
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 04 July 2023
Sec. Bone Research
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1180183
Purpose: Nephrolithiasis is thought to be a risk factor for osteoporosis, but data assessing if osteoporosis predisposes to the risk of nephrolithiasis are lacking. The present study aims to investigate whether patients with nephrolithiasis have a prominently higher prevalence of osteoporosis than the controls and vice versa via a cumulative analysis.
Methods: Four databases were used to detect the eligible studies. We calculated the relative risk (RR) with a 95% confidence interval (CI) to assess the combined effect. The methodologies for conducting this study followed the PRISMA guidelines and were registered in the PROSPERO (ID: CRD42023395875),
Results: Nine case-control or cohort studies with a total of 454,464 participants were finally included. Combined results indicated that there was a significantly higher prevalence of osteoporosis in patients with nephrolithiasis as compared to the general population without nephrolithiasis (overall RR from six studies= 1.204, 95%CI: 1.133 to 1.28, P< 0.001; heterogeneity: I2 = 34.8%, P= 0.162). Conversely, osteoporosis was significantly correlated to an increased risk of nephrolithiasis as compared to the controls without osteoporosis (overall RR from four studies= 1.505, 95%CI: 1.309 to 1.731, P< 0.001; I2 = 89.8%, P< 0.001). Sensitivity analysis on the two categories validated the above findings. No significant publication bias was identified in this study.
Conclusions: The present study highlighted a significantly high prevalence of osteoporosis in patients with nephrolithiasis and vice versa. This reciprocal association reminded the clinicians to conduct a regular follow-up assessment when managing patients with nephrolithiasis or osteoporosis, especially for the elderly.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#searchadvanced, identifier CRD42023395875.
Nephrolithiasis, a common urological disorder, constitutes an important health concern and a common cause of pain and hospitalization. The incidence of nephrolithiasis ranges from 7–13%, 5–9%, and 1–5%, in North America, Europe, and Asia, respectively (1). Various factors such as geography, climate, diet, fluid intake, genetics, gender, occupation, and age can affect the rate of nephrolithiasis (2–4). Without timely treatment, nephrolithiasis may cause serious complications, e.g., infection, nephrorrhagia, hydronephrosis, kidney injury, and even renal failure (5). It was previously reported that $2.1 billion were cost on the medical management of urolithiasis in the United States in 2000 (6), while this fee rose to $5 billion per year by 2030 (7). Nephrolithiasis is a multifactorial disease. Its formation mechanisms have not yet been elucidated. Hypercalciuria is considered to serve as a common etiopathogenesis for nephrolithiasis formation (8). According to several reports, nephrolithiasis formation is correlated with an increased risk of developing hypertension, diabetes mellitus, gallstones, renal cell carcinoma, and transitional cell carcinoma of the upper urinary tract (9, 10). Since both nephrolithiasis and osteoporosis are metabolic diseases, mounting evidence indicates that nephrolithiasis is reciprocally linked to osteoporosis (11). Calcium is the main calculus component, nearly 80% of nephrolithiasis is composed of calcium oxalate (12). It was found that nephrolithiasis patients are liable to suffer from an increased rate of bone resorption and a lower bone mineral content, especially in those with idiopathic calcium stones (12). Therefore, nephrolithiasis may be a key risk factor for osteoporosis.
Osteoporosis is a chronic, metabolic bone disorder in the elderly, manifested by the deterioration of bone mineral density (BMD) and impaired bone micro-architecture (13). It affects about 10% of individuals aged > 50 and up to about 25% of those aged over 80. Osteoporosis is associated with an increased risk of bone fracture, causing disability and mortality of the sufferers (14). Since the incidence of both nephrolithiasis and osteoporosis increases with age and both of them are metabolic disorders, thus it is expected that they may share a common pathogenesis. The pathogenic mechanisms underlying these two diseases may be associated with the intensive interaction between genetic and environmental factors (11). Besides, the natural histories of the two diseases have many similarities, such as morbidity, the characteristics of the disease course, and the adverse consequences without proper management. Based on these findings, a previous meta-analysis (15) summarized the early evidence and demonstrated that nephrolithiasis was associated with dramatically lower values of BMD T-scores in the spine, total hip, and femoral neck. In addition, this meta-analysis also pooled the results from two relevant studies and found that patients with nephrolithiasis have a higher prevalence of osteoporosis than the healthy controls without nephrolithiasis (odds ratio = 4.12, 95% confidence interval: 3.99 to 4.26, P < 0.0001) (15). Interestingly, the investigators have found not only a high prevalence of osteoporosis in nephrolithiasis patients but also a high prevalence of nephrolithiasis in osteoporosis patients, as compared to the controls. Keller et al. (16) showed that patients with osteoporosis were more likely to diagnose with nephrolithiasis when compared to controls (odds ratio=1.66, 95% confidence interval: 1.59 to 1.73, P < 0.05) after adjusting for multiple confounding factors.
Recently, there have been mounting trials demonstrating the reciprocal association between nephrolithiasis and osteoporosis. In the present study, we aimed to quantitatively summarize the current evidence from all the relevant published studies to evaluate the categorical association between nephrolithiasis and osteoporosis.
This cumulative analysis was registered on the PROSPERO (ID: CRD42023395875), an international database of prospectively registered systematic reviews. Moreover, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines when conducting this cumulative analysis (Supplementary Table 1).
To identify the eligible studies, we searched four electronic databases, including MEDLINE (PubMed), the Cochrane Library databases, EMBASE (OVID), and PsychINFO, from their inception until December 1, 2022. Among the studies we included, only those involving human participants reported using English. PubMed search keywords with various combinations were as follows: ((((((((((((((((((((((“Osteoporosis”[Mesh]) OR (Osteoporoses)) OR (Osteoporosis, Post-Traumatic)) OR (Osteoporosis, Post Traumatic)) OR (Post-Traumatic Osteoporoses)) OR (Post-Traumatic Osteoporosis)) OR (Osteoporosis, Senile)) OR (Osteoporoses, Senile)) OR (Senile Osteoporoses)) OR (Osteoporosis, Involutional)) OR (Senile Osteoporosis)) OR (Osteoporosis, Age-Related)) OR (Osteoporosis, Age Related)) OR (Bone Loss, Age-Related)) OR (Age-Related Bone Loss)) OR (Age-Related Bone Losses)) OR (Bone Loss, Age Related)) OR (Bone Losses, Age-Related)) OR (Age-Related Osteoporosis)) OR (Age Related Osteoporosis)) OR (Age-Related Osteoporoses)) OR (Osteoporoses, Age-Related)) AND (((((((((((((((((“Urolithiasis”[Mesh]) OR (Urinary Lithiasis)) OR (Lithiasis, Urinary)) OR (Kidney Calculi)) OR (Calculi, Kidney)) OR (Calculus, Kidney)) OR (Kidney Calculus)) OR (Nephrolith)) OR (Renal Calculus)) OR (Kidney Stones)) OR (Kidney Stone)) OR (Stone, Kidney)) OR (Stones, Kidney)) OR (Renal Calculi)) OR (Calculi, Renal)) OR (Calculus, Renal)) OR (Nephrolithiasis)).
