
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 20 April 2023
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1133958
This article is part of the Research TopicDevelopments in Diagnosis and Management of Thyroid CancerView all 15 articles
 Maria Cristina Campopiano
Maria Cristina Campopiano Arianna Ghirri
Arianna Ghirri Alessandro Prete
Alessandro Prete Loredana Lorusso
Loredana Lorusso Luciana Puleo
Luciana Puleo Virginia Cappagli
Virginia Cappagli Laura Agate
Laura Agate Valeria Bottici
Valeria Bottici Sandra Brogioni
Sandra Brogioni Carla Gambale
Carla Gambale Elisa Minaldi
Elisa Minaldi Antonio Matrone
Antonio Matrone Rossella Elisei*
Rossella Elisei* Eleonora Molinaro
Eleonora MolinaroCurrently, the differentiated thyroid cancer (DTC) management is shifted toward a tailored approach based on the estimated risks of recurrence and disease-specific mortality. While the current recommendations on the management of metastatic and progressive DTC are clear and unambiguous, the management of slowly progressive or indeterminate disease varies according to different centers and different physicians. In this context, active surveillance (AS) becomes the main tool for clinicians, allowing them to plan a personalized therapeutic strategy, based on the risk of an unfavorable prognosis, and to avoid unnecessary treatment. This review analyzes the main possible scenarios in treated DTC patients who could take advantage of AS.
The current knowledge on differentiated thyroid cancer (DTC) requires a shift into precision medicine. Closely tailoring medical decisions, treatments, and practices should be based on individualized risk estimates. Furthermore, the last American Thyroid Association guidelines (1) and Italian Expert Consensus (2) for the diagnosis and management of DTC reviewed the traditional one-size-fits-all approach, turning it into an individualized management of DTC patients, focused on the estimated risks of recurrence (1) and disease-specific mortality (3). DTC usually has a good outcome with a disease-free survival of approximately 98% and a very low rate of disease-specific mortality (4). Because of this, some years ago, experts have advocated a conservative management approach in selected patients with low-volume tumor burden or slow-progressing DTC (5). Following that, current guidelines (1) have expanded the concept of active surveillance (AS) in DTC management, initially applied mainly to the management of intrathyroidal microcarcinoma (mPTC) (6–11).
As defined by the National Cancer Institute, AS is “a treatment plan that involves closely watching a patient condition but not giving any treatment unless there are changes in test results that show the condition is getting worse” (https://www.cancer.gov/publications/dictionaries/cancer-terms). AS consists of periodical programmed physical examinations, blood tests, and imaging tests that are essential to early detect disease progression and to immediately plan therapies, avoiding either under- or overtreatment. According to this definition, AS is clearly different from both the “watchful waiting” approach, which is a relatively passive follow-up strategy with interventions being triggered by symptoms, and “follow-up care” that involves medical checkups over time in cured patients after treatment, with the purpose of checking disease recurrence (12).
While the management of metastatic and progressive DTC patients is much more clear and requires treatment, the main challenge is to identify the DTC patients (with any grade of persistent disease) who do not need active treatment and could benefit from AS. In some cases, immediate treatment could cause more harm than good, and even it could not be curative. While the current guidelines are available since 2016, in some areas, the management of DTC still diverges from the international indications, and in particular, AS is not considered a feasible and safe strategy (13).
In this review, we discuss the main possible scenarios in treated DTC patients, other than mPTC, who could take advantage of AS. Our objective is to show the relevance of AS in clinical practice, underlining its safety, appropriateness, and effectiveness in selected patients.
According to the most recent guidelines, DTC (1) patients who did not obtain an excellent response to the initial treatment (i.e., total thyroidectomy plus remnant radio-ablation) can be classified into three categories as reported in Table 1. These patients should continue with regular checkups but not necessarily have additional treatment. Here, we describe how to tailor the AS according to these categories of DTC patients.
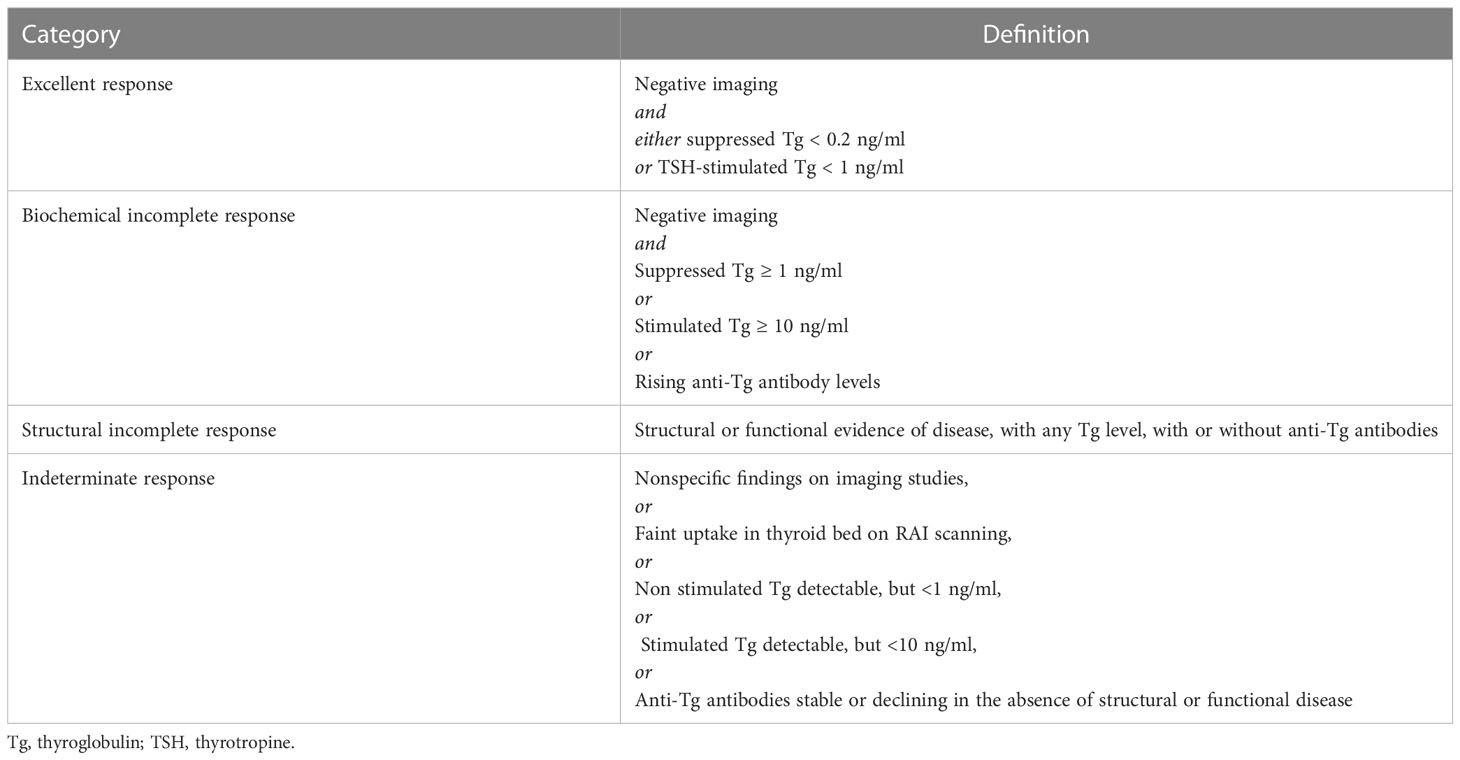
Table 1 Response to therapy reclassification in patients with differentiated thyroid cancer treated with total thyroidectomy and radioiodine remnant ablation (1).
