
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 24 March 2022
Sec. Diabetes: Molecular Mechanisms
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.862545
This article is part of the Research TopicAdvances in the Research of Diabetic NephropathyView all 10 articles
 Yaping Wei1,3
Yaping Wei1,3 Shiyu Han2,3
Shiyu Han2,3 Ruonan Zhou2,3
Ruonan Zhou2,3 Pingyuan Xu2,3
Pingyuan Xu2,3 Lingyan Zhou2,3
Lingyan Zhou2,3 Ziwei Zhu2,3
Ziwei Zhu2,3 Yue Kan2,3
Yue Kan2,3 Xiaoying Yang2,3
Xiaoying Yang2,3 Yingying Xiang2,3
Yingying Xiang2,3 Yue Cao2,3
Yue Cao2,3 Yu Jin3
Yu Jin3 Jing Yan3
Jing Yan3 Xizhong Yu3
Xizhong Yu3 Xin Wang2
Xin Wang2 Wenbin Shang2,3*
Wenbin Shang2,3*Aims/Introduction: Renal function impairment related to type 2 diabetes (T2DM) presents serious threat to public health. Previous studies suggest that vascular endothelial growth factor-B (VEGF-B) might contribute to renal injury. Therefore, this study investigated the association of serum VEGF-B level with the risk of renal function impairment in T2DM patients.
Materials and Methods: Serum VEGF-B levels were measured in 213 patients with type 2 diabetes and 31 healthy participants. Participants with type 2 diabetes were further divided into a group of 112 participants with eGFR<90 mL/min/1.73m2 and 101 participants with eGFR≥ 90 mL/min/1.73m2. Clinical data were collected, and a binary logistic regression model was employed to test the association between potential predictors and eGFR.
Results: Serum VEGF-B levels evaluated in type 2 diabetes patients compared with healthy controls. In patients with type 2 diabetes, serum VEGF-B level was positively correlated with triglyceride, serum creatinine and cystatin C while negatively correlated with HDL-C and eGFR. Binary logistic regression showed that serum VEGF-B level was an independent risk factor of eGFR<90 mL/min/1.73m2.
Conclusions: Serum VEGF-B level is associated with renal function impairment in patients with type 2 diabetes and may be a potential drug target for diabetic kidney disease.
With the global prevalence of type 2 diabetes mellitus (T2DM), diabetic kidney disease (DKD) has become the leading cause of end stage renal disease (ESRD), and poses a great burden to diabetes patients and the health care system (1). Progressing renal function impairment characterized by declining glomerular filtration rate (GFR) runs through the course of T2DM, and eventually results in DKD. Multifactorial pathological processes have been proven to be contributors of diabetes-related renal function impairment, including advanced glycation end products (AGEs), ectopic lipid deposition, hemodynamic perturbations and inflammation (2, 3). However, further study is still required to explore the exact mechanisms underlying renal function impairment in T2DM patients.
Vascular endothelial growth factor-B (VEGF-B) is a member of the vascular endothelial growth factor (VEGF) family expressing in multiple organs, such as heart, adipose, muscle, brain, and kidney (4). Different with other members from VEGF family, VEGF-B shows faint angiogenic effect in vivo, which triggers a widespread speculation of VEGF-B playing its angiogenic role mainly through its recruitment of other VEGFs (5, 6). However, VEGF-B was reported to regulate fatty acid transport proteins (FATPs) in endothelial cells via membrane receptors neuropilin-1(NRP-1) or vascular endothelial growth factor receptor-1 (VEGFR-1), and an improvement of insulin sensitivity along with blood lipid profiles was observed in VEGF-B deficient mice fed with high-fat diet (4, 7). Moreover, the activation of VEGFR-1, one of the VEGF-B receptors, was demonstrated to promote the generation of pro-inflammatory and pro-angiogenic cytokines in macrophages and accelerate the process of inflammatory diseases, for instance, rheumatoid arthritis and retinal injury (8, 9). Seeing the coincidences between VEGF-B biological functions and diabetes-related renal function impairment, several studies reported that VEGF-B could directly impair podocyte insulin sensitivity by promoting ectopic lipid accumulation in podocytes and cause the occurrence of DKD in various diabetic mouse models (10, 11). Furthermore, a newly produced anti-VEGFB/IL22 fusion protein was found to be capable to ameliorate renal dysfunction in db/db mice recently (11).
