- 1Fielding School of Public Health, University of California, Los Angeles, Los Angeles, CA, United States
- 2School of Social Welfare, University of California at Berkeley, Berkeley, CA, United States
- 3UCLA David Geffen School of Medicine, Los Angeles, CA, United States
Introduction: About one in six children in the US, about 17% of the population, have one or more intellectual or developmental disabilities. Increases in disability due to neurodevelopmental or mental health conditions have increased by 21% in the last decade. Early intervention based on developmental screening and provider-initiated monitoring can significantly improve long-term health and cognitive outcomes. This paper assesses whether differences in receipt of developmental screening or monitoring are associated with access to a high-quality primary care medical home and having a provider who shows sensitivity to a family’s customs and values among neurotypical children and children with intellectual and developmental disabilities (IDD).
Methods: We used cross-sectional data from the National Survey of Children’s Health (NSCH) from 2017 to 2019. The NSCH is a nationally representative, parent-completed annual survey of children under 18. Children between 9 months and 5 years with IDD (n = 2,385) and neurotypical children (n = 20,200) were included in the analysis.
Results: Uptake of developmental screening/monitoring in neurotypical children and children with IDD conditions was associated with belonging to minority race/ethnic backgrounds, specifically Black, Asian, and AIAN/NHPI, and single-parent households with lower incomes, being publicly insured or uninsured and not having access to a high-quality medical home. Weighted regression models showed that the odds of neurotypical children receiving developmental monitoring/screening were 53% higher when their healthcare provider always or usually demonstrated cultural sensitivity to the family’s values and customs (OR 1.53, 95% CI, 1.08–2.18, p < 0.05). For children with IDD, the odds of receipt of monitoring/screening increased by 2.1 times when the provider always/usually demonstrated an understanding of the family’s cultural norms (95% CI, 0.99–4.43, p = 0.053). Being female was significantly associated with a lack of screening/surveillance (OR 0.73, 95% CI, 0.58–0.91, p < 0.05).
Discussion: With the rising prevalence of children with IDD conditions, early identification of developmental delays and subsequent access to interventions are crucial steps in supporting children and children with IDD to receive preventive care, services, and reduce disparities in accessing quality care. Implementing culturally sensitive approaches can be a low-cost and effective intervention in improving rates of provider-initiated monitoring and parent-completed screening.
Background
More than one in six children in the US, approximately 17% of the population, have one or more intellectual or developmental disabilities (IDDs) or conditions that may impair their physical activity, learning, language, or behavior (Cogswell et al., 2022). Some examples of these conditions include attention-deficit/hyperactivity disorder, autism spectrum disorder, blindness, cerebral palsy, moderate to profound hearing loss, learning disability, seizures, stuttering or stammering, and/or without intellectual impairment (Hotez et al., 2021). The number of children with physical or neurodevelopmental disabilities in the US has increased over the last decade; in particular, increases in disability due to neurodevelopmental or mental health conditions have increased by 20.9% (Houtrow et al., 2014).
Early intervention can significantly improve the cognitive and behavioral performance of children affected by developmental delays (Kuo et al., 2012); thus, the American Academy of Pediatrics (AAP) recommends that children aged 0 to 5 years receive regular developmental screening and monitoring. Developmental screening involves parent completion of validated, standardized screening tests at specific ages to identify risks for developmental delays and is recommended at 9, 18, and 30 months; improvements in screening mean these tests can identify certain IDDs, such as autism, as early as 18 months. Developmental monitoring involves a healthcare provider asking if a parent has concerns about a child’s learning, development, or behavior as part of routine healthcare visits. If concerns are identified, children can then be referred to early intervention services, such as speech, occupational, or behavioral therapy. In the past decade, the age at which a child can be screened or diagnosed for IDD conditions has improved significantly from early infancy, starting at 18 months of age up to much later in their lifespan.
Several factors are associated with increased screening in recent times, such as more awareness and knowledge about developmental delays in children, improved access to healthcare services, and improved measurement, such as better-quality diagnoses, changes in diagnostic criteria, and increased availability of services (Blumberg et al., 2013). Despite AAP guidelines and studies showing the efficacy of developmental screening and monitoring for early identification and intervention for IDD, rates of developmental screening and monitoring remain low; in 2016, less than one-third of children aged 9–35 months received screening from a parent and only 37% of children received developmental monitoring from a healthcare professional (Bethell et al., 2011; Hirai et al., 2018). Low developmental screening and monitoring rates contribute, in turn, to low receipt of early intervention services, with less than 20% of children with developmental delays receiving appropriate services and support before age 3 (Vitrikas et al., 2017).
Barriers to developmental screening, monitoring, and referral to early intervention
Prior research has documented multiple barriers to providing developmental screening, surveillance, and subsequent referral to appropriate specialists (Radecki et al., 2011; Mention and Heider, 2016; Hirai et al., 2018; Lipkin et al., 2020a,b). At the provider level, barriers to developmental screening and monitoring include lack of time, challenges with implementation in a busy urban clinic, lack of resources for follow-up after positive screens, and lack of provider knowledge regarding how screening can alter outcomes for children with IDD (Carbone et al., 2010; Berger-Jenkins et al., 2019). These barriers may be exacerbated due to provider shortages (Krauss et al., 2003; Chiri and Warfield, 2012). Currently, due to time constraints, many primary care clinics ask parents to complete standardized parent screening tools in advance of scheduled wellness visits rather than relying on busy providers to elicit parental concerns (Morelli et al., 2014; Lordi and Holtby, 2021). However, access to these screening tools is contingent on patients having access to these clinics as a usual source of care.