The indeterminate category has biochemical, morphological, or functional findings that physicians could not classify as either absence or persistence of disease (1). Sub-centimetric thyroid bed nodules or indeterminate cervical lymph nodes (Figure 1A), faint uptake in the thyroid bed (Figure 1B), or non-specific abnormalities on imaging are in this group. Patients with detectable non-stimulated thyroglobulin (Tg) values, but less than 1 ng/ml, stimulated Tg values between 1 and 10 ng/ml, and stable or decreasing Tg antibodies (TgAb)1 in the absence of structural disease are also in this category (1).
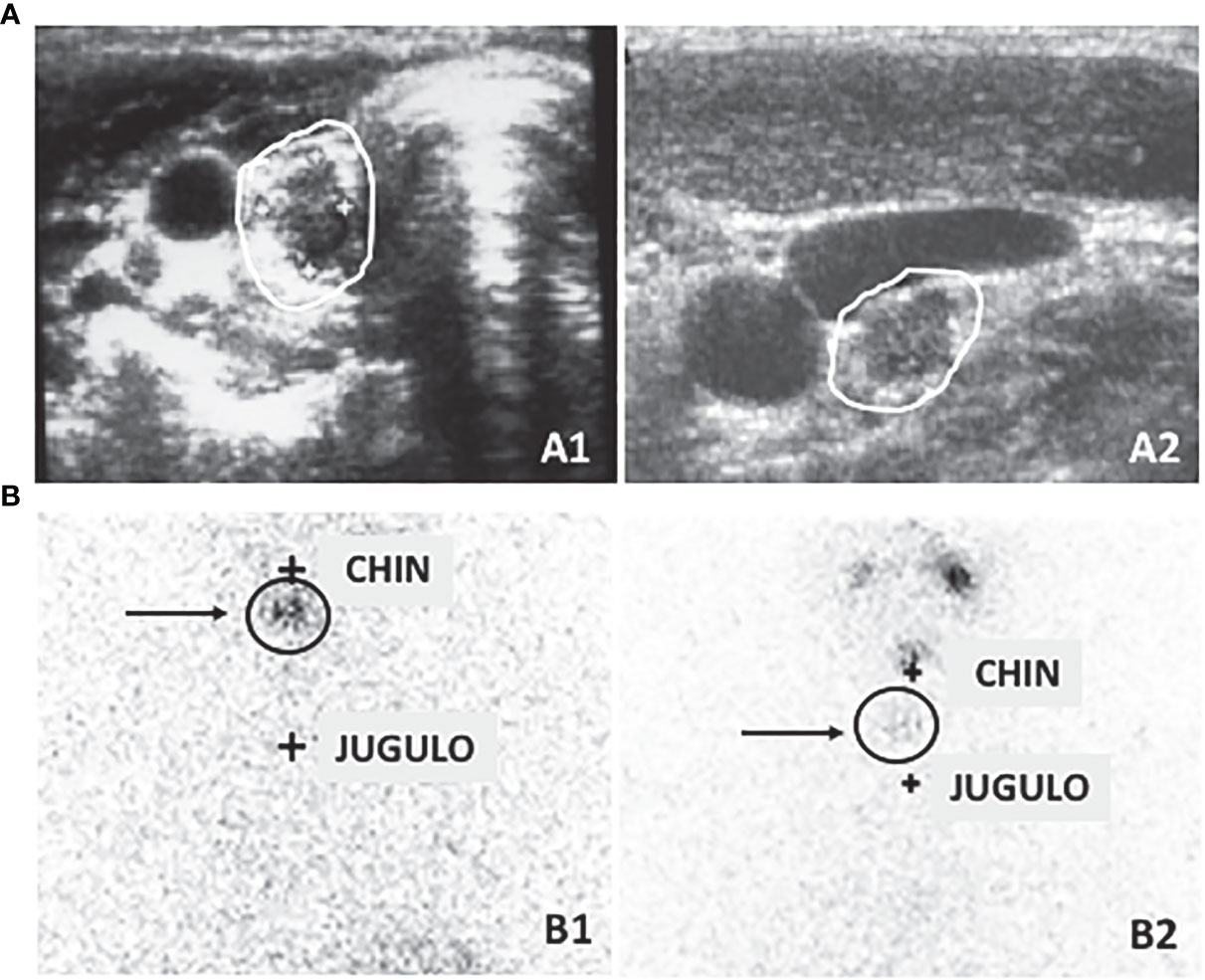
Figure 1 (A) Neck ultrasound can detect small lesions that cannot be clearly interpreted as normal or pathological lesions such as sub-centimetric thyroid bed lesion (A1) referable to either thyroid remnant tissue or cancer persistence and indeterminate small cervical lymph nodes (A2). (B) Similarly, thyroid bed scintigraphy can detect faint uptake in the thyroid bed that can be due to both a normal remnant or a local persistence or recurrence of the disease (B1) or a small lymph node (B2).
