
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 14 July 2022
Sec. Pediatric Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.900325
This article is part of the Research Topic Diabetic Ketoacidosis in Children and Adolescents: From Epidemiological Data to Clinical Aspects View all 5 articles
 Giulio Frontino1,2†
Giulio Frontino1,2† Raffaella Di Tonno1,2†
Raffaella Di Tonno1,2† Valeria Castorani1,2*
Valeria Castorani1,2* Andrea Rigamonti1,2
Andrea Rigamonti1,2 Elisa Morotti1,2
Elisa Morotti1,2 Federica Sandullo1,2
Federica Sandullo1,2 Francesco Scialabba1,2
Francesco Scialabba1,2 Francesca Arrigoni1,2
Francesca Arrigoni1,2 Riccardo Foglino1,2
Riccardo Foglino1,2 Benedetta Dionisi1,2
Benedetta Dionisi1,2 Chiara Irene Carla Ferri1
Chiara Irene Carla Ferri1 Salvatore Zirpoli3
Salvatore Zirpoli3 Graziano Barera1
Graziano Barera1 Franco Meschi1,2
Franco Meschi1,2 Riccardo Bonfanti1,2,4
Riccardo Bonfanti1,2,4Introduction: Despite the use of technology, recurrent diabetic ketoacidosis (DKA) prevention remains an unmet need in children and adolescents with T1D and may be accompanied by life-threatening acute complications. We present a rare case of non-occlusive mesenteric ischemia (NOMI) with overt manifestation after DKA resolution and a discussion of recent literature addressing DKA-associated NOMI epidemiology and pathogenesis in children and adolescents.
Case Presentation: A 13-year-old female with previously diagnosed T1D, was admitted at our emergency department with hypovolemic shock, DKA, hyperosmolar state and acute kidney injury (AKI). Mildly progressive abdominal pain persisted after DKA correction and after repeated ultrasound evaluations ultimately suspect for intestinal perforation, an intraoperative diagnosis of NOMI was made.
Conclusion: The diagnosis of DKA-associated NOMI must be suspected in pediatric patients with DKA, persistent abdominal pain, and severe dehydration even after DKA resolution.
Diabetic ketoacidosis (DKA) is the most serious life-threatening acute complication of Type 1 Diabetes (T1D). (1) DKA may present as the first manifestation of T1D, but may also occur occasionally during the course of the disease, or, more rarely, become a recurrent problem. DKA commonly presents with a short history of symptoms developing over a few weeks. Diagnosis is based on standard biochemical (hyperglycemia and metabolic acidosis) and clinical (dehydration, nausea, vomiting) signs. Additionally, abdominal pain is frequently observed, especially in patients with severe metabolic acidosis. (2)
Although the cause of gastrointestinal involvement has not been fully elucidated, delayed gastric emptying, paralytic ileus, electrolyte disturbances and metabolic acidosis may be involved (3, 4). A lack of resolution within the first 24 hours of treatment should prompt investigation for other causes. (3)
We therefore present a rare case of non-occlusive mesenteric ischemia (NOMI) that presented as mild persistent abdominal pain in the context of progressively resolved severe DKA. An appraisal of recent literature regarding epidemiology and physiopathology of DKA-associated NOMI in children and adolescents will also be provided.
A 13-year-old female with previously diagnosed T1D, was admitted to the emergency department presenting incoercible vomiting since the previous day, oligo-anuria and deteriorating state of consciousness. The girl had been diagnosed with autoimmune T1D at the age of 9 years (no DKA at onset). Past medical history was unremarkable. Sensor-augmented pump therapy (SAP) was started, and her follow-up was characterized by suboptimal glucose control (latest HbA1c of 60 mmol/mol).
