- 1Research Center on Animal Cognition (CRCA), Center for Integrative Biology (CBI); CNRS, Toulouse University, Toulouse, France
- 2CEFE, CNRS, Univ Montpellier, EPHE, IRD, Montpellier, France
Accurate prediction of pollination processes is a key challenge for sustainable food production and the conservation of natural ecosystems. For many plants, pollen dispersal is mediated by the foraging movements of nectarivore animals. While most current models of pollination ecology assume random pollen movements, studies in animal behaviour show how pollinating insects, birds and bats rely on sensory cues, learning and memory to visit flowers, thereby producing complex movement patterns. Building upon a brief review of pollination and movement models, we argue that we need to better consider pollinators’ cognition to improve predictions of animal-mediated pollination across all spatial scales, from individual flowers, to plants, habitat patches and landscapes. We propose a practical roadmap for the integration of behavioural models into pollination models and discuss how this synthesis can refine predictions regarding plant mating patterns and fitness. Such crosstalk between animal behaviour and plant ecology research will provide powerful mechanistic tools to predict and act on pollination services in the context of a looming crisis.
1 Introduction
About 70% of flowering plants rely on animal pollination for reproduction (Ollerton et al., 2011). As pollinators are globally declining and human food demand is booming, a better prediction of pollination services becomes necessary for sustainable food security (Aizen et al., 2022). Additionally, the scarcity of wild pollinators puts many natural ecosystems at risk (Potts et al., 2010). These major stakes have given rise to several modelling approaches aiming to predict the abundance of pollinators at large spatial scales – from agricultural landscapes (Lonsdorf et al., 2009) to entire continents (Zulian et al., 2013). These models are now commonly used for research and commercial purposes alike. For example, the InVEST software serves the Natural Capital Project in more than 60 countries to inform decision-makers about sustainable crop management (Natural Capital Project, 2024).
For most plants, pollinators’ movements are central to the process of pollination, since foraging animals passively disperse pollen across plant populations. Behavioural studies show pollinators rely on learning and memory to guide their movement decisions. For instance, many nectarivore bees, butterflies, bats, and birds follow repetitive foraging routes to revisit sets of profitable flower patches on a regular basis (Lihoreau et al., 2012; Gilbert, 1975; Tello-Ramos et al., 2015; MaChado et al., 1998). However, in current pollination models, these complex pollinators’ movements are highly simplified, sometimes in the form of random movements (Kortsch et al., 2023), or replaced by phenomenological rules that derive pollinator presence from environmental constraints such as the distance to nest and quality of food (Olsson et al., 2015). Such a phenomenological approach might hinder the generalisation of model predictions across different spatial scales and ecological settings. Indeed, their predictions tend to be inaccurate when the spatial distribution of resources is not homogeneous (Nicholson et al., 2019). Moreover, these models are not designed to address the complex mating patterns of plant populations, which are essential to determine pollination quality within plant populations (including, for example, self-pollination rates, mating distance and mate diversity; Ohashi and Thomson, 2009). Such fine-scale patterns could be directly derived from pollinators’ movements.
Adding realistic pollinator behaviours into pollination models would thus provide a more robust mechanistic tool for understanding and predicting pollination, both at the plant and landscape scales. This new line of models could be achieved by integrating current pollination models with pollinator movement models (e.g., Reynolds and Rhodes, 2009), which are based on recent advances in animal navigation and cognition research. Even though such an interdisciplinary synthesis between animal behaviour and plant ecology has been identified as one of the main priorities for pollination ecology in the 21st century (Mayer et al., 2011), too few studies have yet attempted to bridge this gap (e.g., Ohashi and Thomson, 2009; Dorin et al., 2022; Kortsch et al., 2023).
In this mini-review, we briefly describe the current types of models used to predict plant pollination and their assumptions on pollinator-mediated pollen dispersal. Next, we provide an overview of the leading models of pollinator movements. Finally, we discuss how integrating both kinds of models can be achieved and may considerably improve our understanding and prediction of plant pollination patterns and fitness.
2 Plant pollination models
Research in plant ecology uses pollen dispersal models to predict plant mating patterns and fitness at different spatial scales, from the plant level to the landscape level. These models typically assume that pollen dispersal is diffusive, i.e. unbiased and unimpacted by local environmental conditions (e.g., Shaw et al., 2006). Different assumptions are used depending on the spatial scale at which pollination is modelled.
At the plant level, some models (Table 1) predict the outcrossing probability of two plants as a function of the distance between them (Simpson et al., 2006). Others use a dispersal kernel – also called “individual dispersion function” (e.g., Lavigne et al., 1998; Klein et al., 2006). In this latter approach, the dispersal kernel of a donor plant is a 2D function mapping the coordinates in space of a receiver plant with the probability of receiving pollen from the donor plant. Most of these statistical models assume that pollen dispersal kernels are more fat-tailed than the normal distribution and similar in all directions of space (i.e. isotropic) (Austerlitz et al., 2004). Kernel-based models rely on a phenomenological approach: the mechanisms behind pollen dispersal, i.e., pollinator movements, are not represented explicitly. Using a dispersal kernel to model pollen dispersal implicitly assumes that pollen trajectories only depend on the characteristics and the distance separating the donor and receiver flowers (Sasal and Morales, 2013). This approximation is thus likely to be unrealistic when pollen is dispersed by animals relying on perception and cognition for spatial navigation and flower choices (e.g. Morán et al., 2023). Accordingly, some studies show that dispersal kernels do not fit well with real large-distance dispersal data, for instance because they underestimate cross-pollination rates at large distances from the donor flower (Devaux et al., 2008).
