- 1Department of Clinical and Molecular Medicine, Sapienza University of Rome, Rome, Italy
- 2Laboratory of Biomedical Research, Niccolò Cusano University Foundation, Rome, Italy
- 3Unit of Medical Oncology, Sant’Andrea University Hospital, Rome, Italy
- 4Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy
- 5Department of Surgical and Medical Science and Translational Medicine, Sapienza University of Rome, Rome, Italy
- 6Health Management Director, Sant’Andrea University Hospital, Rome, Italy
- 7Scientific Direction, IRCCS Regina Elena National Cancer Institute, Rome, Italy
- 8Unit of Anesthesia, Intensive Care and Pain Medicine, Sant’Andrea University Hospital, Rome, Italy
- 9Department Medical and Surgical Sciences and Biotechnologies, Sapienza University of Rome, Polo Pontino, Latina, Italy
Background and Objective: Nonthyroidal Illness Syndrome (NTIS) occurs in approximately 70% of patients admitted to Intensive Care Units (ICU)s and has been associated with increased risk of death. Whether patients with NTIS should receive treatment with thyroid hormones (TH)s is still debated. Since many interventional randomized clinical trials (IRCT)s were not conclusive, current guidelines do not recommend treatment for these patients. In this review, we analyze the reasons why TH treatment did not furnish convincing results regarding possible beneficial effects in reported IRCTs.
Methods: We performed a review of the metanalyses focused on NTIS in critically ill patients. After a careful selection, we extracted data from four metanalyses, performed in different clinical conditions and diseases. In particular, we analyzed the type of TH supplementation, the route of administration, the dosages and duration of treatment and the outcomes chosen to evaluate the results.
Results: We observed a marked heterogeneity among the IRCTs, in terms of type of TH supplementation, route of administration, dosages and duration of treatment. We also found great variability in the primary outcomes, such as prevention of neurological alterations, reduction of oxygen requirements, restoration of endocrinological and clinical parameters and reduction of mortality.
Conclusions: NTIS is a frequent finding in critical ill patients. Despite several available IRCTs, it is still unclear whether NTIS should be treated or not. New primary endpoints should be identified to adequately validate the efficacy of TH treatment and to obtain a clear answer to the question raised some years ago.
Introduction
Almost fifteen years ago Robin P. Peeters (1) raised the following question: should we treat Nonthyroidal Illness Syndrome (NTIS) or not? We still do not have a definite answer to this question. In Intensive Care Units (ICU)s, this syndrome occurs rather frequently as a complication of critical diseases affecting virtually all systems and organs. NTIS can be diagnosed in up to 70% of the critically ill patients (2–5) of all ages, including preterm neonates, term infants, children and adults (6). NTIS, observed in ICU in association with all these conditions, has a negative prognostic impact on the course of the disease with a relevant increased risk of death (7–10). Moreover, it represents an independent predictor of short- and long-term survival in patients with myocardial infarction, heart failure, or acute stroke also outside the ICU setting (11). This syndrome occurs frequently in COVID-19 patients too (12). We studied COVID-19 patients during two pandemic waves (13–16). Although it has been reported that patient’s characteristics, such as age, sex, occurrence of comorbidities, symptoms and survival time largely differed during the different phases of the pandemic in Italy (17), we found a similar frequency of ~ 60% of NTIS in our patients during two pandemic waves (18, 19). As reported in other critical illness, NTIS was associated with a more severe disease and a higher rate of lethality (18–20).
Even if patients with NTIS show a clear and marked reduction in the serum FT3 levels and, possibly, in the serum FT4 levels too, there is great uncertainty about the benefit of thyroid hormone (TH) replacement therapy. This could also be related to the fact that the syndrome has “different faces” (21). According to L. De Groot, there are many possible conceptual hypotheses to explain the physiological basis of NTIS (22), including TH test artifacts, inhibitors of T4 binding to proteins or to nuclear T3 receptors and enhanced local deiodination activity. However, the question whether the NTIS represents a beneficial, protective and appropriate adaptive response to stress, critical illness and malnutrition or it should be considered a maladaptive response to illness that requires correction still remains unanswered (23). We still do not have convincing data regarding any potential benefit due to TH treatment. Therefore, treatment with TH is not recommended in the absence of clinical signs of hypothyroidism (24). Although the administration of low doses of TH has not been linked to any harm (25–28), there is lack of convincing evidences regarding any clinical benefit (29). One of the reasons could be due to the use of inadequate outcome indicators.
We reviewed the data reported in metanalyses, regarding the interventional randomized clinical trials (IRCT)s aimed to demonstrate the benefit of the treatment with THs in NTIS associated with different conditions and diseases. Conflicting results obtained indicate that more adequate and pathogenic primary outcomes are needed. The possible use of new better endpoints, such as the hydration parameters measured by Bioelectrical Impedance Analysis (BIA) is discussed.
