
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 13 March 2025
Sec. Paleoecology
Volume 13 - 2025 | https://doi.org/10.3389/fevo.2025.1532974
Characterizing millennial and multi-millennial variability in disturbance regimes will be crucial in improving knowledge within the context of a changing climate and the development of sustainable forest management practices in the eastern Canadian mixed boreal forest. The major biotic and abiotic disturbances in the mixed boreal forest are the spruce budworm, and fire, respectively. The ability to reconstruct the variability of these disturbance agents under different climate conditions over long time periods will help elucidate the interaction between the agents and their dynamics in the mixed boreal forest. The objective of this observational study was to reconstruct the frequency of large spruce budworm population (LSBP) and fire disturbance events, and describe their interaction in the mixed boreal forest over the course of the Holocene within the context of changing vegetation and climatic conditions. Lepidopteran scales and sedimentary charcoal were used to reconstruct the local/extra-local disturbance history from lake sediment along with pollen to reconstruct changes in tree species composition. Spruce budworm and fire disturbance events were determined using the CharAnalysis software. Regime shifts in disturbance event frequencies along with changes in tree composition were detected using Sequential T-test Analysis of Regime Shifts. Spearman’s correlation was used to determine the relationship between spruce budworm and fire event frequencies. Over the course of the Holocene, 57 LSBP events and 76 fire events were detected with event frequencies ranging between 0.75-6.30 events*kyr-1 and 1.71-10.5 events*kyr-1 respectively. Nine and 7 regime shifts in LSBP and fire event frequencies were detected respectively, along with 2 shifts in vegetation. A significant negative correlation was observed between LSBP and fire event frequencies from 6000-1000 BP suggestive of a linked disturbance interaction. The first local lake sediment multi-millennial disturbance regime reconstruction comprising both spruce budworm and fire in the mixed forest revealed a very peculiar oscillation in disturbance event frequencies. Each disturbance seemingly establishes a positive disturbance-vegetation feedback that favors itself and inhibits the occurrence of the other. Further, rapid climate change events may act as a key trigger in establishing the respective feedback loops resulting in the observed disturbance event frequency oscillation.
Within the context of increasing variability in temperature and precipitation (Easterling et al., 2000), the effects of forest disturbances are expected to be exacerbated (Dymond et al., 2010; Millar and Stephenson, 2015; McDowell et al., 2020). Greater tree mortality is likely to result from forest disturbances acting synergistically with other drivers (Allen et al., 2010, 2015; Hart et al., 2014, 2017; De Grandpré et al., 2019). Warming temperatures may create conditions favorable to more frequent fire by increasing ignition rates through greater fuel availability which is expected to result in more intense and/or severe fires (see Westerling et al., 2003, 2006, 2011; Flannigan et al., 2009, 2013). Similarly, warming temperatures have the potential of favoring insect development and overwintering survival (Ayres and Lombardo, 2000; Berg et al., 2006; Bentz et al., 2010), resulting in larger populations and more severe insect outbreaks (Murdock et al., 2013; Weed et al., 2013). However, during diapause, prolonged periods of warm temperatures may negatively affect survival (Régnière et al., 2012). Moreover, in response to such changes in temperatures, distribution of insect outbreaks may shift into historically novel habitats in response to a changing climate (Jepsen et al., 2008, 2011; Régnière et al., 2012; Erbilgin et al., 2014), and/or result in feeding on host species that were formerly protected due to phenological asynchronies (Pureswaran et al., 2015, 2019; Fuentealba et al., 2017). Given the uncertainty surrounding disturbance regime behavior under current climatic variability, potential analogs may be found by looking to past climate shifts and their effects on disturbance regimes.
The Holocene is a geological epoch that spans from roughly 11,700 years ago to the present (just after the preindustrial era) that experienced 3 major climate periods (Walker et al., 2012; Wanner et al., 2015; Shuman and Marsicek, 2016). The Early Holocene (EH 11,700 BP-7000 BP; before present; present refers to the year 1950) was a dry period (Lavoie and Richard, 2000; Muller et al., 2003; Shuman and Marsicek, 2016) with rapidly increasing temperature (Wanner et al., 2015; Zhang et al., 2016, 2017; Neil and Gajewski, 2018). The collapse of the Laurentian Ice Sheet (Renssen et al., 2009; Marcott et al., 2013), brought about warm stable temperatures (Viau and Gajewski, 2009; Shuman and Marsicek, 2016; Neil and Gajewski, 2018) during the Holocene Thermal Maximum (HTM; 7000 BP-4200 BP) favoring prompt postglacial vegetation recolonization (Blarquez and Aleman, 2016) despite moisture variability (Lavoie and Richard, 2000; Muller et al., 2003; Viau and Gajewski, 2009). The Neoglacial (4200 BP-present) was generally humid (Lavoie and Richard, 2000; Muller et al., 2003; Shuman and Marsicek, 2016) and underwent cooling (Wanner et al., 2008, 2011; Marsicek et al., 2018) but encompassed a brief dry period of warming (Medieval Climate Anomaly 1000-700 BP; MCA) and cooling (Little Ice Age 550-250 BP; LIA; Mann et al., 2009; Viau et al., 2012; Lafontaine-Boyer and Gajewski, 2014) ending with a rapid rate of warming (Renssen et al., 2012). Punctual rapid significant climate change events, associated with ice raft debris events (Bond et al., 1997, 2001; outbursts of freshwater), occurred within these periods (Mayewski et al., 2004; Wanner et al., 2011), likely affected oceanic (Broecker, 1997, 2003; Törnqvist and Hijma, 2012) and atmospheric circulatory patterns (Smith et al., 2016; Deininger et al., 2017) influencing climate, vegetation, and fire in Europe (Pál et al., 2018; Florescu et al., 2019), and eastern North America (Viau et al., 2002; Viau et al., 2006). The Holocene, given its past climate variability, therefore, is an appropriate period to study potential changes in disturbance regime behavior.
Currently, the mixed boreal forest of Québec is dominated by 2 major forest disturbances: the spruce budworm and fire. The spruce budworm [Chorisoneura fumiferana Clemens] is a native lepidopteran defoliator and is the major biotic disturbance in the mixed boreal forest (MacLean, 2016; Nealis, 2016; Pureswaran et al., 2016). As a larva, the spruce budworm preferentially feeds on current year’s needles of mature balsam fir [Abies balsamea (L.) Mill], its primary host, also feeding on older needles when necessary (Piene, 1989; Hennigar et al., 2008) along with the needles of secondary hosts (Picea spp.; Hennigar et al., 2008). Severe defoliation can result in tree mortality especially in balsam fir (MacLean, 1980, 1984; MacLean and Ostaff, 1989), resulting in the formation of canopy gaps favoring the regeneration of balsam fir (Kneeshaw and Bergeron, 1996, 1998, 1999), along with its establishment in the canopy from pre-established seedlings and/or saplings (Bouchard et al., 2005, 2006, 2007). Incidentally, a greater proportion of balsam fir in a stand will also engender a greater probability of spruce budworm outbreaks, thereby establishing a positive disturbance-vegetation feedback loop (Baskerville, 1975; Morin, 1994) leading to episodic outbreaks (Cooke et al., 2007; Nealis, 2016). This feedback loop has likely existed since the postglacial recolonization of the landscape by balsam fir (Simard I. et al., 2002, 2006; Simard S. et al., 2011; Navarro et al., 2018b).
Fire is the major abiotic disturbance in the mixed boreal forest. Climate and fuels play a substantial role in modulating fire disturbance regimes (Macias Fauria et al., 2010; Ali et al., 2012; Blarquez et al., 2015). Climate influences fuel combustibility through temperature and humidity affecting ignition, fire spread, and intensity (Wotton et al., 2010; Woolford et al., 2014; Molinari et al., 2018). Long-term climate will determine vegetation biomass thereby influencing fuel availability and accumulation (Littel et al., 2016; He and Lamont, 2018; McLauchlan et al., 2020). Further, climate will influence the species composition (i.e., proportion of coniferous and deciduous trees) of an area which can in turn affect subsequent burning (Hély et al., 2000, 2020; Girardin et al., 2013; Blarquez et al., 2015). Burn frequency and severity can also dictate which plant species will be present due to differential species regeneration strategies and requirements (Burns and Honkala, 1990; Keeley et al., 2011; Pausas, 2015). Therefore, there is the potential for the establishment of a positive feedback loop; fire-tolerant species may facilitate fuel structures that favor fire in the stand (e.g., lodgepole pine or black spruce; Rogers et al., 2015; Lamont et al., 2020), and the act of burning at particular frequencies then favors the establishment and propagation of the fire-tolerant species (Dantas et al., 2016; Harrison et al., 2021). In the mixed boreal forest, stand composition will generally be dominated by deciduous species following fire (Bergeron, 2000; Couillard et al., 2021), however this is dependent on fire event frequency or time since last fire, along with the surrounding composition.
In addition to potentially forming their own disturbance-vegetation feedback loops, these two major forest disturbances are able to interact with one another through forest legacies such as changes in forest composition and structure (Buma and Wessman, 2011, 2012, 2013; Buma, 2015). Generally, disturbance interactions can be categorized as being linked or compound (Simard M. et al., 2011; Kleinman et al., 2019; Burton et al., 2020). A linked disturbance interaction implies that the preceding disturbance alters stand structure and/or composition in such a way that the occurrence, extent, frequency and/or severity of the subsequent disturbance is affected (Simard M. et al., 2011). For example, the fuel structure and ensuing fire severity of a bark beetle infested stand is modulated by the time since the outbreak (Page and Jenkins, 2007a, b; Simard M. et al., 2011; Hicke et al., 2012). Similarly, insect defoliation occurring in dry coniferous forests limits available fuel and will reduce fire severity (Lynch and Moorcroft, 2008; Cohn et al., 2014). Alternatively, a compound disturbance interaction generally involves two disturbances occurring simultaneously or in quick succession having a greater effect together than each disturbance acting on its own (Paine et al., 1998; Simard M. et al., 2011). A clear example is the tree mortality resulting from a drought shortly followed by an insect outbreak (Hart et al., 2014, 2017; De Grandpré et al., 2019), or the severity of a fire preceded by a drought (Flannigan et al., 2013; Jolly et al., 2015; Millar and Stephenson, 2015). In the mixed boreal forest, the spruce budworm and fire appear to exhibit a linked disturbance interaction. Over short time-scales, defoliation alters fuel structure in a manner increasing fire hazard (Stocks, 1987; Watt et al., 2018, 2020), fire occurrence (Fleming et al., 2002; Candau et al., 2018), and fire risk (James et al., 2017), meanwhile over decades to centuries, the relationship appears to be antagonistic by decreasing the availability of live ladders fuels (Sturtevant et al., 2012). Similarly, over millennia, the interaction also appears to be negative, where one disturbance would inhibit the other (Navarro et al., 2018b), although the mechanisms behind the interaction has not yet been investigated.
Understanding past variability in disturbance regimes and their interactions given different climate phases and events during the Holocene will be key in elucidating the past disturbance dynamics of the mixed boreal forest ecosystem. For example, fire or spruce budworm events may predominately affect the mixed boreal forest under certain climate and/or vegetation conditions revealing information about possible system thresholds (Scheffer et al., 2001, 2012). Identifying such thresholds help characterize the forest’s ecosystem state landscape (Scheffer and Carpenter, 2003) and potentially reveal factors that may move the ecosystem within this landscape and/or shape this state landscape (Scheffer et al., 2003; van Nes et al., 2007; Scheffer and van Nes, 2007). Furthermore, rapid significant climate change events (Bond et al., 1997, 2001; Mayewski et al., 2004) may modulate disturbance regimes as observed in changes in sedimentary charcoal accumulations and fire frequency in Europe (Florescu et al., 2019), or alter vegetation (Pál et al., 2018) with the potential of changing the interaction between disturbances. Therefore, the long-term reconstruction of past disturbance regime variability may provide insights and reveal conditions that could serve as potential analogs helping guide current and future forest management decisions and practices (Swetnam et al., 1999; Landres et al., 1999; Hennebelle et al., 2018).
The purpose of this observational study is to reconstruct the variability in fire and large spruce budworm population (LSBP) disturbance event frequencies in the mixed boreal forest over the course of the Holocene, and to characterize the long-term interaction between the two agents within the context of potentially changing vegetation and incursions of rapid significant climate change events. In the mixed boreal forest, following postglacial recolonization and the arrival of balsam fir, it is expected that the spruce budworm will be the dominant disturbance due to the near constant availability of host-trees, and the subsequent implementation of a positive feedback between the insect and its host; presence of host-trees favor spruce budworm outbreaks, and spruce budworm outbreaks create favorable conditions for host-tree regeneration and establishment in the canopy. However, prior to the arrival of balsam fir, fire is expected to be the dominant disturbance in the mixed boreal forest; tree species composition prior to the establishment of balsam fir is expected to be more fire-tolerant (Blarquez et al., 2015; Blarquez and Aleman, 2016), and therefore promote more fire-prone conditions (Hély et al., 2000, 2010, 2020; Girardin et al., 2013). Further, cooler and drier conditions are expected to favor to the implementation of the fire disturbance-vegetation feedback loop as such conditions have led to greater fire frequency during the Holocene (Carcaillet et al., 2001a) while likely negatively affecting insect development and survival (Ayres and Lombardo, 2000; Bentz et al., 2010) reducing LSBP events. Finally, an inverse relationship, or negative correlation between the two disturbance agents at millennial and multi-millennial time-scales is expected (e.g., Navarro et al., 2018b) due to ‘competition’ for a limited and changing resource i.e., tree species biomass will vary through time.
