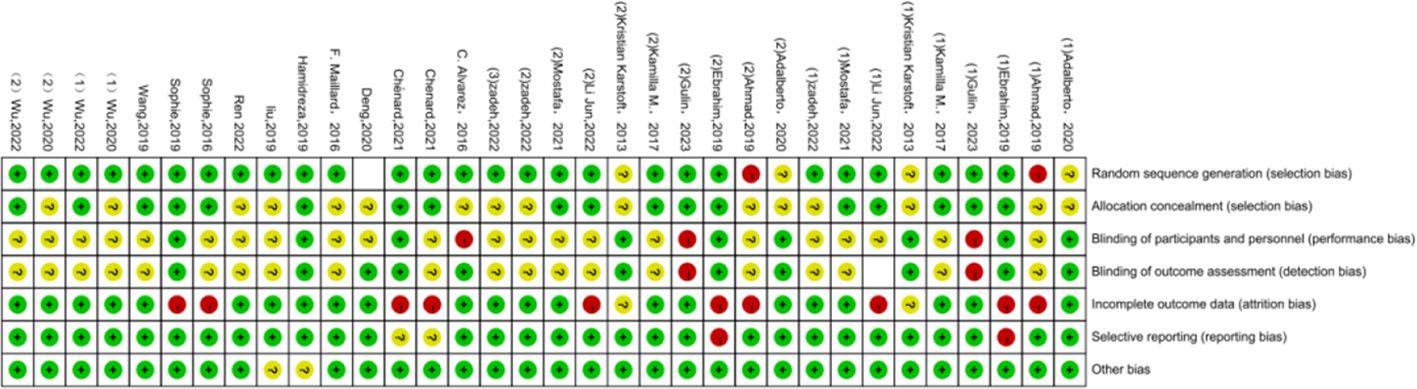
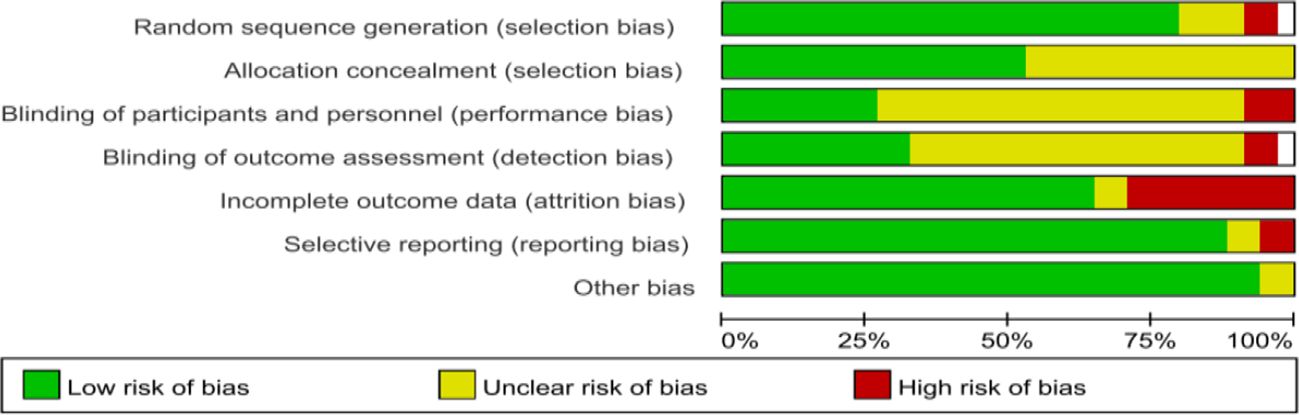
Regarding the quality assessment, 13 studies were identified as having a high risk, and the results are shown in Figures 3, 4. In Figure 3, green means “low risk”, red means “high risk”, and yellow means “unclear”. In Figure 4, green means “low risk”, red means “high risk”, and yellow means “unclear”. The results were as follows: (1) randomization— 2 studies had high risk, and 4 did not describe the random allocation method; (2) allocation concealment, 16 studies did not describe the specific allocation method; (3) blinding— 3 studies did not blind the subjects, 2 did not blind the evaluators, and most of the studies did not describe the method of blinding; (4) incomplete data reporting— 9 studies had case dropout, and 2 did not mention the number of subjects at the beginning; (5) reporting bias— 2 papers had case dropout, and 2 did not mention the number of starting subjects; and (6) the results were as follows: (1) the randomization method was not described. In reporting bias, two papers had biases.
3.3 Primary outcomes (glucose metabolism)
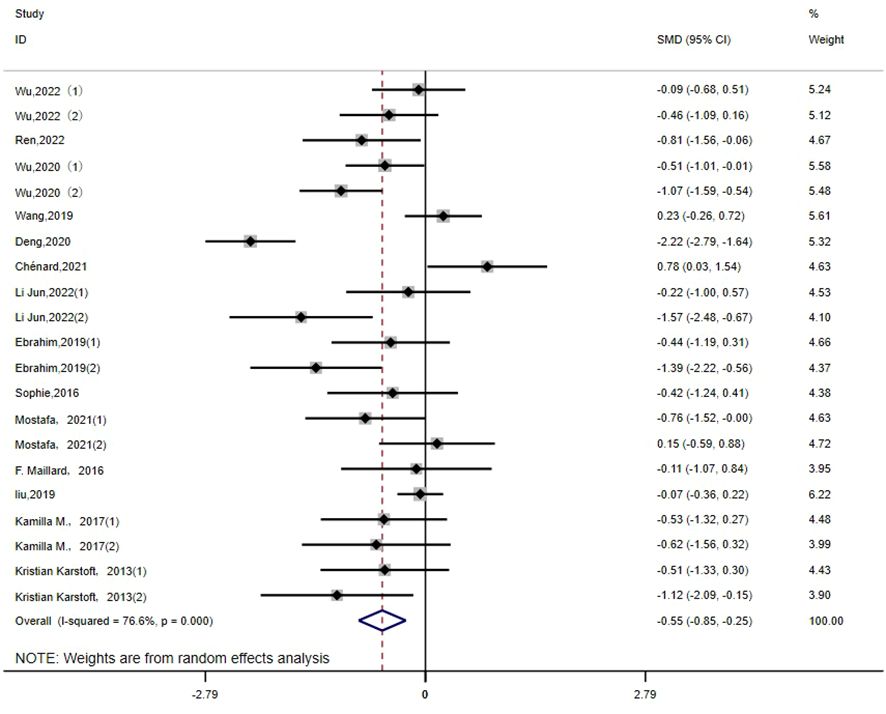
FPG: There are 21 studies (14–16, 18, 21–23, 25–28, 30, 32, 36) that report the effect of HIIT on the FGB index in a type 2 diabetic population. The results show that HIIT had a large effect size on FGB (MD: -0.55; 95% CI: -0.85- -0.25; Hedges’ g = 0.98; p < 0.01). The left side of the center line is “in favor of HIIT”(Figure 5). Because the I2 was 76.6%, a random-effects model was chosen for analysis. Notably, there were notable disparities in the exercise duration and disease duration among the subgroups (Table 3), with effect size values for exercise durations of 30 minutes or less surpassing those for durations exceeding 30 minutes (p < 0.01). Furthermore, individuals with a disease duration of less than 5 years (p<0.01) exhibited a significant effect size in terms of reducing FPG. Conversely, no significant differences were observed in the subgroups based on the exercise period or exercise intensity (p=0.267, p=0.194), and the results of fasting glucose were not affected by them.