Although evidences from animal experiments suggest the vital role of VEGF-B in DKD occurrence, the relationship between circulating VEGF-B level and renal function impairment in T2DM patients still remains unclear. In this study, we tested the serum VEGF-B levels of 213 T2DM patients as well as 31 healthy participants, and analyzed the potential link between serum VEGF-B levels and renal function of patients with T2DM. Our results would provide a better insight into the role of VEGF-B in the pathogenesis of diabetic renal impairment as well as the intervention of diabetic kidney disease.
31 healthy participants were recruited from the health examination center of the affiliated hospital of Nanjing university of Chinese medicine. None of the healthy participants had known metabolic disorders, kidney injury or other kind of diseases. 213 T2DM patients were recruited from the department of endocrinology of the affiliated hospital of Nanjing university of Chinese medicine. All participants with T2DM satisfied the world health organization 1999 criteria when diagnosed (12). This study was approved by the medical research ethics committee of the affiliated hospital of Nanjing university of Chinese medicine and carried out under the principles of the Declaration of Helsinki. Written informed consents were obtained from all participants.
All participants were admitted to the department of endocrinology. Detailed medical history and physical examination data, including gender, age, medicine usage, resting blood pressure, weight and height were obtained upon admission. Venous blood and midstream urine samples were collected at 6:00 a.m. from participants after 12 hours of fasting. The examination of total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL-C), high density lipoprotein (HDL-C), fasting blood-glucose (FBG), fasting C peptide (FCP), glycosylated hemoglobin (HbA1c), serum creatinine (SCr), blood urea nitrogen (BUN), Cystatin C (CysC), urine albumin-to-creatinine ratio (UACR) and 24-hour urine total protein (UTP) were performed at laboratory center of the affiliated hospital of Nanjing university of Chinese medicine. Body mass index (BMI) = body weight (kg)/the square of body height (m2). Homeostasis model of assessment for insulin resistance (HOMA-IR) = fasting glucose (mmol/L)╳fasting insulin (mU/L)/22.5. Estimated glomerular filtration rate (eGFR) was calculated according to CKD-EPI2012scr-cys.
Fresh blood samples were stood at room temperature (22°C) for 30min and then centrifuged at 3000rpm for 10 min. The supernatant were collected as serum samples and stored at -80°C. Serum VEGF-B values were determined by human enzyme-linked immunosorbent assay (ELISA) kits (CB5521, Biorbyt, UK).
Statistical analyses were performed using SPSS 25.0. All data were represented as mean ± standard deviation (SD), median with interquartile range (IQR) or percentage, as appropriate. Data that were not normally distributed, including Diabetes duration, FBG, HbA1c, HOMA-IR, Triglyceride, Total cholesterol, HDL-C, LDL-C, Serum creatinine, Blood urea nitrogen, Cystatin C, UACR, UTP, eGFR, Serum VEGF-B were logarithmically transformed before analysis. ANOVA and Mann-Whitney U tests were used for continuous variables distributed normally and asymmetrically, respectively. Chisquared test (χ2) was employed for comparisons of categorical variables. The relationships between clinical indicators were examined using Pearson correlation, Spearman correlation or partial correlation. Binary logistic regression analysis was used to test the association between potential predictors and eGFR. A p-value of <0.05 was indicated statistically significant.
The study recruited 244 eligible participants, including 213 patients explicitly diagnosed with T2DM at baseline and 31 healthy subjects seeking for routine examination. Fasting blood samples were collected from all participants and serum VEGF-B levels were tested. As shown in Figure 1, compared to the healthy controls, a significantly elevated level of serum VEGF-B was observed in T2DM patients (p<0.001).