Lake Buire (48.16540°N, 70.57077°W) is a small lake 1.3 ha in size, ca. 3.4m deep with limited inflow and outflow (Figure 1). It is found in the Abies balsamea-Betula papyrifera bioclimatic zone (Rowe, 1972; Saucier et al., 1998, 2009) at 244 masl, surrounded by rolling terrain, and is in an area that has sustained heavy spruce budworm defoliation (≥75%) from 1974–1984 and from 2016 to the time of sediment sampling (fall 2018; MFFP (Ministère des forêts, de la faune et des parcs), 2021a). The stand composition around the lake at the time of sampling, in decreasing order of relative abundance, consisted of: trembling aspen [Populus tremuloides Michx.], paper birch [Betula papyrifera Marshall], balsam fir [Abies balsamea (L.) Mill], black spruce [Picea mariana (Mill.) Britton, Sterns & Poggenburg], white spruce [Picea glauca (Moench) Voss], and yellow birch [Betula alleghaniensis Britt.] (MFFP (Ministère des forêts, de la faune et des parcs), 2021b). The sediment column of lake Buire was sampled using a gravity corer (Renberg, 1991; Renberg and Hansson, 2008), and a modified Livingstone corer (Wright et al., 1984) to obtain, respectively, the lake-sediment interface, and the remainder of the column as overlapping 1 m segments. The latter were wrapped in polyethylene plastic and placed in ABS plumbing tubes for transport and storage. Sediment from both core types were sampled at a 1 cm resolution. This was done in the field for the gravity corer segments while the Livingstone segments were divided in the laboratory. All samples were stored at 4°C until they were ready for processing.
The chronological framework of the sediment core was determined using 210Pb and radiocarbon (14C) dates to most accurately reconstruct the recent (last 150 years or so) and deep site history (thousands of years), respectively. 210Pb activity measurements at 6 depths (0-1, 2-3, 5-6, 9-10, 14-15, and 24-25 cm) in the top 25 cm of the gravity core was obtained by Flett Research Ltd (Winnepeg, MN, Canada) from which an age-depth model was derived using the Constant Rate Supply model (Appleby and Oldfield, 1983; Binford, 1990). Macrofossils (leaves, needles, and seeds of terrestrial vegetation) were extracted at 50 cm intervals along the entire sediment profile and sent to the Radiocarbon Laboratory of the André E. Lalonde AMS Laboratory at the University of Ottawa (Ottawa, ON, Canada) to obtain 14C dates. Radiocarbon dates and 210Pb dates were combined in the rbacon package (Blaauw and Christen, 2011; Blaauw et al., 2021) in the R environment (R Core Team, 2021) to derive an age-depth model for the core.
In addition to establishing a chronological framework, core composition along with the successional context of the forest surrounding the lake was determined. Magnetic susceptibility of the sediment along the entire core profile was conducted using the Bartington MS2 System (Dearing, 1999). Magnetic susceptibility helps distinguish organic matter from inorganic matter, where the presence of the latter is suggestive of run-off, erosion, flooding or sediment mixing events (Thompson et al., 1975; Dearing and Flower, 1982; Da Silva et al., 2015). Values will typically fluctuate between 1 and -1 (SI units) where higher positive values indicate a higher proportion of inorganic material present in the sediment while slightly negative values or those occurring around 0 suggests that the core is composed of organic matter (Dearing, 1999). In this case, magnetic susceptibility was used to assess the integrity of the sediment core to identify the point beyond which the sediment core could not be confidently interpreted.
The successional context of the forest was determined by extracting and identifying pollen found in 1 cm3 from the 1 cm core slices corresponding to an approximately 100-year sampling interval for the following species: black spruce, paper birch, balsam fir, eastern white pine [Pinus strobus L.]. These arboreal species were selected as they were most susceptible to show any change in disturbance. Greater abundance of black spruce and paper birch would suggest greater fire influence, whereas greater abundance of balsam fir, and eastern white pine would suggest less fire. Pollen extraction and identification was done using standard procedures (Faegri and Iversen, 1989) at l’Université de Montréal Palynology service laboratory. Pollen count of each species was converted to a percent of the total species sum and visualized using the rioja R package (Juggens, 2020), from which the ratio between the percent of A. balsamea pollen and P. mariana pollen was derived (Supplementary Figure S1) and calculated at each corresponding 100-year interval. An increase in the ratio suggests a larger proportion of balsam fir present relative to black spruce meanwhile a decrease in the ratio suggests either an increase in black spruce or a decrease in balsam fir. Pollen extraction and identification were done in an effort to better interpret the potential changes in disturbance regimes and their interactions through time.
Lepidopteran scale count and charcoal surface area were used as proxies for the occurrence of LSBP events and the occurrence of fire (Supplementary Figure S2), respectively, where two 1 cm3 punches (‘subsamples’ from herein) were extracted from each 1-cm slice along the core profile for lepidopteran scale, and charcoal analysis. Lepidopteran scale sample preparation followed a modified protocol from Navarro et al. (2018a) as described in Leclerc et al. (2024). Briefly, subsamples were deflocculated, wet sieved, and the retained sediment was centrifuged in a sucrose solution. Finally, the pellet was ready for scale identification and count under a microscope once the supernatant was removed. Charcoal subsamples were placed in bleach (10% NaOCl) for a period of at least 24 hours to deflocculate the sediment and to facilitate charcoal particle identification relative to organic matter (Blarquez et al., 2010). The subsamples were sieved using a 150 µm mesh, attempting to retain charcoal remains from local fires (Clark and Royall, 1995; Clark et al., 1996, 1998; Carcaillet et al., 2001b; Higuera et al., 2007). The retained charcoal remains were identified under a dissecting microscope coupled to a camera. The charcoal surface area (mm2) in each subsample was quantified in the WinSEEDLE software (Regent Instruments Inc, 2019). Charcoal surface area, as opposed to charcoal count, was used to reconstruct fire occurrence in an effort to limit the potential of fragmentation that could lead to an erroneous count (Ali et al., 2009).
The CharAnalysis software and procedure (Higuera, 2009; Higuera et al., 2010) was used to reconstruct periods of LSBP and fire event history over the course of the Holocene. The raw accumulation rates were interpolated to a constant time-step using the median sampling resolution (Cint). From the interpolated accumulation rates (Cint) the background accumulation rates (Cback) were determined using a LOWESS robust to outliers with a 500-year smoothing window to differentiate between the low and high frequency signals. The high frequency signal (Cpeak) was isolated by subtracting the background accumulation rates (Cback) from the interpolated accumulation rates (Cint). Noise (Cnoise) found within the high frequency signal was estimated using a Gaussian mixture model (Gavin et al., 2006; Higuera et al., 2010), and in an effort to remove this leftover noise that could result from sediment mixing (Cnoise), a local threshold, within a 500-year window and using the 99th percentile, was applied to identify lepidopteran scale and charcoal peak events (Cfire). The peak events (Cfire) were subjected to a ‘minimum count criterion’ (Higuera et al., 2010), which determined whether two peaks were in fact two individual events, or if the two peaks originated from the same event. Finally, spruce budworm and fire peak event frequencies (number of events/1000 years) were calculated and then smoothed using a LOWESS with a 500-year window.
The 500-year smoothing window used to determine background accumulation rates, local thresholds, and smoothing of peak frequency was applied to both disturbances for comparability between disturbances and among studies. Background accumulation rates have typically been estimated using roughly 3 times the disturbance’s return interval (Carcaillet et al., 2009; Blarquez et al., 2010). The spruce budworm outbreak return interval in recent history has been 30-40 years in the mixed boreal forest (Blais, 1983, 1985; Morin and Laprise, 1990; Boulanger and Arseneault, 2004) which would result in an approximately 100-year smoothing window, while the fire return interval in Abies balsamea-Betula papyrifera type ecosystems appears to be around 300 years based on the estimates of Frégeau et al. (2015), and Couillard et al. (2013, 2021), which would yield a smoothing window of about 900 years. However, to apply a robust local threshold to estimate spruce budworm events, a window of around 400 years would have been required to include at least 30 samples (Higuera et al., 2010). Finally, preliminary analyses revealed that the 500-year window-width yielded the highest Signal-to-Noise Ratio and Goodness of Fit values where shorter or longer widths yielded less or more conservative event estimates respectively. Therefore, the 500-year smoothing window-width used in this study is a trade-off between biological and statistical considerations, and allows for a comparison with the results obtained by Navarro et al. (2018b).
Sequential T-test Analysis of Regime Shifts (STARS; Rodionov, 2004; Rodionov and Overland, 2005; Rodionov, 2006) was used to detect any changes in the observed disturbance event frequencies and vegetation composition over the course of the Holocene in the R environment (R Core Team, 2021). Prior to this analysis, peak spruce budworm and fire event frequencies were estimated using a Gaussian kernel density function with 200-year window width that was bootstrapped with 1000 replicates while applying a correction for edge bias (Mann, 2004; also see Mann, 2008) with the kdffreq function in the paleofire package (Blarquez et al., 2014), based on the median sampling resolution and events identified in CharAnalysis. The 200-year window was selected as preliminary analysis revealed that it was the best compromise between retention of variance and number of samples used to calculate the frequencies within the window (Supplementary Figures S3, S4). A 200-year cut-off was applied at the beginning and the end of the chronology in order to remove any edge effects that could affect subsequent analysis. The interaction between spruce budworm and fire event frequencies at the core of the chronology (6000-1000 BP) was quantified using Spearman’s correlation with a significance level of 0.05.
The STARS method was used to identify change points in the respective disturbance event frequency and pollen accumulation time series over the course of the Holocene by comparing each observation to the previous observations and determining whether a regime shift has occurred (Rodionov, 2004; Stirnimann et al., 2019). This analysis was done using a running window of specified width within which a Student’s t-test was performed determining whether the new observation was part of a new regime or not (Rodionov, 2004; Stirnimann et al., 2019). A potential change point was identified when the mean value of the new regime exceeded the range established by the old regime (Rodionov, 2004; Rodionov and Overland, 2005). If the cumulative sum of the normalized deviations, the Regime Shift Index (RSI), at each potential change point remained positive then a regime shift was detected, and the opposite was true if the RSI became negative (Rodionov, 2004; Rodionov and Overland, 2005).
The rstars function (Stirnimann et al., 2019) was applied to each disturbance regime peak frequency along with the ratio between balsam fir and black spruce (Abies: Picea ratio) with a window-width representing 1001 and 1000 years respectively, and a Huber’s weight parameter of 1. The window-width was selected to be large enough to encompass successional turnover based on the lifespan of the trees found in the mixed boreal forest, typically living no longer than approximately 300 years in the case of balsam fir and black spruce (Burns and Honkala, 1990; Bergeron, 2000), while also remaining short enough to fit within the main known climate periods of the Holocene i.e., the EH, HTM, and Neoglacial which encompassed the MCA, and the LIA and pre-industrial era (Walker et al., 2012). The Huber’s weight parameter weighed observations that fell beyond 1 standard deviation based on their distance from the new regime’s mean, with a further distance resulting in a lower weight (Stirnimann et al., 2019). Neither of the disturbance event peak frequencies nor the Abies: Picea ratio time series were prewhitened. The disturbance series were obtained from the rigorous procedure applied in CharAnalysis, while for the pollen series, using the Inverse Proportionality with 4 corrections (IP4 method) yielded exactly the same result as an analysis without prewhitening (Supplementary Table S1; Supplementary Figure S5). The significance level (α) used to test the RSI was 0.05, Huber’s weight parameter was set to 1.
The Buire sediment core was 741 cm in length dating to just over 8600 cal yr BP (Figure 2). Analysis was restrained to the top 731 cm (339-1069cm) due to the inversion at the bottom of the core (Table 1; Figure 2). Sediment accumulation rate was relatively constant at approximately 10 yr*cm-1, and consisted of homogeneous gyttja (organic sediment); magnetic susceptibility values oscillated around 0 except at around 1005 cm (approximately 8000 BP) with the presence of a gradual gyttja-clay transition beyond which values were greater than 1 revealing an increasing inorganic component. The gradual transition to more clay at the bottom of the core is suggestive of an inorganic input event, likely resulting in the observed inversion in the age-depth model, where the final 14C date was younger than the previous one (Figure 2).
