
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 14 March 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.853494
This article is part of the Research TopicEndocrine and Metabolic Consequences of Childhood ObesityView all 16 articles
 Jiao Fang1
Jiao Fang1 Jingyi Yuan1
Jingyi Yuan1 Dandan Zhang1
Dandan Zhang1 Wanxu Liu1
Wanxu Liu1 Puyu Su1,2
Puyu Su1,2 Yuhui Wan2
Yuhui Wan2 Zhihua Zhang3
Zhihua Zhang3 Fangbiao Tao1,2
Fangbiao Tao1,2 Ying Sun1,2*
Ying Sun1,2*Background: There is an ongoing controversial issue regarding whether onset of puberty is related to childhood BMI.
Objectives: This study aims at investigating the causal association and its shape between prepuberty BMI and early puberty onset.
Methods: Breast development and testicular volume were assessed annually from a population-based prospective cohort of 997 children for consecutive years by professional endocrinologists. Seventeen puberty- and BMI-related SNPs were selected to calculate the polygenic risk score. The two-stage least square method was used to assess and confirm causal effects. A dose–response association between prepuberty BMI and early puberty onset was conducted by using restricted cubic spline Cox regression.
Results: After adjusting for covariates, prepuberty BMI was positively associated with early thelarche among girls (coefficients = 0.18, 95% CI: 0.01, 0.29). A non-linear model suggested an inverted U-shaped relationship between prepuberty BMI and risk for early thelarche (χ2 = 276.3, p < 0.001). The risk for early thelarche increased rapidly from prepuberty BMI at 15.70 kg/m2 (P25) to 20.75 kg/m2 (P85) and gradually decreased afterward. Compared with the P25 of prepuberty BMI, the HRs (95% CI) for early thelarche were 5.08 (1.15, 8.55), 4.48 (1.02, 7.74), 10.15 (3.93, 17.50), and 8.43 (1.91, 13.71) for percentiles P25–P50, P50–P75, P75–P85, and ≥P85 of BMI categories, respectively. In boys, compared with the P25 of prepuberty BMI, boys with BMI between P25 and P50 showed an increased risk of early puberty (HR: 3.94, 95% CI: 1.44, 6.80).
Conclusions: Prepuberty BMI may serve the purpose of identifying the girls at higher risk of early thelarche, which could assist in the adaptation of prevention and intervention strategies targeting childhood obesity. The findings emphasize a non-linear correlation between prepuberty BMI and early puberty onset.
Puberty is a milestone of life phase, with growth and development of all psychological and physiological systems, especially sexual maturation occurrence and reproductive capacity (1). From the middle of the 20th century until now, a temporal trend toward earlier puberty has been observed around the world (2, 3), although it is less certain for gonadarche in boys (4–6). This is a common concern, as early timing of puberty has a wide range of serious health complications, including increased risk of cardiometabolic diseases, depression, cancers, and possible obstetrical and gynecological problems (7, 8). During the same period, the prevalence of obesity in childhood and adolescence has increased substantially worldwide and become a major public health problem (9). Imperial London and the World Health Organization (WHO) reported a ten-fold increase in the number of adolescents and children with obesity aged 5 to 19 years (10). In developed countries during the years of recent economic crises (last decade), the rising trend in body mass index (BMI) or obesity in children and teenagers has been observed to plateau in high-income countries (11), and there is even a small amount of evidence suggesting a statistically significant reduction in overweight and obesity in Greek schoolchildren aged 6–16 years in both sexes (12) but which continues to increase in low- and middle-income countries (LMICs), especially in populous nations like China (11).
Some researchers have suggested the long-term trend of early puberty to be partly attributed to the rise in childhood obesity (13–15). Longitudinal epidemiological studies show that high BMI is related to earlier pubertal maturation in girls (16, 17), specifically that girls with obesity have earlier age at menarche and timing of thelarche than girls with normal weight (14, 15, 18, 19), while a few studies have not found this association (20). In boys, there have been fewer studies; some evidence reported a positive correlation between BMI or obesity and pubertal development in boys (5, 21–23), while some studies indicate a negative association or fail to find any associations (24, 25).
Mendelian randomization (MR) is an alternative means of assessing the causal effect of childhood BMI on early puberty, designed as a quasi-experimental study that is less susceptible to confounding effects. However, to date, applications of MR have been limited to linear models for the associations between exposure and outcome. A clear determination of the shape in the casual relation between childhood BMI and early onset of puberty would elucidate the comparative relevance of higher and lower BMI values on risk for early onset of puberty.
Based on a 4-year prospective cohort with annual objective assessment of pubertal development, the present study aims at validating the causal association and its shape between prepuberty BMI and early onset of puberty in both sexes, by using single-sample MR as well as restricted cubic spline Cox regression.
As illustrated in Supplementary Figure A1, the perspective puberty cohort is a database containing questionnaires and physical examination information on nearly a thousand school-age children from 2 elementary schools, which was established since March 2016 by clustering convenience sampling in Bengbu, Anhui province, China.