The prevalence of indeterminate response (IR) after initial treatment ranges from 4% to 30% and is lower in ATA high-risk patients and increases progressively in intermediate- and low-risk patients (14, 15). Patients treated with either total thyroidectomy or lobectomy alone have a similar rate of IR in different series (16, 17). The nonspecific findings either remain stable or resolve during prolonged observation in most patients, without additional treatment (15, 18). No deaths have been notified in patients with IR up to 10 years of follow-up (14, 16, 17, 19), regardless of the type of initial treatment and the ATA risk category. Conversely, only up to 14% of patients with IR to initial treatment was confirmed to have a persistence of disease, either biochemical or structural, after a median of 6–10 years (16, 17, 19, 20), and this probability increased progressively according to initial risk category (14).
Including a multitude of nonspecific findings, highly different from each other, there is no single strategy to manage patients with IR and they require an even more personalized and tailored approach based on the individual characteristics. In this setting, the trend of serum Tg and TgAb over time (21) should be taken into consideration to identify changes in clinical picture. The evaluation of the serum Tg must also take into consideration the TSH values since there is a strict correlation between these two parameters (21). This is not the case for serum TgAb that can be considered a Tg surrogate marker (22), but independent from TSH values.
Neck ultrasound (US) is the main non-invasive tool in the management of these patients, since the major risk of structural recurrence is in the neck. Unfortunately, by definition, the IR category includes cases with neck lymph nodes not clearly malignant but, at the same time, not clearly inflammatory (Figure 1A). Most of them could be clinically irrelevant, considering the low prevalence of enlarging nodules, the slow growth rate, and the rarity of local complications (23–25). In these cases, routine fine needle aspiration (FNA) is not appropriate. Nevertheless, it should be considered in lymph nodes greater than 1 cm and increasing in size over a couple of evaluations, especially if, in case of a positive FNA result, a modification in management would be applied (1). Whenever a lymph node FNA is planned, a concomitant measurement of Tg in the washout of the needle used for the procedure should also be performed (26).
In these patients, AS is the only available strategy able to identify the minority of patients requiring further therapies. Once-a-year check including clinical and biochemical (i.e., serum Tg, TgAb, TSH; free T4) evaluations plus a neck US should be performed at least for the first 5 years for those at higher risk of recurrence, and then continued every 18–24 months.
Abnormal basal or stimulated Tg or elevated values of TgAb without clear evidence of structural disease define the biochemical incomplete response (BIR) after TT and radioiodine remnant ablation (RRA). Up to 18 months after initial therapy, BIR is found in 10%–20% of DTC patients, and, at variance with IR, the prevalence is similar in all ATA risk categories (1).
Up to 70% of these patients reach the excellent response criteria and the clinical remission over time, without any additional treatment. Moreover, approximately 20%–30% maintain a stable Tg level without structural evidence of disease for many years. Only less than 20% develop a structural disease within 5–10 years (14, 15, 19, 27–29). Moreover, the probability of achieving undetectable Tg could depend on initial risk stratification, which is higher in ATA low-risk patients than in ATA intermediate- and high-risk patients (14). Clinical outcome is generally good in these patients and no deaths have been reported over 10 years of follow-up (14, 15, 19, 27–29). These data suggest that patients with BIR after initial treatment can be managed through AS using Tg and TgAb trends and neck US, without additional interventions to determine whether they will spontaneously reach an excellent response to treatment.
During AS, a sustained increasing Tg trend is strongly suggestive of persistent disease, which should require additional evaluation (1, 30). The doubling time (DT) of serum Tg values is also an important prognostic factor, since prolonged Tg DT (>2 years) is associated with a favorable outcome (31). It is worth pointing out that instead of considering an initial elevated Tg (both basal and stimulated) as a specific marker of persistent disease (30), it should be regarded as an alert sign indicating the need for further evaluation in a patient subset, since the predictive positive value of a single basal value of Tg is low (32–34). Similarly, the increase in the TgAb trend is a warning sign, suggesting the possibility of disease persistence (35, 36). Since TgAb usually disappears 3 years after thyroid ablation (22), its persistence for a long period after initial treatment or its progressive rise, confirmed in at least two to three measurements performed during 1 year of follow up, indicates the presence of a Tg source and consequently could reflect the persistence or recurrence of DTC. In this context, it is crucial to note that Tg or TgAb trends are more helpful than a single determination in predicting disease remission or progression (19, 30, 35). Moreover, Tg and TgAb should always be measured simultaneously, using the same methods over time, to ensure comparability (1).
Recently, after the limitation to RRA use and the promotion to lobectomy for selected patients, the role of Tg and TgAb measurements had to be reassessed (1). In these cases, the Tg level closely depends on the volume of residual thyroid tissue, and it is highly variable from person to person. A Tg value able to discriminate residual thyroid tissue from recurrent or persistent DTC has not been established after surgery alone (1, 18, 37), and the stimulated Tg is worthless in these patients (38). Interestingly, most patients who did not undergo RRA experienced the natural fall of both Tg and TgAb over time in different published series (18, 37, 39–43). Ideally, the residual thyroid tissue could sustain the antigenic stimulus, affecting the disappearance of the serum TgAb. The residual thyroid tissue after TT is probably minimal, and it could not maintain the antigenic stimulus, thus determining the loss of TgAb. Moreover, the initial TgAb levels and degree of lymphocytic infiltration could influence the time to TgAb disappearance (42); thus, the role of Tg and TgAb trend, but not the single value, could also be a surrogate marker in non-ablated patients (1, 16, 18, 37). The disappearance process takes time, especially for TgAb, and during this period, patients should be followed up with AS. The observation of a natural decline or stabilization of serum Tg and/or TgAb represents a positive result, while their progressive increase represents an alert sign, and it should prompt further investigations. Furthermore, in patients treated with lobectomy, Tg and TgAb measurement is useless, because we are not able to determine how much of the total Tg and TgAb depend on residual lobe or on persistent disease (1, 18, 37). In this latter group of patients, neck US becomes fundamental during AS for the early identification of both neck lymph node metastases and/or tumoral foci in the unresected lobe.
Also, in patients with BIR, AS is a useful option to reduce overtreatment and, at the same time, to early identify the few real structural recurrences. Periodic evaluations (every 6–12 months during the first 5 years and then every 18–24 months) based on Tg, TgAb measurement, and neck US allow physicians to discern low and stable Tg or TgAb trend to increasing levels. Additional evaluations should be reserved for those patients showing a rise in Tg or TgAb, shown in two or more consecutive measurements over time. DTC patients treated with TT only but not RRA or even with lobectomy had a good prognosis per se. The concept of BIR is useless in these cases since these patients should be considered cured until proven otherwise and neck US remains the most informative and sensitive tool in their management.