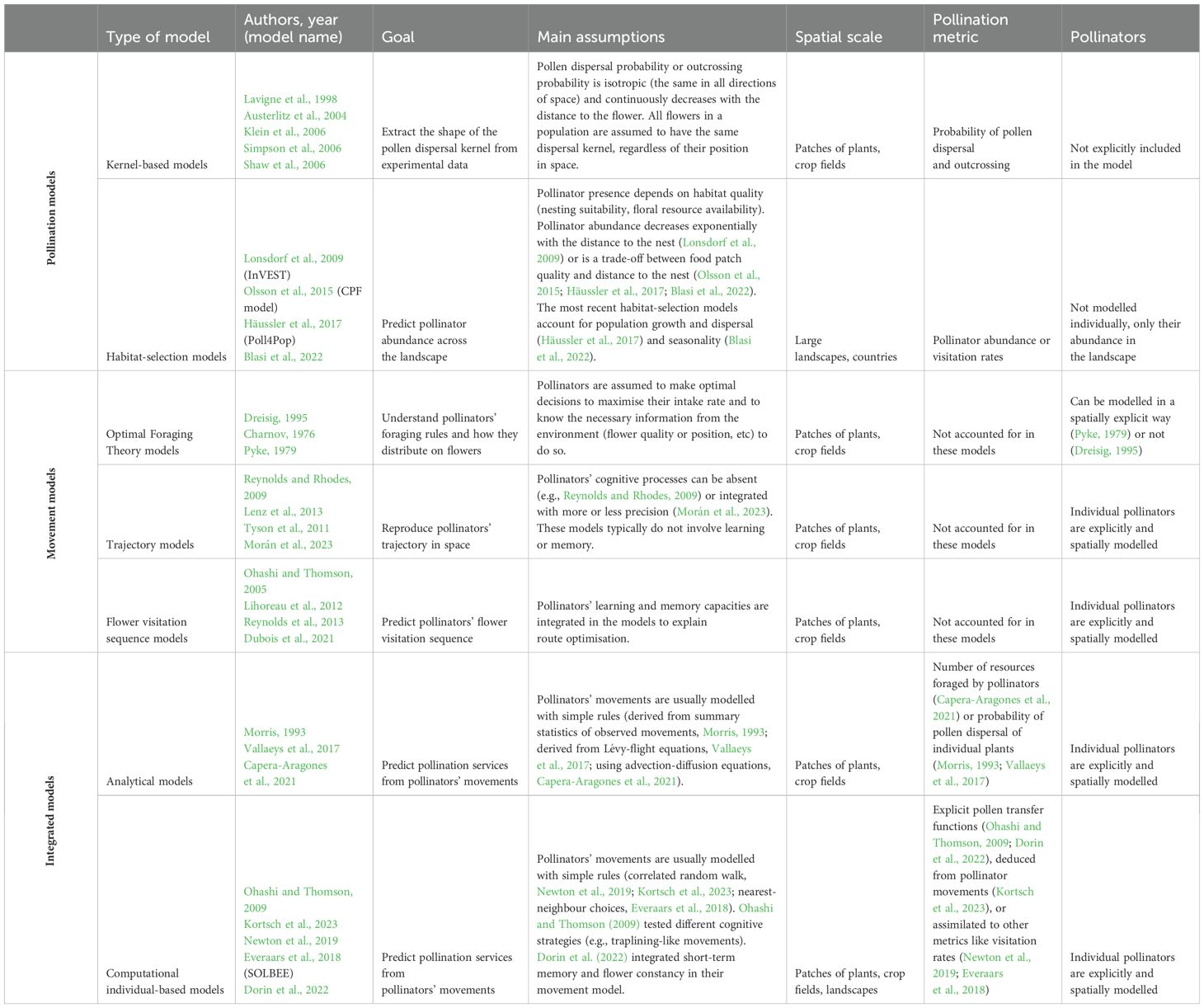
Table 1. Summary of the characteristics of the different types of pollination models, movement models and integrated models, with examples of related publications (authors, year and model name if available).
Other models (Table 1) have been developed to predict pollination at the scale of landscapes (Zhao et al., 2019) or entire countries (Polce et al., 2013). Here, habitat quality (nesting suitability and flower resources) is used to predict the abundance of pollinators in space, a proxy for pollination success and quality. Pollinators’ movements are not modelled explicitly. Instead, visitation rates are typically assumed to decrease similarly in all directions of space and continuously with the distance to the nest in an exponential decay (Lonsdorf et al., 2009, InVEST model). Such approximations implicitly assume that pollinators “diffuse” uniformly around the nest (i.e., isotropically). More behaviourally realistic approaches (Olsson et al., 2015; CPF model) based on optimal foraging theory (Charnov, 1976) assume that pollinator abundance is a trade-off between the distance to the nest and flower patch quality. This more refined assumption better predicts pollinators’ abundance in the landscape (Nicholson et al., 2019), and is now commonly integrated into habitat-selection pollination models (InVEST software; Poll4Pop, Häussler et al., 2017). These tools have recently been adapted to account for population growth and bees’ dispersal (Häussler et al., 2017; Poll4Pop model; Blasi et al., 2022; LandscapePhenoBee). They can also be coupled to species distribution models (SDMs) to estimate pollinators’ spatial distribution from sparse observations (Polce et al., 2013). However, SDMs have been shown to perform poorly in changing conditions (Maguire et al., 2016). Moreover, they do not explicitly model behavioural mechanisms and thus may overlook some key aspects of pollination, such as competition for plant resources (pollen, nectar) and the use of cognition in movement decisions (Pasquaretta et al., 2019). Finally, pollinator abundance may not always be a good predictor of pollination quality (e.g. Ohashi and Thomson, 2009). Factors such as the spatial context of the plant (e.g., density and quality of neighbouring mates; Stehlik et al., 2006), selfing and outcrossing rates (Barrett and Harder, 1996) and mate diversity (Kron and Husband, 2006) can also influence plant reproductive success.