On admission, the child presented in hypovolemic shock with an altered state of consciousness (GCS 12) without evident focal neurological signs, pale skin and Kussmaul respiration. Her physical examination was unremarkable except for decreased and tachycardic distal pulses, cool extremities, prolonged capillary refill (> 2 sec). Venous blood gas analysis and lab tests showed severe DKA, hyperosmolar state, and acute kidney injury (AKI) with hyperkaliemia, confirmed at ECG analysis (Table 1). Two boluses of normal saline were infused (10 mL/kg each) with improvement in vital parameters. No bicarbonates were administered. The patient was then transferred to the intensive care unit (ICU) and DKA correction was carried out according to the most recent guidelines with progressive resolution of the DKA, hyperosmolar state, AKI, and electrolyte imbalances. A brain CT excluded cerebral edema. (2) On arrival the patient’s pump controller showed that the patch pump had expired. Of note, the patch pump is designed to shut-off automatically after 72h, after sounding alarms to alert the user to change the pump when approaching expiration. The child later admitted to not having changed the expiring patch pump while at her grandparents’ due to her not having any replacements and in fear of her mother’s anger. When DKA resolved, the patient was transferred to the pediatrics department and her SAP was reinstated. Since admission she referred modest abdominal pain that had persisted throughout DKA treatment. No other signs or symptoms were present apart from slightly loose stools which were collected for microbiological testing. An abdominal ultrasound only showed slight thickening of the ileal wall. On day 2 since DKA resolution, Clostridium difficile toxin tested positive and oral vancomycin treatment was started. On day 4, follow-up US only confirmed signs of enterocolitis. On day 5, abdominal symptoms and signs worsened alongside onset of fever and elevation of inflammatory indices (CRP 145 mg/L, normal range <6 mg/L). Thus, abdominal ultrasound was repeated, documenting an uneven multi-chambered area of 8x3 cm with a small aerial component (Figure 1). Treatment with metronidazole was started and the patient was transferred to the city’s center of reference for pediatric surgery. Abdominal magnetic resonance imaging revealed a large, fluid-filled pelvic abscess with air bubbles suspect for bowel perforation, with parietal contrast enhancement (Figure 1). Therefore, an urgent ileal resection (44 cm) was performed, and NOMI was diagnosed intraoperatively. Antibiotic treatment with piperacillin-tazobactam and gentamicin was administered for 7 days, and treatment with metronidazole and vancomycin was continued for 10 days. The patient was discharged after 3 weeks of transition from parenteral to enteral feeding. Pre-discharge abdominal ultrasound resulted normal without signs of inflammation or free abdominal fluid.
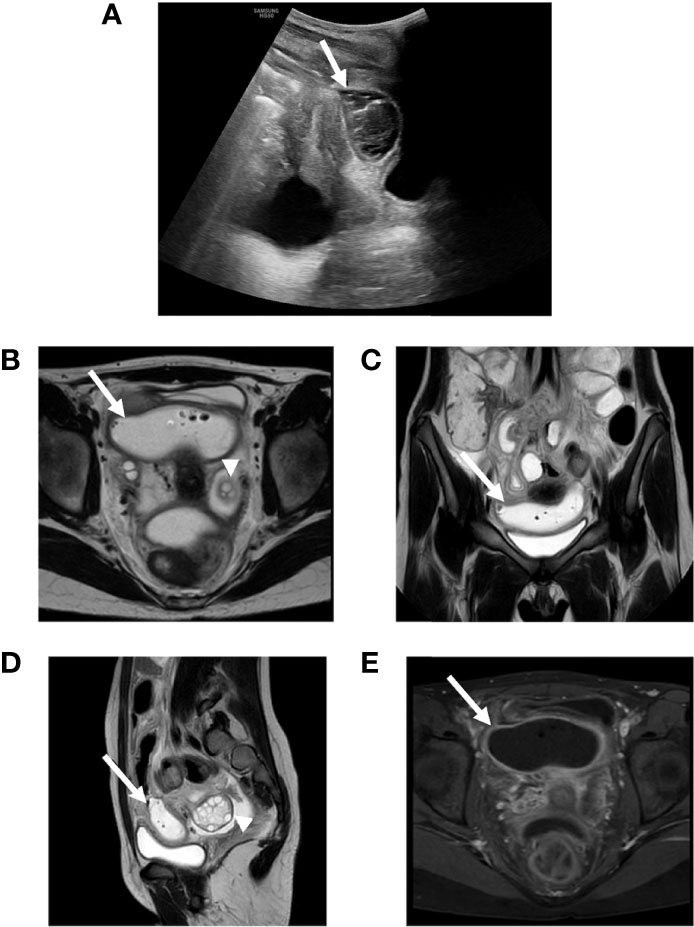
Figure 1 (A) Abdomen ultrasound showing an encapsulated abscess between bladder and uterus (arrow). (B–E) Abdomen MRI showing a pelvic large fluid-filled abscess (arrows) with air bubbles such as for bowel perforation (B–D: T2 weighted) with parietal contrast enhancement (E: T1 weighted) and normal adnexal region (arrowheads).