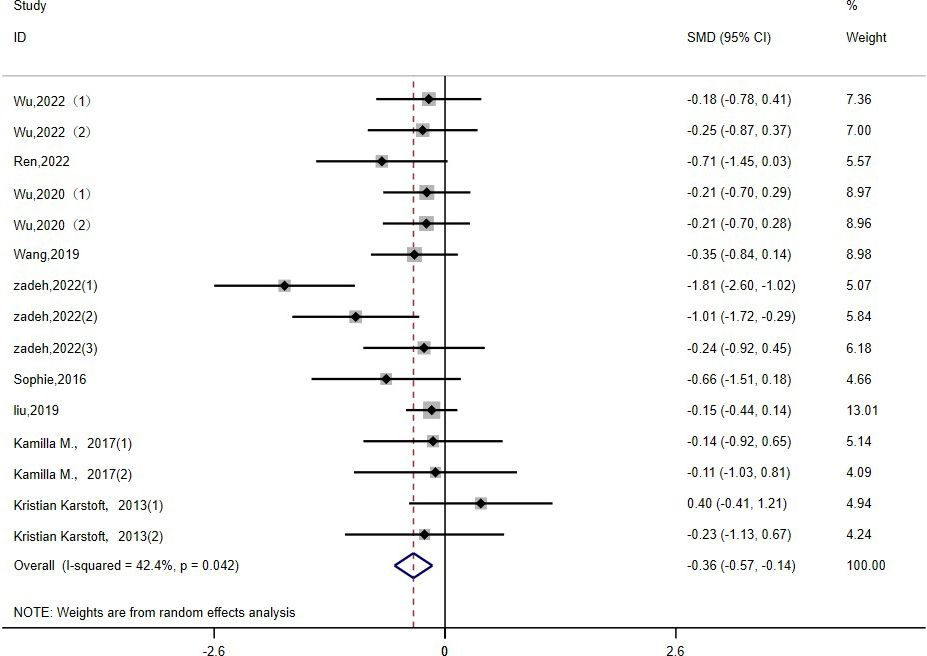
2h-PG: Fifteen studies (15, 16, 19, 22, 23, 26, 30, 36, 39) evaluated the effect of HIIT on 2h-PG metrics in a type 2 diabetic population. The results show that HIIT had a large effect size on 2h-PG (MD: -0.36; 95% CI: -0.57- -0.14; Hedges’ g = 1.05; p<0.01) (Figure 6). Because the I2 was 42.4%, a random-effects model was chosen for analysis. There were no statistically significant differences in the 2h-PG results for the exercise cycle, intensity, duration and disease duration subgroups (p = 0.10, 0.25, 0.32, 0.36, respectively) (Table 3). However, the results were significant for exercise cycles of 8–12 weeks (p = 0.05), exercise intensities of ≥90% (p<0.01), and exercise durations of ≤30 minutes (p <0.01); all had large effect sizes and a good effect on reducing 2h-PG. The effect of 2h-PG reduction was more pronounced for a disease duration of <5 years.
FINS: Fourteen studies (14, 15, 18, 21–23, 30, 35) evaluated the effect of HIIT on FINS metrics in a type 2 diabetes population. The results show that HIIT had a FINS- lowering effect (MD: -0.41; 95% CI: -0.79- -0.03; Hedges’ g =1.07; p<0.01) (Figure 7). Because the I2 was 68.8%, a random-effects model was chosen for the analyses. The subgroup results for exercise intensity and duration were significantly different (p<0.01), but their confidence intervals all overlapped (Table 3). Therefore, there were no significant differences among any of the above subgroups, and the FINS results were not affected by exercise intensity or duration. There was no statistically significant difference in age subgroups (p = 0.447), but the results showed a significant effect of HIIT in reducing FINS in the 40–60 year olds (p < 0.01). F or people over 60 years old, the effect was not significant (p = 0.972).