Patient-level barriers such as inequitable access to primary care medical home or lack of knowledge regarding the importance of screening can also limit screening uptake and contribute to sociodemographic disparities in developmental screening and monitoring and subsequent receipt of early intervention services (Kuo et al., 2012, Schickedanz and Halfon, 2020; Chiam et al., 2021). Black and Latino children from non-English speaking homes are less likely than white children to receive age-appropriate developmental screening and monitoring (Berger, 2018). Prior research also suggests that children living in households with incomes less than 300% of the Federal Poverty Level and lower parental education levels were less likely to receive developmental assessments (Lordi and Holtby, 2021). Low rates of developmental screening among children living in poverty are concerning because the prevalence of disability is highest in this group (Zablotsky et al., 2019).
Despite the inequalities that previous studies have pointed to in rates of screening/monitoring for children, few studies have investigated disparities in accessing these key preventive services in children with IDD. In addition, little research has assessed how access to a high-quality primary care home and having a provider who is culturally sensitive may affect the uptake of screening/monitoring for children with IDD. The recent COVID-19 pandemic has had a disproportionately large impact on parents and caregivers of children with special healthcare needs who shoulder a higher burden of caregiving responsibilities and stress in general (Woodman et al., 2015), and these stressors were exacerbated during the COVID-19 outbreak (Neece et al., 2020). These disparities have likely worsened during the COVID-19 pandemic for children with IDD (Bellomo et al., 2020). Our research aims are to better understand how screening and monitoring fared for these children with IDD before the disruptions brought on by COVID-19.
One such disruption is the upcoming end of the 2020 federal Medicaid continuous coverage requirement, which may cause many eligible children to lose coverage and become uninsured (Georgetown University Report, 2021). Notably, as states consider whether to extend continuous coverage protections for children in the process of redetermining Medi-Cal eligibility, it is important to better understand the effect of having access to a medical home and the role of a culturally sensitive primary care provider in the uptake of screening/monitoring practices for children with IDD.
Methods
Data source and sample
This study uses pooled, cross-sectional data from the 2017–2019 National Survey of Children’s Health (NSCH). The NSCH is a nationally representative survey of children within the United States under 18 years of age, and it is completed by parents. The NSCH is funded by the Health Resources and Services Administration and conducted annually by the US Census Bureau (Child and Adolescent Health Measurement Initiative (CAHMI), 2022).
Data from 2017, 2018, and 2019 were merged for a total of 81,562 observations. In 2017, 21,599 surveys were completed, with an overall weighted response rate of 37.4%. The sample size in 2018 included 30,530 surveys completed, with a response rate of 43.1%. In 2019, 29,433 surveys were completed with a weighted response rate of 42.4%.
We first restricted our study sample to include children between 0 and 5 years, reducing the study sample to 22,585 children. Because children are not eligible for developmental screening until 9 months, we further excluded 5,450 children less than 9 months of age from our analyses, resulting in a final analytic sample of 17,135 children aged 9 months to 5 years. Of these children, 15,028 were neurotypical and 2,107 had IDD. We defined children with IDD as those who responded yes to a question asking about the presence of different neurodevelopmental conditions, including attention-deficit/hyperactivity disorder (ADHD), autism, learning disabilities, intellectual disability, conduct disorders, cerebral palsy, and impairments in vision and hearing (APA, EPA Report, 2015; Lipkin Macias et al., 2020b).
Measures
Developmental screening or monitoring
Our primary outcome measure, whether a child received developmental screening or monitoring by a healthcare provider, was operationalized as a dichotomous variable set =1 if a child received either screening or monitoring, else set = 0. Children were considered to have received developmental screening if a parent or caregiver responded affirmatively to a healthcare professional asking them to complete a questionnaire regarding any specific concerns or observations they may have about their child’s development, communication, or social behavior in the last year. Children were considered to have received developmental surveillance or monitoring if a parent or caregiver responded affirmatively to a survey question checking whether a healthcare professional had asked them if they had any concerns about their child’s learning, development, or behavior in the past 12 months.
Predisposing factors: Predisposing factors included sociodemographic factors such as child’s race [Non-Hispanic White, Non-Hispanic Black, Hispanic, Asian, American Indian/Alaskan Native (AIAN), and Native Hawaiian and Other Pacific Islander (NHOPI)], sex (male or female), parent education (less than high school, high school degree/GED, some college/technical school, and college degree or higher), family structure (two parents, currently married, two parents, not currently married, single parent (mother/father), and grandparent/other family types), the generational status of parents [parent(s) born in the US], any parent born outside of the US, other (child born in the US, parents not listed), and primary household language (English, Spanish and other language).
Enabling factors included the child’s insurance (private insurance, public only, public and private insurance, and uninsured), family income (0–99% FPL, 100–199% FPL, 200–399% FPL, and 400% FPL and above), access to care that meets medical home criteria (yes/no), whether the child had a preventive medical visit in the last year (yes/no), and whether the child’s doctor or healthcare provider showed sensitivity to a family’s values and customs. Access to care that meets medical home criteria was operationalized as a categorical variable reflecting whether the child had access to a usual source of care, had a personal physician or nurse, received family-centered care or specialty care referrals, and received care coordination based on the American Academy of Pediatricians’ guidelines. Positive parent response to the first three components (personal doctor or nurse, usual source of care, and family-centered care) of the variable is coded as yes (1) to the receipt of coordinated, ongoing, comprehensive care within a medical home. Additionally, criteria for a child needing referrals or care coordination needs to be met for parent response to be coded as Yes (1) to the last two components of the medical home composite measure.
