- 1Key Laboratory of Wetland Ecology and Environment, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun, China
- 2Jilin Provincial Engineering Center of CWs Design in Cold Region and Beautiful Country Construction, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun, China
- 3The School of Sciences, Qiqihar University, Qiqihar, China
There are a considerable number of discussions aimed at analyzing microbial communities and their functions during the composting process. However, microbial succession under copper (Cu) and tetracycline (TCH)-stressed conditions has received less attention. Thus, this work analyzed the bacterial and fungal structures with high-throughput sequencing in Cu/TCH-amended composting (Cu: 0, 100, and 500 mg/kg; TCH: 0, 50, and 300 mg/kg), and the dominating controls on microbial diversity were identified using redundancy analysis (RDA) and structural equation models (SEMs). Low-concentration Cu increased the peak temperature (57°C) at the thermophilic phase. Composting phase-derived changes in bacterial and fungal communities were significant, while Cu and TCH showed a remarkable influence on fungi but not on bacteria. Cu and TCH inhibited Firmicutes' activity while promoting Actinobacteriota growth. Low-concentration Cu and TCH had a negative effect on Basidiomycota in the thermophilic phase and a positive influence on Chytridiomycota in the mature phase. TOC and TN were primary controls on the changes in microbial communities. NH-N and NO-N were more beneficial to fungi with a contribution proportion of 42.13 and 16.85%, respectively. These findings could provide theoretical guidance for the directional research on microbial inoculants.
Introduction
Aerobic composting, a microorganism-mediated fermentation technology (Sánchez et al., 2017), was considered a well-developed method to realize the resource utilization associated with agricultural-aquaculture wastes (Biyada et al., 2022), in which organic matter (OM) is decomposed and causes a thermophilic temperature, resulting in well-stabilized and value-added end-products (Deng et al., 2020). Both heavy metals and antibiotics, as non-degradable and sub-metabolic pollutants, respectively, have been introduced into animal feeds to present epidemic diseases and promote animal growth (Cheng et al., 2019; Khadra et al., 2019), 30%−90% of which are excreted in parent compounds or primary metabolites, leading to an inhibition effect on composting process and quality (Deng et al., 2020). For example, Hao et al. (2019) indicated that dissolved organic carbon (DOC) was positively correlated with Cu bound to carbonates and metallic hydroxides. Rensing and Grass (2003) demonstrated that copper (Cu) competed with other ions for adsorption sites, destroying protease activity binding to the corresponding metal cofactors, and thus inhibiting enzymatic reactions. Besides, Cheng et al. (2019) reported that antibiotics (tetracycline, quinolones, and sulfonamides) can restrain OM oxidative decomposition within manure–straw composting. Yang et al. (2013) and Chen et al. (2015) found that the temperature was lower in the antibiotic-amended composting than in the control, and this prolonged the time to research standard sterilization. Furthermore, additives, such as biochar, zeolite, medical stone, and phosphate rock, have been used as bulking agents to passivate heavy metals and degrade antibiotics (Cui et al., 2016; Peng et al., 2018; Akdeniz, 2019) in order to make composting more effective. Composting essence is a microorganism-controlled OM stabilization process in which OM decomposition leads to significant changes in the temperature of composting piles, as well as in the acid–base property (pH), electrical conductivity (EC), and other physico-chemical indicators of composting materials, which in turn determine the influence on the transformation among phosphorus fractions under heavy metal and antibiotic-stressed conditions. Therefore, the above-mentioned parameters in the composting system were considered microorganism-controlled processes, and further efforts into microbial succession potentially enhance the understanding of the composting essence.
Diverse research efforts, aimed at ascertaining microbial communities and functions within the composting systems, have been reported. Firmicutes, a heat-resistant bacterial phylum, often exhibits a relative abundance at different composting stages (Liu et al., 2020; Biyada et al., 2021). Bacteroides shows the capacity to produce an enterotoxin, which could cause watery diarrhea disease in animal and children (Deng et al., 2020; Kheyrodin et al., 2022). Ascomycota and Basidiomycota, as two rich fungal phyla, could tolerate an ill-being environment (i.e., thermal temperature and extreme acid–base property) (Liu et al., 2021; Alavi et al., 2022). Both Neocallimastigomycota and Chytridiomycota were anaerobic fungi, which were only found in the early composting phase (Wang et al., 2018). However, enough information is not available concerning microbial succession: emphasizing bacterial and fungal structures under Cu/Tetracycline (TCH)-stressed conditions. As this report proposed, heavy metals are indispensable micro-elements in microbial metabolism, which can participate in redox reactions through valence conversion, and act as acceptors and donors in the electron transport chain (Yethon et al., 2000). When heavy metals exceed a certain threshold, reactive oxygen species (ROS) will be produced and generate macro-molecules excessive oxidation (Rensing and Grass, 2003). Antibiotics with supernal doses are summarized as following toxic mechanisms: (1) Antibiotics could inhibit protein synthesis and cause bacterial cell metabolism disorders; (2) Antibiotics target bacterial deoxyribonucleotide and restrain their multiplication; (3) Antibiotics inactivate penicillin-binding protein (PBP1b), resulting in microbial expansion and rupture under low-osmotic pressure (Chen et al., 2021b). Taken together, a key research question is: How do heavy metals and antibiotics affect composting process and quality through regulating microbial structures?
To address these research questions, we hypothesized that heavy metals and antibiotics will change the microbial structures and functions in the aerobic composting systems. Thus, this study was conducted (1) to ascertain the Cu/TCH-stressed succession in bacterial and fungal structures with high-throughput sequencing and (2) to identify the dominating controls on microbial alpha-diversity with redundancy analysis (RDA) and structure equation models (SEMs). These results could provide guidance on the biotechnology to improve composting quality under Cu/TCH-stressed conditions.
