
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 25 March 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.836485
This article is part of the Research Topic Understanding the Role of Gut Hormones, Microbiota, and miRNAs in Metabolic Regulation and Glucose Homeostasis in Obesity and Type-2-Diabetes View all 5 articles
 Shuang Liu1,2†
Shuang Liu1,2† Ruying Cao1,3†
Ruying Cao1,3† Luna Liu1,2
Luna Liu1,2 Youyuan Lv1,2
Youyuan Lv1,2 Xiangyu Qi1,2
Xiangyu Qi1,2 Zhongshang Yuan4
Zhongshang Yuan4 Xiude Fan1,2,5
Xiude Fan1,2,5 Chunxiao Yu1,2,5*
Chunxiao Yu1,2,5* Qingbo Guan1,2,5*
Qingbo Guan1,2,5*Objective: This study aimed at investigating the association between testosterone levels and gut microbiota in male patients with type 2 diabetes mellitus (T2DM) and providing a new strategy to elucidate the pathological mechanism of testosterone deficiency in T2DM patients.
Methods: In an observational study including 46 T2DM male patients, the peripheral venous blood and fecal samples of all subjects were collected. The V3–V4 regions of bacterial 16S rDNA were amplified and sequenced. Alpha and beta diversities were calculated by QIIME software. The possible association between gut microbial community and clinical indicators was assessed using the Spearman correlation coefficient. The association between the relative abundance of bacteria and testosterone levels was discovered using linear regression analysis in R language.
Results: There was no substantial difference in alpha and beta diversity. Blautia and Lachnospirales were significantly much higher in the testosterone deficiency group. Linear regression analysis showed that the abundance of Firmicutes at the phylum level and Lachnospirales at the order level were negatively correlated with testosterone level. After correcting for C-reactive protein (CRP) and homeostatic model assessment of insulin resistance (HOMA-IR), the relative abundance of Lachnospirales still had a significant negative correlation with testosterone level. Meanwhile, at the genus level, Lachnoclostridium, Blautia, and Bergeyella had a statistically significant negative association with testosterone level, respectively. Blautia was positively associated with FPG and total cholesterol level. Streptococcus was found positively associated with insulin, connecting peptide, and index of homeostatic model assessment of insulin resistance.
Conclusion: T2DM patients with testosterone deficiency have different gut microbiota compositions compared with T2DM patients alone. Low serum testosterone patients tend to have an increased abundance of opportunistic pathogens, which may be related to the occurrence and development of testosterone deficiency.
Type 2 diabetes mellitus (T2DM) is a global epidemic that is affecting the health of the population (1). As a chronic disease, T2DM has been reported associated with several complications, one of which is hypogonadism, which has a high prevalence and may be linked to a number of comorbidities (2), such as loss of libido, erectile dysfunction, feebleness, despondency, irritability, anemia, and sleep disturbance (3). According to statistics, hypogonadism affects 20%–80.4% of males with T2DM (4), which is often manifested as testosterone deficiency. The high rate of low serum testosterone in type 2 diabetic patients has been linked to defects in the hypothalamic–pituitary–gonadal (HPG) axis in previous studies (5). 10 randomly selected individuals in a small sample of 34 hypogonadal males with diabetes received magnetic resonance imaging that revealed no abnormalities in the hypothalamus or pituitary gland (5). To date, the pathogenesis of decreased serum testosterone levels and the reasons for the individual differences in these patients with type 2 diabetes remain unclear. Therefore, it is important to find other potential risk factors which affect testosterone levels and explore the promising therapy for testosterone deficiency.
In recent years, research has found a close connection between the quantity and activity of the gut microbiota and type 2 diabetes (6). As reported, changes in the gut microbiome composition and their affiliated metabolites, such as trimethylamine N-oxide and lipopolysaccharide, lead to an imbalance of gastrointestinal homeostasis, resulting in aberrant metabolite synthesis, inflammatory state, glycometabolic disruption, and insulin resistance (7, 8). On the other hand, some studies supposed that the gut microbiota may affect the testicular function and regulate androgen production through endotoxin or its metabolites (9). Steroid-17,20-desmolase, a classic steroid-metabolizing enzyme, has been found in some bacteria, including Clostridium (10). In vitro, several bacterial strains have been demonstrated to be able to digest androgens, such as Comamonas testosteroni, which can feed on testosterone (11). The testosterone levels of old male mice might be restored by feeding them purified microorganisms like Lactobacillus reuteri (12). However, the association between gut microbiome composition and androgen deficiency in T2DM male patients is still not clarified.
Accordingly, this study is designed to compare the microbiota diversity and composition in T2DM patients according to its testosterone levels, which help us verify the relationship between specific bacteria and testosterone level in T2DM patients, to explore the promising therapy for male hypogonadism and whether the intestinal bacteria have the capacity to be a potential marker for men hypogonadism.
This cross-sectional study was approved by the Shandong Provincial Hospital ethical committee. All subjects signed a written inform consent. We assessed male patients with diabetes who visited Shandong Provincial Hospital between August 2018 and October 2018. The World Health Organization’s criteria were used to diagnose classic T2DM. Patients were excluded if they (1) have moderate or profound liver and renal dysfunction; (2) have a history of hypogonadotropic hypogonadism, thyroid dysfunction, ramex, testicle, and epididymis injury, abnormal karyotype, or other disease which can affect testosterone levels; (3) had undergone any antibiotic or hormone therapy in the previous 6 months; (4) have diabetic ketoacidosis or diabetic non-ketotic hyperosmolar syndrome; and (5) are >60 years old. According to the US Endocrine Society recommendation (13, 14), a low testosterone level is defined as <2.8 ng/ml. The cohort involved 46 patients. 18 patients had low testosterone levels (LT group), and 28 patients had normal testosterone levels (NT group) (Figure 1).
The age, duration of T2DM, medical history (hypertension, coronary heart disease), and smoking and alcohol status of the participants were all documented. Weight was recorded by adjusting 0.1 kg. Physical status was evaluated by body mass index (BMI). The blood pressure was taken after a sitting posture for 30 min. All individuals had venous blood samples drawn in the morning following an overnight fast of at least 8 h.
The total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), insulin (INS), and connecting peptide (C-P) were tested using electrochemiluminescent procedures (Cobas E601; Roche, Basel, Switzerland). The uric acid (URIC), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), glycosylated hemoglobin A1c (HbA1c), fasting blood glucose (FPG), and C-reactive protein (CRP) levels were measured using enzymatic methods with an ARCHITECT ci16200 Integrated System (Abbott, Abbott Park, IL, USA). HOMA-IR = FPG(mmol/L) × INS(μIU/mL)/22.5. Testosterone secretion index (TSI) = T(nmol/L)/LH(IU/L). (The conversion criterion for testosterone is 1 ng/ml = 0.288184 nmol/l) (15).