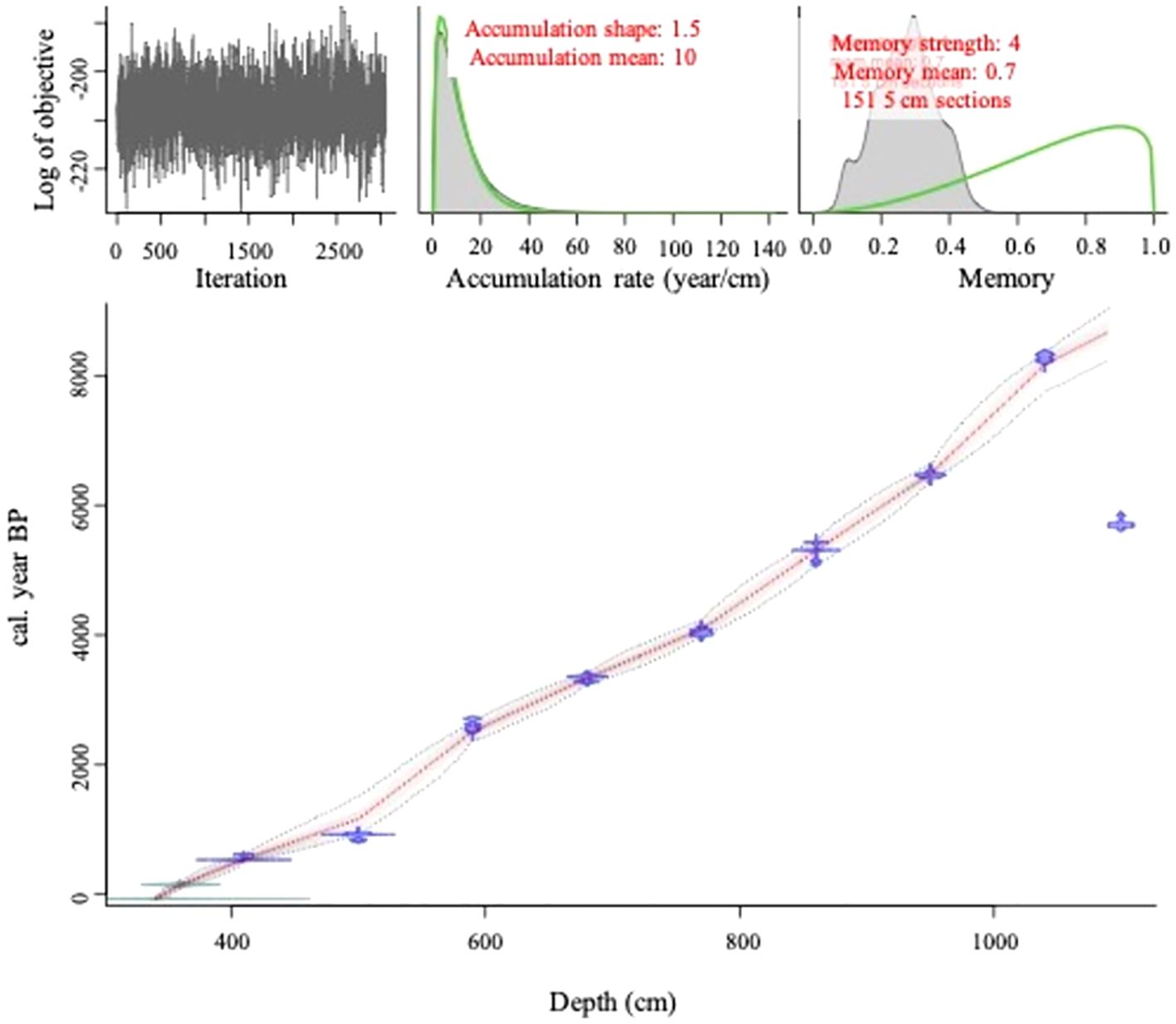
Figure 2. The optimal age-depth model (red line) with 95% confidence interval (grey shading) and associated characteristics for lake Buire. The estimated age-depth model (main panel) with sampling locations (210Pb and radiocarbon dates in green and blue respectively) with their associated estimated errors. Markov Chain Monte Carlo (MCMC) simulations (upper left panel). Modelled accumulation of the core (top center panel) relative to a gamma distribution (green line). Variability in sediment accumulation over time (top right panel; Memory) compared to a beta distribution (green line).
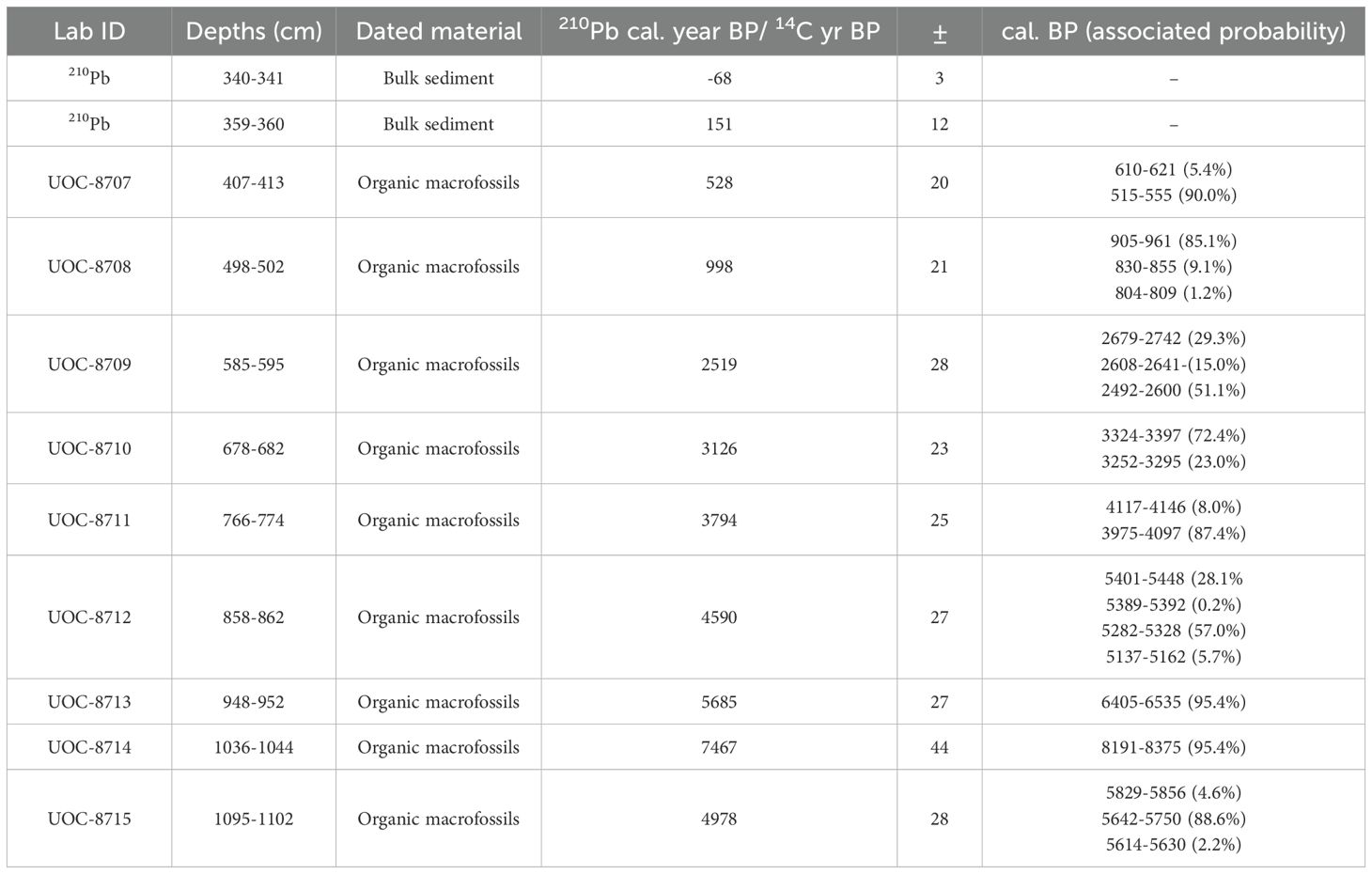
Table 1. The sampling interval and associated dates (cal. year BP ± standard deviation) used to construct the age-depth model for lake Buire.
The CharAnalysis procedure detected 57 lepidopteran scale events over the course of the study period (Figure 3), however one outlying observation in this time series was removed prior to the analysis as it was an abnormally high accumulation (Supplementary Table S2). The frequency of lepidopteran scale events varied between 0.75 events*kyr-1 and 6.30 events*kyr-1 occurring at 7225 BP and 4706 BP, respectively. A total of 76 fire events were detected using the CharAnalysis procedure (Figure 3). The frequency of charcoal peaks varied between 10.5 events*kyr-1 and 1.71 events*kyr-1 occurring at 8655 BP and 7841 BP, respectively. Prior to approximately 6000 BP fire event frequency was generally greater than lepidopteran scale event frequency, however, after this date an oscillation between the disturbance event frequencies is observed (Figure 3).
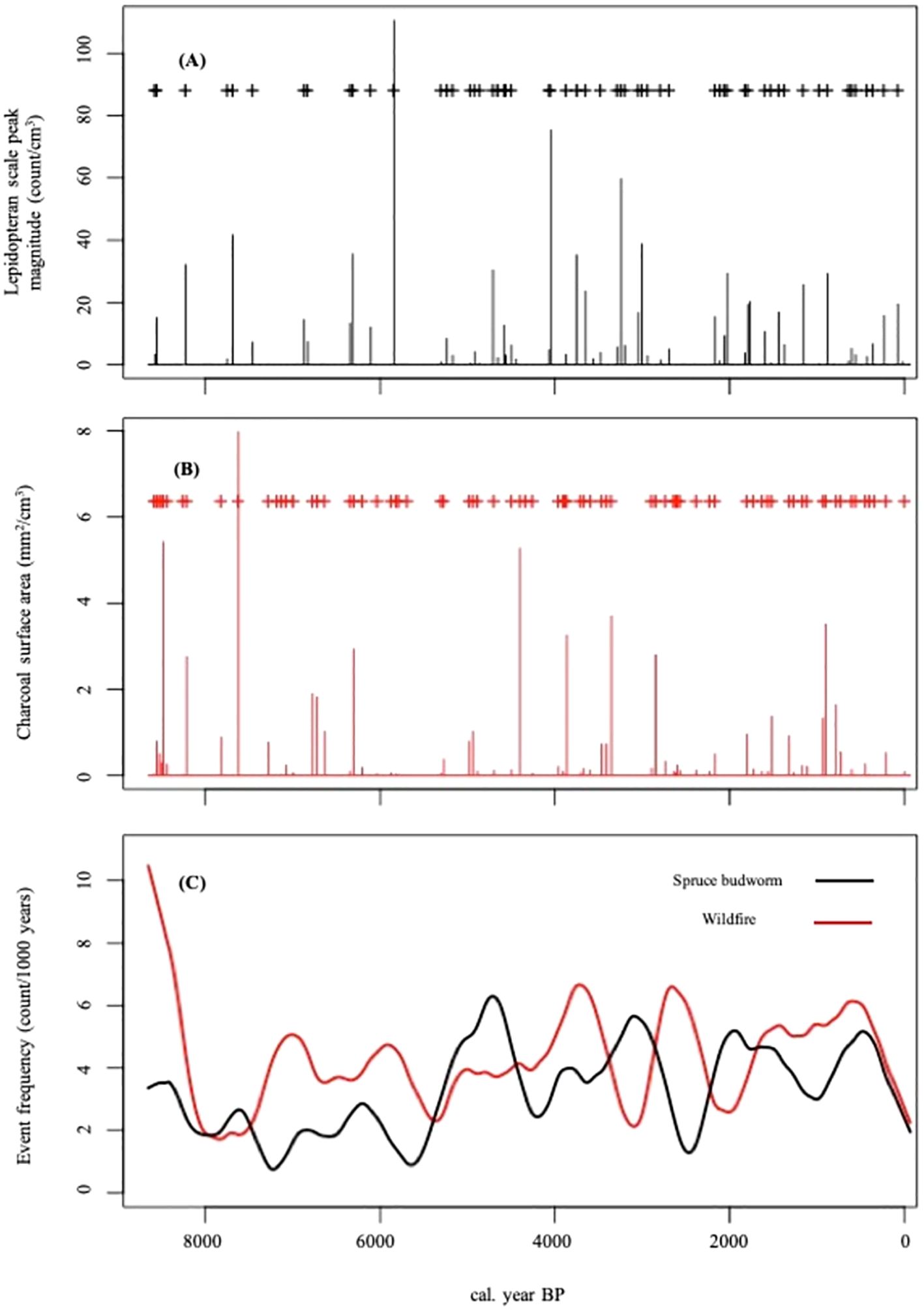
Figure 3. Disturbance event magnitude and frequency as obtained from CharAnalysis using a 500-year smoothing window. Each identified peak (+; diamonds above the respective accumulations) exceeded the low frequency signal (Cback) and the 99th percentile local threshold applied to the high frequency signal (Cpeak). (A) Spruce budworm event peak magnitude; (B) Fire event peak magnitude; (C) disturbance event frequencies.
Multiple regime shifts (i.e., changes in mean) were detected in spruce budworm and fire event frequencies along with the Abies: Picea ratio over the course of the Holocene. Nine shifts in mean spruce budworm event frequency were detected, while 7 shifts in mean fire event frequency were detected (Figure 4). Finally, two regime shifts were detected in the Abies: Picea ratio (Figure 4). One occurred at approximately 6000 BP where there was an increase in the mean ratio, and another shift occurred at around 3500 BP with a decrease in the mean ratio. Further, the regime shift in the Abies: Picea ratio at 6000 BP roughly coincides with a particularly large shift in spruce budworm event frequency (Figure 4). A significant negative correlation (r (452): -0.33, p-value<0.001) was identified between spruce budworm and fire event frequencies from 6000-1000 BP (Figure 5).