Materials and methods
Composting materials and procedure
Swine manure (SM), collected from an industrial-scale breeding base (Changchun, Jilin, China), was chosen as typical livestock manure. Maize straw (MS), obtained from a comprehensive agricultural experiment station (Changchun, Jilin, China), was air-dried and crushed into 1–2-cm pieces and served as a bulking agent to optimize composting conditions. Further information on SM and MS is shown in Table 1. Copper (II) sulfate pentahydrate (CuSO4·5H2O, AR), the most abundant and bio-toxic heavy metal in livestock manure, was purchased from ST. Raman Experimental Mall. Tetracycline hydrochloride (TCH, ≥98%) was achieved from the Layn. Natural Ingredients (Guilin, Guangxi, China).
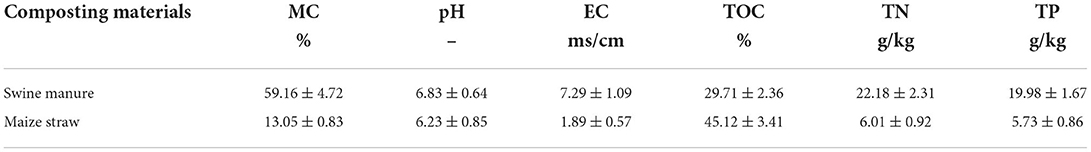
Table 1. The physical and chemical parameters of swine manure and maize straw (dry weight basis, n = 3).
SM and MS were mixed well together (v:v, SM:MS = 1:3; Moisture content, MC ≈ 60%). Given the differences in the Cu and TCH content in livestock manure. Four treatments were amended with 100 mg/kg Cu and 50 mg/kg TCH, 100 mg/kg Cu and 300 mg/kg TCH, 500 mg/kg Cu and 50 mg/kg TCH, and 500 mg/kg Cu and 300 mg/kg TCH, respectively. The control treatment was designed to be 0 mg/kg Cu and TCH. 100-L PVC reactors were used for aerobic fermentation. In the first 10 days, composting mixtures were overturned at a frequency of 2 days and then 5 days apart. The composting temperature was measured using a probe thermometer. Until the composting temperature was close to the surrounding environment, the composting mixtures were considered mature end-products. On days 0, 2, 7, 14, 21, 35, and 52, two samples were collected from the top, middle, and bottom layers; one was refrigerated at an ultra-low temperature (−20°C) until microbial analysis was carried out, and the other was air-dried and sieved (0.15 mm) before physico-chemical analysis.
Microbial high-throughput sequencing
Total microbial DNA was extracted using the FastDNA® SPIN Kit (MP, Biomedicals, Santa, Ana, CA). Bacterial 16S and fungal ITS rRNA gene sequencing were performed at NovoMagic with the Illumina HiSeq platform. The bacterial 16S-(V3 + V4) region was amplified with the primers 341F (CCTAYGGGRBGCASCAG) and 806R (GGACTACNNGGGTATCTAAT). Fungal ITS1-1F was amplified with the primers ITS1-1F-F (CTTGGTCATTTAGAGGAAGTAA) and ITS1-1F-R (GCTGCGTTCTTCATCGATGC). Representative bar-coded PCR products were normalized in equimolar amounts, purified, and sequenced with the HiSeq 2500 (PE 250) following the manufacturer's protocols. Operational taxonomic units (OTUs) were defined at a 97% similarity level.
Physical and chemical analysis
Moisture content was determined according to Chen et al. (2021b). pH and EC were determined as described in Cao et al. (2020). NH-N and NO-N were extracted following Liu et al. (2019). Total organic carbon (TOC) and total nitrogen (TN) were characterized with a Shimadzu TOC-TN analyzer (Shimadzu, Corp, Kyoto, Japan).
Statistical analysis
Data calculation was conducted in Microsoft Excel Ver. 2010. Graphical figures were drawn in Origin Ver. 2017. Microbial analysis was performed using the NovoMagic after-sales platform. RDA used Canoco Ver. 5.0. SEM analysis was carried out in AMOS Ver. 20.0. Statistical significance refers to P < 0.05.
Results and discussion
Physical and chemical indicators
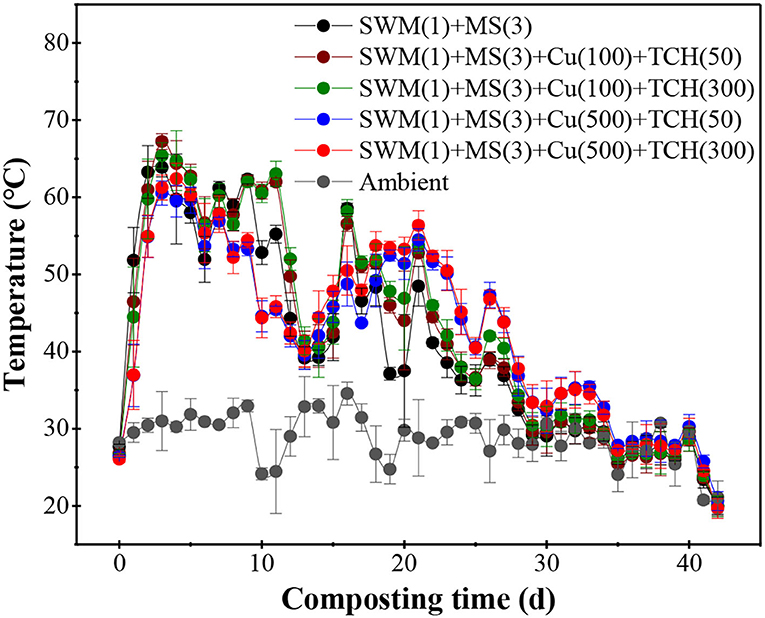