3 Pollinator movement models
Research on animal behaviour uses movement models to study spatial cognition and foraging strategies in nectarivore foragers such as bees, birds and bats. Early models of optimal foraging theory relied on the strong assumption that animals were omniscient about their environment and would exploit resources to maximise net energy intake rate (e.g. Dreisig, 1995; Charnov, 1976; Pyke, 1979). More recently, studies on animal navigation based on new experimental tracking tools such as radars (Riley et al., 1996) and GPS (Goldshtein et al., 2024) have refined our understanding of pollinators’ foraging patterns in the field. Now, different classes of models have emerged that focus on different aspects of pollinator movements.
The first type of models focuses on replicating the flight trajectories of individual foragers (Reynolds and Rhodes, 2009; Lenz et al., 2013; Morán et al., 2023) or of groups (Tyson et al., 2011). These analytical models have been parameterised with the characteristics of real flight trajectories and typically do not integrate cognitive processes. More complex models, such as the one developed by Morán et al. (2023), integrate sensory perception and suggest, for instance, the existence of “masking effects”, by which some plants are visited less than expected from their distance to the nest because foragers are intercepted by a plant lying in between. This effect is also known as the “shadow effect” in the broader ecology literature (Riotte-Lambert and Laroche, 2021). While many of these models explicitly implement aspects of spatial cognition (e.g. path integration), they have overlooked many other aspects of the cognitive abilities used by pollinators to choose the flowers they visit. In particular, when foraging, pollinators learn to localise and recognise flowers and return to familiar locations based on spatial, visual, olfactory and thermal cues (Chittka, 2022). This enables many pollinators to return to the same feeding locations over time, as long as these are not depleted (Ribbands, 1949), sometimes revisiting familiar patches in a repeated order (Janzen, 1971; Thomson et al., 1982; Lihoreau et al., 2012; Buatois and Lihoreau, 2016).
To tackle this problem, a second type of models abstracts from trajectories to focus on the flower visitation sequences resulting from learning and memory. Initially, these models were developed to understand the cognitive mechanisms underlying the formation of repeated foraging routes between flower patches – also known as “traplines” (Thomson et al., 1997). Ohashi and Thomson (2005) modelled different foraging strategies (random, area-restricted search, complete traplining, sample-and-shift traplining) using different probabilities of transitions between flowers. Follow-up models formalised the choice of transitions between flowers through iterative improvement and reinforcement learning, thus adopting a more cognitive approach (Lihoreau et al., 2012; Reynolds et al., 2013; Dubois et al., 2021). So far, these models have been used to simulate only one or very few foragers simultaneously. However, simulating several individuals simultaneously can sometimes give rise to unsuspected patterns (e.g., nonterritorial spatial segregation; Riotte-Lambert et al., 2015; Aarts et al., 2021).
4 Integrating pollinator movement models into plant pollination models
As seen above, models have been developed separately to study pollinators’ movements and plant pollination. As a result, movement models have largely been restricted to the scope of animal behaviour, while pollination models may be imprecise depending on the spatial scale of interest. Here, we argue that the time is ripe for integrating both approaches. Such synthesis will provide more robust mechanistic models of plant reproduction mediated by animals, enabling insightful predictions across a broader range of spatial scales and ecological contexts.
Developing mechanistic models based on a detailed implementation of pollinators’ movements will enable us to cope with the limits of phenomenological approaches as used in current pollination models (Lonsdorf et al., 2009). Although these existing models can predict pollinator abundance in homogeneous landscapes, their predictions are not robust in heterogeneous, complex landscapes (Nicholson et al., 2019). They are also not designed to predict pollination in dynamic environments. As climate change and anthropogenic pressures lead to rapidly changing and ever-more-fragmented environments, mechanistic approaches are more easily generalisable to different contexts as they focus on the processes by which global patterns emerge and not solely on the patterns themselves (Gustafson, 2013; Morin and Lechowicz, 2008).
It is also important to mention that pollinator abundance is often used as a proxy for pollination quality in current models, which is only one part of the full picture. Other factors, such as self-pollination rate, mating distance, and mate diversity, also determine pollination success and quality (Ohashi and Thomson, 2009; Stehlik et al., 2006; Holsinger, 1991). These metrics are defined at the plant level and can be directly derived from predictions of pollinators’ movements. Thus, not only would a mechanistic approach enable the computation of these metrics and the better prediction of pollination quality, but it would also refine our understanding of plant–pollinator interactions. In the long term, we believe such a mechanistic approach can be used to predict pollination processes across a larger range of contexts and, for instance, improve precision pollination in crop fields or greenhouses.