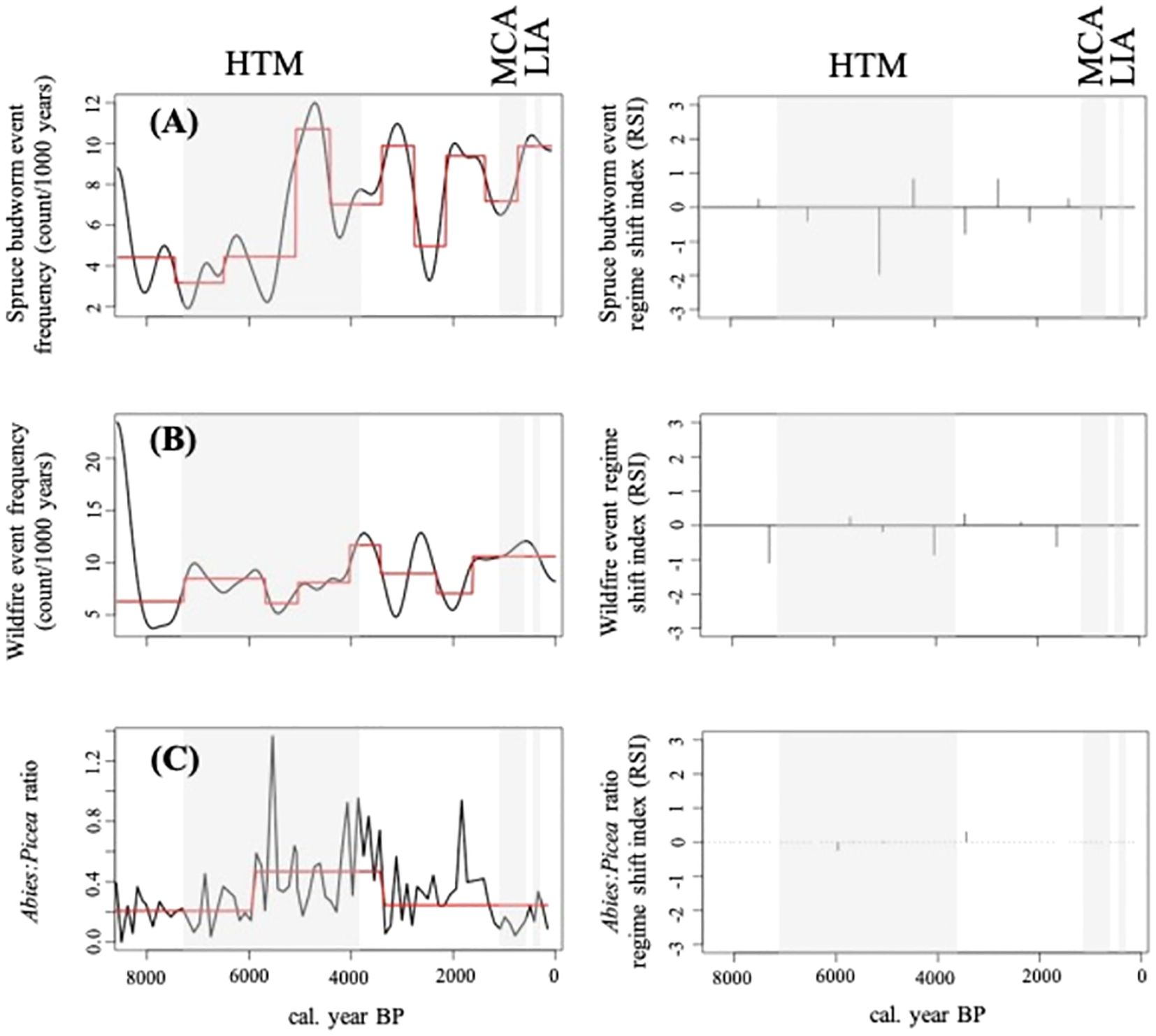
Figure 4. The Sequential T-test Analysis Regime Shift (STARS) output for lake Buire’s spruce budworm and fire event frequencies, and Abies : Picea ratio over the course of the Holocene. The reconstructed disturbance event frequency (black line) and mean (red line) with corresponding Regime Shift Index (bars) for (A) spruce budworm event frequency, (B) fire event frequency, and (C) the Abies : Picea ratio. Known climatic phases identified: the Holocene Thermal Maximum (HTM), Medieval Climate Anomaly (MCA), and the Little Ice Age (LIA).
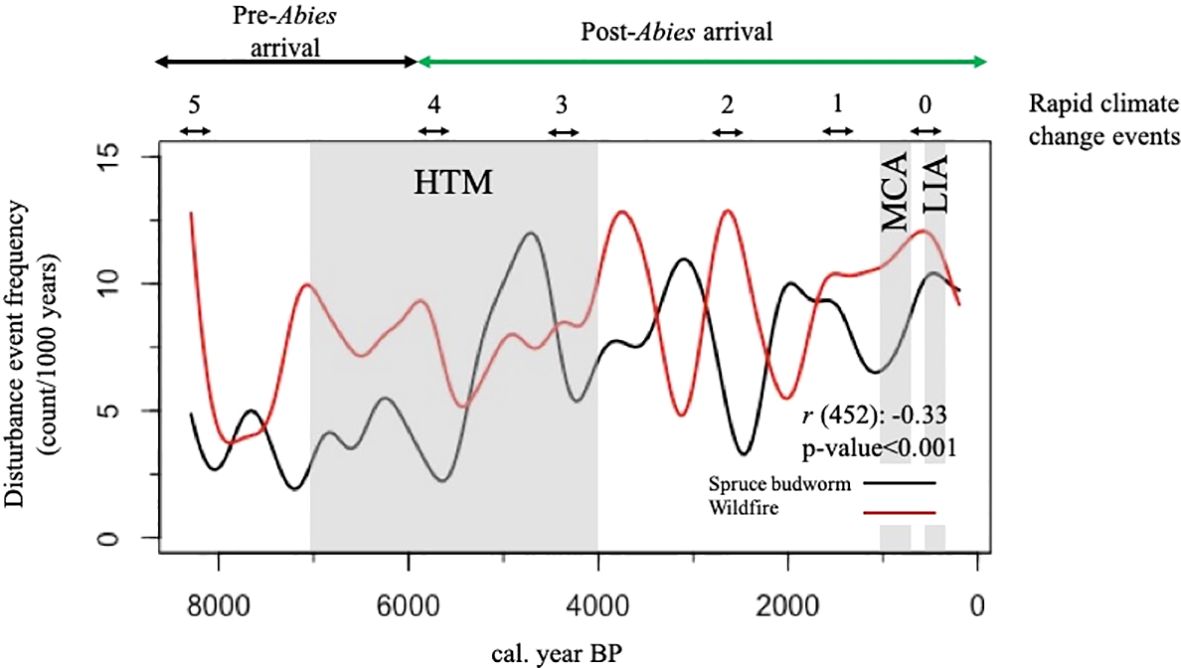
Figure 5. Variability in disturbance event frequencies over the course of the Holocene. Known climatic phases identified: the Holocene Thermal Maximum (HTM), Medieval Climate Anomaly (MCA), and the Little Ice Age (LIA). The approximate timing of the postglacial recolonization arrival of Abies balsamea is identified along with approximate periods of inferred rapid significant climate change events.
To the authors’ knowledge, this is the first local multi-millennial spruce budworm and fire event reconstruction observing their long-term interaction in the mixed boreal forest of central Québec, Canada spanning the different climate phases of the Holocene using lepidopteran scales and sedimentary charcoal. The mixed boreal forest around lake Buire appears to exhibit two distinct regimes: a fire or spruce budworm dominated regime. These can be visually represented by an ecosystem state landscape (see Scheffer and Carpenter, 2003) where the ecosystem, lake Buire depicted as a ball, sits in one of two valleys or basins of attraction corresponding to an ecosystem dominated by a fire disturbance regime or as an ecosystem dominated by a spruce budworm disturbance regime (Figure 6). The hypothesis that the mixed boreal forest shifted from being fire dominated to dominated by the spruce budworm following the increase in abundance of balsam fir on the landscape around 6000 BP was not supported (Figure 6). Instead, following the increased balsam fir abundance, the ecosystem around lake Buire oscillated between the two aforementioned basins, a phenomenon that has not been previously observed in other ecosystems such as the boreal black spruce forest (Navarro et al., 2018b). The oscillatory behavior is best illustrated by the change in disturbance frequencies throughout the Holocene and the many detected regime shifts (Figures 4-6).
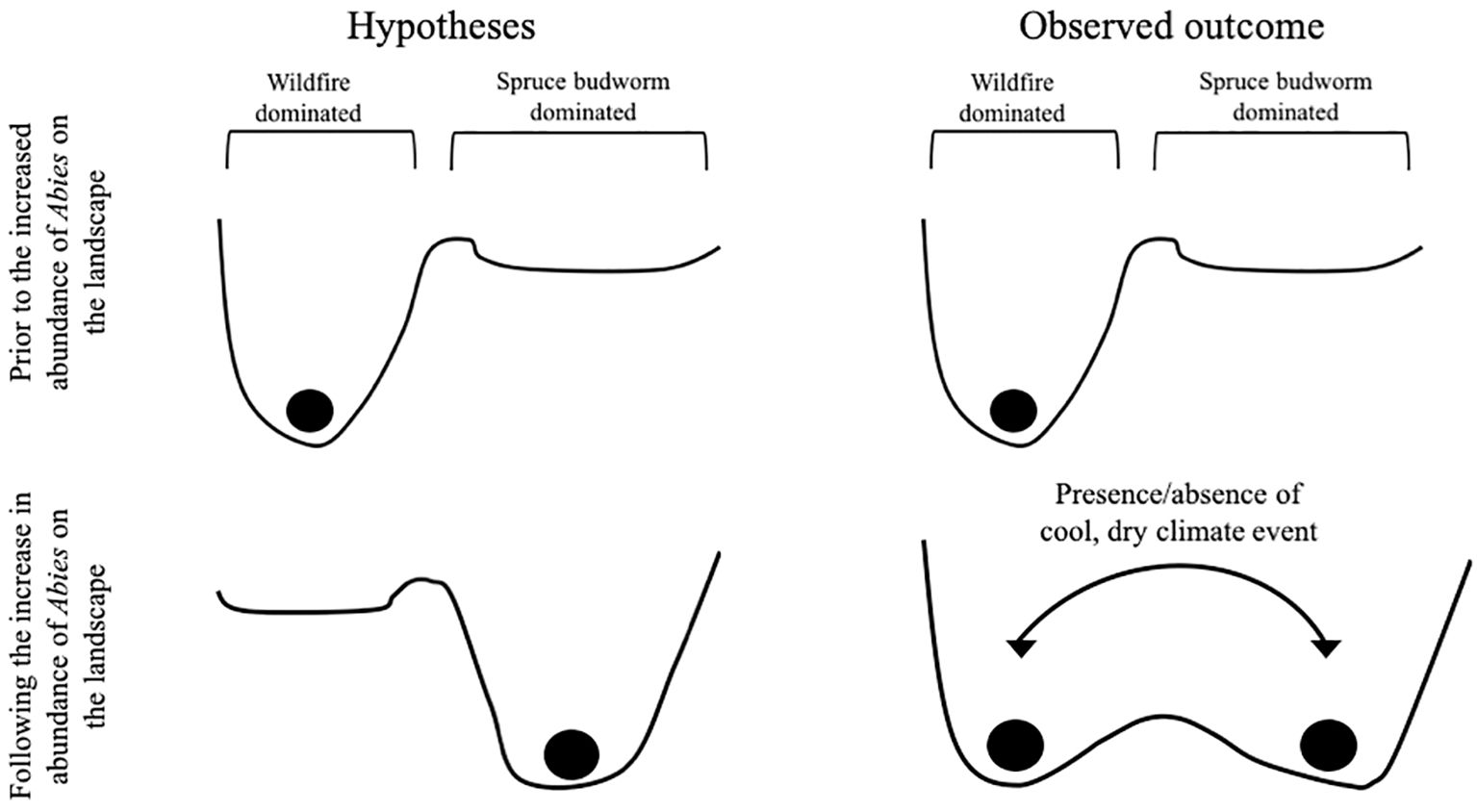
Figure 6. Summary of the mixed boreal forest ecosystem’s position (ball) and ecosystem state landscape (cup) prior and after the postglacial recolonization by balsam fir around lake Buire and the effect of cool, dry conditions on the ecosystem in eastern North America.
The postglacial recolonization by balsam fir appears to be the primary underlying event that allowed for the oscillation between the abiotic and biotic disturbance frequencies by creating basins of attraction of similar size and depth (Figure 6). Pre-8000 BP, fire tends to dominate which also coincides with a low mean Abies: Picea ratio suggesting a greater abundance of black spruce around the lake relative to fir resulting in an ecosystem state landscape favorable to fire. The mean ratio then increases as the warm conditions during the Holocene Thermal Maximum (HTM) allows for postglacial recolonization and increased abundance of balsam fir around the lake at roughly 6000 BP (Blarquez and Aleman, 2016) setting the stage for more frequent LSBP events (Figure 4) due to basins probably becoming of equal depth and size (Figure 6). Following the arrival of balsam fir, there is a decrease in the mean Abies: Picea ratio around 3500 BP due to an increased proportion of black spruce around the lake. This drop in the ratio also coincides with the establishment of an oscillation between the spruce budworm and fire event frequencies as quantified by multiple regime shifts (Figures 4, 5), suggesting movement of the ecosystem between the disturbance basins contrary to our initial hypothesis (Figure 6). Therefore, the changes in relative arboreal species abundance, as measured by the Abies: Picea ratio, likely altered the basin shapes of the ecosystem state landscape facilitating the movement of the ecosystem from one basin to the other given an appropriate trigger.