Structural incomplete response (SIR) identifies a cohort of DTC patients who have not been cured after initial treatment and have evidence of structural disease either immediately after TT or after RRA as shown by post-therapeutic whole-body scan (ptWBS). SIR includes both patients with biopsy-proven disease and patients with structural or functional metastases assessed on clinical scenarios [i.e., positive ptWBS, positron emission tomography with 2-deoxy-2-fluorine-18fluoro-D-glucose (18FDG-PET), computed tomography (CT) scan, etc.].
Fifty percent to 85% of SIR patients continue to have a persistent disease despite additional therapies (15, 19). Consequently, it confirms the highest risk of disease-specific mortality, which is 11% and 57% for lymph node metastases and distant metastases, respectively. The prevalence of SIR is proportional to ATA risk stratification, being higher in ATA high-risk patients than in ATA intermediate- and low-risk patients (1) in whom it is really very rare (44).
Patients with SIR may be led to further therapies or AS, depending on multiple factors, including the size, location, proximity to vital structures, rate of growth, RAI avidity, and a balance between the risks and the efficacy of therapies. Here, we describe the different possibilities and approaches of AS in SIR patients according to the site of the structural disease.
Cervical lymph nodes represent the most common site of persistent and recurrent DTC, occurring in up to 30% of DTC patients and 75% of ATA high-risk patients, especially in those with lymph node metastases at diagnosis (1, 45). Additionally, the number and the size of the involved lymph nodes and the extracapsular extension are risk factors for persistent or recurrent disease (46–48).
Neck US is the most sensitive tool to distinguish persistent or recurrent lymph node metastases (Figure 2A) from enlarged inflammatory lymph nodes (Figure 2B) that are characterized by well-recognized pathological features (Table 2) (49, 50). In particular, neck US can detect small lymph node metastases in the absence of detectable levels of serum Tg, thus being a fundamental tool in the AS of these patients (38, 51). At the same time, it has been demonstrated that the use of neck US increases the incidence of persistent or recurrent lymph node metastases, likely clinically irrelevant, without changing the disease-specific mortality (4, 52). For this reason, neck US interpretation should not be left to the radiologist but a clinical interpretation should always be performed by the specialist who is following the patient and is aware of their disease status.
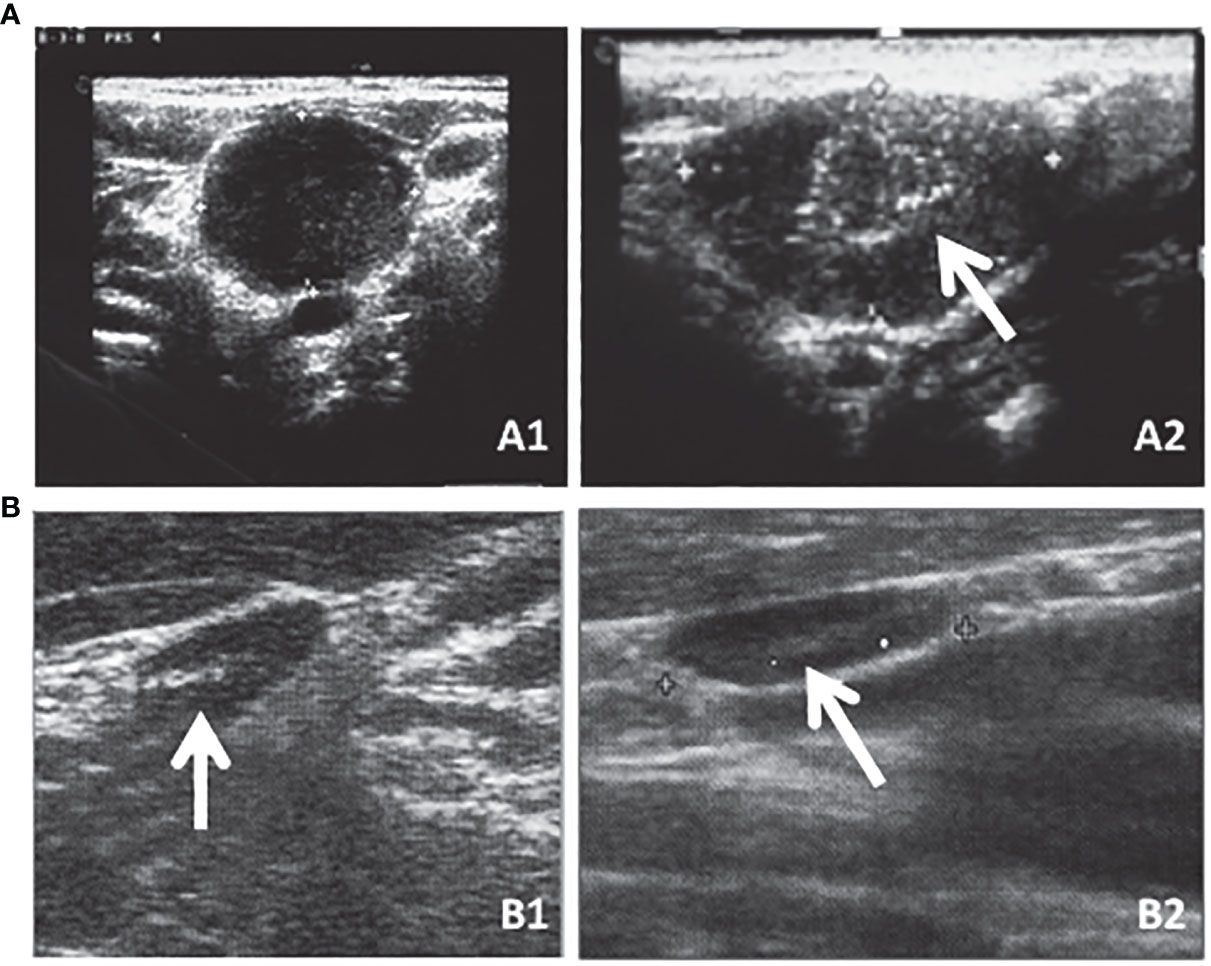
Figure 2 Neck US is the most sensitive tool to distinguish persistent or recurrent lymph node metastases [(A) (A1) transversal and (A2) longitudinal sections] that are characterized by well-recognized pathological features (e.g., round shape as shown in A1 or microcalcifications as indicated by the arrow in A2) from enlarged inflammatory lymph nodes [(B) (B1) transversal and (B2) longitudinal sections] that are oblong in shape and with an evident hylum as indicated by the arrows in both B1 and B2.