Despite a recent Italian study has shown a decrease in the frequency of DKA at T1D diagnosis in children during the last years, DKA frequency remains unacceptably high. (4) Data on the incidence of recurrent DKA in children in Italy are less consistent. Retrospective study conducted in 29 Italian diabetes centers from November 2011 to April 2012 showed an incidence of secondary DKA of 2.4 events/100 py and tended to increase with age. (5) Lower socioeconomic status is associated with a higher risk of DKA and a higher rate of diabetes-related complications. (4, 6–9) The risk of DKA in children after diagnosis of T1D is usually resulting from intentional or inadvertent insulin omission, or sometimes associated with intercurrent illness and increased insulin requirement. (10–12) The classic clinical signs of DKA include polyuria, polydipsia, polyphagia, and weight loss, but they may progress rapidly to vomiting, abdominal pain, dehydration, weakness, and lethargy. Abdominal pain and ileus can result from potassium depletion, acidosis, and poor splanchnic perfusion. (13) These symptoms and signs have been described in 40-75% of the cases of DKA and typically resolve during the first 24 hours of treatment, after administration of fluids and insulin. (2) In the initial phase of DKA abdominal pain may be severe enough to mimic an acute abdomen. Surgery is warranted in 6% of adult cases (mainly for acute cholecystitis or appendicitis). (3, 14).
DKA is characterized by relative or absolute insulin deficiency and increased levels of counterregulatory hormones, such as glucagon, adrenaline, cortisol, growth hormone, and proinflammatory cytokines. (15) High plasma levels of counterregulatory hormone leads to gluconeogenesis and glycogenolysis with increased glucose production and decreased peripheral glucose utilization. This causes hyperglycemia, hyperosmolality, increased lipolysis, and ketogenesis. (13) Glucose-induced osmotic diuresis leads to vascular