Methods
Literature Search and Study Selection
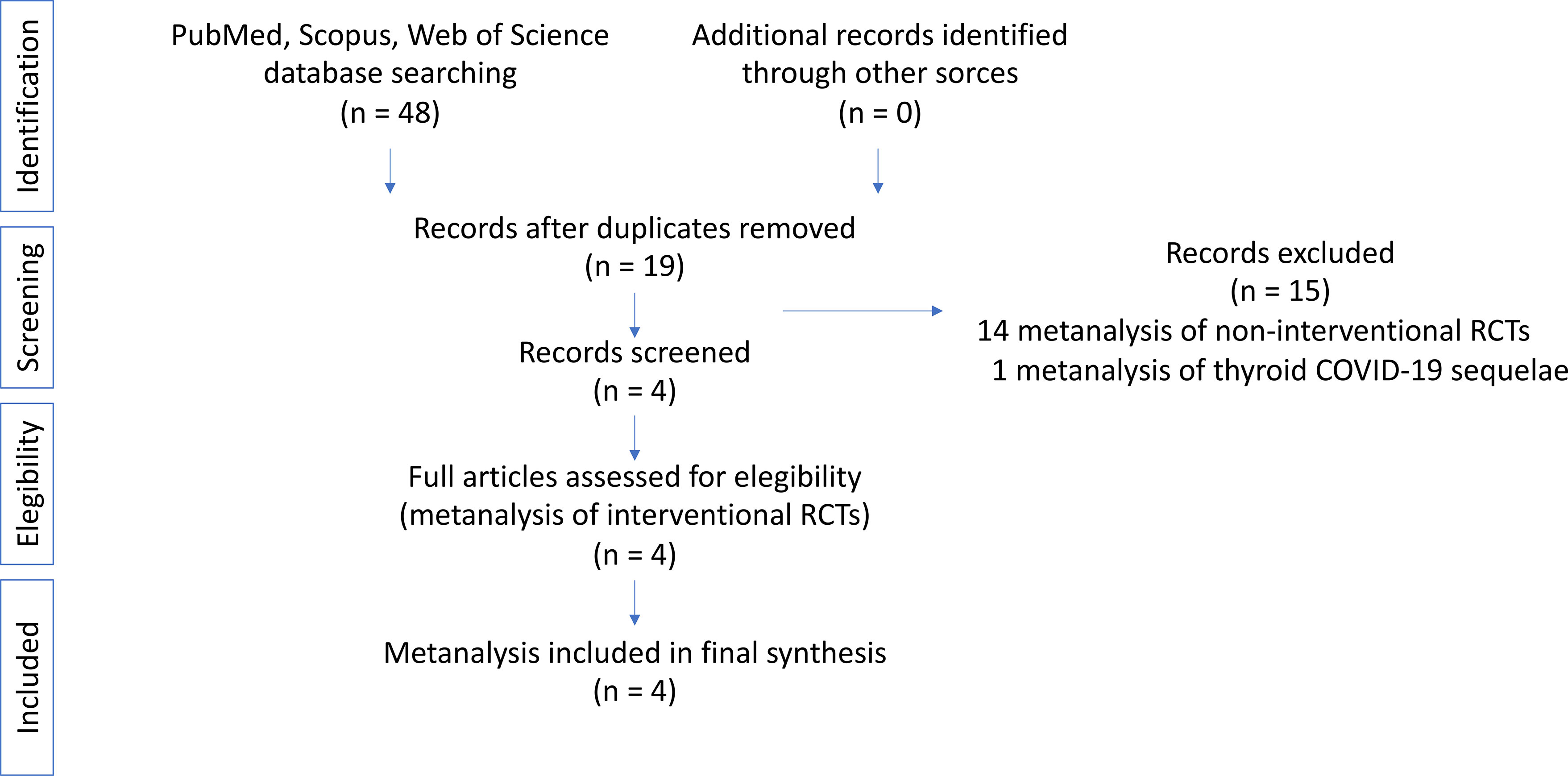
We analyzed the existing published high-quality metanalyses regarding IRCTs performed to treat critical ill patients with NTIS by administrating TH supplementation. For this reason, we undertook a search via PubMed, Scopus and ISI Web of Knowledge until 1st December 2021. The search terms were: “meta-analysis” OR “meta-analyses” AND “Nonthyroidal Illness Syndrome (NTIS)”, “Euthyroid Sick Syndrome (ESS)”, Low T3 Syndrome (LT3S)”. The review was conducted following the guidance proposed by Aromataris et al. (30). Due to the specific goals of this systematic review, specifically focused on metanalyses of IRCTs, data regarding the frequency of NTIS or its role as prognostic factor were excluded. The search strategy and flow of the metanalyses is illustrated in Figure 1. Two authors (FC and SS) carefully reviewed every original article. After this process, another author (CC) repeated the analysis to ensure reliability.
Data Items Collection Process
Having identified the final list of metanalyses, each article contained within each metanalysis was thoroughly reviewed in light of the following questions: 1) which disease/condition was considered; 2) how many patients were included in the metanalysis; 3) what was the type and dosage of THs supplementation; 4) how long was the duration of treatment; 5) what was the primary outcome measured; and 6) did the treatment produce any clinical benefit with respect to the primary outcome.
Assessment of the Quality of the Selected Metanalyses
We used the MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2) to evaluate the methodological quality of the studies included in the selected metanalyses (31). We calculated the AMSTAR score by summing up all yes answers (yes = 1). The reviews were then categorized with respect to their methodological quality and based on their AMSTAR score. Categories of quality were determined as follows: low (score 0 to 4), medium (score 5 to 8), and high (score 9 to 11). The quality of the included metanalyses was assessed by two researchers independently to confirm accuracy of the analysis. Discrepancies were resolved by consensus.
Results
Results of the Literature Search
A total of 48 metanalyses emerged from the initial search process of the literature. Four of them remained after manual removal of duplicates and after a screening process. Fourteen studies were excluded because they refer to non-IRCTs and one because was focused on thyroid sequelae of COVID-19. The selected metanalyses were assessed for eligibility and deemed appropriate for inclusion. A flow diagram representing this search strategy is presented in Figure 1. As a result of the extensive literature search and screening of metanalyses concerning IRCTs in NTIS, we found 4 metanalyses matching our selection criteria. The data extracted from each metanalysis are reported in Table 1.
Quality of the Selected Metanalyses
The results of the evaluation of the quality of the metanalyses included in the review are reported (Supplementary Table 1). The four metanalyses selected showed a medium/high score, according to the 16 points included in AMSTAR 2 check list. However, it should be considered that although the metanalysis on nephrotic syndrome by Liu et al. was based on IRCTs from China, which were written in Chinese and have not been published in peer-reviewed journals available on PubMed, the quality was high according to the AMSTAR 2 score.
Type of Conditions/Diseases
The four metanalyses were focused on NTIS associated with various diseases and conditions. One of them evaluated TH replacement efficacy in 14 IRTCs performed in adult patients who underwent to cardiac surgery (33). In another one the same evaluation was conducted in 9 IRCTs, performed in children after congenital heart surgery (35). The potential benefit of TH replacement was evaluated in a metanalysis focused on 6 IRCTs, performed in patients with nephrotic syndrome (34). Finally, in another metanalysis (32), the potential effect of prophylactic postnatal TH supplementation in preventing morbidity and mortality was evaluated in 4 IRCTs performed in preterm infants. The total number of IRCTs, included in the four metanalyses was 33, with a total of 1,245 patients enrolled in the study group and received treatment with either LT4 or LT3. Results were compared to a total of 1,294 patients enrolled in the control group, which received placebo or no treatment.