Having a provider who shows sensitivity to a family’s values and customs was operationalized as a dichotomous variable set =1 if the parent or caregiver felt that the provider “Always” or “Usually” demonstrated this sensitivity and set =0 if the provider only “Sometimes” or “Never” demonstrated this sensitivity. In this analysis, the variable was created across 2017–2019 as a dichotomous measure by collapsing sometimes/never into 0 (Reference) and always/usually as 1 response options. The receipt of effective care coordination is operationalized as not needing care coordination, receiving needed care coordination, and not receiving needed care coordination. Preventive visit in the last 12 months was measured as categorical with yes/no options.
Need factors included whether the child had IDD (=1 if yes, =0 if no) and complexity of healthcare needs (none, less, and more). The categorical construct measuring a child’s complexity of healthcare needs was characterized as a child having no complex healthcare needs, i.e., were typically developing children and set = 0; being a child with special healthcare needs but having less complex health needs coded as 1; and with more complex needs as 2.
Analysis
We examined bivariate relationships using Pearson’s Chi-squared tests to determine an association between the independent variables and covariates of interest with the receipt of developmental screening or monitoring. Weighted, multiple logistic regression models examined the association between predisposing, enabling, and need-based factors and children’s receipt of developmental screening and or monitoring. We conducted multicollinearity and endogeneity diagnostics to check for an association between having access to care meeting medical home criteria and having a provider who showed sensitivity to a child’s family’s values and customs. In these tests, the magnitude of the effect was small when regressing sensitivity on behalf of the provider on the receipt of developmental screening or monitoring. The VIF factor was less than 10 (approximately 1.24). Furthermore, with weighted data, tests for checking multicollinearity have not been developed yet. Statistical analyses were conducted using Stata statistical software, version 17 from Stata Corp. 2021, College Station, TX, and accounted for the complex survey design and imputation of income-related variables. This study was approved as exempt by the Institutional Review Board of UCLA.
Results
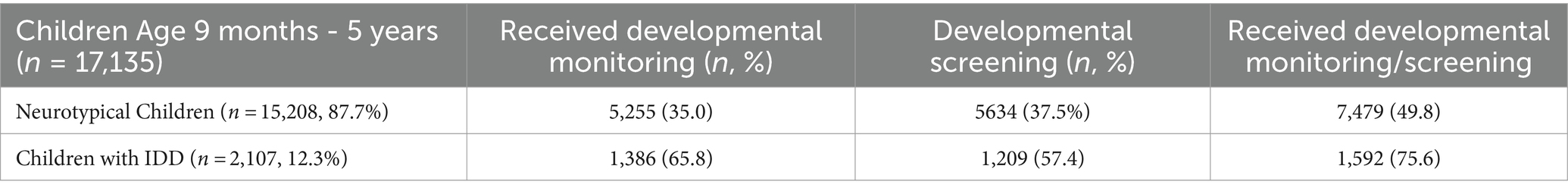
Table 1 shows the descriptive characteristics of children in our sample. Children with IDD conditions comprised 12.3% (n = 2,107) of our sample. Approximately half of neurotypical children (49.8%) in our sample received either developmental screening or monitoring, compared to 75.6% of children with IDD. Neurotypical children and children with IDD in our sample also differed significantly in terms of predisposing, enabling, and need-based factors. For example, about 20.2% of IDD families were single-parent households as compared to 12.2% of neurotypical children. Approximately 38.9% of families with a child with IDD combined had some high school or some college education. A little over one-third of children with IDD (33.8%) belonged to households with income levels less than 200% of the federal poverty level; 31.6% of these children were covered by public insurance or utilized a combination of public and private health insurance (8.2%). Over half of children with IDD were also identified as having more complex healthcare needs (51.8%) as compared to only 3.4% of neurotypical children. One-third of children with IDD were identified as belonging to minority racial/ethnic households (32.6%), compared to only 28.5% of neurotypical children.