All children underwent a questionnaire survey and physical and pubertal development assessment annually. In 2017, buccal cheek swabs were collected from all the children for DNA extraction and genotyping. The final analysis sample of children who had complete and effective data on BMI status, breast Tanner stage, testicular volume, and genetic susceptibility included 997 children (579 girls and 418 boys) aged 9.33 to 12.17 years in the last follow-up (for detailed information, see Sun et al., 2019) (26). The exclusion criteria were as follows: children with organic or chronic diseases that could affect puberty, or those taking oral or inhaled glucocorticoids or human growth hormone, were excluded in this cohort. Ethical approval for this study was obtained from the Institutional Review Board at Anhui Medical University (No. 20180082). Informed consent for all collected data was obtained from parents and schoolteachers, as well as children.
Annual breast Tanner assessments were classified between 1 (prepubescent state) and 5 (full sexual maturity state) (27, 28) through both observation and palpation in primary school while testicular volume was estimated by using a Prader orchidometer by trained pediatric endocrinologists. Pubertal onset was defined as attaining breast Tanner stage 2 (B2, thelarche), or testicular volume more than 3 ml (TV4). The 25th percentile was adopted as the cutoff point (P25 age) for early onset of thelarche and testicular development in Chinese children based on data from the “China Puberty Collaboration Study (29, 30),” which was age at B2 < 8.0 y for girls and age at TV4 ml < 9.7 y (TV3 ml < 8.67 y) for boys.
In the annual follow-up survey, height and weight were measured by trained and certified medical staff. Body height was examined with light clothing to the nearest 0.1 cm by using a portable stadiometer, and weight with an electronic scale (Tanita TI1618) to the nearest 0.1 kg. BMI is a measurement of height-adjusted measure of weight, calculated as weight (in kilograms) divided by the square of height (in meters) (kg/m2). Childhood BMI was derived from all paired height and weight measurements in the age of 6.5 to 12.5 years and age adjusted to 9 years by using a linear regression model. Moreover, it was classified according to percentiles (< P10, P25–P50, P50–P75, P75–P85, and ≥ P85).
Genomic DNA for all children had been extracted from buccal cheek swabs following a standard protocol. PCR-RFLP and real-time PCR were used to extract DNA and then genotyped in the Sequenom MassARRAY. The mean concordance rates of the genotyping system were 98.8%.
The present study identified 11 and 21 single-nucleotide polymorphisms (SNPs) associated with obesity and early puberty at genome-wide significance in the GWAS datasets from 87,802 women and 35,668 children of European ancestry, respectively (31–33). After excluding five SNPs (rs35327298, rs142058842, and rs4237264 for early puberty; s7550711 and rs13387838 for obesity) with MAFs < 5%, three SNPs (rs5932886 for early puberty; rs1310484 and rs987237 for obesity) had a genotyping rate <10%. Additionally, we have imputed genotype data based on the CHB HapMap data (Phase 2 and Phase 3) and further verified that loci were not in a significant linkage disequilibrium (LD) with each other (r2 < 0.3) in the SNP server and Han Chinese (CHB) data. Moreover, 17 puberty-related SNPs and 7 BMI-related SNPs were retained in the final analysis and the polygenic risk score (PRS) was calculated, as shown in the following formula, respectively:
Each SNP was recorded as 0, 1, and 2 according to number of effect alleles (e.g., if the effect allele is T, then TT = 2, CT = 1, CC = 0).
For assessment of potential confounding factors, the current analysis included delivery mode (vaginal or caesarean section), gestational age (>37 weeks or ≤37 weeks), birthweight, and infancy feeding mode (included exclusive breastfeeding, mixed feeding, and formula feeding), as well as parental BMI, education, and household monthly income (obtained from the parents’ questionnaire at baseline).
Statistical calculations were performed with Stata (version 14.0) and R version 3.6.2. We used instrumental variable methods to estimate the causal effect between BMI and early onset of puberty through polygenic risk using the Mendelian randomization design. Instrumental variable regression with two-stage least-square (2SLS) methods was performed using the BMI-related PRS as the instrument (34). MR–Egger is often used in sensitivity analysis, i.e., the causality between obesity and early onset of puberty. The shape of the relationship between prepuberty BMI and early onset of puberty was established by using restricted cubic spline (RCS) Cox regression (35) based on five knots (P5, P25, P50, P75, and P95) of prepuberty BMI and reference BMI of 15.70 kg/m2 (P25) and 16.10 kg/m2 (P25) in girls and boys, respectively, generating hazard ratios (HR) [95% confidence intervals (CI)]. Furthermore, we analyzed the association between prepuberty BMI and early puberty by using categorical BMI (BMI < P10; P25–P50, P50–P75, P75–P85, and ≥ P85 kg/m2) with BMI at P10–P25 as reference.
Final models were adjusted for age, BMI- and puberty-related PRS, parental BMI, parental education, family income, birthweight, delivery mode, infant feeding, and gestational age. 0.05 was defined as a significance threshold of two-tailed p values.
The average cohort follow-up rate from Wave 2 to Wave 4 was more than 90%. Among the 997 children in this cohort, 58% were female, and the mean age was 8.01 (SD, 0.85) years at baseline. The average BMI genetic score (7 SNPs) was 4.61 ± 1.40, 15.32 ± 2.47 for puberty genetic score (17 SNPs), as presented in Supplementary Table A1.
Approximately one-fifth of children were classified as overweight and obese at each wave, respectively. Table 1 shows adiposity and pubertal development in boys and girls during the 4-year follow-up. At wave 1 to wave 4, approximately 9.8%, 14.5%, 22.7%, and 24.5% of girls presented early onset of thelarche, and 0.7%, 1.0%, 6.3%, and 9.9% of boys had early onset of testicular development, respectively.