Types of Interventions and Dosage Strategy
The IRCTs included in the 4 metanalyses showed marked differences in the type of TH supplementation, in the route of administration as well as in the dosages and duration of treatment. In the 4 IRCTs included in the metanalysis reported by Osborn et al. (32), treatment of preterm infants (25-31 weeks’ gestation) consisted in the administration of intravenous (iv) LT4 until tolerating feeds, then orally, starting from 12 - 24 hours of age, at doses ranging from 8 to 20 μg/kg daily for up to six weeks. In some IRCTs, before administration of LT4 a pretreatment with LT3 at 0.5 μg/kg/h was started 24 hours after birth. Treatment with LT4, at doses ranging from 20 to 50 μg/kg/day, was also the treatment of choice given to patients with nephrotic syndrome in the 6 IRCTs included in the metanalysis reported by Liu et al. (34). In the 14 IRCTs included in the metanalysis by Kaptein et al. (33), patients received treatment with iv LT3 at high doses (0.175-0.333 μg/kg/h) or at low doses (0.0275-0.0333 μg/kg/h) or orally at variable doses. Finally, in the eight IRCTs included in the metanalysis by Flores et al. (35), patients that underwent to cardiac surgery were treated post-operatively with LT3, given intravenously or enterally, at doses ranging from 0.4 to 5.0 μg/kg/h.
Outcomes
Since the disease associated with NTIS were different and affected several apparatuses, the outcomes were extremely variable too, reflecting the aim of each IRCT to demonstrate improvement in the function of the specific organ/system involved. In IRCTs designed to evaluate the efficacy of prophylactic LT3 treatment in preterm infants, the chosen outcomes were the following: prevention of neurological alterations, reduction of oxygen requirements, restoration of endocrinological and clinical parameters and reduction of mortality. In adult patients that underwent to cardiac surgery, the efficacy of TH supplementation was assessed based on the effects on cardiovascular parameters, such as the cardiac index, the systemic vascular resistance, the heart rate, the occurrence of atrial fibrillation, the use of inotropes and, finally, mortality. The primary outcome in patient with nephrotic syndrome was the remission of the disease. In pediatric patients that were surgically treated for cardiac congenital heart defects, the outcomes were mostly related to cardiac function and consisted in the cardiac index, the inotrope score, the duration of mechanical ventilation (MV), the length of stay in ICU and in the hospital after operation, and mortality.
Effects of Interventions With THs on the Chosen Outcomes
Analysis of the results reported by the metanalyses and concerning the effects of LT3 in both pediatric and adult patients that underwent cardiac surgery are inconclusive. An effect of LT3 on cardiac index has been reported in adult patients but not in pediatric ones. No clear effects on mortality were reported in all IRCTs, performed both in children and adult patients. In children that were surgically treated for congenital heart defects, LT3 supplementation did not significantly alter also the postoperative course of the disease in any of the parameters considered, namely duration of mechanical ventilation, duration of postoperative hospital stays, cardiac index at 24 hours postoperatively, and inpatient mortality. A limited effect was reported in the inotrope score and in the duration of ICU stay. In one IRCT, perioperative LT3 administration decreased the incidence and need for antiarrhythmic treatment of postoperative atrial fibrillation (40). Better results have been reported in the IRCTs performed in nephrotic patients, where LT4 treatment produced a significant increase in the total remission rate of the disease. Analysis of the results reported in the IRCTs performed in preterm infants showed no evidence of any beneficial effects of the prophylactic administration of LT4, with respect of neurodevelopment, oxygen need, restoration of endocrine and clinical parameters and on mortality. Heterogeneity of the diseases considered in each RCT and of the therapeutic approaches used as well as of the measures chosen to evaluate the potential beneficial effects make difficult to draw any general conclusion.
Discussion
Metanalyses of IRCTs With THs in Patients NTIS
There have been many attempts in the past to demonstrate the benefit of treatment with THs in NTIS. Treatment with LT4 proved to be not beneficial (67) and treatment with LT3 was ineffective too (68) or showed appreciable improvement (69–73). Heart failure, cardiac surgery and acute myocardial infarction appear to be interesting areas of investigation. NTIS is frequently detected in these patients and many trials have analyzed the possible favorable effects of either LT4 or LT3. Some results have been obtained by treating with LT3 patients with cardiac diseases or those undergoing cardiothoracic or coronary bypass procedures (25, 27, 45, 74–77). However, after an initial enthusiasm the results of the trials were disappointing (46). In 2 metanalyses of IRCTs performed in both pediatric and adult patients that underwent to cardiac surgery, treatment with LT3, administered either intravenously at low or high doses or enterally, proved to have beneficial effects only with regard to some endpoints, namely the cardiac index, the use on antiarrhythmic drug for atrial fibrillation and the inotrope score, but it was not effective in two major outcomes, namely mortality and duration of mechanical ventilation. Since THs are widely recognized as potent stimulators of fetal lung maturation and of surfactant production in the newborns, the use of TH supplementation has, therefore, been suggested in preterm infants. We retrieved 1 metanalysis, including 4 IRCTs in which the potential benefit of LT4 was assessed with respect to two outcomes i.e., oxygen requirement and mortality. In addition, the occurrence of late sequelae, consisting in endocrinological and neurodevelopmental alterations, were also analyzed. The 4 IRCTs considered in this metanalysis, failed to demonstrate any beneficial effects of prophylactic LT4 treatment in these subjects. Conversely, the administration of LT4 proved to be effective in inducing the remission of the NTIS and of the nephrotic syndrome, indicating a therapeutic effect of THs on hydroelectrolytic homeostasis and on renal hemodynamics.
However, after many years and many published papers as well as many IRCTs, the question whether NTIS should be considered an euthyroid condition that doesn’t require treatment or a truly hypothyroid disorder that may benefit from treatment with thyroid hormone remains unanswered. We are still waiting for additional IRCTs, based on adequate doses of THs and proper time frame to evaluate the effects of treatment and, most important, based on more appropriate primary outcome measures and on valid pathogenic biomarkers to assess the potential benefit.
There are several possible reasons why many of the IRCTs failed: i) the treatment was not appropriate and LT4 was used instead of LT3 or a combination of both; ii) the treatment was given at dosage unappropriated; or iii) the chosen primary endpoint was not adequate to evaluate the efficacy of the treatment. The last point is crucial. In many cases, in fact, it is difficult to find congruence of the conclusions regarding the effects of the treatments and there is lack of evidence regarding a strong pathophysiological mechanism of action or the final target of the hormone. Selection of non-appropriate endpoints could result in failure of the trial in achieving statistical significance (78). Decision whether to treat or not to treat should be based on evidences obtained using the best possible and hard clinical endpoint and not on surrogates (79, 80). The availability of a suitable and clear gold-standard endpoint would certainly facilitate the analysis of the benefit of TH treatment in future IRCTs in patients with NTIS.
