- 1Delhi Public School Electronic City, Bangalore, India
- 2Home School, Pune, India
- 3SymphonyTech Biologics, Pune, India
- 4Jasper High School, Plano, TX, United States
- 5River Hill High School, Clarksville, MD, United States
- 6Department of Cell Biology, University of Maryland, College Park, MD, United States
Although vaccines are being developed and administered to people for more than a century, the understanding of the steps involved in vaccine development is a relatively new subject to the general public. During the current pandemic, there has been an explosion of non-validated news about COVID-19 and vaccines. To enhance the understanding of this critical societal science, there is an urgent need to teach these topics in the early education systems. Defining the essential subjects and courses for high school and developing syllabi for undergraduate courses in immunology and vaccinology can be difficult, as students choose diverse career options after their studies. To define these curricula, understanding the current level of awareness regarding vaccinology and immunology among students becomes essential. Thus, we have undertaken an exploratory survey of 650 high school and undergraduate college students in India on their awareness of the processes of vaccine development. Our results confirmed our hypothesis that there is a very limited understanding of this topic among school-going students. In this article, we propose an outline for a course for teaching in high schools. We recommend that this course should be interdisciplinary and a mix and match of majors and minors. It should train students with soft skills and prepare them for their careers in biomedical research.
Introduction
We routinely use easy-to-use medicines available at a local pharmacy for everyday ailments such as headaches and sores, and we rarely think about how these medicines are made. With the constant bombardment of news in public media about COVID-19 and vaccines, there is a huge amount of information and misinformation on how the COVID-19 vaccines were developed in <1 year (Dror et al., 2020). We, as high school and college students, are not aware of what it takes to develop these vaccines. These subjects are not taught in high schools, and it can be challenging to develop age/class-appropriate courses, as this field is rapidly advancing and expanding (Chatterjea, 2020). The multidisciplinary nature of the vaccine development process requires defining the key components of the curriculum. In this article, we have conducted an exploratory survey of high school and college students and teachers across India on the awareness of vaccine development processes. We have hypothesized that there is a very limited understanding of these processes among high school and college students as well as teachers. Using the data obtained through our survey and taking COVID-19 vaccines as an example, we have curated a novel course outline that can be utilized as a framework for developing a curriculum that can enable teaching the multidisciplinary aspects of vaccine development in high schools and colleges.
In December 2019, there was an outbreak of an unknown respiratory disease in Wuhan, China, characterized by dry cough, difficulty in breathing, loss of taste and smell, chest pain, and fever, which could potentially lead to multiple organ failure (Trojánek et al., 2020). The disease was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was later coined “COVID-19” by the WHO (2020). On 11 March 2020, the WHO declared it a pandemic. As of this submission, there are more than 590 million infected and more than 6.4 million dead. It is predicted that the virus originated in bats and was transmitted to humans via Pangolin, which are the intermediate hosts of the virus.
Severe acute respiratory syndrome coronavirus 2 is an enveloped RNA virus, with a ~30 kilobase long single-stranded RNA as a genetic material. It is capable of infecting a variety of host species (Vabret et al., 2020). Most infected people recover from COVID-19 with mild-to-moderate symptoms and do not need any medical intervention. However, older patients, immunocompromised individuals, and those with underlying conditions including diabetes, chronic respiratory disease, or cancer experience more severe symptoms such as difficulty in breathing, chest pain, or pressure. SARS-CoV-2 infects host cells by the binding of its spike protein to the ACE2 and TMPRSS2 receptors present on various cells in the nasal cavity, trachea, and lungs (Hirano and Murakami, 2020; Hoffmann et al., 2020; Lukassen et al., 2020). Once the virus enters the cell, it uses the enzyme RNA-dependent RNA polymerase (RdRp) to transcribe viral proteins. These proteins assemble in the cell and new viral particles emerge, which infect other cells (Figure 1). Neutralizing antibodies to the spike protein can block viral entry (Poeschla, 2020). Drugs, such as remdesivir and molnupiravir, bind to RdRp and can block viral replication (Felsenstein et al., 2020). Several SARS-CoV-2 variants have been identified, which include Alpha, Beta, Gamma, Epsilon, Eta, Iota, Kappa, B.1.617.3, Mu, Zeta, and Omicron. The understanding of the pathogenesis of COVID-19 continues to evolve as new large-scale studies are being conducted, which will provide insights into the long-term effects of COVID-19 on human health (Lopez-Leon et al., 2021).