The BMD T-score criteria are commonly used to diagnose osteoporosis. A T-score ≤ −2.5 in any of the bones was applied for defined osteoporosis. Nephrolithiasis could be confirmed by ultrasound and radiograph examination (i.e., X-rays, computerized tomography).
This cumulative analysis included all epidemiologic studies that met the prior inclusion criteria. The question guiding the present study was: Does nephrolithiasis increase the risk of osteoporosis and vice versa? The Patient, Intervention, Comparison, Outcome, and Study Design (PICOS) framework was applied. The components for this PICOS evidence contained the following factors: patients diagnosed with osteoporosis or nephrolithiasis (P); a history of nephrolithiasis or osteoporosis (I); compared with the healthy controls (C); the prevalence of osteoporosis or nephrolithiasis (O); any study designs (S). Furthermore, those articles that provided relative risk (RR) or odds ratios (OR) with the corresponding 95% confidence intervals (CI) were also considered to be eligible.
The exclusion criteria of this cumulative analysis were: (a) non-English studies, (b) lack of a control group; (c) review articles, comments, and case reports; (d) duplicated data; (e) experimental studies (i.e., in vivo or in vitro studies); (f) meta-analysis researches. Under the inclusion and exclusion criteria, two authors independently screened the potential included studies. The corresponding author or the third author was expected to resolve the ambiguities during the selection of the eligible studies.
By using a standardized data collection table, two authors independently evaluated and extracted the data, including the names of the first author, the publication year of the included studies, study location, study design, age of the participants, the number of cases of osteoporosis or nephrolithiasis in both the study and the control group, the RR accompanied with the 95%CI generated in each included study, and the assessments of osteoporosis or nephrolithiasis ascertainment.
Two independent authors assessed the methodological quality of the eligible studies. Any ambiguities were resolved by consensus or the third author. The methodological quality of case-control and cohort studies was followed by the Newcastle–Ottawa Scale (NOS). Within this scale, nine domains were assessed and the score of conformity gained one score. Studies were assigned a score between 0 and 3 to indicate low quality, 4 to 6 to indicate moderate quality, and 7 to 9 to indicate high quality.
We conducted the current cumulative analysis by using the STATA (version 13.0, Stata Corp LP, College Station, Texas, USA). The combined RR with the corresponding 95% CI was applied to quantitatively evaluate the strength of the association between nephrolithiasis and osteoporosis. Two-tail P values of 0.05 were assumed to be statistically significant. The heterogeneity test was conducted using I2 statistics and the Cochrane Q statistic. Significant heterogeneity was defined as I2 > 50%. In the Q test, a P-value < 0.10 is considered statistically significant. Considering the high likelihood of between-study variance for differences in the study design and demographics, a random-effect model is used in this cumulative analysis rather than a fixed-effect model. Additionally, we performed a sensitivity analysis to investigate the sources of heterogeneity. In sensitivity analyses, one study was eliminated at a time and the effect of this elimination was subsequently evaluated. An assessment of publication bias was conducted using the funnel plot and Begg’s rank correlation test.
The selection process for screening the potential inclusions is shown in Figure 1. A total of 1456 publications were screened during the initial search within the databases of MEDLINE, Cochrane Library databases, EMBASE, and PsychINFO. There were 71 articles were retrieved for full-text review after eliminating duplicates and those studies out of the aforementioned inclusion criteria. Among the 71 potential trials, 21 studies were excluded due to without a control group; 18 studies did not meet the inclusion criteria; 15 studies for inappropriate grouping; 8 studies for insufficient outcome data. Finally, nine studies (16–24) were included in this cumulative analysis. Among these eligible studies, one study conducted by Kim et al. (22) provided the prevalence of osteoporosis in nephrolithiasis patients as well as the prevalence of nephrolithiasis in patients with osteoporosis. So, six eligible studies reported nephrolithiasis and the risk of osteoporosis and four eligible studies reported osteoporosis and the risk of nephrolithiasis were conducted for synthesizing the overall RR for the two topics.
Table 1 showed the characteristics of the six included studies of nephrolithiasis and risk of osteoporosis and four included studies of osteoporosis and the risk of nephrolithiasis (one study provided two independent data). Among the nine included studies, five of them were designed for case-control and four studies were cohort design. These studies were published between 2002 and 2022. In terms of geographical distribution, five studies were developed in Europe and four studies were conducted in Asia. These studies involved participants ranging in mean age from 39.2 to 66.7. The sample size ranged from 64 to 135,622. The confirmation of osteoporosis mainly depended on the T-score and International Classification of Disease (ICD), while nephrolithiasis was diagnosed by ultrasound and radiograph, etc.
Following NOS’ methodological quality checker, six included studies gained a score of 7-9, so they were considered high quality. The remainder three included studies were judged to a score of 6, indicating these studies were of moderate quality. As a result, the proportion of high methodological quality in this cumulative analysis was 67% (6/9). The detail-specific scoring was listed in Supplementary Table 2.
As shown in Figure 2, the pooled result derived from six eligible studies (one study provided both the hip and spine data) demonstrated that there was a significantly higher prevalence of osteoporosis in patients with nephrolithiasis as compared to the general population without nephrolithiasis by using a random-effects model (overall RR from 7 studies= 1.204, 95%CI: 1.133 to 1.28, P< 0.001; heterogeneity test: I2 = 34.8%, P= 0.162).
