- National Demonstration Center for Experimental Fisheries Science Education, Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Key Laboratory of Freshwater Aquatic Genetic Resources, Ministry of Agriculture, Shanghai Engineering Research Center of Aquaculture, Shanghai Ocean University, Shanghai, China
Red pigment concentrating hormone (RPCH) and pigment dispersing hormone (PDH) are crustacean neuropeptides involved in broad physiological processes including body color changes, circadian rhythm, and ovarian growth. In this study, the full-length cDNA of RPCH and PDH were identified from the brain of the Chinese mitten crab Eriocheir sinensis. The deduced RPCH and PDH mature peptides shared identical sequence to the adipokinetic hormone/RPCH peptides family and the β-PDH isoforms and were designated as Es-RPCH and Es-β-PDH, respectively. Es-RPCH and Es-β-PDH transcripts were distributed in the brain and eyestalks. The positive signals of Es-RPCH and Es-β-PDH were localized in the neuronal clusters 6, 8, 9, 10, and 17 of the brain as revealed by in situ hybridization. The expression level of Es-RPCH and Es-β-PDH mRNA in nervous tissues were all significantly increased at vitellogenic stage, and then decreased at the final meiotic maturation stage. The administrated with synthesized Es-RPCH peptide results in germinal vesicles shift toward the plasma membrane in vitellogenic oocyte, and significant decrease of the gonad-somatic index (GSI) and mean oocyte diameter as well as the expression of vitellogenin mRNA at 30 days post injection in vivo. Similar results were also found when injection of the Es-β-PDH peptide. In vitro culture demonstrated that Es-RPCH and Es-β-PDH induced germinal vesicle breakdown of the late vitellogenic oocytes. Comparative ovarian transcriptome analysis indicated that some reproduction/meiosis-related genes such as cdc2 kinase, cyclin B, 5-HT-R and retinoid-X receptor were significantly upregulated in response to Es-RPCH and Es-β-PDH treatments. Taken together, these results provided the evidence for the inductive effect of Es-RPCH and Es-β-PDH on the oocyte meiotic maturation in E. sinensis.
Introduction
In most animals, ovarian maturation contains two major cellular events: vitellogenesis and the final meiotic maturation of oocytes, which were precisely regulated by elaborate endocrine system (1–3). Crustacean neuropeptides are important neuroendocrine factors generally synthesized and secreted from the central nerve system (CNS) such as the brain and the X-organ/sinus gland complex in eyestalks, then transported to the target tissue through hemolymph to govern a variety of critical physiological processes including metabolism, molting, growth, and reproduction (4–7). As unique neuroendocrine factors, many crustacean neuropeptides have attracted wide attention in the regulation of ovarian development (1, 4, 8). It has been well known that the neuropeptides in eyestalks exhibit inhibitory effect on the gonadal development. For instances, the crustacean hyperglycemic hormone (CHH) family in eyestalks were well-characterized in the inhibition of vitellogenin (Vg) expression (9). The neuropeptide F inhibits vitellogenesis and oocyte maturation in the mud crab Scylla paramamosain (5). Vitellogenesis-inhibiting hormone (VIH) suppresses vitellogenin production directly in the target tissues and ultimately inhibits the ovarian maturation in the white shrimp Litopenaeus vannamei (10). Thus, the removal of eyestalk has become an alternative method for promoting the ovarian maturation in the artificial reproduction of decapod crustaceans (11).
The red pigment concentrating hormone (RPCH) and pigment dispersing hormone (PDH) are another two important crustacean neuropeptides mainly generated in the eyestalks. They have implications in various biological processes including pigment granules concentration or dispersion, body color changes, circadian rhythm and light-dark adaption in crustaceans (12–16). RPCH has been identified as an octapeptide with identical sequence pQLNFSPGWamide in several decapod species such as Callinectes sapidus (17), Scylla olivacea (18), Litopenaeus vannamei (19) and Penaeus monodon (20), suggesting it structural conservation among decapod crustaceans. Unlike previously characterized inhibitory hormones in eyestalks, however, functional studies showed that RPCH stimulated the synthesis and release of methyl farnesoate to induce ovarian development in the crayfish Procambarus clarkii (21–23). Administrated with RPCH significantly increase the gonad-somatic index and Vg expression in the mud crab S. paramamosain (24) and the white shrimp L. vannamei (19).
In contrast to RPCH, PDH is an octadecapeptide present in multiple forms. Based on the difference of the third amino acid residue at the N-terminus, PDH can be divided into two major categories: α-PDH for the Gly and β-PDH for the Glu (25, 26). To date, the β-PDHs were identified more popular than α-PDH in crustaceans (13, 25, 27–29). Unfortunately, no direct evidence has been provided for the potential role of PDH in ovarian maturation so far, even though the higher expression of PDH mRNA level were found in the ovary at vitellogenic stage as compared with other stages in S. paramamosain (28). In the present study, we identified the Es-RPCH and Es-β-PDH transcripts from the brain in the Chinese mitten crab Eriocheir sinensis, one of the most important economically aquaculture species in China, and then artificially synthesized Es-RPCH and Es-β-PDH peptides to further functionally characterized their role in regulating ovarian maturation. Our results provide the first evidence for the possible role of Es-RPCH and Es-β-PDH in the oocyte meiotic maturation thereby providing new potential applications in the mitten crab breeding in aquaculture.
Materials and Methods
Animals and Tissue Sampling
Female individuals (body weight 80-110g) were collected from a local farm in Pudong District, Shanghai. The crabs were kept in a freshwater circulation system supplying sufficient dissolved oxygen and were fed on commercial diet pellets (SC-9011, South Ranch, China) once a day. Various tissues including the muscle, gill, heart, hepatopancreas, thoracic ganglia, brain, eyestalks and ovaries were dissected on ice, quickly frozen in liquid nitrogen, and then stored at -80°C for gene expression analysis. For histological observation or in situ hybridization analysis, the ovarian and brain tissues were fixed in 4% paraformaldehyde solution at 4°C overnight. The ovarian development was divided into three main stages: previtellogenesis (Pvt), vitellogenesis (Vt) (early, Evt; middle, Mvt; late, Lvt), and the final meiotic maturation (30). Germinal vesicle breaks down (GVBD) in the oocyte at the final meiotic maturation was detected in a clearing solution (31).
Total RNA Extraction
Total RNA was extracted from tissues using RNAiso Plus reagent (Takara, Kusatsu, Japan) according to the manufacturer’s instructions. The concentration and purity of RNA were quantified by NanoDrop 2000 Spectrophotometers (Thermo Fisher, Massachusetts, USA).
Rapid Amplification of cDNA Ends (RACE) of Es-RPCH and Es-β-PDH
The first strand of cDNA was synthesized with 1 μg of total RNA using the SMARTer RACE cDNA Amplification Kit (Clontech, Kusatsu, Japan). Gene-specific primers (Table S1) were designed by Primer Premier 5.0 software based on the homologous sequence in the brain transcriptome database (32). RACE-PCR amplification was conducted with initial denaturation at 94°C for 30 s, followed by 35 cycles at 94°C for 5 s, 60°C for 30 s, and 72°C for 2 min, then a final elongation at 72°C for 10 min. PCR products were gel-purified (Tiangen, Beijing, China) and ligated to the pGEM®-T Easy vector (Promega, Madison, WI, USA) at 4°C overnight, then transformed into competent Escherichia coli (DH5α) cells. Eight positive clones were picked for Sanger sequencing.
Bioinformatics Analysis
The open reading frame (ORF) of Es-RPCH and Es-β-PDH were determined using the ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/). The basic physical and chemical properties of the deduced amino acid sequences were analyzed using the ExPASy Molecular Biology server (https://web.expasy.org/protparam/). The signal peptide was scanned using the SignalP 5.0 Server program (http://www.cbs.dtu.dk/services/SignalP/). Multiple sequence alignment was performed by DNAman software. A phylogenetic tree was generated by MEGAX 64 software using the neighbor-joining methods. Bootstrap analysis of 1000 replicates was carried out to compute the tree branch position confidence (Latin name and protein id are given in Figure S2).
