- 1U.S. Geological Survey, Western Fisheries Research Center, Seattle, WA, United States
- 2U.S. Geological Survey, Forest and Rangeland Ecosystem Science Center, Seattle, WA, United States
- 3Olympic National Park, National Park Service, Port Angeles, WA, United States
- 4Western Washington Fish and Wildlife Conservation Office, U.S. Fish and Wildlife Service, Lacey, WA, United States
- 5Washington Department of Fish and Wildlife, Port Angeles, WA, United States
- 6Fish Ecology Program, Northwest Fisheries Science Center, National Oceanic and Atmospheric Administration, Seattle, WA, United States
- 7Department of Geography, University of Zurich, Zurich, Switzerland
- 8Shreffler Environmental, Sequim, WA, United States
- 9Trout Unlimited, Port Angeles, WA, United States
- 10Lower Elwha Klallam Tribe, Port Angeles, WA, United States
The removal of two large dams on the Elwha River was completed in 2014 with a goal of restoring anadromous salmonid populations. Using observations from ongoing field studies, we compiled a timeline of migratory fish passage upstream of each dam. We also used spatially continuous snorkeling surveys in consecutive years before (2007, 2008) and after (2018, 2019) dam removal during summer baseflow to assess changes in fish distribution and density over 65 km of the mainstem Elwha River. Before dam removal, anadromous fishes were limited to the 7.9 km section of river downstream of Elwha Dam, potamodromous species could not migrate throughout the river system, and resident trout were the most abundant species. After dam removal, there was rapid passage into areas upstream of Elwha Dam, with 8 anadromous species (Chinook, Coho, Sockeye, Pink, Chum, Winter Steelhead, Summer Steelhead, Pacific Lamprey, and Bull Trout) observed within 2.5 years. All of these runs except Chum Salmon were also observed in upper Elwha upstream of Glines Canyon Dam within 5 years. The spatial extent of fish passage by adult Chinook Salmon and Summer Steelhead increased by 50 km and 60 km, respectively, after dam removal. Adult Chinook Salmon densities in some previously inaccessible reaches in the middle section of the river exceeded the highest densities observed in the lower section of the river prior to dam removal. The large number (>100) of adult Summer Steelhead in the upper river after dam removal was notable because it was among the rarest anadromous species in the Elwha River prior to dam removal. The spatial extent of trout and Bull Trout remained unchanged after dam removal, but their total abundance increased and their highest densities shifted from the lower 25 km of the river to the upper 40 km. Our results show that reconnecting the Elwha River through dam removal provided fish access to portions of the watershed that had been blocked for nearly a century.
Introduction
Societies around the world are confronting the many interacting—at times conflicting—demands placed on rivers. Rivers provide valuable ecosystem services like food, drinking water, and recreational opportunity, as well as important infrastructure for human activities like transportation and electricity generation (Palmer and Ruhi, 2019). At the same time, rivers are home to or provide essential habitat for aquatic organisms, including spawning, rearing, and migratory habitat for fish species. Many of the world’s large and medium sized rivers are dammed or otherwise fragmented (Lehner et al., 2011; Grill et al., 2015, 2019), impacting anadromous and potamodromous fish species by eliminating riverine habitat inundated by reservoirs, inhibiting connectivity and within-river migrations, and altering flow, sediment, temperature, and nutrient regimes in downstream areas (Petts, 1984; Poff et al., 1997; Bunn and Arthington, 2002; Poff, 2018). Coupled with introductions of non-native species and other anthropogenic impacts, this has led to significant changes to biodiversity of the world’s rivers (Su et al., 2021), a situation expected to intensify in the coming decades due to climate change (Pecl et al., 2017).
One tool to mitigate these impacts is improving connectivity through the removal of dams and other barriers, which can restore within river movements by resident and migratory fish species (Bednarek, 2001; Kemp and O’Hanley, 2010; Branco et al., 2014; Foley et al., 2017a). Ecological responses to dam removal are context dependent, varying with biogeographic setting, the size of the dam, historical and current watershed conditions, and reservoir sediment volume and composition (Foley et al., 2017b; Bellmore et al., 2019). To date, most studies examining responses of fish populations to dam removal have shown positive and rapid effects (Pess et al., 2014). For example, Hitt et al. (2012) found an increase in the abundance of American Eels (Anguilla rostrata) in headwater streams of Shenandoah National Park following the removal of a downstream dam. Others have shown that anadromous fish can return to and spawn in upstream areas following dam removal (Burdick and Hightower, 2006; Hogg et al., 2015; Allen et al., 2016; Battle et al., 2016; Liermann et al., 2017).
Resident fish assemblages can change, as fish species found downstream of a dam can move into upstream areas following dam removal (Catalano et al., 2007; but see Muha et al., 2021). In some cases, life history diversity can increase as fish reestablish within new habitats and fill vacant ecological niches (Quinn et al., 2017; Brenkman et al., 2019). Changes in fish assemblages after reconnection of formerly dammed rivers can be detrimental, especially if non-native or invasive species are part of the reassembly (e.g., Kornis et al., 2014). In fact, maintaining dams and other barriers has been necessary to protect upstream habitats (Jones et al., 2021), for example in the Laurentian Great Lakes where barrier removal would expose upstream habitats to spawning by non-native Sea Lamprey (Petromyzon marinus) that management agencies are spending millions of dollars annually to control (e.g., McLaughlin et al., 2007).
Most documented dam removal projects have occurred in the developed world, where old, mostly small dams (i.e., <10 m, but averaging around 3 m in height) built for a variety of purposes became obsolete or unsafe, prompting their removal (O’Connor et al., 2015; Bellmore et al., 2017; American Rivers, 2019). In many cases, coexisting opportunities to restore important ecological functions, like hydrological connectivity and sediment supply, provided additional motivation to remove dams (Pohl, 2002; Magilligan et al., 2016).
The removal of larger dams has increased recently, despite challenging and complex political and socio-economic factors (Lejon et al., 2009; Poff and Schmidt, 2016; Roy et al., 2018). The response of fish populations — often the target of restoration efforts via dam removal—needs to be better understood so that decision makers have a clear idea of the possible outcomes when weighing the costs and benefits of potential dam removal and river restoration projects (Whitelaw and MacMullan, 2002; Duda and Bellmore, in press).
One of the earliest proposed and most prominent large dam removal projects involved two hydroelectric dams on the Elwha River in Washington State, United States (Wunderlich et al., 1994; Duda et al., 2008). Following their construction without fish passage facilities in the early 1900s to provide hydroelectric power, the dams had significant negative effects on Pacific salmon populations (Pess et al., 2008). The decision to remove the Elwha River dams was based, in large part, upon facilitating the restoration of the Elwha River anadromous fish populations (Winter and Crain, 2008). Because both dams lacked fish passage, connectivity was severely disrupted; over 90% of the presumed historical distribution of salmon was unavailable (Pess et al., 2008), and the remaining salmon populations downstream of Elwha Dam were in decline.