volume depletion, severe dehydration and low cardiac output; moreover, the high catecholaminergic expression may induce hypoperfusion. Therefore, a physiological mechanism is established to maintain the perfusion of vital organs at the expense of mesenteric perfusion. A mismatch between supply and demand develops in the intestine, due to persistent mesenteric vasoconstriction, resulting in reduced blood flow and oxygen supply to the intestine, particularly to the vulnerable superficial mucosa. (16) This represents the main pathological mechanism involved in the genesis NOMI which refers to acute mesenteric ischemia without occlusion of the mesenteric arteries (Figure 2). (17, 18) Tissue damage from NOMI usually begins with mesenteric vasospasm, however, blood flow restoration (such as during DKA fluid treatment) exacerbates injury due to ischemia/reperfusion. (19) After the onset of ischemia, three different processes may be distinguished: the ischemic phase, reperfusion and the injury phase. In acute arterial occlusion these processes occur sequentially; in non-occlusive ischemia different stages can occur simultaneously, intermittently, or even repeatedly. The re-establishment of oxygenated blood flow produces oxygen-free radical metabolites with local inflammation in previously hyperperfused regions of the intestine. Cell damage induced by prolonged ischemia-reperfusion injury may lead to apoptosis, autophagy, necrosis, and necroptosis. (20) The integrity of the membrane is lost, with further cell necrosis. Not only is the damaging effect of Reactive Oxygen Species (ROS) greater than that of pure ischemia, but the damage is also no longer limited to the ischemic area. (21) NOMI accounts for about 20-30% of all cases of mesenteric ischemia and represents a potentially lethal condition with mortality rates up to 50%. (17, 22–25)
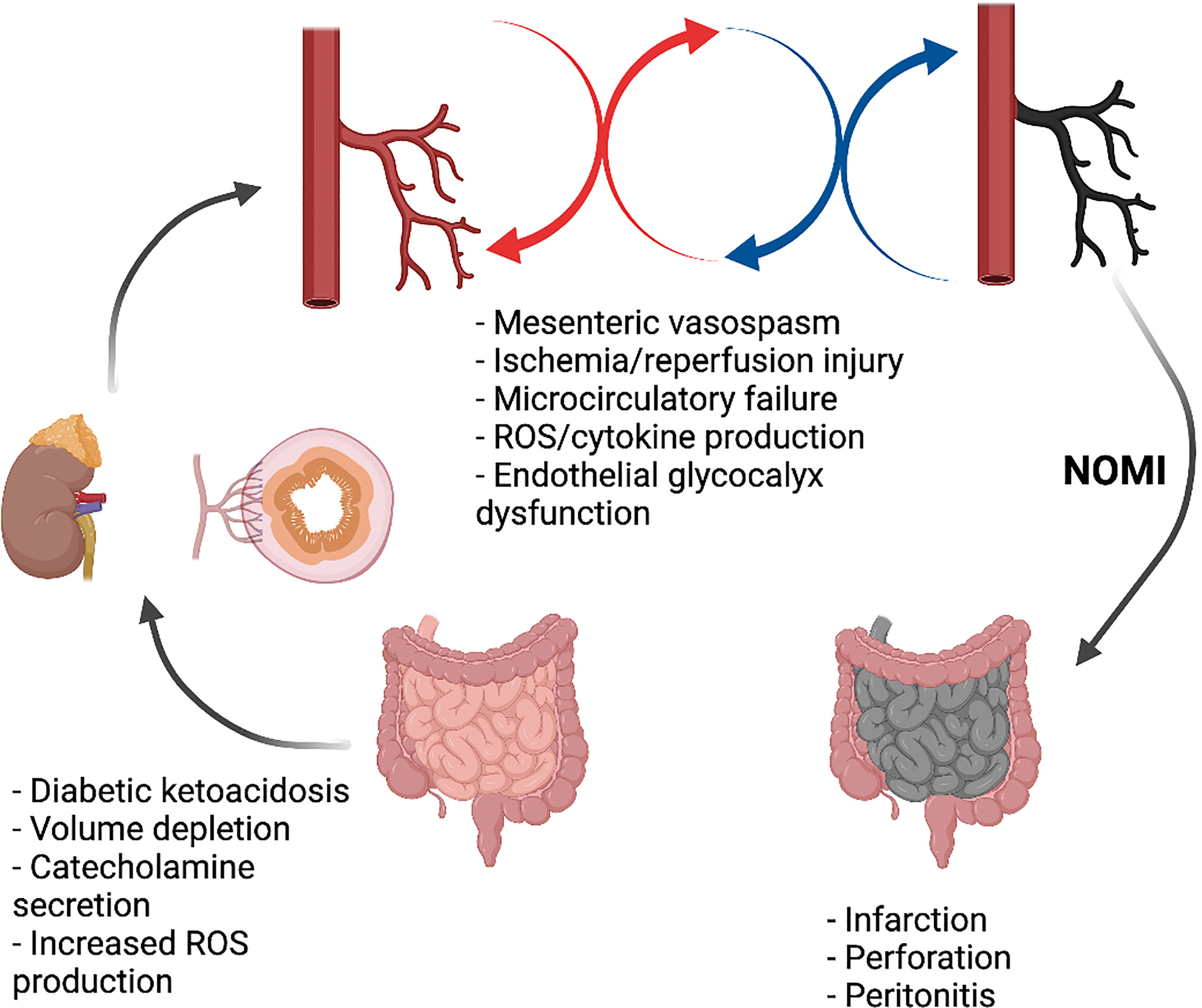
Figure 2 Non-occlusive mesenteric ischemia in DKA. Created with BioRender.com.