Some studies have begun to bridge this gap. For instance, analytical trajectory models have been used to infer pollen dispersal functions (Morris, 1993; Vallaeys et al., 2017). Pollination services have been inferred from pollinators’ quantity of resources collected (Capera-Aragones et al., 2021). Computational agent-based models have been developed to simulate individual pollinators’ movements and their consequences on pollination. Here again, pollen transfers can be integrated explicitly (Ohashi and Thomson, 2009; Dorin et al., 2022), deduced from pollinator movements (Kortsch et al., 2023), or pollination can be assimilated to other metrics such as visitation rates (Newton et al., 2019; Everaars et al., 2018). While most of these models still rely on relatively simplistic movement rules (e.g. diffusion equations, Morris, 1993; correlated random walk, Newton et al., 2019), some studies also integrated cognitive-based movements: Ohashi and Thomson (2009) suggested that informed movements lead to higher flower mating distances and mate diversity and lower selfing rates than more random pollinator movement. Recently, Dorin et al. (2022) improved upon a long line of agent-based models (Waser, 1978; Dyer et al., 2012; Bukovac et al., 2013; Dyer et al., 2014; Bukovac et al., 2017; Dorin et al., 2018) by integrating mechanisms such as flower constancy (i.e. the tendency of individual pollinators to specialise on one plant species) and short-term memory into a random movement model.
While this is an important first step, real pollinators exhibit much more diverse movement patterns and dynamically modify their foraging sequences as they gain experience (Lihoreau et al., 2012). They continually alternate between route-following and exploration, even when they developed stable and efficient routes, presumably to sample information about potential new profitable resources in their environment (Woodgate et al., 2017). We thus argue that future research should focus on integrating the latest findings in pollinator behaviour into pollination models for more robust predictions. Several existing models could readily be used as a starting point for implementing different modules to be assembled within a common platform (Figure 1). This integrated model could have the following modules:
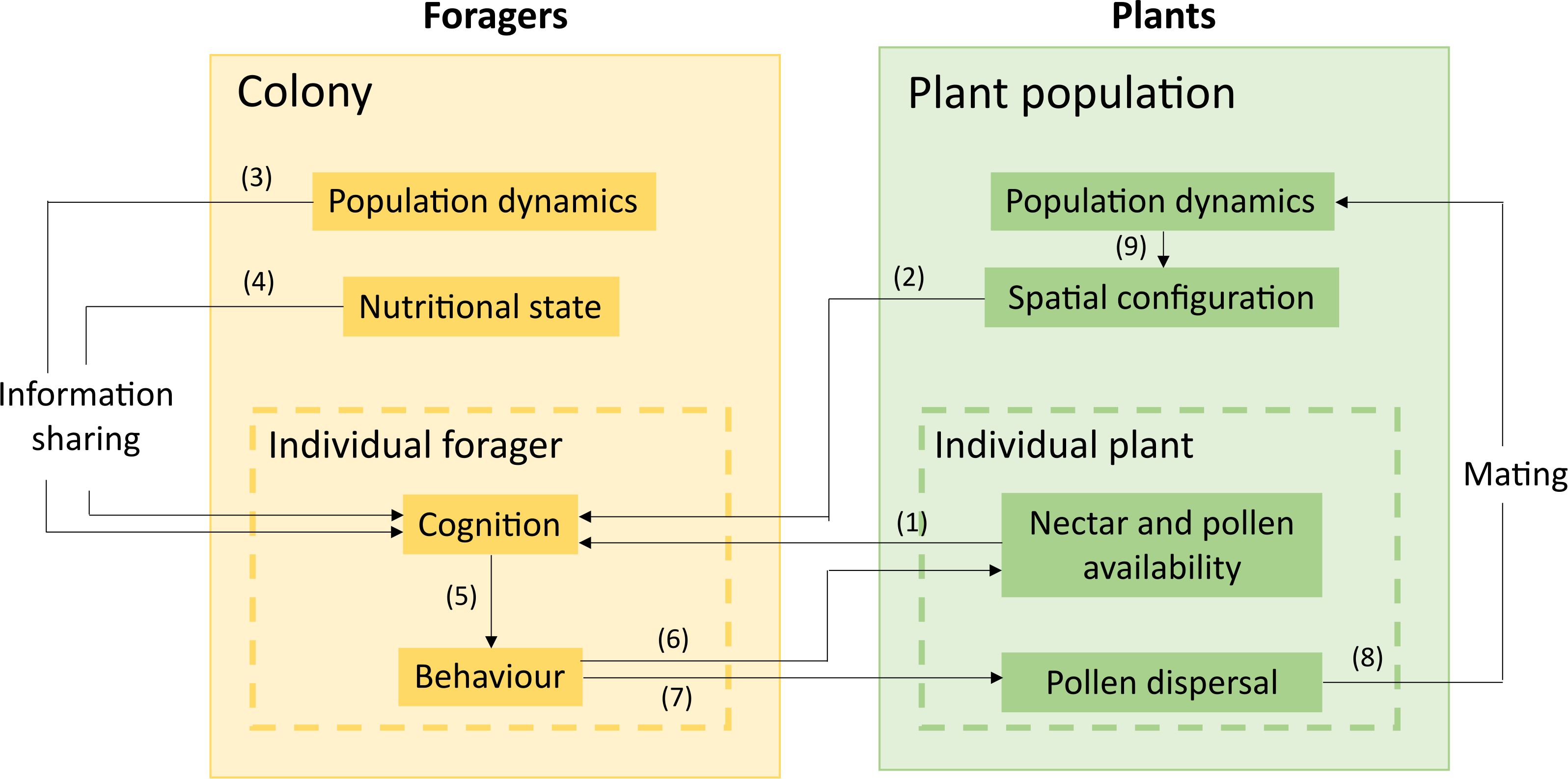
Figure 1. Conceptual diagram of a behaviourally explicit agent-based model for social bees integrating pollinators’ movements and plant reproduction. The simulated environment contains plants whose nectar and pollen resources (1) and coordinates in space (2) influence pollinators’ decisions, learning, memory, perception, etc. The colony’s population dynamics (3) and current nutritional state (4) are also integrated into the cognitive processes of the foragers. These cognitive capacities drive pollinators’ behaviour (5). They move from flower to flower, causing floral resources to deplete (6) and pollen dispersal (7). Pollen dispersal causes plant mating (8), which influences the plant population’s dynamics and, thus, the spatial configuration of the next generation of plants (9).