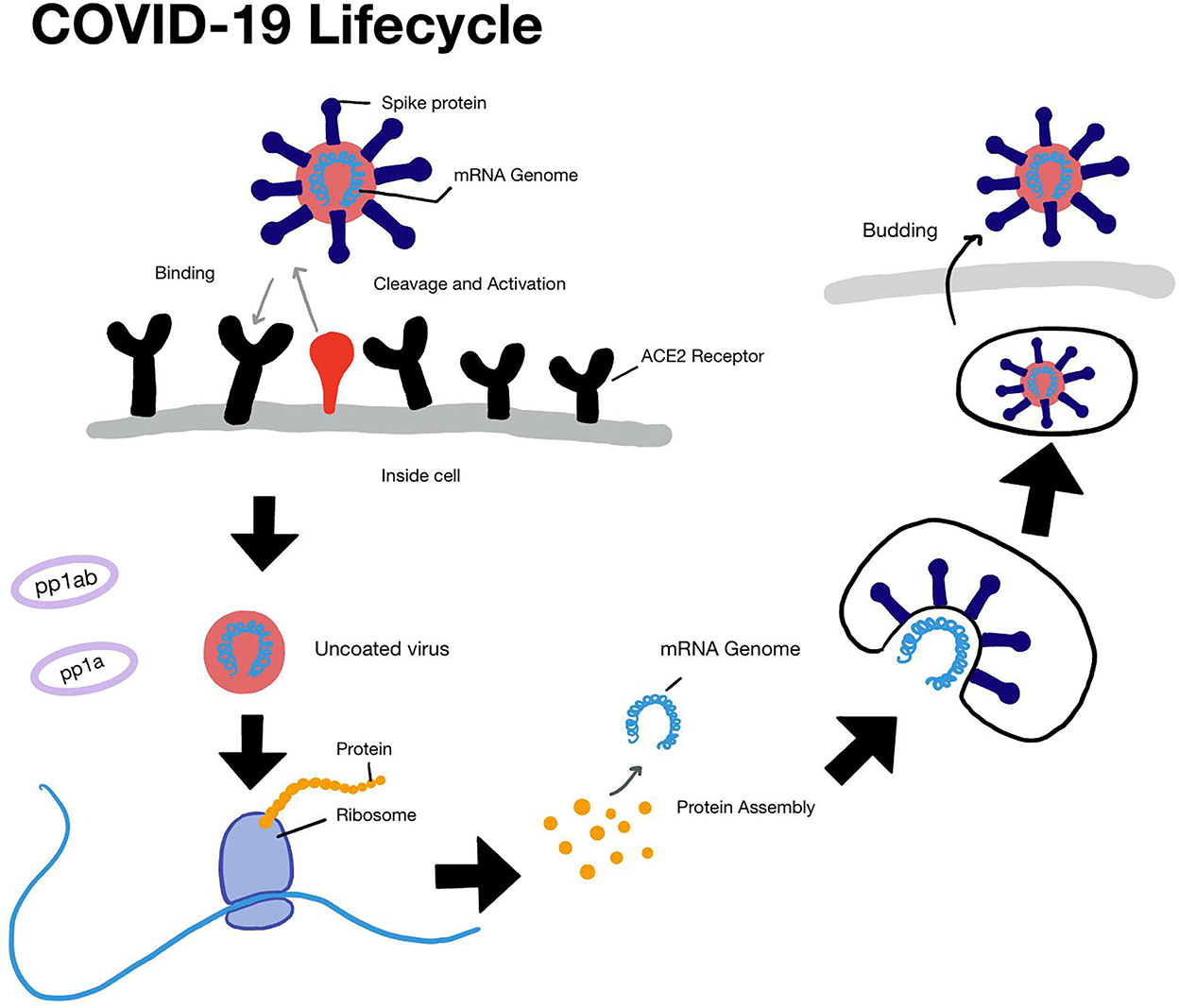
Figure 1. The life cycle of SARS-COV2. The steps involved from entry to replication and budding are shown in this figure.