Here, we describe spatial and temporal patterns of fish before and after dam removal in the Elwha River. As restoring anadromous fish populations was a primary goal of dam removal, their utilization of upstream habitats following dam removal is a key metric of project success and potentially a major driver of ecological changes in those areas (Bellmore et al., 2019). Using a riverscape approach to evaluate fish response to dam removal can be useful because it provides spatially continuous, high-resolution data for the entire river, allowing patterns to be explored across multiple spatial scales from meters to tens of kilometers (McMillan et al., 2013; Torgersen et al., in press). In particular, we were interested in assessing changes in salmonid distribution, abundance, and density after dam removal. We report two main findings that describe both the timing and extent of fish reentering formerly inaccessible sections of the watershed. First, for migratory fish species, we report the date of the first observed fish upstream of each dam (ascertained from several ongoing field investigations using multiple techniques), to build a timeline of passage past each dam from 2011 through 2019. Second, we utilize a before-after, continuous “riverscape survey” approach (Fausch et al., 2002; Brenkman et al., 2012) to quantify patterns of adult and juvenile salmonids over a 12-year period.
Study Area and Dam Removal Background
The Elwha River flows 72 km from the interior of Washington’s Olympic Mountains to the Strait of Juan de Fuca in the northwestern United States (Figure 1). The annual hydrograph is bimodal, driven by wet winters and spring snowmelt, with a long-term average annual discharge of 43 m3/s (Duda et al., 2011). The majority of the river basin (83%) occurs in protected, largely roadless wilderness areas of Olympic National Park, while the remaining downstream portion of the river flows through a mixture of public, private, and tribal lands. The river consists of three main sections (Figure 1): the Lower Elwha (mouth to Elwha Dam, hereafter abbreviated as LE), the Middle Elwha (Elwha Dam to Glines Canyon Dam, ME), and the Upper Elwha (upstream of Glines Canyon Dam, UE).
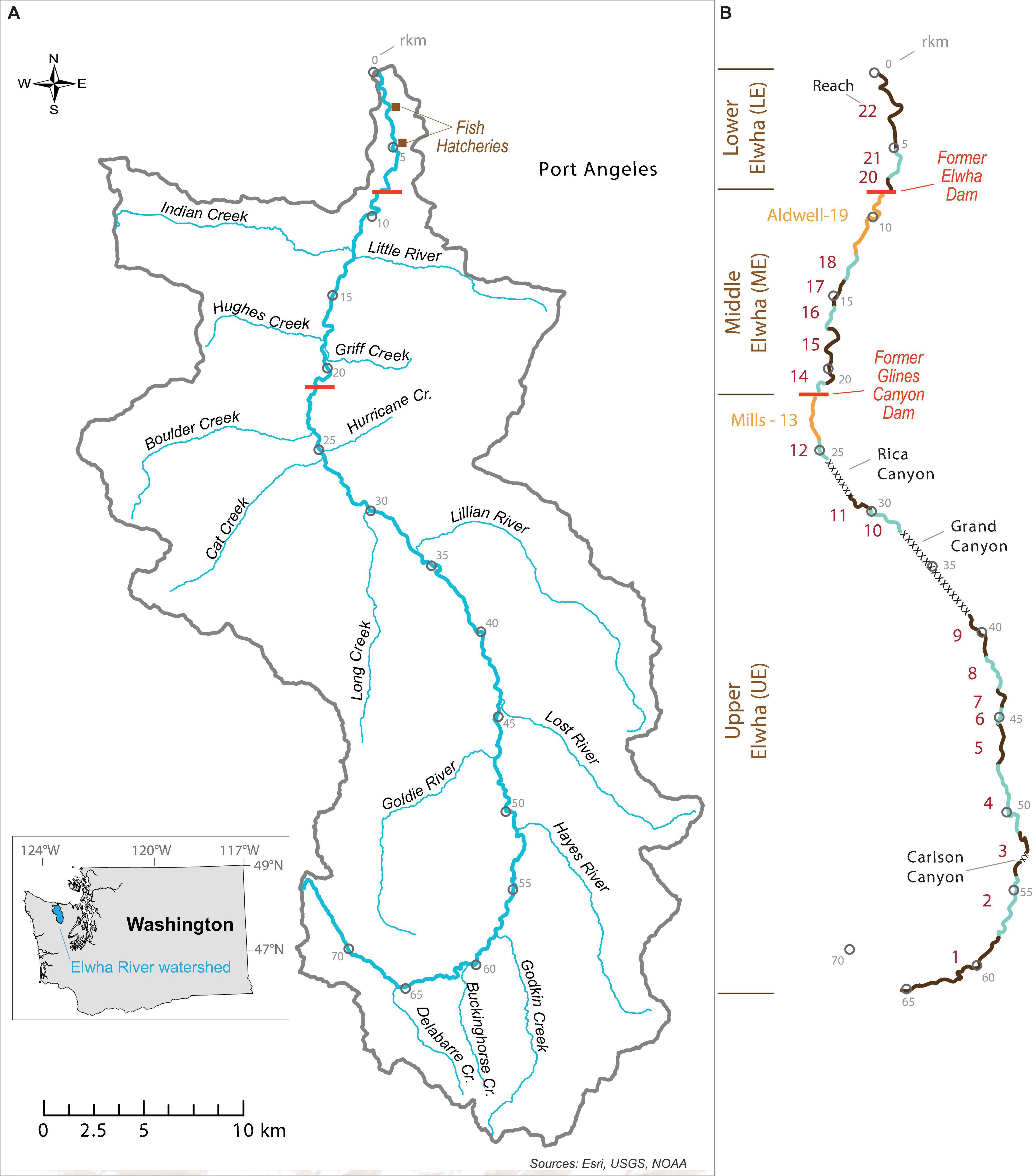
Figure 1. (A) Map of the Elwha River watershed and post-dam removal hydrography showing the two former dams, two fish hatcheries, and (B) the 22 riverscape survey reaches numbered in longitudinal order and alternating color. Distances upstream from the mouth of the Elwha River are indicated with open circles and corresponding river km (rkm) measures. Canyon reaches not surveyed and the former reservoir reaches (Lake Aldwell and Lake Mills) are indicated by cross-hatch and yellow, respectively.