The oscillating disturbance frequencies revealed an inverse relationship or negative interaction between the two disturbance agents in the mixed boreal forest from 6000-1000 BP at lake Buire and could be interpreted as competition for a limited resource. A negative correlation between disturbance frequencies was observed, confirming the relationship described by Navarro et al. (2018b) in the boreal black spruce forest, and is suggestive of a linked disturbance interaction (Simard M. et al., 2011; Kleinman et al., 2019), at local or extra-local (roughly 1km-10km area around a lake), and at multi-millennial scales. This interaction could be viewed as a trophic interaction where disturbances are ‘organisms’ competing for a food resource (vegetation) while also creating conditions that favor their own survival (Pausas and Bond, 2020a, b, Pausas and Bond, 2022). Fire is an ancient process (He and Lamont, 2018), that is part of the ecosystem (Pausas and Bond, 2019; McLauchlan et al., 2020; Harrison et al., 2021), and as an ‘organism’ is an herbivore generalist (McCullough et al., 1998), with the ability of consuming all available fuel (Bond and Keeley, 2005; Pausas and Bond, 2019, 2020) competing with the spruce budworm, an herbivore specialist (Hennigar et al., 2008; Nealis, 2016). Fire would negatively affect spruce budworm host-tree abundance by consuming the budworm’s preferred food source along with all other vegetation (McCullough et al., 1998) resulting in food scarcity limiting LSBPs. Further, over long time periods fire may create more fire-prone conditions by favoring growth of fire-tolerant species (Rogers et al., 2015) that more easily re-establish post-fire via semi-serotinous cones, or sprouting (see Burns and Honkala, 1990; Bergeron, 2000). Conversely, through differential canopy host-tree mortality (Martin et al., 2019, 2020) creating variable canopy gap sizes resulting in complex regeneration patterns (Kneeshaw and Bergeron, 1998, 1999; D’Aoust et al., 2004; Couillard et al., 2021), LSBP events appear to favor the regeneration and establishment of balsam fir in the canopy subsequently predisposing the forest to further spruce budworm events (Baskerville, 1975; Morin, 1994; Bouchard et al., 2005, 2006, 2007). As such, transitioning from a spruce budworm or fire disturbance-vegetation feedback loop would likely require some sort of external forcing, such as a rapid climate change event.
Given the postglacial recolonization by balsam fir creating basins of attraction of similar dimensions, appropriate climate conditions could then influence the initiation and establishment of the above mentioned positive disturbance-vegetation feedback loops by moving the ecosystem into the different basins of attraction at lake Buire. It is possible that the alternating disturbance frequencies may be influenced by the periodic occurrence of punctual rapid climate change events (Bond et al., 1997, 2001; Mayewski et al., 2004) that appear to coincide with the switch in the dominant disturbance (Figures 4, 5). Such rapid climate change events have been associated with ice-raft debris events that altered oceanic thermohaline (Broecker, 1997, 2003; Alley and Ágústsdóttir, 2005; Törnqvist and Hijma, 2012) and atmospheric circulatory patterns (Smith et al., 2016; Deininger et al., 2017) resulting in particularly dry, cool conditions (Willard et al., 2005; Li et al., 2007; Springer et al., 2008; Orme et al., 2020), which during the Holocene have been correlated with changes in sedimentary charcoal accumulations in Europe (Florescu et al., 2019), and have resulted in higher fire frequencies in eastern North America (Carcaillet et al., 2001a). It is these arid conditions that have likely favored the observed increases in fire frequencies (Molinari et al., 2018) by facilitating ignitions via drying of fuels (Flannigan and Harrington, 1988; Macias Fauria and Johnson, 2008; Macias Fauria et al., 2010). Simultaneously, cooler conditions are likely to have had a negative effect on insect development and survival (Ayres and Lombardo, 2000; Bentz et al., 2010; Pureswaran et al., 2018) resulting in fewer LSBP events. It is possible that the presence/absence of such rapid climate change events may: primarily influence the presence/absence of fire events, or primarily influence the presence/absence of the spruce budworm or a more complex interaction (see Kefi et al., 2016) may result where both event types are simultaneously affected by these climate events. It is possible then, that rapid climate change events may mediate the interaction between the two disturbance agents potentially explaining the observed oscillation, however this requires further investigation.
Around lake Buire the spruce budworm and fire have been key ecosystem processes in the mixed boreal forest of central Québec over the past roughly 8000 years. Over the course of the Holocene, the two disturbances appear to exhibit an inverse relationship and have varied in frequency. Similar to the black spruce forest, an inverse relationship between disturbance frequencies was observed, however, the recurring oscillation between disturbance frequencies at lake Buire was not (Navarro et al., 2018b). At lake Buire, host-tree availability and abundance appears to be the primary determinant of spruce budworm population fluctuations, while climate effects may play a more secondary role, although it is difficult to pinpoint the more influential factor since they are not mutually exclusive (Buma et al., 2019). Conversely, fire as an herbivore generalist and a more stochastic physical process appears to be primarily driven by climate (Bessie and Johnson, 1995; Riley et al., 2019; Halofsky et al., 2020), and subsequently modulated by the vegetation present on the landscape (Hély et al., 2000, 2010, 2020; Girardin et al., 2013; Blarquez et al., 2015). Therefore, the peculiar oscillatory pattern between disturbance event frequencies may be the result of the presence of balsam fir around lake Buire and the subsequent effect of the punctual rapid significant climate change events.
Since this is the first reconstruction of its kind using lepidopteran scales and charcoal to reconstruct local Holocene disturbance frequencies and their interaction in the mixed boreal forest, the observed interaction needs to be confirmed to determine whether the observed pattern is due to site-level effects or may reflect a more general long-term regional behavior (for an example in the Mediterranean region see Furia et al., 2024). With a greater number of sediment profiles analyzing both spruce budworm population fluctuations and fire during the Holocene in the mixed boreal forest, a more accurate and precise picture of site-level variability of these disturbances can be attained which may elucidate the role of local and extra-local species composition on disturbance event frequency. Additionally, as more and more sediment profiles are analyzed there is also the opportunity to disentangle the effects of climate and/or vegetation composition on disturbance regimes. Finally, by combining multiple sediment profiles, a regional composite may be created to gain a broader and more general picture of spruce budworm and fire variability through time along with potential changes in their interactions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
M-AL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HM: Conceptualization, Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing. MS: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The authors received funding from the ‘NSERC Industrial Research Chair on black spruce growth and the effect of the spruce budworm on landscape heterogeneity in the boreal forest’ grant number 499381-15.
The authors would like to thank the 2 reviewers for their constructive feedback in ameliorating the original manuscript. Major thanks go out to Dr. Olivier Blarquez for providing invaluable advice pertaining to methodology, analysis, and interpretation prior to an abrupt career change. Thank you to Marika Tremblay and Guillaume Vigneault for help in the laboratory, and Hugues Terreaux de Félice and Cassy Berguet for help in the field. A big thank you to Claire Fournier and Mireille Boulianne for lending equipment and preparing sucrose solution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1532974/full#supplementary-material
Ali A. A., Blarquez O., Girardin M. P., Hély C., Tinquaut F., El Guellab A., et al. (2012). Control of the multimillennial wildfire size in boreal North America by spring climatic conditions. Proc. Natl. Acad. Sci. U.S.A. 109, 20966–20970. doi: 10.1073/pnas.1203467109
Ali A. A., Higuera P. E., Bergeron Y., Carcaillet C. (2009). Comparing fire-history interpretations based on area, number and estimated volume of macroscopic charcoal in lake sediments. Quat Res. 73, 462–468. doi: 10.1016/j.yqres.2009.07.002
Allen C. D., Breshears D. D., McDowell N. G. (2015). On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, 129. doi: 10.1890/ES15-00203.1
Allen C. D., Macalady A. K., Chenchouni H., Bachelet D., McDowell N., Vennetier M., et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol. Manage 259, 660–684. doi: 10.1016/j.foreco.2009.09.001
Alley R. B., Ágústsdóttir A. M. (2005). The 8k event: cause and consequences of a major Holocene abrupt climate change. Quat Sci. Rev. 24, 1123–1149. doi: 10.1016/j.quascirev.2004.12.004
Appleby P. G., Oldfield F. (1983). The assessment of 210Pb data from sites with varying sediment accumulation rates. Hydrobiologia 103, 29–35. doi: 10.1007/BF00028424
Ayres M. P., Lombardo M. J. (2000). Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 262, 263–286. doi: 10.1016/S0048-9697(00)00528-3
Baskerville G. L. (1975). Spruce budworm- Super silviculturist. For Chron 51, 138–140. doi: 10.5558/tfc51138-4
Bentz B. J., Régnière J., Fettig C. J., Hansen E. M., Hayes J. L., Hicke J. A., et al. (2010). Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. Bioscience 60, 602–613. doi: 10.1525/bio.2010.60.8.6
Berg E. E., Henry J. D., Fastie C. L., De Volder A. D., Matsuoka S. M. (2006). Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: Relationship to summer temperatures and regional differences in disturbance regimes. For Ecol. Manage 227, 291–232. doi: 10.1016/j.foreco.2006.02.038
Bergeron Y. (2000). Species and stand dynamics in the mixed woods of Quebec’s southern boreal forest. Ecology 81, 1500–1516. doi: 10.1890/0012-9658(2000)081[1500:SASDIT]2.0.CO;2
Bessie W. C., Johnson E. A. (1995). The relative importance of fuels and weather on fire behavior in subalpine forests. Ecology 76, 747–762. doi: 10.2307/1939341
Binford M. W. (1990). Calculation and uncertainty analysis of 210Pb dates for PIRLA project lake sediment cores. J. Paleolimnol 3, 253–267. doi: 10.1007/BF00219461
Blaauw M., Christen A. (2011). Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 6, 457–474. doi: 10.1214/11-BA618
Blaauw M., Christen A., Aquino Lopez M. A. (2021). rbacon: Age-depth modelling using Bayesian statistics. R package version 2.5.7. Available online at: https://CRAN.R-project.org/package=rbacon (Accessed May 25, 2022).
Blais J. R. (1983). Trends in the frequency, extent, and severity of spruce budworm outbreaks in eastern Canada. Can. J. For Res. 13, 539–547. doi: 10.1139/x83-079
Blais J. R. (1985). Epidemiology of the spruce budworm in western Ontario: A discussion. For Chron 61, 494–498. doi: 10.5558/tfc61494-6
Blarquez O., Aleman J. C. (2016). Tree biomass reconstruction shows no lag in postglacial afforestation of eastern Canada. Can. J. For Res. 46, 485–498. doi: 10.1139/cjfr-2015-0201
Blarquez O., Ali A. A., Girardin M. P., Grondin P., Fréchette B., Bergeron Y., et al. (2015). Regional paleofire regimes affected by non-uniform climate, vegetation and human drivers. Sci. Rep. 5, 13356. doi: 10.1038/srep13356
Blarquez O., Bremond L., Carcaillet C. (2010). Holocene fires and a herb-dominated understorey track wetter climates in subalpine forests. J. Ecol. 98, 1358–1368. doi: 10.1111/j.1365-2745.2010.01721.x
Blarquez O., Vannière B., Marlon J. R., Daniau A.-L., Power M. J., Brewer S., et al. (2014). Paleofire: An R package to analyse sedimentary charcoal records from the Global Charcoal Database to reconstruct past biomass burning. Comput. Geosci 72, 255–261. doi: 10.1016/j.cageo.2014.07.020
Bond W. J., Keeley J. E. (2005). Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 20, 387–394. doi: 10.1016/j.tree.2005.04.025
Bond G., Kromer B., Beer J., Muscheler R., Evans M. N., Showers W., et al. (2001). Persistent solar influence on north Atlantic climate during the Holocene. Science 294, 2130–2136. doi: 10.1126/science.1065680
Bond G., Showers W., Cheseby M., Lotti R., Almasi P., deMenocal P., et al. (1997). A pervasive millennial-scale cycle in north Atlantic Holocene and glacial climates. Science 278, 1257–1266. doi: 10.1126/science.278.5341.1257
Bouchard M., Kneeshaw D., Bergeron Y. (2006). Forest dynamics after successive spruce budworm outbreaks in mixedwood forests. Ecology 87, 2319–2329. doi: 10.1890/0012-9658(2006)87[2319:FDASSB]2.0.CO;2
Bouchard M., Kneeshaw D., Bergeron Y. (2007). Forest dynamics following spruce budworm outbreaks in the northern and southern mixedwoods of central Quebec. Can. J. For Res. 37, 763–772. doi: 10.1139/X06-278
Bouchard M., Kneewshaw D., Bergeron Y. (2005). Mortality and stand renewal patterns following the last spruce budworm outbreak in mixed forests of western Quebec. For Ecol. Manage 204, 297–313. doi: 10.1016/j.foreco.2004.09.017
Boulanger Y., Arseneault D. (2004). Spruce budworm outbreaks in eastern Québec over the last 450 years. Can. J. For Res. 34, 1035–1043. doi: 10.1139/x03-269
Broecker W. S. (1997). Thermohaline circulation, the Achilles heel of our climate system: will man-made CO2 upset the current balance? Science 278, 1582–1588. doi: 10.1126/science.278.5343.1582
Broecker W. S. (2003). Does the trigger for abrupt climate change reside in the ocean or in the atmosphere? Science 300, 1519–1522. doi: 10.1126/science.1083797
Buma B. (2015). Disturbance interactions: characterization, prediction, and the potential for cascading effects. Ecosphere 6, 1–15. doi: 10.1890/ES15-00058.1
Buma B., Harvey B. J., Gavin D. G., Kelly R., Loboda T., McNeil B. E., et al. (2019). The value of linking paleoecological and neoecological perspectives to understand spatially-explicit ecosystem resilience. Landsc Ecol. 34, 17–33. doi: 10.1007/s10980-018-0754-5
Buma B., Wessman C. A. (2011). Disturbance interactions can impact resilience mechanisms of forests. Ecosphere 2, 1–13. doi: 10.1890/ES11-00038.1
Buma B., Wessman C. A. (2012). Differential species responses to compounded perturbations and implications for landscape heterogeneity and resilience. For Ecol. Manage 266, 25–33. doi: 10.1016/j.foreco.2011.10.040
Buma B., Wessman C. A. (2013). Forest resilience, climate change, opportunities for adaptation: a specific case of a general problem. For Ecol. Manag 306, 216–225. doi: 10.1016/j.foreco.2013.06.044
Burns R. M., Honkala B. H. (1990). “Silvics of North America volume 1: conifers and volume 2: hardwoods,” in United States Department of Agriculture Forest Service Agriculture Handbook. US Government Printing Office: Washington, DC, 654.