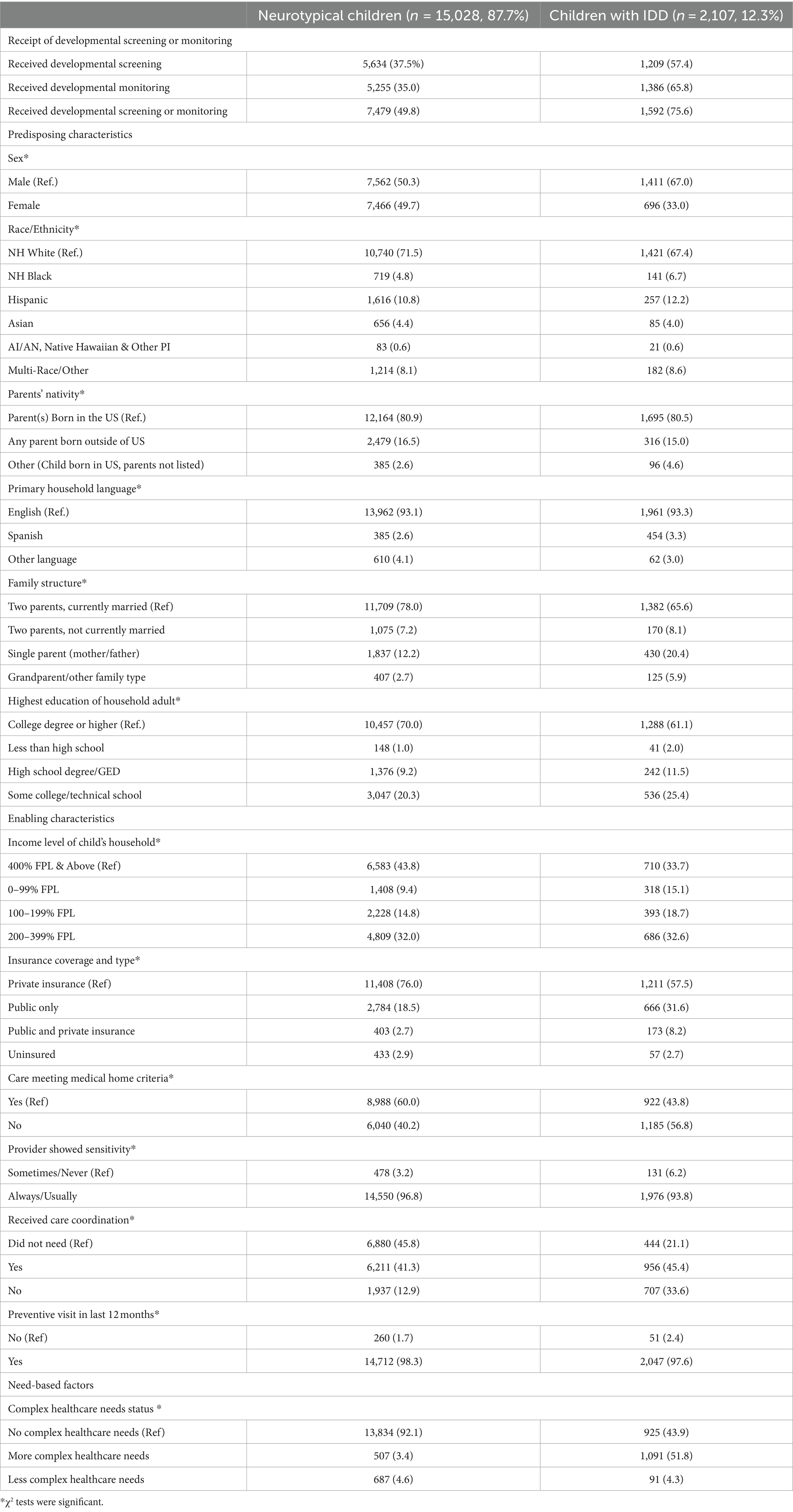
Table 1. Descriptive characteristics of neurotypical children and children with IDD aged 9 months to 5 years.
Pearson’s Chi-squared test results
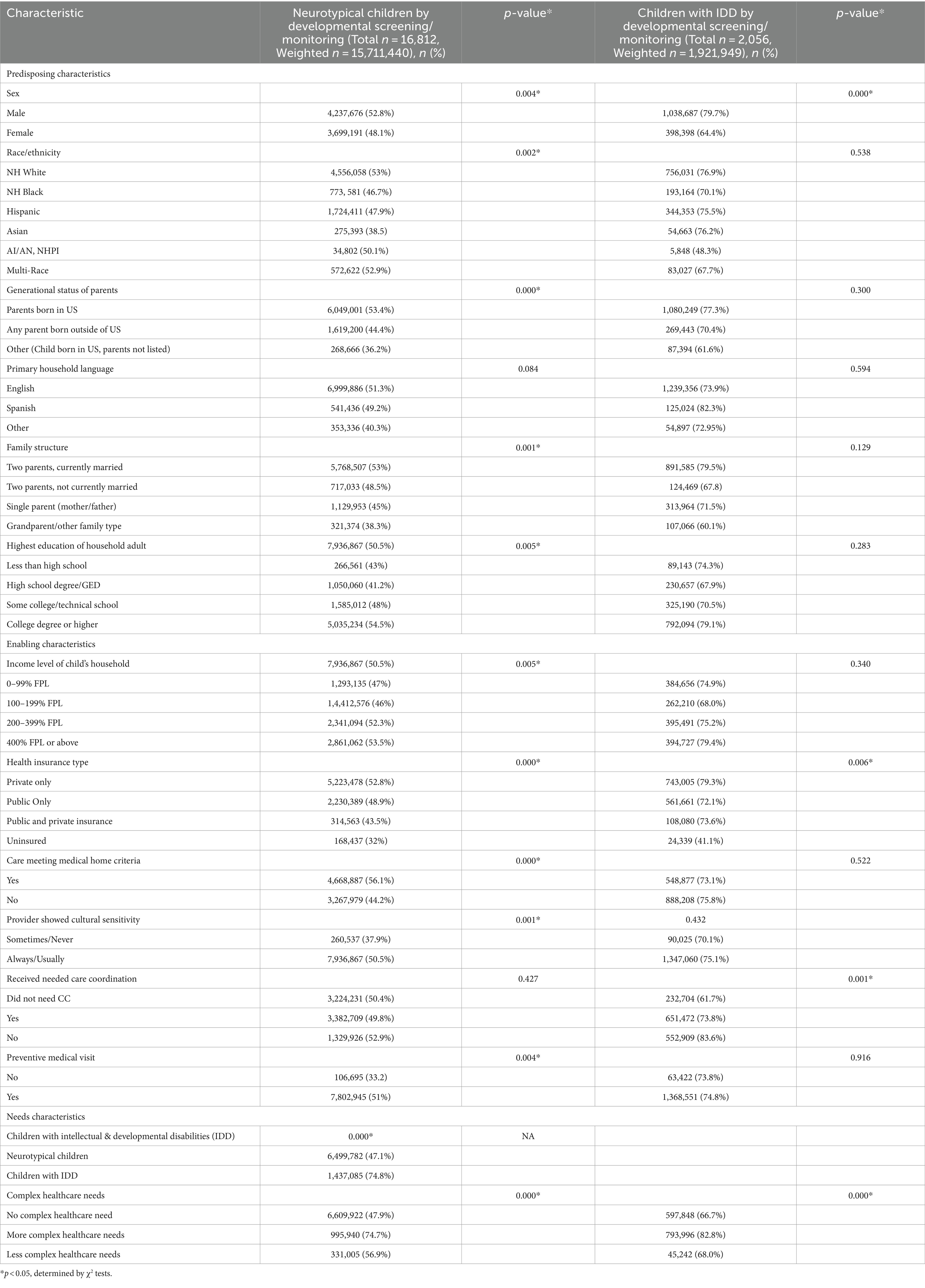
Pearson’s Chi-squared tests (see Table 2) identified several predisposing, enabling, and need-based factors significantly associated with the receipt of developmental screening and monitoring. Among neurotypical children, all predisposing, enabling, and need-based characteristics except household language were significantly associated with the receipt of developmental screening and monitoring. By contrast, among children with IDD, only gender, health insurance type, receipt of care coordination, and complexity of healthcare needs were significantly associated with the receipt of developmental screening or monitoring.