In Situ Hybridization (ISH)
DIG-labeled probes of anti-sense and sense RNA were synthesized by DIG RNA Labeling Kit (Roche Diagnostics, Mannheim, Germany) with the SP6, and T7 RNA polymerases (Takara), respectively. ISH was performed as previously described (33). In brief, the brain tissue sections (~6 µm) were dehydrated and deparaffinized with xylene (three times for 5 min each). After rinsing, each section was treated with 3-5 µg/mL proteinase K at 37°C for 10 min, and then hybridized with DIG-labeled antisense or sense RNA probes two hours at 55°C for 2 hours. After serial washing, the DIG was visualized using colorimetric substrates NBT/BCIP (Roche, Germany) following the manufacturer’s instructions.
In Vivo Injection of the Es-RPCH and Es-β-PDH Peptides
The Es-RPCH and Es-β-PDH peptides were chemically synthesized (Jier Biosciences, China). Female individuals were randomly divided into four groups: crabs in group 1 were untreated and served as the blank control group, sacrificed after day 0 post injection. Crabs in group 2, 3 and 4 were respectively injected with 100 μL of PBS (control group), Es-RPCH, and Es-β-PDH every seven days at the base of the fifth walking leg, and then sacrificed after 30 days post injection. The body weight and gonad weight were measured to calculate the gonadosomatic index (GSI) (gonad weight/body weight × 100%). Ovary tissues from each group were sampled for measuring the relative Vg mRNA expression levels and mean oocyte diameter and histological observation.
In Vitro Culture of Oocyte With Es-RPCH and Es-β-PDH Peptides
Ovarian tissues at Lvt stage were selected from the sexually mature crabs (n = 36) and then were dispersed with a pipette to release oocytes. The oocytes were rinsed with DPBS and placed in medium 199 in 24-well culture plates as previously described (34) (containing 100 g/mL of BSA, 100 g/mL of streptomycin, and 100 IU/mL of penicillin G). In the experimental group, the synthesized Es-RPCH or Es-β-PDH was added at the different concentrations of 10-11 M, 10-10 M, 10-9 M, 10-8 M and 10-7 M, respectively, while equal volume of PBS was added to the wells in the control group. All the plates were incubated in dark at 24°C. The number of oocytes in GVBD from each well were determined in clearing solution (formaldehyde, ethyl alcohol, acetic acid, 30:60:1) (31).
Quantitative Real-Time PCR
Quantitative real-time PCR (qRT-PCR) was performed with gene-specific primers (Table S1) using SYBR® Premix Ex Taq™ II kit (Takara) in a 20 µL reaction mix containing 4 µL 5× iScript reaction mix (Bio-Rad), 1 μL cDNA template, 0.5 μL forward primer (10 μM), 0.5 μL reverse primer (10 μM) and 14 μL RNase-free ddH2O. Thermal cycling included an initial denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 55°C for 30 s. To examine product specificity, PCR products were verified by sequencing and melting curve analyses (60°C to 95°C in increments of 0.2°C/s), and a negative control was set without cDNA template. Each reaction was conducted in triplicate. The crab β-actin gene was employed as an internal reference to estimate the relative mRNA expression levels using the 2−δδCt method (35). The amplification efficiencies for the target gene and the β-Actin gene were approximated 100%.
Statistical Analysis
All data in this study were presented as mean + Standard Deviation (SD), and “n” represents the number of replicates. Kolmogorov-Smirnov and Cochran tests were performed and Student’s t-test or Tukey’s test were utilized to analyze the normally distributed data. One-way analysis of variance (ANOVA) and paired sample T-test were performed to calculate statistically significant values respectively using SPSS statistical software (version 20.0). In all cases, statistical differences among groups were accepted at p <0.05 and indicated with asterisks or double asterisks.
RNA-Seq Analysis
The administrated female crabs with PBS, Es-RPCH and Es-β-PDH were sacrificed at 24 hours post injection for sampling ovarian tissues. Three individuals were sampled in each administrated group for RNA-seq libraries construction. RNA-seq were conducted using an Illumina HiSeq™ 2500/MiSeq (Novogene Biotech,Tianjin, China).
Clean reads were obtained after filtering and removing low quality raw reads. The differentially expressed genes (DEGs) between the Es-RPCH and PBS (Es-β-PDH and PBS) groups were screened by DESeq 2 software using DESeq and BH methods with the negative binomial distribution model methods. The screening criteria used for target DEGs is as follows|log2Fold change|≥0, P ≤ 0.05. The putative target DEGs were annotated by the Gene Ontology (GO) enrichment analysis (http://www.geneontology.org/), and the pathway was deduced using the Kyoto Encyclopedia of Gene and Genomes (KEGG) database (https://www.kegg.jp/kegg/pathway.html).
Results
Molecular Characterization of Es-RPCH and Es-β-PDH
Two full-length cDNA sequences of Es-RPCH and Es-β-PDH were cloned by RACE method from the brain of E. sinensis as shown in Figure 1. The 650-base pair (bp) Es-RPCH (GenBank accession number: OK315660) contains a 75 bp 5′-untranslated region (UTR), a 245 bp 3′-UTR including a potential polyadenosine (ATTAAA), and a 330 bp ORF encoding a 109 amino acid with a calculated molecular weight of ~11.08 kDa and a predicted isoelectric point of 7.84. Sequence analysis predicted the ORF of Es-RPCH containing a 24-residue signal peptide, a 8-residue mature peptide, and a 73-residue precursor related peptide (RPRP) (Figure 1A). Multiple sequence alignments showed that the Es-RPCH amino acid sequence has high identities with other crustacean RPCHs, sharing the identical mature RPCH peptides, glycine residue preceding the dibasic cleavage site, dibasic cleavage site (KR) and the amidation site (Figure S1A). Phylogenetic analysis showed that Es-RPCH is more closely related to crustacean RPCHs and clustered with insects AKHs, grouped into one GnRH superfamily, which is consistent with traditional classification (Figure S2A).

Figure 1 The full-length cDNA and deduced amino acid sequences of Es-RPCH (A) and Es-β-PDH (B) in E. sinensis. The initiation codon (ATG) and stop codon (TGA/TAA) are indicated in bold and asterisk (*) respectively. The predicted signal peptides are underlined. The putative precursor-related peptides are in italics, and the mature peptides are in bold. The amidation site and potential dibasic cleavage site (KR) of Es-RPCH and two proteolytic sites (KR and RR) of Es-β-PDH are highlighted by white, black triangles and boxes, respectively. The polyadenylation signal (ATTAAA) are indicated by wave lines.
The Es-β-PDH cDNA (GenBank accession number: OK315661) was 535 bp in length, including a 5′-UTR of 36 bp, a 3′-UTR of 253 bp with a potential polyadenosine (ATTAAA), and an ORF of 246 bp encoding a 81 amino acid with a calculated molecular weight of ~9.03 kDa and a predicted isoelectric point of 10.11. The deduced amino acid sequence of Es-β-PDH containing a 25-residue signal peptide, a 33-residue precursor related peptide (PPRP), two proteolytic sites (KR, RR) at positions 59 and 80, and a 18-residue mature peptide (Figure 1B). Sequence alignments showed that the amidation site and two proteolytic sites are identical in different crustacean species, and the PDH mature peptides are conserved in crustaceans (Figure S1B). Phylogenetic analysis showed that Es-β-PDH is more closely related to crustacean β-PDHs than α-PDH, followed by pigment dispersing factor, which is also consistent with traditional classification (Figure S2B).
Tissue Distribution of Es-RPCH and Es-β-PDH mRNA
QRT-PCR detection showed high expression of Es-RPCH and Es-β-PDH mRNA in the eyestalks, and moderate in brain. While weak expression of the Es-RPCH and Es-β-PDH was found in thoracic ganglia and ovary, respectively. No expression was found in other tested tissues (Figure 2).