1. A map of individual plants in space (as in Kortsch et al., 2023) with complementary information about their attractivity for the pollinators (e.g. signalling, nutritional values), self-compatibility, and genetic diversity.
2. A movement module relying on cognitive assumptions, which can be used to predict the flower visitation sequence (as in Dubois et al., 2021). This module would drive the foraging behaviour, which causes nectar and pollen depletion and, therefore, competition between individuals. It can also be refined by considering the variation of quality across nectars and pollen of different plants and the nutritional needs of pollinators, for instance, using models of nutritional ecology (Lihoreau et al., 2015).
3. A colony-level population dynamics module that simulates the colony’s growth and dynamics over time depending on nutrient intake (e.g., Becher et al., 2014). Such a module could be adapted to the pollinator species. It should also be dependent on the nutritional state of the colony. Several colonies of the same or different species could be included to model more complex community-level plant-pollinator interactions (Burkle and Alarcón, 2011).
4. A module of pollen dispersal by the moving pollinators. It should account for pollen carryover (i.e., how much pollen is dragged to the subsequent flowers in the visit sequence; as in Bateman, 1947). This module should also clarify which pollination events will result in a successful reproduction.
Such an integrative model could then be used to measure the fitness of both plants and pollinators. For this, several metrics could be outputted from the model, both at the plant level (quality of pollination visits or pollination success, parenting outcomes, gene flow, etc.) and at the pollinator level (foraging efficiency, the nutritional state of the colony, population growth, etc).
5 Concluding remarks
Recent conceptual advances in animal behaviour and plant ecology enable a theoretical synthesis that could help predict and study patterns of animal-mediated pollination with unprecedented details. Several models have been developed and could readily be integrated into a unifying platform connecting animal behaviour and pollination. Insights from these new kinds of models will help refine expectations of pollinator visit function and pollen dispersal kernels. Ultimately, these outputs could be integrated back into landscape-level models (such as Poll4Pop or InVEST), which are also used to predict population dynamics and dispersal.
We believe that the fine-scale resolution of the model outputs in terms of pollination metrics (crossing patterns, gene flow) has the potential to provide new lines of investigation with highly detailed predictions about the genetic structures and population dynamics of plant populations. Such a modelling approach would thus constitute a unique tool to address key questions in pollination ecology. For instance, pollen dispersal could be studied at the scale of several colonies. Current trending models, such as habitat-scale models (Lonsdorf et al., 2009), do not encompass competition between colonies – more colonies simply result in more pollination. However, competition might act as a regulating mechanism and might put a cap on pollinator abundance in disputed areas. Notably, we might expect resource depletion to promote spatial segregation between colonies (e.g. Aarts et al., 2021 in marine birds and mammals; Morinay et al., 2023 in lesser kestrels). These predictions might help optimise the number and position of commercially introduced pollinator colonies in crops (such as honey bees or bumblebees). Even at small spatial scales, studying pollen dispersal through an integrated model could help understand how individual movements and competitive and social interactions can shape pollen dispersal (Mailly et al., 2024). Both the tools and the new knowledge derived from this integrative approach in pollination ecology thus have the potential to revolutionise our usage of pollinators by providing more accurate predictions to inform actions for precision conservation and crop pollination.
Author contributions
JM: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. LR: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the European Research Council to ML (ERC Cog Bee Move, grant number 101002644).
Acknowledgments
We kindly thank Dr. Fabien Laroche and Dr. Anouk Glad for their article recommendations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarts G., Mul E., Fieberg J., Brasseur S., van Gils J. A., Matthiopoulos J., et al. (2021). Individual-level memory is sufficient to create spatial segregation among neighboring colonies of central place foragers. Am. Nat. 198, E37–E52. doi: 10.1086/715014
Aizen M. A., Garibaldi L. A., Harder L. (2022). Myth and reality of a global crisis for agricultural pollination. Ecol. Austral 32, 698–715. doi: 10.25260/EA.22.32.2.1.1875
Austerlitz F., Dick C. W., Dutech C., Klein E. K., Oddou-Muratorio S., Smouse P. E., et al. (2004). Using genetic markers to estimate the pollen dispersal curve. Mol. Ecol. 13, 937–954. doi: 10.1111/j.1365-294X.2004.02100.x