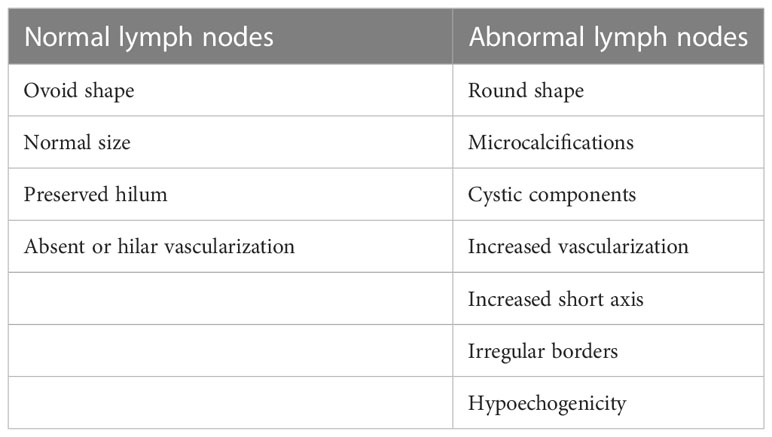
Table 2 Ultrasound features of normal vs. abnormal lymph nodes (49).
Current ATA guidelines (1) recommend performing neck US to evaluate cervical lymph nodes, and their progression, at 6 to 12 months and then periodically. While resection of large, clinically apparent loco-regional metastases often provides a clinical benefit, it remains unclear whether resection of persistent or recurrent small-volume disease, identified using highly sensitive tools, provides any meaningful clinical benefit. The prevalence of increasing lymph nodes is low, the growth rate is slow as well, and the local complication during AS is rare (53). Moreover, the cure rate after lymph node metastasis re-operation ranges from 20% to 50% in different series, and some cases are required to repeat more than one surgery to achieve disease remission (24, 54–60). Furthermore, up to 30% of patients experience recurrent or persistent lymph node metastases after the second neck dissection (56–58, 60). On the other hand, the surgery failed to remove lymph node metastasis, especially the smallest ones, in up to 10% of patients, despite having biopsy-proven metastases (54, 60). Moreover, up to 15% of patients showed a negative surgical exploration (57, 58). Although neck surgery is relatively safe in expert hands, each surgery carries a significant risk of complications, set as 9% of permanent complications in referral centers (55, 58, 59, 61). Re-operation for recurrent or persistent lymph node metastases is associated with high risks of major and permanent complications, due to the fibrotic tissue and the disruption of the normal anatomic planes after the initial surgery (62).
For all the above-mentioned reasons, abnormal lymph nodes less than 1 cm in the smallest diameter at neck US are candidates for AS and no treatment. Neck US evaluation should be performed every 6–12 months according to the rate of growth, if any, and simultaneously, a biochemical evaluation of TSH, Free T4, and mainly Tg and TgAb is indicated. Routine FNA is not appropriate, but it might be useful in well-proven progressive lymph nodes greater than 1.0–1.5 cm, in which the FNA results, combined with the results of the Tg measurement in the washout of the needle used for FNA (26), will lead to an appropriate and reasonable therapeutic intervention.
Up to 5%–10% of SIR patients may have distant metastasis at diagnosis and 5%–10% may develop distant metastases during follow-up. Almost all distant metastases are in the lungs, while a smaller number is in the bones, brain, and liver. Distant metastases are the most frequent cause of disease-specific mortality, especially in older patients. While 10-year survival rates >95% have been documented in young patients with distant metastases, a median 10-year survival rate of <50% can be expected in older patients with distant metastases (63–66). Ten-year overall survival drops to 10% when distant metastases are not responsive to radioiodine therapy (67) and overall survival is significantly worse in patients with bone or brain metastases (68, 69).
Patients with SIR should be regularly assessed by biochemical and appropriate imaging evaluation to plan a personalized strategy. In this setting, Tg could estimate the tumor burden, and Tg doubling time (DT) may represent a prognostic factor since a short Tg DT (<6 months) is associated with a poor outcome (31). In contrast, an incongruous reduction in serum Tg with no concomitant decrease or with an increase in tumor size could be due to a dedifferentiation of the tumoral cells and suggests a radioiodine refractory disease. Cross-sectional imaging provides the most precise information on tumor burden, proximity to contiguous structures, and tumor growth. DT of distant metastases is also a good prognostic indicator of overall survival in patients with metastatic DTC: a shorter DT correlates with a worse overall survival (70). Positron emission tomography (18FDG) may provide additional prognostic information, since 18FDG-PET positive lesions usually have a more aggressive behavior (71, 72).
Two-thirds of patients with metastatic disease demonstrate substantial uptake of radioiodine, but only 42% of them demonstrate structural resolution of disease, and fewer than 10% demonstrate complete resolution of both biochemical and structural disease (67, 68, 73). Moreover, patients who are not responsive to radioiodine treatment have a poor prognosis and a reduced life expectancy (67). For these patients, the probability of obtaining a remission of disease with further radioiodine treatment is low and other strategies should be evaluated.
Radioiodine refractory metastatic patients require a multi-disciplinary approach, since a myriad of aspects should be assessed to guarantee a tailored and integrated management, based on the high risk of adverse outcome. However, not all patients with structural radioiodine refractory disease require immediate treatment. Patients with asymptomatic, stable, or slowly progressive diseases are candidates for AS since they may not require initiation of therapy until tumors reach a critical volume, patients experience symptoms, or vital structures are involved. Treating a small, stable, and asymptomatic disease could expose patients to treatment’s adverse events without giving them a real prognostic improvement. These patients are candidates for AS with 6-month evaluations including the measurement of serum Tg and calculation of its DT (74), as well as the measurement of TgAb especially for those cases with elevated levels whose trend must be accurately evaluated (74). According to the increasing trend of these serum markers, total body CT scans associated in some cases with 18-FDG-PET should be performed to verify the tumor burden increase (74). Further therapy can be considered when tumor burden becomes clinically significant and tumor progression needs to be documented (70), because the benefit of treatment is demonstrated only in this setting (1, 75–77), and it justifies the potential related adverse events of therapy (78, 79).
In case of stable disease without symptoms, with a slow progression, and without life-threatening lesions, risk about systemic treatment outweighs the benefit and AS can provide a prolonged period of symptom-free disease, without side effects of treatments.