To combat the pandemic, several pharmaceutical companies began the development of novel COVID-19 vaccines as early as February 2020 (Cohen, 2021). Vaccines prepare the body to combat viral pathogens and significantly reduce the chances of infection and adverse effects. The major platforms to develop vaccines include mRNA, DNA, protein, viral vectors, inactivated viral proteins, and live attenuated viruses (Francis et al., 2022). Table 1 shows the characteristics of these platforms. Several reviews have summarized the efficacy and effectiveness of these vaccine platforms in preventing COVID-19 disease (Krammer, 2020; Cai et al., 2021; Fiolet et al., 2022; Francis et al., 2022).
Prior to the development of a vaccine, it is imperative to understand the pathogenesis of the disease, and hence, the first step involves research on how the disease is caused and progresses. We have described the vaccine development process in seven major steps (Stern, 2020) (Figure 2). (i) discovery: experiments that enable understanding the mechanism by which the vaccine can prevent the disease; (ii) process development and scale up: methods to manufacture the vaccine on a large scale; (iii) pharmacology: dose and frequency of immunization required to enable efficacy; (iv) toxicology: study the side effects of the vaccine; (v) clinical trials: phases 1, 2, and 3 stages of testing the vaccine in humans to define the efficacy and toxicity; (vi) quality management: processes involved in ensuring that vaccines manufactured are reproducible in terms of safety and efficacy, stable, and maintain potency through time; and (vii) regulatory approval: processes by which government bodies review and approve the vaccine to be administered to millions of people.
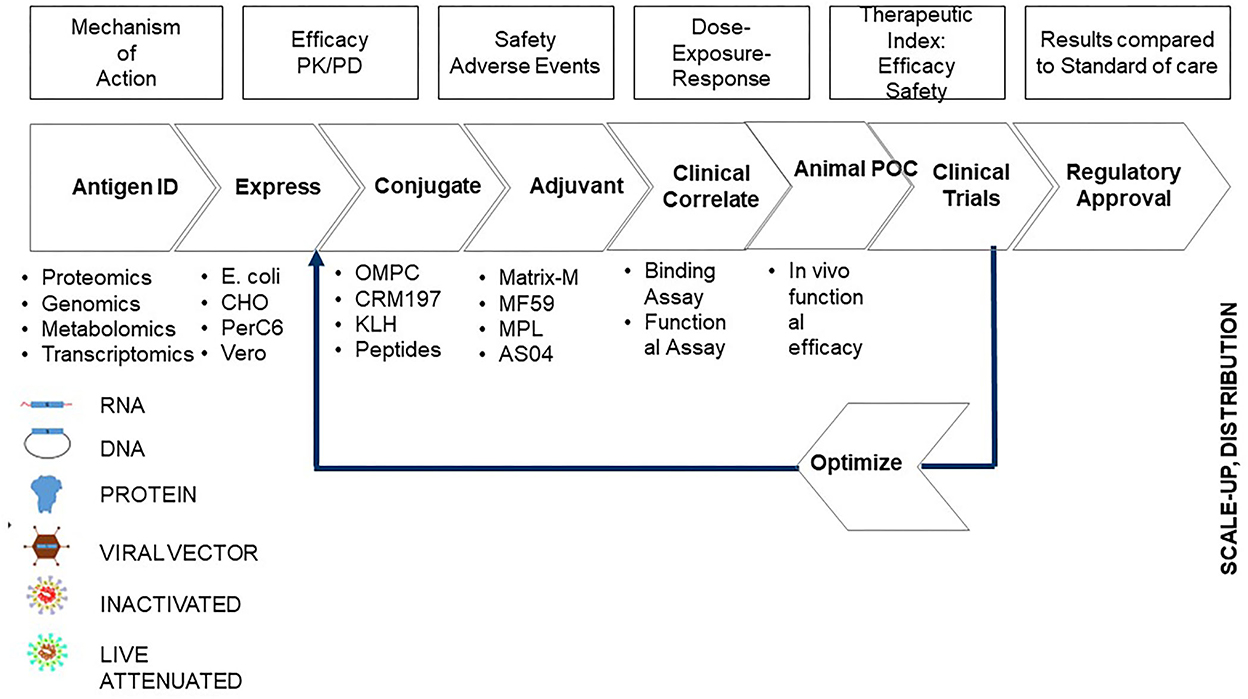
Figure 2. Steps involved in vaccine development. Chemistry, Manufacturing, and Controls (CMC) is the step that involves process development and scale-up of the method of developing the vaccine.