The construction of two dams on the Elwha River led to several changes to the ecology of the river and its fish populations. Before the construction of Elwha Dam in 1912, the river was among the most productive salmon rivers in the Salish Sea, hosting a diverse migratory fish assemblage: all five Pacific salmon species (Oncorhynchus spp.), Eulachon (Thaleichthys pacificus) Pacific Lamprey (Lampetra tridentata), Bull Trout (Salvelinus confluentus), Coastal Cutthroat Trout (O. clarkii clarkii), and two runs of Steelhead Trout (O. mykiss) (Wunderlich et al., 1994; Brenkman et al., 2008; Pess et al., 2008). Both dams were built within the historical homeland of the Lower Elwha Klallam Tribe, who opposed the dams, suffered from reduced subsistence and commercial fishing opportunities, and experienced the inundation of culturally significant lands and resources. The two hydroelectric dams (operated as run-of-river for the last four decades before removal), provided the first municipal electricity for the growing population of the North Olympic Peninsula, including the frontier town of Port Angeles, Washington. Elwha Dam was a 32-m-tall concrete gravity dam built 7.9 km from the river mouth. The lack of fish passage at the dam had immediate effects on the size of salmon runs. In 1927, Glines Canyon Dam (64-m-tall concrete arch) was constructed 21.4 km from the river mouth, and its reservoir Lake Mills became the main sediment trap in the system, sequestering significant quantities of smaller sized sand and gravels. Over the following decades, the sequestration of this sediment supply contributed significantly to an increase of bed grain size and armoring (Pohl, 2004; Draut et al., 2011), reducing spawning area downstream of Elwha Dam where salmon still had access (Peters et al., 2017). Because both dams lacked fish passage, they blocked salmonids from accessing over 90% of their historical range and reduced the size of anadromous salmon runs (Pess et al., 2008). In response to a decades-long decline of salmon populations, two hatchery facilities were established in 1976 and 1978, respectively, by the Washington Department of Fish and Wildlife and the Lower Elwha Klallam Tribe to continue hatchery operations that had been started and stopped since the early 1900s (Duda et al., 2018). The state hatchery produces Chinook Salmon, while the tribal hatchery focuses on Coho Salmon and Steelhead. At the start of dam removal, the hatcheries were using native Elwha River stocks. During and following dam removal, the hatcheries have been part of an integrated salmon recovery program that includes hatcheries, adult relocations, and volitional movements into upstream areas (Ward et al., 2008; Peters et al., 2014; Liermann et al., 2017).
After passage of a 1992 federal law, the Elwha River Ecosystem and Fisheries Restoration Act (PL 102-495) and years of studying alternatives, it was determined that full removal of both dams provided the best opportunity to restore anadromous fish populations and their ecosystem (see Winter and Crain, 2008 and references therein). Both dams were removed simultaneously in a phased process from 2011 to 2014. After drawing down its reservoir Lake Aldwell ∼4 m starting in June 2011, active removal of Elwha Dam started in September 2011, with reservoir drawdown and dam removal lasting until May 2012. A re-emergent floodplain channel topography and river channel developed in the former Lake Aldwell, as the river eroded and transported reservoir sediments (Randle et al., 2015). Removal of Glines Canyon Dam also began in September 2011, with a similar controlled drawdown of Lake Mills reservoir acting to limit the release of sediment downstream and promote the transport and redistribution of reservoir sediments. Removal of Glines Canyon Dam was completed in August 2014. During the 5 years following the start of dam removal, over 20 Mt of sediment (approximately 65% of the original 21 × 106 m3 of stored sediment) was transported downstream, about 90% of which reached marine waters and contributed to rebuilding coastal habitats and beaches (Gelfenbaum et al., 2015; Warrick et al., 2015; Foley et al., 2017c; Ritchie et al., 2018).
In October 2014, a few months following dam removal, several large boulders that had become detached from the wall of Glines Canyon (presumably slabs of rock weakened by blasting during dam construction) became unstable during high flows and fell into the river channel. Situated downstream of the former dam site, these large boulders created a barrier to upstream fish migration. To restore fish passage, demolition of the rockfall occurred in October 2015 and September 2016 (Ertle et al., 2019).
Materials and Methods
Documenting Fish Passage
We assembled a timeline illustrating when each migratory fish species was first observed upstream of the dam sites after dam removal. This required compiling published and unpublished data from ongoing monitoring conducted by the Lower Elwha Klallam Tribe, Washington Department of Fish and Wildlife, National Oceanic and Atmospheric Administration (NOAA) Fisheries, National Park Service, and U.S. Fish and Wildlife Service (see details in Supplementary Table 1). Sampling efforts by these long-term monitoring programs upstream of each dam site were opportunistic and non-standardized among years. Nonetheless, they provided the first reported sightings of adults upstream of each dam site.
Conducting Riverscape Surveys
Teams of snorkelers and shore-based support crew conducted spatially continuous fish surveys during summer base flows (Table 1) from an area presumed to be the upstream limit of anadromous habitat (Pess et al., 2008) to the river mouth 65 km downstream. Before dam removal, the two reservoirs (8 km in total length) were not surveyed due to their large size (>500 m wide and >10 m deep), poor visibility (<2 m), and the incongruence of snorkeling methods for lake habitat. Two hazardous canyons with whitewater (∼6 km) were not snorkeled before or after dam removal. In 2008, a section of the river at river kilometer (rkm) 44–47 was not surveyed due to high flows and unsafe diving conditions. In 2019, the lowermost ∼3 km of the river was not surveyed for fish due to high turbidity and poor visibility (see Supplementary Figure 1 for maps of surveyed reaches in 2007, 2008, 2018, and 2019).
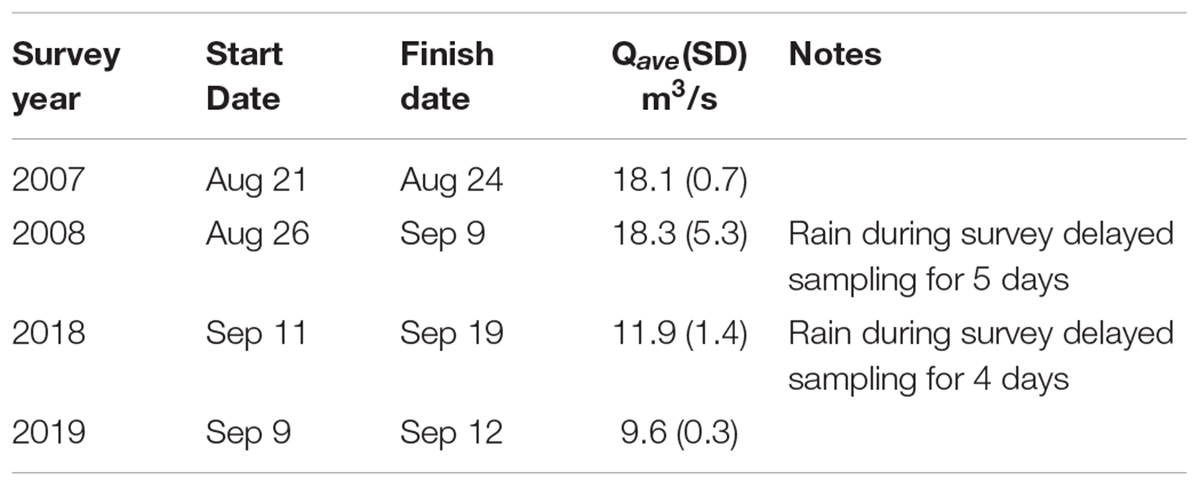
Table 1. Start and finish dates and average (SD) daily discharge of the Elwha River during the four riverscape surveys. Discharge data (Qave in m3/s) from USGS Elwha River at McDonald Bridge near Port Angeles, WA, United States (USGS, 2021).