Regression analysis
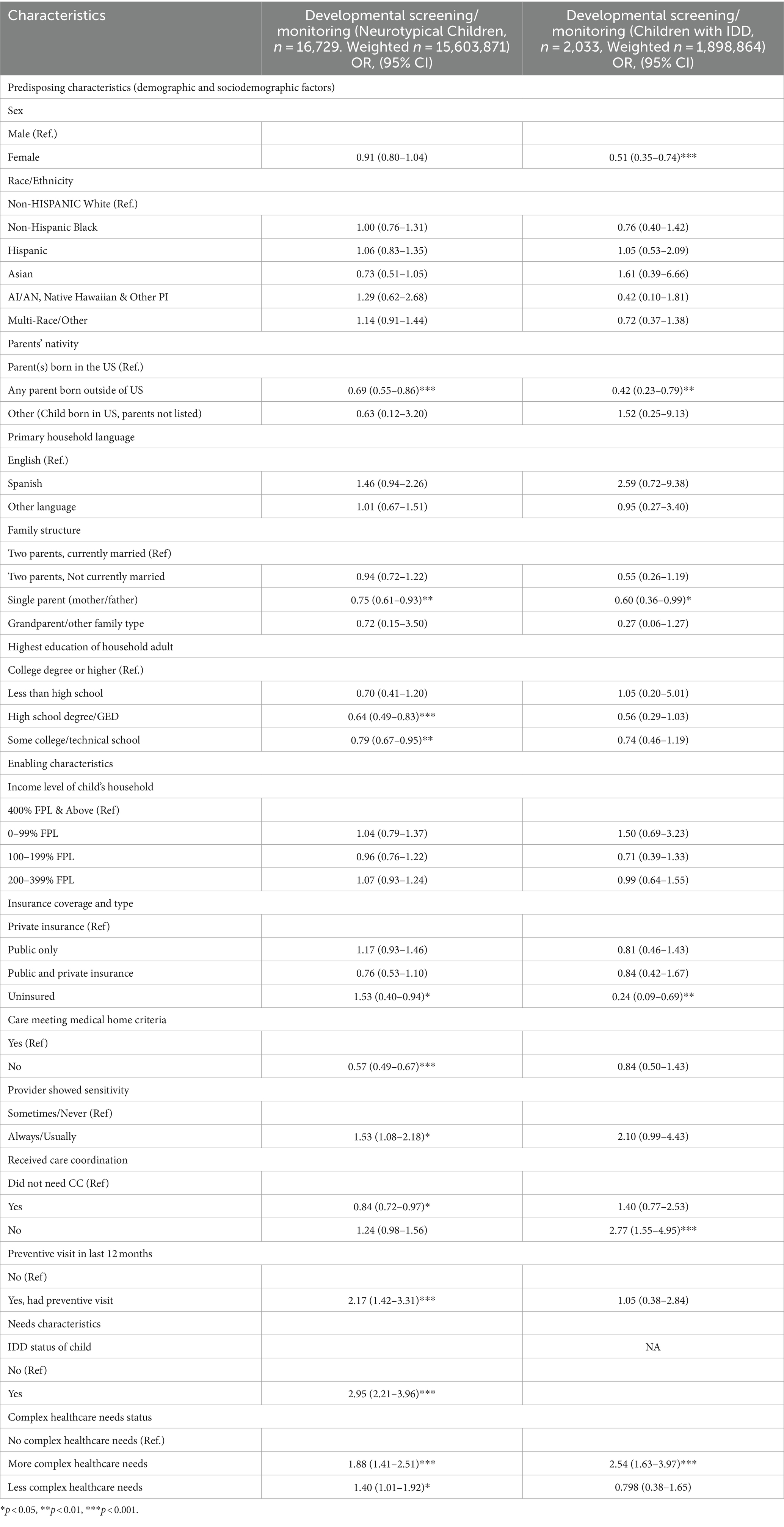
Table 3 provides weighted, logistic regression results identifying predisposing, enabling, and need-based factors associated with the receipt of developmental screening or monitoring among neurotypical children and those with IDD. To facilitate the interpretation of results, Table 4 presents discrete changes in the receipt of developmental screening or monitoring given different values of categorical independent variables identified as significant in Table 3.