A survey to understand the awareness of vaccines in high schools and colleges
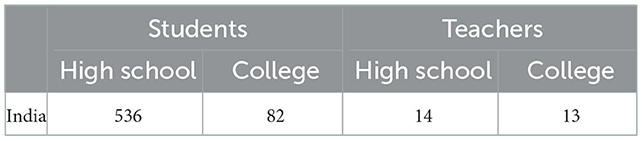
To understand the awareness of vaccine development processes among students beyond the student authors of this article, we conducted an exploratory survey among high school students, teachers, and administrators. The survey sought to examine several aspects of awareness of the processes involved in vaccine development, particularly COVID-19 vaccine development. We received a total of 645 responses through the authors' peer networks, colleagues, friends, and acquaintances. The dissemination was not random, and therefore, the authors do not claim that the data are representative of the population. The respondents included high school or college students or teachers in India (Table 2). Out of the total respondents, 83.1% were high school students and 12.7% were college students.
While examining and understanding the concepts of vaccine development among respondents, the pattern of responses from students and teachers pointed to the following key areas of teaching that could be implemented in high schools and colleges:
Awareness of vaccine development
The awareness of vaccine development was assessed by a rating score ranging from 1 to 10, with one having the least understanding (having taken 0 classes) to 10 being the higher (having taken three more classes). Figures 3A, B compare levels of high school (HS) & college education (CS) for the rating of awareness about the development of vaccines. The response to the survey suggests that the students with a higher level of education scored lower on their awareness of the vaccine development processes, whereas students of lower classes scored higher. To