Barrett S. C. H., Harder L. D. (1996). Ecology and evolution of plant mating. Trends Ecol. Evol. 11, 73–79. doi: 10.1016/0169-5347(96)81046-9
Becher M. A., Grimm V., Thorbek P., Horn J., Kennedy P. J., Osborne J. L. (2014). BEEHAVE: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 51, 470–482. doi: 10.1111/1365-2664.12222
Blasi M., Clough Y., Jönsson A. M., Sahlin U. (2022). A model of wild bee populations accounting for spatial heterogeneity and climate-induced temporal variability of food resources at the landscape level. Ecol. Evol. 12, e9014. doi: 10.1002/ece3.9014
Buatois A., Lihoreau M. (2016). Evidence of trapline foraging in honeybees. J. Exp. Biol. 219, 2426–2429. doi: 10.1242/jeb.143214
Bukovac Z., Dorin A., Dyer A. (2013). “A-bees see: A simulation to assess social bee visual attention during complex search tasks,” in Proceedings of the ECAL 2013: The Twelfth European Conference on Artificial Life. ECAL 2013: The Twelfth European Conference on Artificial Life, September 2–6, 2013. 276–283 (Sicily, Italy: ASME). doi: 10.1162/978-0-262-31709-2-ch042
Bukovac Z., Dorin A., Finke V., Shrestha M., Garcia J., Avarguès-Weber A., et al. (2017). Assessing the ecological significance of bee visual detection and colour discrimination on the evolution of flower colours. Evol. Ecol. 31, 153–172. doi: 10.1007/s10682-016-9843-6
Burkle L. A., Alarcón R. (2011). The future of plant–pollinator diversity: Understanding interaction networks across time, space, and global change. Am. J. Bot. 98, 528–538. doi: 10.3732/ajb.1000391
Capera-Aragones P., Foxall E., Tyson R. C. (2021). Differential equation model for central-place foragers with memory: implications for bumble bee crop pollination. J. Math. Biol. 83, 1–24. doi: 10.1007/s00285-021-01676-1
Charnov E. L. (1976). Optimal foraging, the marginal value theorem. Theor. popul. Biol. 9, 129–136. doi: 10.1016/0040-5809(76)90040-X
Devaux C., Klein E. K., Lavigne C., Sausse C., Messéan A. (2008). Environmental and landscape effects on cross-pollination rates observed at long distance among French oilseed rape Brassica napus commercial fields. J. Appl. Ecol. 45, 803–812. doi: 10.1111/j.1365-2664.2007.01400.x
Dorin A., Dyer A., Taylor T., Bukovac Z. (2018). “Simulation-governed design and tuning of greenhouses for successful bee pollination,” in Proceedings of the ALIFE 2018: The 2018 Conference on Artificial Life. ALIFE 2018: The 2018 Conference on Artificial Life, July 23–27, 2018. 171–178 (Tokyo, Japan: ASME). doi: 10.1162/isal_a_00038
Dorin A., Taylor T., Dyer A. G. (2022). Goldilocks’ quarter-hectare urban farm: An agent-based model for improved pollination of community gardens and small-holder farms. PloS Sustainabil. Transform. 1, 1–23. doi: 10.1371/journal.pstr.0000021
Dreisig H. (1995). Ideal free distributions of nectar foraging bumblebees. Oikos 72, 161–172. doi: 10.2307/3546218
Dubois T., Pasquaretta C., Barron A. B., Gautrais J., Lihoreau M. (2021). A model of resource partitioning between foraging bees based on learning. PloS Comput. Biol. 17, 1–19. doi: 10.1371/journal.pcbi.1009260
Dyer A. G., Dorin A., Reinhardt V., Garcia J. E., Rosa M. G. (2014). Bee reverse-learning behavior and intra-colony differences: simulations based on behavioral experiments reveal benefits of diversity. Ecol. Model. 277, 119–131. doi: 10.1016/j.ecolmodel.2014.01.009
Dyer A., Dorin A., Reinhardt V., Rosa M. (2012). Colour reverse learning and animal personalities: the advantage of behavioural diversity assessed with agent-based simulations. Nat. Preceed. doi: 10.1038/npre.2012.7037.1
Everaars J., Settele J., Dormann C. F. (2018). Fragmentation of nest and foraging habitat affects time budgets of solitary bees, their fitness and pollination services, depending on traits: Results from an individual-based model. PloS One 13, 1–28. doi: 10.1371/journal.pone.0188269
Gilbert L. E. (1975). “Ecological consequences of a coevolved mutualism between butterflies and plants,” in Coevolution of animals and plants. Eds. Gilbert L. E., Raven P. H. (University of Texas Press, New(York), 210–240. doi: 10.7560/710313-011
Goldshtein A., Chen X., Amichai E., Boonman A., Harten L., Yinon O., et al. (2024). Acoustic cognitive map–based navigation in echolocating bats. Science 386, 561–567. doi: 10.1126/science.adn6269
Gustafson E. J. (2013). When relationships estimated in the past cannot be used to predict the future: using mechanistic models to predict landscape ecological dynamics in a changing world. Landscape Ecol. 28, 1429–1437. doi: 10.1007/s10980-013-9927-4
Häussler J., Sahlin U., Baey C., Smith H. G., Clough Y. (2017). Pollinator population size and pollination ecosystem service responses to enhancing floral and nesting resources. Ecol. Evol. 7, 1898–1908. doi: 10.1002/ece3.2765
Holsinger K. E. (1991). Mass-action models of plant mating systems: the evolutionary stability of mixed mating systems. Am. Nat. 138, 606–622. doi: 10.1086/285237