Temperature, an important factor controlling microbial activity, can reflect the micro-environment in the composting system (Wei et al., 2018). The composting process was characterized by four stages (Figure 1). (1) First is the temperature-heating phase (<50°C, days 0–1), during which mesophilic microorganisms utilized bio-degradable OM to generate exothermic reactions (Sánchez et al., 2017), which is similar to present results that show composting temperature sharply increased in treatment without Cu and TCH, followed by Cu-amended treatments with fewer doses and finally Cu-amended treatments with supernal doses. However, an obvious difference in the temperature among TCH-amended treatments with various doses was not found. (2) Second is the thermophilic phase (>50°C, days 2–11), which is considered an effective stage for sterilization and disinfection (Jiang et al., 2019); it causes thermophilic microorganisms to become more competitive. The composting temperature reached above 50°C on day 2 in all treatments and was maintained for 10 days with a maximum of 63.88 ± 1.21, 67.23 ± 1.02, 65.40 ± 1.68, 60.55 ± 1.67, and 61.28 ± 1.62°C on day 3, respectively. This result indicates that composting satisfied standard sterilization, which reported that the thermophilic temperature must be maintained at 50–55°C for 5–7 days (Liu et al., 2017). Compared to treatment without Cu and TCH, the composting temperature was higher in Cu-amended treatments with fewer doses, but the opposite effect was found in Cu-amended treatments with supernal doses. However, composting temperature showed a distinctive difference in TCH-amended treatments. Together, low-concentration Cu could promote composting process and increase the composting temperature at the thermophilic phase, while high-concentration Cu inhibited this. These results occur in accordance with Hu et al. (2011) and Shehata et al. (2021), who reported that Cu-amended treatment made composting less effective, and tetracycline showed insignificant influence on the changes in temperature within the manure–straw co-composting. (3) Third is the temperature-cooling phase (40–50°C, days 12–24) in which the composting temperature is reduced to below 50°C, during which organic carbon contains only macro-molecular OM and humic substances (Ezzariai et al., 2018). As a result, microorganisms lack the carbon sources to sustain metabolic activities. (4) Fourth is the mature phase (<40°C, days 25–42) in which the composting temperature decreases to match the surrounding environment.
Next to temperature, the pH level also determines microbial structures and functions, and a pH between 7 and 8 is optimal in the composting system (Chen et al., 2021a). During the temperature-heating and thermophilic phases, the pH level quickly increased from 6.15 ± 0.51 to 6.66 ± 0.05 on day 0 to 8.66 ± 0.08 to 8.94 ± 0.03 on day 7, whereafter it remained steady with a negligible fluctuation (7.76 ± 0.03 to 8.78 ± 0.04) in all treatments (Figure 2A). An increase in pH level could be ascribed to the ammonium accumulation during the nitrogen-containing OM mineralization process (Li et al., 2020), and the evaporation associated with organic acids owing to elevated-temperature at the thermophilic phase (Wei et al., 2016). EC, a factor indicating the salinity degree in the composting system, reflects the possible phytotoxic-inhibitory effect on seed growth (Wang et al., 2016). On the contrary, EC values sharply decreased and reached a minimum on day 7 before fluctuating from 5.98 ± 0.10 to 7.83 ± 0.43 ms/cm in all treatments (Figure 2B). A reasonable explanation could be that complex OM was decomposed into water-soluble components, then micromolecular organic acids were converted as macromolecular humic substances (Wang et al., 2016). MC content influences microbial metabolism, and ventilation, and helps to maintain the thermophilic phase (Chen et al., 2021a), which decreased first and then increased within 0–21 days, and reached the minimum on day 14 (Figure 2C). MC content in Cu-amended treatments with supernal doses was lower than that in Cu-amended treatments with fewer doses, while a significant difference was not found in TCH-amended treatments, as it may contribute to the thermal temperature in treatments with low-concentration Cu.
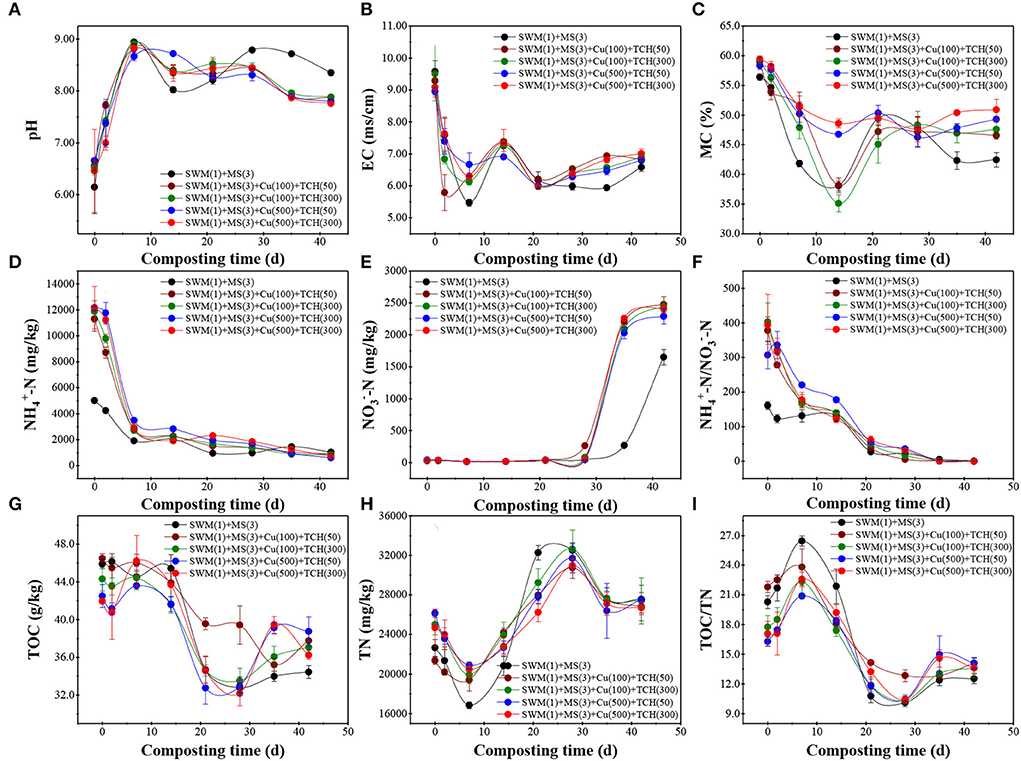
Figure 2. (A–I) Dynamic changes in the physical and chemical parameters during the composting process.