Future Perspectives
Our review does make it apparent that it is difficult to draw any conclusions and this represents an area that remains to be explored. New adequate outcomes are needed to evaluate potential beneficial effects of THs in NTIS. During the last COVID-19 pandemic wave, we analyzed critical ill patients, admitted to the ICU of our Hospital, by means of BIA and we demonstrated that low FT3 serum values were associated with profound changes in the hydroelectrolytic equilibrium at the periphery, responsible for marked water and salt retention and generalized edema (18). The development of NTIS occurred acutely in these patients. However, the picture was similar to that observed in myxedema due to chronic, longstanding and progressive hypothyroidism. We observed an increase in the total body water (TBW) as well as in the fraction of free fat mass (FFM) as water, expressed as the TBW/FFM ratio, also indicated as hydration, in COVID-19 patients with NTIS, compared to those with normal FT3 serum values. Such changes were associated with an increase in the Nae : Ke exchangeable ratio, always measured by BIA and with a reduced mRNA expression levels of the two genes coding for the two major subunits of the Na+/K+ pump, in the PBMCs obtained from COVID-19 patients during the acute phase of the disease (18).
These results suggest that the primary change in NTIS could possibly be the reduced expression/activity of the Na+/K+ pump. It is well known that THs are key determinants of cellular metabolism in many target tissues (81). The Na+/K+ pump has been known for a long time as a target of T3 transcriptional as well as non-transcriptional activity (82–85). In cultured chick cardiac myocytes, inhibition of Na+/K+ pump promotes the efflux of ions and water, causing cell shrinkage (86). Na+ efflux is an energy consuming process that has been attributed to the ATP-dependent Na+/K+ pump. Activation of this pump leads to the exit of 3 Na+ ions for every 2 K+ ions entering the cell. According to the pump-leak hypothesis (87, 88), inhibition of the Na+/K+ pump activity should result in cell swelling. However, it has been demonstrated that the effect of Na+/K+ pump inhibition is, indeed, not swelling, but apoptotic cell shrinkage (86, 89). The regulation, mechanism of action as well as the role of Na+/K+ pump in many human diseases, however, is not fully understood yet (90). We may speculate that Na+/K+ pump downregulation, due to an acute decrease in LT3 serum concentration, would lead to an efflux of ions and other molecules outside the cellular membrane. Water would follow as a consequence of osmotic attraction. The LT3-mediated downregulation of the Na+/K+ pump could be the likely pathogenic mechanisms, responsible for the cellular damage observed in NTIS.
Conclusion
We performed an extensive search in the literature, looking for metanalyses of IRCTs performed in NTIS affecting critically ill patients in the ICU setting. We investigated the reasons why several clinical trials failed and why there is still lacking of convincing data regarding the efficacy of TH supplementation in patients with NTIS. We found extreme variability in terms of conditions or diseases associated with NTIS, types, dosages and routes of administration and duration of TH supplementation used in the various IRCTs. This may reflect the peculiarity of NTIS, considered a “syndrome with many different faces”. In particular, we found that in each IRCT included in the selected metanalysis the chosen primary outcomes seem to be not appropriate or not accurately defined for all the pathological conditions associated with NTIS. Our experience in COVID-19 patients with NTIS indicates that BIA parameters, and in particular hydration and Nae : Ke ratio, are suitable markers for the assessment of the hydroelectrolytic balance at the periphery in NTIS and may represent adequate clinical endpoints to evaluate the efficacy of TH treatment in future IRCTs. We may, therefore, be close to yield a definite answer to the question concerning the potential benefit of treatment with LT3 in critically ill patients with NTIS.
Author Contributions
SS principal investigator. SS, CN, and PA designed and supervised the review. CV and VS performed the literature search. SS, CC, and FC carefully reviewed every article. SS and FC performed the analysis of the quality through the AMSTAR 2 score system. SS, FC, RM, and MR wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by: (1) Italian Association for Cancer Research (AIRC) grants IG24451 to RM, (2) the LazioInnova grant 2018 n.85-2017-13750 to RM, (3) the Sapienza University Research Projects of National Relevance - PRIN 2017 (Prot. 2017HWTP2K) to RM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.850328/full#supplementary-material
Abbreviations
TH, thyroid hormone; T3, 3,5,3′-triiodothyronine; T4, thyroxine; LT3, levo-triiodothyronine; LT4, levothyroxine; FT3, free triiodothyronine; FT4, free thyroxine; NTIS, nonthyroidal illness syndrome; ESS, euthyroid sick syndrome; LT3S, low T3 syndrome; IRCT, interventional randomized clinical trial; MV, mechanical ventilation; BIA, bioelectrical impedance analysis; Rz, resistance; Xc, reactance; PhA, phase angle; Nae : Ke, sodium/potassium exchangeable ratio; BCM, body cell mass; BMI, body mass index; TBW, total body water; ECW, extra cellular water; ICW, intra cellular water; FFM, fat-free mass; FM, fat mass; PBMCs, peripheral blood mononuclear cells.
