
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 23 February 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.768028
This article is part of the Research Topic Targeted Therapy in Advanced Thyroid Cancer View all 9 articles
Objectives: Our aim was to describe our experience in using apatinib as treatment for radioiodine-refractory differentiated thyroid carcinoma (RAIR-DTC).
Methods: Forty-seven patients undergoing apatinib treatment for RAIR-DTC were prospectively enrolled in this study. The study endpoints were objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and rate of adverse events.
Results: No patients achieved complete response, while 36 (76.6%) and 8 (17.0%) patients achieved partial response and stable disease, respectively. The ORR and DCR were 76.6% and 93.6%, respectively. The median PFS and OS were 18 and 59 months, respectively. A total of 91 adverse events occurred, of which 21 were graded as grade 3 or higher. There were no drug-related deaths.
Conclusions: Apatinib has distinct anti-RAIR-DTC efficacy in terms of ORR, PFS, and OS and has a favorable safety profile. It is a feasible treatment option for RAIR-DTC.
Differentiated thyroid carcinoma (DTC) is the most common endocrine malignancy. The mainstay of treatment is surgical resection, occasionally followed by iodine-131 therapy (1). Most patients treated accordingly have good prognoses. However, disease recurrence may occur in a minor subset of DTC patients (2). If complete surgical excision is not possible, or if tumor progression has occurred and distant metastases are present, iodine-131 treatment is used (3). Nevertheless, some recurrent/metastatic lesions lose their ability of iodine concentration, which is known as radioiodine-refractory DTC (RAIR-DTC) (4). RAIR-DTC is associated with poor survival and contributes to most DTC-related deaths (5).
Treatment of RAIR-DTC is challenging in clinics as the therapeutic options are limited (6). Sorafenib and lenvatinib were approved for the treatment of progressive, metastatic RAIR-DTC by the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA), owing to the clinical benefits of these drugs reported in two phase III clinical trials (7, 8). However, both drugs are too expensive for most Chinese patients. Apatinib (Jiangsu Hengrui Medicine, Lianyungang, China) is another small-molecule tyrosine kinase inhibitor that has similar targets to sorafenib and lenvatinib. The effectiveness of apatinib has been demonstrated in a phase II trial by Lin et al., who reported an objective response rate (ORR) of 80% and a disease control rate (DCR) of 95% (9). Zhang et al. (10) have recently reported a case of inoperable DTC treated with apatinib. Research works regarding the application of apatinib in DTC are limited. Therefore, in the current study, we aimed to describe our experience in using apatinib for the treatment of RAIR-DTC.
This study was approved by the Henan Cancer Hospital Institutional Research Committee. All participants provided written informed consent. All procedures involving human participants were conducted according to the principles of the Declaration of Helsinki.
From January 2015 to December 2020, a total of 1,094 patients with recurrent/metastatic thyroid cancer presented to our department in need of medical treatment. Among them, 99 patients were confirmed as having unresectable RAIR-DTC based on the following criteria: no uptake of iodine in the target lesion; progressive disease 12–16 months after iodine-131 therapy; and progressive disease after receiving a cumulative iodine-131 dose of above 22.3 GBq (11). All of the 99 patients had at least one measurable lesion and had not received prior tyrosine kinase inhibitor treatment or chemotherapy. After the potential benefits and complications were clearly explained, 30 patients refused enrollment owing to the possibility of adverse complications, 12 patients refused enrollment due to poor economy, and 10 patients abandoned further treatment of their metastatic thyroid cancer prior to initiating apatinib. A total of 47 patients finally agreed to participate in our study (Figure 1).