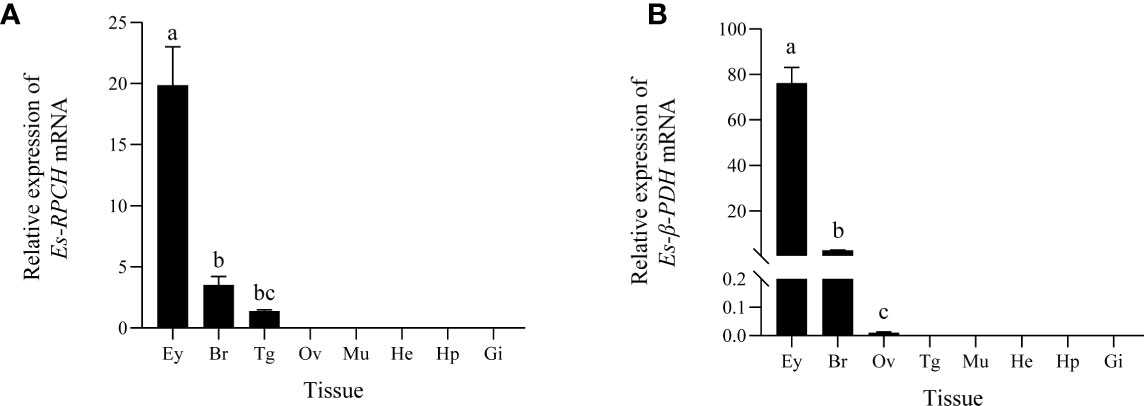
Figure 2 QRT-PCR detection of the tissue distribution of Es-RPCH (A) and Es-β-PDH (B) mRNAs in E. sinensis. Ey, eyestalks; Br, brain; Tg, thoracic ganglia; Ov, ovary; Mu, muscle; He, heart; Hp, hepatopancreas; Gi, gill. Different letters indicate significant differences (p <0.05)
ISH Localization of Es-RPCH and Es-β-PDH mRNA in the Brain
Histological section showed that the mitten crab brain can be classified into protocerebrum, deutocerebrum, and tritocerebrum (36). Middle horizontal section of the brain in Figure 3A showed the locations of neuronal clusters (numbers) and neuropils. A strong positive signal of Es-RPCH was visualized in the cell clusters 6 in the protocerebrum, middle signal in cell clusters 8 in the protocerebrum, 9 and 10 in the deutocerebrum, but a weaker signal in cell clusters 17 in the tritocerebrum (36) (Figures 3B, F). In addition, a strong positive signal of Es-β-PDH was visualized in the cell clusters 8, 9, and 10, but weaker in cell clusters 6 and 17 (Figures 3D, G–I). No positive signal was detected in negative control sections with the sense-strand RNA probes (Figures 3C, E).
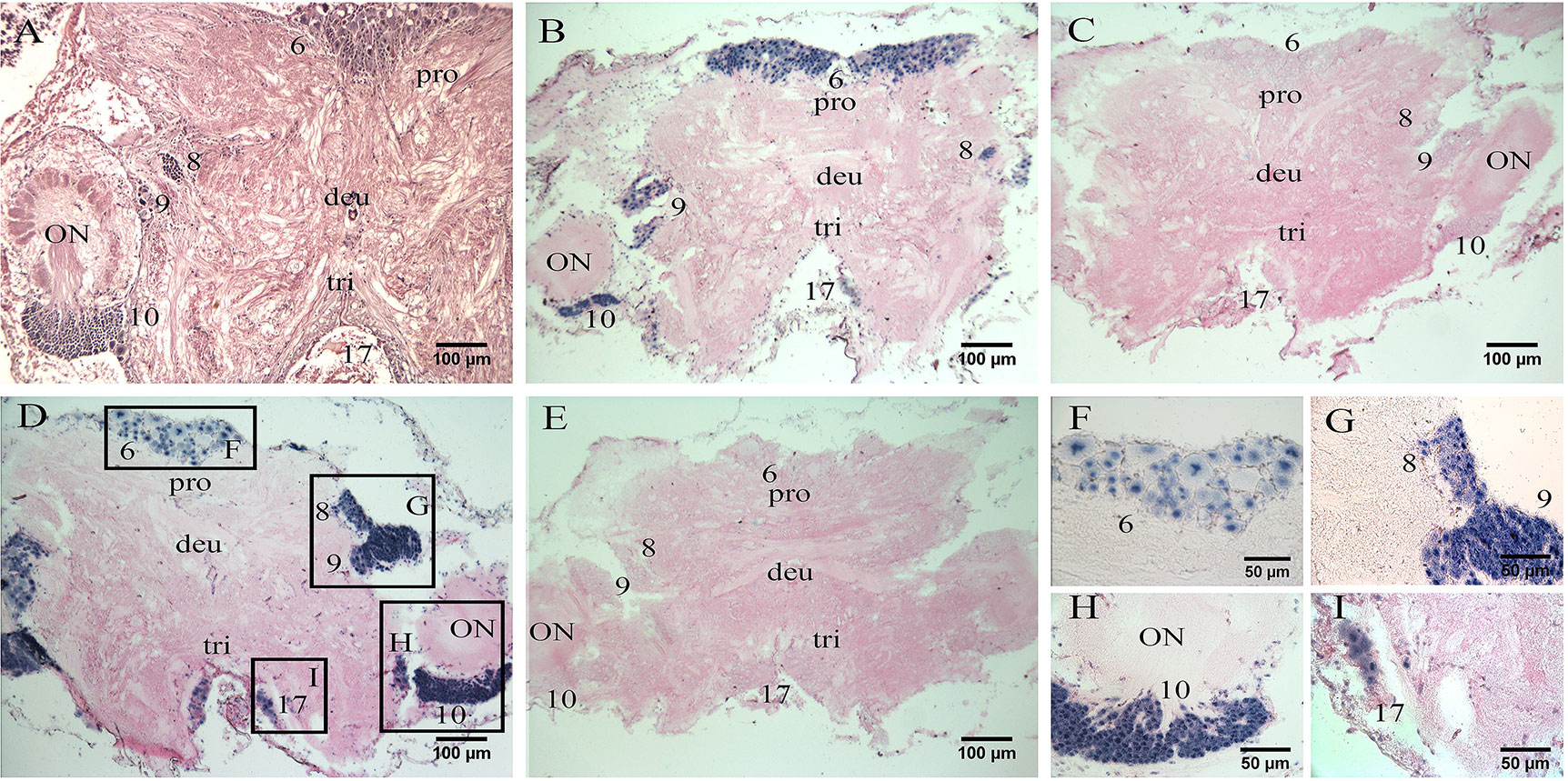
Figure 3 In situ hybridization (ISH) localization of Es-RPCH and Es-β-PDH mRNAs in the brain of E. sinensis. (A) Hematoxylin-eosin staining of a horizontal section of the brain showing the locations of neuronal clusters (numbers) and neuropils. (B) ISH using Es-RPCH antisense probes showing positive signals (blue) in various neuronal clusters of the brain. (C) Negative control of ISH using Es-RPCH sense probes. (D) ISH using Es-β-PDH antisense probes showing positive signals (blue) in various neuronal clusters of the brain. (E) Negative control of ISH using Es-β-PDH sense probes. Scale bars = 100 µm. (F, I) High magnification micrographs showing positive ISH signals in the cluster 6 (F), clusters 8 and 9 (G), cluster 10 (H), and cluster 17 (I). Scale bars= 50 µm. ON, olfactory neuropil; pro, protocerebrum; deu, deutocerebrum; tri, tritocerebrum.
Quantitative Expression of Es-RPCH and Es-β-PDH mRNA During Ovarian Development
As shown in Figure 4, qRT-PCR indicated that the abundance of Es-RPCH and Es-β-PDH mRNA were the highest in the eyestalks, and moderate in the brain at each ovarian developmental stage (Figure 4A). The relative expression level of Es-RPCH and Es-β-PDH mRNA were all dramatically increased in the eyestalks and brain and reach the highest at Vt stage, and then decrease at GVBD stage (P <0.01) (Figure 4B).