References
1. Peeters RP. Non Thyroidal Illness: To Treat or Not to Treat? Ann Endocrinol (Paris) (2007) 68(4):224–8. doi: 10.1016/j.ando.2007.06.011
2. Chopra IJ. Clinical Review 86: Euthyroid Sick Syndrome: Is it a Misnomer? J Clin Endocrinol Metab (1997) 82(2):329–34. doi: 10.1210/jcem.82.2.3745
3. Bermudez F, Surks MI, Oppenheimer JH. High Incidence of Decreased Serum Triiodothyronine Concentration in Patients With Nonthyroidal Disease. J Clin Endocrinol Metab (1975) 41(1):27–40. doi: 10.1210/jcem-41-1-27
4. Kaplan MM, Larsen PR, Crantzf R, Dzau VJ, Rossing TH, Haddow JE. Prevalence of Abnormal Thyroid Function Test Results in Patients With Acute Medical Illnesses. Am J Med (1982) 72(1):9–16. doi: 10.1016/0002-9343(82)90565-4
5. Plikat K, Langgartner J, Buettner R, Bollheimer LC, Woenckhaus U, Schölmerich J, et al. Frequency and Outcome of Patients With Nonthyroidal Illness Syndrome in a Medical Intensive Care Unit. Metabolism (2007) 56(2):239–44. doi: 10.1016/j.metabol.2006.09.020
6. Langouche L, Jacobs A, Van den Berghe G. Nonthyroidal Illness Syndrome Across the Ages. J Endocr Soc (2019) 3(12):2313–25. doi: 10.1210/js.2019-00325
7. Chinga-Alayo E, Villena J, Evans AT, Zimic M. Thyroid Hormone Levels Improve the Prediction of Mortality Among Patients Admitted to the Intensive Care Unit. Intensive Care Med (2005) 31(10):1356–61. doi: 10.1007/s00134-005-2719-9
8. Iglesias P, Díez JJ. Thyroid Dysfunction and Kidney Disease. Eur J Endocrinol (2009) 160(4):503–15. doi: 10.1530/EJE-08-0837
9. Bello G, Pennisi MA, Montini L, Silva S, Maviglia R, Cavallaro F, et al. Nonthyroid Illness Syndrome and Prolonged Mechanical Ventilation in Patients Admitted to the ICU. Chest (2009) 135(6):1448–54. doi: 10.1378/chest.08-1816
10. Sharshar T, Bastuji-Garin S, Polito A, De Jonghe B, Stevens RD, Maxime V, et al. Groupe De Réflexion Et D’Etude Des Neuromyopathies En RéanimationHormonal Status in Protracted Critical Illness and in-Hospital Mortality. Hormonal Status in Protracted Critical Illness and in-Hospital Mortality. Crit Care (2011) 15(1):R47. doi: 10.1186/cc10010
11. Iervasi G, Nicolini G. Thyroid Hormone and Cardiovascular System: From Basic Concepts to Clinical Application. Intern Emerg Med (2013) 8(Suppl 1):S71–4. doi: 10.1007/s11739-013-0911-4
12. Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID-19 on the Thyroid Gland: An Update. Rev Endocr Metab Disord (2021) 22(4):803–15. doi: 10.1007/s11154-020-09615-z
13. D’ascanio M, Innammorato M, Pasquariello L, Pizzirusso D, Guerrieri G, Castelli S, et al. Age is Not the Only Risk Factor in COVID-19: The Role of Comorbidities and of Long Staying in Residential Care Homes. BMC Geriatr (2021) 21(1):63. doi: 10.1186/s12877-021-02013-3
14. Ricci A, Pagliuca A, D’Ascanio M, Innammorato M, De Vitis C, Mancini R, et al. Circulating Vitamin D Levels Status and Clinical Prognostic Indices in COVID-19 Patients. Respir Res (2021) 22(1):76. doi: 10.1186/s12931-021-01666-3
15. Sciacchitano S, Sacconi A, De Vitis C, Blandino G, Piaggio G, Salvati V, et al. H-Ras Gene Takes Part to the Host Immune Response to COVID-19. Cell Death Discov (2021) 7(1):158. doi: 10.1038/s41420-021-00541-w
16. Ciliberto G, Mancini R, Paggi MG. Drug Repurposing Against COVID-19: Focus on Anticancer Agents. J Exp Clin Cancer Res (2020) 39(1):86. doi: 10.1186/s13046-020-01590-2
17. Palmieri L, Palmer K, Lo Noce C, Meli P, Giuliano M, Floridia M, et al. Italian National Institute of Health COVID-19 Mortality GroupDifferences in the Clinical Characteristics of COVID-19 Patients Who Died in Hospital During Different Phases of the Pandemic: National Data From Italy. Aging Clin Exp Res (2021) 33(1):193–9. doi: 10.1007/s40520-020-01764-0
18. Sciacchitano S, De Vitis C, D’Ascanio M, Giovagnoli S, De Dominicis C, Laghi A, et al. Gene Signature and Immune Cell Profiling by High-Dimensional, Single-Cell Analysis in COVID-19 Patients, Presenting Low T3 Syndrome and Coexistent Hematological Malignancies. J Transl Med (2021) 19(1):139. doi: 10.1186/s12967-021-02805-6
19. Sciacchitano S, Capalbo C, Napoli C, Negro A, De Biase L, Marcolongo A, et al. Nonthyroidal Illness Syndrome (NTIS) in Severe COVID-19 Patients: Role of T3 on the Na/K Pump Gene Expression and on Hydroelectrolytic Equilibrium. J Transl Med (2021) 19:491. doi: 10.1186/s12967-021-03163-z
20. Llamas M, Garo ML, Giovanella L. Low Free-T3 Serum Levels and Prognosis of COVID-19: Systematic Review and Meta-Analysis. Clin Chem Lab Med (2021) 59(12):1906–13. doi: 10.1515/cclm-2021-0805
21. Van den Berghe G. Non-Thyroidal Illness in the ICU: A Syndrome With Different Faces. Thyroid (2014) 24(10):1456–65. doi: 10.1089/thy.2014.0201
22. De Groot LJ. Dangerous Dogmas in Medicine: The Nonthyroidal Illness Syndrome. J Clin Endocrinol Metab (1999) 84(1):151–64. doi: 10.1210/jcem.84.1.5364
23. Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid Function in Critically Ill Patients. Lancet Diabetes Endocrinol (2015) 3(10):816–25. doi: 10.1016/S2213-8587(15)00225-9
24. Economidou F, Douka E, Tzanela M, Orfanos S, Kotanidou A. Thyroid Function in Critical Illness. In: Rajendram R, Preedy VR, Patel VB, editors. Diet and Nutrition in Critical Care. New York, NY: Springer (2015). doi: 10.1007/978-1-4614-7836-2_2
25. Klemperer JD, Klein I, Gomez M, Helm RE, Ojamaa K, Thomas SJ, et al. Thyroid Hormone Treatment After Coronary-Artery Bypass Surgery. N Engl J Med (1995) 333(23):1522–7. doi: 10.1056/NEJM199512073332302
26. Bennett-Guerrero E, Jimenez JL, White WD, D’Amico EB, Baldwin BI, Schwinn DA. Cardiovascular Effects of Intravenous Triiodothyronine in Patients Undergoing Coronary Artery Bypass Graft Surgery. A Randomized, Double-Blind, Placebo-Controlled Trial. Duke T3 Study Group. JAMA (1996) 275(9):687–92. doi: 10.1001/jama.1996.03530330031025