further analyze their awareness, we evaluated their rating based on the subject they were studying. Students studying subjects such as finance, humanities, architecture, arts, natural science, and management scored lower than those studying engineering and medicine (Figure 3C). We find this observation intriguing; the data suggest that students pursuing engineering and medicine have a higher level of awareness for vaccine development compared to other subject groups.
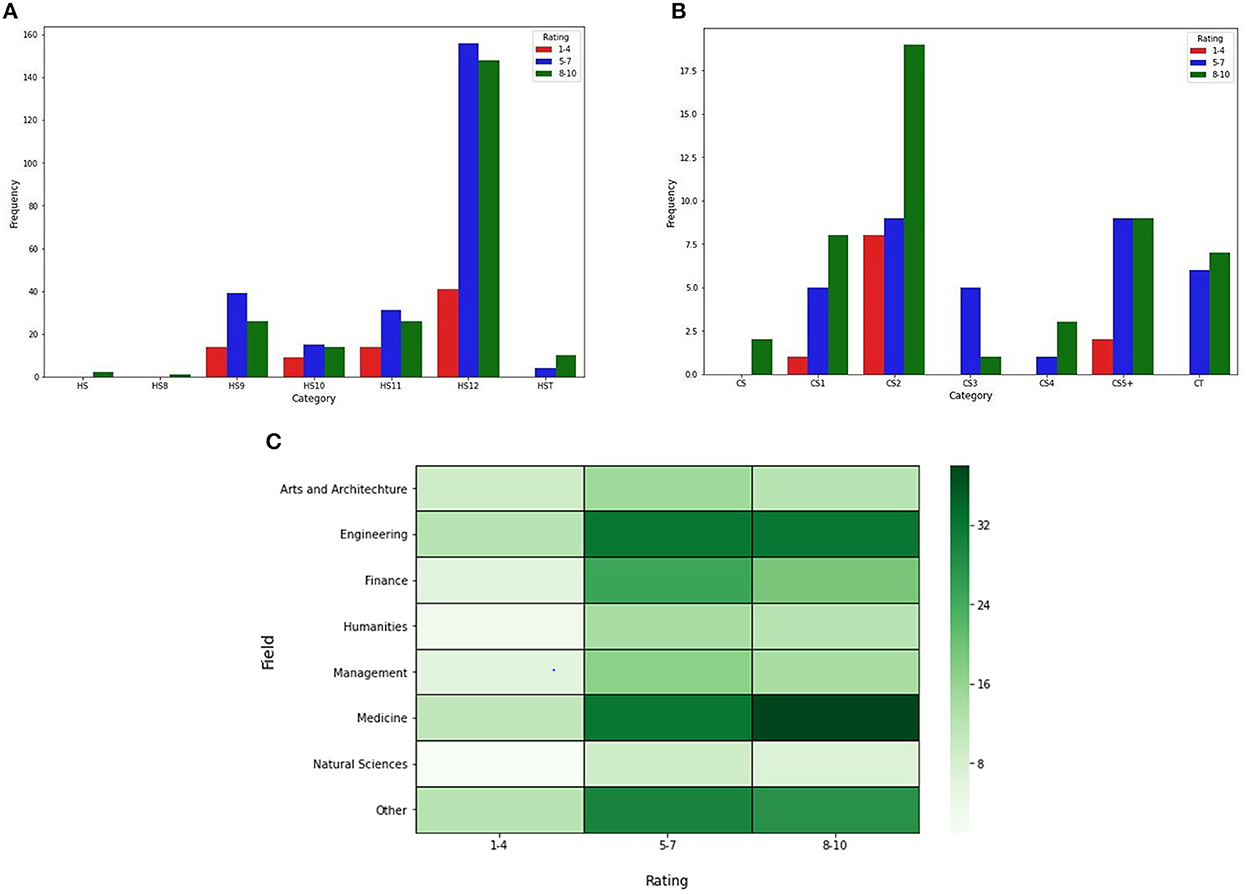
Figure 3. Awareness of vaccine development. (A, B) Bar graphs comparing the class level of high school (HS) students (B) year of college students (college) based on their education and rating of awareness of vaccine development. The bars correspond to the respondents' understanding of vaccine development on a scale of 1–10; by overall ranges. (C) Heat maps comparing the subject of interest of the individual with the rating of awareness of vaccine development.
Knowledge of vaccines approved
To ascertain the awareness of the students on vaccine development, the survey asked each respondent to list three diseases for which vaccines were approved. Figure 4 shows the list of the various diseases that the respondents listed for vaccines that have been approved. The results indicate that there is some understanding of vaccines and infectious diseases, which is an encouraging sign of knowledge awareness in schools.
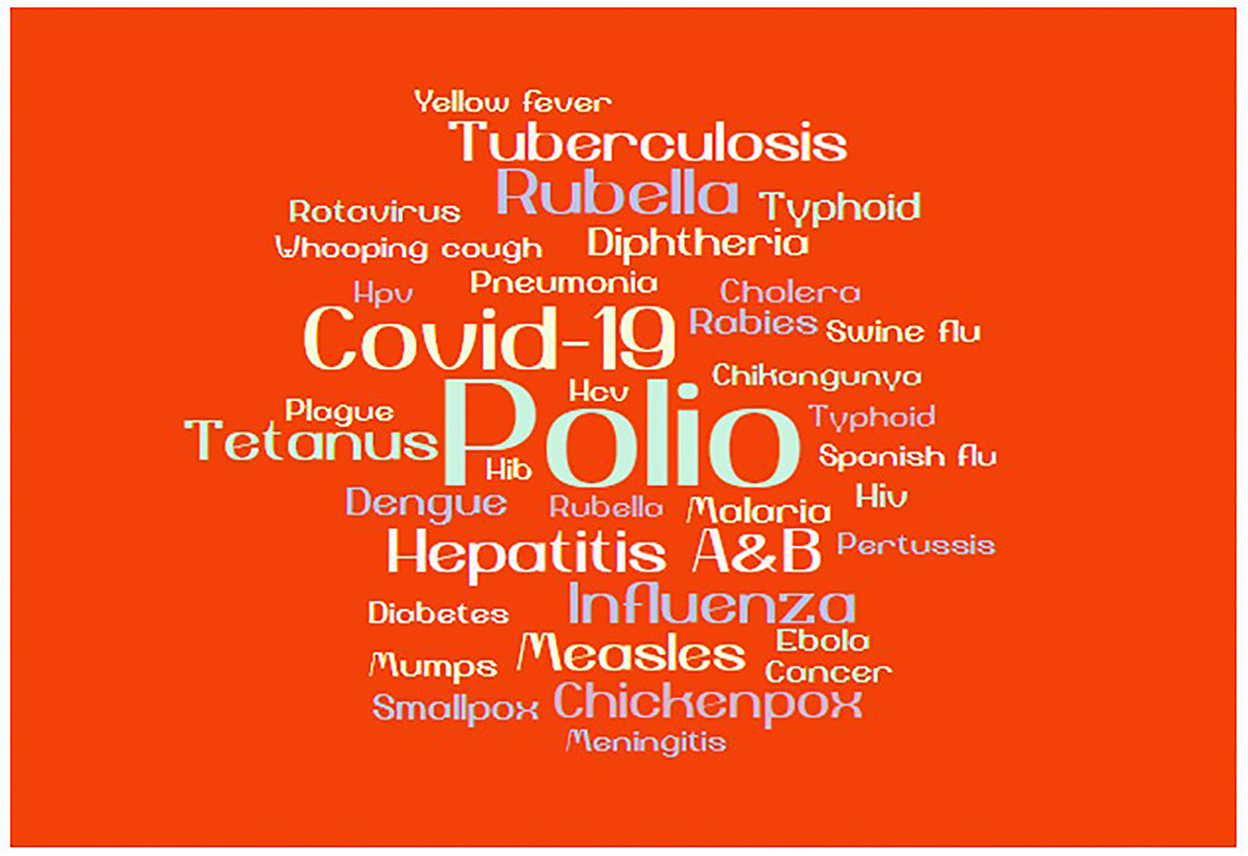
Figure 4. Knowledge of vaccines. The word cloud demonstrates the frequency of people who responded to the mentioned diseases against which they knew how vaccines were approved. In the word cloud, the size of each disease (word) is proportional to its frequency.