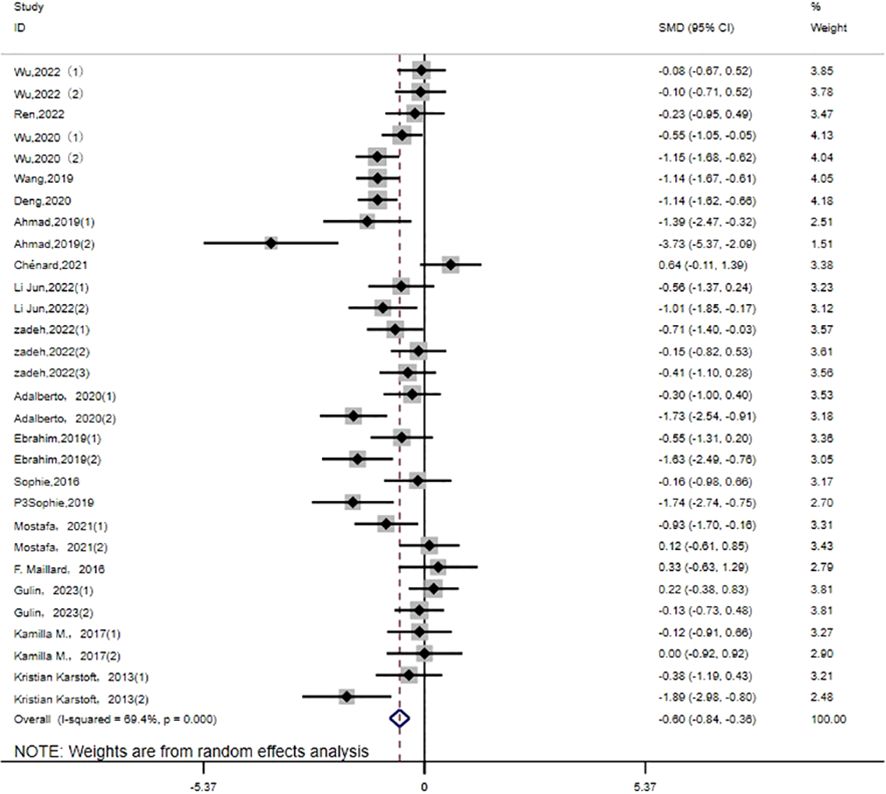
HbA1c: Thirty studies (14–27, 30–32, 35) evaluated the effect of HIIT on HbA1c metrics in a type 2 diabetic population. The results show that HIIT had a large effect size on HbA1c (MD: -0.60; 95% CI: -0.84- -0.36; Hedges’ g = 2.69; p<0.01) (Figure 8). Because the I2 was 69.4%, a random-effects model was chosen for analysis. There were significant differences in the exercise cycle subgroups (Table 3), with larger effect size results for ≤8 weeks compared to 8–12 weeks and >12 weeks (p < 0.01). There were no significant differences in the subgroup results for exercise intensity, age or disease duration, so the HbA1c results were not affected by these. From the results, it can be concluded that the group with an age of < 40 years (p < 0.01) had a large effect size, and the results were greater for exercise intensity of 80%-89%. HbA1c reduction was more pronounced for disease duration of <5 years.
3.4 Secondary outcomes (lipid metabolism)
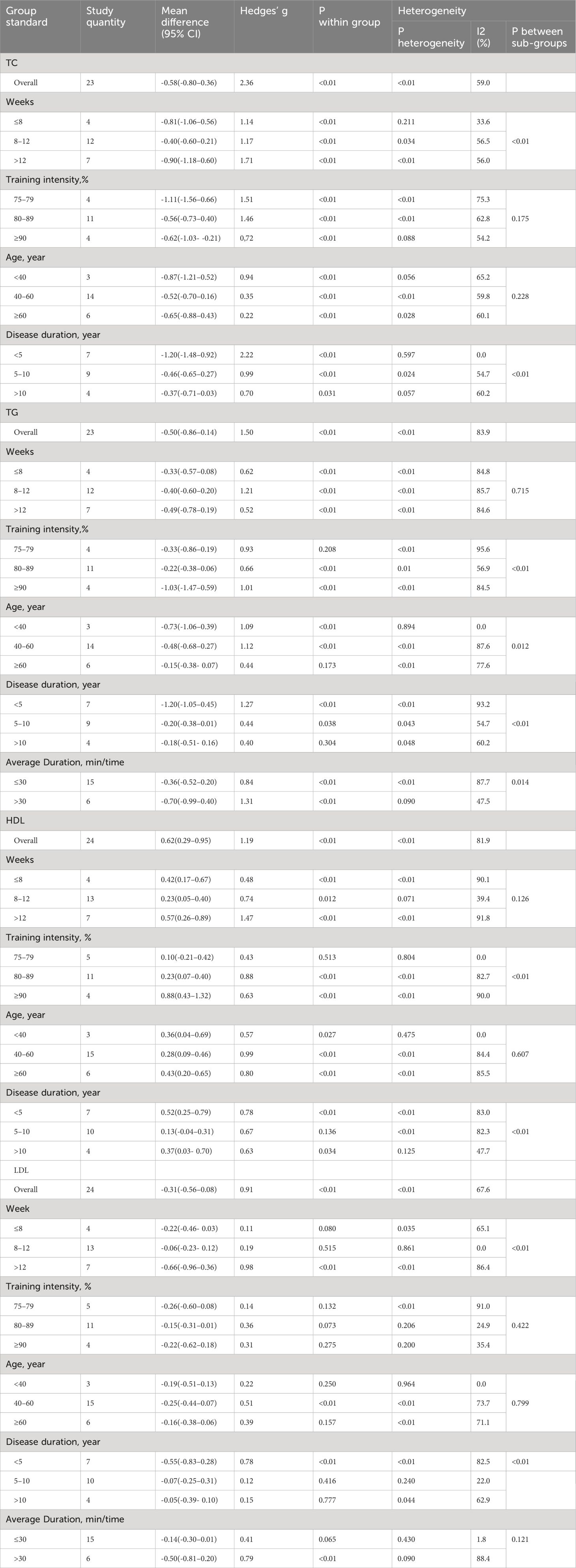
TC: Twenty-three studies (14–16, 19, 20, 22–25, 29, 32, 33, 35) assessed the effects of HIIT on markers of TC in a type 2 diabetic population. The results showed that HIIT had a large effect size on TC (MD: -0.58; 95% CI: -0.80- -0.36; Hedges’ g =2.36; p<0.01) (Figure 9). Because the I2 was 59%, a random-effects model was chosen for analysis. There was a significant difference in the subgroup results for exercise cycle and disease duration (Table 4), with an exercise cycle >12 weeks having a greater effect size compared to the other two subgroups. The effect size for a disease duration of less than 5 years was greater than that for the subgroup with >5 years of disease duration. However, there were no significant differences between the exercise intensity and age subgroups (p = 0.175, 0.228) and the TC values were not affected by the intensity of exercise or age, but the results show that the exercise intensity 75%-79% and 80%-89% both had large effect sizes.
TG: Twenty-three studies (14–16, 19, 20, 22–25, 29, 32, 33, 35) evaluated the effect of HIIT on markers of TG in type 2 diabetic population. We found that there was a large effect size of HIIT intervention on TG (MD: -0.50; 95% CI: -0.86- -0.14; Hedges’ g =1.50; p<0.01) (Figure 10). Because the I2 was 83.9%, subgroup analyses were performed for the exercise period, intensity, duration, age, and disease duration. There were significant differences in the exercise duration subgroups (Table 4), with durations >30 min reducing TG values more than durations ≤30 min. There were no significant differences between the subgroups for the remaining groups, and the TG values were not affected by the exercise cycle, intensity, age, or disease duration. An exercise cycle of 8 to 12 weeks was found to have a large effect size. An age of 40–60 years (p<0.01) had a better effect size for exercise, and an age of >60 years had no significant effect size for exercise (p=0.173). The effect sizes were larger for illness durations of less than 5 years.