Continuous data with a normal distribution are provided as mean values with standard deviation (SD), whereas variables without a normal distribution are shown as median values with an interquartile range (IQR; 25th–75th percentiles). The percentages are used to represent categorical variables. A t-test for continuous variables was performed for variables having a normal distribution. For variables without a normal distribution, the non-parametric Mann–Whitney U-test was used. Fisher exact tests were used to compare the constituent ratio. All results were analyzed by R software (Version 4.1.2). p < 0.05 was considered to be statistically significant.
Fecal samples collected in centrifuge tubes were stored at –20°C and then transferred to a –80°C freezer within 24 h. DNA was extracted from the fecal samples, and sequencing libraries were generated using TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA) to amplify the V3–V4 regions (Novogene Co., Ltd., Beijing, China).
Alpha diversity was reflected by Chao1, Observed-species, Simpson, and Shannon indexes. The Chao1 index was chosen to determine Community richness, while the Simpson and Shannon indexes were utilized to determine Community diversity. All these indexes in our samples were calculated with QIIME software (Version 1.9.0) and displayed with the ggpubr package in R software (Version 4.1.2).
The beta diversity analysis was performed to assess how different samples differed in terms of species complexity. Beta diversity was calculated on QIIME software. Principal coordinate analysis (PCoA) was performed based on Bray–Curtis distance to get principal coordinates and visualize multidimensional data. A distance matrix of previously collected sample data was translated into a new set of orthogonal axes, with the first primary coordinate displaying the largest variance factor, the second main coordinate displaying the second maximum factor, etc. PCoA analysis was displayed by WGCNA package, stat packages, and ggplot2 package in R software.
The effect size (LEfSe) of linear discriminant analysis (LDA) was utilized to find the bacterial taxa and metabolism-related clinical factors that differed significantly across groups. Logarithmic LDA values > 2.0 were set as the threshold for differential flora identification.
Linear regression analysis was used to analyze the relationship between testosterone and the abundance of microbiota in T2DM. Stepwise linear regression analysis was used to identify the association between testosterone levels and related risk factors. All results were analyzed by R software. p < 0.05 was considered to be statistically significant.
A comparison of the random forest model based on taxa composition and the correlation between gut microbiota and clinical manifestations (Spearman’s rank correlation coefficient) was completed using the Wekemo Bioincloud (https://www.bioincloud.tech).
Table 1 summarizes the medical history and clinical and metabolic data of the participants. The age (p = 0.68), BMI (p = 0.67), LDL-C (p = 0.59), TC (p = 0.95), TG (p = 0.12), URIC (p = 0.14), HbA1c (p = 0.50), FPG (p = 0.13), INS (p = 0.08), CRP (p = 0.29), FSH (p = 0.88), LH (p = 0.47), and PRL (p = 0.77) were similar between the two groups. Compared with the NT group, C-P is significantly higher in the LT group (p = 0.002).
We analyzed the diversity and relative abundance of taxon in the two groups to determine the distribution of intestinal microbiota in T2D males included in this study. At the phylum level, the predominant microbiota taxa in samples from two groups were Bacteroidetes, Firmicutes, and Proteobacteria (Supplemental Figure 1A). Some bacteria genera, such as Bacteroides, Lactobacillus, Bifidobacterium, Blautia, and Escherichia–Shigella, dominated the gut microbiota of the two groups at the genus level.
To determine the differences in community construction and richness between the two groups, we checked the microflora diversity in the groups (using Chao1, observed species, Shannon, and Simpson indexes). However, in these indicators, there was no significant difference between the two groups (Figure 2A). We further compared the differences in community membership between the different groups by PCoA (Figure 2B). Between the NT and LT groups, there was no discernible difference in gut flora.
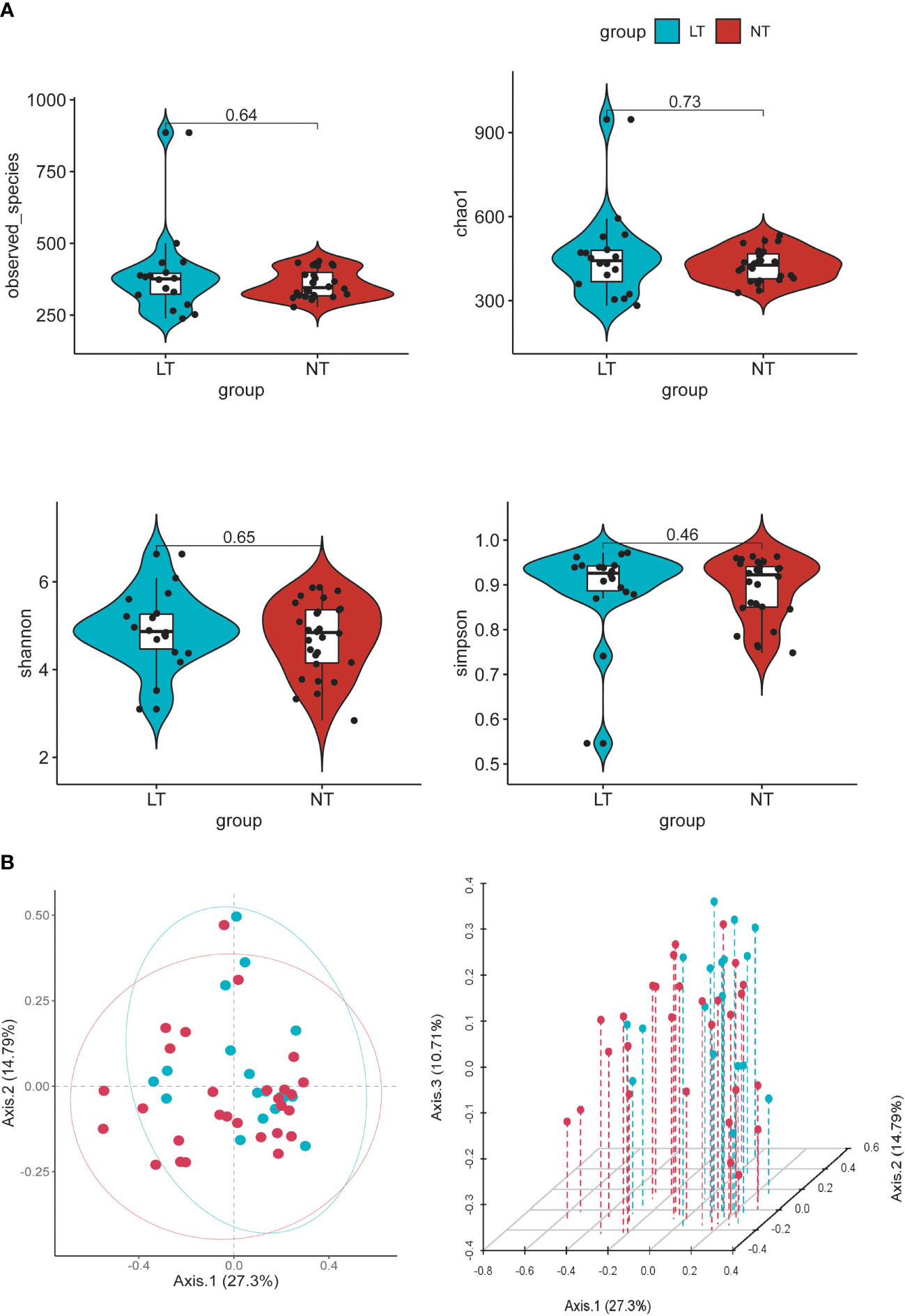
Figure 2 Alpha and beta diversity of the gut microbial communities from participants. (A) Comparison of observed OUT Numbers, chao1, shannon and simpson indexes between two groups. (B) Principal coordinate analysis (PCoA) of fecal microbiota, each dot represents the bacterial community composition of one individual stool sample, and the axis titles indicates the percentage variation explained (27.3% and 14.79 %, respectively).