Both carbon and nitrogen, which served as energy sources and essential nutrients, show an important impact on microbial metabolism and composting quality (Yu et al., 2019). During the thermophilic phase, TOC rapidly declined until a moderate tendency appeared on day 21, and this was higher in Cu/TCH-amended treatments. From the short review above, key findings emerge: Cu and TCH inhibited OM decomposition during the composting process. During the temperature-cooling phase, TOC content was higher in low-concentration Cu/TCH-amended treatment than that in other treatments (Figure 2G). This supports the conclusion of Chen et al. (2021a), who reported that Cu and TCH could promote microbial metabolic activity, thus accelerating the OM decomposition, and this phenomenon was more obvious at high-concentration Cu/TCH-amended treatments than that in low-concentration Cu/TCH-amended treatments in selected Cu/TCH concentration range. TN content also dropped within 0–7 days (Figure 2H), which is in accordance with the findings of Liu et al. (2019), who reported that nitrogen-containing OM mineralization causes an increase in NH-N, thus resulting in NH3 volatilization at thermal temperature and high pH, in agreement with that NH-N sharply decreased to a stable level on day 7 in this study (Figure 2D). Subsequently, TN quickly increased during the temperature-cooling and mature phases, which was because ammonifying and denitrifying microorganisms transformed NH-N into microbial substances (proteins, amino acids, etc.) and NO-N, respectively. The above-mentioned conclusion also verified the present finding that NO-N sharply increased duiring temperature-cooling and mature phases (Figure 2E). Within 0–28 composting days, TOC/TN showed a unimodal trend and peaked on day 7 (Figure 2I), while NH-N/NO-N keep going down (Figure 2F). Afterward, TOC/TN and NH-N/NO-N were stable between 12.51 ± 0.49 and 14.10 ± 0.55, as well as 0.26 ± 0.03 and 0.35 ± 0.04, respectively. Together, the present results confirmed that the composting end-products were well-stabilized and eco-friendly for agricultural development. TOC/TN < 15 and NH-N/NON < 0.5 indicate mature compost (Awasthi et al., 2018).
Microbial communities
Bacterial (Figure 3A) and fungal (Figure 3B) successions and their composting time and selective pressure (Cu and TCH) were identified. In the initial mixtures (Day 0), bacterial communities showed significant differences among all treatments, while fungal communities presented high-degree uniformity. A reason for this could be the special character of bacteria between SM and MS, which were mixed with various volume ratios. During the composting process, bacteria were concentrated at a distance among all treatments and were distributed discretely with composting time, while fungi were always alienated from each other with diverse treatments and composting time. These results indicate that composting phase-derived changes in bacterial and fungal communities were significant, while Cu and TCH showed a remarkable influence on fungi but not on bacteria. Cao et al. (2020) also reported that bacterial communities obviously changed with composting time, and bacteria can acquire resistance to antibiotics via horizontal gene transformation (Ezzariai et al., 2018).
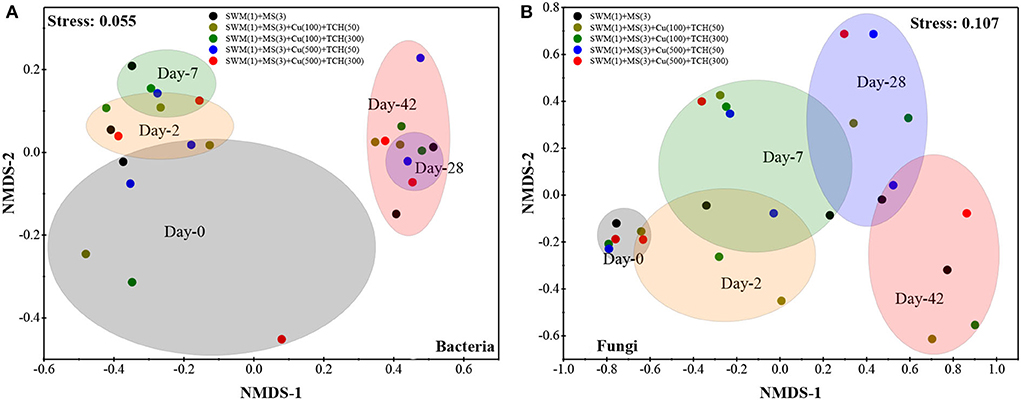
Figure 3. Non-metric multidimensional scaling (NMDS) analysis in bacterial (A) and fungal (B) communities based on OUTs during the composting process.
Alpha indexes and microbial richness were estimated with Chao1 and ace indexes, while the Shannon and Simpson's indexes were used to calculate the microbial diversity (Chen et al., 2021a). Within 0–7 composting days, Chao1 and ace indexes in bacterial (Figure 4A) and fungal (Figure 4B) communities increased in TCH-amended treatments with fewer doses but decreased in TCH-amended treatments with supernal doses; thereafter two indexes increased in all treatments. For bacterial communities, the Shannon and Simpson indexes first increased and then decreased and reached the minimum on day 7 during all treatments, while two indexes in fungal communities remained steady until a decrease occurred on day 28. These results indicate that (1) low-concentration TCH enhanced bacterial and fungal richness at the thermophilic phase, while high-concentration TCH reduced it; (2) the temperature-cooling and mature phases were more conducive to microbial growth; (3) thermal temperature presented a insignificant inhibitory influence on fungal diversity but not bacteria. These conclusions were consistent with previous findings that iproved that low-concentration antibiotics promoted microbial diversity, while high-concentration antibiotics resulted in the opposite effect (Chen et al., 2021a), and fungi have the capacity to tolerate an ill-being environment (i.e., thermal temperature and extreme pH) and re-product (Liu et al., 2021).