The only predisposing characteristics significantly associated with the receipt of developmental screening or monitoring for both neurotypical children and children with IDD were parental nativity status and family structure. Specifically, children of parents born outside the US had significantly lower odds of receiving developmental screening or monitoring (OR 0.69, p < 0.001 for neurotypical children and OR 0.42, p < 0.01 for children with IDD), as were children in single-parent households (OR 0.75, p < 0.01 for neurotypical children and OR 0.60, p < 0.01 for children with IDD). Among children with IDD, girls were less likely than boys to receive developmental screening or monitoring (OR 0.51, 95% CI, 0.35–0.74, p < 0.001).
Enabling characteristics significantly associated with the receipt of developmental screening or monitoring for neurotypical children and children with IDD included being uninsured, having a culturally sensitive provider, and receipt of care coordination. Contrary to the hypothesis, uninsured neurotypical children were more likely to receive developmental screening or monitoring than their privately insured peers (OR 1.53, p < 0.05), perhaps due to the small sample size of uninsured children; however, uninsured children with IDD were significantly less likely to receive developmental screening or monitoring than typically developing uninsured children (OR 0.24, p < 0.05). As hypothesized, children with providers perceived as being more sensitive to their needs were more likely to receive developmental screening and monitoring (OR 1.53, p < 0.05 for neurotypical children and OR 2.1, p = 0.053 for children with IDD). Contrary to the hypothesis, children with IDD who did not receive needed care coordination were more likely to have received developmental screening or monitoring (OR 2.77, p < 0.001), which may be a result of variable measurement constraints such as the majority of children being categorized as not needing care coordination due to seeing a single healthcare provider.
Finally, need-based factors associated with the receipt of developmental screening and monitoring included the complexity of healthcare needs. Specifically, compared to children with no complex healthcare needs, children with more complex healthcare needs were significantly more likely to receive developmental screening or monitoring (OR 1.88 p < 0.001 among neurotypical children, and OR 2.54 p < 0.001 among children with IDD).
Discussion
Acknowledging the importance of screening and early intervention for cognitive and behavioral performance of children affected by developmental delays, in 2006, the AAP recommended administering a validated, developmental screening tool as part of pediatric preventive care visits for children at 9, 18, 24, or 30 months of age, and autism-specific screening for all children at 18, 24, or 30 months. In addition, many national and local organizations and programs have incorporated these guidelines and steps for early monitoring and screening, including the National Quality Forum (NQF) and Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents (Hagan et al., 2008).
Despite these recommendations, developmental screening and monitoring rates have not improved significantly over the last two decades. In our study, we found that less than half of neurotypical children received developmental screening or monitoring. Approximately 38% of children received physician-ordered, parent-completed screening, and approximately 36% of parents received developmental monitoring from a healthcare professional, i.e., informal conversations between parents and providers about the child’s wellbeing. In recent times, parent-completed screening has become the preferred method of screening (Squires et al., 1997; Sices et al., 2003; Sices, 2007); however, subsequent communication between parents and providers regarding screening results is still important for connecting children to early intervention when needs are identified.
Study findings also highlight predisposing, enabling, and need-based characteristics associated with the receipt of developmental screening and monitoring. Consistent with prior research identifying gender-based and socioeconomic disparities in developmental screening and monitoring (Hirai et al., 2018; Zablotsky et al., 2019), we found that children were less likely to receive screening or monitoring if they belonged to single-parent households, had a parent born outside of the US, or who was not legally documented. We also found that children with IDD were less likely to receive developmental screening or monitoring if they were uninsured as opposed to privately insured. This finding is important to highlight as more than 6.7 million children may have lost health insurance through Medicaid or the Children’s Health Insurance Program (CHIP) in 2023 (Georgetown University Report, 2021). These children were insured through Medicaid during the COVID-19 pandemic as part of a federal continuous coverage requirement; however, following the expiration of this requirement, states have varied in whether they have extended these protections and their eligibility redetermination policies. Our findings reinforce prior research suggesting that children with IDD who are uninsured may fail to receive critical preventive services such as developmental screening, which may, in turn, impact their ability to access early intervention services such as speech, language, or behavioral therapies that could improve their long-term health and cognitive outcomes.
As hypothesized, our study also highlights the importance of having a culturally sensitive healthcare professional or provider to improve the utilization of recommended care. Although majority of children and children with IDD received culturally-sensitive care, however our findings show that odds of parents completing developmental screening or receiving monitoring were much higher as compared to when the provider was only sometimes or usually sensitive to their cultural norms. These findings reinforce research suggesting the importance of using culturally sensitive approaches when interacting with families. Families that belong to diverse cultural backgrounds with alternative belief systems, or those that are less trusting of the healthcare system, may more likely utilize services based on their relationship with their provider (Stevens et al., 2003; Garg et al., 2017).