The coefficients of the bidirectional MR analysis are presented in Table 2. The BMI-PRS created from 7 SNPs showed a positive association with prepuberty BMI (coefficient = 0.17, 95% CI: 0.02, 0.33; p = 0.031). The BMI-PRS served as a strong instrument for adiposity, with F statistics of 2.424. The results of 2SLS analysis revealed that prepuberty BMI was associated with early thelarche onset in girls (coefficient = 0.18, 95% CI: 0.01, 0.29; p = 0.005). In MR sensitivity analyses, the IVW, Egger, and Median methods provided results similar to those of the 2SLS analysis of early puberty based on TV4 assessment (Supplementary Table A2). No causal association was observed between prepuberty BMI and early onset of testicular development in boys (coefficient = 0.07, 95% CI: -0.08, 1.92; p = 0.113). Furthermore, we also performed an MR sensitivity analysis based on early puberty in boys assessed by TV3, and again no significant association was observed (coefficient = 0.08, 95% CI: -0.07, 1.88; p = 0.365) (Supplementary Table A3).
As illustrated in Figure 1, the restricted cubic spline shows that compared to reference groups (prepuberty BMI = 15.70 kg/m2, P25), girls with elevated percentile of prepuberty BMI before the peak risk point (prepuberty BMI = 20.75 kg/m2) are at increased risk for earlier onset of thelarche. After that, the risk for earlier onset of thelarche decreases with a continued increase in prepuberty BMI (pnon-linear < 0.001).
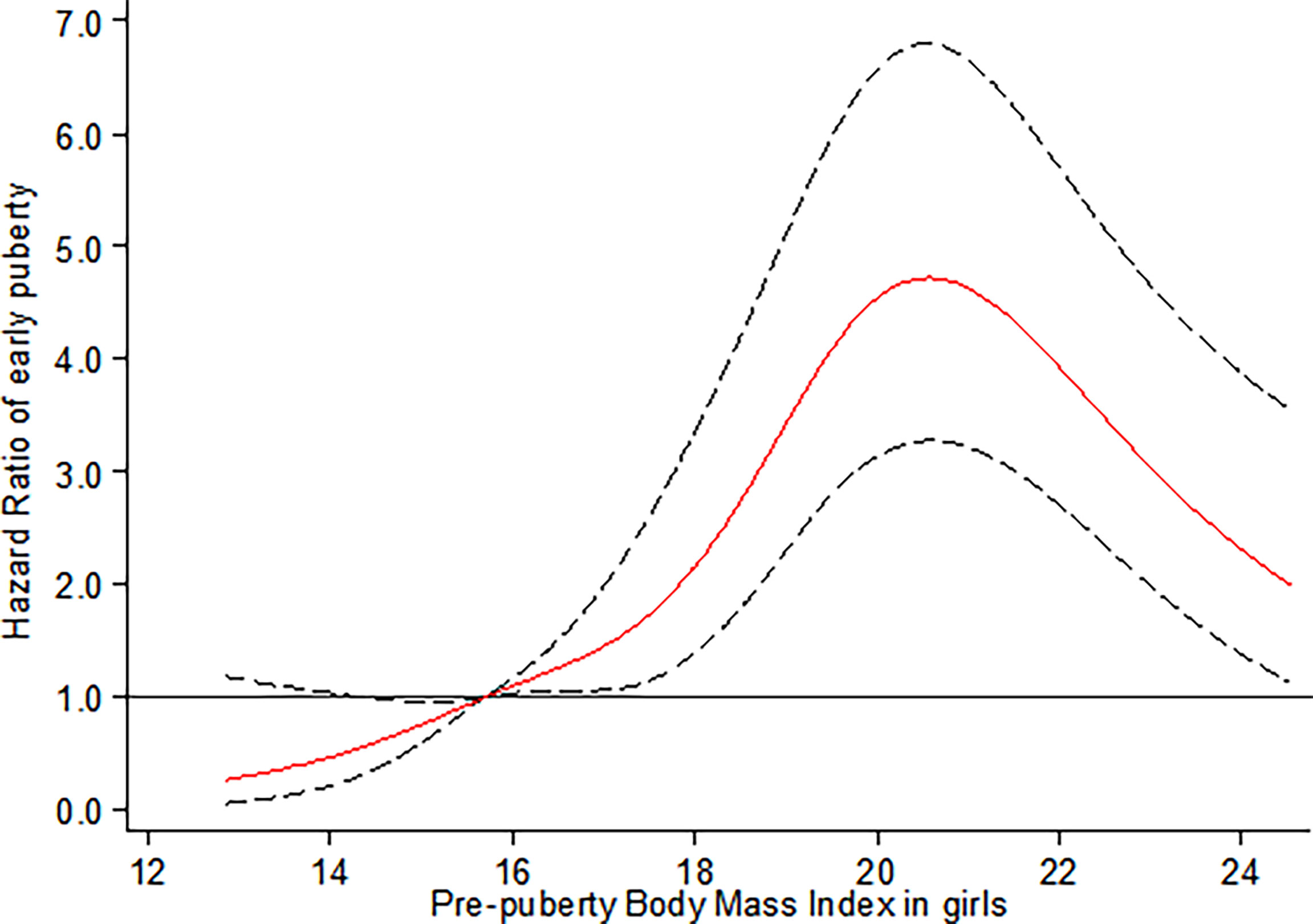
Figure 1 Restricted cubic spline for the association between prepuberty BMI and the HR for early breast development among girls. The curves are based on restricted cubic spline Cox regression with five knots of BMI and a reference BMI of 15.70 kg/m2 (P25). Individuals with BMI below the 1st or above the 99th percentiles were excluded. Analyses were adjusted for age, BMI- and puberty-related PRS, birthweight, delivery mode, infant feeding, family income, parental BMI, and education.