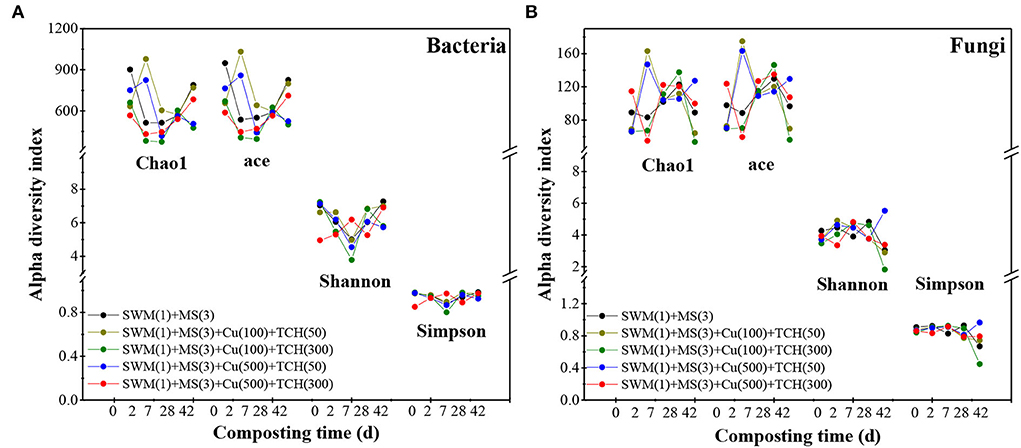
Figure 4. Alpha-diversity indexes related to bacterial (A) and fungal (B) communities based on OUTs during the composting process.
Microbial relative abundance at the phylum level showed significant variation among all treatments (Figures 5, 6). The bacterial community Firmicutes, as an important contributor to hydrolyze polysaccharides and degrade cellulose (Xu et al., 2017), was a dominant phylum (42.24%−92.92%) during the temperature-heating and thermophilic phases, confirming the well-established inference that Firmicutes could grow at extreme temperature and widely distributed at the thermophilic phase (Wei et al., 2018). Firmicutes was lower in the Cu/TCH-amended treatments as contrasted to the treatment without Cu and TCH according to Liu et al. (2020), who reported that Firmicutes was less abundant in antibiotics-amended treatments. During the temperature-cooling and mature phases, Bacteroidota and Proteobacteria replaced Firmicutes as the primary bacterial phylum (Jiang et al., 2019). Actinobacteriota also showed more competitiveness at the mature phase, higher in the treatments amended with Cu and TCH, in agreement with how Actinobacteriota can secrete various antibiotics to inhibit pathogenic microorganisms in the composting system (Tian et al., 2013). The fungal communities Ascomycota and Neocallimastigomycota were the main phyla in initial mixtures. Then, Ascomycota increased throughout the composting process, while Neocallimastigomycota was not detected from the thermophilic phase onwards. Identical results were obtained in studies, where Ascomycota had always been the dominating phylum among all examined phases and Neocallimastigomycota survived in the temperature-heating phase (Wang et al., 2018). Basidiomycota, which co-occurred in anaerobic conditions and aerobic surroundings (Liu et al., 2021), was higher in the temperature-heating phase during treatment without Cu and TCH and showed richness in the thermophilic phase iduring Cu/TCH-amended treatments. This result at least hints that Cu and TCH promoted Basidiomycota growth in the thermophilic phase. Chytridiomycota was characterized as a decomposer and pathogen as well as a main component in complex environments (Mao et al., 2020). Relatively, Chytridiomycota was slightly abundant in Cu/TCH-amended treatments with supernal doses but showed considerable richness in Cu/TCH-amended treatment with fewer doses, which results in a novel conclusion that low-concentration Cu and TCH stimulated Chytridiomycota metabolism during the composting process.
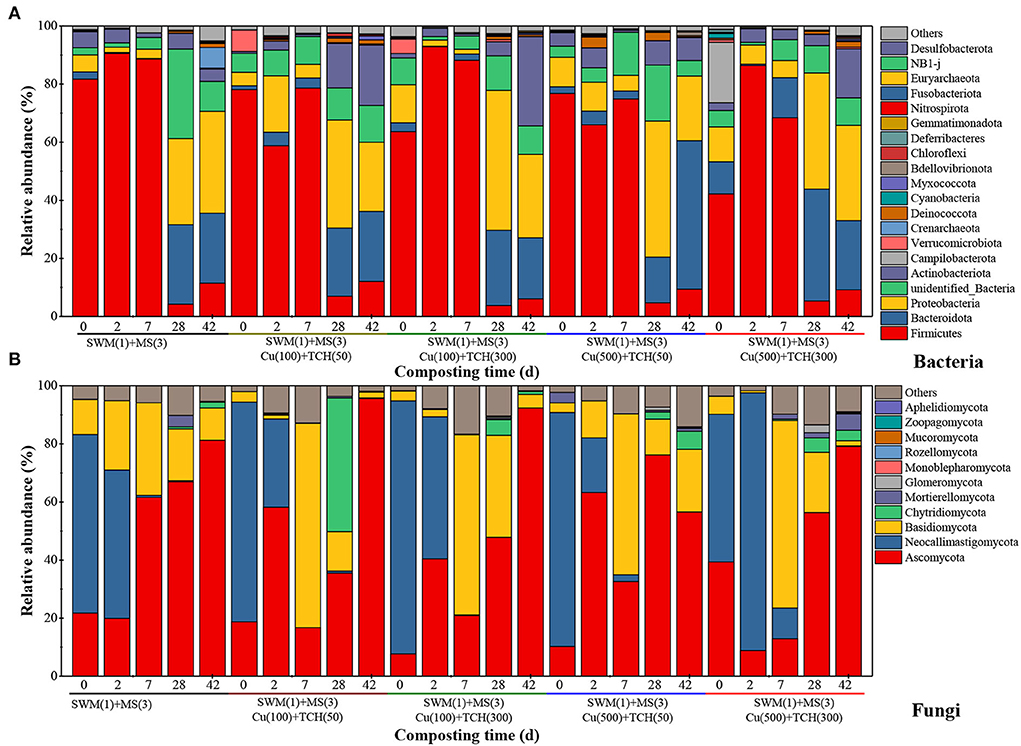
Figure 5. Dynamic changes in the bacterial (A) and fungal (B) communities at the phylum level during the composting process.