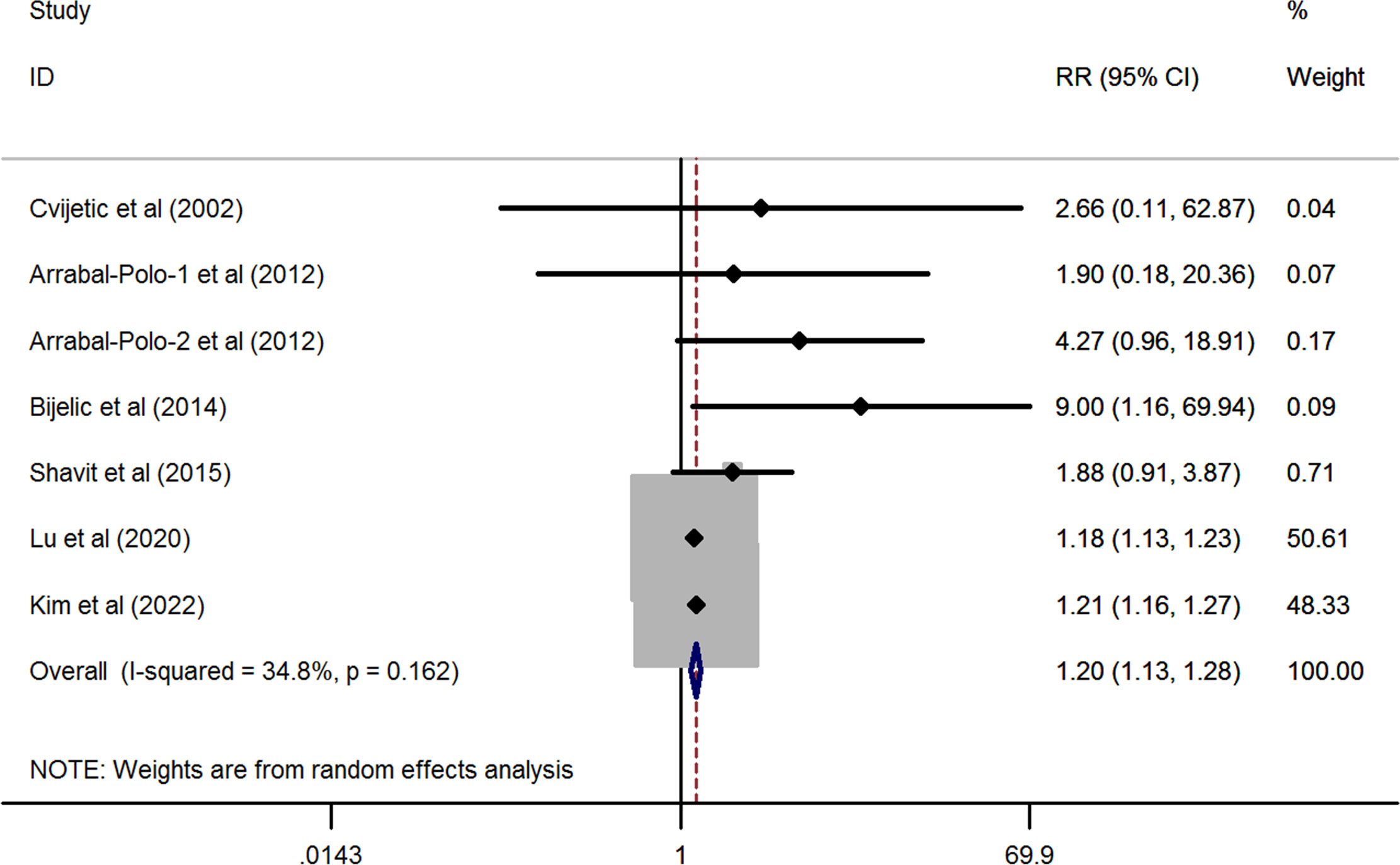
Figure 2 Forest plots of the cumulative analysis of the six included studies on the association between nephrolithiasis and the risk of osteoporosis.
In line with the above findings, the prevalence of nephrolithiasis was also significantly higher in patients with osteoporosis than in the healthy control without osteoporosis (pooled RR from four studies = 1.505, 95%CI: 1.309 to 1.731, P< 0.001). However, substantial heterogeneity was observed in this synthesized analysis (I2 = 89.8%, P< 0.001) (Figure 3).
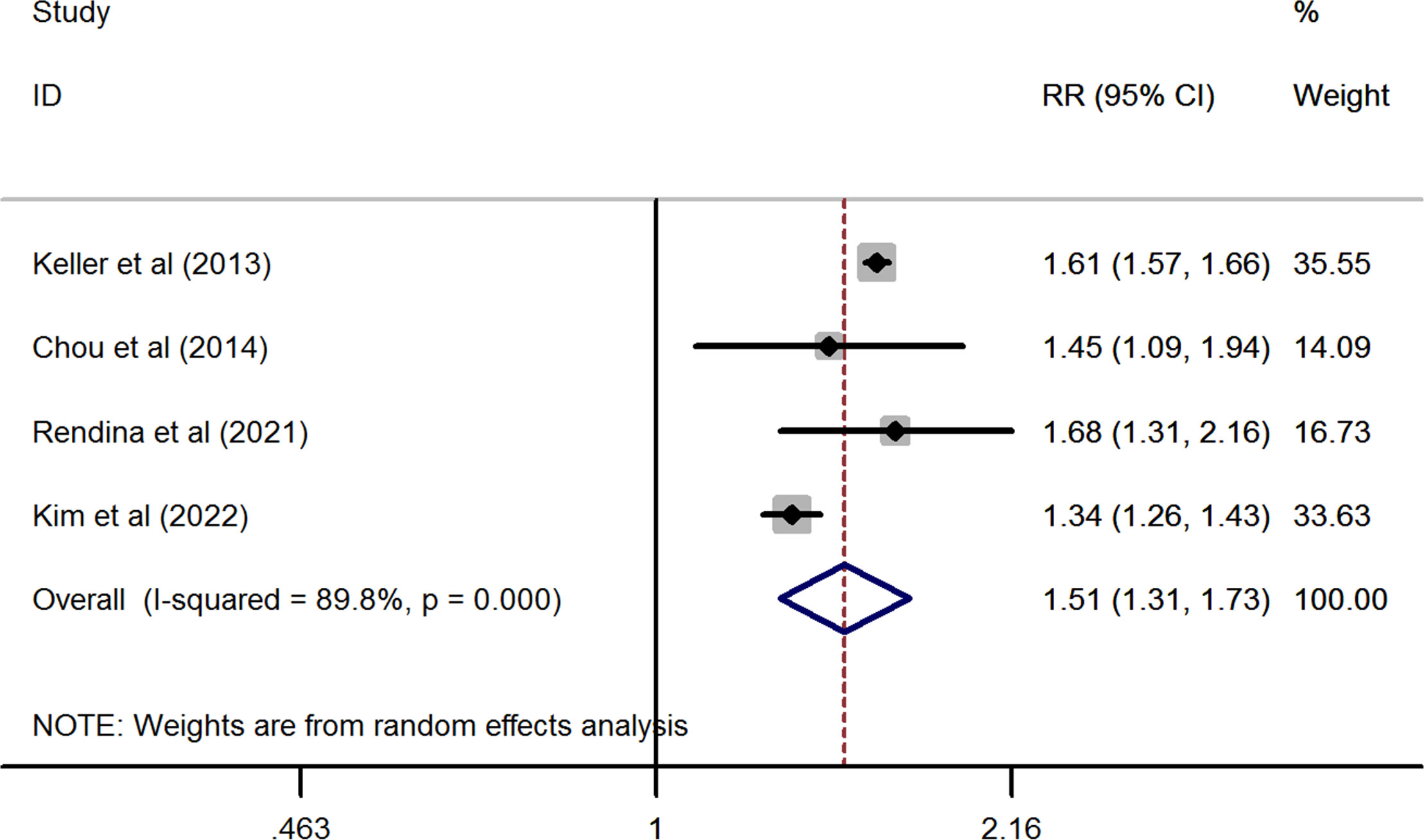
Figure 3 Forest plots of the cumulative analysis of the four included studies on the association between osteoporosis and the risk of nephrolithiasis.