It tends to occur mostly in elderly patients who have low-cardiac output and other risk factors, but several pediatric cases have been previously described. These children usually present with underlying diseases such as familial dysautonomia, Addison’s disease, situs inversus, burns, chemotherapy for hematological malignancies, encephalitis, and septic shock. (26–34) Although rare, NOMI is a complication of DKA observed in adult diabetic patients. (18) Most described cases are patients older than 50 years of age and their rapid worsening condition required surgery to remove necrotizing intestine. (35–39) Two pediatric cases of NOMI in the context of DKA have been documented (Table 2). (40, 41) The general clinical conditions and abdominal symptoms of these patients, despite adequate fluid resuscitation and insulin administration, suggest a concurrent complicating event. When distinctive signs of ischemia were revealed during imaging assessment, both children underwent laparotomy and subsequent intestinal resection. The clinical evolution of the adolescent male described by Chan Chua et al. was probably driven by underlying neurological comorbidities (autonomic neuropathy). (41) Presently, the 3-year-old girl described by Di Meglio et al. is the only reported case of NOMI in a previously healthy child at T1D onset. (40) The pathogenesis of NOMI in DKA remains elusive although the two conditions most probably involve at least partially shared underlying mechanisms. Several previous studies have suggested that mesenteric artery spasm may be promoted by catecholamine, renin-angiotensin system and vasopressin activation during DKA. (42) Nieuwdorp M et al. have also suggested that endothelial glycocalyx (EG) dysfunction may be one of the possible mechanisms of NOMI onset in DKA. (43) EG is a layer of proteoglycans that covers the vascular endothelium, consisting of a core protein that carries one or more covalently attached glycosaminoglycan chains; it regulates vascular tone and permeability, inflammation and coagulation. (18, 44) EG damage during hyperglycemic conditions may cause vasospasm, hypoperfusion, as well as promoting inflammation and microthrombi formation due to hypercoagulability, ultimately resulting in NOMI. (18)
Despite the use of technology, recurrent DKA prevention remains an unmet need in children and adolescents with T1D and may be accompanied by life-threatening acute complications. The diagnosis of DKA-associated NOMI must be suspected in pediatric patients with DKA, persistent abdominal pain, and severe dehydration. Importantly, our case underlines how NOMI may exhibit a very subtle onset with mildly progressive abdominal pain, and unremarkable ultrasound imaging over days until overt symptoms and signs of complications (ie. Intestinal perforation) manifest.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
GF and RT share first authorship due to equal contribution. GF, RT and VC reviewed the case data and literature and contributed to manuscript drafting. GF and RT are responsible for final revision of the manuscript and significant intellectual content. All authors issued final approval for the version to be submitted. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ghetti S, Lee JK, Sims CE, DeMaster DM, Glaser NS. Diabetic Ketoacidosis and Memory Dysfunction in Children With Type 1 Diabetes. J Pediatr (2010) 156(1):109–14. doi: 10.1016/j.jpeds.2009.07.054
2. Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic Ketoacidosis and the Hyperglycemic Hyperosmolar State. Pediatr Diabetes (2018) 19(27):155–77. doi: 10.1111/pedi.12701