The complexity of these situations and the availability of prospective clinical trials should encourage physicians to refer such patients to tertiary centers with specific expertise.
In clinical practice, AS is the main tool enabling physicians to optimize therapy planning and prevent side effects from unnecessary treatments. AS can ensure appropriate timing and a personalized approach for an active treatment and, at the same time, avoids unnecessary treatment for patients who would not require additional therapy.
AS is indicated as first-line management for patients with a detectable Tg value or TgAb without structural disease and also for those with small, isolated cervical lymph node metastases, especially in cases previously submitted to nodal compartment resection. AS can also be applied in cases of slowly progressing and asymptomatic distant metastases given that the risk of progression or local invasion is low, and the risk of adverse events outweighs the benefit of treatment.
All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
2. Pacini F, Basolo F, Bellantone R, Boni G, Cannizzaro MA, De Palma M, et al. Italian Consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest (2018) 41(7):849–76. doi: 10.1007/s40618-018-0884-2
3. AJCC - American joint committee on cancer [Internet] . Available at: https://cancerstaging.org/Pages/default.aspx.
4. Thyroid cancer — cancer stat facts [Internet]. Available at: https://seer.cancer.gov/statfacts/html/thyro.html.
5. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid (2009) 19(11):1167–214. doi: 10.1089/thy.2009.0110
6. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid (2014) 24(1):27–34. doi: 10.1089/thy.2013.0367
7. Molinaro E, Campopiano MC, Pieruzzi L, Matrone A, Agate L, Bottici V, et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at a single Italian center. J Clin Endocrinol Metab (2020) 105(3):172–80. doi: 10.1210/clinem/dgz113
8. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg (2017) 143(10):1015–20. doi: 10.1001/jamaoto.2017.1442
9. Oh HS, Ha J, Kim HI, Kim TH, Kim WG, Lim DJ, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid (2018) 28(12):1587–94. doi: 10.1089/thy.2018.0263
10. Sanabria A. Active surveillance in thyroid microcarcinoma in a Latin-American cohort. JAMA Otolaryngol Head Neck Surg (2018) 144(10):947–8. doi: 10.1001/jamaoto.2018.1663
11. Kim HI, Jang HW, Ahn HS, Ahn S, Park SY, Oh YL, et al. High serum TSH level is associated with progression of papillary thyroid microcarcinoma during active surveillance. J Clin Endocrinol Metab (2017) 103(2):446–51. doi: 10.1210/jc.2017-01775/4677373
12. Comprehensive cancer information - national cancer institute. Available at: https://www.cancer.gov/.
13. Or K, Benbassat C, Koren S, Shteinshneider M, Koren R, Cantrell D, et al. Adherence to ATA 2015 guidelines in the management of unifocal non-invasive papillary thyroid cancer: a clinical survey among endocrinologists and surgeons. Eur Arch Oto-Rhino-Laryngol. (2018) 275(11):2851–9. doi: 10.1007/s00405-018-5126-x
14. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American thyroid association staging system. Thyroid (2010) 20(12):1341–9. doi: 10.1089/thy.2010.0178
15. Vaisman F, Momesso D, Bulzico DA, Pessoa CHCN, Dias F, Corbo R, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf) (2012) 77(1):132–8. doi: 10.1111/j.1365-2265.2012.04342.x
16. Park S, Kim WG, Song E, Oh HS, Kim M, Kwon H, et al. Dynamic risk stratification for predicting recurrence in patients with differentiated thyroid cancer treated without radioactive iodine remnant ablation therapy. Thyroid (2017) 27(4):524–30. doi: 10.1089/thy.2016.0477
17. Momesso DP, Vaisman F, Yang SP, Bulzico DA, Corbo R, Vaisman M, et al. Dynamic risk stratification in patients with differentiated thyroid cancer treated without radioactive iodine. J Clin Endocrinol Metab (2016) 101(7):2692–700. doi: 10.1210/jc.2015-4290
18. Vaisman F, Shaha A, Fish S, Michael Tuttle R. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin Endocrinol (Oxf) (2011) 75(1):112–9. doi: 10.1111/j.1365-2265.2011.04002.x
19. Vaisman F, Tala H, Grewal R, Tuttle RM. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid (2011) 21(12):1317–22. doi: 10.1089/thy.2011.0232
20. Malandrino P, Tumino D, Russo M, Marescalco S, Fulco RA, Frasca F. Surveillance of patients with differentiated thyroid cancer and indeterminate response: a longitudinal study on basal thyroglobulin trend. J Endocrinol Invest (2019) 42(10):1223–30. doi: 10.1007/s40618-019-01044-3
21. Matrone A, Faranda A, Latrofa F, Gambale C, Donati DS, Molinaro E, et al. Thyroglobulin changes are highly dependent on TSH in low-risk DTC patients not treated with radioiodine. J Clin Endocrinol Metab (2020) 105(8):E2845–52. doi: 10.1210/clinem/dgaa297
22. Chiovato L, Latrofa F, Braverman LE, Pacini F, Capezzone M, Masserini L, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med (2003) 139(5 I):346–351+I75. doi: 10.7326/0003-4819-139-5_part_1-200309020-00010
23. Rondeau G, Fish S, Hann LE, Fagin JA, Tuttle RM. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid (2011) 21:845–53. doi: 10.1089/thy.2011.0011
24. Robenshtok E, Fish S, Bach A, Domínguez JM, Shaha A, Tuttle RM. Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab (2012) 97(8):2706–13. doi: 10.1210/jc.2012-1553
25. Lamartina L, Grani G, Biffoni M, Giacomelli L, Costante G, Lupo S, et al. Risk stratification of neck lesions detected sonographically during the follow-up of differentiated thyroid cancer. J Clin Endocrinol Metab (2016) 101(8):3036–44. doi: 10.1210/jc.2016-1440
26. Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab (1992) 74(6):1401–4. doi: 10.1210/jcem.74.6.1592886
27. Alzahrani AS, Mohamed G, Al Shammary A, Aldasouqi S, Abdal Salam S, Shoukri M. Long-term course and predictive factors of elevated serum thyroglobulin and negative diagnostic radioiodine whole body scan in differentiated thyroid cancer. J Endocrinol Invest (2005) 28(8):540–6. doi: 10.1007/BF03347243