Figure 6. Constructed phylogenetic trees related to bacterial (A) and fungal (B) communities at the genus level.
Primary factors controlling the bacterial and fungal communities
Distance-based redundancy analysis (RDA) was performed to ascertain the correlations between physical and chemical parameters as well as the bacterial (Figure 7A) and fungal (Figure 7B) phylum. The first two axes show 34.65–39.11 and 22.61–30.96% of the total variability, respectively. A positive relationship between TOC and Firmicutes was found. Tang et al. (2019) also demonstrated that Firmicutes was the primary OM decomposer. There was a significant negative correlation between temperature and bacterial communities, such as Crenarchaeota and Unidentified-Bacteria, and fungal communities, including Rozellomycota and Mucoromycota. On this basis, the inferred conclusion was that four of them maybe were non-heat-resistant microbial phylum. NH-N was positively related to Neicallimastigomycota. NO-N presented a positive relationship with the bacterial community (Actinobacteria) and fungal communities (Mucoromycota and Mortierellomycota). TN was positively correlated with Mucoromycota and Mortierellomycota. From the results, it is clear that temperature and NO-N were dominating factors controlling the microbial communities during the composting process.

Figure 7. RDA ordination biplots between bacterial (A), fungal (B) communities and physico-chemical parameters.
Until now, not enough information on the succession mechanism associated with microbial communities is available. Thus, this work established multiple interaction pathways between physico-chemical parameters as well as bacterial (Figure 8A) and fungal (Figure 8B) alpha-indexes with structural equation models (SEMs). Both TOC and TN showed a significantly negative effect (P < 0.01) on bacterial richness and diversity. Likewise, there was a significant negative effect (P < 0.01) and negative affect (P < 0.05) between fungal richness and diversity as well as TOC and TN, respectively. Moreover, a significantly negative effect between temperature as well as TOC and TN was found in bacterial and fungal systems. These results supported the idea that temperature-controlled TOC decomposition and TN mineralization affect microbial activity in the composting system. Both NH-N and NO-N presented a negative effect on fungal richness and diversity but not on bacteria, which is in line with Cleveland and Liptzin (2007), who indicated that fungi are important dominators in nutrient-poor soil because their nutrient requirements and metabolic activity are lower compared to bacteria.
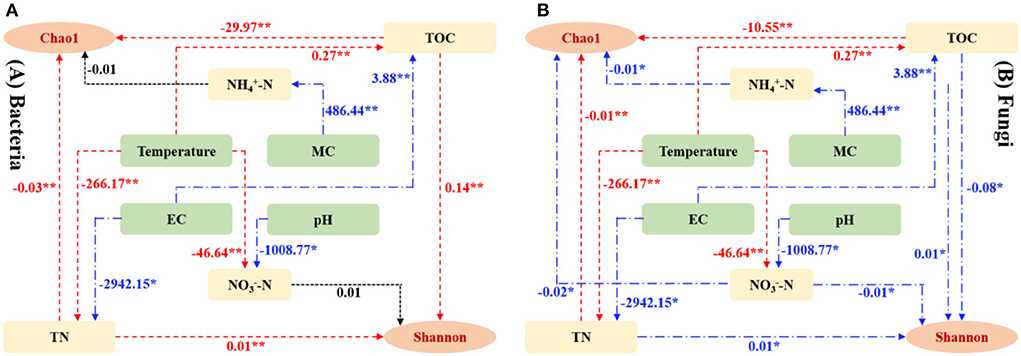
Figure 8. Structure equation models (SEMs), representing hypothesized causal correlations between bacterial (A) and fungal (B) communities as well as physico-chemical parameters.
Conclusion
Composting quality and microbial succession were evaluated during the Cu/TCH-amended composting process. Low-concentration Cu could promote the composting process and increase the composting temperature during thermophilic phase but this is not true of TCH. When it came to microbial succession, composting phase-derived changes in bacterial and fungal communities were significant, while Cu and TCH showed a remarkable influence on fungi but not on bacteria. Low-concentration TCH enhanced bacterial and fungal richness during the thermophilic phase, while high-concentration TCH reduced it. With respect to microbial abundance, Firmicutes was the dominating phylum during the thermophilic phases; thereafter, Bacteroidota and Proteobacteria became more competitive during temperature-cooling and mature phases. In terms of fungal communities, Ascomycota was always detected among all examined phases, and Neocallimastigomycota was only found during the early composting stage. Cu and TCH inhibited Firmicutes activity while promoting Actinobacteriota growth during the composting process. Meanwhile, low-concentration of Cu and TCH showed an inhibition effect on Basidiomycota in the thermophilic phase and a stimulative influence on Chytridiomycota at temperature-cooling and mature phases. During the composting process, TOC and TN were the primary factors controlling bacterial and fungal communities. Compared to bacteria, NH-N and NO-N were more beneficial to fungi. These results might provide a scientific basis to develop a virus-free and value-added composting technology.
Data availability statement
The raw paired-end sequences have been deposited in the Sequence Read Archive Database under the accession number PRJNA896743. Further queries can be directed to the corresponding author.