The above results suggested that there was a cross-correlation between nephrolithiasis and osteoporosis. Nephrolithiasis might elevate the risk of osteoporosis. Conversely, patients with osteoporosis might also be susceptible to suffering from nephrolithiasis.
To evaluate the impact of individual studies on the overall RR, sensitivity analysis was conducted on the two topics. First, as shown in Table 2; Figure 4A, no significant change in the newly generated pooled RR was observed for studies that reported nephrolithiasis and the risk of osteoporosis. The RR ranged from 1.815 (95%CI: 1.067-3.184, P= 0.028) to 1.206 (95%CI: 1.128-1.289, P< 0.001). On the other hand, no substantial heterogeneity was observed after eliminating any one of the 7 included studies. The I2 ranged from 8.8% to 44.8% and all P>0.1.
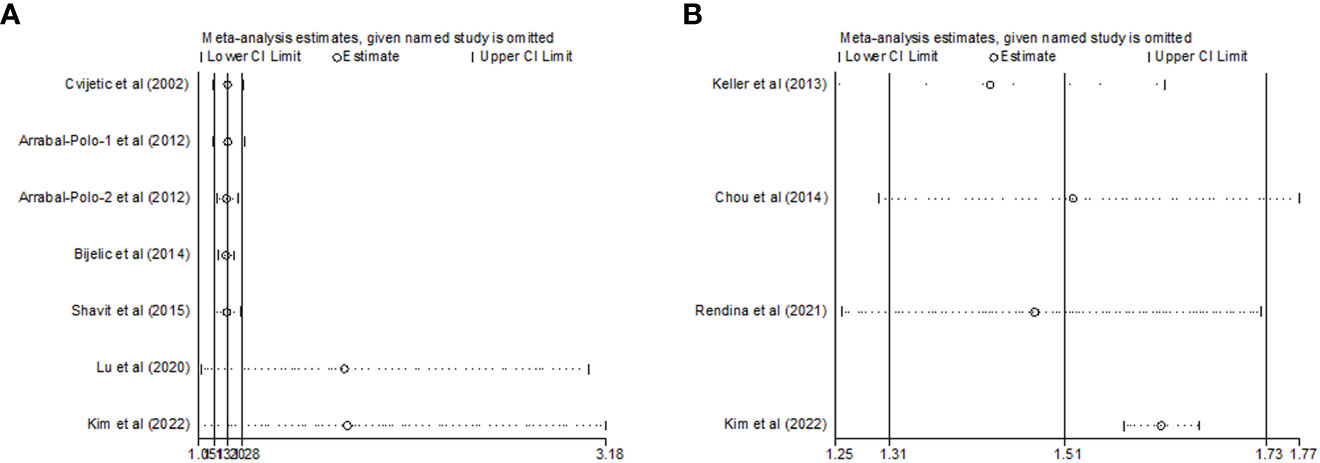
Figure 4 Sensitivity analysis after each study was excluded by turns in the categories (A) nephrolithiasis and risk of osteoporosis; (B) osteoporosis and risk of nephrolithiasis).
As regards to those studies reporting osteoporosis and the risk of nephrolithiasis, there was no substantial change in the new synthesized overall RR, which ranged from 1.422 (95%CI: 1.249-1.618, P< 0.001) to 1.614 (95%CI: 1.572-1.656, P< 0.001) after eliminating any of the four included studies. However, the substantial heterogeneity remarkably disappeared after excluding Kim et al.’s study (22) (I2 = 0.0%, P= 0.742) (Table 2; Figure 4B). These results demonstrated that Kim et al.’s study might be the source of the great heterogeneity (I2 = 89.8%) in this cumulative analysis.
According to Begg’s rank-correlation test, there was no significant publication bias in the seven studies reporting the association between nephrolithiasis and the risk of osteoporosis (Begg’s, P > |z| = 0.548) (Figure 5A). Consistent with this finding, Begg’s rank-correlation test also revealed no significant publication bias within the analysis of the relationship between osteoporosis and the risk of nephrolithiasis (Begg’s, P > |z| = 0.734) (Figure 5B).
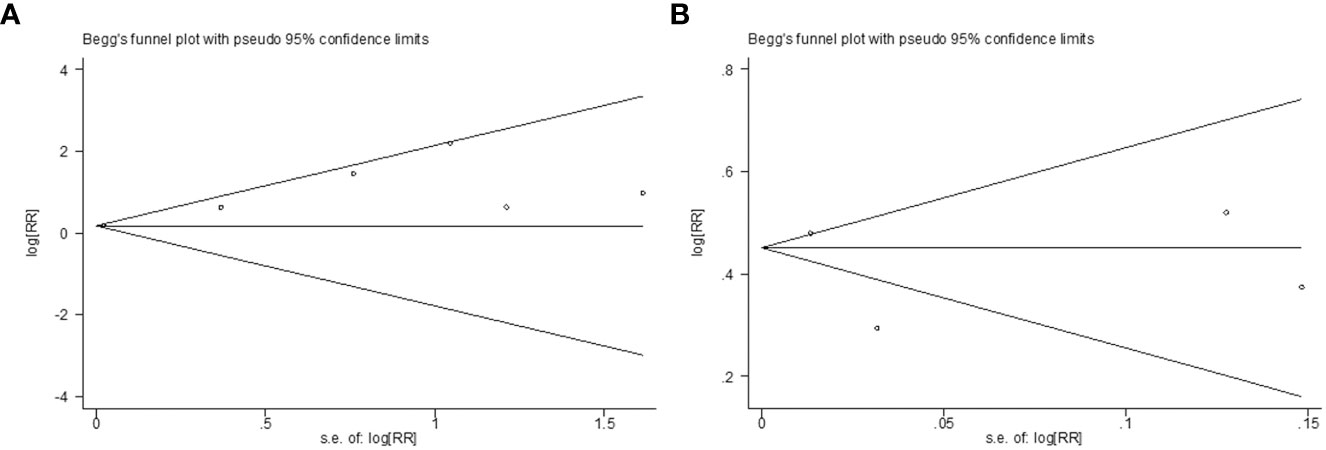
Figure 5 (A) Begg’s test for detecting publication bias on the topic of nephrolithiasis and risk of osteoporosis; (B) Begg’s test for detecting publication bias on the topic of and osteoporosis risk of nephrolithiasis.