We used the LEfSe method to investigate the microbe difference between the LT and NT groups. Compared with the NT group, T2D men with low testosterone had a greater abundance of Massilia, Gemella, Lachnospiraceae_UCG_001, Actinoplanes, Allorhizobium_Neorhizobium_Pararhizobiu, Solobacterium, Lachnoclostridium, Parvimonas, Bergeyella, and Blautia at the genus level, yet Candidatus_Saccharimonas, Paludicola, and Allisonella were the most abundant species in the NT group (Figure 3). These findings revealed that the LT and NT groups had statically significantly different microbial species.
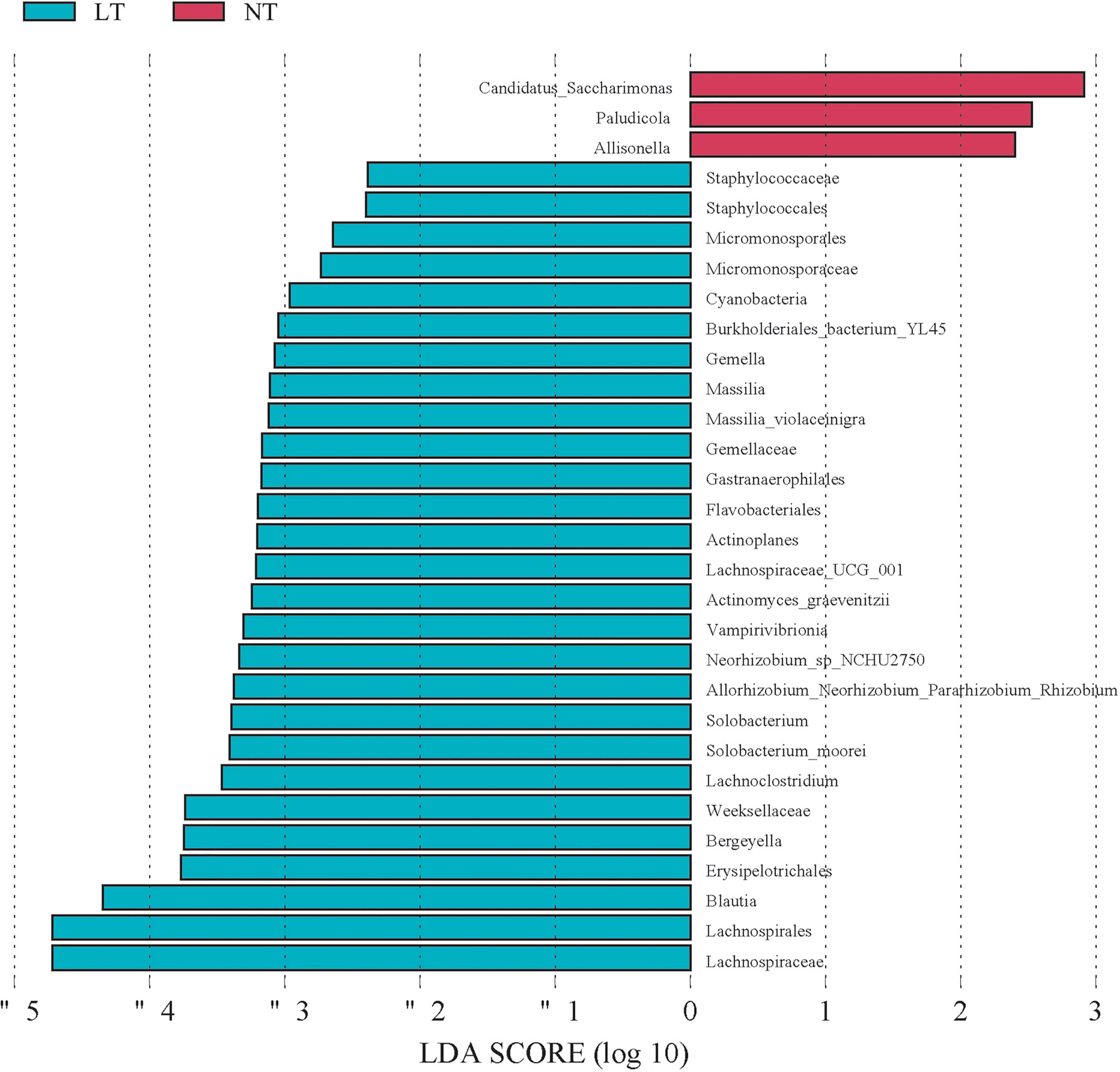
Figure 3 Microbial species differed between LT group and NT group. Linear discrimination analysis (LAD) effect size (LEfSE). Histogram of the LAD scores computed for differentially abundant species between the LT group and control (NT) group. The LAD scores (Log 10)>2 are listed.
In terms of variable importance, intestinal bacteria, including Butyricicoccus, Blautia, CAG-56, Lachnoclostridium, Actinomyces, Fusicatenibacter, Bergeyella, Streptococcus, and Solobacterium were the most associated with the LT group, and Lactobacillus, Bifidobacterium, Christensenellaceae_R-7_group, Allisonella, UGC-009, and NK4A214_group were the most associated with the NT group (Figure 4).