27. Hsu RB, Huang TS, Chu SH. Effects of Triiodothyronine Administration in Experimental Myocardial Injury. J Endocrinol Invest (1995) 18(9):702–9. doi: 10.1007/BF03349792
28. Hamilton MA, Stevenson LW. Thyroid Hormone Abnormalities in Heart Failure: Possibilities for Therapy. Thyroid (1996) 6(5):527–9. doi: 10.1089/thy.1996.6.527
29. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. American Thyroid Association Task Force on Thyroid Hormone ReplacementGuidelines for the Treatment of Hypothyroidism. Thyroid (2014) 24(12):1670–751. doi: 10.1089/thy.2014.0028
30. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing Systematic Reviews: Methodological Development, Conduct and Reporting of an Umbrella Review Approach. Int J Evid Based Healthc (2015) 13(3):132–40. doi: 10.1097/XEB.0000000000000055
31. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or non-Randomised Studies of Healthcare Interventions, or Both. BMJ (2017) 358:j4008. doi: 10.1136/bmj.j4008
32. Osborn DA, Hunt RW. Prophylactic Postnatal Thyroid Hormones for Prevention of Morbidity and Mortality in Preterm Infants. Cochrane Database Syst Rev (2007) 1):CD005948. doi: 10.1002/14651858.CD005948.pub2
33. Kaptein EM, Sanchez A, Beale E, Chan LS. Clinical Review: Thyroid Hormone Therapy for Postoperative Nonthyroidal Illnesses: A Systematic Review and Synthesis. J Clin Endocrinol Metab (2010) 95(10):4526–34. doi: 10.1210/jc.2010-1052
34. Liu H, Yan W, Xu G. Thyroid Hormone Replacement for Nephrotic Syndrome Patients With Euthyroid Sick Syndrome: A Meta-Analysis. Ren Fail (2014) 36(9):1360–5. doi: 10.3109/0886022X.2014.949559
35. Flores S, Loomba RS, Checchia PA, Graham EM, Bronicki RA. Thyroid Hormone (Triiodothyronine) Therapy in Children After Congenital Heart Surgery: A Meta-Analysis. Semin Thorac Cardiovasc Surg (2020) 32(1):87–95. doi: 10.1053/j.semtcvs.2019.05.020
36. Smith LM, Leake RD, Berman N, Villanueva S, Brasel JA. Postnatal Thyroxine Supplementation in Infants Less Than 32 Weeks!"Gestation: Effects on Pulmonary Morbidity. J Perinatol (2000) 20:427–31. doi: 10.1038/sj.jp.7200417
37. Valerio PG, van Wassenaer AG, de Vijlder JJ, Kok JH. A Randomized, Masked Study of Triiodothyronine Plus Thyroxine Administration in Preterm Infants Less Than 28 Weeks of Gestational Age: Hormonal and Clinical Effects. Pediatr Res (2004) 55:248–53. doi: 10.1203/01.PDR.0000104153.72572.F5
38. van Wassenaer AG, Kok JH, de Vijlder JJ, Briet JM, Smit BJ, Tamminga P, et al. Effects of Thyroxine Supplementation on Neurologic Development in Infants Born at Less Than 30 Weeks>:"gestation. N Engl J Med (1997) 336(1):21–6. doi: 10.1056/NEJM199701023360104
39. Vanhole C, Aerssens P, Naulaers G, Casneuf A, Devlieger H, Van den Berghe G, et al. L-Thyroxine Treatment of Preterm Newborns: Clinical and Endocrine Effects. Pediatr Res (1997) 42:87–92. doi: 10.1203/00006450-199707000-00014
40. Klemperer JD, Klein IL, Ojamaa K, Helm RE, Gomez M, Isom OW, et al. Triiodothyronine Therapy Lowers the Incidence of Atrial Fibrillation After Cardiac Operations. Ann Thorac Surg (1996) 61(5):1323–7. doi: 10.1016/0003-4975(96)00102-6
41. Mullis-Jansson SL, Argenziano M, Corwin S, Homma S, Weinberg AD, Williams M, et al. A Randomized Double Blind Study of the Effect of Triiodothyronine on Cardiac Function and Morbidity After Coronary Bypass Surgery. J Thorac Cardiovasc Surg (1999) 117:1128–34. doi: 10.1016/s0022-5223(99)70249-7
42. Güden M, Akpinar B, Sagğbaş E, Sanisoğlu I, Cakali E, Bayindir O. Effects of Intravenous Triiodothyronine During Coronary Artery Bypass Surgery. Asian Cardiovasc Thorac Ann (2002) 10(3):219–22. doi: 10.1177/021849230201000306
43. Ranasinghe AM, Quinn DW, Pagano D, Edwards N, Faroqui M, Graham TR, et al. Glucose-Insulin-Potassium and Tri-Iodothyronine Individually Improve Hemodynamic Performance and are Associated With Reduced Troponin I Release After on-Pump Coronary Artery Bypass Grafting. Circulation (2006) 114. doi: 10.1161/CIRCULATIONAHA.105.000786
44. Acker CG, Flick R, Shapiro R, Scantlebury VP, Jordan ML, Vivas C, et al. Thyroid Hormone in the Treatment of Post-Transplant Acute Tubular Necrosis (ATN). Am J Transplant (2002) 2:57–61. doi: 10.1034/j.1600-6143.2002.020110.x
45. Novitzky D, Cooper DK, Barton CI, Greer A, Chaffin J, Grim J, et al. Triiodothyronine as an Inotropic Agent After Open Heart Surgery. J Thorac Cardiovasc Surg (1989) 98(5 Pt 2):972–7. doi: 10.1016/S0022-5223(19)34281-3
46. Teiger E, Menasché P, Mansier P, Chevalier B, Lajeunie E, Bloch G, et al. Triiodothyronine Therapy in Open-Heart Surgery: From Hope to Disappointment. Eur Heart J (1993) 14(5):629–33. doi: 10.1093/eurheartj/14.5.629
47. Vavouranakis I, Sanoudos G, Manios A, Kalogeropoulou K, Sitaras K, Kokkinos C. Triiodothyronine Administration in Coronary Artery Bypass Surgery: Effect on Hemodynamics. J Cardiovasc Surg (Torino) (1994) 35:383–9.
48. Spratt DI, Frohnauer M, Cyr-Alves H, Kramer RS, Lucas FL, Morton JR, et al. Physiological Effects of Nonthyroidal Illness Syndrome in Patients After Cardiac Surgery. Am J Physiol Endocrinol Metab (2007) 293:E310–5. doi: 10.1152/ajpendo.00687.2006
49. Sirlak M, Yazicioglu L, Inan MB, Eryilmaz S, Tasoz R, Aral A, et al. Oral Thyroid Hormone Pretreatment in Left Ventricular Dysfunction. Eur J Cardiothorac Surg (2004) 26:720–5. doi: 10.1016/j.ejcts.2004.07.003
50. Magalhães AP, Gus M, Silva LB, Schaan BD. Oral Triiodothyronine for the Prevention of Thyroid Hormone Reduction in Adult Valvular Cardiac Surgery. Braz J Med Biol Res (2006) 39:969–78. doi: 10.1590/s0100-879x2006000700015
51. Choi YS, Kwak YL, Kim JC, Chun DH, Hong SW, Shim JK. Peri-Operative Oral Triiodothyronine Replacement Therapy to Prevent Postoperative Low Triiodothyronine State Following Valvular Heart Surgery. Anaesthesia (2009) 64:871–7. doi: 10.1111/j.1365-2044.2009.05984.x
52. Duan GS, Cheng XH, Xu WW. Treatment of Nephrotic Syndrome Combined With Hypothyroidism. J Clin Nephrol (2008) 8(3):123–4.