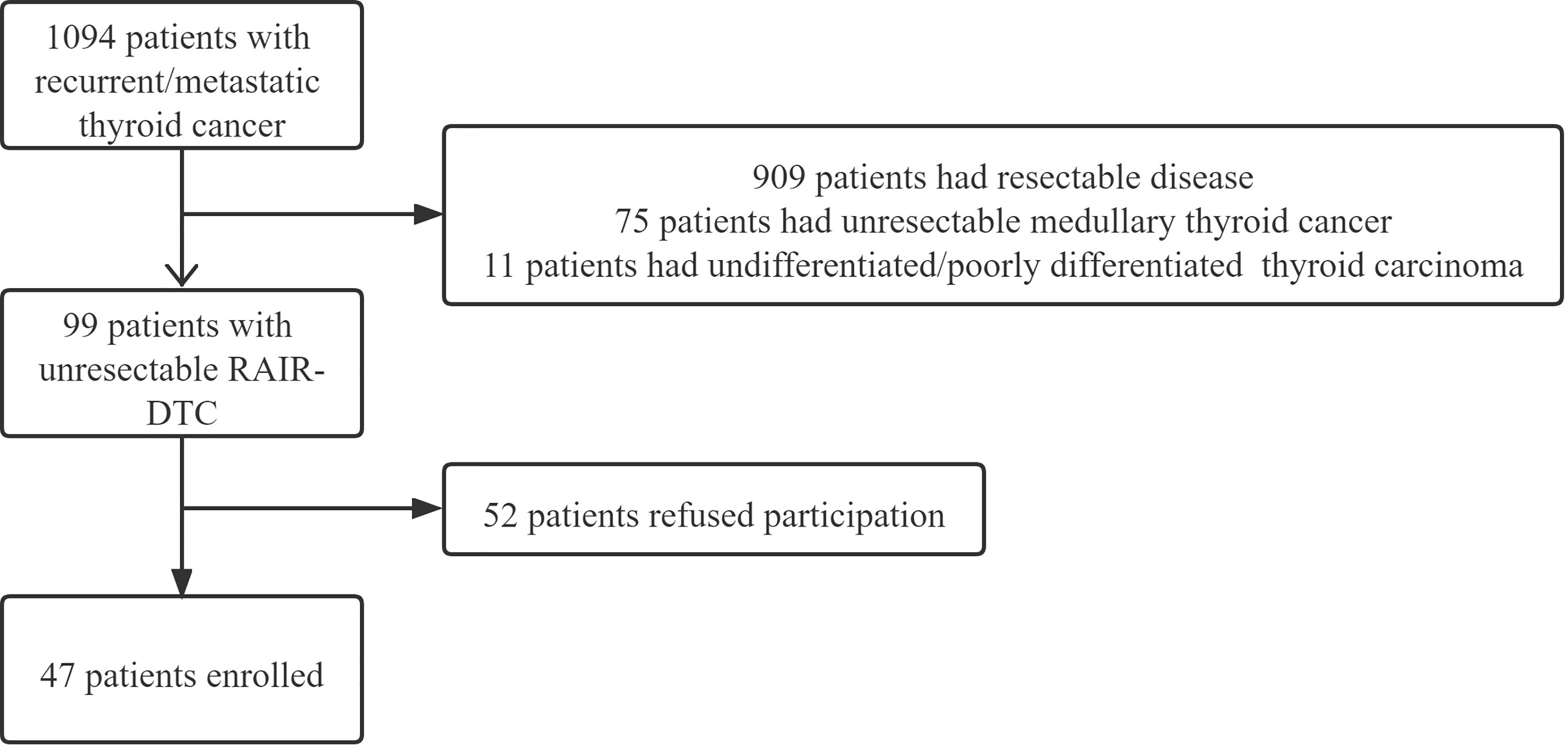
Figure 1 Flowchart of the enrollment of radioiodine-refractory differentiated thyroid carcinoma (RAIR-DTC) patients.
The 47 participants received treatment of 500 mg of apatinib daily for a 4-week cycle. The patients were followed every 4 weeks for the first 8 weeks and every 8 weeks thereafter with CT or MRI imaging. The dosage of apatinib was reduced in the instance of a grade 3 or higher adverse event. Treatment continued until the occurrence of disease progression, drug intolerance, or withdrawal of consent to participate in the study.
The primary endpoints in this study were ORR and DCR. The ORR refers to the percentage of patients who showed complete response (CR) or partial response (PR), while the DCR refers to the percentage of patients who showed CR, PR, or stable disease (SD), according to the Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 (12). The secondary endpoints included progression-free survival (PFS) and overall survival (OS). PFS was calculated from the date of initial treatment to the date of disease progression or death from any cause. OS was calculated from the date of initial treatment to the date of the last follow-up or death from any cause (13). The third endpoint was the rate of adverse reactions, which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) (14).