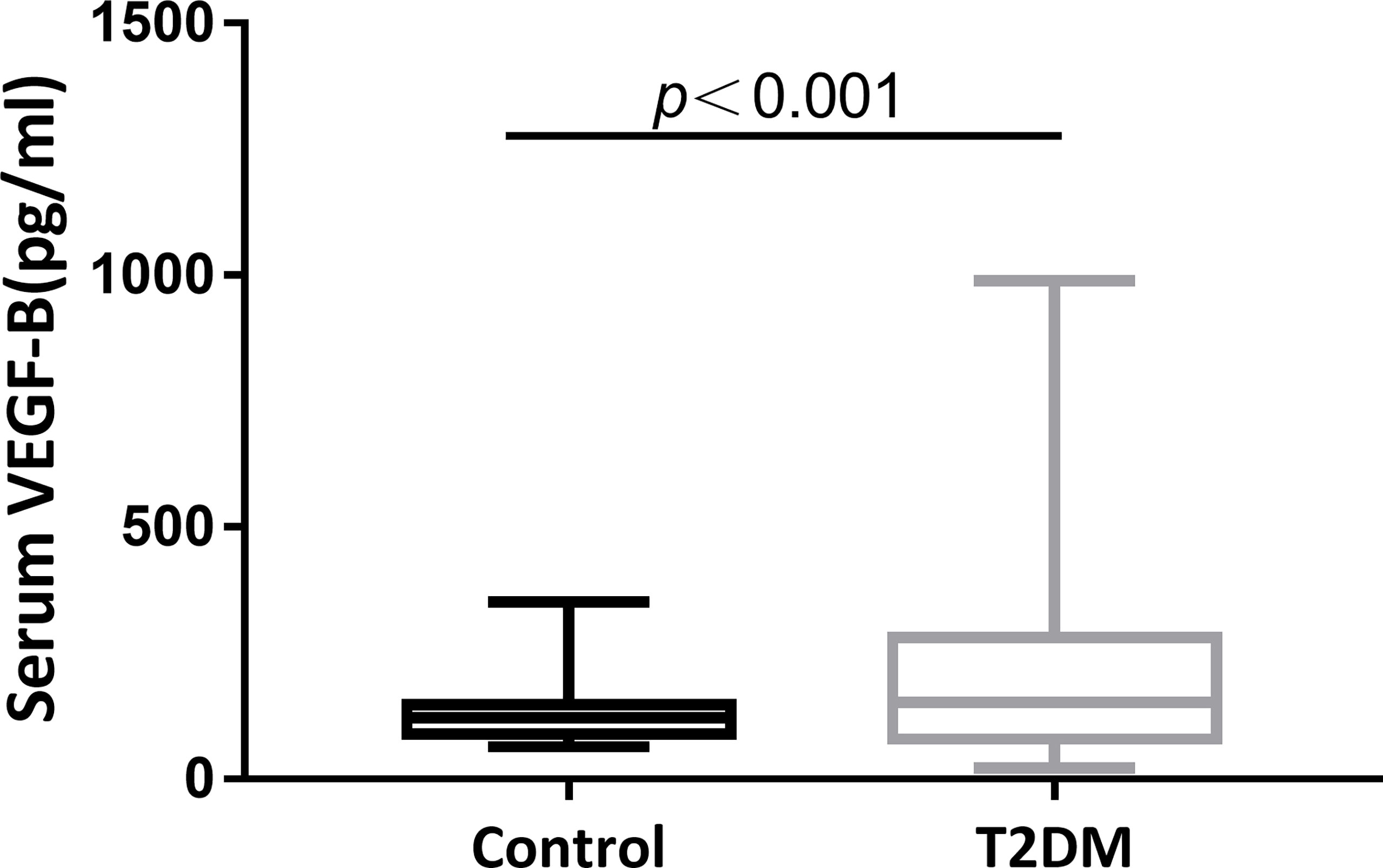
Figure 1 Serum VEGF-B level elevates in T2DM patients. A significantly increase of serum VEGF-B levels in T2DM patients compared to healthy control subjects was observed (p < 0.001).
Based on the accepted eGFR cutoff value (90 mL/min/1.73m2), we further divided the enrolled T2DM patients into a group of 112 participants with eGFR<90 mL/min/1.73m2 and 101 participants with eGFR≥ 90 mL/min/1.73m2 (13). Gender distribution, drinking history, smoking history, BMI, SBP, HbA1c, FPG, HOMA-IR, TC, TG, HDL-C, LDL-C, UCR and UTP were similar between the groups (all p>0.05). However, the eGFR<90 mL/min/1.73m2 group had older age (p<0.001), longer diabetes duration (p=0.004), lower DBP (p=0.003), as well as worse renal dysfunction characterized by higher levels of SCr (p<0.001), BUN (p<0.001), CysC (p<0.001) and UACR (p=0.005). Most importantly, eGFR<90 mL/min/1.73m2 group also showed a significantly increased level of serum VEGF-B (p=0.002) (Table 1). Upon the usage of medications, no significant difference was observed between 2 groups on the usage of metformin, sulfonylureas, pioglitazone, glucosidase inhibitors, GLP1rA (glucagon-like peptide 1 receptor agonists), DPP4 inhibitors, SGLT2 inhibitors, statins, insulin and ACEI/ARB (all p>0.05). But, a higher percentage of patients using beta-blockers (p=0.007) and Calcium channel blockers (p=0.039) in eGFR<90 mL/min/1.73m2 group was showed by Chisquared test (Table 2).