For boys, a higher risk of earlier onset of testicular development with prepuberty BMI was observed in the range of 16.10 kg/m2 (P25) to 17.35 kg/m2 (P40), compared to the reference group (prepuberty BMI = 16.10 kg/m2, pnon-linear < 0.001) (Figure 2).
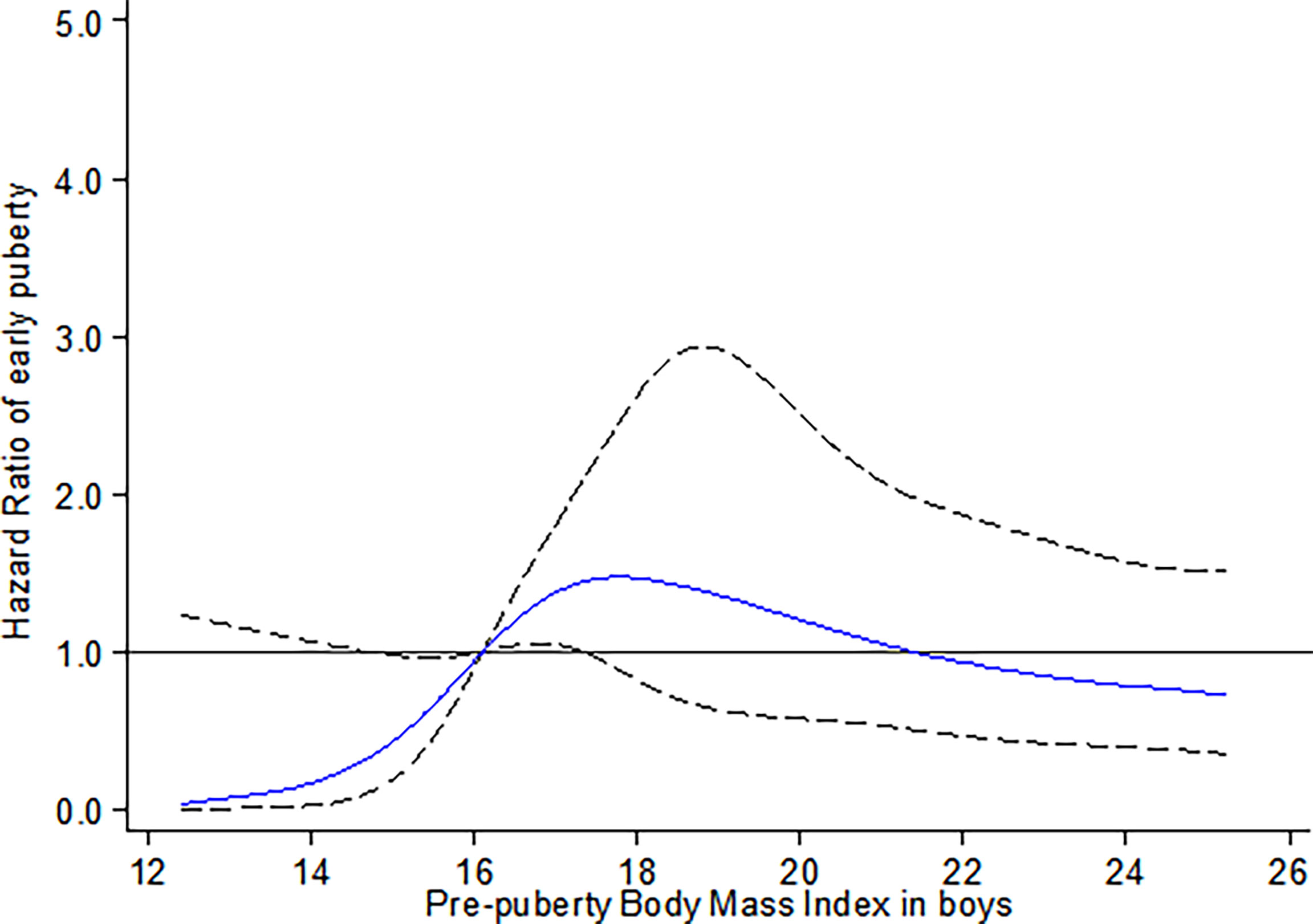
Figure 2 Restricted cubic spline for the association between prepuberty BMI and the HR for early testicular development among boys. The curves are based on restricted cubic spline Cox regression with five knots of BMI and a reference BMI of 16.10 kg/m2 (P25). Individuals with BMI below the 1st or above the 99th percentiles were excluded. Analyses were adjusted for age, BMI- and puberty-related PRS, birthweight, delivery mode, infant feeding, family income, parental BMI, and education.