We explored the relationship between these individuals’ gut microbiome and their clinical data; Lachnoclostridium, Blautia, and Bergeyella had a statistically significant negative association with testosterone level, respectively. Furthermore, Lachnoclostridium is also negatively correlated with FSH and positively correlated with TG, and Blautia was positively associated with fasting blood glucose (FPG) and TC level. Streptococcus was found to have a positive association with INS, C-P, and HOMA-IR. By contrast, the microbiota taxa related to the NT group, such as Christensenellaceae_R-7_group, was found positively correlated with the serum levels of testosterone and LH and negatively correlated with BMI, INS, C-P, HOMA-IR, and CRP levels. Bifidobacterium had a negative association with the FPG level (Figure 5).
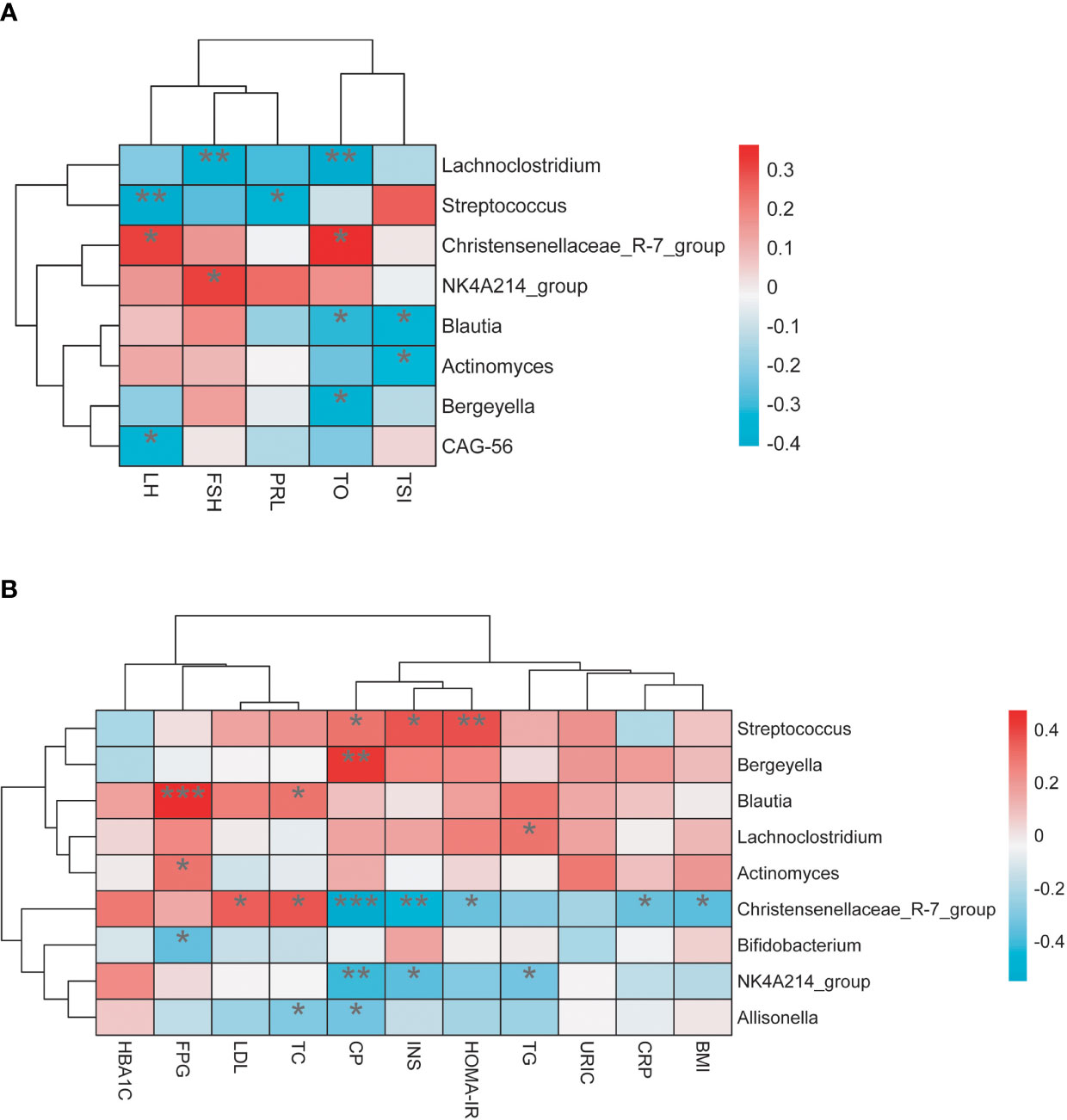
Figure 5 (A) The relationship between gut microbiota and sex hormone. (B) The relationship between gut microbiota and related metabolic indicators. *P < 0.05, **P < 0.01, ***P < 0.001.
Based on the above results, we found that most of the intestinal flora associated with the testosterone level group (Blautia, CAG_56, Lachnoclostridium, Fusicatenibacter) belongs to Lachnospirales at the order level and Firmicutes at the phylum level. We use linear regression analysis to assess the association between them and found that Lachnospirales is negatively correlated with testosterone levels (p = 0.015) (Figure 6A). We further analyzed the relationship between Firmicutes and testosterone level and found that at phylum levels, the relative abundance of Firmicutes is significantly negatively associated with testosterone level (p = 0.011) (Figure 6B).