Janzen D. H. (1971). Euglossine bees as long-distance pollinators of tropical plants. Science 171, 203–205. doi: 10.1126/science.171.3967.203
Klein E. K., Lavigne C., Picault H., Renard M., Gouyon P.-H. (2006). Pollen dispersal of oilseed rape: estimation of the dispersal function and effects of field dimension. J. Appl. Ecol. 43, 141–151. doi: 10.1111/j.1365-2664.2005.01108.x
Kortsch S., Saravia L., Cirtwill A. R., Timberlake T., Memmott J., Kendall L., et al. (2023). Landscape composition and pollinator traits interact to influence pollination success in an individual-based model. Funct. Ecol. 37, 2056–2071. doi: 10.1111/1365-2435.14353
Kron P., Husband B. C. (2006). The effects of pollen diversity on plant reproduction: Insights from apple. Sex. Plant Reprod. 19, 125–131. doi: 10.1007/s00497-006-0028-2
Lavigne C., Klein E. K., Vallée P., Pierre J., Godelle B., Renard M. (1998). A pollen-dispersal experiment with transgenic oilseed rape. Estimation of the average pollen dispersal of an individual plant within a field. Theor. Appl. Genet. 96, 886–896. doi: 10.1007/s001220050816
Lenz F., Chechkin A. V., Klages R. (2013). Constructing a stochastic model of bumblebee flights from experimental data. PloS One 8, 1–7. doi: 10.1371/journal.pone.0059036
Lihoreau M., Buhl C., Charleston M. A., Sword G. A., Raubenheimer D., Simpson S. J. (2015). Nutritional ecology beyond the individual: a conceptual framework for integrating nutrition and social interactions. Ecol. Lett. 18, 273–286. doi: 10.1111/ele.12406
Lihoreau M., Raine N. E., Reynolds A. M., Stelzer R. J., Lim K. S., Smith A. D., et al. (2012). Radar tracking and motion-sensitive cameras on flowers reveal the development of pollinator multi-destination routes over large spatial scales. PloS Biol. 10, 1–13. doi: 10.1371/journal.pbio.1001392
Lonsdorf E., Kremen C., Ricketts T., Winfree R., Williams N., Greenleaf S. (2009). Modelling pollination services across agricultural landscapes. Ann. Bot. 103, 1589–1600. doi: 10.1093/aob/mcp069
MaChado I. C. S., Sazima I., Sazima M. (1998). Bat pollination of the terrestrial herb Irlbachia alata (Gentianaceae) in northeastern Brazil. Plant System. Evol. 209, 231–237. doi: 10.1007/BF00985230
Maguire K., Nieto-Lugilde D., Blois J., Fitzpatrick M., Williams J., Ferrier S., et al. (2016). Controlled comparison of species- and community-level models across novel climates and communities. Proc. R. Soc. B: Biol. Sci. 283, 20152817. doi: 10.1098/rspb.2015.2817
Mailly J., Brebner J., Doussot C., Riotte-Lambert L., Lihoreau M. (2024). Modelling bee movements to improve pollination. The Project Repository J. 19 (1), 24–27. doi: 10.54050/PRJ1921089
Mayer C., Adler L., Armbruster W. S., Dafni A., Eardley C., Huang S.-Q., et al. (2011). Pollination ecology in the 21st century: key questions for future research. J. Pollin. Ecol. 3, 8–23. doi: 10.26786/1920-7603(2011)1
Morán A., Lihoreau M., Pérez-Escudero A., Gautrais J. (2023). Modeling bee movement shows how a perceptual masking effect can influence flower discovery. PloS Comput. Biol. 19, 1–21. doi: 10.1371/journal.pcbi.1010558
Morin X., Lechowicz M. J. (2008). Contemporary perspectives on the niche that can improve models of species range shifts under climate change. Biol. Lett. 4, 573–576. doi: 10.1098/rsbl.2008.0181
Morinay J., Riotte-Lambert L., Aarts G., De Pascalis F., Imperio S., Morganti M., et al. (2023). Within-colony segregation of foraging areas: from patterns to processes. Oikos 8, e09926. doi: 10.1111/oik.09926
Morris W. F. (1993). Predicting the consequence of plant spacing and biased movement for pollen dispersal by honey bees. Ecology 74, 493–500. doi: 10.2307/1939310
Natural Capital Project (2024). InVEST 0.0 (Stanford University, University of Minnesota, Chinese Academy of Sciences, The Nature Conservancy, World Wildlife Fund, Stockholm Resilience Centre and the Royal Swedish Academy of Sciences). Available online at: https://naturalcapitalproject.stanford.edu/software/invest (Accessed September 14, 2024).
Newton A. C., Boscolo D., Ferreira P. A., Lopes L. E., Evans P. (2019). Impacts of deforestation on plant-pollinator networks assessed using an agent based model. PloS One 13, 1–17. doi: 10.1371/journal.pone.0209406
Nicholson C. C., Ricketts T. H., Koh I., Smith H. G., Lonsdorf E. V., Olsson O. (2019). Flowering resources distract pollinators from crops: Model predictions from landscape simulations. J. Appl. Ecol. 56, 618–628. doi: 10.1111/1365-2664.13333
Ohashi K., Thomson J. D. (2005). Efficient harvesting of renewing resources. Behav. Ecol. 16, 592–605. doi: 10.1093/beheco/ari031
Ohashi K., Thomson J. D. (2009). Trapline foraging by pollinators: its ontogeny, economics and possible consequences for plants. Ann. Bot. 103, 1365–1378. doi: 10.1093/aob/mcp088
Ollerton J., Winfree R., Tarrant S. (2011). How many flowering plants are pollinated by animals? Oikos 120, 321–326. doi: 10.1111/j.1600-0706.2010.18644.x
Olsson O., Bolin A., Smith H. G., Lonsdorf E. V. (2015). Modeling pollinating bee visitation rates in heterogeneous landscapes from foraging theory. Ecol. Model. 316, 133–143. doi: 10.1016/j.ecolmodel.2015.08.009