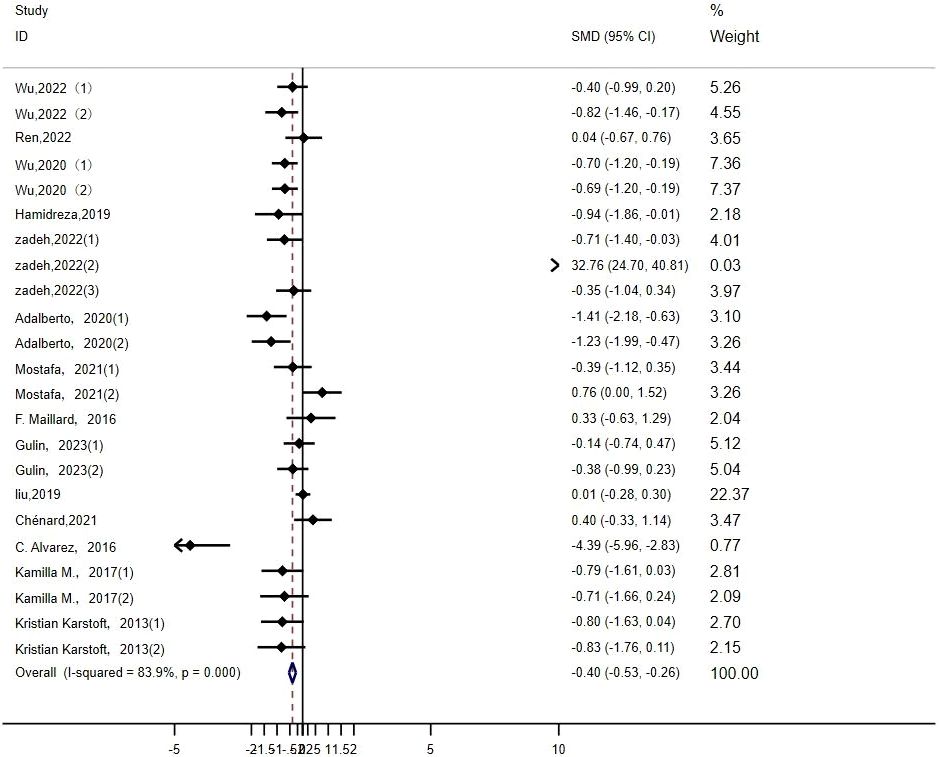
HDL: Twenty-four studies (14–16, 19, 20, 22–26, 29, 32, 33, 35, 36) assessed the impact of HIIT on HDL indicators in a type 2 diabetes population. The r esults showed that HIIT promoted HDL with a large effect size (MD: 0.62; 95% CI: 0.29–0.95; Hedges’ g =1.19; p<0.01) (Figure 11). Because the I2 was 81.9%, a random-effects model was chosen for analysis. Although the differences between the subgroups for exercise intensity and disease duration were significant (p<0.01) (Table 4), there was an overlap in the confidence intervals, so none of the above subgroups were significantly different, suggesting that the HDL results were not affected by the exercise cycle, intensity, age or disease duration. The results show that exercise cycles of >12 weeks were more effective in promoting HDL in people than exercise cycles of ≤ 8 weeks. Disease durations of less than 5 years had a large effect size. An exercise intensity of 80%-89% (p<0.01) had a large effect size. The group aged 40–60 (p<0.01) and the group over 60 (p<0.01) were both highly effective groups, with greater contributions to HDL.
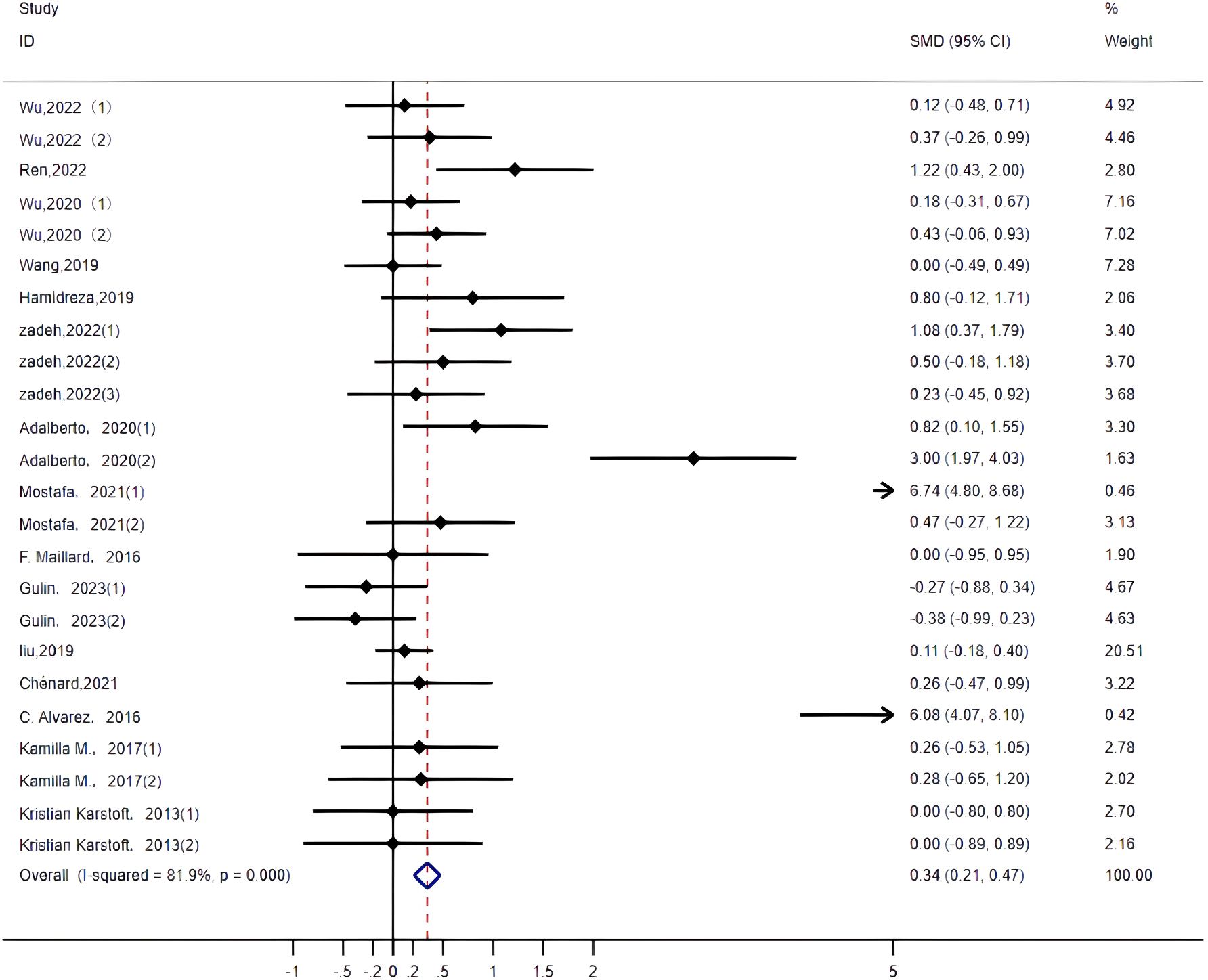
LDL: Twenty-four studies (14–16, 19, 20, 22–26, 29, 32, 33, 35, 36) assessed the impact of HIIT on LDL metrics in a type 2 diabetes population. The results showed that HIIT had a large effect size on LDL (MD: -0.31; 95% CI: -0.56–0.08; Hedges’ g = 0.91; p<0.01) (Figure 12). Because the I2 was 67.6%, a random-effects model was chosen for analysis. Although there was a significant difference between the exercise cycle and disease duration subgroups (p<0.01) (Table 4), the confidence intervals overlapped, so there was no significant difference between the subgroups, suggesting that the LDL results were not influenced by the exercise cycle, intensity, duration, age or disease duration. The results show that an exercise period of >12 weeks (p<0.01) had a large effect size. Exercise durations of >30 min (p<0.01) had a better exercise effect. An age of 40–60 years of age (p<0.01) significantly reduced LDL values with moderate effect size. A disease duration of <5 years (p<0.01) had a large effect size.
3.5 Sensitivity analysis
Sensitivity analysis of the findings for each indicator showed that the fasting glucose indicator produced a large bias due to the removal of the article by Deng, 2020 (27); the HDL indicator produced a large bias due to the removal of the articles by Adalberto, 2020(2) (20), Mostafa, 2021(1) (14), and Alvarez, 2016 (33); the 2h-PG indicator produced a large bias due to the removal of the articles by Zadeh, 2022 (19); and the FINS indicator produced a large bias due to the removal of the articles by Kamilla, 2017(1) (23) and Kamilla, 2017(2) (23). Therefore, the combined results of the above indicators may be unstable, and the combined results of the other indicators may be more stable (Annex 1).
3.6 Publication bias
We examined the publication bias for each indicator based on the funnel plot analysis. It was found that the study sites were found to be basically distributed on either side of the x=0 vertical line, but there was still a small number of study sites scattered regarding the FPG index, LDL index, and TC index. This suggests that there may be some publication bias (Annex 2).
4 Discussion