Some limitations of this study are that the NSCH captures cross-sectional, self-reported data; hence, inferences about causality cannot be made. The survey data are also likely to be biased based on the nature and type of respondents that the NSCH was able to reach, including non-response and recall biases. As a result of variable measurement constraints, we might be missing information about important components of the medical home model that are key to characterizing care quality as high and other access measures as well. Additionally, small sample sizes within uninsured neurotypical children could explain some counterintuitive results, such as uninsured children being more likely to receive screening or monitoring and not receiving care coordination. The NSCH uses imputation and sequential regression methods to account for missing demographic characteristics such as race/ethnicity, income, and education; however, this indicates that data are not missing completely at random. It is also important to note that our measures were focused on developmental screening and monitoring by healthcare professionals and may not reflect screening and monitoring conducted in other settings, such as early childcare or schools.
Conclusion
With the rising prevalence of children with IDD conditions, early identification of developmental delays and subsequent access to interventions are crucial steps in supporting children and children with IDD to access preventive care and services and reduce disparities in accessing quality care. Parents of 38% of neurotypical children reported receiving a screening questionnaire regarding their child’s developmental trajectory, while 36% of parents were asked by a healthcare provider if they had any concerns about their child’s development. Our results show that parents of neurotypical children were 53% more likely to complete a developmental screening questionnaire or to have received monitoring when the healthcare provider usually or always understood their values and customs. The magnitude of these results was amplified for children with IDD, with parents being twice as likely to complete screening or receive monitoring by a culturally sensitive healthcare provider.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
PM had full access to all the data in the study and took responsibility for data integrity, data analysis, and drafting of manuscript. PM, EC, JN, and AK: concept and design, statistical analysis, acquisition, analysis, and interpretation of data. PM, EC, JN, KR, and AK: revision of manuscript for content. PM and KR: administrative, technical, and material support. EC, JN, and AK: supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the AHRQ T-32 predoctoral fellowship (grant no: AHRQ2T32HS000046), UCLA Graduate Mentorship Program, and UCLA’s Center of Excellence in Maternal & Child Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bellomo, T. R., Prasad, S., Munzer, T., and Laventhal, N. (2020). The impact of the COVID-19 pandemic on children with autism spectrum disorders. J. Pediatr. Rehabil. Med. 13, 349–354. doi: 10.3233/PRM-200740
Berger, S. (2018). Rates of developmental and behavioral screening of young children: Implications for health care policy and practice.
Berger-Jenkins, E., Monk, C., D’onfro, K., Sultana, M., Brandt, L., Ankam, J., et al. (2019). Screening for both child behavior and social determinants of health in pediatric primary care. J. Dev. Behav. Pediatrics 40, 415–424. doi: 10.1097/DBP.0000000000000676
Bethell, C., Reuland, C., Schor, E., Abrahms, M., and Halfon, N. (2011). Rates of parent-centered developmental screening: disparities and links to services access. Pediatrics 128, 146–155. doi: 10.1542/peds.2010-0424
Blumberg, S. J., Bramlett, M. D., Kogan, M. D., Schieve, L. A., Jones, J. R., and Lu, M. C. (2013). Changes in prevalence of parent-reported autism Spectrum disorder in school-aged U.S. Children: 2007 to 2011–2012. Hyattsville, MD: National Center for Health Statistics.
Carbone, P. S., Behl, D. D., Azor, V., and Murphy, N. A. (2010). The medical home for children with autism Spectrum disorders: parent and pediatrician perspectives. J. Autism Dev. Disord. 40, 317–324. doi: 10.1007/s10803-009-0874-5
Chiam, M., Rojas, E., Bergey, M. R., and Mackie, T. I. (2021). The effect of medical home on shared decision-making for caregivers of children with emotional, developmental, or behavioral health conditions. Matern. Child Health J. 25, 1285–1295. doi: 10.1007/s10995-021-03148-w
Child and Adolescent Health Measurement Initiative (CAHMI) (2022). 2017, 2018, 2019 National Survey of Children’s health: Child and family health measures, National Performance and outcome measures, and subgroups, Stata codebook, version 17, data resource Center for Child and Adolescent Health supported by the U.S. Department of Health and Human Services, Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau (MCHB).
Chiri, G., and Warfield, M. E. (2012). Unmet need and problems accessing Core health Care Services for Children with autism Spectrum disorder. Matern. Child Health J. 16, 1081–1091. doi: 10.1007/s10995-011-0833-6
Cogswell, M. E., Coil, E., Tian, L. H., Tinker, S. C., Ryerson, A. B., Maenner, M. J., et al. (2022). Health needs and use of services among children with developmental disabilities — United States, 2014–2018. MMWR Morb. Mortal Wkly. Rep. 71, 453–458. doi: 10.15585/mmwr.mm7112a3
Garg, P., Ha, M. T., Eastwood, J., Harvey, S., Woolfenden, S., Murphy, E., et al. (2017). Explaining culturally and linguistically diverse (CALD) parents’ access of healthcare services for developmental surveillance and anticipatory guidance: qualitative findings from the ‘watch me Grow’ study. BMC Health Serv. Res. 17, 1–12. doi: 10.1186/s12913-017-2143-1
Georgetown University Report. (2021). Georgetown University McCourt School of Public Policy Center for Children and Families analysis of February 2020–June 2021 Centers for Medicare and Medicaid Services state Medicaid and CHIP applications, eligibility determinations, and enrollment data.
Hagan, J. F., Shaw, J. S., and Duncan, P. M. (2008). Bright futures: Guidelines for health supervision of infants, children, and adolescents. Itasca, IL: American Academy of Pediatrics.