Source of information
Finally, the survey also asked each respondent to provide the source of information on vaccines. Few students including both high school and college level selected more than two options for the question asked, and therefore, the responses were segregated, which were then considered as individual responses for the analysis. Figure 5 shows that 32.5% (351) of the students mentioned that communication with friends and family is the main source of information, whereas 20.89% (225) opted for headlines and news as a source of information, 18.9% (204) opted for newspaper articles, and 7.7% (83) did not put any efforts for collecting information. In total, 11.3% (122) read scientific articles.
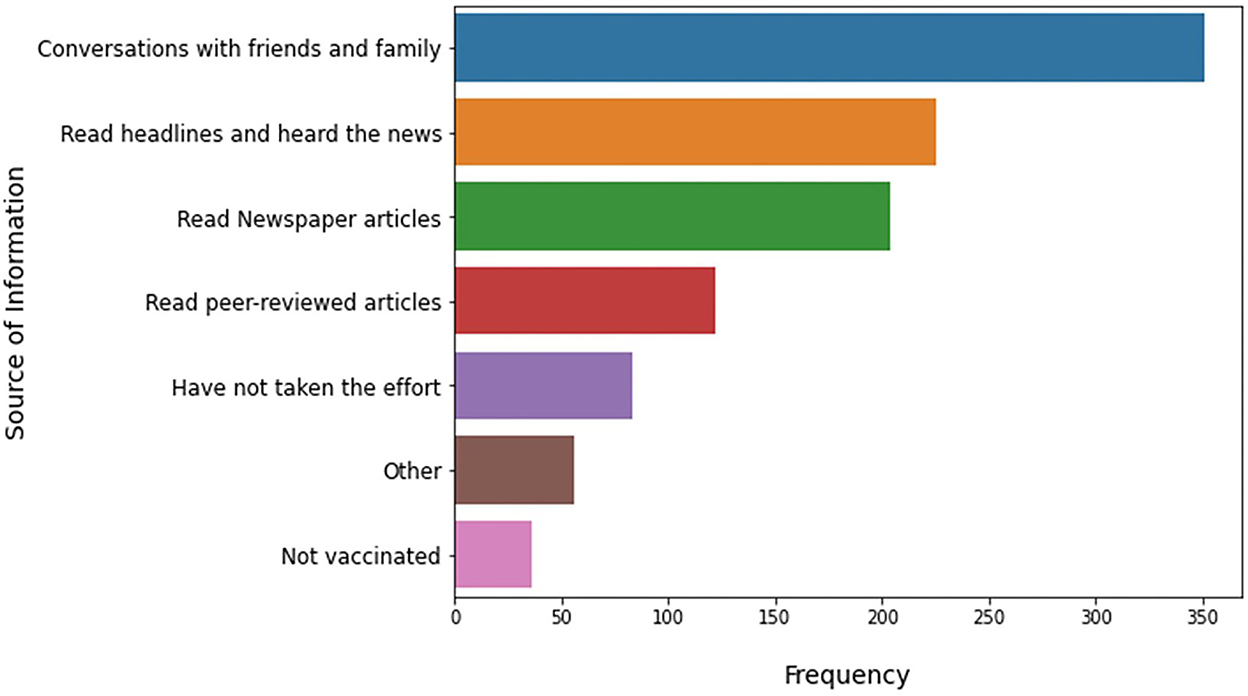
Figure 5. Sources of information. The bar graphs illustrate the various commonly used media through which respondents got their information about topics such as vaccines, vaccine development, and diseases. The list of sources was predefined in the survey.