As shown in Table 3, in T2DM patients, serum VEGF-B level was positively correlated with triglyceride (r=0.172, p=0.013), serum creatinine (r=0.150, p=0.031) and cystatin C (r=0.245, p<0.001) while inversely correlated with HDL-C (r=-0.138, p=0.047) and eGFR (r=-0.205, p=0.003) after adjusting gender, age, smoking history, drinking history, diabetes duration and BMI. However, although partial correlation confirmed that VEGF-B level significantly associates with several renal function indicators (Figures 2A–C), no significant correlation was observed between serum VEGF-B and UACR nor UTP (both p>0.05).
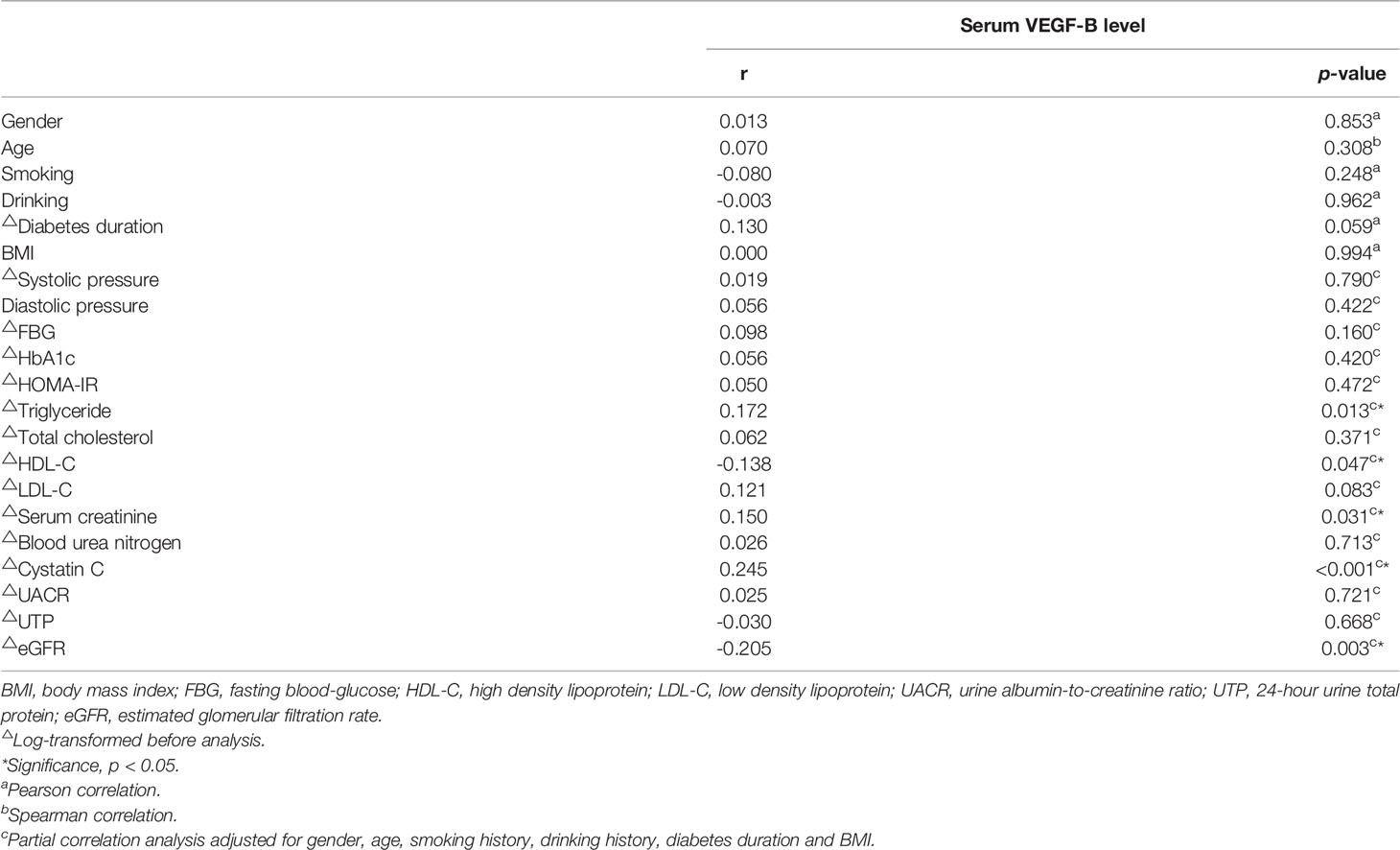
Table 3 The correlation of serum VEGF-B (log-transformed) with clinical indicators in T2DM patients.