The river was surveyed twice prior to dam removal in 2007 and 2008 (Brenkman et al., 2012) and twice after dam removal in 2018 and 2019. The 4-year period between the completion of dam removal in 2014 and the 2018 survey allowed the effects of dam removal (e.g., increased turbidity and river geomorphic change to the river channel; see details in East et al., 2015; Magirl et al., 2015; Randle et al., 2015; Ritchie et al., 2018) to dissipate from the former reservoirs and areas downstream of the two dams. By 2018, underwater visibility was sufficient (>2 m) for the visual observation and identification of salmonids.
During each survey year, we used four teams, each consisting of at least four field biologists. Two were divers with extensive experience conducting salmonid snorkel surveys and the other two recorded data and georeferenced the survey. Each team had an assigned section to survey, with start and end points defined by mapped landmarks (e.g., river confluence, start of canyon) that were established during the first survey in 2007. In each year, the entire river was surveyed within 4–14 days depending on weather. Two teams traveled on foot up to 40 km to reach remote start points. In 2008 and 2018, rain immediately prior to or during the survey increased river discharge and decreased visibility for divers, causing delays of 4 or 5 days (Table 1).
The spatial extents of the four surveys were the same, but the resolution of sampling units varied among surveys. In the 2007 survey, 22 river reaches (1–8 km in length) were demarcated based on tributaries and known landmarks and fish counts were tallied for each reach. In subsequent years, fish data were collected for each channel unit (5–200 m in length). Channel units were assigned one of four types based on water depth and velocity: riffle, glide-like riffle, glide-like pool, and pool (Bisson et al., 2017). We used global positioning system (GPS) units (accuracy ± 10 m) to map the path walked by surveyors and collect point coordinates of the start and end of each channel unit. These GPS track logs and point coordinates were later used to georeference the survey data in a geographic information system (GIS; see Section “Geographic Information System Analysis”). One of the surveyors measured the length of each unit with a laser range finder. In some cases, channel units longer than 200 m were divided into two or more contiguous units of the same type (usually riffles, glide-like riffles or glide-like pools) and GPS coordinates were collected at the point where channel units were divided. In this manner, we recorded geographic benchmarks for the survey at a spatial resolution of ≤200 m even though some channel units were longer than 200 m.
Channel unit boundaries were identified longitudinally and surveyed in a downstream direction. Starting at the upstream point, the two divers partitioned the river channel laterally from the center of the thalweg to the right or left river edge (see similar methods described by O’Neal et al., 2007). Each diver generally floated downstream and searched for fish while proceeding to the downstream boundary of the channel unit, unless fluvial morphology and flow necessitated an approach from downstream (i.e., behind boulders or within log jams). Divers progressed downstream parallel to one another and coordinated their movement to minimize duplicate counts. Narrow channel units, especially those near the headwaters, were surveyed with a single diver, whereas wider channel units near the mouth required up to three divers. When large aggregations of salmonids were encountered, or large wood jams were surveyed, divers made two passes and averaged counts.
Divers identified fish to species and noted life stage in all channels that had at least an estimated 10% of the mainstem channel flow. During August and September, salmon runs consisted of Chinook Salmon (O. tshawytscha), Summer Steelhead (O. mykiss) and Pink Salmon (O. gorbuscha), with occasional observations of Sockeye Salmon (O. nerka), Coho Salmon (O. kisutch), and Chum Salmon (O. keta). Divers also counted adult resident and potamodromous fish species, including Bull Trout, Rainbow Trout, and Coastal Cutthroat Trout. However, counts for Rainbow Trout and Coastal Cutthroat Trout were combined because of the difficulty in distinguishing these two species while snorkeling. For clarity, we refer to these counts as “trout” but note that previous studies suggest Coastal Cutthroat Trout were in low abundance in the Elwha’s mainstem prior to dam removal (Brenkman and Connolly, 2008; Brenkman et al., 2008), environmental DNA results after dam removal also show a greater occupancy in tributaries than in the mainstem (Duda et al., 2021). Therefore, it is likely that most “trout” were Rainbow Trout. Observers also identified the presence of juvenile salmon (young-of-year Coho Salmon and young-of-year/yearling Chinook Salmon) or trout (<70 mm). Because the Elwha River contains Steelhead, our designation of “trout” juveniles may have been Rainbow Trout, Steelhead, or Cutthroat Trout. At that small size, it is impossible to know whether an observed O. mykiss will follow a resident or anadromous life history when these forms are sympatric (Kendall et al., 2015).
Geographic Information System Analysis
Linear Referencing
Fish data were georeferenced in a GIS using a combination of mapped landmarks, GPS track logs, and point coordinates. In 2007, landmarks (e.g., tributary junctions, bridge crossings, and named features) were used to position the data linearly along the digitized channel (Brenkman et al., 2012). Subsequent surveys relied on GPS for more precise “linear referencing” of individual channel units. The same channel map was used for 2007 and 2008 (i.e., derived from publicly available spatial data layers that had been updated based on concurrent aerial photography as described in Brenkman et al., 2012) but had to be revised substantially for the surveys after dam removal. We used high-resolution aerial photography (<1 m resolution) from 2017 to (1) manually digitize new stream lines for sections of the river that previously had been inundated by reservoirs, and (2) update other reaches that had changed since 2008. This updated hydrography layer was used for georeferencing survey data from 2018 and 2019 and for calculating distance upstream from the river mouth (i.e., rkm; Figure 1). The general process of linear referencing of the survey data was similar for data from 2008, 2018, and 2019. Welty et al. (2015) describe the method and associated software for positioning linear features (i.e., channel units) along stream lines according to their length in a GIS. Coordinates from the GPS at the start and end points of each channel unit served as ground control points between which channel units were spatially rectified along the digitized stream channel based on their field-measured length (ESRI, 2021).
Common Reaches Across the Four Survey Years
We overlaid the linearly referenced data from all four survey years in a GIS and manually assigned common reaches for aggregating the data and making comparisons among years (Figure 1B). This set of common reaches was necessary to account for (1) the coarser resolution of survey data in 2007, (2) differences in hydrography before and after dam removal, and (3) slight differences in the channel units surveyed in each year. The differences among years in channel units surveyed were due to logistical difficulties associated with high-flow events (e.g., navigating portions of canyons, poor visibility from high turbidity), shifts in tributary junctions resulting from natural meander patterns, changes in channel unit endpoints, and obstructions from in-river construction associated with dam removal (e.g., water treatment plant construction in LE during 2008) (see summary of surveyed sections in Table 2). Linearly referenced data from the 2007 survey were used to establish endpoints in the GIS for a total of 22 reaches that could be compared before and after dam removal (Figure 1B). Channel-unit-scale data collected in 2008, 2018, and 2019 were then aggregated to these reaches using a process that minimized data loss and maximized comparability among survey years. Reaches were named numerically in increasing order from upstream to downstream (Figure 1B).