All statistical analyses were performed using SPSS version 20.0 (IBM Inc., Chicago, IL, USA), and the Kaplan–Meier method was used to calculate the survival rates. Statistical significance was defined as p < 0.05.
Of the 47 participants enrolled in our study, 19 (40.4%) were men and 28 (59.6%) were women, and the mean age was 55.8 years (range = 48–68 years). Diagnosis of papillary thyroid carcinoma was confirmed with fine needle aspiration, and the mean time between previous treatment and diagnosis of RAIR-DTC was 2.5 ± 1.2 years. Thirty-two (68.1%) patients were found to have the BRAFV600E mutation. The Eastern Cooperative Oncology Group (ECOG) performance status scores were 0 in 15 (31.9%) patients, 1 in 30 (63.8%) patients, and 2 in 2 (4.3%) patients. All patients had previously received surgical treatment and iodine-131 therapy, and the mean cumulative dose of iodine-131 was 406 ± 116 mCi. Distant metastases were present in all patients, namely, lung metastasis in 35 (74.5%), bone metastasis in 16 (34.0%), liver metastasis in 7 (14.9%), and brain metastasis in 4 (8.5%) cases (Table 1).
During apatinib treatment, no patients achieved CR, while 36 (76.6%) and 8 (17.0%) patients achieved PR and SD, respectively; progressive disease occurred in 3 (6.4%) patients (Figure 2, Supplementary Data, and Supplementary Figure S1). The ORR and DCR were 76.6% and 93.6%, respectively. The mean time to objective response was 2.5 ± 1.4 months, and the mean duration of response was 17.7 ± 8.6 months.
Dose reduction was necessary for 20 patients. Of these 20 patients, PR, SD, and progressive disease occurred in 14, 5, and 1, respectively. Two patients withdrew from this study because of serious adverse reaction and disease progression.
At the last follow-up, with a median time of 35 months, all patients showed disease progression, and the median PFS was 18 months (Figure 3A). In patients with the BRAFV600E mutation, the median PFS was 18 months. This was comparable to the PFS of 16 months in patients without the BRAFV600E mutation (p = 0.567).
A total of 11 patients died, and the median OS was 59 months (Figure 3B). In patients with the BRAFV600E mutation, the median OS was 59 months, which was the same as that of patients without the BRAFV600E mutation (p = 0.554).
Ninety-one adverse events occurred in the 47 patients, of which 21 events were of grade 3 or higher. There were no drug-related deaths. The most common hematological toxicity was leucopenia, which occurred in 10.6% of patients. The three most common non-hematological toxicities were hypertension, proteinuria, and hand–foot skin reaction, which accounted for nearly 50% of all adverse events and 70% of the grade 3 and 4 adverse events (Table 2).
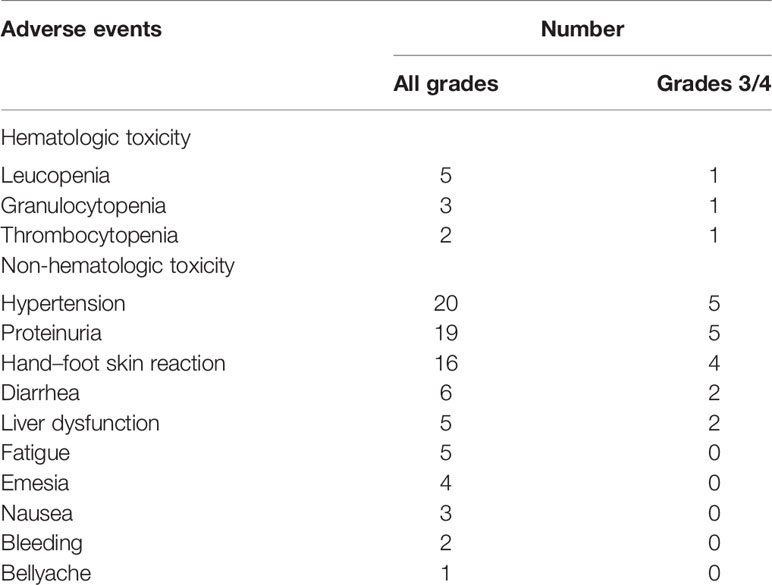
Table 2 Adverse events in the 47 patients undergoing apatinib for radioiodine-refractory thyroid cancer.