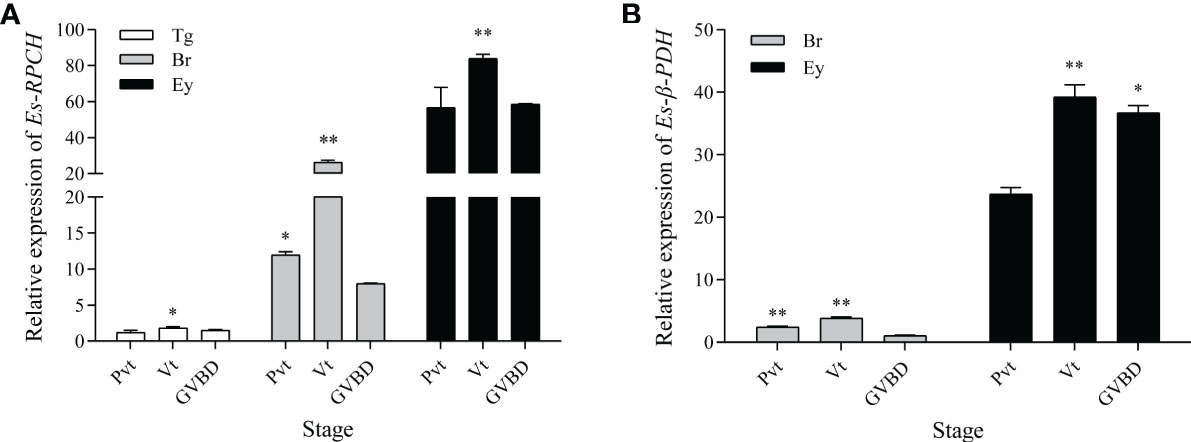
Figure 4 qPCR assay of the relative expression level of Es-RPCH (A) and Es-β-PDH (B) mRNA in the thoracic ganglia (Tg), brain (Br);, eyestalks (Ey) during ovarian development in E. sinensis. Ovarian stages are divided into previtellogenic (Pvt), vitellogenic (Vt) and germinal vesicle breakdown (GVBD) stages. The data are presented as the means + SD (n = 3). * represents significant difference (P < 0.05), and ** represents highly significant difference (P < 0.01).
Effect of Es-RPCH and Es-β-PDH Peptides on Ovarian Maturation In Vivo
To investigate the exact role of the Es-RPCH and Es-β-PDH in the ovarian maturation in E. sinensis, the female individuals at Pvt stage were selected for injection of the synthesized Es-RPCH and Es-β-PDH peptides. At the beginning of experiment, the nucleus in the Pvt oocytes were transparent and were termed germinal vesicle (GV), while the ooplasm and nucleolus were notably stained in dark blue color by hematoxylin (Figure 5A). After administrated with injection of 30 days, the oocytes were visualized at Mvt stage in PBS, Es-RPCH, and Es-β-PDH groups. Due to the appearance of yolk, the ooplasm was stained in red by eosin (Figures 5B–D). Notably, in the Es-RPCH injection group, the GV visually migrated toward peripheral cytoplasmic membrane as compared with that of the Es-β-PDH and PBS groups (Figures 5C, D), in which GV situated in the center of oocyte. The GSI index and Vg expression in the ovary were all significantly decreased in the Es-RPCH and Es-β-PDH injection group when compared with the PBS control group (Figures 5E, G). The mean oocytes diameter was also smaller in the Es-RPCH group, but no significant differences between Es-β-PDH and the control groups (Figure 5F).
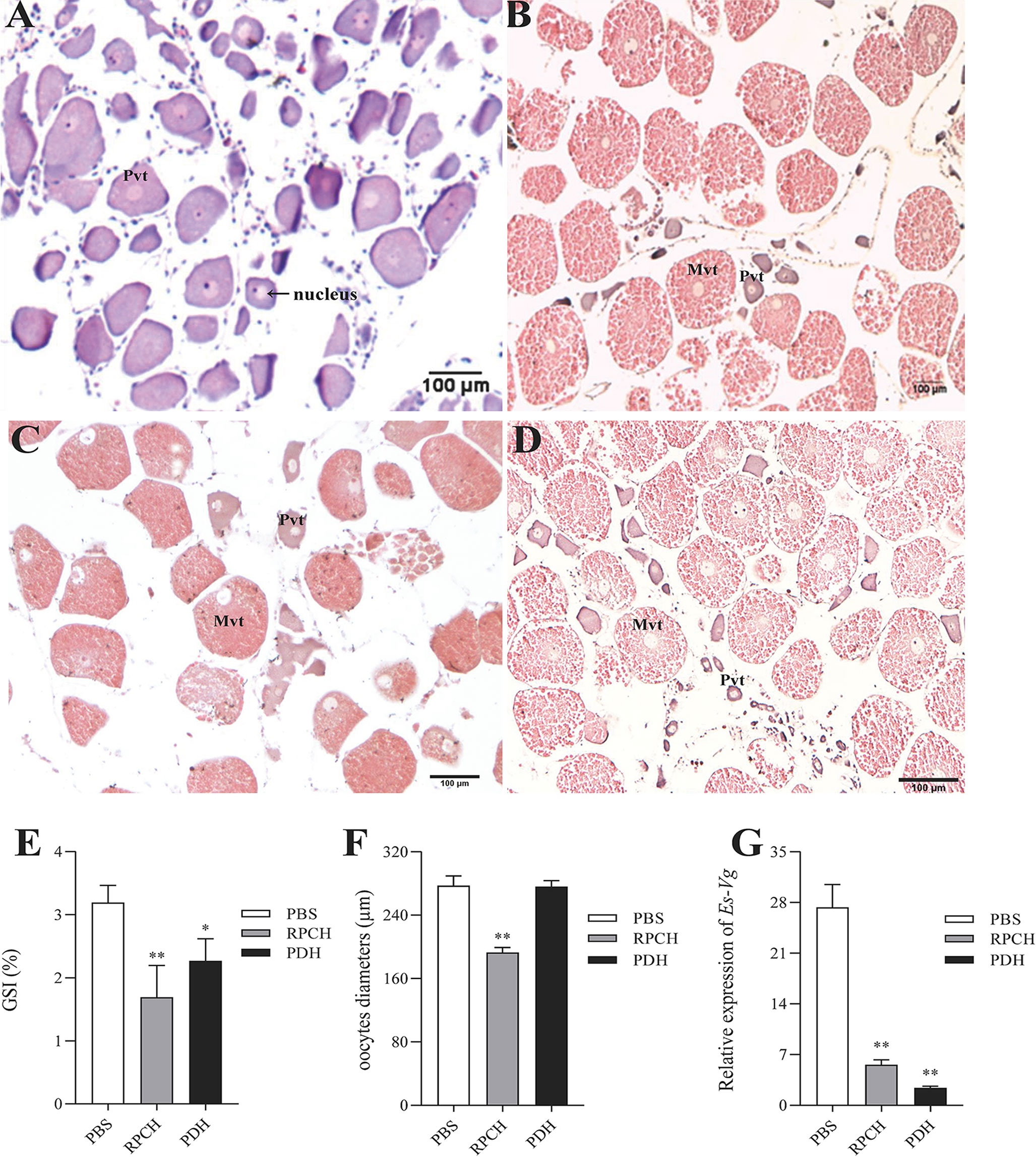
Figure 5 Effects of in vivo injection of Es-RPCH and Es-β-PDH peptides on the ovarian development in E. sinensis. Histological observation: Ovarian tissue sections at the first day (A) and the 30 st day (B) post injection of PBS; ovarian tissue sections at the 30 st day post injection of the Es-RPCH peptides (C) and Es-β-PDH peptides (D). Inhibitory effects on the gonadosomatic index (E), mean oocytes diameters (F), and relative expression level of Es-Vg mRNA as revealed by qPCR (G). Pvt, previtellogenesis. Mvt, middle vitellogenesis. The data are presented as means + SD (n = 5). Asterisks (*) and double asterisks (**) represent statistically significant difference as compared with PBS control (P < 0.05 and P < 0.01). Scale bar = 100 μm.
Effect of Es-RPCH and Es-β-PDH Peptides on GVBD of Oocytes In Vitro
To determine whether the Es-RPCH and Es-β-PDH can induce GVBD, oocytes at Lvt stage were selected to culture with the synthesized Es-RPCH and Es-β-PDH peptides. Results showed that the GVBD index in the Es-RPCH and Es-β-PDH group were all dramatically increased at the concentration of 10-11 M, 10-10 M, 10-9 M, 10-8 M, and 10-7 M as compared with control groups (p < 0.05). There was a significant dosage effect with the increasing concentration of Es-RPCH or Es-β-PDH. In addition, the GVBD index in Es-RPCH group was higher than in the Es-β-PDH in each concentration gradient (Figure 6).