Table 3 presents the relationship between categories of prepuberty BMI and risk of early onset of puberty in both sexes. Compared with the reference group (P10–P25), the HRs (95% CI) for early thelarche were 5.08 (1.15, 8.55), 4.48 (1.02, 7.74), 10.15 (3.93, 17.50), and 8.43 (1.91, 13.71) for BMI categories at P25–P50, P50–P75, P75–P85, and ≥P85, respectively, but not in the BMI < P10 group. In boys, compared with the reference (P10–P25), only boys at P25–P50 of BMI categories showed an increased risk of early onset of testicular development [3.94 (1.44, 6.80)].
To our knowledge, this study is the first prospective longitudinal cohort study simultaneously evaluating the causal relationship and its shape between prepuberty BMI with early puberty in both genders. By using Mendelian randomization analysis, we provided robust evidence to support the causal association between increased prepubertal BMI with early onset of thelarche in girls. However, a similar association was not confirmed in boys. For non-linear relations, an inverted U-shaped curve was observed between prepuberty BMI and earlier onset of thelarche. Specifically, girls with elevated percentile of prepuberty BMI before the peak risk point (20.75 kg/m2) are at increased risk for earlier onset of thelarche. After that, the risk for early thelarche decreases with a continued increase in BMI. In contrast, for boys, the risk for early onset of testicular development was observed with the prepuberty BMI in the range of 25th to 40th percentile, equal to 16.10 kg/m2 to 17.35 kg/m2, respectively.
Previous longitudinal epidemiological studies have shown that high childhood BMI is related to earlier pubertal maturation in girls (16–19), while potential correlations among boys are controversial (20–25). Additionally, although BMI and timing of puberty are known to be closely linked, the causality and its effects between these traits in boys and girls remain poorly studied (36).
Our finding of MR analysis in girls is consistent with results from the Taiwan Children Health Study (TCHS) (16), supporting the causal effects of higher adiposity accumulation during childhood on earlier onset of puberty. However, TCHS is limited by using a self-reported questionnaire to define breast Tanner stages. Their measurements of pubertal development starting at ages 11 and 12 lead to underestimation of the true age at pubertal onset in girls. In comparison, our study was based on a 4-year cohort with objective annual breast development assessment since childhood (around 6–8 years) and no more than 2% of girls enrolled at baseline initiated puberty, helping to observe and capture the process of pubertal onset.
The results of the present MR analysis indicated no casual association between prepuberty BMI and early pubertal onset in boys, which is inconsistent with two previous MR studies in boys. The Copenhagen Puberty Study (2006–2014) using a mixed longitudinal cohort (n = 93) and cross-sectional study (n = 637) of 730 healthy Danish boys determined the possible causal link between higher BMI and earlier timing of voice break in boys (37). A longitudinal analysis from the Taiwan Children Health Study (TCHS) also revealed that prepuberty BMI (overweight/obesity) predicted early onset of self-reported pubertal development among male adolescents (17). The heterogeneities could be largely explained by the differences in male pubertal assessment. Although voice break, as a measure of puberty, is frequently used in epidemiological studies, it represents a late pubertal milestone and the validity of self-reported voice break remains a question. As male pubertal onset is manifested by the gradual enlargement of genital and testicular size, direct measurement of testicular volume by palpation is likewise preferable to recalled age at voice break, providing a more accurate estimate of age at attaining the milestones of puberty for boys.
The current study, herein, further extended the non-linear dose–response relationship between prepuberty BMI and onset of puberty by using the restricted cubic spline (RCS) Cox regression model of five knots and indicated a significant non-linear dose–response association of prepubertal BMI with early onset of puberty in both genders. Our finding of an inverted U-shaped correlation with an inflection point in the risk function at 20.75 kg/m2 (equal to BMI threshold for obesity at 9 years of age) in girls further complements existing evidence of non-linear associations between prepubertal BMI with early onset of puberty. This is in line with conclusions from the Danish National Birth Cohort (DNBC) and sibling-matched study (38). The DNBC study indicated that childhood overweight (between 85th and 95th percentiles of BMI) and obesity (≥ 95th percentile of BMI ) were associated with earlier puberty timing (self-reported pubertal milestones) in both sexes in a dose-dependent manner by using restricted cubic splines with three knots. A confirmatory analysis of the association was conducted in a sibling-matched study of 1,700 brothers of DNBC, and it reported that a higher BMI was associated with earlier age at attaining most milestones of puberty among girls, but only a tendency toward earlier timing of puberty was observed in boys. Despite the small sample size of the present study, our results highlight the association between objectively assessed BMI and early breast development, which is more convincing than parental report data in the DNBC study.
Our findings in boys supported the results of Bygdell et al. (39) and a DNBC sibling-matched study (38), suggesting that the risk for early testicular development increased with an increasing prepuberty BMI within the range of 16.10 to 17.35 kg/m2 (equivalent to the range of normal weight). Bygdell et al. (39) demonstrated that prepubertal BMI associated with early timing of puberty (indicated by age at PHV) in normal-weight but not overweight boys. However, the piecewise linear regression model used in their study cannot observe the shape of the non-linear correlation on both sides of the threshold.