Author contributions
HC wrote the original draft. LW and JZ provided suggestions to improve the writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA28080400) and the Fundamental Research Funds in Heilongjiang Provincial Universities (No. 135109227).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akdeniz, N. (2019). A systematic review of biochar use in animal waste composting. Waste Manage. 88, 291–300. doi: 10.1016/j.wasman.2019.03.054
Alavi, M., Rai, M., Martinez, F., Kahrizi, D., Khan, H., Rose Alencar de Menezes, I., et al. (2022). The efficiency of metal, metal oxide, and metalloid nanoparticles against cancer cells and bacterial pathogens: different mechanisms of action. Cell. Mol. Biomed. Rep. 2, 10–21. doi: 10.55705/cmbr.2022.147090.1023
Awasthi, M. K., Wang, Q., Awasthi, S. K., Li, R. H., Zhao, J. C., Ren, X. N., et al. (2018). Feasibility of medical stone amendment for sewage sludge co-composting and production of nutrient-rich compost. J. Environ. Manage. 216, 49–61. doi: 10.1016/j.jenvman.2018.01.032
Biyada, S., Merzouki, M., Démčénko, T., Vasiliauskien,é, D., Ivanec-Goranina, R., Urbonavičius, J., et al. (2021). Microbial community dynamics in the mesophilic and thermophilic phases of textile waste composting identified through next-generation sequencing. Sci. Rep. 11, 23624. doi: 10.1038/s41598-021-03191-1
Biyada, S., Merzouki, M., Démčénko, T., Vasiliauskiené, D., Marčiulaitiené, E., Vasarevičius, S., et al. (2022). The effect of feedstock concentration on the microbial community dynamics during textile waste composting. Front. Ecol. E10, 813448. doi: 10.3389/fevo.2022.813488
Cao, R. K., Ben, W. W., Qiang, Z. M., and Zhang, J. Y. (2020). Removal of antibiotic resistance genes in pig manure composting influenced by inoculation of compound microbial agents. Bioresour. Technol. 317, 123966. doi: 10.1016/j.biortech.2020.123966
Chen, Z., Li, Y. Z., Peng, Y. Y., Ye, C. S., and Zhang, S. H. (2021a). Effects of antibiotics on hydrolase activity and structure of microbial community during aerobic co-composting of food waste with sewage sludge. Bioresour. Technol. 321, 124506. doi: 10.1016/j.biortech.2020.124506
Chen, Z., Li, Y. Z., Ye, C. S., He, X., and Zhang, S. H. (2021b). Fate of antibiotics and antibiotic resistance genes during aerobic co-composting of food waste with sewage sludge. Sci. Total Environ. 784, 146950. doi: 10.1016/j.scitotenv.2021.146950
Chen, Z. Q., Zhang, S. H., Wen, Q. X., and Zheng, J. (2015). Effect of aeration rate on composting of penicillin mycelial dreg. J. Environ. Sci. 37, 172–178. doi: 10.1016/j.jes.2015.03.020
Cheng, D. M., Feng, Y., Liu, Y. W., Xue, J. M., and Li, Z. J. (2019). Dynamics of oxytetracycline, sulfamerazine, and ciprofloxacin and related antibiotic resistance genes during swine manure composting. J. Environ. Manage. 230, 102–109. doi: 10.1016/j.jenvman.2018.09.074
Cleveland, C. C., and Liptzin, D. (2007). C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85, 235–252. doi: 10.1007/s10533-007-9132-0
Cui, E. P., Wu, Y., Zuo, Y. R., and Chen, H. (2016). Effect of different biochars on antibiotic resistance genes and bacterial community during chicken manure composting. Bioresour. Technol. 203, 11–17. doi: 10.1016/j.biortech.2015.12.030
Deng, W. W., Zhang, A. Y., Chen, S. J., He, X. P., Jin, L., Yu, X. M., et al. (2020). Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. J. Environ. Manage. 257, 109980. doi: 10.1016/j.jenvman.2019.109980
Ezzariai, A., Hafidi, M., Khadra, A., Aemig, Q., Fels, L. E., Barret, M., et al. (2018). Human and veterinary antibiotics during composting of sludge or manure: global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 359, 465–481. doi: 10.1016/j.jhazmat.2018.07.092
Hao, J. K., Wei, Z. M., Wei, D., Mohamed, T. A., Yu, H. M., Xie, X. Y., et al. (2019). Roles of adding biochar and montmorillonite alone on reducing the bioavailability of heavy metals during chicken manure composting. Bioresour. Technol. 294, 122199. doi: 10.1016/j.biortech.2019.122199
Hu, Z. H., Liu, Y. L., Chen, G. W., Gui, X. Y., Chen, T. H., Zhan, X. M., et al. (2011). Characterization of organic matter degradation during composting of manure–straw mixtures spiked with tetracyclines. Bioresour. Technol. 102, 7329–7334. doi: 10.1016/j.biortech.2011.05.003
Jiang, Z. W., Lu, Y. Y., Xu, J. Q., Li, M. Q., Shan, G. C., Li, Q. L., et al. (2019). Exploring the characteristics of dissolved organic matter and succession of bacterial community during composting. Bioresour. Technol. 292, 121942. doi: 10.1016/j.biortech.2019.121942
Khadra, A., Ezzariai, A., Merlina, G., Capdeville, M. J., Budzinski, H., Hamdi, H., et al. (2019). Fate of antibiotics present in a primary sludge of WWTP during their co-composting with palm wastes. Waste Manage. 84, 13–19. doi: 10.1016/j.wasman.2018.11.009
Kheyrodin, H., Jami, R., and Rehman, F. U. (2022). Cellular structure and molecular functions of plants, animals, bacteria, and viruses. Cell. Mol. Biomed. Rep. 2, 33–41. doi: 10.55705/cmbr.2022.330941.1021
Li, H. H., Zhang, T., Tsang, D. C. W., and Li, G. X. (2020). Effects of external additives: biochar, bentonite, phosphate, on co-composting for swine manure and corn straw. Chemosphere 248, 125927. doi: 10.1016/j.chemosphere.2020.125927