Data Analysis
We analyzed changes in adult salmonid densities and juvenile salmonid presence before and after dam removal to assess responses to dam removal (Torgersen et al., 2021). Adult salmonid densities (number/km) were calculated for each reach by dividing total fish count by the GIS-derived channel unit length (2007) or the total channel length snorkeled in each reach (2008, 2018, 2019). The percentages of each reach occupied by juvenile Chinook, Coho, and trout in 2018 and 2019 were calculated by dividing the total length of channel units in which juveniles were observed by the total length of all channel units. Fish densities and percentages of juvenile occurrence in reaches were plotted longitudinally with respect to their GIS-derived distance upstream (rkm) from the river mouth, which was based on the hydrography layer used to georeference data from the surveys conducted in 2018 and 2019. We used Pearson’s correlation between pairs of years before and after dam removal for Chinook Salmon, Steelhead, Bull Trout, and trout to assess interannual patterns in reach density. All calculations were completed in R software (R Core Team, 2020).
Results
Temporal Patterns of Fish Migration
We created a timeline of the first detections of 8 anadromous fish species (9 anadromous runs) upstream of each removed dam site (Figure 2). Details of each observation and attribution to personal communications and/or technical documents are given in Supplementary Table 1. After the removal of Elwha Dam, anadromous fish species progressively returned to areas upstream: Winter Steelhead were first observed within 2 months, followed by four other anadromous species within the first year (April 2012 to March 2013). Pacific Lamprey and all 7 anadromous salmonids (including two runs of Steelhead) had ascended past the former Elwha Dam within 2.5 years of dam removal.
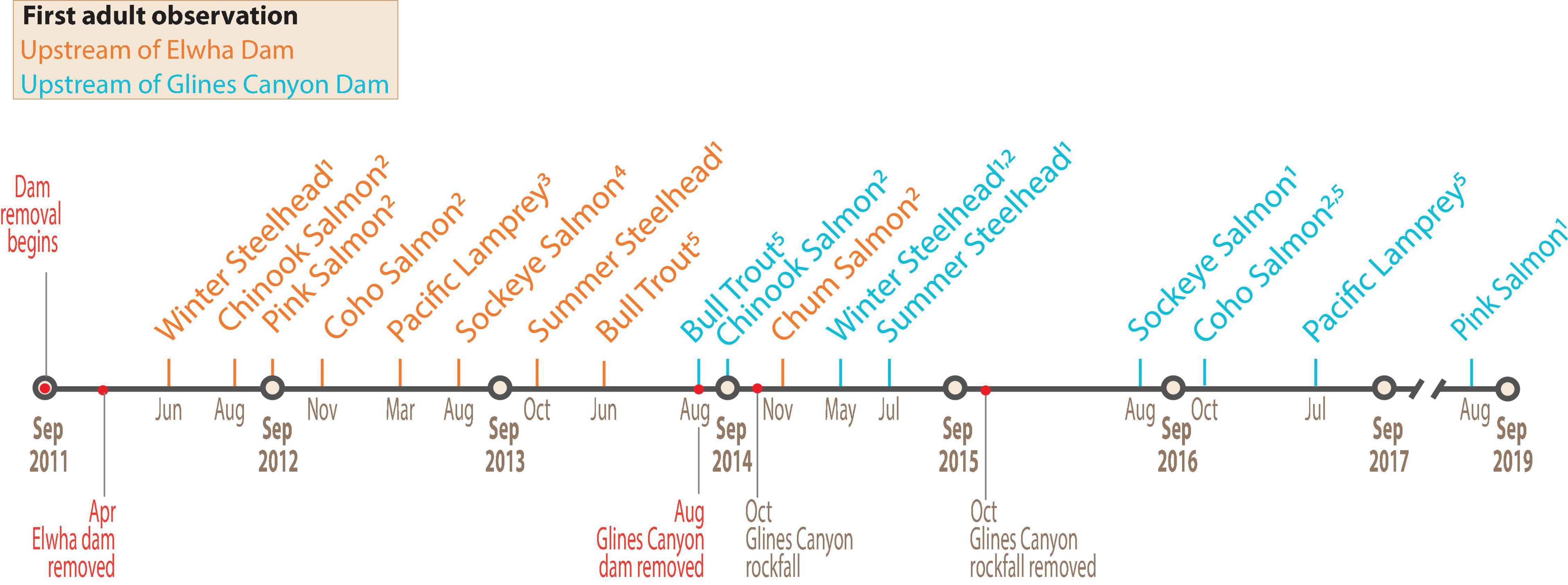
Figure 2. Timeline showing the dates of dam removal and first observations of adult migratory fish upstream of Elwha Dam (orange lines) and Glines Canyon Dam (blue lines). Observations were compiled from ongoing field studies indicated by superscripts: (1) snorkeling surveys; (2) redd surveys; (3) smolt trapping; (4) tangle netting; and (5) radio-telemetry (Supplementary Table 1). A recent environmental DNA study (Duda et al., 2021) detected some species earlier upstream of Elwha Dam (Chum Salmon in Aug 2014) and Glines Canyon Dam (Pink Salmon in August 2014, Pacific Lamprey and Sockeye Salmon in September 2014, and Chum Salmon in January of 2016), but this method cannot distinguish between adult and juvenile.
The first anadromous salmonid observed upstream of the former Glines Canyon Dam site was an adult Bull Trout, detected via radio-telemetry while dam removal was still underway. In the days following the removal, biologists conducting spawner and snorkeling surveys in the former Lake Mills observed the first Chinook Salmon adults and redds. The rockslide in October 2014 presumably delayed upstream fish passage from October 2014 to August 2016, when Winter Steelhead and Summer Steelhead were the only species detected upstream of the former Glines Canyon Dam. Four additional anadromous species ascended past the upper dam site from August 2016 to August 2019 (Figure 2). By September 2019, Chum Salmon were the only species not observed upstream of Glines Canyon Dam. Collectively, these first observations provide a general timeline of fish passage for anadromous species in the lower 21.4 rkm and establish that the reoccupation of areas upstream of the dams started prior to our riverscape surveys in 2018 and 2019.
Spatial Extent and Density Patterns of Salmonids
In each of the annual riverscape surveys, the total length of river snorkeled ranged 6.0–10.4 km in LE (Lower Elwha, downstream of the former Elwha Dam), 8.7–16.9 km in ME (Middle Elwha, between the two former dams), and 29.2–36.7 km in UE (Upper Elwha, upstream of the former Glines Canyon Dam) (Table 2 and Supplementary Figure 1). In LE, there were three reaches in all 4 years, but in ME and UE the number of reaches changed after 2008. After dam removal, two small portions of river upstream of the Lake Mills inlet were no longer surveyable due to geomorphic changes, and a single long reach in UE was split in two for logistical reasons. Also, the former reservoirs were replaced by approximately 8 km of free-flowing river which we surveyed in 2018 and 2019: 2.3 and 2.6 km in the former Lake Mills reservoir and 5.3 and 5.8 km in the former Lake Aldwell reservoir, respectively. The total number of reaches surveyed changed from 5 to 6 and 11 to 13 in ME and UE, respectively. We collected snorkeling data between established reference points across the four survey years in a total of 22 common reaches ranging in length from 0.57 to 7.8 km.