The possible mechanisms and relevance of our present findings in terms of sex divergence of early pubertal timing by prepubertal BMI merit further investigation. Understanding of the neurobiological basis of puberty in general, especially the underlying mechanisms for its metabolic regulation in particular, has substantially expanded in recent years. Sanchez Garrido et al. (40) evaluated sexually dimorphic responses in a metabolic programming of puberty to nutritional challenges in rats of both gender. Their study found that male puberty is more sensitive to postnatal nutritional stress (overfeeding) and female puberty is more vulnerable to peripubertal nutritional stress (high-fat feeding) (41). On the other hand, overfeeding before and after puberty leads to an increase in hypothalamic Kiss1 expression and advances the onset of puberty in female rats, whereas sustained excess energy and obesity are associated with inhibition of Kiss1 expression and lower expression levels of key limiting factors of testicular steroidogenesis in male rodents (42).
Human studies further note that excessive adiposity, in the absence of substantial sex steroid surge, may partly suppress hypothalamic–pituitary–gonadal function in girls with earlier puberty, although it is known to accelerate pubertal onset, which indirectly elucidated the decreased risk of early pubertal onset in obese girls than overweight ones, but the mechanism is unclear in boys. Future population-based studies to better characterize the association between body fat and onset of puberty in boys are needed and will require focus on the neuroendocrine basis of the physiological control of puberty and its deviations, as well as epigenetic regulation and metabolic cues, to better understand the mechanisms that trigger pubertal initiation and progression in both sexes.
Although the present study is based on a rigorous longitudinal design with repeated objective assessment of puberty, there are some limitations that should be acknowledged. First, this study was conducted among Chinese children; differences between race/ethnicities should be considered for generalizing our findings. Secondly, our analysis used BMI as the primary indicator to measure child obesity, which may not fully reflect body fat level as its calculation only considers height and weight. Further studies are recommended using skinfold thickness or other measures such as dual-energy X-ray absorptiometry to examine the association between body fat and timing of puberty. Third, we only considered the prepubertal BMI of children, which can explain the effects of prepubertal weight status on early puberty; it is necessary to clarify the influence of weight changes during follow-up on puberty in future analysis. In addition, due to the small size of sample, the number of our candidate SNPs representing exposure and outcome phenotype were relatively small compared with large GWAS, which may affect the stable and reasonable estimates of the MR model. Therefore, we also did a further sensitivity analysis using MR–Egger. Given the difficulty of conducting population surveys, another limitation of this study would be that there has been no evaluation of the individual growth charts and the children’s growth patterns related to their target heights and their bone age of course combined at least with basal LH < 08:00 h. Furthermore, although there seems to be no evidence of a secular trend for gonadarche in boys, such a trend seems to be evident for pubarche, and future studies should consider the effect of obesity on adrenarche, especially in boys (4). Some evidence indicated that the physical changes of puberty require a concerted effort from many organs and that these changes are independent of each other, although adrenal maturation often coincides with HPG axis maturation; it is important to note that pubarche itself is not the best indicator of pubertal development. Finally, nearly half of the boys in the cohort had not reached puberty yet at the last follow-up, which may have reduced the power of studies.
The current study provided robust evidence on the casual and inverted U-shaped relationship between prepubertal BMI with early thelarche in girls. For boys, although no similar causal association was observed, our finding identified a non-linear relationship between BMI and early onset of testicular development in normal-weight boys. Further studies are warranted to elucidate the mechanisms behind the observed associations, which might aid future interventional studies targeting the prevention of childhood obesity and precocious puberty.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
We secured approval from the Institutional Review Boards at Anhui Medical University (No. 20180082). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
YS, PS, YW, and ZZ conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. JF, JY, DZ, and WL designed the data collection instruments, collected the data, carried out the initial analyses, and reviewed and revised the manuscript. FT conceptualized and designed the study, coordinated and supervised the data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