Liu, N., Hou, T., Yin, H. J., Han, L. J., and Huang, G. Q. (2019). Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. J. Hazard. Mater. 362, 258–265. doi: 10.1016/j.jhazmat.2018.09.028
Liu, T., Awasthi, M. K., Jiao, M. N., Awasthi, S. K., Qin, S. Y., Zhou, Y. W., et al. (2021). Changes of fungal diversity in fine coal gasification slag amendment pig manure composting. Bioresour. Technol. 325, 124703. doi: 10.1016/j.biortech.2021.124703
Liu, W., Huo, R., Xu, J. X., Liang, S., Li, J. J., Zhao, T. K., et al. (2017). Effects of biochar on nitrogen transformation and heavy metals in sludge composting. Bioresour. Technol. 235, 43–49. doi: 10.1016/j.biortech.2017.03.052
Liu, Y. W., Cheng, D. M., Xue, J. M., Weaver, L., Wakelin, S. A., Feng, Y., et al. (2020). Changes in microbial community structure during pig manure composting and its relationship to the fate of antibiotics and antibiotic resistance genes. J. Hazard. Mater. 389, 122082. doi: 10.1016/j.jhazmat.2020.122082
Mao, H. L., Wang, K., Wang, Z., Peng, J., and Ren, N. Q. (2020). Metabolic function, trophic mode, organics degradation ability and influence factor of bacterial and fungal communities in chicken manure composting. Bioresour. Technol. 302, 122883. doi: 10.1016/j.biortech.2020.122883
Peng, S., Li, H. J., Song, D., Lin, X. G., and Wang, Y. M. (2018). Influence of zeolite and superphosphate as additives on antibiotic resistance genes and bacterial communities during factory-scale chicken manure composting. Bioresour. Technol. 263, 393–401. doi: 10.1016/j.biortech.2018.04.107
Rensing, C., and Grass, G. (2003). Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27, 197–213. doi: 10.1016/S0168-6445(03)00049-4
Sánchez, Ó. J., Ospina, D. A., and Montoya, S. (2017). Compost supplementation with nutrients and microorganisms in composting process. Waste Manage. 69, 136–153. doi: 10.1016/j.wasman.2017.08.012
Shehata, E., Cheng, D. M., Ma, Q. Q., Li, Y. L., Liu, Y. W., Feng, Y., et al. (2021). Microbial community dynamics during composting of animal manures contaminated with arsenic, copper, and oxytetracycline. J. Integr. Agric. 20, 1649–1659. doi: 10.1016/S2095-3119(20)63290-7
Tang, Z. R., Xi, B. D., Huang, C. H., Tan, W. B., Xia, X. Q., Yang, T. X., et al. (2019). Linking phytoavailability of heavy metals with microbial community dynamics during municipal sludge composting. Process Saf. Environ. Prot. 130, 288–296. doi: 10.1016/j.psep.2019.08.026
Tian, W., Sun, Q., Xu, D. B., Zhang, Z. H., Chen, D., Li, C. Y., et al. (2013). Succession of bacterial communities during composting process as detected by 16S rRNA clone libraries analysis. Int. Biodeter. Biodegr. 78, 58–66. doi: 10.1016/j.ibiod.2012.12.008
Wang, K., Yin, X. B., Mao, H. L., Chu, C., and Tian, Y. (2018). Changes in structure and function of fungal community in cow manure composting. Bioresour. Technol. 255, 123–130. doi: 10.1016/j.biortech.2018.01.064
Wang, Q., Wang, Z., Awasthi, M. K., Jiang, Y. H., Li, R. H., Ren, X. N., et al. (2016). Evaluation of medical stone amendment for the reduction of nitrogen loss and bioavailability of heavy metals during pig manure composting. Bioresour. Technol. 220, 297–304. doi: 10.1016/j.biortech.2016.08.081
Wei, H. W., Wang, L. H., Hassan, M., and Xie, B. (2018). Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresour. Technol. 256, 333–341. doi: 10.1016/j.biortech.2018.02.050
Wei, Y. Q., Wei, Z. M., Cao, Z. Y., Zhao, Y., Zhao, X. Y., Lu, Q., et al. (2016). A regulating method for the distribution of phosphorus fractions based on environmental parameters related to the key phosphate-solubilizing bacteria during composting. Bioresour. Technol. 211, 610–617. doi: 10.1016/j.biortech.2016.03.141
Xu, S., Lu, W. J., Liu, Y. T., Ming, Z. Y., Liu, Y. J., Meng, R. H., et al. (2017). Structure and diversity of bacterial communities in two large sanitary landfills in China as revealed by high-throughput sequencing (MiSeq). Waste Manage. 63, 41–48. doi: 10.1016/j.wasman.2016.07.047
Yang, F., Li, G. X., Yang, Q. Y., and Luo, W. H. (2013). Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 93, 1393–1399. doi: 10.1016/j.chemosphere.2013.07.002
Yethon, J. A., Vinogradov, E., Perry, M. B., and Whitfield, C. (2000). Mutation of the lipopolysaccharide core glycosyltransferase encoded by waaG destabilizes the outer membrane of Escherichia coli by interfering with core phosphorylation. J. Bacteriol. 182, 5620–5623. doi: 10.1128/JB.182.19.5620-5623.2000
Keywords: co-composting, copper, tetracycline hydrochloride, bacteria, fungi
Citation: Cui H, Wang L and Zhang J (2022) Synergistic influence on microbial communities ascribed to copper and tetracycline during aerobic composting: Insights into bacterial and fungal structures. Front. Ecol. Evol. 10:1019494. doi: 10.3389/fevo.2022.1019494
Received: 15 August 2022; Accepted: 28 September 2022;
Published: 03 November 2022.
Edited by:
Ravindra Soni, Indira Gandhi Krishi Vishva Vidyalaya, IndiaReviewed by:
Bahman Fazeli-Nasab, University of Zabol, IranJayani J. Wewalwela, University of Colombo, Sri Lanka
Copyright © 2022 Cui, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixia Wang, bHh3YW5nQGlnYS5hYy5jbg==
 Hu Cui1,2
Hu Cui1,2 Lixia Wang
Lixia Wang