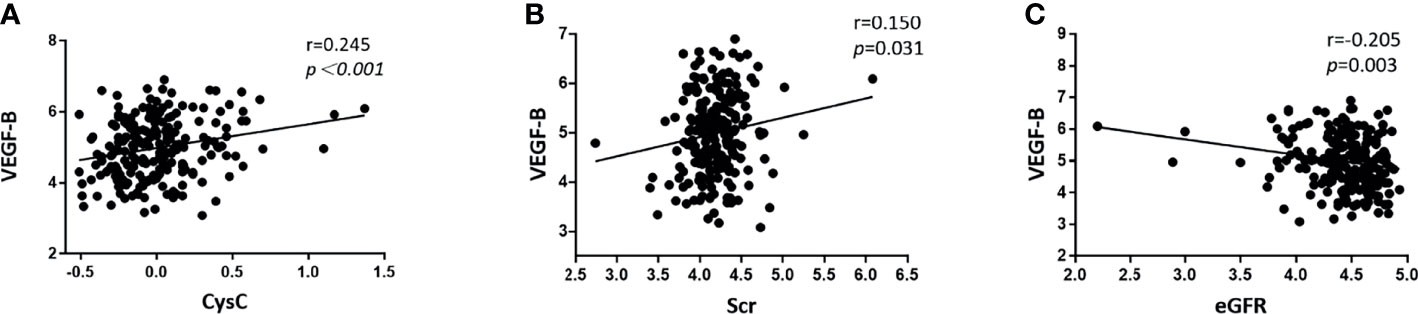
Figure 2 Scatter diagrams showing the significant partial correlations between serum VEGF-B levels and renal function indicators in T2DM patients after adjusting for gender, age, smoking history, drinking history, diabetes duration and BMI. (A) The serum VEGF-B level was positively correlated with Cystatin C (r=0.245, p < 0.001); (B) The serum VEGF-B level was positively correlated with Scr (r=0.150, p=0.031); (C) The serum VEGF-B level was negatively correlated with eGFR (r=-0.205, p=0.003). All data was log-transformed before analysis.
We further employed binary logistic regression analysis to analyze the associations between serum VEGF-B and the risk of eGFR<90 mL/min/1.73m2. We found that serum VEGF-B level (p=0.002) was significant in predicting eGFR<90 mL/min/1.73m2, even after controlling age, UACR, diabetes duration and diastolic pressure (based on the results from Table 1). Of the risk factors above, age (p<0.001) showed a significant, positive relationship with eGFR<90 mL/min/1.73m2 as well (Table 4 and Figure 3).

Figure 3 Serum VEGF-B level is an independent risk factor for eGFR<90 mL/min/1.73m2. As shown in the forest plots, binary logistic regression demonstrated that serum VEGF-B level and age are independent risk factors for eGFR<90 mL/min/1.73m2. *Significance, p<0.05. △Log-transformed before analysis.
Diabetic kidney disease is one of the leading complications of type 2 diabetes. Currently, the mechanisms underlying T2DM-related renal function impairment remains unclear, and the strategies of DKD prediction are still limited (14). In this study, we assessed whether serum VEGF-B, a biomarker from vascular endothelial growth factor family, was associated with progression of diabetes-related renal function impairment in 213 patients with established T2DM. Our results showed that elevated level of VEGF-B in T2DM patients was significantly correlated with markers of lipid metabolism and glomerular function. Regression model also indicated that serum VEGF-B was one of the independent risk factors of eGFR<90 mL/min/1.73m2 in T2DM patients.