There is now a growing body of literature suggesting that the HIIT exercise model is more compatible with people undertaking self-directed interventions, that it is more feasible relative to MICT, and that it helps to improve exercise adherence (13, 40). Additionally, there is new evidence for its effects on human health (40–43). The latest physical activity guidelines emphasize, for the first time, the value of intermittent short-duration physical activity in building up the recommended amount of physical activity. Achieving weekly vigorous physical activity (VPA) goals with 10 minutes of VPA 3–4 times a day, 2–3 days a week, is beneficial for glycolipid metabolism and can increase life expectancy (41). Mengyun Luo et al. noted that longer durations of exercise are associated with a lower risk of T2DM, and that sustained exercise of 68.4 minutes or more reduced the incidence of T2DM by approximately 74% (42). Dong Hoon Lee et al. found that when exercise time was increased to two to four times the amount recommended by the World Health Organization, i.e., 150–299 minutes of VPA per week, the participants experienced a 21–23% reduction in all-cause mortality, a 27–33% reduction in cardiovascular disease mortality, and a 19% reduction in non-cardiovascular disease mortality (43).
Previous studies (6, 9, 10) have not reached consistent conclusions on whether HIIT improves glycolipid metabolism in patients with type 2 diabetes and there is a lack of data from studies involving HIIT intervention protocols (e.g., training intensity, training frequency, and total duration) and patient characteristics (age, duration of diabetes). To evaluate this type of exercise and obtain higher-level evidence, we performed this meta-analysis.
The results from this study indicate that HIIT has a positive effect on glucose-lipid metabolism in patients with type 2 diabetes mellitus. In terms of glucose metabolism, for the FBG, 2h-PG, and HbA1c indexes, HIIT was more advantageous than MICT for lowering their levels. In terms of lipid metabolism, HIIT was more favorable than MICT for in lowering TC, and there were no significant difference in the rest of the indicators. Furthermore, the results of the study show that an exercise program with a medium to long duration (> 8 weeks) and medium to high intensity (80%-89%) had a greater effect on most glycolipid indices. An exercise duration of > 30 min was more effective in lowering lipid indices, while sustained exercise for ≤ 30 min had a significant effect on lowering blood glucose levels. The majority of the indicators showed no significant effect of exercise in people > 60 years of age, but for the HDL indicator, people aged > 60 years of age had increased HDL values instead.
The effect sizes of the findings indicate that HIIT can reduce four types of glycemic indicators: FPG, 2h-PG, FINS, and HbA1c. Due to the high heterogeneity, we analyzed subgroups of relevant information for each indicator. In all age subgroups, the glycemic- lowering effect of HIIT was not significant for those aged over 60 years, and those aged 40–60 years were able to obtain more benefits from exercise training. All disease duration subgroups demonstrated that those with less than 5 years of disease duration could obtain better exercise results from HIIT than those with more than 5 years of disease. The r esults showed that a moderate-to-high intensity, moderate-to-long duration exercise program had a more significant effect on lowering blood glucose. For lowering HbA1c, the effect size results were greater for exercise cycles of <8 weeks and exercise intensities of 80%-89% or even higher. Previous studies (15, 26, 37) have shown HIIT to be effective in improving fasting blood glucose and HbA1c levels, and to facilitating glycemic control compared to other exercises. Winding et al. showed that HbA1c, fasting blood glucose, postprandial blood glucose, glycemic variability, and HOMA-IR were all reduced after HIIT (23). The results of the present study are consistent with the results of previous studies.