53. Hu ZJ, Li HJ, Niu K, Liu B. Treatment of Primary Nephrotic Syndrome Complicated by Hypothyroidism. Clin Misdiagn Misther (2005) 18(2):77–9.
54. Li LS, Zhou ZH, Liu LX. Curative Effects of Levothyroxine in Patients With Primary Nephrotic Syndrome Followed With Hypothyroidism. J Med Forum (2005) 31(19):21–3.
55. Nie JR, Gao QY, Cheng XM. Study the Serum Levels of Thyroid Hormone on Patients With Primary Nephritic Syndrome and the Efficacy of Thyroid Hormone Treatment. Chin J Clin Health (2008) 11(1):33–4.
56. Zhang HZ, Xu JH, Ren YY. Curative Effects of Small Dose of Thyroxine on Nephrotic Syndrome. The Paper Collection of the Chinese Medical Association 2004 Annual Meeting of Renal Disease. Beijing, China.
57. Xie MB. Curative Effect in Patients With Nephrotic Syndrome Combined With Hypothyroidism. Chin J Health Lab Tech (2009) 19(8):1841–2.
58. Bettendorf M, Schmidt KG, Grulich-Henn J, Ulmer HE, Heinrich UE. Tri-Iodothyronine Treatment in Children After Cardiac Surgery: A Double-Blind, Randomised, Placebo-Controlled Study. Lancet (2000) 356:529–34. doi: 10.1016/S0140-6736(00)02576-9
59. Chowdhury D, Ojamaa K, Parnell VA, McMahon C, Sison CP, Klein I. A Prospective Randomized Clinical Study of Thyroid Hormone Treatment After Operations for Complex Congenital Heart Disease. J Thorac Cardiovasc Surg (2001) 122:1023–5. doi: 10.1067/mtc.2001.116192
60. Mackie AS, Booth KL, Newburger JW, Gauvreau K, Huang SA, Laussen PC, et al. A Randomized, Double-Blind, Placebo-Controlled Pilot Trial of Triiodothyronine in Neonatal Heart Surgery. J Thorac Cardiovasc Surg (2005) 130:810–6. doi: 10.1016/j.jtcvs.2005.04.025
61. Mainwaring RD, Capparelli E, Schell K, Acosta M, Nelson JC. Pharmacokinetic Evaluation of Triiodothyronine Supplementation in Children After Modified Fontan Procedure. Circulation (2000) 101:1423–9. doi: 10.1161/01.cir.101.12.1423
62. Marwali EM, Boom CE, Sakidjan I, Santoso A, Fakhri D, Kartini A, et al. Oral Triiodothyronine Normalizes Triiodothyronine Levels After Surgery for Pediatric Congenital Heart Disease. Pediatr Crit Care Med (2013) 14:701–8. doi: 10.1097/PCC.0b013e3182917f87
63. Marwali EM, Boom CE, Budiwardhana N, Fakhri D, Roebiono PS, Santoso A, et al. Oral Triiodothyronine for Infants and Children Undergoing Cardiopulmonary Bypass. Ann Thorac Cardiovasc Surg (2017) 104:688–95. doi: 10.1016/j.athoracsur.2017.01.001
64. Portman MA, Fearneyhough C, Ning XH, Duncan BW, Rosenthal GL, Lupinetti FM. Triiodothyronine Repletion in Infants During Cardiopulmonary Bypass for Congenital Heart Disease. J Thorac Cardiovasc Surg (2000) 120:604–8. doi: 10.1067/mtc.2000.108900
65. Portman MA, Slee A, Olson AK, Cohen G, Karl T, Tong E, et al. Triiodothyronine Supplementation in Infants and Children Undergoing Cardiopulmonary Bypass (TRICC): A Multicenter Placebo-Controlled Randomized Trial: Age Analysis. Circulation (2010) 122(11 suppl):S224–33. doi: 10.1161/CIRCULATIONAHA.109.926394
66. Talwar S, Bhoje A, Khadagawat R, Chaturvedi P, Sreenivas V, Makhija N, et al. Oral Thyroxin Supplementation in Infants Undergoing Cardiac Surgery: A Double-Blind Placebocontrolled Randomized Clinical Trial. J Thorac Cardiovasc Surg (2018) 156(3):1209–17.e3. doi: 10.1016/j.jtcvs.2018.05.044
67. Brent GA, Hershman JM. Thyroxine Therapy in Patients With Severe Nonthyroidal Illnesses and Low Serum Thyroxine Concentration. J Clin Endocrinol Metab (1986) 63(1):1–8. doi: 10.1210/jcem-63-1-1
68. Becker RA, Vaughan GM, Ziegler MG, Seraile LG, Goldfarb IW, Mansour EH, et al. Hypermetabolic Low Triiodothyronine Syndrome of Burn Injury. Crit Care Med (1982) 10(12):870–5. doi: 10.1097/00003246-198212000-00014
69. Hesch RD, Husch M, Kodding R, Hoffken B, Meyer T. Treatment of Dopamine-Dependent Shock With Triiodothyronine. Endocr Res Commun (1981) 8(4):229–37. doi: 10.3109/07435808109045741
70. Meyer T, Husch M, van den Berg E, Kodding R, Hoffken B, Hesch RD. Behandlung Des Dopaminabhängigen Schocks Mit TrijodthyroninVorläufige Mitteilung [Treatment of Dopamine-Dependent Shock With Triiodothyronine: Prelimary Results (Author’s Transl)]. Dtsch Med Wochenschr (1979) 104(48):1711–4. doi: 10.1055/s-0028-1129177
71. Dulchavsky SA, Maitra SR, Maurer J, Kennedy PR, Geller EG, Dreis DJ. Beneficial Effects of Thyroid Hormone Administration on Metabolic and Hemodynamic Function in Hemorrhagic Shocwk. FASEB J (1990) 4:A952.