Previously, several studies have observed increased VEGF-B levels in DKD patients compared to healthy controls (10, 11). Our study further proved that, not only in DKD patients, just in T2DM population (only 28 of 213 T2DM patients fit the diagnostic criteria of DKD), a significantly rising level of serum VEGF-B could be marked. This may provide evidence for the hypothesis that VEGF-B might be a cause for T2DM-related renal function impairment rather than a result. A genome-wide association study demonstrated that the up-regulation of vegf-b is related to chronic kidney disease, T2DM, hypertension and hyperlipidemia (15). In animal models of diabetes, VEGF-B increment was found in various type of cells, such as choroidal cells and podocytes (10, 16). Furthermore, vegf-b overexpression impaired whereas vegf-b knockout rescued the insulin sensitivity and blood lipid profile of gene-edited mouse models (7, 17). Hence, we can assume that VEGF-B may be a future drug target of T2DM and the complications related with huge potential. In addition, a cross-sectional study involving 45 patients with newly diagnosed T2DM also showed an elevation of circulating VEGF-B level in newly diagnosed T2DM patients compared with healthy subjects, and the study exhibited a significantly correlation between circulating VEGF-B and markers of glucose metabolism as well (18). However, in our study, no significant correlation between serum VEGF-B and FBP nor HOMA-IR was observed, which may on account of the anti-diabetic drug usage. We will further expand the sample size and refine the groups to see if more evidence could be provided.
By correlation analysis, we also demonstrated that serum VEGF-B in T2DM patients was correlated with triglyceride and HDL-C, besides renal function markers. Consistent with our findings, Ye etc. found that circulating VEGF-B level was positively correlated with HDL-C and negatively correlated with eGFR in patients with non-alcoholic fatty liver disease (19). Disorder of lipid metabolism is an acknowledged contributor of diabetes-related renal function impairment (20). It has been proven that VEGF-B could destroy glomerular filtration barrier by inducing ectopic lipid deposition in podocyte, and VEGF-B antibody injection prevented renal lipotoxicity and further improved the renal function of db/db mice (10). Our results confirmed that serum VEGF-B is positively correlated with triglyceride and HDL-C while negatively correlated with eGFR, which supports the point that VEGF-B affects renal function of T2DM patients at least partially via regulating lipid metabolism.
It is well known that the classic characteristics of DKD are descending GFR and progressing albuminuria. However, epidemiological investigation exhibits a gradually increasing incidence rate of chronic kidney disease with normoalbuminuria in diabetic patients, especially type 2 diabetes, and the disease was recently referred to as normoalbuminuric diabetic kidney disease (NADKD) (21). Our data also showed that after grouping T2DM patients by eGFR, no difference could be seen in UTP between eGFR≥ 90 mL/min/1.73m2 group and eGFR<90 mL/min/1.73m2 group (the slight decline of UACR in eGFR<90 mL/min/1.73m2 group may depend on the increase of creatinine). This could be partially explained by the limited sample size, but also, the phenomenon was consistent with the epidemic of NADKD in T2DM. Moreover, traditionally, albuminuria and diabetic retinopathy are important components of early DKD prediction and diagnosis. But increasing studies point out that substantial numbers of T2DM patients with DKD do not have retinopathy nor abnormal albuminuria, and normoalbuminuria T2DM patients with low GFR (<60 mL/min/1.73m2) are more likely to show diffuse lesions, nodular lesions, tubulointerstitial lesions or vascular lesions compared to those with preserved GFR (≥60 mL/min/1.73m2) (22, 23). And as such, more sensitive indicators are urgently needed for early diagnosis and intervention of DKD. In our research, we found that serum VEGF-B level in T2DM patients was positively correlated with SCr and CysC, while negatively correlated with eGFR and is an independent predictor of eGFR<90 mL/min/1.73m2. Interestingly, no significant correlation between serum VEGF-B and UTP nor UACR was observed, which suggested that the relationship between serum VEGF-B level and renal function impairment in T2DM patients was independent of albuminuria, and the VEGF-B up regulation is likely to be a new mechanism underlying NADKD in T2DM patients.
Overall, in this study, we demonstrated that T2DM patients exhibited an elevated serum VEGF-B level, which was associated with renal function impairment. The increased serum VEGF-B level was an independent risk factor of eGFR<90 mL/min/1.73m2 in patients with type 2 diabetes and it was irrelevant to albuminuria. However, certain limitations of this study should be noted. Firstly, owing to the difficulty in obtaining renal samples from T2DM patients, VEGF-B levels in serum were tested only, thus we are unable to trace the accurate source of the increasing VEGF-B. Secondly, the persuasion as well as the reproducibility of our findings might be blunted by the limited sample size. Current chronic kidney disease nomenclature used by KDIGO is based on eGFR (G1-G5) along with albuminuria (A1-A3), but most of our cases were restricted at G1 and G2 stage, which made it far too difficult to further stage the patients by KDIGO standard. 90 mL/min/1.73m2 is an eGFR cutoff value widely accepted by guidelines (13, 24). eGFR<90 mL/min/1.73m2 usually indicates renal function impairment. By dividing T2DM patients into eGFR≥ 90 mL/min/1.73m2 group and eGFR<90 mL/min/1.73m2 group, we found a strong link between serum VEGF-B level and renal function impairment in T2DM patients. However, we will further expand the sample size in the future to explore the role of serum VEGF-B level in different stages of DKD. Lastly, as a cross-sectional research, this study was constrained in inferring causal relationships, and usage of medications was unavoidable in patients recruited. Although Chisquared test showed no significant differences in the usage of RAS inhibitors, SGLT2 inhibitors and statins between eGFR≥ 90 mL/min/1.73m2 group and eGFR<90 mL/min/1.73m2 group, which sharpens our observations, future studies designed in cohorts are still required to confirm the findings and to determine the role of VEGF-B in DKD pathology as well as whether serum VEGF-B level could be used for early prediction of DKD or NADKD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the medical research ethics committee of the affiliated hospital of Nanjing university of Chinese medicine. The patients/participants provided their written informed consent to participate in this study.