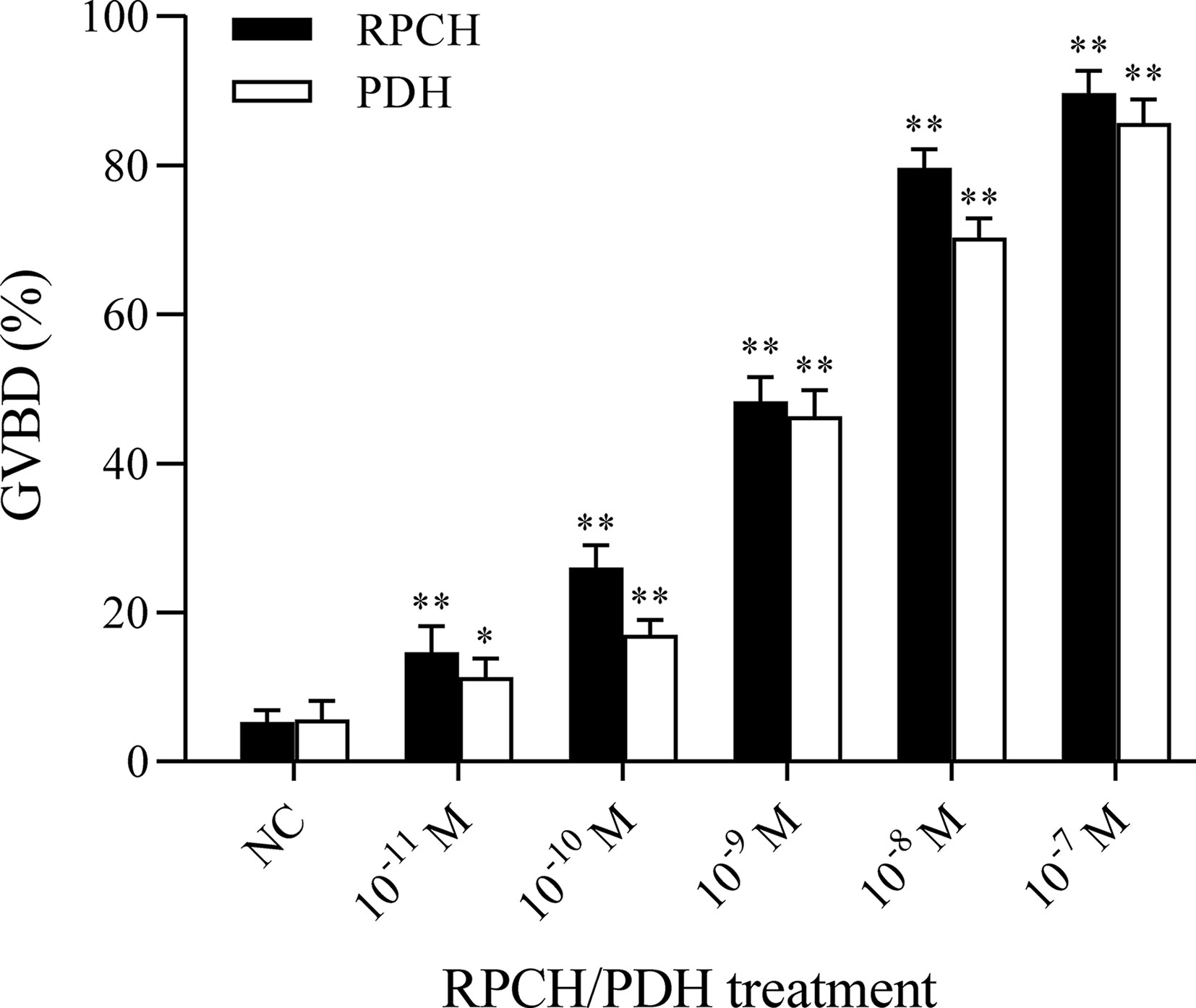
Figure 6 Effects of in vitro culture of the oocytes with the Es-RPCH and Es-β-PDH peptides on the germinal vesicle breakdown index in E. sinensis. A replicate from the control group (NC) was used as a calibrator. Bars represent mean + SD (n = 5). Asterisks (*) and double asterisks (**) represent values statistically different from the control group (P < 0.05 and P < 0.01).
Ovarian Transcriptome Analysis
To probe the molecular basis of induction of GVBD by Es-RPCH and Es-β-PDH, nine ovarian cDNA libraries from the crabs administrated with PBS, Es-RPCH and Es-β-PDH were constructed and sequenced by Illumina HiSeq 4000 platform. More than 42 million raw reads were generated, and then about 33 million clean reads of every group were obtained after removing adapters as well as filtering the low-quality sequences. The Q20 percentage of each sample was over 97%, the Q30 percentage was over 92%, and the CG content was below 53.96% (Table S2), indicating that the sequencing data are high quality and credibility.
In comparison with PBS group, a total of 1976 differentially expressed genes (DEGs) including 1002 up- and 974 down-regulated genes were identified in the Es-RPCH group, as well as1714 DEGs including 885 up- and 829 down-regulated genes in the Es-β-PDH group (Figure 7). GO analysis indicated DEGs from the Es-RPCH and Es-β-PDH groups were all classified into three ontologies: molecular function, biological process, and cellular component. Respectively, DEGs in the Es-RPCH group were mainly assigned into DNA binding, DNA integration and biosynthetic processes (Figure S3A), while in the Es-β-PDH group DEGs were mainly assigned into DNA integration, small molecule metabolism process, and monosaccharide metabolism process (Figure S3C). In addition, KEGG pathway analysis showed that DEGs in the Es-RPCH group were statistically enriched some pathways, including TGF‐β signaling pathway, oocyte meiosis, and Wnt signaling pathway, and so on. (Figure S3B), while in the Es-β-PDH group the DEGs were mainly enriched into TGF‐β signaling pathway, pyruvate metabolism and insect hormone biosynthesis, and so on (Figure S3D). Interestingly, some DEGs related to meiosis such as cell division cycle 2 kinase (Cdc2) and cyclin proteins as well as 5-HT-R were identified to be highly expressed in the Es-RPCH and Es-β-PDH groups as compared with the PBS control (Figure 8A). Additionally, the DEGs related to gonadal maturation such as foxl2, retinoid-X receptor (RXR), neuropeptide F-R, and GnRHR were also detected to be highly expressed in the Es-RPCH and Es-β-PDH groups (Figure 8B).
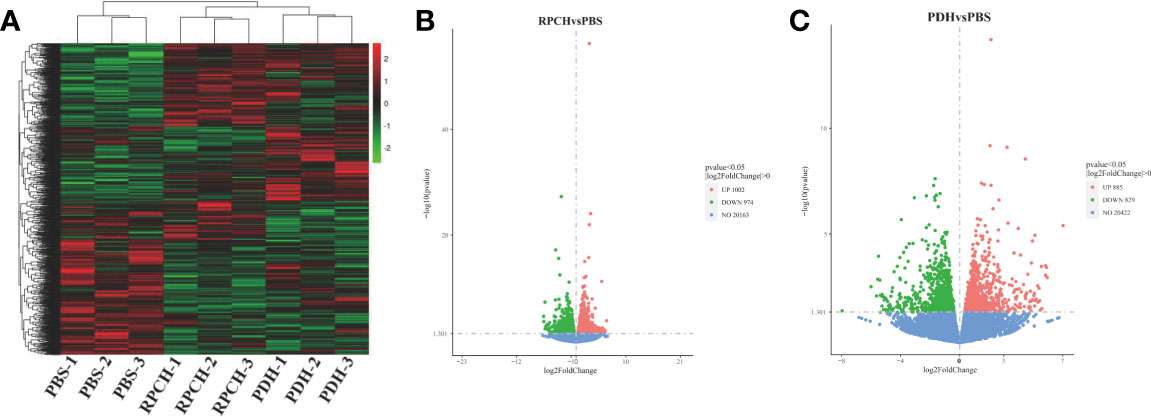
Figure 7 Comparative transcriptome analysis of the ovaries from the individuals (n = 3) injected with PBS, Es-RPCH and Es-β-PDH peptides. (A) A heat map of differentially expression genes (DEGs) in the ovaries administrated with PBS, Es-RPCH and Es-β-PDH peptides. The color scale indicates that the log10‐fold change from high (red) to low (green). (B) A volcano plot of DEGs between the Es-RPCH administrated and PBS control groups. (C) A volcano plot of DEGs between the Es-β-PDH administrate and PBS control groups. The upregulated, downregulated and non-regulated genes were indicated by red, green and blue dots, respectively.