In this study, we aimed to determine the efficacy of apatinib in RAIR-DTC. We found that apatinib had reliable oncologic efficacy in RAIR-DTC patients, with limited adverse events.
RAIR-DTC is relatively uncommon, as shown in a previous study in which it only occurred in 2.2% of the 5,163 DTC patients treated (4). Nonetheless, RAIR-DTC was associated with a low 10-year OS rate (10%) (15); therefore, there is a critical need for effective treatment. Sorafenib was the first tyrosine kinase inhibitor approved by the FDA for RAIR-DTC. In the phase III DECISION trial, the sorafenib arm had an ORR of 12% and a longer PFS (by 5 months) compared with the placebo arm (7). Lenvatinib was the second tyrosine kinase inhibitor approved by the FDA and EMA for RAIR-DTC. In the phase III SELECT study, the lenvatinib arm showed an ORR of 64.8% and a median PFS of 18.3 months, both of which were higher than those of patients receiving placebo (8). A recently published phase III study has revealed the high efficacy of lenvatinib in controlling RAIR-DTC in Chinese patients (16). However, sorafenib has only been approved for advanced renal cell carcinoma and inoperable hepatocellular carcinoma in China. Since both sorafenib and lenvatinib are expensive, other feasible strategies are needed for RAIR-DTC therapy.
Apatinib, another tyrosine kinase inhibitor, is made in China. It was first used for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma. Patients treated with apatinib showed significant improvements in PFS and OS (17). The application of apatinib to RAIR-DTC has only been explored by Lin et al. (9, 10, 18–20). Lin et al. reported overall ORR and DCR of approximately 80% and 90%, respectively. This finding is consistent with the findings of the present study. The ORR and DCR in our study were significantly higher than those in the DECISION trial and slightly higher than those in the SELECT study. There are at least three considerations that may explain the differences in the findings: 1) research has shown that the migration and proliferation of endothelial cells are significantly regulated by vascular endothelial growth factor receptor-2 (VEGFR-2) (21); 2), apatinib has shown highly selective VEGFR-2 inhibition, with an IC50 of 1 nM in vitro, which is apparently lower than the IC50 values of lenvatinib and sorafenib (18, 22); and 3) the demographic and genomic differences between the Western and Chinese populations might also be partially responsible for the differences in the findings between studies.
The median PFS and OS in this study were 18 and 59 months, respectively. These findings are consistent with those in the study by Lin et al. (9). Koehler et al. (23) recently conducted a study in the real-world setting, at six German referral centers, and found median PFS rates of 9 and 12 months with the use of sorafenib and lenvatinib, respectively; the median OS rates with the use of sorafenib and lenvatinib were 37 and 47 months, respectively. Ahn et al. (24) showed that the median OS and PFS of 40 patients treated with sorafenib were 34.3 and 14.8 months, respectively. Apatinib appears to have the most oncological benefits among the three tyrosine kinase inhibitors; however, direct comparisons of apatinib, sorafenib, and lenvatinib are impossible due to the differences in patient characteristics and follow-up frequencies, among other factors.
The time to objective response is an important factor to consider when analyzing drug efficacy. We found that the time to objective response with apatinib treatment was 2.5 ± 1.4 months, which was shorter than that in the study by Lin et al. (9). A possible explanation is the difference in the inclusion standards between the studies: poorly differentiated thyroid cancer patients were also enrolled in the study by Lin et al. More importantly, the time to objective response found in our study was shorter than the time of 20% additional tumor volume increase in the placebo arms of the DECISION and SELECT trials (8, 9). This shows the potential role of apatinib in neoadjuvant treatment due to its rapid tumor burden reduction effect. Zhang et al. (10) found that an inoperable tumor shrank from 56 × 37 to 29 × 26 mm after 6 weeks of apatinib treatment; the tumor could then be completely excised with minimal complications.
The pathogenic role of the BRAFV600E mutation in DTC is well established (25). Lin et al. (9) noted that patients with the BRAFV600E mutation had longer PFS than those without the BRAFV600E mutation. A similar finding was also described by Brose et al. (7). However, in our study, we did not detect this association, most likely due to our limited sample size.