This work was funded from the National Natural Science Foundation of China (grant number 82173537), National Natural Science Foundation of China (grant number 81872638), Scientific Promotion Project of Anhui Medical University (grant number 2021xkjT012), and Natural Science Foundation of Anhui Province for Distinguished Young Scholars (grant number 1908085J26).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the participants for their dedication and contribution to the research.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.853494/full#supplementary-material
1. Patton GC, Viner R. Pubertal Transitions in Health. Lancet (2007) 369:1130–9. doi: 10.1016/S0140-6736(07)60366-3
2. Ong KK, Ahmed ML, Dunger DB. Lessons From Large Population Studies on Timing and Tempo of Puberty (Secular Trends and Relation to Body Size): The European Trend. Mol Cell Endocrinol (2006) 254-255:8–12. doi: 10.1016/j.mce.2006.04.018
3. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The Timing of Normal Puberty and the Age Limits of Sexual Precocity: Variations Around the World, Secular Trends, and Changes After Migration. Endocr Rev (2003) 24(5):668–93. doi: 10.1210/er.2002-0019
4. Papadimitriou A, Douros K, Kleanthous K, Papadimitriou DT, Attilakos A, Fretzayas A. Pubertal Maturation of Contemporary Greek Boys: No Evidence of a Secular Trend. J Adolesc Health (2011) 49(4):434–6. doi: 10.1016/j.jadohealth.2010.12.022
5. Chen C, Zhang Y, Sun W, Chen Y, Jiang Y, Song Y, et al. Investigating the Relationship Between Precocious Puberty and Obesity: A Cross-Sectional Study in Shanghai, China. BMJ Open (2017) 7(4):e014004. doi: 10.1136/bmjopen-2016-014004
6. Juul A, Magnusdottir S, Scheike T, Prytz S, Skakkebaek NE. Age at Voice Break in Danish Boys: Effects of Pre-Pubertal Body Mass Index and Secular Trend. Int J Androl (2007) 30:537–42. doi: 10.1111/j.1365-2605.2007.00751.x
7. Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty Timing Associated With Diabetes, Cardiovascular Disease and Also Diverse Health Outcomes in Men and Women: The UK Biobank Study. Sci Rep (2015) 5:11208. doi: 10.1038/srep11208
8. Graber JA. Pubertal Timing and the Development of Psychopathology in Adolescence and Beyond. Horm Behav (2013) 64:262–69. doi: 10.1016/j.yhbeh.2013.04.003
9. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults During 1980-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8
10. NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Bod Mass Index, Underweight, Overweight, and Obesity From 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies In128.9 Million Children, Adolescents, and Adults. Lancet (2017) 390:2627–42. doi: 10.1016/S0140-6736(17)32129-3
11. Fang J, Gong C, Wan Y, Xu Y, Tao F, Sun Y. Polygenic Risk, Adherence to a Healthy Lifestyle, and Childhood Obesity. Pediatr Obes (2019) 14(4):e12489. doi: 10.1111/ijpo.12489
12. Kleanthous K, Dermitzaki E, Papadimitriou DT, Papaevangelou V, Papadimitriou A. Overweight and Obesity Decreased in Greek Schoolchildren From 2009 to 2012 During the Early Phase of the Economic Crisis. Acta Paediatr (2016) 105(2):200–5. doi: 10.1111/apa.13143
13. Ebbeling CB, Pawlak DB, Ludwig DS. Childhood Obesity: Public-Health Crisis, Common Sense Cure. Lancet (2002) 360:473–82. doi: 10.1016/S0140-6736(02)09678-2
14. Biro FM, Khoury P, Morrison JA. Influence of Obesity on Timing of Puberty. Int J Androl (2006) 29:272–77;discussion 86-90. doi: 10.1111/j.1365-2605.2005.00602.x
15. Ahmed ML, Ong KK, Dunger DB. Childhood Obesity and the Timing of Puberty. Trends Endocrinol Metab (2009) 20:237–42. doi: 10.1016/j.tem.2009.02.004
16. Chen YC, Fan HY, Yang C, Hsieh RH, Pan WH, Lee YL. Assessing Causality Between Childhood Adiposity and Early Puberty: A Bidirectional Mendelian Randomization and Longitudinal Study. Metabolism (2019) 100:153961. doi: 10.1016/j.metabol.2019.153961
17. Lee Y. Is Obesity Associated With Early Sexual Maturation? A Comparison of the Association in American Boys Versus Girls. Pediatrics (2002) 110:903–10. doi: 10.1542/peds.110.5.903
18. Juul F, Chang VW, Brar P, Parekh N. Birth Weight, Early Life Weight Gain and Age at Menarche: A Systematic Review of Longitudinal Studies. Obes Rev (2017) 18:1272–88. doi: 10.1111/obr.12587
19. Li WY, Liu Q, Deng X, Chen YW, Liu SD, Story M. Association Between Obesity and Puberty Timing: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health (2017) 14(10):1266. doi: 10.3390/ijerph14101266
20. Mouritsen A, Aksglaede L, Soerensen K, Hagen CP, Petersen JH, Main KM, et al. The Pubertal Transition in 179 Healthy Danish Children: Associations Between Pubarche, Adrenarche, Gonadarche, and Body Composition. Eur J Endocrinol (2012) 168(2):129–36. doi: 10.1530/EJE-12-0191
21. Buyken AE, Karaolis-Danckert N, Remer T. Association of Pre-Pubertal Body Composition in Healthy Girls and Boys With the Timing of Early and Late Pubertal Markers. Am J Clin Nutr (2009) 89:221–30. doi: 10.3945/ajcn.2008.26733
22. Sorensen K, Aksglaede L, Petersen JH, Juul A. Recent Changes in Pubertal Timing in Healthy Danish Boys: Associations With Body Mass Index. J Clin Endocrinol Metab (2010) 95:263–70. doi: 10.1210/jc.2009-1478