Approximately 40 years ago, an earlier report conducted by Fuss et al. (25) demonstrated that a significant decreased bone mineral content (BMC) was observed in renal stone formers as compared to the normal (P<0.001). During the last 40 years, a considerable proportion of investigators found a reduction in both BMC and BMD in patients with calcium nephrolithiasis (26–29). In these studies, the prevalence of osteoporosis in stone-forming patients ranged from 6 to 60% (30). In a more recent study developed by Ganesan et al. in 2021, the authors reported that 20% of the 531,431 patients with kidney stone disease were subsequently diagnosed with osteoporosis (31). However, most of the above studies have a key limitation which is the lack of a control group. On the other hand, more and more authors have described that patients with osteoporosis are susceptible to nephrolithiasis (16, 19). Therefore, it would seem likely a reciprocal relationship exists between osteoporosis and nephrolithiasis. To better explore the association between nephrolithiasis and osteoporosis via a scientific method, we conducted this cumulative analysis focused on the two research questions. As a result of comprehensive searches for the included studies, six observational trials reporting nephrolithiasis and the risk of osteoporosis and three retrospective studies reporting osteoporosis and the risk of nephrolithiasis were eventually included in this pooled analysis. Synthetic RR from seven studies described a 1.2-fold elevated risk of osteoporosis in patients with nephrolithiasis compared to the controls without nephrolithiasis (a total of 218,198 participants, overall RR= 1.2, 95%CI: 1.13 to 1.28, P< 0.001). On the other hand, combined RR from four studies reporting the relationship between osteoporosis and risk of nephrolithiasis also yielded a 1.51-fold increased risk of nephrolithiasis in patients with osteoporosis as compared to the healthy population without osteoporosis (a total of 236,266 participants, overall RR= 1.51, 95%CI: 1.31 to 1.73, P< 0.001). In this pooled analysis, the methodological quality of the nine included studies was either moderate or high quality. And the proportion of high methodological quality among the eligible studies was up to 67%. Besides, sensitivity analysis suggested that no single study dominated the overall RR in the two independent cumulative analyses. Moreover, no remarkable publication bias was detected when combining the RR from the included studies. Based on the above facts, our study is highly reliable and the evidence is robust.
Though there has been a positive association between nephrolithiasis and osteoporosis found in the current cumulative analysis and other uncontrolled studies, a clear etiology for such a correlation is still elusive. Theoretically, both nephrolithiasis and osteoporosis are calcium metabolic disorders, indicating there are some commonalities between the two diseases. According to the findings from some relevant studies, the predominant etiological agents underlying the relationship between nephrolithiasis and osteoporosis might be hypercalciuria and dietary calcium intake (15).
It is known that patients with hypercalciuria may be predisposed to bone demineralization (32). It has been reported that 20 to 30% of patients initially diagnosed with osteoporosis suffer from idiopathic hypercalciuria, a subclinical condition that contributes most to bone loss (15, 33, 34). The pathogeny of the loss of BMD in patients with idiopathic hypercalciuria is not well-understood. Hypercalciuria has been described to correlate to calcium-phosphate homeostasis, promotion of cytokines predisposing to bone loss, the elevation of parathormone, and calcitriol-mediated cascades. Hypercalciuria can alter the calcium-phosphate homeostasis of the body. In patients with hypercalciuria, this condition may cause tubular dysfunction of the Na-P cotransporters, which is associated with renal phosphate leak and thus predisposing to bone demineralization (35). Patients with hypercalciuria are susceptible to suffering from the disorders of calcium–phosphate and vitamin D homeostasis. Since vitamin D or calcium is essential for bone health, osteoporosis may be attributable to deficiencies in these two nutrients (36). Hypercalciuria was found to be associated with low serum vitamin D levels (37), while low vitamin D significantly influenced bone loss. Vitamin D functions to increase the intestinal absorption of calcium and phosphorous. Also, Lacey et al. (38) reported that vitamin D could regulate IL-1 and IL-6 production and thus affect bone metabolism in a dose-dependent way. As a result, adequate calcium and vitamin D intakes were considered to be beneficial for osteoporosis (39). On the other hand, however, calcium and vitamin D supplementations are risk factors for stone formation. Therefore, patients who received calcium and vitamin D treatment may induce nephrolithiasis (36). It showed a 17% elevated risk of nephrolithiasis in patients treated with calcium and vitamin D (40). But this speculation did not support by some investigators. Penniston et al. (41) found that vitamin D supplements did not elevate urine calcium and the risk of nephrolithiasis. Similar to this study, Arrabal-Polo et al. (42) demonstrated that patients with nephrolithiasis presented higher calcium excretion in 24-hour urine, irrespective of vitamin D excretion rates. In terms of genetic determinants, a high level of intestinal vitamin D receptors was found in the hypercalciuric animal model (43). It was reported that BMD could be regulated by a simple allelic substitution in the vitamin D receptor gene (44). And the vitamin D receptor polymorphisms were found to be associated with calcium kidney stone disease (12). Some investigators found an increase in vitamin D receptors’ sensitivity affecting bone resorption (12, 30), indicating a role of vitamin D in the development of osteoporosis. In addition to hypercalciuria, hyperuricosuria may also play a role in the relationship between nephrolithiasis and osteoporosis. Hyperuricosuria was found to not only promote the formation of calcium oxalate stones but also associated with lower BMD (17, 45, 46). Though the relationship among hypercalciuria, vitamin D, and bone health is still unclear, several studies pointed out that thiazides might be more beneficial than calcium and vitamin D supplements in treating patients with osteoporosis and hypercalciuria (47, 48). The underlying mechanisms that might be correlated to thiazides can elevate renal tubular reabsorption of calcium and increase the levels of serum calcium, thus reducing lithogenic risk as well as improving BMD (49).
In addition to vitamin D, intact parathyroid hormone (PTH) is also one of the pivotal hormones responsible for the metabolism of calcium and phosphorous. A parathyroid-independent pathologic process may cause bone Ca efflux. Excess PTH levels induce additional loss of calcium in the sufferers, which leads to the formation of calcium stones (50, 51). In individuals with hyperparathyroidism, urinary tract stones, and vertebral fractures occur frequently (52). Higher PTH levels may decline the circulating 25-hydroxyvitamin D by facilitating renal conversion to 1,25-dihydroxyvitamin D (53). A surplus of PTH was found to induce calcium loss with a consequential creation of calcium stones as well as lessen BMD and develop osteopenia and osteoporosis (54). Interestingly, Reid et al. (53) showed that the occurrence of osteoporosis was high in hyperparathyroidism patients, but the rate of nephrolithiasis was not high in these patients. This finding suggested that PTH disturbance-mediated osteoporosis might not be relevant to nephrolithiasis development. Thus, the potential role of PTH in the correlation between nephrolithiasis and osteoporosis needs prospective evaluation.