These results indicate that majority of the students obtain their scientific information from newspapers, family and friends, rather than scientific articles. These observations indicate the necessity of the school systems to provide access to appropriate scientific journals and publications to students.
Developing a training and education system for high schools and colleges
The subject of biotechnology being taught in academia over the past decade has not been aligned with industry requirements. This gap has resulted in the formation of “finishing schools,” which provide a bridge to the industry requirements. Having a detailed understanding of different processes of vaccine development in the pharmaceutical and biotechnology industry requires a multifactorial process that involves diverse subjects such as immunology, chemical engineering, pharmacology, veterinary sciences, and clinical and regulatory studies. Skill development in these areas is critical to the success of novel vaccine development in the future.
We have used a methodical approach to capture the key topics for inclusion in a course that can be curated to teach various aspects of vaccine development. The use of analogies in the pedagogy of education is a powerful tool for explaining complex concepts. The process of vaccine development can be quite similar to designing a car: from design, developing a prototype, scaling up, testing its performance, and ensuring that it is safe and efficient. It is a systematic process with numerous moving parts. Moreover, there is variability associated with this high-risk-high-reward product in terms of health and economic returns.
Since the understanding of the vaccine development process is multifunctional and involves several diverse activities, the course starts with a role-play workshop, in which three students are assigned the roles of CEO, CFO, and head of R&D of an industry. The goal of the team is to develop a vaccine from discovery, through process development, pharmacology, toxicology, clinical trials, and quality management of manufacturing to regulatory approval. At each key milestone of the development process, the team must make decisions based on an analysis of the cost, time, and risks involved prior to proceeding to the next step. A video recording of such a process is depicted in a YouTube video (Chirmule, 2020a). At the end of the workshop, there is a group analysis of the lessons learned during the vaccine development process. These lessons seek answers to questions such as (i) what could have been done to prevent the mistake? (ii) how could costs be reduced? (iii) why did the study not perform as predicted?
After the completion of the workshop, there will be a detailed presentation of the various steps involved in the drug development process, each of which should be studded with case studies and examples. Participants should be taught the key soft skills, such as the ability to ask important and meaningful questions (Chirmule, 2020b), a process to make decisions (Chirmule, 2020c), and several other soft skills important for various aspects of life. Table 2 shows a list of topics that can be considered to develop a curriculum. It consists of scientific and operational aspects of the steps and understanding the application of statistical concepts. The intent of this outline of the course is to provide administrators, teachers, and students with a framework to develop a course that can teach the multifactorial processes involved in the development of vaccines and, in fact, in the development of any medicine.
In summary, our exploratory survey confirmed the observation that there is extremely limited knowledge in the understanding of vaccine development in high schools and colleges. The limited information manifests in a lack of knowledge of drug development in society at large, resulting in the spreading of misinformation. Systematic education on the process of vaccine development will enable society to understand the multifactorial aspects, high degree of complexity, and risks. Understanding these processes can bring more awareness to the general population and provide informed decision-making that can ultimately provide high-quality informed debates on the risks and benefits of vaccines and drugs currently in use in society.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AG and SP researched and wrote the section on the pathogenesis of COVID-19 and participated in the survey planning and execution activities. PK and RZ researched and wrote the section on the COVID-19 vaccines and participated in the survey, planning, and execution activities. AL, SS, and NK conceived, planned, executed, analyzed the survey, and contributed to discussions on COVID-19 pathogenesis and vaccine. NC directed the concepts and oversaw all aspects of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Vihang Ghalsasi for their help with editing the manuscript and Professor Manohar Rao for advice and help with the design of the survey.
Conflict of interest
NC and SS are employed by SymphonyTech biologics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cai, C., Peng, Y., Shen, E., Huang, Q., Chen, Y., Liu, P., et al. (2021). A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol. Ther. 29, 2794–2805. doi: 10.1016/j.ymthe.2021.08.001
Chatterjea, D. (2020). Teaching immunology as a liberal art. Front. Immunol. 11, 1462. doi: 10.3389/fimmu.2020.01462
Chirmule, N. (2020a). Gene Therapy and Drug Development. Available online at: https://www.youtube.com/watch?v=GuVa588tdaI (accessed December 12, 2022).