HbA1c levels are closely related to microvascular complications in diabetes, with studies showing that a 1% reduction in HbA1c levels is associated with a 14% reduction in myocardial infarction rates and a 21% reduction in the risk of diabetes-related death (12, 14). Therefore, HbA1c is an important indicator for evaluating diabetes therapies. The type, intensity and volume of exercise affect the degree of reduction in HbA1c levels (12). The results of this study suggest that HIIT with exercise cycles of <8 weeks and moderate intensity is more effective in reducing HbA1c, and in previous studies (12, 38) it was concluded that short intervals, medium to long cycles (11–16 weeks), and moderate-intensity HIIT exercise regimens were more beneficial for patients with T2DM. HbA1c is a blood marker that quantifies the three-month average glucose concentration (12), and it may take more than 12 weeks for an exercise program to demonstrate an effect on HbA1c, but many studies have durations of 12 weeks or less. Therefore, a greater amount of the literature needs to be included and further studies should be conducted to demonstrate the effects of different HIIT programs on HbA1c.
The results of this study show that HIIT can effectively reduce the three types of lipid metabolic indicators—TC, TG, and LDL— while increasing HDL indexes. Feng Chen et al. found that HIIT can reduce TC, TG, and LDL-C levels while increasing HDL-C levels in patients (44). Yajing et al. noted that patients’ glycemic and lipid indices were significantly improved after implementing a HIIT exercise program (26). The results of this study are consistent with the above studies. Due to the high heterogeneity, we performed subgroup analyses with relevant information (exercise cycle, intensity, duration, disease duration, and age) for each indicator. The results show that exercise regimens with a long cycle (>12 weeks), moderate to high intensity (80%-89% or ≥90%), and >30 min duration had more pronounced effects on lipid indicators, and that people aged 40–60 years and with a disease duration of less than 5 years were able to derive greater benefits from HIIT. However, for the TC values, the effect of exercise intensity of 75%-79% was shown to be more significant, and due to the small number of included studies for the relevant criteria in this paper, further research on the effect of exercise intensity on TC values is required.
The results of this study show that HIIT improves glycemic control and lipid metabolism and has more potential to facilitate the implementation of completion in the lives of patients. Comparing the experimental and control groups, the daily energy expenditure control was balanced between the two groups. Compared to MICT, HIIT exercise is more intense, but the duration of exercise is shorter, and its feasibility is higher because targeted training is easier to accomplish in a short period of time. This advantage may make HIIT an effective strategy for improving clinical application in patients. At the same time, many studies have confirmed that resistance training can also enhance the effect of insulin (39, 45), and several studies have shown that HIIT combined with resistance training may provide additional benefits for patients with T2DM; whether HIIT paired with resistance exercise has a greater improvement in type 2 diabetes mellitus or not, more studies are needed to confirm this.
Strengths and limitations: This review evaluated the interventional effects of HIIT on glycolipid metabolism in a type 2 diabetic population. However, there is still a lack of convincing studies on evaluating HbA1c as well as TC; therefore, there is a need to include more of the literature for additional studies to demonstrate the effects of different HIIT regimens on HbA1c and TC. The strengths of this meta-analysis are as follows. The first is the more systematic and complete data extraction. We searched for target studies from nine countries and in two languages (English/Chinese), which further minimized regional bias and language bias. The subjects’ personal information and the intervention programs were extracted more comprehensively. Secondly, we analyzed two methods (Hedges’ g and mean difference) for the effect of exercise training. For example, Hedges’ g reflects the actual clinical effect, while the mean difference reflects the statistical effect, thus providing a clear understanding of the impact of different factors on the intervention. The optimized plan can provide personalized exercise prescriptions for T2DM patients; for example, patients aged 40–60 years and with a disease duration of less than 5 years can benefit more from HIIT. Overall, exercise programs of moderate to high intensity (80%-90% or >90%) and exercise cycles (8–12 weeks or >12 weeks) have more significant effects. The main limitation of this study is that we did not differentiate between the pre-illness and post-illness stages of the included T2DM patients, and the severity and progression of the disease may have affected the study outcomes.
5 Conclusions
HIIT has been shown to improve glucose and lipid metabolism in patients with type 2 diabetes, especially in HbA1c, TC, TG, and HDL. For patients between the ages of 40 and 60 with less than 5 years of disease, exercise programs of a moderate to longer duration or moderate to high intensity will produce more favorable results. Whether HIIT paired with resistance training provides greater improvement in the treatment of type 2 diabetes is subject to verification via future supplementary studies.
Author contributions
JF: Writing – review & editing, Writing – original draft, Software, Methodology, Data curation. QZ: Writing – review & editing, Supervision, Methodology. BC: Writing – review & editing, Supervision, Methodology. JC: Writing – review & editing, Software. WW: Writing – review & editing, Software, Investigation, Data curation. YH: Writing – review & editing, Software, Investigation. JY: Writing – review & editing, Software, Methodology. HH: Writing – review & editing, Validation, Software, Project administration, Methodology, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Social Science Foundation of China (grant 18BTY100).
Acknowledgments
We thank all the participants for participating in all included studies.
Conflict of interest
Author JC was employed by the company Nanjing Kuanyue Health Technology Co., Ltd. Author WW was employed by the company Ningbo New Fitness Health Technology Co., Ltd,.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1360998/full#supplementary-material
Abbreviations
HIIT, high-intensity interval training; FBG,fasting glucose; FINS, fasting insulin; 2h-PG, 2-hour postprandial glucose; HbA1c, cglycosylated hemoglobin; TG,triglyceride; TC,total cholesterol; HDL-C, high density lipid-cholesterol; LDL-C, low density lipoprotein-cholesterol; ADA, American Diabetes Association; T2DM, type 2 diabetes mellitus; EASD, European Association for the Study of Diabetes; MICT, Moderate-Intensity Continuous Training; HOMA- IR, Homeostatic Model Assessment of Insulin Resistance; MD, mean difference; CI, confidence interval; VO2max, maximal oxygen uptake; HRmax, maximal heart rate; RCT, Randomized Controlled trial; EG, experiment Group; CG: Control Group; VPA, vigorous physical activity; CVD, cardiovascular disease; RT, Resistance training; AT, aerobic training; Combined A+R trainings, Combined aerobic+resistance training; CWT, continuous-walking training; CNKI, China National Knowledge Infrastructure.