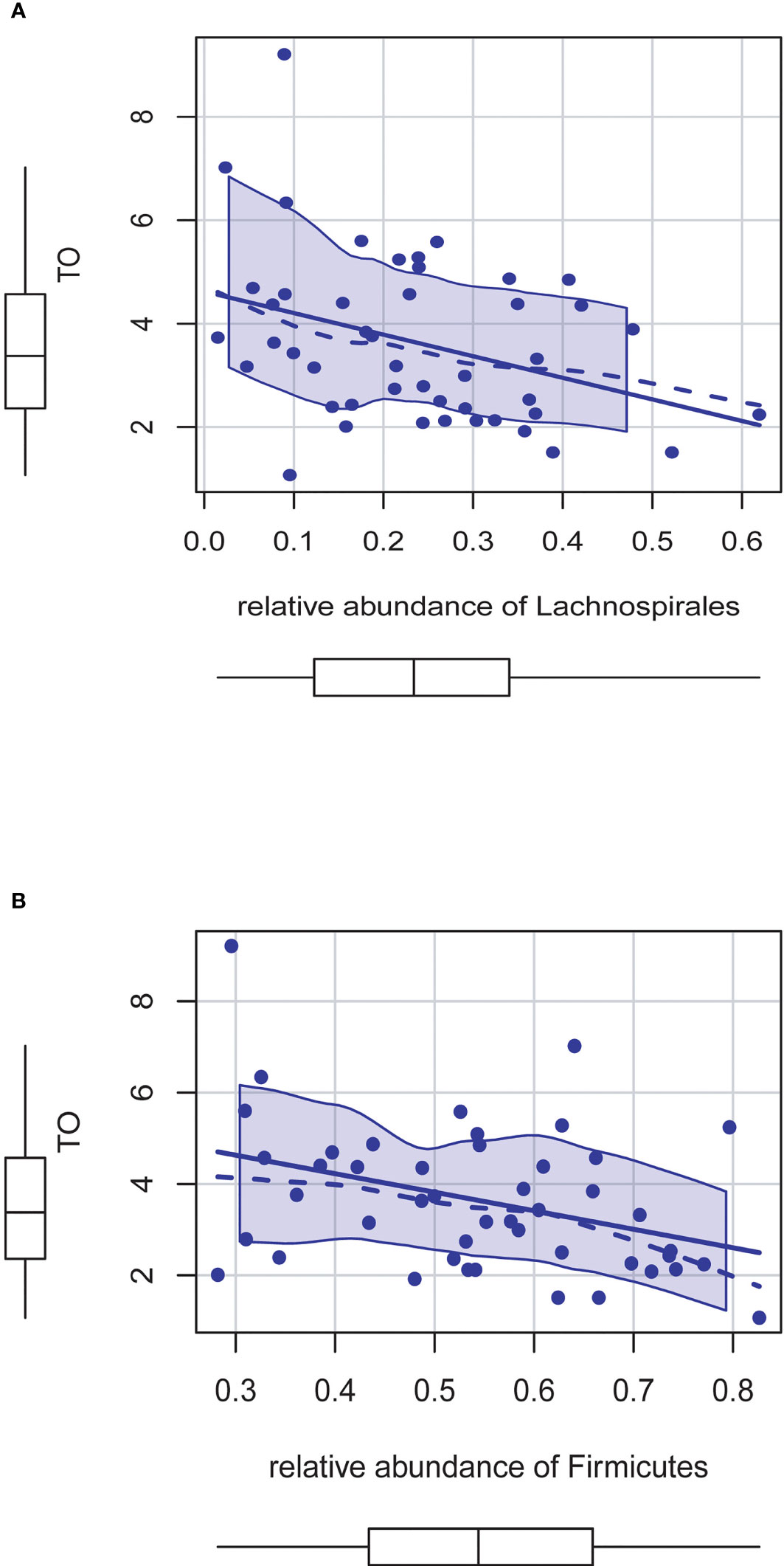
Figure 6 (A) The relative abundance of Lachnospirales is negatively correlated with testosterone levels (P = 0.015). (B) The relative abundance of Firmicutes is significantly negatively associated with testosterone level (P = 0.011).
The stepwise linear regression analysis was used to identify the association between testosterone levels and related risk factors. After adjustment for HOMA-IR and CRP, the relative abundance of Lachnospirales still had a significant negative correlation with testosterone level (Table 2). The relative weight model of Jeff Johnson (16) was used to evaluate the degree to which each predictive variable explained the model variance (R2 = 30.8%). HOMA-IR explained 34.97% of R2, and CRP explained 32.22% of R2, followed by Lachnospirales (32.22%) (Supplemental Figure 2).
Correlation analysis was used to decipher the relationship of microbiota taxon in the LT group. These findings were then used to build a network for detecting microbial taxon intragroup correlations (Spearman’s rank correlation coefficient > 0.6, adjusted p < 0.05). Erysipclotrichaccae_UCG_003, Megamonas, Faecalibacterium, Agathobacter, and Roseburia which belong to Firmicutes show a closely positive connection. Ruminococcus_torques_group had a significant positive correlation with Streptococcus. Ruminococcus_gnavus_group which belongs to Firmicutes and Bacteroidota shows a closely negative connection (Figure 7).
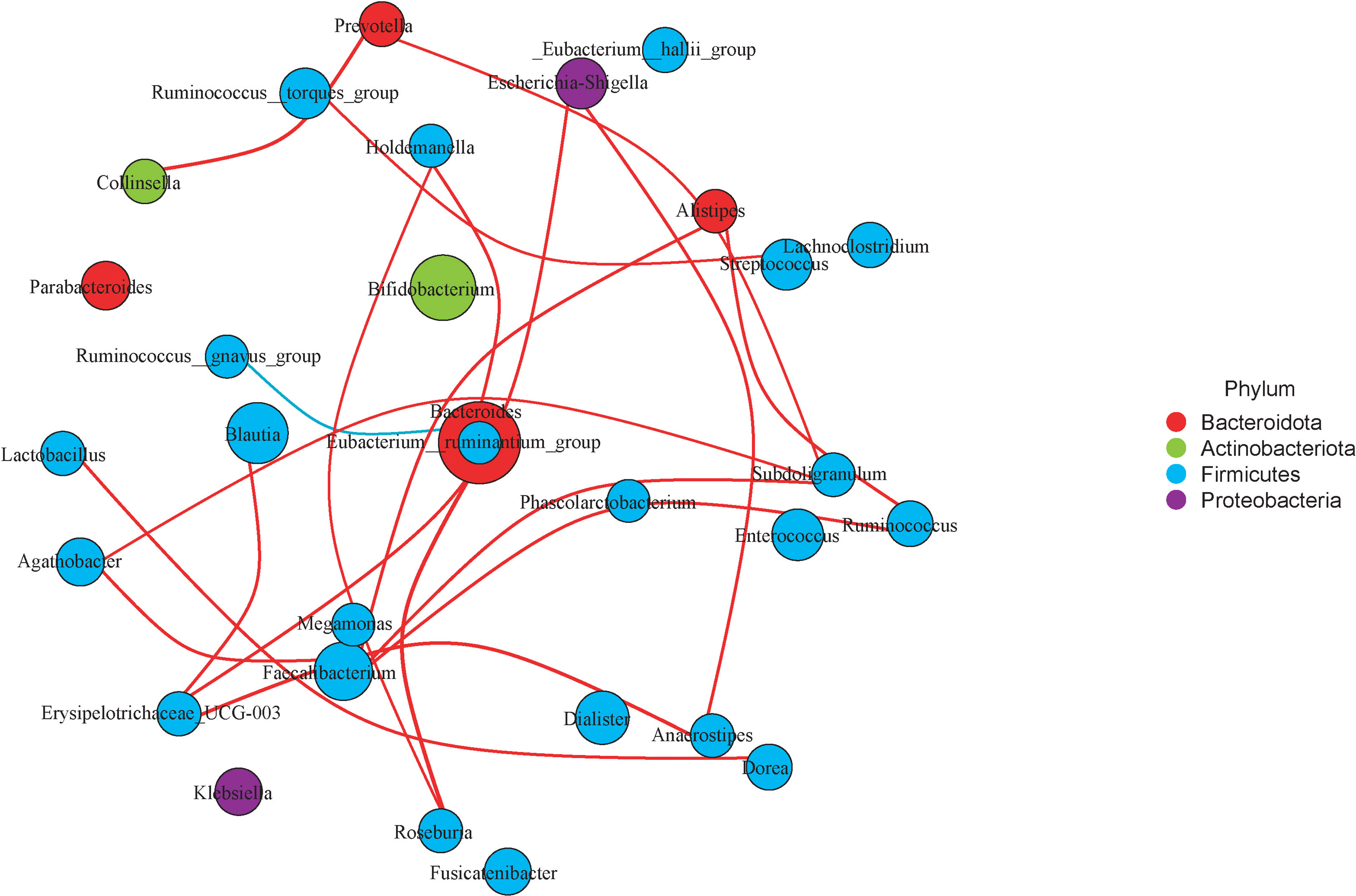
Figure 7 Microbiota network of LT group. Choose the top 30 microbiota taxon of LT group and detect correlations of them (Spearman’s rank correlation coefficient>0.6, adjusted P<0.05). The red lines represent a positive correlation, and the blue lines represent negative correlation.