Alternatively, the diet has also been postulated to be an important factor underlying the correlation between osteoporosis and nephrolithiasis. Theoretically, nephrolithiasis patients may restrict calcium and vitamin D intake to prevent the recurrence of urinary tract stones, and this may contribute to the development of osteoporosis (55). Limited calcium intake may cause a negative calcium balance that increases the risk of bone loss and BMD depletion over time. Conversely, patients with osteoporosis are more likely to be treated with calcium and vitamin D, which may increase the risk of renal stone formation. This is due to vitamin D, calcium, or combined supplementation was found to be associated with an increase in the incidence of kidney stones (36). Calcium dietary intake was previously found to promote oxalate absorption and excretion. Of note, however, many investigators observed that both vitamin D and calcium supplementation did not elevate the risk of nephrolithiasis (56, 57). In addition to hypercalciuria, both nephrolithiasis and osteoporosis may share other common pathogenic factors, e.g. unhealthy eating habits. It was reported that patients with nephrolithiasis or osteoporosis tended to have high salt, high sugar intake, and low physical activity (11, 58, 59). Nouvenne et al. (60) revealed that high sodium in the diet might elevate the risk of nephrolithiasis and osteoporosis on account of a higher urinary calcium excretion and lower citrate excretion. However, diets higher in potassium intake are inversely correlated with both nephrolithiasis and osteoporosis (52). Therefore, dietary patterns may involve the reciprocal association between osteoporosis and nephrolithiasis in the time period.
Nephrolithiasis is thought to be a risk factor for osteoporosis, but data assessing if osteoporosis predisposes to the risk of nephrolithiasis are lacking. In this study, we clarified the two phenomena underlying nephrolithiasis and osteoporosis by summarizing all the data from the relevant studies. To the best of our knowledge, the present study is the first study to quantify the association between nephrolithiasis and the risk of osteoporosis and vice versa through a cumulative analysis. Some strengths existed in this study, including large sample size (a total of 454,464 participants), high methodological qualities of the relevant studies, fewer heterogeneities among the included studies, and an evidence-based etiological theory for the association between nephrolithiasis and risk of osteoporosis and vice versa. However, some inherent limitations should be noted when interpreting the present results. First, only nine relevant studies were included in the analysis, which may yield biased data. Second, all the included studies were retrospectively designed, which may downgrade the evidence of this cumulative analysis. Third, different geographic regions, sample sizes, mean age, study designs, variable factors, and characteristics of participants among the eligible studies might cause potential heterogeneities. Fourth, higher age, gender, hyperlipidemia, hypertension, diabetes mellitus, hyperuricemia, smoking, and being overweight might be the independent risk factors for nephrolithiasis so as well for osteoporosis. Given the inability to adjust for these potential confounders, it’s not possible to conclude that the causal relationship exists between nephrolithiasis and osteoporosis. Based on these facts, additional high-quality rigorous and prospective cohorts are still warranted to better illustrate the reciprocal correlation between nephrolithiasis and the risk of osteoporosis and vice versa.
The present cumulative analysis provides evidence supporting the reciprocal relationship between nephrolithiasis and the high risk of osteoporosis and vice versa. However, the evidence of the causal relationship between nephrolithiasis and osteoporosis is not so strong due to the study design and the confounding factors. Further study of these relationships is still required, given the possibilities to screen for these two conditions if necessary. Our study highlights that regular follow-up assessments are needed when managing patients with nephrolithiasis or osteoporosis, especially for the elderly.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization and methodology: SJ and WX. Software and formal analysis: YW and WZ. Writing original draft and editing: SJ, JJ and WX. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Planning Project of Taizhou City, Zhejiang Province (Grant No: 22ywa42); Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant No.2020KY367); The Science and Technology Program of Zhejiang Province (Grant No. 2018ZB136).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1180183/full#supplementary-material
Supplementary Table 1 | PRISMA Checklist.
Supplementary Table 2 | Newcastle-Ottawa Scale assessment of the quality of the case-control and cohort studies.
1. Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol (2017) 35:1301–20. doi: 10.1007/s00345-017-2008-6
2. Chen X, Zhang XB, Li DJ, Qi GN, Dai YQ, Gu J, et al. miR-155 facilitates calcium oxalate crystal-induced HK-2 cell injury via targeting PI3K associated autophagy. Exp Mol Pathol (2020) 115:104450. doi: 10.1016/j.yexmp.2020.104450
3. Jonassen JA, Cao LC, Honeyman T, Scheid CR. Mechanisms mediating oxalate-induced alterations in renal cell functions. Crit Rev Eukaryot Gene Expr (2003) 13:55–72. doi: 10.1615/critreveukaryotgeneexpr.v13.i1.50
4. Sorokin I, Pearle MS. Medical therapy for nephrolithiasis: state of the art. Asian J Urol (2018) 5:243–55. doi: 10.1016/j.ajur.2018.08.005
5. Hou J, Lv Z, Wang Y, Wang X, Wang Y, Wang K. Knowledge-map analysis of percutaneous nephrolithotomy (PNL) for urolithiasis. Urolithiasis (2023) 51:34. doi: 10.1007/s00240-023-01406-w
6. Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: urolithiasis. J Urol (2005) 173:848–57. doi: 10.1097/01.ju.0000152082.14384.d7
7. Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the national health and nutrition examination survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol (2014) 66:724–9. doi: 10.1016/j.eururo.2014.06.036
8. Khan A. Prevalence, pathophysiological mechanisms and factors affecting urolithiasis. Int Urol Nephrol (2018) 50:799–806. doi: 10.1007/s11255-018-1849-2
9. Lin BB, Huang RH, Lin BL, Hong YK, Lin ME, He XJ. Associations between nephrolithiasis and diabetes mellitus, hypertension and gallstones: a meta-analysis of cohort studies. Nephrology (2020) 25:691–9. doi: 10.1111/nep.13740
10. Cheungpasitporn W, Thongprayoon C, O'Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, et al. The risk of kidney cancer in patients with kidney stones: a systematic review and meta-analysis. QJM (2015) 108:205–12. doi: 10.1093/qjmed/hcu195
11. Rendina D, De Filippo G, Iannuzzo G, Abate V, Strazzullo P, Falchetti A. Idiopathic osteoporosis and nephrolithiasis: two sides of the same coin? Int J Mol Sci (2020) 21:8183. doi: 10.3390/ijms21218183
12. Bilic-Curcic I, Milas-Ahic J, Smolic M, Smolic R, Mihaljevic I, Tucak-Zoric S. Urolithiasis and osteoporosis: clinical relevance and therapeutic implications. Coll Antropol (2009) 33 Suppl 2:189–92.