Drug safety is a key issue that requires attention. In this study, although all patients experienced adverse events, most of them were of grade 1 or 2, and there were no drug-related deaths. Lin et al. (18) reported that the toxicity of apatinib was associated with the dose: the 500-mg dose protocol achieved similar oncological control and was associated with fewer adverse events compared with the 750-mg dose protocol; this concept was also applied in the present study. In the DECISION and SELECT trials, the rates of grade 3 and 4 adverse events for the use of sorafenib and lenvatinib were 82.1% and 75.9%, respectively (5). In a recent trial involving Chinese patients, the rate of grade 3 and 4 adverse events was 85.4%, with 10 fatal treatment-related adverse events (16). This finding suggests the high safety profile of apatinib for RAIR-DTC treatment.
In conclusion, apatinib has distinct anti-RAIR-DTC efficacy in terms of ORR, PFS, and OS and has a favorable safety profile. It is a feasible treatment option for RAIR-DTC.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
This study was approved by Henan Cancer Hospital Institutional Research Committee. All participants signed a written informed consent agreement. The patients/participants provided their written informed consent to participate in this study.
All authors made a contribution to the study design, manuscript writing, study selection, data analysis, study quality evaluation, and manuscript revision. All authors have read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.768028/full#supplementary-material
1. Sutherland R, Tsang V, Clifton-Bligh RJ, Gild ML. Papillary Thyroid Microcarcinoma: Is Active Surveillance Always Enough? Clin Endocrinol (Oxf) (2021) 95:811–7. doi: 10.1111/cen.14529
2. Park J, Kim K, Lim DJ, Bae JS, Kim JS. Male Sex is Not an Independent Risk Factor for Recurrence of Differentiated Thyroid Cancer: A Propensity Score-Matching Study. Sci Rep (2021) 11:14908. doi: 10.1038/s41598-021-94461-5
3. Juweid ME, Tulchinsky M, Mismar A, Momani M, Zayed AA, Al Hawari H, et al. Contemporary Considerations in Adjuvant Radioiodine Treatment of Adults With Differentiated Thyroid Cancer. Int J Cancer (2020) 147:2345–54. doi: 10.1002/ijc.33020
4. Li G, Lei J, Song L, Jiang K, Wei T, Li Z, et al. Radioiodine Refractoriness Score: A Multivariable Prediction Model for Postoperative Radioiodine-Refractory Differentiated Thyroid Carcinomas. Cancer Med (2018) 7:5448–56. doi: 10.1002/cam4.1794
5. de la Fouchardiere C, Alghuzlan A, Bardet S, Borget I, Borson Chazot F, Do Cao C, et al. The Medical Treatment of Radioiodine-Refractory Differentiated Thyroid Cancers in 2019. A TUTHYREF® Network Review. Bull Cancer (2019) 106:812–9. doi: 10.1016/j.bulcan.2019.04.012
6. Russell MD, Kamani D, Randolph GW. Modern Surgery for Advanced Thyroid Cancer: A Tailored Approach. Gland Surg (2020) 9:S105–19. doi: 10.21037/gs.2019.12.16
7. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in Radioactive Iodine-Refractory, Locally Advanced or Metastatic Differentiated Thyroid Cancer: A Randomised, Double-Blind, Phase 3 Trial. Lancet (2014) 384:319–28. doi: 10.1016/S0140-6736(14)60421-9
8. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib Versus Placebo in Radioiodine-Refractory Thyroid Cancer. N Engl J Med (2015) 372:621–30. doi: 10.1056/NEJMoa1406470
9. Lin YS, Zhang X, Wang C, Liu YQ, Guan WM, Liang J. Long-Term Results of a Phase II Trial of Apatinib for Progressive Radioiodine Refractory Differentiated Thyroid Cancer. J Clin Endocrinol Metab (2021) 106:e3027–36. doi: 10.1210/clinem/dgab196
10. Zhang Y, Deng X, Ding Z, Kang J, Wu B, Guo B, et al. Preoperative Neoadjuvant Targeted Therapy With Apatinib for Inoperable Differentiated Thyroid Cancer: A Case Report. Med (Baltimore) (2021) 100:e25191. doi: 10.1097/MD.0000000000025191
11. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
12. Fakhry C, Lacchetti C, Rooper LM, Jordan RC, Rischin D, Sturgis EM, et al. Human Papillomavirus Testing in Head and Neck Carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J Clin Oncol (2018) 36:3152–61. doi: 10.1200/JCO.18.00684
13. Li X, Fang Q, Du W, Zhang X, Dai L, Qiao Y. Induction Chemotherapy Combined With Immunotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma. BMC Cancer (2021) 21:622. doi: 10.1186/s12885-021-08373-8
14. National Cancer Institute. Common Terminology Criteria for Adverse Events v.4.0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
15. Jones H, Green V, England J, Greenman J. Current Understanding of Nonsurgical Interventions for Refractory Differentiated Thyroid Cancer: A Systematic Review. Future Sci OA (2021) 7:FSO738. doi: 10.2144/fsoa-2021-0041
16. Zheng X, Xu Z, Ji Q, Ge M, Shi F, Qin J, et al. A Randomized, Phase 3 Study of Lenvatinib in Chinese Patients With Radioiodine-Refractory Differentiated Thyroid Cancer. Clin Cancer Res (2021) 27:5502–9. doi: 10.1158/1078-0432.CCR-21-0761
17. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol (2016) 34:1448–54. doi: 10.1200/JCO.2015.63.5995
18. Lin Y, Wang C, Gao W, Cui R, Liang J. Overwhelming Rapid Metabolic and Structural Response to Apatinib in Radioiodine Refractory Differentiated Thyroid Cancer. Oncotarget (2017) 8:42252–61. doi: 10.18632/oncotarget.15036
19. Zhang X, Wang C, Lin Y. Pilot Dose Comparison of Apatinib in Chinese Patients With Progressive Radioiodine-Refractory Differentiated Thyroid Cancer. J Clin Endocrinol Metab (2018) 103:3640–6. doi: 10.1210/jc.2018-00381
20. Wang C, Zhang X, Yang X, Li H, Cui R, Guan W, et al. PET Response Assessment in Apatinib-Treated Radioactive Iodine-Refractory Thyroid Cancer. Endocr Relat Cancer (2018) 25:653–63. doi: 10.1530/ERC-18-0007
21. Meng X, Wang H, Zhao J, Hu L, Zhi J, Wei S, et al. Apatinib Inhibits Cell Proliferation and Induces Autophagy in Human Papillary Thyroid Carcinoma via the PI3K/Akt/Mtor Signaling Pathway. Front Oncol (2020) 10:217. doi: 10.3389/fonc.2020.00217
22. Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, et al. YN968D1 Is a Novel and Selective Inhibitor of Vascular Endothelial Growth Factor Receptor-2 Tyrosine Kinase With Potent Activity In Vitro and In Vivo. Cancer Sci (2011) 102:1374–80. doi: 10.1111/j.1349-7006.2011.01939.x
23. Koehler VF, Berg E, Adam P, Weber GL, Pfestroff A, Luster M, et al. Real World Efficacy and Safety of Multi-Tyrosine Kinase Inhibitors in Radioiodine Refractory Thyroid Cancer. Thyroid (2021) 31:1531–41. doi: 10.1089/thy.2021.0091
24. Ahn J, Song E, Kim WG, Kim TY, Kim WB, Shong YK, et al. Prognostic Role of the Lymphocyte-to-Monocyte Ratio for Clinical Outcomes of Patients With Progressive Radioiodine-Refractory Differentiated Thyroid Carcinoma Treated by Sorafenib. Clin Endocrinol (Oxf) (2020) 92:71–6. doi: 10.1111/cen.14120
Keywords: differentiated thyroid carcinoma, apatinib, radioiodine refractory differentiated thyroid carcinoma, targeted treatment, drug efficacy
Citation: Du W, Shi X, Fang Q, Zhang X and Liu S (2022) Feasibility of Apatinib in Radioiodine-Refractory Differentiated Thyroid Carcinoma. Front. Endocrinol. 13:768028. doi: 10.3389/fendo.2022.768028
Received: 31 August 2021; Accepted: 18 January 2022;
Published: 23 February 2022.
Edited by:
Laura Boucai, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Peng Huang, Central South University, ChinaCopyright © 2022 Du, Shi, Fang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanting Liu, bGl1c2hhbnRpbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.