In the past 50 years, with the decline in physical activity and a shift to diets with high-fat content high-calorie foods, T2DM is increasing in prevalence (17, 18). These led to changes in metabolism and hormonal regulation (18), which lead to many complications. T2DM concomitant with testosterone deficiency may significantly affect sexual function and multiple-organ systems, leading to sarcopenia, obesity, frailty, cognitive complaints, and cardiovascular diseases (19–21). Such problems seriously undermine the mental and physical health as well as life quality of men. Subnormal testosterone levels are found inside one to two-thirds of all males with T2DM (19). The pathophysiological mechanism underpinning T2DM-induced hypogonadism has not been identified. Gut microbiota dysbiosis is a feature that is shared by both T2DM and hypogonadism (22, 23). However, the role of gut dysbiosis in testosterone deficiency with T2DM remains unclear. Our findings demonstrated that T2DM patients with testosterone insufficiency had more severe gut dysbiosis than T2DM groups, which is manifested as an increase in opportunistic pathogens and gram-negative bacteria. Furthermore, some taxa at the genus level, such as Lachnoclostridium, Blautia, and Bergeyella, were linked to testosterone and had strong correlations with metabolic indicators such as HOMA-IR, FPG, TC, and TG.
According to the research done in this work on the structural composition of intestinal flora, we showed that the composition of gut microbiota of testosterone deficiency patients and the NT group is different. However, there was no significant difference between the two groups in terms of α-diversity and β-diversity. At present, some studies show that the species diversity was positively associated with testosterone levels (24), but some research has not found a connection between them (25). It might be due to the limited sample size, and increasing the sample size may result in a more exact response.
LEfSe multilevel species discrimination was used to identify the important bacterial genus in the digestive tract of individuals with low testosterone levels. The microbes enriched in the NT group were critical to a stable intestinal environment. For example, Candidatus_Saccharimonas may help to reduce intestinal inflammation and prevent intestinal barrier dysfunction (26). By comparison, the LT group was enriched with opportunistic pathogens such as Gemella, Bergeyella, Parvimonas, and Actinomyces. According to earlier research, these infections may be linked to metabolic problems, immunological modulation, and inflammation (27, 28). Furthermore, Gram-negative bacteria such as Massilia and Allorhizobium, which were plentiful with LPS, were increased in LT patients (29, 30). LPS was associated with an increase in a number of metabolic illnesses, which are among the strongest elicitors of pro-inflammatory signaling (31). According to some studies, injecting LPS into mice reduces the synthesis and metabolism of Cyp3a11, a steroidogenesis enzyme (32); other studies showed that LPS promoted infectious orchitis (33). The various characteristics and metabolic processes of these pathogens may increase the host’s susceptibility to inflammation, resulting in testosterone deficiency. This is in line with Tremellen’s view that metabolism endotoxemia caused by gut microbiota dysbiosis might impact androgen production (29).
The impact of gut dysbiosis on testosterone levels may not be limited to inflammation. Previous research has proposed that gut microbiota may affect testosterone levels via the HPG axis (9). According to our results, the microbiota taxa with a higher importance in the LT group, such as Lachnoclostridium and Streptococcus, showed a stronger negative correlation with FSH and LH, respectively. It may suggest a possible mechanism that gut microbiota may affect testosterone by regulating the HPG axis. Moreover, other taxa related to the LT group, such as Lachnoclostridium and Blautia, are significantly positively correlated with HOMA-IR, TG, TC, and FPG which represent metabolic disorders, which is consistent with the results of Ren (34) and Xie (25). According to previous reports, insulin resistance and hyperlipidemia were independently associated with testosterone levels (35). We proposed that the neuroendocrine disorders caused by gut microbiota dysbiosis, together with previous findings, may indicate an underlying mechanism that explained testosterone deficiency.
We found that most of the intestinal flora associated with testosterone levels belongs to Lachnospirales at the order level. Further analysis shows that after adjustment for HOMA-IR and CRP, the relative abundance of Lachnospirales had a significant negative correlation with testosterone level. Previous research has found that the quantity of Lachnospirales including Blautia is enhanced in a variety of disorders, such as diabetes and non-alcoholic fatty liver disease. Inflammation, hyperglycemia, and obesity have all been linked to the activity of Lachnospirales (36–38). In germ-free (GF) mice, Lachnospirales were identified from the hyperglycemic obese mice, indicating its role in the development of metabolic disorders. The aforesaid species colonized GF mice, causing substantial increases in plasma glucose, as well as reduced serum insulin (36). At present, there is no research that reported that Lachnospirales could directly affect the synthesis or metabolism of testosterone. We suspect that the increased abundance of Lachnospirales may cause or represent a metabolic disorder status. Identification of Lachnospirales dysbiosis may be an important predictor of testosterone deficiency in T2DM male patients.