Burton P. J., Jentsch A., Walker L. R. (2020). The ecology of disturbance interactions. Bioscience 70, 854–870. doi: 10.1093/biosci/biaa088
Candau J.-N., Fleming R. A., Wang X. (2018). Ecoregional patterns of spruce budworm-wildfire interactions in central Canada’s forests. Forests 9, 137. doi: 10.3390/f9030137
Carcaillet C., Ali A. A., Blarquez O., Genries A., Mourier B., Bremond L. (2009). Spatial variability of fire history in subalpine forests: From natural to cultural regimes. Ecoscience 16, 1–12. doi: 10.2980/16-1-3189
Carcaillet C., Bergeron Y., Richard P. J. H., Féchette B., Gauthier S., Prairie Y. T. (2001a). Changes of fire frequency in the eastern Canadian boreal forests during the Holocene: does vegetation composition or climate trigger the fire regime? J. Ecol. 89, 930–946. doi: 10.1111/j.1365-2745.2001.00614.x
Carcaillet C., Bouvier M., Fréchette B., Larouche A. C., Richard P. J. H. (2001b). Comparison of pollen-slide and sieving methods in lacustrine charcoal analyses for local and regional fire history. Holocene 11, 467–476. doi: 10.1191/095968301678302904
Clark J. S., Lynch J., Stocks B. J., Goldammer J. G. (1998). Relationships between charcoal particles in air and sediments in west-central Siberia. Holocene 8, 19–29. doi: 10.1191/095968398672501165
Clark J. S., Royall P. D. (1995). Particle-size evidence for source areas of charcoal accumulation in late Holocene sediments of eastern North American lakes. Quat Res. 43, 80–89. doi: 10.1006/qres.1995.1008
Clark J. S., Royall P. D., Chumbley C. (1996). The role of fire during climate change in an eastern deciduous forest at Devil’s Bathtub, New York. Ecology 77, 2148–2166. doi: 10.2307/2265709
Cohn G. M., Parsons R. A., Heyerdahl E. K., Gavin D. G., Flower A. (2014). Simulated western spruce budworm defoliation reduces torching and crowning potential: a sensitivity analysis using a physics-based fire model. Int. J. Wildland Fire 23, 709–720. doi: 10.1071/WF13074
Cooke B. J., Nealis V. G., Régnière J. (2007). “Insect defoliators as periodic disturbances in northern forest ecosystems,” in Plant disturbance ecology: the process and the response. Eds. E.A. Johnson E. A., Miyanishi K. (Elsevier), 487–525.
Couillard P.-L., Payette S., Grondin P. (2013). Long-term impact of fire on high-altitude balsam fir (Abies balsamea) forests in south-central Quebec deduced from soil charcoal. Can. J. For Res. 43, 188–199. doi: 10.1139/cjfr-2012-0414
Couillard P.-L., Payette S., Lavoie M., Frégeau M. (2021). Precarious resilience of the boreal forest of eastern North American during the Holocene. For Ecol. Manage 485, 118954. doi: 10.1016/j.foreco.2021.118954
D’Aoust V., Kneeshaw D., Bergeron Y. (2004). Characterization of canopy openness before and after a spruce budworm outbreak in the southern boreal forest. Can. J. For Res. 34, 339–352. doi: 10.1139/x03-278
Dantas V. L., Hirota M., Oliveira R. S., Pausas J. G. (2016). Disturbance maintains alternative biome states. Ecol. Lett. 19, 12–19. doi: 10.1111/ele.12537
Da Silva A. C., Whalen M. T., Hladil J., Chadimova L., Chen D., Spassov S., et al. (2015). Magnetic susceptibility application: a window onto ancient environments and climatic variations: foreword. Geol Soc. Spec Publ 414, 1–13. doi: 10.1144/SP414.12
Dearing J. A. (1999). Environmental magnetic susceptibility: using the Bartington MS2 System 2nd edition (Kenilworth: Chi Publishing).
Dearing J. A., Flower R. J. (1982). The magnetic susceptibility of sediment material trapped in Lough Neagh, northern Ireland, and its erosional significance. Limnol Oceanogr 27, 969–975. doi: 10.4319/lo.1982.27.5.0969
De Grandpré L., Kneeshaw D. D., Perigon S., Boucher D., Marchand M., Pureswaran D., et al. (2019). Adverse climatic periods precede and amplify defoliator-induced tree mortality in eastern boreal North America. J. Ecol. 107, 452–467. doi: 10.1111/1365-2745.13012
Deininger M., McDermott F., Mudelsee M., Werner M., Frank N., Mangini A. (2017). Coherency of late Holocene European speleothem δ18O records linked to north Atlantic ocean circulation. Clim Dyn 49, 595–619. doi: 10.1007/s00382-016-3360-8
Dymond C. C., Neilson E. T., Stinson G., Porter K., MacLean D. A., Gray D. R., et al. (2010). Future spruce budworm outbreak may create a carbon source in eastern Canadian forests. Ecosystems 13, 917–931. doi: 10.1007/s10021-010-9364-z
Easterling D. R., Meehl G. A., Parmesan C., Changnon S. A., Karl T. R., Mearns L. O. (2000). Climate extremes: observations, modelling and impacts. Science 289, 2068–2074. doi: 10.1126/science.289.5487.2068
Erbilgin N., Ma C., Whitehouse C., Shan B., Najar A., Evenden M. (2014). Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naïve host ecosystem. New Phytol. 201, 940–950. doi: 10.1111/nph.12573
Faegri K., Iversen J. (1989). Textbook of pollen analysis IV edition. Chichester, UK: John Wiley and Sons.
Flannigan M., Cantin A. S., de Groot W. J., Wotton M., Newbery A., Gowman L. M. (2013). Global wildland fire season severity in the 21st century. For Ecol. Manage 294, 54–61. doi: 10.1016/j.foreco.2012.10.022
Flannigan M. D., Harrington J. B. (1988). A study of the relation of meteorological variables to monthly provincial area burned by wildfire in Canada, (1953-1980). J. Appl. Meteorol Climatol 27, 441–452. doi: 10.1175/1520-0450(1988)027%3C0441:ASOTRO%3E2.0.CO;2
Flannigan M., Stocks B., Turetsky M., Wotton M. (2009). Impacts of climate change on fire activity and fire management in the circumboreal forest. Glob Chang Biol. 15, 549–560. doi: 10.1111/j.1365-2486.2008.01660.x
Fleming R. A., Candau J.-N., McApline R. S. (2002). Landscape-scale analysis of interactions between insect defoliation and forest fire in central Canada. Clim Change 55, 251–272. doi: 10.1023/A:1020299422491
Florescu G., Brown K. J., Carter V. A., Kunes P., Veski S., Feurdean A. (2019). Holocene rapid climate changes and ice-rafting debris events reflected in high-resolution European charcoal records. Quat Sci. Rev. 222, 105877. doi: 10.1016/j.quascirev.2019.105877
Frégeau M., Payette S., Grondin P. (2015). Fire history of the central boreal forest in eastern North America reveals stability since the mid-Holocene. Holocene 25, 1912–1922. doi: 10.1177/0959683615591361
Fuentealba A., Pureswaran D., Bauce É., Despland E. (2017). How does synchrony with host plant affect the performance of an outbreaking insect defoliator? Oecologia 184, 847–857. doi: 10.1007/s00442-017-3914-4
Furia E., Clò E., Florenzano A., Mercuri A. M. (2024). Human-induced fires and land use driven changes in tree biodiversity on the northern Tyrrhenian coast. Quat Int. 705, 37–52. doi: 10.1016/j.quaint.2023.12.002
Gavin D. G., Hu F. S., Lertzman K., Corbett P. (2006). Weak climatic control of stand-scale fire history during the late Holocene. Ecology 87, 1722–1732. doi: 10.1890/0012-9658(2006)87[1722:WCCOSF]2.0.CO;2
Girardin M. P., Ali A. A., Carcaillet C., Blarquez O., Hély C., Terrier A., et al. (2013). Vegetation limits the impact of a warm climate on boreal wildfires. New Phytol. 199, 1001–1011. doi: 10.1111/nph.12322
Halofsky J. E., Peterson D. L., Harvey B. J. (2020). Changing wildfire, changing forests: the effects of climate change on fire regimes and vegetation in the Pacific Northwest, USA. Fire Ecol. 16, 1–26. doi: 10.1186/s42408-019-0062-8
Harrison S. P., Prentice I. C., Bloomfield K. J., Dong N., Forkel M., Forrest M., et al. (2021). Understanding and modelling wildfire regimes: an ecological perspective. Environ. Res. Lett. 16, 125008. doi: 10.1088/1748-9326/ac39be
Hart S. J., Veblen T. T., Eisenhart K. S., Jarvis D., Kulakowski D. (2014). Drought induces spruce beetle (Dendroctonus rufipennis) outbreaks across northwestern Colorado. Ecology 95, 930–939. doi: 10.1890/13-0230.1
Hart S. J., Veblen T. T., Schneider D., Molotch N. P. (2017). Summer and winter drought drive the initiation and spread of spruce beetle outbreak. Ecology 98, 2698–2707. doi: 10.1002/ecy.1963
He T., Lamont B. B. (2018). Baptism by fire: the pivotal role of ancient conflagrations in evolution of the Earth’s flora. Natl. Sci. Rev. 5, 237–254. doi: 10.1093/nsr/nwx041
Hély C., Bergeron Y., Flannigan M. D. (2000). Effects of stand composition on fire hazard in mixed-wood Canadian boreal forest. J. Veg Sci. 11, 813–824. doi: 10.2307/3236551
Hély C., Chaste E., Girardin M. P., Remy C. C., Blarquez O., Bergeron Y., et al. (2020). A Holocene perspective of vegetation controls on seasonal boreal wildfire sizes using numerical paleo-ecology. Front. For Glob Change 3. doi: 10.3389/ffgc.2020.511901
Hély C., Girardin M. P., Ali A. A., Carcaillet C., Brewer S., Bergeron Y. (2010). Eastern boreal North American wildfire risk of the past 7000 years: a model-data comparison. Geophys Res. Lett. 37, L14709. doi: 10.1029/2010GL043706
Hennebelle A., Grondin P., Aleman J. C., Ali A. A., Bergeron Y., Borcard D., et al. (2018). Using paleoecology to improve reference conditions for ecosystem-based management in western spruce-moss subdomain of Québec. For Ecol. Manage 430, 157–165. doi: 10.1016/j.foreco.2018.08.007
Hennigar C. R., MacLean D. A., Quiring D. T., Kershaw J. A. (2008). Differences in spruce budworm defoliation among balsam fir, and white, red, and black spruce. For. Sci. 54, 158–166. doi: 10.1093/forestscience/54.2.158
Hicke J., Johnson M., Hayes J. L., Preisler H. K. (2012). Effects of bark beetles-caused tree mortality on wildfire. For Ecol. Manage 271, 81–90. doi: 10.1016/j.foreco.2012.02.005
Higuera P. E. (2009). CharAnalysis 0.9: Diagnostic and analytical tools for sediment-charcoal analysis. Available online at: https://github.com/phiguera/CharAnalysis (Accessed November 2, 2021).
Higuera P. E., Gavin D. G., Bartlein P. J., Hallet D. J. (2010). Peak detection in sediment-charcoal records: Impacts of alternative data analysis methods on fire-history interpretations. Int. J. Wildland Fire 19, 996–1014. doi: 10.1071/WF09134
Higuera P. E., Peters M. E., Brubaker L. B., Gavin D. G. (2007). Understanding the origin and analysis of sediment-charcoal records with a simulation model. Quat Sci. Rev. 26, 1790–1809. doi: 10.1016/j.quascirev.2007.03.010
James P. M. A., Robert L.-E., Wotton B. M., Martell D. L., Fleming R. A. (2017). Lagged cumulative spruce budworm defoliation affects the risk of fire ignition in Ontario, Canada. Ecol. Appl. 27, 532–544. doi: 10.1002/eap.1463
Jepsen J. U., Hagen S. B., Ims R. A., Yoccoz N. G. (2008). Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: Evidence of a recent outbreak range expansion. J. Anim. Ecol. 77, 257–264. doi: 10.1111/j.1365-2656.2007.01339.x
Jepsen J. U., Kapari L., Hagen S. B., Schott T., Vinstad O. P. L., Nilssen A. C., et al. (2011). Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub-Arctic birch. Glob Chang Biol. 17, 2071–2083. doi: 10.1111/j.1365-2486.2010.02370.x
Jolly W. M., Cochrane M. A., Freeborn P. H., Holden Z. A., Brown T. J., Williamson G. J., et al. (2015). Climate-induced variations in global wildfire danger from 1979 to 2013. Nat. Commun. 6, 7537. doi: 10.1038/ncomms8537
Juggens S. (2020). Rioja: analysis of quaternary science data. Available online at: https://cran.r-project.org/package=rioja (Accessed February 17, 2022).