13. Verdonck C, Willems R, Borgermans L. Implementation and operationalization of integrated people-centred health services delivery strategies in integrated osteoporosis care (IOC) initiatives: a systematic review. Osteoporos Int (2023) 34:841-865. doi: 10.1007/s00198-023-06678-x
14. Yu F, Xia W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch Osteoporos (2019) 14:32. doi: 10.1007/s11657-018-0549-y
15. Lucato P, Trevisan C, Stubbs B, Zanforlini BM, Solmi M, Luchini C, et al. Nephrolithiasis, bone mineral density, osteoporosis, and fractures: a systematic review and comparative meta-analysis. Osteoporos Int (2016) 27:3155–64. doi: 10.1007/s00198-016-3658-8
16. Keller JJ, Lin CC, Kang JH, Lin HC. Association between osteoporosis and urinary calculus: evidence from a population-based study. Osteoporos Int (2013) 24:651–7. doi: 10.1007/s00198-012-2019-5
17. Cvijetic S, Furedi-Milhofer H, Babic-Ivancic V, Tucak A, Galic J, Dekanic-Ozegovic D. Bone mineral density loss in patients with urolithiasis: a follow-up study. Arch Med Res (2002) 33:152–7. doi: 10.1016/s0188-4409(01)00367-8
18. Arrabal-Polo MA, Arrabal-Martin M, Giron-Prieto MS, Poyatos-Andujar A, Garrido-Gomez J, Zuluaga-Gomez A, et al. Osteopenia/osteoporosis in patients with calcium nephrolithiasis. Urol Res (2012) 40:709–16. doi: 10.1007/s00240-012-0497-8
19. Bijelic R, Milicevic S, Balaban J. Incidence of osteoporosis in patients with urolithiasis. Med Arch (2014) 68:335–8. doi: 10.5455/medarh.2014.68.335-338
20. Shavit L, Girfoglio D, Vijay V, Goldsmith D, Ferraro PM, Moochhala SH, et al. Vascular calcification and bone mineral density in recurrent kidney stone formers. Clin J Am Soc Nephrol (2015) 10:278–85. doi: 10.2215/CJN.06030614
21. Lu YM, Li CC, Juan YS, Lee YC, Chien TM. Urolithiasis increases the risk of subsequent onset of osteoporosis. J Bone Miner Metab (2020) 38:38–43. doi: 10.1007/s00774-019-01022-y
22. Kim SY, Chung J, Park DS, Yoo DM, Bang WJ, Choi HG. The reciprocal relationship between osteoporosis and renal stones. J Clin Med (2022) 11:6614. doi: 10.3390/jcm11226614
23. Chou PS, Kuo CN, Hung KS, Chang WC, Liao YC, Chi YC, et al. Osteoporosis and the risk of symptomatic nephrolithiasis: a population-based 5-year follow-up study in Taiwan. Calcif Tissue Int (2014) 95:317–22. doi: 10.1007/s00223-014-9895-y
24. Rendina D, D'Elia L, Evangelista M, De Filippo G, Giaquinto A, Barone B, et al. Osteoporosis is a predictive factor for nephrolithiasis in an adult free-living Caucasian population from southern Italy: a longitudinal retrospective study based on a general practice database. Calcif Tissue Int (2020) 107:446–52. doi: 10.1007/s00223-020-00737-9
25. Fuss M, Gillet C, Simon J, Vandewalle JC, Schoutens A, Bergmann P. Bone mineral content in idiopathic renal stone disease and in primary hyperparathyroidism. Eur Urol (1983) 9:32–4. doi: 10.1159/000474039
26. Bataille P, Achard JM, Fournier A, Boudailliez B, Westeel PF, El EN, et al. Diet, vitamin d and vertebral mineral density in hypercalciuric calcium stone formers. Kidney Int (1991) 39:1193–205. doi: 10.1038/ki.1991.151
27. Pietschmann F, Breslau NA, Pak CY. Reduced vertebral bone density in hypercalciuric nephrolithiasis. J Bone Miner Res (1992) 7:1383–8. doi: 10.1002/jbmr.5650071205
28. Trinchieri A, Nespoli R, Ostini F, Rovera F, Zanetti G, Pisani E. A study of dietary calcium and other nutrients in idiopathic renal calcium stone formers with low bone mineral content. J Urol (1998) 159:654–7. doi: 10.1016/S0022-5347(01)63694-2
29. Caudarella R, Vescini F, Buffa A, Sinicropi G, Rizzoli E, La Manna G, et al. Bone mass loss in calcium stone disease: focus on hypercalciuria and metabolic factors. J Nephrol (2003) 16:260–6.
30. Caudarella R, Vescini F, Buffa A, La Manna G, Stefoni S. Osteoporosis and urolithiasis. Urol Int (2004) 72(Suppl 1):17–9. doi: 10.1159/000076585
31. Ganesan C, Thomas IC, Romero R, Song S, Conti S, Elliott C, et al. Osteoporosis, fractures, and bone mineral density screening in veterans with kidney stone disease. J Bone Miner Res (2021) 36:872–8. doi: 10.1002/jbmr.4260
32. Cerda GD, Peris P, Monegal A, Albaladejo C, Martinez MA, Muxi A, et al. Search for hidden secondary causes in postmenopausal women with osteoporosis. Menopause (2010) 17:135–9. doi: 10.1097/gme.0b013e3181ade8e5
33. Giannini S, Nobile M, Dalle CL, Lodetti MG, Sella S, Vittadello G, et al. Hypercalciuria is a common and important finding in postmenopausal women with osteoporosis. Eur J Endocrinol (2003) 149:209–13. doi: 10.1530/eje.0.1490209
34. Letavernier E, Traxer O, Daudon M, Tligui M, Hubert-Brierre J, Guerrot D, et al. Determinants of osteopenia in male renal-stone-disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol (2011) 6:1149–54. doi: 10.2215/CJN.10191110
35. Prie D, Beck L, Friedlander G, Silve C. Sodium-phosphate cotransporters, nephrolithiasis and bone demineralization. Curr Opin Nephrol Hypertens (2004) 13:675–81. doi: 10.1097/00041552-200411000-00015
36. Kahwati LC, Weber RP, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, et al. Calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US preventive services task force. Jama (2018) 319:1600–12. doi: 10.1001/jama.2017.21640
37. Eller-Vainicher C, Cairoli E, Zhukouskaya VV, Morelli V, Palmieri S, Scillitani A, et al. Prevalence of subclinical contributors to low bone mineral density and/or fragility fracture. Eur J Endocrinol (2013) 169:225–37. doi: 10.1530/EJE-13-0102
38. Lacey DL, Grosso LE, Moser SA, Erdmann J, Tan HL, Pacifici R, et al. IL-1-induced murine osteoblast IL-6 production is mediated by the type 1 IL-1 receptor and is increased by 1,25 dihydroxyvitamin D3. J Clin Invest (1993) 91:1731–42. doi: 10.1172/JCI116383
39. Zhou W, Langsetmo L, Berger C, Poliquin S, Kreiger N, Barr SI, et al. Longitudinal changes in calcium and vitamin d intakes and relationship to bone mineral density in a prospective population-based study: the Canadian multicentre osteoporosis study (CaMos). J Musculoskelet Neuronal Interact (2013) 13:470–9.
40. Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin d supplementation and the risk of fractures. N Engl J Med (2006) 354:669–83. doi: 10.1056/NEJMoa055218
41. Penniston KL, Jones AN, Nakada SY, Hansen KE. Vitamin d repletion does not alter urinary calcium excretion in healthy postmenopausal women. Bju Int (2009) 104:1512–6. doi: 10.1111/j.1464-410X.2009.08559.x
42. Arrabal-Polo MA, Giron-Prieto MS, Cano-Garcia MC, Poyatos-Andujar A, Quesada-Charneco M, Abad-Menor F, et al. Retrospective review of serum and urinary lithogenic risk factors in patients with osteoporosis and osteopenia. Urology (2015) 85:782–5. doi: 10.1016/j.urology.2015.01.019
43. Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin d receptor in genetic hypercalciuric rats. a cause of intestinal calcium hyperabsorption. J Clin Invest (1993) 91:661–7. doi: 10.1172/JCI116246
44. Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, et al. Prediction of bone density from vitamin d receptor alleles. Nature (1994) 367:284–7. doi: 10.1038/367284a0
45. Jaeger P, Lippuner K, Casez JP, Hess B, Ackermann D, Hug C. Low bone mass in idiopathic renal stone formers: magnitude and significance. J Bone Miner Res (1994) 9:1525–32. doi: 10.1002/jbmr.5650091004
46. Tugcu V, Ozbek E, Aras B, Ozbay B, Islim F, Tasci AI. Bone mineral density measurement in patients with recurrent normocalciuric calcium stone disease. Urol Res (2007) 35:29–34. doi: 10.1007/s00240-006-0074-0
47. Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int (2011) 79:393–403. doi: 10.1038/ki.2010.473
48. O'Neill S, MacLennan A, Bass S, Diamond T, Ebeling P, Findlay D, et al. Guidelines for the management of postmenopausal osteoporosis for GPs. Aust Fam Physician. (2004) 33:910–9.
49. Giron-Prieto MS, Arias-Santiago S, Del CCM, Poyatos-Andujar A, de Haro-Munoz T, Abad-Menor F, et al. Bone remodeling markers as lithogenic risk factors in patients with osteopenia-osteoporosis. Int Urol Nephrol (2016) 48:1777–81. doi: 10.1007/s11255-016-1361-5
50. Bijelic R, Milicevic S, Balaban J. Correlation of osteoporosis and calcium urolithiasis in adult population. Med Arch (2016) 70:66–8. doi: 10.5455/medarh.2016.70.66-68
51. Milicevic S, Bijelic R, Jakovljevic B. Correlation of parathormone and the serum values of acidum uricum with calcium nephrolithiasis examined by three different methods of diagnostics. Acta Inform Med (2015) 23:132–4. doi: 10.5455/aim.2015.23.132-134
52. Cipriani C, Biamonte F, Costa AG, Zhang C, Biondi P, Diacinti D, et al. Prevalence of kidney stones and vertebral fractures in primary hyperparathyroidism using imaging technology. J Clin Endocrinol Metab (2015) 100:1309–15. doi: 10.1210/jc.2014-3708
53. Reid LJ, Muthukrishnan B, Patel D, Seckl JR, Gibb FW. Predictors of nephrolithiasis, osteoporosis, and mortality in primary hyperparathyroidism. J Clin Endocrinol Metab (2019) 104:3692–700. doi: 10.1210/jc.2018-02483
54. Bijelic R, Balaban M, Milicevic S. Gender representation of osteoporosis in patients with urolithiasis. Med Arch (2015) 69:331–3. doi: 10.5455/medarh.2015.69.331-333
55. Carbone LD, Hovey KM, Andrews CA, Thomas F, Sorensen MD, Crandall CJ, et al. Urinary tract stones and osteoporosis: findings from the women's health initiative. J Bone Miner Res (2015) 30:2096–102. doi: 10.1002/jbmr.2553
56. Malihi Z, Wu Z, Stewart AW, Lawes CM, Scragg R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin d supplementation: a systematic review and meta-analysis. Am J Clin Nutr (2016) 104:1039–51. doi: 10.3945/ajcn.116.134981
57. Heaney RP. Calcium supplementation and incident kidney stone risk: a systematic review. J Am Coll Nutr (2008) 27:519–27. doi: 10.1080/07315724.2008.10719734
58. Fatahi S, Namazi N, Larijani B, Azadbakht L. The association of dietary and urinary sodium with bone mineral density and risk of osteoporosis: a systematic review and meta-analysis. J Am Coll Nutr (2018) 37:522–32. doi: 10.1080/07315724.2018.1431161
59. Gambaro G, Croppi E, Coe F, Lingeman J, Moe O, Worcester E, et al. Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: a consensus statement. J Nephrol. (2016) 29:715–34. doi: 10.1007/s40620-016-0329-y
Keywords: osteoporosis, nephrolithiasis, prevalence, risk, cumulative analysis
Citation: Jia S, Liao J, Wang Y, Zheng W, Jin J, Xu W and Zheng Q (2023) Prevalence of osteoporosis in patients with nephrolithiasis and vice versa: a cumulative analysis. Front. Endocrinol. 14:1180183. doi: 10.3389/fendo.2023.1180183
Received: 05 March 2023; Accepted: 24 May 2023;
Published: 04 July 2023.
Edited by:
Jonathan H. Tobias, University of Bristol, United KingdomReviewed by:
Kuanjun He, Inner Mongolia Minzu University, ChinaCopyright © 2023 Jia, Liao, Wang, Zheng, Jin, Xu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifang Xu, d2VpZmFuZ3h1MjAxOUAxNjMuY29t; Qi Zheng, ZHJ6aGVuZ3FpMzU1NEAxNjMuY29t
†These authors share first authorship
‡ORCID: Weifang Xu, orcid.org/0000-0003-4803-8748
Qi Zheng, orcid.org/0009-0005-7202-708X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.