Another interesting feature of our research is that taxonomic groups of similar microorganisms like to clump together. This was in line with prior findings (39, 40). Erysipclotrichaccae_UCG_003, Megamonas, Faecalibacterium, Agathobacter, and Roseburia which belong to Firmicutes show a closely positive connection. The Ruminococcus_torques_group had a significant positive correlation with Streptococcus. These microorganisms, which have a favorable correlation in testosterone deficit individuals, may collaborate in certain pathologic situations.
This study has some limitations. Firstly, this study included participants of various ages, regions, and lifestyles, potentially resulting in heterogeneity. Secondly, because of the cross-sectional character of the study, the findings are mostly associations. Thirdly, we put much emphasis on serum testosterone to explore whether gut dysbiosis is associated with testosterone deficiency, and did not measure the contents of testosterone metabolites in the fecal samples. As a result, a well-designed randomized study investigating the clinical and metabolic consequences of hypogonadism in male T2DM patients is suggested. Despite these flaws, we believe that our research is worthwhile. Previous studies usually focused on comparing the changes of microbiota between diabetic patients and health but ignored the differences in their dietary structure. T2DM patients tend to prefer a high-fat diet. Thus, choosing T2DM patients in our study could exclude the effect of lifestyle on microbes as much as possible.
Our findings may help to better understand the effects of gut microbiota on testosterone metabolism, as well as the most effective way to prevent hypogonadism in diabetic male patients. In the future, evaluating the relative metabolites in fecal samples is helpful to explore the underlying reasons for the correlation between microbiota and testosterone level. Focusing on the exploration of potential probiotics, microbiota prescription, and microbial regulator to treat males with andrological diseases will be an appealing prospect.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This cross-sectional study was approved by the Shandong Provincial Hospital ethical committee. Written informed consent was obtained from all participants.
Conceptualization, SL and RC. Data curation, RC. Analysis, SL and ZY. Validation and visualization, SL, LL, and XF. Writing—original draft, SL and RC. Writing—review and editing, SL, RC, YL, XQ, CY, and QG. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation (81770860) and the Key Research and Development Plan of Shandong Province (2017CXGC1214).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.836485/full#supplementary-material
1. Perreault L, Skyler JS, Rosenstock J. Novel Therapies With Precision Mechanisms for Type 2 Diabetes Mellitus. Nat Rev Endocrinol (2021) 17(6):364–77. doi: 10.1038/s41574-021-00489-y
2. Halpern JA, Brannigan RE. Testosterone Deficiency. JAMA (2019) 322(11):1116. doi: 10.1001/jama.2019.9290
3. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N Engl J Med (2010) 363(2):123–35. doi: 10.1056/NEJMoa0911101
4. Herrero A, Marcos M, Galindo P, Miralles JM, Corrales JJ. Clinical and Biochemical Correlates of Male Hypogonadism in Type 2 Diabetes. Andrology (2018) 6(1):58–63. doi: 10.1111/andr.12433
5. Cheung KK, Luk AO, So WY, Ma RC, Kong AP, Chow FC, et al. Testosterone Level in Men With Type 2 Diabetes Mellitus and Related Metabolic Effects: A Review of Current Evidence. J Diabetes Investig (2015) 6(2):112–23. doi: 10.1111/jdi.12288
6. Canfora EE, Meex RCR, Venema K, Blaak EE. Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat Rev Endocrinol (2019) 15(5):261–73. doi: 10.1038/s41574-019-0156-z
7. Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients (2020) 12(12):3719. doi: 10.3390/nu12123719
8. Xia F, Wen LP, Ge BC, Li YX, Li FP, Zhou BJ. Gut Microbiota as a Target for Prevention and Treatment of Type 2 Diabetes: Mechanisms and Dietary Natural Products. World J Diabetes (2021) 12(8):1146–63. doi: 10.4239/wjd.v12.i8.1146
9. Li X, Cheng W, Shang H, Wei H, Deng C. The Interplay Between Androgen and Gut Microbiota: Is There a Microbiota-Gut-Testis Axis. Reprod Sci (2021). doi: 10.1007/s43032-021-00624-0
10. Devendran S, Mythen SM, Ridlon JM. The desA and desB Genes From Clostridium Scindens ATCC 35704 Encode Steroid-17,20-Desmolase. J Lipid Res (2018) 59(6):1005–14. doi: 10.1194/jlr.M083949
11. Chen YL, Wang CH, Yang FC, Ismail W, Wang PH, Shih CJ, et al. Identification of Comamonas Testosteroni as an Androgen Degrader in Sewage. Sci Rep (2016) 6:35386. doi: 10.1038/srep35386
12. Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, et al. Probiotic Microbes Sustain Youthful Serum Testosterone Levels and Testicular Size in Aging Mice. PloS One (2014) 9(1):e84877. doi: 10.1371/journal.pone.0084877
13. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2018) 103(5):1715–44. doi: 10.1210/jc.2018-00229
14. Seftel AD, Kathrins M, Niederberger C. Critical Update of the 2010 Endocrine Society Clinical Practice Guidelines for Male Hypogonadism: A Systematic Analysis. Mayo Clin Proc (2015) 90(8):1104–15. doi: 10.1016/j.mayocp.2015.06.002
15. Muerkoster AP, Frederiksen H, Juul A, Andersson AM, Jensen RC, Glintborg D, et al. Maternal Phthalate Exposure Associated With Decreased Testosterone/LH Ratio in Male Offspring During Mini-Puberty. Odense Child Cohort. Environ Int (2020) 144:106025. doi: 10.1016/j.envint.2020.106025
16. Johnson JW. A Heuristic Method for Estimating the Relative Weight of Predictor Variables in Multiple Regression. Multivar Behav Res (2000) 35(1):1–19. doi: 10.1207/S15327906MBR3501_1
17. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 Diabetes Mellitus in Older Adults: Clinical Considerations and Management. Nat Rev Endocrinol (2021) 17(9):534–48. doi: 10.1038/s41574-021-00512-2
18. SantaCruz-Calvo S, Bharath L, Pugh G, SantaCruz-Calvo L, Lenin RR, Lutshumba J, et al. Adaptive Immune Cells Shape Obesity-Associated Type 2 Diabetes Mellitus and Less Prominent Comorbidities. Nat Rev Endocrinol (2021) 18(1):23–42. doi: 10.1038/s41574-021-00575-1
19. Russo V, Chen R, Armamento-Villareal R. Hypogonadism, Type-2 Diabetes Mellitus, and Bone Health: A Narrative Review. Front Endocrinol (Lausanne) (2020) 11:607240. doi: 10.3389/fendo.2020.607240
20. Saad F. The Relationship Between Testosterone Deficiency and Frailty in Elderly Men. Horm Mol Biol Clin Investig (2010) 4(1):529–38. doi: 10.1515/HMBCI.2010.060
21. Cherrier M. Testosterone Effects on Cognition in Health and Disease. Front Horm Res (2009) 37:150–62. doi: 10.1159/000176051
22. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature (2012) 490(7418):55–60. doi: 10.1038/nature11450
23. Collden H, Landin A, Wallenius V, Elebring E, Fändriks L, Nilsson ME, et al. The Gut Microbiota is a Major Regulator of Androgen Metabolism in Intestinal Contents. Am J Physiol Endocrinol Metab (2019) 317(6):E1182–92. doi: 10.1152/ajpendo.00338.2019
24. Mayneris-Perxachs J, Arnoriaga-Rodríguez M, Luque-Córdoba D, Priego-Capote F, Pérez-Brocal V, Moya A, et al. Gut Microbiota Steroid Sexual Dimorphism and Its Impact on Gonadal Steroids: Influences of Obesity and Menopausal Status. Microbiome (2020) 8(1):136. doi: 10.1186/s40168-020-00913-x
25. Xie Y, Sun J, Wei L, Jiang H, Hu C, Yang J, et al. Altered Gut Microbiota Correlate With Different Immune Responses to HAART in HIV-Infected Individuals. BMC Microbiol (2021) 21(1):11. doi: 10.1186/s12866-020-02074-1
26. Huang C, Chen J, Wang J, Zhou H, Lu Y, Lou L, et al. Dysbiosis of Intestinal Microbiota and Decreased Antimicrobial Peptide Level in Paneth Cells During Hypertriglyceridemia-Related Acute Necrotizing Pancreatitis in Rats. Front Microbiol (2017) 8:776. doi: 10.3389/fmicb.2017.00776
27. Sciavilla P, Strati F, Di Paola M, Modesto M, Vitali F, Cavalieri D, et al. Gut Microbiota Profiles and Characterization of Cultivable Fungal Isolates in IBS Patients. Appl Microbiol Biotechnol (2021) 105(8):3277–88. doi: 10.1007/s00253-021-11264-4
28. Shi M, Wei Y, Hu W, Nie Y, Wu X, Lu R. The Subgingival Microbiome of Periodontal Pockets With Different Probing Depths in Chronic and Aggressive Periodontitis: A Pilot Study. Front Cell Infect Microbiol (2018) 8:124. doi: 10.3389/fcimb.2018.00124
29. Tremellen K. Gut Endotoxin Leading to a Decline IN Gonadal Function (GELDING) - A Novel Theory for the Development of Late Onset Hypogonadism in Obese Men. Basic Clin Androl (2016) 26:7. doi: 10.1186/s12610-016-0034-7
30. Habbadi K, Meyer T, Vial L, Gaillard V, Benkirane R, Benbouazza A, et al. Essential Oils of Origanum Compactum and Thymus Vulgaris Exert a Protective Effect Against the Phytopathogen Allorhizobium Vitis. Environ Sci Pollut Res Int (2018) 25(30):29943–52. doi: 10.1007/s11356-017-1008-9
31. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes (2007) 56(7):1761–72. doi: 10.2337/db06-1491
32. Goralski KB, Abdulla D, Sinal CJ, Arsenault A, Renton KW. Toll-Like Receptor-4 Regulation of Hepatic Cyp3a11 Metabolism in a Mouse Model of LPS-Induced CNS Inflammation. Am J Physiol Gastrointest Liver Physiol (2005) 289(3):G434–43. doi: 10.1152/ajpgi.00562.2004
33. Deng SL, Zhang BL, Reiter RJ, Liu YX. Melatonin Ameliorates Inflammation and Oxidative Stress by Suppressing the P38mapk Signaling Pathway in LPS-Induced Sheep Orchitis. Antioxidants (Basel) (2020) 9(12):1277. doi: 10.3390/antiox9121277
34. Ren SM, Mei L, Huang H, Cao SF, Zhao RH, Zheng PY. Correlation Analysis of Gut Microbiota and Biochemical Indexes in Patients With Non-Alcoholic Fatty Liver Disease. Zhonghua Gan Zang Bing Za Zhi (2019) 27(5):369–75. doi: 10.3760/cma.j.issn.1007-3418.2019.05.009
35. Fernandez-Miro M, Chillaron JJ, Pedro-Botet J. Testosterone Deficiency, Metabolic Syndrome and Diabetes Mellitus. Med Clin (Barc) (2016) 146(2):69–73. doi: 10.1016/j.medcli.2015.06.020
36. Mu Z, Yang Y, Xia Y, Wang F, Sun Y, Yang Y, et al. Probiotic Yeast BR14 Ameliorates DSS-Induced Colitis by Restoring the Gut Barrier and Adjusting the Intestinal Microbiota. Food Funct (2021) 12(18):8386–98. doi: 10.1039/D1FO01314A
37. Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms (2020) 8(4):573. doi: 10.3390/microorganisms8040573
38. Zeng H, Ishaq SL, Zhao FQ, Wright AG. Colonic Inflammation Accompanies an Increase of Beta-Catenin Signaling and Lachnospiraceae/Streptococcaceae Bacteria in the Hind Gut of High-Fat Diet-Fed Mice. J Nutr Biochem (2016) 35:30–6. doi: 10.1016/j.jnutbio.2016.05.015
39. Chu W, Han Q, Xu J, Wang J, Sun Y, Li W, et al. Metagenomic Analysis Identified Microbiome Alterations and Pathological Association Between Intestinal Microbiota and Polycystic Ovary Syndrome. Fertil Steril (2020) 113(6):1286–98.e4. doi: 10.1016/j.fertnstert.2020.01.027
Keywords: T2DM, gut microbiota, dysbiosis, testosterone deficiency, male
Citation: Liu S, Cao R, Liu L, Lv Y, Qi X, Yuan Z, Fan X, Yu C and Guan Q (2022) Correlation Between Gut Microbiota and Testosterone in Male Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 13:836485. doi: 10.3389/fendo.2022.836485
Received: 15 December 2021; Accepted: 18 February 2022;
Published: 25 March 2022.
Edited by:
Thozhukat Sathyapalan, Hull York Medical School, United KingdomReviewed by:
Tarique Hussain, Nuclear Institute for Agriculture and Biology, PakistanCopyright © 2022 Liu, Cao, Liu, Lv, Qi, Yuan, Fan, Yu and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxiao Yu, eXVjaHgwOEAxNjMuY29t; Qingbo Guan, ZG9jdG9yZ3VhbnFpbmdib0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.