3. Umpierrez G, Freire AX. Abdominal Pain in Patients With Hyperglycemic Crises. J Crit Care (2002) 17(1):63–7. doi: 10.1053/jcrc.2002.33030
4. Gesuita R, Maffeis C, Bonfanti R, Cardella F, Citriniti F, D'Annunzio G, et al. Socioeconomic Inequalities Increase the Probability of Ketoacidosis at Diagnosis of Type 1 Diabetes: A 2014–2016 Nationwide Study of 2,679 Italian Children. Front Pediatr (2020) 8. doi: 10.3389/fped.2020.575020
5. Cherubini V, Pintaudi B, Rossi MC, Lucisano G, Pellegrini F, Chiumello G, et al. Severe Hypoglycemia and Ketoacidosis Over One Year in Italian Pediatric Population With Type 1 Diabetes Mellitus: A Multicenter Retrospective Observational Study. Nutrition Metab Cardiovasc Dis (2014) 24:538–46. doi: 10.1016/j.numecd.2013.11.004
6. Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors Associated With the Presence of Diabetic Ketoacidosis at Diagnosis of Diabetes in Children and Young Adults: A Systematic Review. BMJ (2011) 343:d4092. doi: 10.1136/bmj.d4092
7. Shulman R, Stukel TA, Miller FA, Newman A, Daneman D, Wasserman JD, et al. Low Socioeconomic Status is Associated With Adverse Events in Children and Teens on Insulin Pumps Under a Universal Access Program: A Population-Based Cohort Study. BMJ Open Diabetes Res Care (2016) 4(1):e000239. doi: 10.1136/bmjdrc-2016-000239
8. Shulman R, Stukel TA, Miller FA, Newman A, Daneman D, Guttmann A. Insulin Pump Use and Discontinuation in Children and Teens: A Population-Based Cohort Study in Ontario, Canada. Pediatr Diabetes (2017) 18(1):33–44. doi: 10.1111/pedi.12353
9. Zuijdwijk CS, Cuerden M, Mahmud FH. Social Determinants of Health on Glycemic Control in Pediatric Type 1 Diabetes. J Pediatr (2013) 162(4):730–5. doi: 10.1016/j.jpeds.2012.12.010
10. Cengiz E, Xing D, Wong JC, Wolfsdorf JI, Haymond MW, Rewers A, et al. Severe Hypoglycemia and Diabetic Ketoacidosis Among Youth With Type 1 Diabetes in the T1D Exchange Clinic Registry. Pediatr Diabetes (2013) 14(6):447–54. doi: 10.1111/pedi.12030
11. Mays JA, Jackson KL, Derby TA, Behrens JJ, Goel S, Molitch ME, et al. An Evaluation of Recurrent Diabetic Ketoacidosis, Fragmentation of Care, and Mortality Across Chicago, Illinois. Diabetes Care (2016) 39(10):1671–6. doi: 10.2337/dc16-0668
12. Al-Hayek AA, Robert AA, Braham RB, Turki AS, Al-Sabaan FS. Frequency and Associated Risk Factors of Recurrent Diabetic Ketoacidosis Among Saudi Adolescents With Type 1 Diabetes Mellitus. Saudi Med J (2015) 36(2):216–20. doi: 10.15537/smj.2015.2.10560
13. Cashen K, Petersen T. Diabetic Ketoacidosis. Pediatr In Rev (2019) 40(8):412–20. doi: 10.1542/pir.2018-0231
14. Freire AX, Umpierrez GE, Afessa B, Latif KA, Bridges L, Kitabchi AE. Predictors of Intensive Care Unit and Hospital Length of Stay in Diabetic Ketoacidosis. J Crit Care (2002) 17(4):207–11. doi: 10.1053/jcrc.2002.36755
15. Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic Crises in Adult Patients With Diabetes. Diabetes Care (2006) 29(12):2739–48. doi: 10.2337/dc06-9916
16. Al-Diery H, Phillips A, Evennett N, Pandanaboyana S, Gilham M, Windsor JA. The Pathogenesis of Nonocclusive Mesenteric Ischemia: Implications for Research and Clinical Practice. J Intensive Care Med (2019) 34(10):771–81. doi: 10.1177/0885066618788827
17. Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-Occlusive Mesenteric Ischemia: Etiology, Diagnosis, and Interventional Therapy. Eur Radiol (2002) 12:1179–87. doi: 10.1007/s00330-001-1220-2
18. Takiguchi T, Arai M, Kim S, Ishii H, Ogasawara T, Shigeta K, et al. Nonocclusive Mesenteric Ischemia Associated With a Hyperosmolar Hyperglycemic State: Hepatic Portal Venous Gas as an Indicator of Mesenteric Ischemia. Acute Med Surg vol (2021) 8:e673. doi: 10.1002/ams2.673
19. Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of Mesenteric Ischemia/Reperfusion: A Review. Acta Cirurgica Bras (2005) 20:336–43. doi: 10.1590/S0102-86502005000400013
20. Wu M-Y, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, et al. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem (2018) 46:1650–67. doi: 10.1159/000489241
21. Kolkman JJ, Mensink PBF. Non-Occlusive Mesenteric Ischaemia: A Common Disorder in Gastroenterology and Intensive Care. Best Pract Res Clin Gastroenterol (2003) 17:457–73. doi: 10.1016/S1521-6918(03)00021-0
22. Acosta S, Ogren M, Sternby N-H, Bergqvist D, Bjorck M. Fatal Nonocclusive Mesenteric Ischaemia: Population-Based Incidence and Risk Factors. J Internal Med (2006) 259:305–13. doi: 10.1111/j.1365-2796.2006.01613.x
23. Bassiouny HS. Nonocclusive Mesenteric Ischemia. Surg Clin North Am (1997) 77(2):319–26. doi: 10.1016/S0039-6109(05)70551-X
24. Florim S, Almeida A, Rocha D, Portugal P. Acute Mesenteric Ischaemia: A Pictorial Review. Insights into Imaging (2018) 9:673–82. doi: 10.1007/s13244-018-0641-2
25. ENDE N. Infarction of the Bowel in Cardiac Failure. New Engl J Med (1958) 258:879–81. doi: 10.1056/NEJM195805012581804
26. Ghatak T, Singh RK, Baronia AK. An Unusual Case of Nonocclusive Mesenteric Ischemia in a Young Girl. Indian J Crit Care Med (2012) 16:28–30. doi: 10.4103/0972-5229.94423
27. Jeican II, Ichim G, Gheban D. Intestinal Ischemia in Neonates and Children. Clujul Med (2016) 89:347–51. doi: 10.15386/cjmed-600
28. Yoshida M, Kataoka N, Miyauchi K, Ohe K, Iida K, Yoshida S, et al. Rectifier of Aberrant mRNA Splicing Recovers tRNA Modification in Familial Dysautonomia. Proc Natl Acad Sci (2015) 112:2764–9. doi: 10.1073/pnas.1415525112
29. Roldan-Martin MB, Rodriguez-Ogando A, Sanchez-Galindo AC, Parente-Hernandez A, Luengo-Herrero V, Sanchez-Sanchez C. Rare Presentation of Shock and Acute Mesenteric Ischaemia Secondary to Acute Adrenal Insufficiency in an 11-Year-Old Male. J Paediatrics Child Health (2013) 49:498–500. doi: 10.1111/j.1440-1754.2012.02556.x
30. Mirza B, Iqbal S, Talat N, Saleem M, Ahmad S. Intraoperative Acute Mesenteric Ischemia: A Hard Nut to Crack. J Indian Assoc Pediatr Surgeons (2014) 19:247. doi: 10.4103/0971-9261.142026
31. Wilson MD, Dziewulski P. Severe Gastrointestinal Haemorrhage and Ischaemic Necrosis of the Small Bowel in a Child With 70% Full-Thickness Burns: A Case Report. Burns (2001) 27:763–6. doi: 10.1016/s0305-4179(01)00044-4
32. Groger A, Bozkurt A, Franke E, Hornchen H, Steinau G, Piatkowski A, et al. Ischaemic Necrosis of Small and Large Intestine in a 2-Year-Old Child With 20% Partial Thickness Burns: A Case Report. Burns (2005) 31:930–2. doi: 10.1016/j.burns.2005.02.011
33. Hirabayashi K, Takatsuki M, Motobayashi M, Kurata T, Saito S, Shigemura T, et al. Nonocclusive Mesenteric Ischemia After Chemotherapy in an Adolescent Patient With a History of Three Allogeneic Hematopoietic Stem Cell Transplantations for Acute Lymphoblastic Leukemia. Pediatr Neonatology (2017) 58:81–4. doi: 10.1016/j.pedneo.2014.07.008
34. Oyachi N, Emura T, Numano F, Tando T, Saito T, Goto Y. Non-Occlusive Intestinal Ischemia in the Ascending Colon and Rectum: A Pediatric Case Occurring During Encephalitis Treatment. Surg Case Rep (2019) 5:23. doi: 10.1186/s40792-019-0592-y
35. Soravia C, Höhn L, Mentha G, Chevrolet JC, Suter P, Rohner A, et al. [Non Occlusive Mesenteric Ischemia: A Late Complication of Cardiogenic Shock]. Annales chirurgie (1994) 48(11):1029–31.