References
1. Zhang R. Comparative study on the effects of circuit resistance training and high-intensity interval training on glycolipid metabolism in patients with type 2 diabetes mellitus. Chongqing: Southwest University (2022). p. 002336.
2. Norby FL, Reinier K, Uy-Evanado A. Sudden cardiac death in patients with type 1 versus type 2 diabetes. Mayo Clin Proc. (2022) 97:2271–81. doi: 10.1016/j.mayocp.2022.05.021
3. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. (2022) 45:2753–86. doi: 10.2337/dci22-0034
4. Wang MJ, Chen LQ. Meta-analysis of the effects of HIIT on glucose-lipid metabolism in people with type 2 diabetes mellitus and prediabetes. Sports Res Educ. (2020) 35:89–96. doi: 10.16207/j.cnki.2095-235x.2020.02.017
5. American College of Sports Medicine, Pescatello LS, Arena R, et al. ACSM ‘s Guide-lines for exercise testing and prescription. 9th ed. Philadelphia: Wolters Kluwer/Lippincott Williams &Wilkins Health (2014).
6. Chen BL, Guo JB. Meta-analysis of the effect of high-intensity interval exercise on type 2 diabetes mellitus intervention. Chin Rehabil Theory Pract. (2018) 24:353–62. doi: 10.3969/j.issn.1006-9771.2018.03.020
7. Reindell H, Roskamm H. Ein Beitrag zu den physiologischen Grundlagen des Intervall training unter besonderer Berücksichtigung des Kreilaufes. Schweiz Z Sportmed. (1959) 7:1–8.
8. Khurshid S, Al-Alusi MA, et al. Accelerometer-derived “Weekend warrior” Physical activity and incident cardiovascular disease. JAMA. (2023) 330:247–52. doi: 10.1001/jama.2023.10875
9. Liu JX, Zhu L, Li PJ, et al. Effectiveness of high-intensity interval training on glycemic control and cardiorespiratory fitness in patients with type 2 diabetes: a systematic review and meta-analysis. Aging Clin Exp Res. (2019) 31:575–93. doi: 10.1007/s40520-018-1012-z
10. Han CJ. A study on the effect of high-intensity interval training on cardiovascular disease risk factors in type 2 diabetes mellitus. Shanghai, China: Journal of Shanghai Institute of Physical Education (2020).
11. Lora-Pozo I, Lucena-Anton D, Salazar A. Anthropometric, cardiopulmonary and metabolic benefits of the high-intensity interval training versus moderate, low-intensity or control for type 2 diabetes: systematic review and meta-analysis. Int J Environ Res Public Health. (2019) 16:4524. doi: 10.3390/ijerph16224524
12. Yang P, Yiran O, Ke W, et al. The effect of low volume high-intensity interval training on metabolic and cardiorespiratory outcomes in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 13:1098325. doi: 10.3389/fendo.2022.1098325
13. Jung ME, Bourne JE, Beauchamp MR, et al. High-intensity interval training as an efficacious alternative to moderate-intensity continuous training for adults with prediabetes. Diabetes Res. (2015) 2015:191595. doi: 10.1155/2015/191595
14. Sabouri M, Hatami E, Pournemati P. Inflammatory, antioxidant and glycemic status to different mode of high-intensity training in type 2 diabetes mellitus. Mol Biol Rep. (2021) 48:5291–304. doi: 10.1007/s11033-021-06539-y
15. Wu YP, Lei YY, Shen JB, et al. Effects of different exercise modalities on glycemic control and risk factors for complications in T2DM patients. Chin J Gerontology. (2022) 42:2165–9. doi: 10.3969/J.ISSN.1005-9202.2022.09.036
16. Wu MQ. Efficacy of high-intensity interval training in young obese patients with type 2 diabetes mellitus Vol. 08. Jilin, China: Jilin University (2020). p. 50.
17. Ahmad AM. Moderate-intensity continuous training: is it as good as high-intensity interval training for glycemic control in type 2 diabetes? J Exercise Rehabil. (2019) 15:327–33. doi: 10.12965/jer.1836648.324
18. Li J, Cheng W, Ma HF. A comparative study of health efficacy indicators in subjects with T2DM applying power cycling to 12 weeks of lowVolume high-intensity interval training and moderate-intensity continuous training. J Diabetes Res. (2022), 9273830. doi: 10.1155/2022/9273830
19. Mohammadi zadeh MA, Afrasyabi S, Mohamadi ZA. The effects of exercise training induced calories expenditure on type 2 diabetes related cardio metabolic physiological parameters and adipocytokines. J Diabetes Metab Disord. (2022) 21:1219–31. doi: 10.1007/s40200-021-00808-0
20. Louzada Júnior A, da Silva JM, da Silva VF, Castro ACM, de Freitas RE, Cavalcante JB, et al. Multimodal HIIT is more efficient than moderate continuousTraining for management of body composition, lipid profile and glucose metabolism in the diabetic elderly. Int J Morphol. (2020) 38:392–9.
21. Banitalebi E, Faramarzi M, et al. Effects of different exercise modalities on novel hepatic steatosis indices in overweight women with type 2 diabetes. Clin Mol Hepatol. (2019) 25:294–304. doi: 10.3350/cmh.2018.0086
22. Karstoft K, Winding K, Knudsen SH, Nielsen JS, Thomsen C, Pedersen BK, et al. The effects of free-living intervalWalking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients. Diabetes Care. (2013) 36:228–36. doi: 10.2337/dc12-0658
23. Winding KM, Munch GW, Parikh J, Hollingsworth KG, Taylor R. The effect on glycemic control of low-volume high-intensity interval training versus endurance training in individuals with type 2 diabetes. Diabetes Obes Metab. (2018) 20:1131–9. doi: 10.1111/dom.13198
24. Findikoglu G, Altinkapak A, Yaylali GF. Is isoenergetic high-intensity interval exercise superior to moderate-intensity continuous exercise for cardiometabolic risk factors in individuals with type 2 diabetes mellitus? A single blinded randomized controlled study. Eur J Sport Sci. (2023) 5:1–12. doi: 10.1080/17461391.2023.2167238
25. Ren N. A study of the effect of high-intensity interval training versus moderate-intensity continuous aerobic training on T2DM intervention Vol. 11. Tianjin, China: Tianjin Sports Institute (2022). p. 63.