Bull Trout were observed in most of the survey reaches before and after dam removal (Figures 3A,B). We observed fewer Bull Trout in both years before dam removal (117 in 2007 and 86 in 2008) than after (264 in 2018 and 399 in 2019). The lowest densities of Bull Trout consistently were observed in LE (Figures 3A,B). In ME, the peak density of Bull Trout immediately downstream of Glines Canyon Dam (reach 14) before dam removal was no longer present following dam removal. A similar peak in UE before dam removal in reach 12, located just upstream of the former Lake Mills, also was not present after dam removal. After dam removal, the greatest densities of Bull Trout in UE were seen upstream of the Grand Canyon in reaches 2–9 (rkm 40–60). Following dam removal, every surveyed reach upstream of UE except reach 1 had a higher density of Bull Trout. Bull Trout were also detected in the two former reservoir reaches after dam removal. More Bull Trout were observed in the Aldwell Reach (reach 19) than the Mills Reach (reach 13), with the maximum number (n = 27) seen in the Aldwell Reach in 2019. The correlation of fish counts between years was greater before dam removal (Pearson’s r = 0.94; p < 0.001) than after dam removal (Pearson’s r = 0.50; p = 0.02).
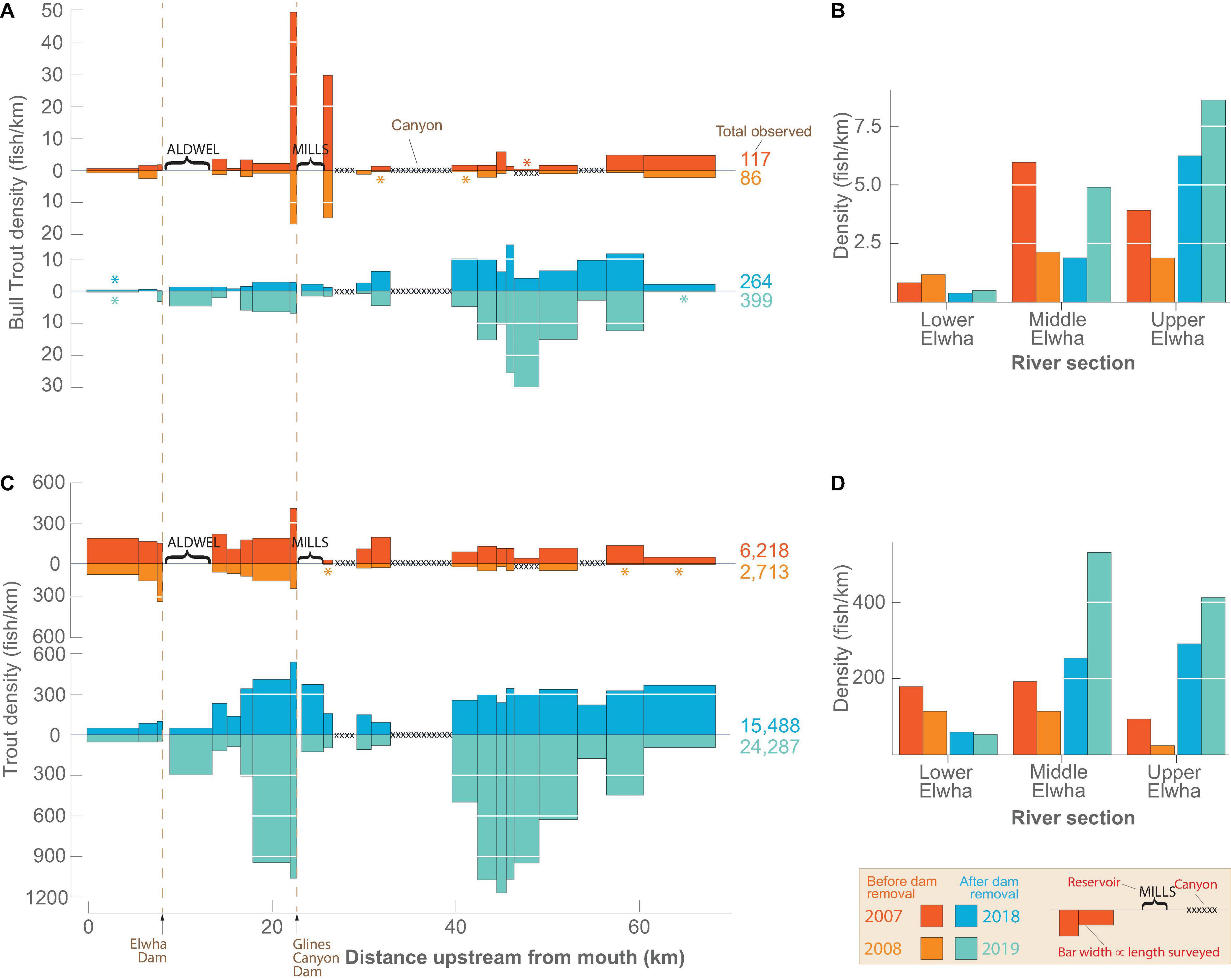
Figure 3. Snorkeling survey results showing the density (number/km surveyed) of Bull Trout and trout (Oncorhynchus spp. including Rainbow Trout and Coastal Cutthroat Trout) observed during four annual riverscape surveys. Densities are shown for reaches (A,C) and river sections (B,D), with demarcations of reaches and sections shown in Figure 1. The 2007 and 2008 surveys (warm colors) occurred prior to dam removal and the 2018 and 2019 surveys (cool colors) occurred after most dam removal effects (e.g., sediment release, turbidity, channel geomorphology; Ritchie et al., 2018) had dissipated. The start and end locations of each river section were standardized across surveys (see section “Common reaches across the four survey years”) and the width of each bar is proportional to the length of the survey measured in the field. Unsurveyed reservoir and canyon sections are indicated by labels and cross-hatch, respectively. Asterisks above and below bars indicate very low densities (>0) that are not clearly visible due to the scale of the y-axis.
Trout (Rainbow and Coastal Cutthroat combined) were detected in all 22 survey reaches both before and after dam removal (Figures 3C,D). In LE, their density was greater before dam removal, but in ME and UE density was greater after dam removal. A peak in density downstream of Glines Canyon Dam (reach 14) before dam removal also occurred after dam removal. As with Bull Trout, trout densities in the upper 9 reaches of the Elwha increased, with several reaches showing an order of magnitude increase (Figure 3C). Trout were observed in both reservoir reaches after dam removal, in some cases with densities exceeding levels in adjacent upstream and downstream reaches. The correlation of fish counts between years was similar before (Pearson’s r = 0.53; p = 0.02) and after (Pearson’s r = 0.61; p = 0.003) dam removal.