Pasquaretta C., Jeanson R., Pansanel J., Raine N. E., Chittka L., Lihoreau M. (2019). A spatial network analysis of resource partitioning between bumblebees foraging on artificial flowers in a flight cage. Move. Ecol. 7, 1–10. doi: 10.1186/s40462-019-0150-z
Polce C., Termansen M., Aguirre-Gutiérrez J., Boatman N. D., Budge G. E., Crowe A., et al. (2013). Species distribution models for crop pollination: A modelling framework applied to Great Britain. PloS One 8, 1–12. doi: 10.1371/journal.pone.0076308
Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Pyke G. H. (1979). Optimal foraging in bumblebees: rule of movement between flowers within inflorescences. Anim. Behav. 27, 1167–1181. doi: 10.1016/0003-3472(79)90064-2
Reynolds A. M., Lihoreau M., Chittka L. (2013). A simple iterative model accurately captures complex trapline formation by bumblebees across spatial scales and flower arrangements. PloS Comput. Biol. 9, 1–10. doi: 10.1371/journal.pcbi.1002938
Reynolds A. M., Rhodes C. J. (2009). The Lévy flight paradigm: random search patterns and mechanisms. Ecology 90, 877–887. doi: 10.1890/08-0153.1
Ribbands C. R. (1949). The foraging method of individual honey-bees. J. Anim. Ecol. 18, 47–66. doi: 10.2307/1581
Riley J. R., Smith A. D., Reynolds D. R., Edwards A. S., Osborne J. L., Williams I. H., et al. (1996). Tracking bees with harmonic radar. Nature 379, 29–30. doi: 10.1038/379029b0
Riotte-Lambert L., Benhamou S., Chamaillé-Jammes S. (2015). How memory-based movement leads to nonterritorial spatial segregation. Am. Nat. 185, E103–E116. doi: 10.1086/680009
Riotte-Lambert L., Laroche F. (2021). Dispersers’ habitat detection and settling abilities modulate the effect of habitat amount on metapopulation resilience. Landscape Ecol. 36, 675–684. doi: 10.1007/s10980-021-01197-8
Sasal Y., Morales J. M. (2013). Linking frugivore behaviour to plant population dynamics. Oikos 122, 95–103. doi: 10.1111/j.1600-0706.2012.20669.x
Shaw M. W., Harwood T. D., Wilkinson M. J., Elliott L. (2006). Assembling spatially explicit landscape models of pollen and spore dispersal by wind for risk assessment. Proc. R. Soc. B: Biol. Sci. 273, 1705–1713. doi: 10.1098/rspb.2006.3491
Simpson E., McRoberts N., Sweet J. (2006). Out-crossing between genetically modified herbicide-tolerant and other winter oilseed rape cultivars. Plant Genet. Resour. 4, 96–107. doi: 10.1079/PGR2005103
Stehlik I., Caspersen J. P., Barrett S. C. H. (2006). Spatial ecology of mating success in a sexually polymorphic plant. Proc. R. Soc. B: Biol. Sci. 273, 387–394. doi: 10.1098/rspb.2005.3317
Tello-Ramos M. C., Hurly T. A., Healy S. D. (2015). Traplining in hummingbirds: flying short-distance sequences among several locations. Behav. Ecol. 26, 812–819. doi: 10.1093/beheco/arv014
Thomson J. D., Maddison W. P., Plowright R. C. (1982). Behavior of bumble bee pollinators of Aralia hispida Vent.(Araliaceae). Oecologia 54, 326–336. doi: 10.1007/BF00380001
Thomson J. D., Slatkin M., Thomson B. A. (1997). Trapline foraging by bumble bees: II. Definition and detection from sequence data. Behav Ecol. 8, 199–210. doi: 10.1093/beheco/8.2.199
Tyson R. C., Wilson J. B., Lane W. D. (2011). Beyond diffusion: Modelling local and long-distance dispersal for organisms exhibiting intensive and extensive search modes. Theor. Popul. Biol. 79, 70–81. doi: 10.1016/j.tpb.2010.11.002
Vallaeys V., Tyson R. C., Lane W. D., Deleersnijder E., Hanert E. (2017). A Lévy-flight diffusion model to predict transgenic pollen dispersal. J. R. Soc. Interface 14, 20160889. doi: 10.1098/rsif.2016.0889
Waser N. M. (1978). Interspecific pollen transfer and competition between co-occurring plant species. Oecologia 36, 223–236. doi: 10.1007/BF00349811
Woodgate J. L., Makinson J. C., Lim K. S., Reynolds A. M., Chittka L. (2017). Continuous radar tracking illustrates the development of multi-destination routes of bumblebees. Sci. Rep. 7, 17323. doi: 10.1038/s41598-017-17553-1
Zhao C., Sander H. A., Hendrix S. D. (2019). Wild bees and urban agriculture: assessing pollinator supply and demand across urban landscapes. Urban Ecosyst. 22, 455–470. doi: 10.1007/s11252-019-0826-6
Keywords: agent-based models, pollination ecology, foraging patterns, pollen dispersal, pollination models
Citation: Mailly J, Riotte-Lambert L and Lihoreau M (2025) Integrating pollinators’ movements into pollination models. Front. Ecol. Evol. 13:1504480. doi: 10.3389/fevo.2025.1504480
Received: 30 September 2024; Accepted: 28 January 2025;
Published: 14 February 2025.
Edited by:
Alan Dorin, Monash University, AustraliaReviewed by:
Thilo Gross, Helmholtz Institute for Functional Marine Biodiversity (HIFMB), GermanyTim Taylor, University of London, United Kingdom
Copyright © 2025 Mailly, Riotte-Lambert and Lihoreau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juliane Mailly, anVsaWFuZS5tYWlsbHlAdW5pdi10bHNlMy5mcg==
†These authors have contributed equally to this work and share last authorship
 Juliane Mailly
Juliane Mailly Louise Riotte-Lambert
Louise Riotte-Lambert Mathieu Lihoreau
Mathieu Lihoreau