28. Padovani RP, Robenshtok E, Brokhin M, Tuttle RM. Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid (2012) 22(8):778–83. doi: 10.1089/thy.2011.0522
29. Pacini F, Agate L, Elisei R, Capezzone M, Ceccarelli C, Lippi F, et al. Outcome of differentiated thyroid cancer with detectable serum tg and negative diagnostic 131I whole body scan: comparison of patients treated with high 131I activities versus untreated patients. J Clin Endocrinol Metab (2001) 86(9):4092–7. doi: 10.1210/jcem.86.9.7831
30. Baudin E, Do CC, AF C, Leboulleux S, Travagli JP, Schlumberger M. Positive predictive value of serum thyroglobulin levels, measured during the first year of follow-up after thyroid hormone withdrawal, in thyroid cancer patients. J Clin Endocrinol Metab (2003) 88(3):1107–11. doi: 10.1210/jc.2002-021365
31. Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid (2011) 21(7):707–16. doi: 10.1089/thy.2010.0355
32. Lamartina L, Montesano T, Trulli F, Attard M, Torlontano M, Bruno R, et al. Papillary thyroid carcinomas with biochemical incomplete or indeterminate responses to initial treatment: repeat stimulated thyroglobulin assay to identify disease-free patients. Endocrine (2016) 54(2):467–75. doi: 10.1007/s12020-015-0823-3
33. Malandrino P, Latina A, Marescalco S, Spadaro A, Regalbuto C, Fulco RA, et al. Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J Clin Endocrinol Metab (2011) 96(6):1703–9. doi: 10.1210/jc.2010-2695
34. Wong KCW, Ng TY, Yu KS, Kwok JSS, Chan KCA, Suen JJS, et al. The use of post-ablation stimulated thyroglobulin in predicting clinical outcomes in differentiated thyroid carcinoma – what cut-off values should we use? Clin Oncol (2019) 31(2):e11–20. doi: 10.1016/j.clon.2018.10.009
35. Won GK, Jong HY, Won BK, Tae YK, Eui YK, Jung MK, et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab (2008) 93(12):4683–9. doi: 10.1210/jc.2008-0962
36. Chung JK, Park YJ, Kim TY, So Y, Kim SK, Park DJ, et al. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf) (2002) 57(2):215–21. doi: 10.1046/j.1365-2265.2002.01592.x
37. Vaisman F, Momesso D, Bulzico DA, Pessoa CHCN, Domingos Gonçalves Da Cruz M, Dias F, et al. Thyroid lobectomy is associated with excellent clinical outcomes in properly selected differentiated thyroid cancer patients with primary tumors greater than 1 cm. J Thyroid Res (2013) 2013:5. doi: 10.1155/2013/398194
38. Torlontano M, Crocetti U, Augello G, D’Aloiso L, Bonfitto N, Varraso A, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab (2006) 91(1):60–3. doi: 10.1210/jc.2005-1185
39. Durante C, Montesano T, Attard M, Torlontano M, Monzani F, Costante G, et al. Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab (2012) 97(8):2748–53. doi: 10.1210/jc.2012-1123
40. Tsushima Y, Miyauchi A, Ito Y, Kudo T, Masuoka H, Yabuta T, et al. Prognostic significance of changes in serum thyroglobulin antibody levels of pre-and post-total thyroidectomy in thyroglobulin antibody-positive papillary thyroid carcinoma patients. Endocr J (2013) 60(7):871–6. doi: 10.1507/endocrj.EJ12-0410
41. Ernaga-Lorea A, Hernández-Morhain MC, Anda-Apiñániz E, Pineda-Arribas JJ, Migueliz-Bermejo I, Eguílaz-Esparza N, et al. Prognostic value of change in anti-thyroglobulin antibodies after thyroidectomy in patients with papillary thyroid carcinoma. Clin Trans Oncol (2018) 20(6):740–4. doi: 10.1007/s12094-017-1782-3
42. Matrone A, Latrofa F, Torregrossa L, Piaggi P, Gambale C, Faranda A, et al. Changing trend of thyroglobulin antibodies in patients with differentiated thyroid cancer treated with total thyroidectomy without 131 I ablation. Thyroid (2018) 28(7):871–9. doi: 10.1089/thy.2018.0080
43. Bueno F, Falcone MGG, Peñaloza MA, Abelleira E, Pitoia F. Dynamics of serum antithyroglobulin antibodies in patients with differentiated thyroid cancer. Endocrine (2020) 67(2):387–96. doi: 10.1007/s12020-019-02112-7
44. Matrone A, Gambale C, Piaggi P, Viola D, Giani C, Agate L, et al. Postoperative thyroglobulin and neck ultrasound in the risk restratification and decision to perform 131I ablation. J Clin Endocrinol Metab (2017) 102(3):893–902. doi: 10.1210/jc.2016-2860
45. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med (1994) 97(5):418–28. doi: 10.1016/0002-9343(94)90321-2
46. Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab (2005) 90(10):5723–9. doi: 10.1210/jc.2005-0285
47. Chéreau N, Buffet C, Trésallet C, Tissier F, Leenhardt L, Menegaux F. Recurrence of papillary thyroid carcinoma with lateral cervical node metastases: predictive factors and operative management. Surgery (2016) 159(3):755–62. doi: 10.1016/j.surg.2015.08.033
48. Randolph GW, Duh QY, Heller KS, Livolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid (2012) 22(11):1144–52. doi: 10.1089/thy.2012.0043
49. Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab (2007) 92(9):3590–4. doi: 10.1210/jc.2007-0444
50. Leenhardt L, Erdogan MF, Hegedus L, Mandel SJ, Paschke R, Rago T, et al. 2013 European Thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J (2013) 2(3):147–59. doi: 10.1159/000354537
51. Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab (2003) 88(8):3668–73. doi: 10.1210/jc.2002-021925
52. Davies L, Welch HG. Current thyroid cancer trends in the united states. JAMA Otolaryngol Head Neck Surg (2014) 140(4):317–22. doi: 10.1001/jamaoto.2014.1
53. Tomoda C, Sugino K, Matsuzu K, Uruno T, Ohkuwa K, Kitagawa W, et al. Cervical lymph node metastases after thyroidectomy for papillary thyroid carcinoma usually remain stable for years. Thyroid (2016) 26(12):1706–11. doi: 10.1089/thy.2016.0225
54. Al-Saif O, Farrar WB, Bloomston M, Porter K, Ringel MD, Kloos RT. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab (2010) 95(5):2187–94. doi: 10.1210/jc.2010-0063