Before dam removal, adult anadromous salmon were restricted to areas downstream of Elwha Dam, but their distribution expanded into upstream areas following dam removal (Figure 4). The timing of our surveys corresponded with the peak upstream migrations of Chinook Salmon and Pink Salmon. Total numbers of Chinook Salmon decreased initially after the first survey year (548 in 2007 and 316 in 2008), but after dam removal, their numbers increased (1,609 in 2018 and 1,937 in 2019) (Figures 4A,B). Both years before dam removal, Chinook Salmon densities were greatest in reach 22 near the mouth of the river and decreased progressively upstream where they ended abruptly at Elwha Dam. After dam removal, Chinook Salmon persisted in the three reaches of LE, but also migrated past both former dam sites and were observed in 10 additional reaches (10 – 19) upstream of Elwha Dam in both years. A few individuals were observed upstream of the Grand Canyon (i.e., upstream of rkm 38) in reaches 9 (n = 4), 7 (n = 1), 4 (n = 1), and 3 (n = 2). These low numbers of fish in the upper reaches suggest that passage through Grand Canyon was possible for Chinook Salmon, but as of 2019 few pioneers had started to reoccupy these upper reaches for spawning. The densities of Chinook Salmon in the former Lake Aldwell reach were the fourth and third highest in the river in 2018 and 2019, respectively. In contrast, Chinook Salmon densities in the former Lake Mills reach were among the three lowest densities in the river for these years. During the 2018 survey, 998 adult Chinook salmon being held at the Washington Department of Fish and Wildlife hatchery holding pond (until egg-take goals were met) were released below the former Glines Canyon Dam (reach 14). This reach had the highest density of Chinook Salmon in 2018, potentially due in part to this management action. The correlation between Chinook Salmon counts between the two post-dam removal years was high (Pearson’s r = 0.94, p < 0.001), but there were insufficient data to assess the relationship before dam removal. A few Pink Salmon (26 in 2007 and 95 in 2019), Coho Salmon (a total of 21 in all four surveys) and Sockeye Salmon (a total of 9 in all four surveys) were observed both before and after dam removal.
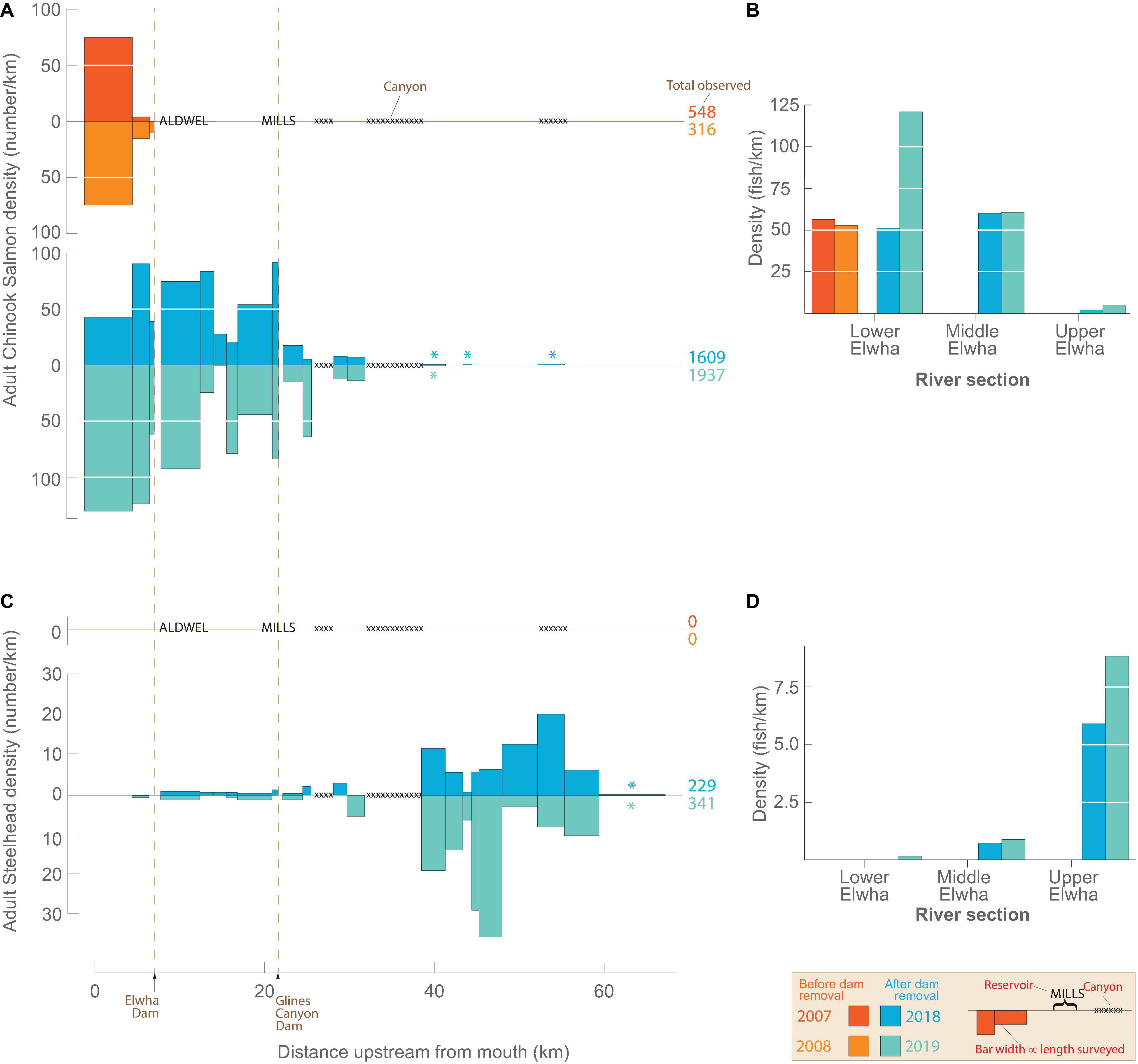
Figure 4. Snorkeling survey results showing the density (number/km surveyed) of Chinook Salmon and Summer Steelhead observed during 4 riverscape surveys. Densities are shown for reaches (A,C) and river sections (B,D), with demarcations of reaches and sections shown in Figure 1. The 2007 and 2008 surveys (warm colors) occurred prior to dam removal and the 2018 and 2019 surveys (cool colors) occurred after most dam removal effects (e.g., sediment release, turbidity, channel geomorphology; Ritchie et al., 2018) had dissipated. The start and end locations of each river section were standardized across surveys (see section “Common reaches across the four survey years”) and the width of each bar is proportional to the length of the survey measured in the field. Unsurveyed reservoir and canyon sections indicated by labels and cross-hatch, respectively. Asterisks above and below bars indicate very low densities (>0) that are not clearly visible due to the scale of the y-axis.