Chirmule, N. (2020b). A Process on How to Ask Questions. Available online at: https://chirmule.wordpress.com/2022/02/02/a-process-on-how-to-ask-questions/ (accessed December 12, 2022).
Chirmule, N. (2020c). A Process of Make Decisions. Available online at: https://chirmule.wordpress.com/2019/05/30/a-process-to-make-decisions/ (accessed December 12, 2022).
Dror, A. A., Eisenbach, N., Taiber, S., Morozov, N. G., Mizrachi, M., Zigron, A., et al. (2020). Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 35, 775–779. doi: 10.1007/s10654-020-00671-y
Felsenstein, S., Herbert, J. A., McNamara, P. S., and Hedrich, C. M. (2020). COVID-19: Immunology and treatment options. Clin. Immunol. 215, 108448. doi: 10.1016/j.clim.2020.108448
Fiolet, T., Kherabi, Y., MacDonald, C. J., Ghosn, J., and Peiffer-Smadja, N. (2022). Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin. Microbiol. Infect. 28, 202–221. doi: 10.1016/j.cmi.2021.10.005
Francis, A. I., Ghany, S., Gilkes, T., and Umakanthan, S. (2022). Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad. Med. J. 98, 389–394. doi: 10.1136/postgradmedj-2021-140654
Hirano, T., and Murakami, M. (2020). COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity 52, 731–733. doi: 10.1016/j.immuni.2020.04.003
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically. Cell 181, 271–280.e278. doi: 10.1016/j.cell.2020.02.052
Krammer, F. (2020). SARS-CoV-2 vaccines in development. Nature 586, 516–527. doi: 10.1038/s41586-020-2798-3
Lopez-Leon, S., Wegman-Ostrosky, T., Perelman, C., Sepulveda, R., Rebolledo, P. A., Cuapio, A., et al. (2021). More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv. doi: 10.21203/rs.3.rs-266574/v1
Lukassen, S. A.-O., Chua, R. L., Trefzer, T., Kahn, N. C., Schneider, M. A., Muley, T., et al. (2020). SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient. EMBO J. 39, e105114. doi: 10.15252/embj.2020105114
Stern, P. L. (2020). Key steps in vaccine development. Ann. Allergy Asthma Immunol. 125, 17–27. doi: 10.1016/j.anai.2020.01.025
Trojánek, M., Grebenyuk, V., Herrmannová, K., Nečas, T., Gregorová, J., Kucbel, M., et al. (2020). A novel coronavirus (SARS-CoV-2) and COVID-19. Cas. Lek. Cesk. 159, 55–66.
Vabret, N., Britton, G. J., Gruber, C., Hegde, S., Kim, J., Kuksin, M., et al. (2020). Immunology of COVID-19: current state of the science. Immunity 52, 910–941. doi: 10.1016/j.immuni.2020.05.002
WHO (2020). Coronavirus. Available online at: https://covid19.who.int/ (accessed December 12, 2022).
Keywords: COVID-19 vaccines, toxicology, pharmacology, clinical trials, survey, awareness
Citation: Ghosh A, Lalsare A, Chirmule N, Khare N, Kalakuntla P, Zarkar R, Pawar S and Sheth S (2023) Teaching vaccine development in schools: Learnings from a survey and curriculum design for a course. Front. Educ. 7:935683. doi: 10.3389/feduc.2022.935683
Received: 04 May 2022; Accepted: 25 November 2022;
Published: 05 January 2023.
Edited by:
Jeffrey Buckley, Athlone Institute of Technology, IrelandReviewed by:
Becky Sparks-Thissen, University of Southern Indiana, United StatesUjjwala Khare, Savitribai Phule Pune University, India
Gaganjyot Kaur, Guru Nanak Khalsa College of Art, Science and Commerce Autonomous, India
Copyright © 2023 Ghosh, Lalsare, Chirmule, Khare, Kalakuntla, Zarkar, Pawar and Sheth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Narendra Chirmule,  Y2hpcm11bGVAZ21haWwuY29t
Y2hpcm11bGVAZ21haWwuY29t
 Aishani Ghosh1
Aishani Ghosh1 Narendra Chirmule
Narendra Chirmule Smritie Sheth
Smritie Sheth