23. Lundeen EA, Norris SA, Martorell R, Suchdev PS, Mehta NK, Richter LM, et al. Early Life Growth Predicts Pubertal Development in South African Adolescents. J Nutr (2016) 146:622–29. doi: 10.3945/jn.115.222000
24. Lee JM, Kaciroti N, Appugliese D, Corwyn RF, Bradley RH, Lumeng JC. Body Mass Index and Timing of Pubertal Initiation in Boys. Arch Pediatr Adolesc Med (2010) 164:139–44. doi: 10.1001/archpediatrics.2009.258
25. Lee JM, Wasserman R, Kaciroti N, Gebremariam A, Steffes J, Dowshen S, et al. Timing of Puberty in Overweight Versus Obese Boys. Pediatrics (2016) 137(2):e20150164. doi: 10.1542/peds.2015-0164
26. Sun Y, Fang J, Wan Y, Su P, Tao F. Role of Polygenic Risk in Susceptibility to Accelerated Pubertal Onset Following Chronic Stress Exposure. Eur J Endocrinol (2019) 181(2):129–37. doi: 10.1530/EJE-19-0033
27. Marshall WA, Tanner JM. Variations in Pattern of Pubertal Changes in Girls. Arch Dis Child (1969) 44(235):291–303. doi: 10.1136/adc.44.235.291
28. Marshall WA, Tanner JM. Variations in the Pattern of Pubertal Changes in Boys. Arch Dis Child (1970) 45(239):13–23. doi: 10.1136/adc.45.239.13
29. Sun Y, Tao FB, Su PY, Mai JC, Shi HJ, Han YT, et al. National Estimates of the Pubertal Milestones Among Urban and Rural Chinese Girls. J Adolesc Health (2012) 51(3):279–84. doi: 10.1016/j.jadohealth.2011.12.019
30. Sun Y, Tao F, Su PY, China Puberty Research Collaboration. National Estimates of Pubertal Milestones Among Urban and Rural Chinese Boys. Ann Hum Biol (2012) 39(6):461–7. doi: 10.3109/03014460.2012.712156
31. Elks CE, Perry JR, Sulem P, Chasman DI, Franceschini N, He C, et al. Thirty New Loci for Age at Menarche Identified by a Meta-Analysis of Genome-Wide Association Studies. Nat Genet (2010) 42(12):1077–85. doi: 10.1038/ng.714
32. Ong KK, Elks CE, Li S, Zhao JH, Luan J, Andersen LB, et al. Genetic Variation in LIN28B Is Associated With the Timing of Puberty. Nat Genet (2009) 41(6):729–33. doi: 10.1038/ng.382
33. Felix JF, Bradfield JP, Monnereau C, van der Valk RJ, Stergiakouli E, Chesi A, et al. Genome-Wide Association Analysis Identifies Three New Susceptibility Loci for Childhood Body Mass Index. Hum Mol Genet (2016) 25:389–403. doi: 10.1093/hmg/ddv472
34. Sjolander A, Dahlqwist E, Martinussen T. Ivtools: Instrumental Variables. Technical Report (2019). Available at: https://CRAN.R-project.org/package=ivtools. R package version 2.2. 0, 2019.
35. Durrleman S, Simon R. Flexible Regression Models With Cubic Splines. StatMed (1989) 8:551–61. doi: 10.1002/sim.4780080504
36. Reinehr T, Roth CL. Is There a Causal Relationship Between Obesity and Puberty? . Lancet Child Adolesc Health (2019) 3(1):44–54. doi: 10.1016/S2352-4642(18)30306-7
37. Busch AS, Hollis B, Day FR, Sørensen K, Aksglaede L, Perry JRB, et al. Voice Break in Boys-Temporal Relations With Other Pubertal Milestones and Likely Causal Effects of BMI. Hum Reprod (2019) 34(8):1514–22. doi: 10.1093/humrep/dez118
38. Brix N, Ernst A, Lauridsen LLB, Parner ET, Arah OA, Olsen J, et al. Childhood Overweight and Obesity and Timing of Puberty in Boys and Girls: Cohort and Sibling-Matched Analyses. Int J Epidemiol (2020) 49(3):834–44. doi: 10.1093/ije/dyaa056
39. Bygdell M, Kindblom JM, Celind J, Nethander M, Ohlsson C. Childhood BMI is Inversely Associated With Pubertal Timing in Normal-Weight But Not Overweight Boys. Am J Clin Nutr (2018) 108(6):1259–63. doi: 10.1093/ajcn/nqy201
40. Sanchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Garcia-Galiano D, Castaño JP, et al. Obesity-Induced Hypogonadism in the Male: Premature Reproductive Neuroendocrine Senescence and Contribution of Kiss1-Mediated Mechanisms. Endocrinology (2014) 155(3):1067–79. doi: 10.1210/en.2013-1584
41. Sánchez-Garrido MA, Castellano JM, Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, Leon S, et al. Metabolic Programming of Puberty: Sexually Dimorphic Responses to Early Nutritional Challenges. Endocrinology (2013) 154(9):3387–400. doi: 10.1210/en.2012-2157
42. Castellano JM, Bentsen AH, Sánchez-Garrido MA, Ruiz-Pino F, Romero M, Garcia-Galiano D, et al. Early Metabolic Programming of Puberty Onset: Impact of Changes in Postnatal Feeding and Rearing Conditions on the Timing of Puberty and Development of the Hypothalamic Kisspeptin System. Endocrinology (2011) 152(9):3396–408. doi: 10.1210/en.2010-1415
Keywords: body mass index, puberty, Mendelian randomization, causal effects, restricted cubic spline
Citation: Fang J, Yuan J, Zhang D, Liu W, Su P, Wan Y, Zhang Z, Tao F and Sun Y (2022) Casual Associations and Shape Between Prepuberty Body Mass Index and Early Onset of Puberty: A Mendelian Randomization and Dose–Response Relationship Analysis. Front. Endocrinol. 13:853494. doi: 10.3389/fendo.2022.853494
Received: 12 January 2022; Accepted: 10 February 2022;
Published: 14 March 2022.
Edited by:
Malgorzata Wojcik, Jagiellonian University Medical College, PolandReviewed by:
Dimitrios T. Papadimitriou, National and Kapodistrian University of Athens, GreeceCopyright © 2022 Fang, Yuan, Zhang, Liu, Su, Wan, Zhang, Tao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Sun, yingsun@ahmu.edu.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.