55. Schuff KG, Weber SM, Givi B, Samuels MH, Andersen PE, Cohen JI. Efficacy of nodal dissection for treatment of persistent/recurrent papillary thyroid cancer. Laryngoscope (2008) 118(5):768–75. doi: 10.1097/MLG.0b013e318162cae9
56. Yim JH, Kim WB, Kim EY, Kim WG, Kim TY, Ryu JS, et al. The outcomes of first reoperation for locoregionally recurrent/persistent papillary thyroid carcinoma in patients who initially underwent total thyroidectomy and remnant ablation. J Clin Endocrinol Metab (2011) 96(7):2049–56. doi: 10.1210/jc.2010-2298
57. Hughes DT, Laird AM, Miller BS, Gauger PG, Doherty GM. Reoperative lymph node dissection for recurrent papillary thyroid cancer and effect on serum thyroglobulin. Ann Surg Oncol (2012) 19(9):2951–7. doi: 10.1245/s10434-012-2380-9
58. Lamartina L, Borget I, Mirghani H, Al Ghuzlan A, Berdelou A, Bidault F, et al. Surgery for neck recurrence of differentiated thyroid cancer: outcomes and risk factors. J Clin Endocrinol Metab (2017) 102(3):1020–31. doi: 10.1210/jc.2016-3284
59. Lang BHH, Lee GCC, Ng CPC, Wong KP, Wan KY, Lo CY. Evaluating the morbidity and efficacy of reoperative surgery in the central compartment for persistent/recurrent papillary thyroid carcinoma. World J Surg (2013) 37(12):2853–9. doi: 10.1007/s00268-013-2202-7
60. Onuma AE, Beal EW, Nabhan F, Hughes T, Farrar WB, Phay J, et al. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer: 13-year follow-up. Ann Surg Oncol (2019) 26(6):1737–43. doi: 10.1245/s10434-019-07263-5
61. Shah MD, Harris LD, Nassif RG, Kim D, Eski S, Freeman JL. Efficacy and safety of central compartment neck dissection for recurrent thyroid carcinoma. Arch Otolaryngol - Head Neck Surg (2012) 138(1):33–7. doi: 10.1001/archoto.2011.223
62. Gopalakrishna Iyer N, Shaha AR. Complications of thyroid surgery: prevention and management. Minerva Chir (2010) 65(1):71–82.
63. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer (1998) 83(12):2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1
64. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid (2006) 16(12):1229–42. doi: 10.1089/thy.2006.16.1229
65. Haugen BR, Sherman SI. Evolving approaches to patients with advanced differentiated thyroid cancer. Endocr Rev (2013) 34(3):439–55. doi: 10.1210/er.2012-1038
66. Mazzaferri EL. An overview of the management of thyroid cancer. In: Practical management of thyroid cancer. Springer-Verlag (2006). p. 1–28.
67. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab (2006) 91(8):2892–9. doi: 10.1210/jc.2005-2838
68. Sabra MM, Ghossein R, Tuttle RM. Time course and predictors of structural disease progression in pulmonary metastases arising from follicular cell-derived thyroid cancer. Thyroid (2016) 26(4):518–24. doi: 10.1089/thy.2015.0395
69. Hirsch D, Levy S, Tsvetov G, Gorshtein A, Slutzky-Shraga I, Akirov A, et al. Long-term outcomes and prognostic factors in patients with differentiated thyroid cancer and distant metastases. Endocrine Practice (2017) 23(10):1193–200. doi: 10.4158/EP171924.OR
70. Sabra MM, Sherman EJ, Tuttle RM. Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell-derived thyroid carcinoma. Cancer (2017) 123(15):2955–64. doi: 10.1002/cncr.30690
71. Zhu X, Wu S, Yuan X, Wang H, Ma C. Progression free survival related to 18F-FDG PET/CT uptake and 131I uptake in lung metastases of differentiated thyroid cancer. Hell J Nucl Med (2019) 22(2):123–30. doi: 10.1967/s002449911005
72. Kang SY, Bang JI, Kang KW, Lee Hy, Chung JK. FDG PET/CT for the early prediction of RAI therapy response in patients with metastatic differentiated thyroid carcinoma. PloS One (2019) 14(6):e0218416. doi: 10.1371/journal.pone.0218416
73. Sabra MM, Dominguez JM, Grewal RK, Larson SM, Ghossein RA, Tuttle RM, et al. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J Clin Endocrinol Metab (2013) 98(5):E829–36. doi: 10.1210/jc.2012-3933
74. Fugazzola L, Elisei R, Fuhrer D, Jarzab B, Leboulleux S, Newbold K, et al. 2019 European Thyroid association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur Thyroid J (2019) 8(5):227–45. doi: 10.1159/000502229
75. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic diff erentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet (2014) 384(9940):319–28. doi: 10.1016/S0140-6736(14)60421-9
76. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Engl J Med (2015) 372(7):621–30. doi: 10.1056/NEJMoa1406470
77. Sabra MM, Sherman E, Tuttle RM. Prolongation of tumour volume doubling time (midDT) is associated with improvement in disease-specific survival in patients with rapidly progressive radioactive iodine refractory differentiated thyroid cancer selected for molecular targeted therapy. Clin Endocrinol (Oxf) (2019) 90(4):617–22. doi: 10.1111/cen.13941
78. Berdelou A, Borget I, Godbert Y, Nguyen T, Garcia ME, Chougnet CN, et al. Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid (2018) 28(1):72–8. doi: 10.1089/thy.2017.0205
Keywords: thyroid cancer, active surveillance, rate of growth, recurrence, radioiodine
Citation: Campopiano MC, Ghirri A, Prete A, Lorusso L, Puleo L, Cappagli V, Agate L, Bottici V, Brogioni S, Gambale C, Minaldi E, Matrone A, Elisei R and Molinaro E (2023) Active surveillance in differentiated thyroid cancer: a strategy applicable to all treatment categories response. Front. Endocrinol. 14:1133958. doi: 10.3389/fendo.2023.1133958
Received: 29 December 2022; Accepted: 04 April 2023;
Published: 20 April 2023.
Edited by:
Joana Simões-Pereira, Instituto Português de Oncologia de Lisboa Francisco Gentil, PortugalReviewed by:
Sana Ghaznavi, University of Calgary, CanadaCopyright © 2023 Campopiano, Ghirri, Prete, Lorusso, Puleo, Cappagli, Agate, Bottici, Brogioni, Gambale, Minaldi, Matrone, Elisei and Molinaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rossella Elisei, cm9zc2VsbGEuZWxpc2VpQG1lZC51bmlwaS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.