Figure 8 Heat maps of the DEGs related to oocyte meiosis and maturation. (A) The DEGs relative expression levels between the Es-RPCH administrated and PBS control groups (n = 3). (B) The DEGs relative expression levels between the Es-β-PDH administrated and PBS control groups (n = 3). The color reflects relative expression levels from high (red) to low (green).
Discussion
In the present study, the full-length cDNA of Es-RPCH and Es-β-PDH were identified and characterized in the brain of E. sinensis. Phylogenetic analysis indicated that the Es-RPCH belongs to the adipokinetic hormone (AKH)/RPCH family proteins and grouped into GnRHs superfamily (Figure S2A). This result was also supported by several structural characteristics of RPCH. Firstly, the signal peptide was demonstrated critical for guiding the AKH precursor into the secretory pathway, cleaved post-translationally, and then convert the prohormone into a prohormone, which was predicted in Es-RPCH (37). Secondly, the glycine residue (G) preceding the dibasic cleavage site (KR) as an amine donor yielding an amidated tryptophan (W-NH2) to be amidation at the C-terminus ends in AKHs, which is important for increasing binding affinity to the receptor and were also predicted in Es-RPCH (13, 38, 39). Insect AKHs was proposed as the cognate ligand of a GnRH receptor in C. elegans involving in egg laying behavior (40). Owing to the co-evolution of members of the GnRH superfamily and their receptors, their diverse functions seem to overlap including reproduction (41). Thirdly, the Es-RPCH mature peptide shares the 100% identity with those of other crustacean RPCHs and shares the 75% identity with insect AKHs (Figure S1A), indicating that the structure and function of RPCHs could be conserved during evolution. In contrast, the Es-β-PDH has the conserved PDH mature peptides sequence with identical amidation and proteolytic sites (Figure S1B). Phylogenetic analysis showed that Es-β-PDH is clustered with insects pigment dispersing factor (PDF)-commonly considered an equivalent of crustaceans PDH (Figure S2A), which has implication in regulating reproduction and ovarian maturation (42).
Many pioneer studies suggested that RPCH and PDH are generated in the eyestalks (18, 19, 28). Our present study revealed the Es-RPCH and Es-β-PDH were also produced in the brain besides eyestalks (Figure 2). The hybridization signal of Es-RPCH were widely distributed in the neuronal clusters 6, 8, 9, 10, and 17 of the brain (Figure 3), which is identical with the expression pattern of RPCH in the brain of Scylla olivacea (18). The ubiquitously distribution in neuronal clusters of the brain indicated that Es-RPCH may perform various important physiological functions in crustaceans, which refer to the neuronal clusters 9 and 10 of brain playing an important role in visual and chemical sensations reception, as well as feeding and reproductive behaviors regulation in crustaceans (43, 44). Interestingly, similarly results were also found in Es-β-PDH indicated that their biological function maybe overlapping.
To explore whether Es-RPCH and Es-β-PDH are involved in the ovarian maturation in E. sinensis, we firstly examined the expression profiles of Es-RPCH and Es-β-PDH in neural tissues during ovarian development. QPCR results shows both Es-RPCH and Es-β-PDH are significantly increased and highest in vitellogenic stage, decreased in germinal vesicle breakdown stage (Figure 4), suggesting the potential role of Es-RPCH and Es-β-PDH in ovarian maturation. Then the female individuals at Pvt stage were selected for injection of synthetic Es-RPCH and Es-β-PDH peptides. The results showed Es-RPCH can induce the germinal vesicles shifting from center to peripheral plasma membrane in vitellogenic oocyte (Figure 5). In vitro culture experiments further revealed that Es-RPCH peptides can induce germinal vesicle breakdown in the oocytes at Lvt stage (Figure 6). The mean oocyte diameter, EsVg expression and GSI, however, were all significantly decreased after Es-RPCH peptides injection (Figure 5), indicating that the Es-RPCH inhibit the vitellogenesis and induce oocyte meiotic maturation. At the end of vitellogensis, the oogenesis enters into the final meiotic maturation stage. The vitellogenesis is terminated and the germinal vesicles begin to migrate toward peripheral plasma membrane and ultimately break down. Thus, we deduced that Es-RPCH could function at the transition from vitellogenesis to final meiotic maturation of oocytes. Like Es-RPCH, the Vg expression and GSI were also notably decreased when injection of Es-β-PDH peptide in vivo. Although no shifting of germinal vesicle was observed, the Es-β-PDH peptide can also induce germinal vesicle breakdown of oocyte at Lvt stage when culture in vitro (Figure 6). It is worth to note that our results are inconsistent with the previous studies in S. paramamosain (24), P. clarkii (22), and L. vannamei (19), in which the injection of RPCH can increase mean oocyte diameter, Vg expression and GSI. As an eyestalk hormone, to our knowledge, the inductive effect of RPCH in vitellogenesis is unexplained and the inhibitory effect is more reasonable since it is well known that removal of eyestalks can induce the vitellogensis.
To better understand the molecular regulatory basis of the Es-RPCH and Es-β-PDH in ovarian maturation, we next performed ovarian transcriptome analysis. KEGG pathway analysis showed that reproduction-related pathways such as the Wnt and TGF‐β signaling pathway were enriched (45, 46) (Figure S3). Interestingly, some key genes involved in meiosis and ovarian development, such as cyclin B, Cdc2, 5-HT-R, RXR and FOXL2, were detected to be upregulated in Es-RPCH group (Figure 8). Cdc2 kinase and cyclin B are components of M-phase promoting factor (MPF), a heterodimer responsible for the final meiotic maturation of oocyte (30, 47–49). Furthermore, 5-HT binding to 5HT-R can induce GVBD in mud crab S. paramamosain (50). RXR and FOXL2 can regulate Vg expression and ovarian development in S. paramamosain and P. trituberculatus (51–54). Similar results were also found in the comparative transcriptome analysis of Es‐β-PDH injection group, suggesting that Es-RPCH and Es-β-PDH might have similar regulatory pathway for inducing oocyte meiotic maturation and ovarian maturation in E. sinensis.
Conclusion
The full-length cDNA of Es-RPCH and Es-β-PDH were identified and functionally characterized in E. sinensis. The synthestic Es-RPCH and Es-β-PDH peptides can induce the germinal vesicles breakdown in vitro, but downregulate the Vg expression and decreased the GSI in vivo. In addition, comparative ovarian transcriptome analysis further revealed that meiosis-related genes were significantly upregulated in response to Es-RPCH and Es-β-PDH peptides. Our study provided the first evidence of the stimulating effect of Es-RPCH and Es-β-PDH in the oocyte meiotic maturation in E. sinensis.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in NCBI. RPCH group; SRR16603372 PDH group; SRR16603373 control group; SRR16603374.
Ethics Statement
Ethical review and approval was not required for the animal study because the mitten crab E. sinensis is not an endangered or protected species, and permission to perform experiments involving this species is not required in China. Written informed consent for participation was not obtained from the owners because the mitten crab E. sinensis is not an endangered or protected species, and permission to perform experiments involving this species is not required in China.