In this study, YW and WS designed the project, analyzed the data and wrote portions of the manuscript. SH, RZ, PX, LZ, ZZ, YK, XYY, YX, YC, and XW performed the collection of samples and data. YJ, JY and XZY performed the measurement of serum VGEF-B. WS guided the project and wrote portions of the manuscript. All authors are in agreement with the content of the manuscript, and the authors declare no conflict of interest. All authors contributed to the article and approved the submitted version.
The present study was supported by the open projects of the discipline of Chinese Medicine of Nanjing University of Chinese Medicine supported by the subject of academic priority discipline of Jiangsu higher education institutions (ZYX03KF058).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Khan NU, Lin J, Liu X, Li H, Lu W, Zhong Z, et al. Insights Into Predicting Diabetic Nephropathy Using Urinary Biomarkers. Biochim Biophys Acta Proteins Proteom (2020) 1868(10):140475. doi: 10.1016/j.bbapap.2020.140475
2. Chen X, Yin Q, Ma L, Fu P. The Role of Cholesterol Homeostasis in Diabetic Kidney Disease. Curr Med Chem (2021) 28(36):7413–26. doi: 10.2174/0929867328666210419132807
3. Ricciardi CA, Gnudi L. Kidney Disease in Diabetes: From Mechanisms to Clinical Presentation and Treatment Strategies. Metab Clin Exp (2021) 124:154890. doi: 10.1016/j.metabol.2021.154890
4. Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, et al. Vascular Endothelial Growth Factor B Controls Endothelial Fatty Acid Uptake. Nature (2010) 464(7290):917–21. doi: 10.1038/nature08945
5. Uemura A, Fruttiger M, D'Amore PA, De Falco S, Joussen AM, Sennlaub F, et al. VEGFR1 Signaling in Retinal Angiogenesis and Microinflammation. Prog Retin Eye Res (2021) 84:100954. doi: 10.1016/j.preteyeres.2021.100954
6. Bry M, Kivelä R, Leppänen V-M, Alitalo K. Vascular Endothelial Growth Factor-B in Physiology and Disease. Physiol Rev (2014) 94(3):779–94. doi: 10.1152/physrev.00028.2013
7. Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, et al. Targeting VEGF-B as a Novel Treatment for Insulin Resistance and Type 2 Diabetes. Nature (2012) 490(7420):426–30. doi: 10.1038/nature11464
8. Murakami M, Iwai S, Hiratsuka S, Yamauchi M, Nakamura K, Iwakura Y, et al. Signaling of Vascular Endothelial Growth Factor Receptor-1 Tyrosine Kinase Promotes Rheumatoid Arthritis Through Activation of Monocytes/Macrophages. Blood (2006) 108(6):1849–56. doi: 10.1182/blood-2006-04-016030
9. Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, et al. Sustained Inflammation After Pericyte Depletion Induces Irreversible Blood-Retina Barrier Breakdown. JCI Insight (2017) 2(3):e90905. doi: 10.1172/jci.insight.90905
10. Falkevall A, Mehlem A, Palombo I, Heller Sahlgren B, Ebarasi L, He L, et al. Reducing VEGF-B Signaling Ameliorates Renal Lipotoxicity and Protects Against Diabetic Kidney Disease. Cell Metab (2017) 25(3):713–26. doi: 10.1016/j.cmet.2017.01.004
11. Shen Y, Chen W, Han L, Bian Q, Fan J, Cao Z, et al. VEGF-B Antibody and Interleukin-22 Fusion Protein Ameliorates Diabetic Nephropathy Through Inhibiting Lipid Accumulation and Inflammatory Responses. Acta Pharm Sin B (2021) 11(1):127–42. doi: 10.1016/j.apsb.2020.07.002
12. Alberti KG, Zimmet PZ . Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation. Diabet Med (1998) 15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
13. Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, De Jong PE, et al . Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl (2013) 3:1–150. doi: 10.1038/kisup.2012.73
14. Sugahara M, Pak WLW, Tanaka T, Tang SCW, Nangaku M. Update on Diagnosis, Pathophysiology, and Management of Diabetic Kidney Disease. Nephrol (Carlton) (2021) 26(6):491–500. doi: 10.1111/nep.13860
15. Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New Genetic Loci Link Adipose and Insulin Biology to Body Fat Distribution. Nature (2015) 518(7538):187–96. doi: 10.1038/nature14132
16. Huang D, Zhao C, Ju R, Kumar A, Tian G, Huang L, et al. VEGF-B Inhibits Hyperglycemia- and Macugen-Induced Retinal Apoptosis. Sci Rep (2016) 6:26059. doi: 10.1038/srep26059
17. Karpanen T, Bry M, Ollila HM, Seppänen-Laakso T, Liimatta E, Leskinen H, et al. Overexpression of Vascular Endothelial Growth Factor-B in Mouse Heart Alters Cardiac Lipid Metabolism and Induces Myocardial Hypertrophy. Circ Res (2008) 103(9):1018–26. doi: 10.1161/CIRCRESAHA.108.178459
18. Wu J, Wei H, Qu H, Feng Z, Long J, Ge Q, et al. Plasma Vascular Endothelial Growth Factor B Levels Are Increased in Patients With Newly Diagnosed Type 2 Diabetes Mellitus and Associated With the First Phase of Glucose-Stimulated Insulin Secretion Function of β-Cell. J Endocrinol Invest (2017) 40(11):1219–26. doi: 10.1007/s40618-017-0677-z
19. Ye X, Kong W, Zafar MI, Zeng J, Yang R, Chen L-L. Plasma Vascular Endothelial Growth Factor B Is Elevated in Non-Alcoholic Fatty Liver Disease Patients and Associated With Blood Pressure and Renal Dysfunction. EXCLI J (2020) 19:1186–95. doi: 10.17179/excli2020-2647
20. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic Kidney Disease: A Report From an ADA Consensus Conference. Am J Kidney Dis (2014) 64(4):510–33. doi: 10.1053/j.ajkd.2014.08.001
21. Chen C, Wang C, Hu C, Han Y, Zhao L, Zhu X, et al. Normoalbuminuric Diabetic Kidney Disease. Front Med (2017) 11(3):310–8. doi: 10.1007/s11684-017-0542-7
22. Shimizu M, Furuichi K, Toyama T, Kitajima S, Hara A, Kitagawa K, et al. Long-Term Outcomes of Japanese Type 2 Diabetic Patients With Biopsy-Proven Diabetic Nephropathy. Diabetes Care (2013) 36(11):3655–62. doi: 10.2337/dc13-0298
23. Shimizu M, Furuichi K, Yokoyama H, Toyama T, Iwata Y, Sakai N, et al. Kidney Lesions in Diabetic Patients With Normoalbuminuric Renal Insufficiency. Clin Exp Nephrol (2014) 18(2):305–12. doi: 10.1007/s10157-013-0870-0
Keywords: vascular endothelial growth factor B, type 2 diabetes mellitus, diabetic kidney disease, glomerular filtration rate, cystatin C
Citation: Wei Y, Han S, Zhou R, Xu P, Zhou L, Zhu Z, Kan Y, Yang X, Xiang Y, Cao Y, Jin Y, Yan J, Yu X, Wang X and Shang W (2022) Increased Serum VEGF-B Level Is Associated With Renal Function Impairment in Patients With Type 2 Diabetes. Front. Endocrinol. 13:862545. doi: 10.3389/fendo.2022.862545
Received: 26 January 2022; Accepted: 02 March 2022;
Published: 24 March 2022.
Edited by:
Jehad Ahmed Abubaker, Dasman Diabetes Institute, KuwaitReviewed by:
Junfeng Han, Shanghai Jiao Tong University, ChinaCopyright © 2022 Wei, Han, Zhou, Xu, Zhou, Zhu, Kan, Yang, Xiang, Cao, Jin, Yan, Yu, Wang and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Shang, d2JzaGFuZ0BuanVjbS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.