26. Wang YJ, Zhang C. Study on the effect of high-intensity interval exercise on glycemic and lipid metabolism indexes in patients with type 2 diabetes mellitus. Chin J Nursing. (2019) 54:1605–9. doi: 10.3761/j.issn.0254-1769.2019.11.001
27. Deng CJ, Chen XY, Chen XJ, Cai X, Liu Y, Deng HTF, et al. A study of high intensity interval exercise in blood glucose and negative emotions in type 2 diabetes mellitus patients. Modern Hosp. (2020) 20:100–102+106. doi: 10.3969/j.issn.1671-332X.2020.01.029
28. Marcotte-Chénard A, Tremblay D, Mony M-M, et al. Acute and chronic effects of low-volume high-intensity interval training compared to moderate-intensity continuous training on glycemic control and body composition in older women with type 2 diabetes. Obesities. (2021) 1:72–87. doi: 10.3390/Obesities1020007
29. Malekinezhad H, Moflehi D, et al. Effect of the low or high volume of highIntensity interval training protocols on the leptin and lipid profile in men with type 2 diabetes. J Community Health Res. (2019) 8:228–36. doi: 10.18502/jchr.v8i4.2078
30. Cassidy S, Thoma C, Hallsworth K, Parikh J, Hollingsworth KG, Taylor R, et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomized controlled trial. Diabetologia. (2016) 59:56–66. doi: 10.1007/s00125-015-3741-2
31. Cassidy S, Vaidya V, Houghton D, Zalewski P, Seferovic JP, Hallsworth K, et al. Unsupervised high-intensity interval training improves glycemic control but not cardiovascular autonomic function in type 2 diabetes patients: A randomized controlled trial. Diabetes Vasc Dis Res. (2019) 16:69–76. doi: 10.1177/1479164118816223
32. Maillard F, Rousset S, Pereira B, Traore A, de Pradel Del Amaze P, Boirie Y, et al. High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes Metab. (2016) 42:433–41. doi: 10.1016/j.diabet.2016.07.031
33. Alvarez C, Ramirez-Campillo R, Martinez-Salazar C, Mancilla R, Flores-Opazo M, Cano-Montoya J, et al. Low-volume high-intensity interval training as a therapy for type 2 diabetes. Int J Sports Med. (2016) 37:723–9. doi: 10.1055/s-0042-104935
34. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. (1981) 6:107–28. doi: 10.3102/10769986006002107
35. Marcotte-Chenard A, Tremblay D, Mony M-M, Brochu M, Dionne IJ, Langlois MF, et al. Low-volume walking HIIT: Efficient strategy to improve physical capacity and reduce the risk of cardiovascular disease in older women with type 2 diabetes. Diabetes Metab Syndrome: Clin Res Rev. (2021) 15:102233. doi: 10.1016/j.dsx.2021.102233
36. Liu XC, Wang GF. The effect of high-intensity interval training on physical parameters, metabolomic indexes and serum ficolin-3 levels in patients with prediabetes and type 2 diabetes Exp Clin Endocrinol Diabetes. (2020) 13:01.
37. Shen YL. Net Meta-analysis of the Effects and Applicability of Different Exercise Modalities on Glycemic Control in Type II Diabetes Mellitus Patients. Shandong, China: Shandong Normal University. (2022) 12:125.
38. Brondani de Mello M, Camponogara Righi N, Schuch FB. Effect of high-intensity interval training protocols on VO2max and HbA1c level in people with type 2 diabetes: A systematic review and meta-analysis. Ann Phys Rehabil Med. (2022) 65:101586. doi: 10.1016/j.rehab.2021.101586
39. Yan ZY, Yan PP. Research progress on the effects of continuous and high-intensity interval exercise on blood glucose regulation in type 2 diabetes mellitus. Chin Family Med. (2021) 24:1575–80.
40. Khurshid S, Al-Alusi M, et al. Accelerometer-derived "Weekend warrior" Physical activity and incident cardiovascular disease. JAMA. (2023) 330:247–52. doi: 10.1001/jama.2023.10875
41. Ahmadi MN, Clare PJ, Katzmarzyk PT, Del Pozo Cruz B, Lee MI. Vigorous physical activity, incident heart disease, and cancer: how little is enough? Eur Heart J. (2022) 43:4801–14. doi: 10.1093/eurheartj/ehac572
42. Luo M, Yu C, et al. Accelerometer-measured intensity-specific physical activity, genetic risk and incident type 2 diabetes: a prospective cohort study. Br J Sports Med. (2023) 57:1257–64. doi: 10.1136/bjsports-2022-106653
43. Lee DH, Rezende LFM, Joh H-K, Keum NN, Ferrari G, Rey-Lopez JP, et al. Long-term leisure-time physical activity intensity and all-cause and cause-specific mortality: A prospective cohort of US adults. Circulation. (2022) 146:523–34. doi: 10.1161/CIRCULATIONAHA.121.058162
44. Feng C, Yao JM. Effectiveness of high-intensity interval training on exercise intervention for patients with type 2 diabetes mellitus: based on the WHO Guidelines on Physical Activity and Sedentary Behavior and WHO-FICs. Chin Rehabil Theory Pract. (2022) 28:646–52.
45. Du L, Wang M. Meta-analysis of the effect of different modalities of exercise on intervention in patients with prediabetes. Compilation of Abstracts of the 12th National Congress of Sport Science - Wall Communication (Exercise Physiology and Biochemistry Division). Chin Soc Sport Sci. (2022), 372–4.
Keywords: high-intensity interval exercise, type 2 diabetes mellitus, glucose metabolism, lipid metabolism, meta-analysis
Citation: Feng J, Zhang Q, Chen B, Chen J, Wang W, Hu Y, Yu J and Huang H (2024) Effects of high-intensity intermittent exercise on glucose and lipid metabolism in type 2 diabetes patients: a systematic review and meta-analysis. Front. Endocrinol. 15:1360998. doi: 10.3389/fendo.2024.1360998
Received: 24 December 2023; Accepted: 23 May 2024;
Published: 24 June 2024.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Evelyn Frias-Toral, Catholic University of Santiago de Guayaquil, EcuadorEwa Śliwicka, Poznan University of Physical Education, Poland
Copyright © 2024 Feng, Zhang, Chen, Chen, Wang, Hu, Yu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiming Huang, aHVhbmdodWltaW5nQG5idS5lZHUuY24=
†These authors have contributed equally to this work
 Jingwen Feng1†
Jingwen Feng1† Jiabin Yu
Jiabin Yu Huiming Huang
Huiming Huang