Keeley J. E., Pausas J. G., Rundel P. W., Bond W. J., Bradstock R. A. (2011). Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 16, 406–411. doi: 10.1016/j.tplants.2011.04.002
Kefi S., Holmgren M., Scheffer M. (2016). Mechanisms and consequences of facilitation in plant communities: when can positive interactions cause alternative stable states in ecosystems? Funct. Ecol. 30, 88–97. doi: 10.1111/1365-2435.12601
Kleinman J. S., Goode J. D., Fries A. C., Hart J. L. (2019). Ecological consequences of compound disturbances in forest ecosystems: a systematic review. Ecosphere 10, e02962. doi: 10.1002/ecs2.2962
Kneeshaw D. D., Bergeron Y. (1996). Ecological factors affecting the abundance of advance regeneration in Quebec’s southwestern boreal forest. Can. J. For Res. 26, 888–898. doi: 10.1139/x26-097
Kneeshaw D. D., Bergeron Y. (1998). Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology 79, 783–794. doi: 10.1890/0012-9658(1998)079[0783:CGCATR]2.0.CO;2
Kneeshaw D. D., Bergeron Y. (1999). Spatial and temporal patterns of seedling and sapling recruitment within canopy gaps caused by spruce budworm. Écoscience 6, 214–222. doi: 10.1080/11956860.1999.11682522
Lafontaine-Boyer K., Gajewski K. (2014). Vegetation dynamics in relation to late Holocene climate variability and disturbance, Outaouais, Québec, Canada. Holocene 24, 1515–1526. doi: 10.1177/0959683614544054
Lamont B. B., Pausas J. G., He T., Witkowski E. T. F., Hanley M. E. (2020). Fire as a selective agent for both serotiny and nonserotiny over space and time. Crit. Rev. Plant Sci. 39, 140–172. doi: 10.1080/07352689.2020.1768465
Landres P. B., Morgan P., Swanson F. J. (1999). Overview of the use of natural variability concepts in managing ecological systems. Ecol. Appl. 9 (4), 1179–1188. doi: 10.1890/1051-0761(1999)009[1179:OOTUON]2.0.CO;2
Lavoie M., Richard P. J. H. (2000). Postglacial water-level changes of a small lake in southern Québec, Canada. Holocene 10, 621–634. doi: 10.1191/095968300672141865
Leclerc M.-A., Simard M., Blarquez O., Morin H. (2024). Lepidopteran scales in lake sediments as a reliable proxy for spruce budworm outbreak events in the boreal forest of Eastern Canada. Holocene 34, 978–986. doi: 10.1177/09596836241236326
Li Y.-X., Yu Z., Kodama K. P. (2007). Sensitive moisture response to Holocene millennial-scale climate variations in the Mid-Atlantic region, USA. Holocene 17, 3–8. doi: 10.1177/0959683606069386
Littell J. S., Peterson D. L., Riley K. L., Liu Y., Luce C. H. (2016). A review of the relationships between drought and forest fire in the United States. Glob Chang Biol. 22, 2353–2369. doi: 10.1111/gcb.13275
Lynch H. J., Moorcroft P. R. (2008). A spatiotemporal Ripley’s K-function to analyze interactions between spruce budworm and fire in British Columbia, Canada. Can. J. For Res. 38, 3112–3119. doi: 10.1139/X08-143
Macias Fauria M., Johnson E. A. (2008). Climate and wildfires in the north american boreal forest. Phil Trans. R Soc. B 363 (1501), 2317–2329. doi: 10.1098/rstb.2007.2202
Macias Fauria M., Michaletz S. T., Johnson E. A. (2010). Predicting climate change effects on wildfires requires linking processes across scales. Clim Change 2, 99–112. doi: 10.1002/wcc.92
MacLean D. A. (1980). Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks- A review and discussion. For Chron 56, 213–221. doi: 10.5558/tfc56213-5
MacLean D. A. (1984). Effects of spruce budworm outbreaks on the productivity and stability of balsam fir forests. For Chron 60, 273–279. doi: 10.5558/tfc60273-5
MacLean D. A. (2016). Impacts of insect outbreaks on tree mortality, productivity, and stand development. Can. Entomol 148, S138–S159. doi: 10.4039/tce.2015.24
MacLean D. A., Ostaff D. P. (1989). Patterns of balsam fir mortality caused by an uncontrolled spruce budworm outbreak. Can. J. For Res. 19, 1087–1095. doi: 10.1139/x89-165
Mann M. E. (2004). On smoothing potentially non-stationary time series. Geophys Res. Lett. 31, L07214. doi: 10.1029/2004GL019569
Mann M. E. (2008). Smoothing of climate time series revisited. Geophys Res. Lett. 35, L16708. doi: 10.1029/2008GL034716
Mann M. E., Zhang Z., Rutherford S., Bradley R. S., Hughes M. K., Shindell D., et al. (2009). Global signatures and dynamical origins of the Little Ice Age and Medieval Climate Anomaly. Science 326, 1256–1260. doi: 10.1126/science.1177303
Marcott S. A., Shakun J. D., Clark P. U., Mix A. C. (2013). A reconstruction of regional and global temperature for the past 11,300 years. Science 339, 1198–1201. doi: 10.1126/science.1228026
Marsicek J., Shuman B. N., Bartlein P. J., Shafer S. L., Brewer S. (2018). Reconciling divergent trends and millennial variations in Holocene temperatures. Nature 554, 92–96. doi: 10.1038/nature25464
Martin M., Krause C., Morin H. (2020). Linking radial growth patterns and moderate-severity disturbance dynamics in boreal old-growth forests driven by recurrent insect outbreaks: a tale of opportunities, successes, and failures. Ecol. Evol. 11, 566–586. doi: 10.1002/ece3.7080
Martin M., Morin H., Fenton N. J. (2019). Secondary disturbances of low and moderate severity drive the dynamics of eastern Canadian boreal old-growth forests. Ann. For Sci. 76, 108. doi: 10.1007/s13595-019-0891-2
Mayewski P. A., Rohling E. E., Stager J. C., Karlen W., Maasch K. A., Meeker L. D., et al. (2004). Holocene climate variability. Quat Res. 62, 243–255. doi: 10.1016/j.yqres.2004.07.001
McCullough D. G., Werner R. A., Neumann D. (1998). Fire and insects in northern and boreal forest ecosystems of North America. Annu. Rev. Entomol 43, 107–127. doi: 10.1146/annurev.ento.43.1.107
McDowell N. G., Allen C. D., Anderson-Teixeira K., Aukema B. H., Bond-Lamberty B., Chini L., et al. (2020). Pervasive shifts in forest dynamics in a changing world. Science 368, eaaz9463. doi: 10.1126/science.aaz9463
McLauchlan K. K., Higuera P. E., Miesel J., Rogers B. M., Schweitzer J., Shuman J. K., et al. (2020). Fire as a fundamental ecological process: research advances and frontiers. J. Ecol. 108, 2047–2069. doi: 10.1111/1365-2745.13403
MFFP (Ministère des forêts, de la faune et des parcs) (2021a). Shapefiles of the aerial surveys done to assess the damage caused by the spruce budworm from 1967-present. Available online at: https://www.donneesquebec.ca/recherche/fr/dataset/donnees-sur-les-perturbations-naturelles-insecte-tordeuse-des-bourgeons-de-lepinette (Accessed May 15, 2020).
MFFP (Ministère des forêts, de la faune et des parcs) (2021b). Shapefiles of the forest inventory data including forest composition and previous disturbances. Available online at: https://www.donneesquebec.ca/recherche/dataset/carte-ecoforestiere-avec-perturbations (Accessed May 15, 2020).
Millar C. I., Stephenson N. L. (2015). Temperate forest health in an era of emerging megadisturbance. Science 349, 823–826. doi: 10.1126/science.aaa9933
Molinari C., Lehsten V., Blarquez O., Carcaillet C., Davis B. A. S., Kaplan J. O., et al. (2018). The climate, the fuel and the land use: long-term regional variability of biomass burning in boreal forests. Glob Chang Biol. 24, 4929–4945. doi: 10.1111/gcb.14380
Morin H. (1994). Dynamics of balsam fir forests in relation to spruce budworm outbreaks in the boreal zone of Québec. Can. J. For Res. 24, 730–741. doi: 10.1139/x94-097
Morin H., Laprise D. (1990). Histoire récente des épidémies de la Tordeuse des bourgeons de l’épinette au nord du lac-Saint-Jean (Québec): une analyse dendrochronologique. Can. J. For Res. 20, 1–8. doi: 10.1139/x90-001
Muller S. D., Richard P. J. H., Guiot J., de Beaulieu J.-L., Fortin D. (2003). Postglacial climate in the St. Lawrence lowlands, southern Québec: pollen and lake-level evidence. Palaeogeogr Palaeoclimatol Palaeoecol 193, 51–72. doi: 10.1016/S0031-0182(02)00710-1
Murdock T. Q., Taylor S. W., Flower A., Mehlenbacher A., Montenegro A., Zwiers F. W., et al. (2013). Pest outbreak distribution and forest management impacts in a changing climate in British Columbia. Environ. Sci. Policy 26, 75–89. doi: 10.1016/j.envsci.2012.07.026
Navarro L., Harvey A. E., Ali A. A., Bergeron Y., Morin H. (2018b). A Holocene landscape dynamic multiproxy reconstruction: How do interactions between fire and insect outbreaks shape an ecosystem over long time scales? PloS One 13, e0204316. doi: 10.1371/journal.pone.0204316
Navarro L., Harvey A. E., Morin H. (2018a). Lepidoptera wing scales: a new paleocological indicator for reconstructing spruce budworm abundance. Can. J. For Res. 48, 302–308. doi: 10.1139/cjfr-2017-0009
Nealis V. G. (2016). Comparative ecology of conifer-feeding spruce budworms (Lepidoptera: Tortricidae). Can. Entomol 148, S33–S57. doi: 10.4039/tce.2015.15
Neil K., Gajewski K. (2018). An 11,000-yr record of diatom assemblage responses to climate and terrestrial vegetation changes, southwestern Quebec. Ecosphere 9, e02505. doi: 10.1002/ecs2.2505
Orme L. C., Miettinen A., Seidenkrantz M.-S., Tuominen K., Pearce C., Divine D., et al. (2020). Mid to late-Holocene sea-surface temperature variability off north-eastern Newfoundland and its linkage to the North Atlantic Oscillation. Holocene 31, 3–15. doi: 10.1177/0959683620961488
Page W. G., Jenkins M. J. (2007a). Mountain pine beetle-induced changes to selected lodgepole pine fuel complexes within the Intermountain region. For. Sci. 53, 507–518. doi: 10.1093/forestscience/53.4.507
Page W., Jenkins M. J. (2007b). Predicted fire behavior in selected mountain pine beetle-infested lodgepole pine. For. Sci. 53, 662–674. doi: 10.1093/forestscience/53.6.662
Paine R. T., Tegner M. J., Johnson E. A. (1998). Compounded perturbations yield ecological surprises. Ecosystems 1, 535–545. doi: 10.1007/s100219900049
Pál I., Buczkó K., Vincze I., Finsinger W., Braun M., Biró T., et al. (2018). Terrestrial and aquatic ecosystem responses to early Holocene rapid climate change (RCC) events in the South Carpathian Mountains, Romania. Quat Int. 477, 79–93. doi: 10.1016/j.quaint.2016.11.015
Pausas J. G. (2015). Evolutionary fire ecology: lessons learned from pines. Trends Plant Sci. 20, 318–324. doi: 10.1016/j.tplants.2015.03.001
Pausas J. G., Bond W. J. (2019). Humboldt and the reinvention of nature. J. Ecol. 107, 1031–1037. doi: 10.1111/1365-2745.13109
Pausas J. G., Bond W. J. (2020a). On the three major recycling pathways in terrestrial ecosystems. Trends Ecol. Evol. 35, 767–775. doi: 10.1016/j.tree.2020.04.004
Pausas J. G., Bond W. J. (2020b). Alternative biome states in terrestrial ecosystems. Trends Plant Sci. 25, 250–263. doi: 10.1016/j.tplants.2019.11.003
Pausas J. G., Bond W. J. (2022). Feedbacks in ecology and evolution. Trends Ecol. Evol. 37, 637–644. doi: 10.1016/j.tree.2022.03.008
Piene H. (1989). Spruce budworm defoliation and growth loss in young balsam fir: defoliation in space and unspaced stands and individual tree survival. Can. J. For Res. 19, 1211–1217. doi: 10.1139/x89-185
Pureswaran D. S., De Grandpré L., Paré D., Taylor A., Barrette M., Morin H., et al. (2015). Climate-induced changes in host tree-insect phenology may drive ecological state-shift in boreal forests. Ecology 96, 1480–1491. doi: 10.1890/13-2366.1
Pureswaran D. S., Johns R., Heard S. B., Quiring D. (2016). Paradigms in eastern spruce budworm (Lepidopteran: Tortricidae) population ecology: a century of debate. Environ. Entomol 45, 1333–1342. doi: 10.1093/ee/nvw103
Pureswaran D. S., Neau M., Marchard M., De Grandpré L., Kneeshaw D. (2019). Phenological synchrony between eastern spruce budworm and its host trees increases with warmer temperatures in the boreal forest. Ecol. Evol. 9, 576–586. doi: 10.1002/ece3.4779
Pureswaran D. S., Roques A., Battisti A. (2018). Forest insects and climate change. Curr. For Rep. 4, 35–50. doi: 10.1007/s40725-018-0075-6
R Core Team. (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/ (Accessed December 12, 2022).