36. Sharieff GQ, Shad JA, Garmel G. An Unusual Case of Mesenteric Ischemia in a Patient With New-Onset Diabetes Mellitus. Am J Emergency Med (1997) 15(3):282–4. doi: 10.1016/S0735-6757(97)90016-4
37. Hohmann C, Teuteberg S, Aschenbrenner I, Kaag N, Heizmann O. Nicht-Okklusive Mesenterialischämie Bei Diabetischem Koma. DMW - Deutsche Medizinische Wochenschrift (2019) 144(23):1638–41. doi: 10.1055/a-0974-1877
38. Itoh Y, Sagawa R, Kinoshita H, Tamba S, Yamamoto K, Yamada Y, et al. Small-Intestinal Necrosis Due to non-Occlusive Mesenteric Ischemia With Diabetic Ketoacidosis After Quetiapine Treatment. Diabetol Int (2019) 10(3):225–30. doi: 10.1007/s13340-018-0386-7
39. Gocho N, Aoki E, Okada C, Omura K, Hirashima T, Suzuki N, et al. Non-Occlusive Mesenteric Ischemia With Diabetic Ketoacidosis and Lactic Acidosis Following the Administration of a Sodium Glucose Co-Transporter 2 Inhibitor. Internal Med (2016) 55(13):1755–60. doi: 10.2169/internalmedicine.55.6338
40. DiMeglio LA, Chaet MS, Quigley CA, Grosfeld JL. Massive Ischemic Intestinal Necrosis at the Onset of Diabetes Mellitus With Ketoacidosis in a Three-Year-Old Girl. J Pediatr Surg (2003) 38(10):1537–9. doi: 10.1016/S0022-3468(03)00510-4
41. Chan-Cua S, Jones KL, Lynch FP, Freidenberg GR. Necrosis of the Ileum in a Diabetic Adolescent. J Pediatr Surg (1992) 27(9):1236–8. doi: 10.1016/0022-3468(92)90798-C
42. Worly JM, Fortenberry JD, Hansen I, Chambliss CR, Stockwell J. Deep Venous Thrombosis in Children With Diabetic Ketoacidosis and Femoral Central Venous Catheters. Pediatrics (2004) 113(1):e57–60. doi: 10.1542/peds.113.1.e57
43. Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, et al. Loss of Endothelial Glycocalyx During Acute Hyperglycemia Coincides With Endothelial Dysfunction and Coagulation Activation In Vivo. Diabetes (2006) 55(2):480–6. doi: 10.2337/diabetes.55.02.06.db05-1103
Keywords: non-occlusive mesenteric ischemia (NOMI), DKA (diabetic ketoacidosis), insulin pump (CSII: continuous subcutaneous insulin infusion), acute kidney injury, hyperosmolar (hyperglycemic) coma, T1DM (type 1 diabetes mellitus)
Citation: Frontino G, Di Tonno R, Castorani V, Rigamonti A, Morotti E, Sandullo F, Scialabba F, Arrigoni F, Foglino R, Dionisi B, Ferri CIC, Zirpoli S, Barera G, Meschi F and Bonfanti R (2022) Non-Occlusive Mesenteric Ischemia in Children With Diabetic Ketoacidosis: Case Report and Review of Literature. Front. Endocrinol. 13:900325. doi: 10.3389/fendo.2022.900325
Received: 20 March 2022; Accepted: 13 June 2022;
Published: 14 July 2022.
Edited by:
Andrea Enzo Scaramuzza, Istituti Ospitalieri di Cremona, ItalyReviewed by:
Theocharis Koufakis, University General Hospital of Thessaloniki AHEPA, GreeceCopyright © 2022 Frontino, Di Tonno, Castorani, Rigamonti, Morotti, Sandullo, Scialabba, Arrigoni, Foglino, Dionisi, Ferri, Zirpoli, Barera, Meschi and Bonfanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Castorani, Y2FzdG9yYW5pLnZhbGVyaWFAaHNyLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.