Author Contributions
L-LW performed the experiments and analyzed data. T-TC wrote the manuscript and analyzed data. B-YL instructed oocytes culture experiments. G-FQ designed this study, analyzed data and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Key R&D Program of China (project number 2018YFD0900201) and the Natural Science Foundation of China (project number 41976103, 41476130).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.802768/full#supplementary-material
References
1. Nagaraju GPC. Reproductive Regulators in Decapod Crustaceans: An Overview. J Exp Biol (2011) 214(1):3–16. doi: 10.1242/jeb.047183
2. Jayasankar V, Tomy S, Wilder MN. Insights on Molecular Mechanisms of Ovarian Development in Decapod Crustacea: Focus on Vitellogenesis-Stimulating Factors and Pathways. Front Endocrinol (2020) 11:577925. doi: 10.3389/fendo.2020.577925
3. Tsukimura B. Crustacean Vitellogenesis: Its Role in Oocyte Development. Am Zool (2001) 41(3):465–76. doi: 10.1668/0003-1569(2001)041[0465:Cvirio]2.0.Co;2
4. Chen H-Y, Toullec J-Y, Lee C-Y. The Crustacean Hyperglycemic Hormone Superfamily: Progress Made in the Past Decade. Front Endocrinol (2020) 11:578958. doi: 10.3389/fendo.2020.578958
5. Bao C, Yang Y, Huang H, Ye H. Inhibitory Role of the Mud Crab Short Neuropeptide F in Vitellogenesis and Oocyte Maturation via Autocrine/Paracrine Signaling. Front Endocrinol (2018) 9:390. doi: 10.3389/fendo.2018.00390
6. Chang ES, Mykles DL. Regulation of Crustacean Molting: A Review and Our Perspectives. Gen Comp Endocrinol (2011) 172(3):323–30. doi: 10.1016/j.ygcen.2011.04.003
7. Liu L, Laufer H, Wang Y, Hayes T. A Neurohormone Regulating Both Methyl Farnesoate Synthesis and Glucose Metabolism in a Crustacean. Biochem Biophys Res Commun (1997) 237(3):694–701. doi: 10.1006/bbrc.1997.6977
8. Liu X, Ma K, Liu Z, Feng J, Ye B, Qiu G. Transcriptome Analysis of the Brain of the Chinese Mitten Crab, Eriocheir sinensis, for Neuropeptide Abundance Profiles During Ovarian Development. Anim Reprod Sci (2019) 201:63–70. doi: 10.1016/j.anireprosci.2018.12.010
9. Tsutsui N, Nagakura-Nakamura A, Nagai C, Ohira T, Wilder MN, Nagasawa H. The Ex Vivo Effects of Eyestalk Peptides on Ovarian Vitellogenin Gene Expression in the Kuruma Prawn Marsupenaeus japonicus. Fish Sci (2012) 79(1):33–8. doi: 10.1007/s12562-012-0566-5
10. Chen T, Zhang L-P, Wong N-K, Zhong M, Ren C-H, Hu C-Q. Pacific White Shrimp (Litopenaeus vannamei) Vitellogenesis-Inhibiting Hormone (VIH) Is Predominantly Expressed in the Brain and Negatively Regulates Hepatopancreatic Vitellogenin (VTG) Gene Expression. Biol Reprod (2014) 90(3). doi: 10.1095/biolreprod.113.115030
11. Amankwah BK, Wang C, Zhou T, Liu J, Shi L, Wang W, et al. Eyestalk Ablation, a Prerequisite for Crustacean Reproduction: A Review. Isr J Aquac (2019) 71.
12. Josefsson L. Structure and Function of Crustacean Chromatophorotropins. Gen Comp Endocrinol (1975) 25(2):199–202. doi: 10.1016/0016-6480(75)90189-6
13. Rao KR. Crustacean Pigmentary-Effector Hormones: Chemistry and Functions of RPCH, PDH, and Related Peptides. Am Zool (2001) 41(3):364–79. doi: 10.1668/0003-1569(2001)041[0364:Cpehca]2.0.Co;2
14. Verde MA, Barriga-Montoya C, Fuentes-Pardo B. Pigment Dispersing Hormone Generates a Circadian Response to Light in the Crayfish, Procambarus clarkii. Comp Biochem Physiol A Mol Integr Physiol (2007) 147(4):983–92. doi: 10.1016/j.cbpa.2007.03.004
15. Sullivan JM, Genco MC, Marlow ED, Benton JL, Beltz BS, Sandeman DC. Brain Photoreceptor Pathways Contributing to Circadian Rhythmicity in Crayfish. Chronobiol Int (2009) 26(6):1136–68. doi: 10.1080/07420520903217960
16. Milograna SR, Bell FT, McNamara JC. Signaling Events During Cyclic Guanosine Monophosphate-Regulated Pigment Aggregation in Freshwater Shrimp Chromatophores. Biol Bull (2012) 223(2):178–91. doi: 10.1086/BBLv223n2p178
17. Martinez-Perez F, Valdes J, Zinker S, Arechiga H. The Genomic Organization of the Open Reading Frame of the Red Pigment Concentrating Hormone Gene in the Blue Crab Callinectes sapidus. Peptides (2002) 23(4):781–6. doi: 10.1016/s0196-9781(01)00648-9
18. Kornthong N, Chotwiwatthanakun C, Chansela P, Tinikul Y, Cummins SF, Hanna PJ, et al. Characterization of Red Pigment Concentrating Hormone (RPCH) in the Female Mud Crab (Scylla olivacea) and the Effect of 5-HT on Its Expression. Gen Comp Endocrinol (2013) 185:28–36. doi: 10.1016/j.ygcen.2013.01.011
19. Chen H-Y, Kang BJ, Sultana Z, Wilder MN. Molecular Cloning of Red Pigment-Concentrating Hormone (RPCH) From Eyestalks of the Whiteleg Shrimp (Litopenaeus vannamei): Evaluation of the Effects of the Hormone on Ovarian Growth and the Influence of Serotonin (5-HT) on Its Expression. Aquaculture (2018) 495:232–40. doi: 10.1016/j.aquaculture.2018.04.027
20. Sathapondecha P, Panyim S, Udomkit A. Molecular Characterization of a cDNA Encoding Red Pigment-Concentrating Hormone in Black Tiger Shrimp Penaeus monodon: Implication of Its Function in Molt and Osmoregulation. Comp Biochem Physiol A Mol Integr Physiol (2014) 175:124–30. doi: 10.1016/j.cbpa.2014.06.001
21. Landau M, Laufer H, Homola E. Control of Methyl Farnesoate Synthesis in the Mandibular Organ of the Crayfish Procambarus Clarkii: Evidence for Peptide Neurohormones With Dual Functions. Invertebr Reprod Dev (1989) 16(1-3):165–8. doi: 10.1080/07924259.1989.9672073
22. Sarojini R, Nagabhu S, Fingerman M. A Neurotransmitter Role for Red-Pigment-Concentrating Hormone in Ovarian Maturation in the Red Swamp Crayfish Procambarus Clarkii. J Exp Biol (1995) 198:1253–7. doi: 10.1242/jeb.198.6.1253
23. Fingerman M. Roles of Neurotransmitters in Regulating Reproductive Hormone Release and Gonadal Maturation in Decapod Crustaceans. Invertebr Reprod Dev (1997) 31(1-3):47–54. doi: 10.1080/07924259.1997.9672562
24. Zeng H, Bao C, Huang H, Ye H, Li S. The Mechanism of Regulation of Ovarian Maturation by Red Pigment Concentrating Hormone in the Mud Crab Scylla paramamosain. Anim Reprod Sci (2016) 164:152–61. doi: 10.1016/j.anireprosci.2015.11.025
25. Rao KR, Mohrherr CJ, Riehm JP, Zahnow CA, Norton S, Johnson L, et al. Primary Structure of an Analog of Crustacean Pigment-Dispersing Hormone From the Lubber Grasshopper Romalea microptera. J Biol Chem (1987) 262(6):2672–5. doi: 10.1016/S0021-9258(18)61559-5
26. Yang WJ, Aida K, Nagasawa H. Characterization of Chromatophorotropic Neuropeptides From the Kuruma Prawn Penaeus japonicus. Gen Comp Endocrinol (1999) 114(3):415–24. doi: 10.1006/gcen.1999.7266