Regent Instruments Inc. (2019). WinSEEDLE: Seed and needle morphology and count v 2019a. Québec: Regent Instruments Inc.
Régnière J., St-Amant R., Duval P. (2012). Predicting insect distributions under climate change from physiological responses: spruce budworm as an example. Biol. Invasions 14, 1571–1586. doi: 10.1007/s10530-010-9918-1
Renberg I., Hansson H. (2008). The HTH sediment corer. J. Paleolimnol 40, 655–659. doi: 10.1007/s10933-007-9188-9
Renssen H., Seppa H., Crosta X., Goosse H., Roche D. M. (2012). Global characterization of the holocene thermal maximum. Quat Sci. Rev. 48, 7–19. doi: 10.1016/j.quascirev.2012.05.022
Renssen H., Seppa H., Heiri O., Roche D. M., Goosse H., Fichefet T. (2009). The spatial and temporal complexity of the Holocene thermal maximum. Nat. Geosci 2, 411–414. doi: 10.1038/ngeo513
Riley K. L., Williams A. P., Urbanski S. P., Calkin D. E., Short K. C., O’Connor C. D. (2019). Will landscape fire increase in the future? A systems approach to climate, fire, fuel and human drivers. Curr. pollut. Rep. 5, 9–24. doi: 10.1007/s40726-019-0103-6
Rodionov S. N. (2004). A sequential algorithm for testing climate regime shifts. Geophys Res. Lett. 31, L09204. doi: 10.1029/2004GL019448
Rodionov S. N. (2006). Use of prewhitening in climate regime shift detection. Geophys Res. Lett. 33, L12707. doi: 10.1029/2006GL025904
Rodionov S., Overland J. E. (2005). Application of a sequential regime shift detection method to the Bering Sea ecosystem. ICES J. Mar. Sci. 62, 328–332. doi: 10.1016/j.icesjms.2005.01.013
Rogers B. M., Soja A. J., Goulden M. L., Randerson J. T. (2015). Influence of tree species on continental differences in boreal fires and climate feedbacks. Nat. Geosci 8, 228–234. doi: 10.1038/ngeo2352
Rowe J. S. (1972). Forest Regions of Canada (Ottawa, Canada: Department of the Environment, Canadian Forestry Service). Publication No. 1300.
Saucier J.-P., Bergeron J.-F., Grondin P., Robitaille A. (1998). Les régions écologiques du Québec méridional (3e version): un des éléments du système hiérarchique de classification écologique du territoire mis au point par le ministère des Ressources naturelles du Québec. Supplément de l’Aubelle. 124, 12p.
Saucier J.-P., Robitaille A., Grondin P. (2009). “Cadre bioclimatique du Québec,” in Manuel de foresterie, 2nd édition (Éditions MultiMondes, Québec), 186–205. ed. Ordre des ingénieurs forestiers du Québec.
Scheffer M., Carpenter S. R. (2003). Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol. 18, 648–656. doi: 10.1016/j.tree.2003.09.002
Scheffer M., Carpenter S., Foley J. A., Folke C., Walker B. (2001). Catastrophic shifts in ecosystems. Nature 413, 591–596. doi: 10.1038/35098000
Scheffer M., Hirota M., Holmgren M., Van Nes E. H., Chapin III F.S. (2012). Thresholds for boreal biome transitions. Proc. Natl. Acad. Sci. U.S.A. 109, 21384–21389. doi: 10.1073/pnas.1219844110
Scheffer M., Szabo S., Gragnani A., van Nes E. H., Rinaldi S., Kautsky N., et al. (2003). Floating plant dominance as a stable state. Proc. Natl. Acad. Sci. U.S.A. 100, 4040–4045. doi: 10.1073/pnas.0737918100
Scheffer M., van Nes E. H. (2007). Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584, 455–466. doi: 10.1007/s10750-007-0616-7
Shuman B. N., Marsicek J. (2016). The structure of Holocene climate change in mid-lattitude North America. Quat Sci. Rev. 141, 38–51. doi: 10.1016/j.quascirev.2016.03.009
Simard S., Morin H., Krause C. (2011). Long-term spruce budworm outbreak dynamics reconstructed from subfossil trees. J. Quat Sci. 26, 734–738. doi: 10.1002/jqs.1492
Simard I., Morin H., Lavoie C. (2006). A millennial-scale reconstruction of spruce budworm abundance in Saguenay, Québec, Canada. Holocene 16, 31–37. doi: 10.1191/0959683606hl904rp
Simard I., Morin H., Potelle B. (2002). A new paleoecological approach to reconstruct long-term history of spruce budworm outbreaks. Can. J. For Res. 32, 428–438. doi: 10.1139/x01-215
Simard M., Romme W. H., Griffin J. M., Turner M. G. (2011). Do mountain pine beetle outbreaks change the probability of active crown fire in lodgepole pine forests? Ecol. Monogr. 81, 3–24. doi: 10.1890/10-1176.1
Smith A. C., Wynn P. M., Barker P. A., Leng M. J., Noble S. R., Tych W. (2016). North Atlantic forcing of moisture delivery to Europe throughout the Holocene. Sci. Rep. 6, 24745. doi: 10.1038/srep24745
Springer G. S., Rowe H. D., Hardt B., Edwards R. L., Cheng H. (2008). Solar forcing of Holocene droughts in a stalagmite record from West Virginia and east-central North America. Geophys Res. Lett. 35, L17703. doi: 10.1029/2008GL034971
Stirnimann L., Conversi A., Marini S. (2019). Detection of regime shifts in the environment: testing ‘STARS’ using synthetic and observed time series. ICES J. Mar. Sci. 76, 2286–2296. doi: 10.1093/icesjms/fsz148
Stocks B. J. (1987). Fire potential in the spruce budworm-damaged forests of Ontario. For Chron 63, 8–14. doi: 10.5558/tfc63008-1
Sturtevant B. R., Miranda B. R., Shinneman D. J., Gustafson E. J., Wolter P. T. (2012). Comparing modern and presettlement forest dynamics of a subboreal wilderness: Does spruce budworm enhance fire risk? Ecol. Appl. 22, 1278–1296. doi: 10.1890/11-0590.1
Swetnam T. W., Allen C. D., Betancourt J. L. (1999). Applied historical ecology: using the past to manage for the future. Ecol. Appl. 9 (4), 1189–1206. doi: 10.1890/1051-0761(1999)009[1189:AHEUTP]2.0.CO;2
Thompson R., Battarbee R. W., O’Sullivan P. E., Oldfield F. (1975). Magnetic susceptibility of lake sediments. Limnol Oceanogr 20, 687–698. doi: 10.4319/lo.1975.20.5.0687
Törnqvist T. E., Hijma M. P. (2012). Links between early Holocene ice-sheet decay, sea-level rise and abrupt climate change. Nat. Geosci 115, 601–606. doi: 10.1038/ngeo1536
van Nes E. H., Rip W. J., Scheffer M. (2007). A theory for cyclic shifts between alternative states in shallow lakes. Ecosystems 10, 17–27. doi: 10.1007/s10021-006-0176-0
Viau A. E., Gajewski K. (2009). Reconstructing millennial-scale, regional paleoclimates of boreal Canada during the Holocene. J. Clim 22, 316–330. doi: 10.1175/2008JCLI2342.1
Viau A. E., Gajewski G., Fines P., Atkinson D. E., Sawada M. C. (2002). Widespread evidence of 1500 yr climate variability in north america during the past 14000 yr. Geology 30 (5), 455–458. doi: 10.1130/0091-7613(2002)030%3C0455:WEOYCV%3E2.0.CO;2
Viau A. E., Gajewski K., Sawada M. C., Fines P. (2006). Millennial-scale temperature variations in North America during the Holocene. J. Geophys Res. 111, D09102. doi: 10.1029/2005JD006031
Viau A. E., Ladd M., Gajewski K. (2012). The climate of North America during the past 2000 years reconstructed from pollen data. Glob Planet Change 84-85, 75–83. doi: 10.1016/j.gloplacha.2011.09.010
Walker M. J. C., Berkelhammer M., Björck S., Cwynar L. C., Fisher D. A., Long A. J., et al. (2012). Formal subdivision of the Holocene series/Epoch: a discussion paper by a working group of INTIMATE (Integration of ice-core, marine and terrestrial records) and the Subcommission on Quaternary Stratigraphy (International Commission on Stratigraphy). J. Quat Sci. 27, 649–659. doi: 10.1002/jqs.2565
Wanner H., Beer J., Bütikofer J., Crowley T. J., Cubasch U., Flückiger J., et al. (2008). Mid to late Holocene climate change: an overview. Quat Sci. Rev. 27, 1791–1828. doi: 10.1016/j.quascirev.2008.06.013
Wanner H., Mercolli L., Grosjean M., Ritz S. P. (2015). Holocene climate variability and change; a data-based review. J. Geol Soc. 172, 254–263. doi: 10.1144/jgs2013-101
Wanner H., Solomina O., Grosjean M., Ritz S. P., Jetel M. (2011). Structure and origin of Holocene cold events. Quat Sci. Rev. 30, 3109–3123. doi: 10.1016/j.quascirev.2011.07.010
Watt G. A., Fleming R. A., Smith S. M., Fortin M.-J. (2018). Spruce budworm (Choristoneura fumiferana Clem.) defoliation promotes vertical fuel continuity in Ontario’s boreal mixedwood forest. Forests 9, 256. doi: 10.3390/f9050256
Watt G. A., Stocks B. J., Fleming R. A., Smith S. M. (2020). Stand breakdown and surface fuel accumulation due to spruce budworm (Choristoneura fumiferana) defoliation in the boreal mixedwood forest of central Canada. Can. J. For Res. 50, 533–541. doi: 10.1139/cjfr-2019-0076
Weed A. S., Ayres M. P., Hicke J. A. (2013). Consequences of climate change for biotic disturbance in North American forests. Ecol. Monogr. 83, 441–470. doi: 10.1890/13-0160.1
Westerling A. L., Gershunov A., Brown T. J., Cayan D. R., Dettinger M. D. (2003). Climate and wildfire in the western United States. Bull. Am. Meteorol Soc. 84, 595–604. doi: 10.1175/BAMS-84-5-595
Westerling A. L., Hidalgo H. G., Cayan D. R., Swetnam T. W. (2006). Warming and earlier spring increase western U.S. forest wildfire activity. Science 313, 940–943. doi: 10.1126/science.1128834
Westerling A. L., Turner M. G., Smithwick E. A. H., Romme W. H., Ryan M. G. (2011). Continued warming could transform Greater Yellow stone fire regimes by mid-21st century. Proc. Natl. Acad. Sci. U.S.A. 108, 13165–13170. doi: 10.1073/pnas.1110199108
Willard D. A., Bernhardt C. E., Korejwo D. A., Meyers S. R. (2005). Impacts of millennial-scale Holocene climate variability on eastern North American terrestrial ecosystems: pollen-based climatic reconstruction. Glob Planet Change 47, 17–35. doi: 10.1016/j.gloplacha.2004.11.017
Woolford D. G., Dean C. B., Martell D. L., Cao J., Wotton B. M. (2014). Lightning-caused forest fire risk in Northwestern Ontario, Canada, is increasing and associated with anomalies in fire weather. Environmetrics 25, 496–416. doi: 10.1002/env.2278
Wotton B. M., Nock C. A., Flannigan M. D. (2010). Forest fire occurrence and climate change in Canada. Intl J. Wildland Fire 19, 253–271. doi: 10.1071/WF09002
Wright H. E., Mann D. H., Glaser P. H. (1984). Piston corers for peat and lake sediments. Ecology 65, 657–659. doi: 10.2307/1941430
Zhang Y., Renssen H., Seppä H. (2016). Effects of melting ice sheets and orbital forcing on the early Holocene warming in the extratropical Northern Hemisphere. Climate Past 12, 1119–1135. doi: 10.5194/cp-12-1119-2016
Keywords: Choristoneura fumiferana, spruce budworm, fire, disturbance interaction, mixed boreal forest, Holocene
Citation: Leclerc M-A, Simard M and Morin H (2025) Holocene reconstruction of the spruce budworm outbreak-fire interaction in the mixed boreal forest reveals a peculiar oscillation. Front. Ecol. Evol. 13:1532974. doi: 10.3389/fevo.2025.1532974
Received: 22 November 2024; Accepted: 24 February 2025;
Published: 13 March 2025.
Edited by:
Anna Maria Mercuri, University of Modena and Reggio Emilia, ItalyReviewed by:
Yawen Ge, Hebei Normal University, ChinaCopyright © 2025 Leclerc, Simard and Morin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc-Antoine Leclerc, bGVjbGVyY21hcmNhbnRvaW5lQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.