72. Novitzky D, Cooper DK, Reichart B. Hemodynamic and Metabolic Responses to Hormonal Therapy in Brain-Dead Potential Organ Donors. Transplantation (1987) 43(6):852–4. doi: 10.1097/00007890-198743060-00016
73. Dulchavsky SA, Hendrick SR, Dutta S. Pulmonary Biophysical Effects of Triidothyronine (T3) Augmentation During Sepsis-Induced Hypothyroidism. J Trauma (1993) 35(1):104–8. doi: 10.1097/00005373-199307000-00017
74. Novitzky D, Cooper DKC, Zuhdi N. Triiodothyronine Therapy in the Cardiac Transplant Recipient. Transplant Proc (1988) 20(5 Suppl 7):65–8.
75. Novitzky D, Cooper DK, Chaffin JS, Greer AE, DeBault LE, Zuhdi N. Improved Cardiac Allograft Function Following Triiodothyronine Therapy to Both Donor and Recipient. Transplantation (1990) 49(2):311–6. doi: 10.1097/00007890-199002000-00017
76. Orlowski JO, Spees EK. Improved Cardiac Transplant Survival With Thyroxine Treatment of Hemodynamical Unstable Donors: 95.2% Graft Survival at 6 and 30 Months. Transplant Proc (1993) 25(1 Pt 2):1535.
77. Jeevanandam V, Todd B, Hellman S, Eldridge C, McClurken J, Addonizio VP. Use of Triiodothyronine Replacement Therapy to Reverse Donor Myocardial Dysfunction: Creating a Larger Donor Pool. Transplant Proc (1993) 25(6):3305–6.
78. Pocock SJ, Stone GW. The Primary Outcome Fails — What Next? N Engl J Med (2016) 375(9):861–70. doi: 10.1056/NEJMra1510064
79. Begg CB. Justifying the Choice of Endpoints for Clinical Trials. J Natl Cancer Inst (2013) 105(21):1594–5. doi: 10.1093/jnci/djt289
80. Kluft C. Principles of Use of Surrogate Markers and Endpoints. Maturitas (2004) 47(4):293–8. doi: 10.1016/j.maturitas.2003.11.011
81. Cicatiello AG, Di Girolamo D, Dentice M. Metabolic Effects of the Intracellular Regulation of Thyroid Hormone: Old Players, New Concepts. Front Endocrinol (Lausanne) (2018) 9:474. doi: 10.3389/fendo.2018.00474
82. Kamitani T, Ikeda U, Muto S, Kawakami K, Nagano K, Tsuruya Y, et al. Regulation of Na,K-ATPase Gene Expression by Thyroid Hormone in Rat Cardiocytes. Circ Res (1992) 71(6):1457–64. doi: 10.1161/01.res.71.6.1457
83. Brodie C, Sampson SR. Characterization of Thyroid Hormone Effects on Na-K Pump and Membrane Potential of Cultured Rat Skeletal Myotubes. Endocrinology (1988) 123(2):891–7. doi: 10.1210/endo-123-2-891
84. Zhiqin L, Langhans SA. Transcriptional Regulators of Na,K-ATPase Subunits. Front Cell Dev Biol (2015) 3:66. doi: 10.3389/fcell.2015.00066
85. Lei J, Nowbar S, Mariash CN, Ingbar DH. Thyroid Hormone Stimulates Na-K-ATPase Activity and its Plasma Membrane Insertion in Rat Alveolar Epithelial Cells. Am J Physiol Lung Cell Mol Physiol (2003) 285(3):L762–72. doi: 10.1152/ajplung.00376.2002
86. Smith TW, Rasmusson RL, Lobaugh LA, Lieberman M. Na+/K+ Pump Inhibition Induces Cell Shrinkage in Cultured Chick Cardiac Myocytes. Basic Res Cardiol (1993) 88(5):411–20. doi: 10.1007/BF00795408
87. MacKnight ADC, Leaf A. Regulation of Cellular Volume. Physiol Rev (1977) 57(3):510–73. doi: 10.1152/physrev.1977.57.3.510
88. Tosteson DC, Hoffman JF. Regulation of Cell Volume by Active Cation Transport in High and Low Potassium Sheep Red Cells. J Gen Physiol (1960) 44(1):169–94. doi: 10.1085/jgp.44.1.169
89. Nobel CSI, Aronson JK, van den Dobbelsteen DJ, Slater AFG. Inhibition of Na+/K+-ATPase may be One Mechanism Contributing to Potassium Efflux and Cell Shrinkage in CD95-Induced Apoptosis. Apoptosis (2000) 5(2):153–63. doi: 10.1023/a:1009684713784
Keywords: nonthyroidal illness syndrome, SARS-CoV-2 (2019-nCoV) coronavirus, bioelectrical impedance analysis, hydration, sodium/potassium exchangeable ratio
Citation: Sciacchitano S, Capalbo C, Napoli C, Anibaldi P, Salvati V, De Vitis C, Mancini R, Coluzzi F and Rocco M (2022) Nonthyroidal Illness Syndrome: To Treat or Not to Treat? Have We Answered the Question? A Review of Metanalyses. Front. Endocrinol. 13:850328. doi: 10.3389/fendo.2022.850328
Received: 07 January 2022; Accepted: 16 March 2022;
Published: 10 May 2022.
Edited by:
Salman Razvi, Newcastle University, United KingdomReviewed by:
Lorenzo Scappaticcio, University Hospital “Luigi Vanvitelli”, ItalyCopyright © 2022 Sciacchitano, Capalbo, Napoli, Anibaldi, Salvati, De Vitis, Mancini, Coluzzi and Rocco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Flaminia Coluzzi, ZmxhbWluaWEuY29sdXp6aUB1bmlyb21hMS5pdA==
†ORCID: Salvatore Sciacchitano, orcid.org/0000-0003-1492-5365
Carlo Capalbo, orcid.org/0000-0001-8445-6782
Christian Napoli, orcid.org/0000-0002-5775-2276
Valentina Salvati, orcid.org/0000-0002-3843-6235
Claudia De Vitis, orcid.org/0000-0001-8899-2347
Rita Mancini, orcid.org/0000-0002-5491-2449
Flaminia Coluzzi, orcid.org/0000-0001-7184-5519
Monica Rocco, orcid.org/0000-0001-8380-3607
 Salvatore Sciacchitano
Salvatore Sciacchitano Carlo Capalbo
Carlo Capalbo Christian Napoli
Christian Napoli Paolo Anibaldi6
Paolo Anibaldi6 Claudia De Vitis
Claudia De Vitis Flaminia Coluzzi
Flaminia Coluzzi