Hirai, A. H., Kogan, M. D., Kandasamy, V., Reuland, C., and Bethell, C. (2018). Prevalence and variation of developmental screening and surveillance in early childhood. JAMA Pediatr. 172, 857–866. doi: 10.1001/jamapediatrics.2018.1524
Hotez, E., Hotez, P. J., Rosenau, K. A., and Kuo, A. A. (2021). Prioritizing COVID-19 vaccinations for individuals with intellectual and developmental disabilities. EClinicalMedicine 32:100749. doi: 10.1016/j.eclinm.2021.100749
Houtrow, A. J., Larson, K., Olson, L. M., Newacheck, P. W., and Halfon, N. (2014). Changing trends of childhood disability, 2001–2011. Pediatrics 134, 530–538. doi: 10.1542/peds.2014-0594
Krauss, M. W., Gulley, S., Sciegaj, M., and Wells, N. (2003). Access to specialty medical care for children with mental retardation, autism, and other special health care needs. Ment. Retard. 41, 329–339. doi: 10.1352/0047-6765(2003)41<329:ATSMCF>2.0.CO;2
Kuo, A. A., Etzel, R. A., Chilton, L. A., Watson, C., and Gorski, P. A. (2012). Primary care pediatrics and public health: meeting the needs of today’s children. Am. J. Public Health 102, e17–e23. doi: 10.2105/AJPH.2012.301013
Lipkin, P. H., Macias, M. M., Baer Chen, B., Coury, D., Gottschlich, E. A., Hyman, S. L., et al. (2020a). Trends in pediatricians’ developmental screening: 2002–2016. Pediatrics 145:e20190851:145. doi: 10.1542/peds.2019-0851
Lipkin, P. H., and Macias, M. M.Council on Children With Disabilities, Section on Developmental and Behavioral Pediatrics (2020b). Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics 145:3449. doi: 10.1542/peds.2019-3449
Lordi, N., and Holtby, S. (2021). Developmental screening among children ages 1-5 in California. Los Angeles, CA: UCLA Center for Health Policy Research.
Mention, N., and Heider, F. (2016) The nuts and bolts of Medicaid reimbursement for developmental screening: Insights from Georgia, Minnesota, and North Carolina – The National Academy for state health policy.
Morelli, D. L., Pati, S., Butler, A., Blum, N. J., Gerdes, M., Pinto-Martin, J., et al. (2014). Challenges to implementation of developmental screening in urban primary care: a mixed methods study. BMC Pediatr. 14:16. doi: 10.1186/1471-2431-14-16
Neece, C., McIntyre, L. L., and Fenning, R. (2020). Examining the impact of COVID-19 in ethnically diverse families with young children with intellectual and developmental disabilities. J. Intellect. Disabil. Res. 64, 739–749. doi: 10.1111/jir.12769
Radecki, L., Sand-Loud, N., O'Connor, K. G., Sharp, S., and Olson, L. M. (2011). Trends in the use of standardized tools for developmental screening in early childhood: 2002–2009. Pediatrics 128, 14–19. doi: 10.1542/peds.2010-2180
Schickedanz, A., and Halfon, N. (2020). Evolving roles for health Care in Supporting Healthy Child Development. Futur. Child. 30, 143–164. doi: 10.1353/foc.2020.a807755
Sices, L. (2007). Developmental screening in primary care: The effectiveness of current practice and recommendations for improvement. Washington, DC: The Commonwealth Fund.
Sices, L., Feudtner, C., McLaughlin, J., Drotar, D., and Williams, M. (2003). How do primary care physicians identify young children with developmental delays? A national survey. J. Dev. Behav. Pediatr. 24, 409–417. doi: 10.1097/00004703-200312000-00002
Squires, J., Bricker, D., and Potter, L. (1997). Revision of a parent-completed development screening tool: ages and stages questionnaires. J. Pediatr. Psychol. 22, 313–328. doi: 10.1093/jpepsy/22.3.313
Stevens, G. D., Shi, L., and Cooper, L. A. (2003). Patient-provider racial and ethnic concordance and parent reports of the primary care experiences of children. Ann. Fam. Med. 1, 105–112. doi: 10.1370/afm.27
Vitrikas, K., Savard, D., and Bucaj, M. (2017). Developmental delay: when and how to screen. Am. Fam. Physician 96, 36–43
Woodman, A. C., Mawdsley, H. P., and Hauser-Cram, P. (2015). Parenting stress and child behavior problems within families of children with developmental disabilities: transactional relations across 15 years. Res. Dev. Disabil. 36, 264–276. doi: 10.1016/j.ridd.2014.10.011
Keywords: healthcare disparities, developmental screening, monitoring, culturally sensitive provider, children with intellectual and developmental disabilities, primary care medical home model, developmental surveillance, children
Citation: Mudnal PS, Chuang E, Needleman J, Rosenau K and Kuo AA (2024) Disparities in prevalence of screening/monitoring in children with intellectual and developmental disabilities: culturally sensitive provider can mitigate effects. Front. Educ. 9:1224720. doi: 10.3389/feduc.2024.1224720
Edited by:
Geoff Anthony Lindsay, University of Warwick, United KingdomReviewed by:
Veronica Vidal, Universidad de los Andes, ChileKiley Mclean, Drexel University, United States
Wei Song, Drexel University, United States
Copyright © 2024 Mudnal, Chuang, Needleman, Rosenau and Kuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Purnima S. Mudnal, cG11ZG5hbEB1Y2xhLmVkdQ==
 Purnima S. Mudnal
Purnima S. Mudnal Emmeline Chuang1,2
Emmeline Chuang1,2