27. Rao KR, Riehm JP. Pigment-Dispersing Hormones. Ann NY Acad Sci (1993) 680:78–88. doi: 10.1111/j.1749-6632.1993.tb19676.x
28. Huang X, Ye H, Huang H, Yu K, Huang Y. Two Beta-Pigment-Dispersing Hormone (Beta-PDH) Isoforms in the Mud Crab, Scylla Paramamosain: Implication for Regulation of Ovarian Maturation and a Photoperiod-Related Daily Rhythmicity. Anim Reprod Sci (2014) 150(3-4):139–47. doi: 10.1016/j.anireprosci.2014.09.004
29. Jorenby WH, Riehm JP, Rao KR. Position 3 Analogues of a Crustacean Pigment-Dispersing Hormone: Synthesis And Biological Activity. Biochem Biophys Res Commun (1987) 143(2):652–7. doi: 10.1016/0006-291x(87)91403-3
30. Fang J-J, Qiu G-F. Molecular Cloning of Cyclin B Transcript With an Unusually Long 3’ Untranslation Region and Its Expression Analysis During Oogenesis in the Chinese Mitten Crab, Eriocheir sinensis. Mol Biol Rep (2009) 36(6):1521–9. doi: 10.1007/s11033-008-9346-9
31. Liu Z, Feng J, Qiu G. Clearing Solution’s Ingredients for Oocyte in Chinese Mitten Crab (Eriocheir sinensis) (in Chinese). J Fisheries China (2019) 43(4):852–7. doi: 10.11964/jfc.20171111021
32. Liu X, Ma K, Liu Z, Feng J, Ye B, Qiu G. Transcriptome Analysis of the Brain of the Chinese Mitten Crab, Eriocheir sinensis, for Neuropeptide Abundance Profiles During Ovarian Development. Anim Reprod Sci (2019) 201:63–70. doi: 10.1016/j.anireprosci.2018.12.010
33. Ma K-Y, Chen J, Liu Z-Q, Qiu G-F. Inhibitory Effects of RNAi-Mediated Knockdown of EsDmrt-Like Gene on Testicular Development in the Chinese Mitten Crab Eriocheir sinensis. Aquaculture (2016) 463:217–23. doi: 10.1016/j.aquaculture.2016.06.003
34. Liu M, Xie X, Tao T, Jiang Q, Shao J, Zhu D. Molecular Characterization of Methoprene-Tolerant Gene (Met) in the Swimming Crab Portunus trituberculatus: Its Putative Role in Methyl Farnesoate-Mediated Vitellogenin Transcriptional Activation. Anim Reprod Sci (2016) 174:132–42. doi: 10.1016/j.anireprosci.2016.09.017
35. Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(T)(-Delta Delta C) Method. Methods (2001) 25(4):402–8. doi: 10.1006/meth.2001.1262
36. Liang X, Shen C-Y, Liu Z-Q, Qiu G-F. Histological Observation of the Brain and Thoracic Ganglion Mass in Chinese Mitten Crab, Eriocheir sinensis (in Chinese). J Shanghai Ocean Univ (2014) 23(2):173–8.
37. O’Shea M, Rayne RC. Adipokinetic Hormones: Cell and Molecular Biology. Experientia (1992) 48(5):430–8. doi: 10.1007/bf01928161
38. Bradbury AF, Smyth DG. Biosynthesis of the C-Terminal Amide in Peptide Hormones. Biosci Rep (1987) 7(12):907–16. doi: 10.1007/bf01122123
39. Eipper BA, Stoffers DA, Mains RE. The Biosynthesis of Neuropeptides: Peptide Alpha-Amidation. Annu Rev Neurosci (1992) 15:57–85. doi: 10.1146/annurev.ne.15.030192.000421
40. Lindemans M, Liu F, Janssen T, Husson SJ, Mertens I, Gäde G, et al. Adipokinetic Hormone Signaling Through the Gonadotropin-Releasing Hormone Receptor Modulates Egg-Laying in Caenorhabditis elegans. Proc Natl Acad Sci USA (2009) 106(5):1642–7. doi: 10.1073/PNAS.0809881106
41. Roch GJ, Busby ER, Sherwood NM. Evolution of GnRH: Diving Deeper. Gen Comp Endocrinol (2011) 171(1):1–16. doi: 10.1016/j.ygcen.2010.12.014
42. Hamanaka Y, Yasuyama K, Numata H, Shiga S. Synaptic Connections Between Pigment-Dispersing Factor-Immunoreactive Neurons and Neurons in the Pars Lateralis of the Blow Fly Protophormia terraenovae. J Comp Neurol (2005) 491(4):390–9. doi: 10.1002/cne.20712
43. Sullivan JM, Beltz BS. Neural Pathways Connecting the Deutocerebrum and Lateral Protocerebrum in the Brains of Decapod Crustaceans. J Comp Neurol (2001) 441(1):9–22. doi: 10.1002/cne.1394
44. Thongbuakaew T, Sumpownon C, Engsusophon A, Kornthong N, Chotwiwatthanakun C, Meeratana P, et al. Characterization of Prostanoid Pathway and the Control of Its Activity by the Eyestalk Optic Ganglion in the Female Giant Freshwater Prawn, Macrobrachium rosenbergii. Heliyon (2021) 7(1):e05898–e. doi: 10.1016/j.heliyon.2021.e05898
45. Xue R, Lin W, Sun J, Watanabe M, Xu A, Akira M, et al. The Role of Wnt Signaling in Male Reproductive Physiology and Pathology. Mol Hum Reprod (2021). doi: 10.1093/molehr/gaaa085
46. Perrot CY, Javelaud D, Mauviel A. Overlapping Activities of TGF-Beta and Hedgehog Signaling in Cancer: Therapeutic Targets for Cancer Treatment. Pharmacol Ther (2013) 137(2):183–99. doi: 10.1016/j.pharmthera.2012.10.002
47. Nurse P. Universal Control Mechanism Regulating Onset of M-Phase. Nature (1990) 344(6266):503–8. doi: 10.1038/344503a0
48. Pines J. Four-Dimensional Control of the Cell Cycle. Nat Cell Biol (1999) 1(3):E73–9. doi: 10.1038/11041
49. Yamashita M. Molecular Mechanisms of Meiotic Maturation and Arrest in Fish and Amphibian Oocytes. Semin Cell Dev Biol (1998) 9(5):569–79. doi: 10.1006/scdb.1998.0251
50. Yang Y, Lin D, Bao C, Huang H, Ye H. Serotonergic Mechanisms of Oocyte Germinal Vesicle Breakdown in the Mud Crab, Scylla paramamosain. Front Physiol (2019) 10:797. doi: 10.3389/fphys.2019.00797
51. Tang J, Zhu DF, Cui XY, Xie X, Qiu XE. Molecular Cloning, Characterization and Expression Analysis of the Retinoid X Receptor in the Swimming Crab, Portunus TRITUBERCULATUS (Miers, 1876) (Decapoda, Portunidae). Crustaceana (2014) 87(3):312–27. doi: 10.1163/15685403-00003286
52. Gong J, Huang C, Shu L, Bao C, Huang H, Ye H, et al. The Retinoid X Receptor From Mud Crab: New Insights Into Its Roles in Ovarian Development and Related Signaling Pathway. Sci Rep (2016) 6. doi: 10.1038/srep23654
53. Wan H, Zhong J, Zhang Z, Xie Y, Wang Y. Characterization of the Foxl2 Gene Involved in the Vtg Expression in Mud Crab (Scylla paramamosain). Gene (2021) 798. doi: 10.1016/j.gene.2021.145807
Keywords: Eriocheir sinensis, RPCH, PDH, oocyte meiosis, ISH, transcriptome
Citation: Wei L-L, Chen T-T, Luo B-Y and Qiu G-F (2021) Evidences for Red Pigment Concentrating Hormone (RPCH) and Beta-Pigment Dispersing Hormone (β-PDH) Inducing Oocyte Meiotic Maturation in the Chinese Mitten Crab, Eriocheir sinensis. Front. Endocrinol. 12:802768. doi: 10.3389/fendo.2021.802768
Received: 27 October 2021; Accepted: 30 November 2021;
Published: 16 December 2021.
Edited by:
Shubo Jin, Chinese Academy of Fishery Sciences, ChinaReviewed by:
Zhengfei Wang, Yancheng Teachers University, ChinaHui Qiao, Chinese Academy of Fishery Sciences, China
Copyright © 2021 Wei, Chen, Luo and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gao-Feng Qiu, Z2ZxaXVAc2hvdS5lZHUuY24=
†These authors have contributed equally to this work
 Ling-Ling Wei†
Ling-Ling Wei† Bi-Yun Luo
Bi-Yun Luo Gao-Feng Qiu
Gao-Feng Qiu