We did not observe Summer Steelhead in the two surveys before dam removal. Following dam removal, however, we observed 229 and 339 Summer Steelhead (Figures 4C,D) in ME and UE, with the highest densities in UE upstream of Grand Canyon (reaches 2–9). Downstream of the former Elwha Dam, only 2 Summer Steelhead were observed.
Juvenile Salmonid Occurrences After Dam Removal
The spatial extent and frequency of juvenile salmonid occurrence varied by species during 2018 and 2019 (Figure 5). Juvenile Coho Salmon and Chinook Salmon were observed upstream of the former dam sites during both years but had differing patterns. Percentages of channel units with juvenile Coho Salmon and Chinook Salmon occurrence were highest in ME in both years, but juvenile Chinook Salmon were present farther upstream and were more common than juvenile Coho Salmon, particularly in 2019 (Figures 5A,B). Juvenile trout were more broadly distributed and more ubiquitous than Coho and Chinook (Figure 5C). Their longitudinal patterns of occurrence were similar during the 2 years, except at rkm 17–37, which had lower percentages of occurrence in 2019.
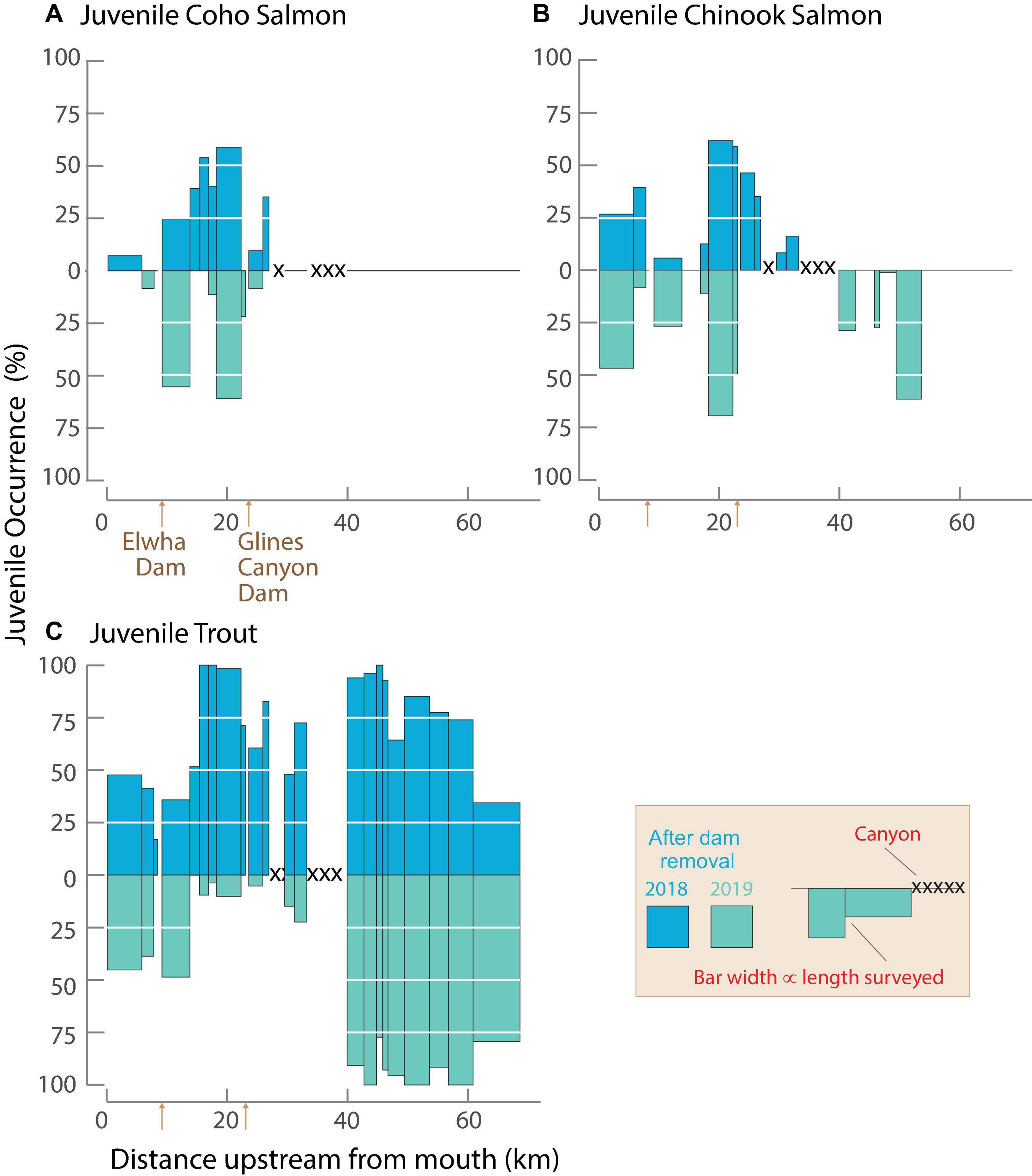
Figure 5. Percentage occurrence of juvenile Coho Salmon (A), Chinook Salmon (B), and trout (C; potentially includes Rainbow Trout, Steelhead, and Coastal Cutthroat Trout) in riverscape survey reaches. Percentages of reaches occupied by juvenile salmonids are based on channel unit length. Juvenile surveys were not consistently conducted in 2007 and 2008 before dam removal period and are not shown. Unsurveyed canyon sections are indicated with crosshatch.
Discussion
A primary goal of the Elwha River dam removal project was to restore the river’s anadromous fish runs by allowing them to ascend into upstream areas once connectivity of the river was reestablished after nearly a century of blocked passage. To assess the effects of dam removal and restored connectivity on fish migrations, we (1) compiled a timeline of anadromous fishes (five species of Pacific salmon, Pacific Lamprey, Bull Trout, and two runs of Steelhead) migrating past the former dams and (2) conducted high-scope riverscape surveys (Torgersen et al., in press) of adult and juvenile salmonids to assess changes in their spatial extent, relative abundance, and density. Bull Trout and Rainbow Trout occurred throughout the river during all four surveys (2007, 2008, 2018, 2019) but were more abundant overall and their highest densities shifted farther upstream following dam removal. Chinook Salmon also moved upstream past the two former dams, and their abundance increased threefold from 2007 to 2019. Most of these Chinook Salmon were detected downstream of the former Glines Canyon Dam, with few individuals in the upper reaches of the watershed. Population sizes of Summer Steelhead in the Elwha River prior to dam removal were severely depressed—the most critically low of all the salmonids (see Table 1 in Pess et al., 2008)—and were undetected in our surveys before dam removal. In the fourth and fifth years after dam removal, we observed 229 and 341 (respectively) adult Summer Steelhead in 19 of 22 survey reaches spanning approximately 50 km. The presence of juvenile Chinook Salmon and Coho Salmon confirmed that adults were not only passing upstream of the former dams but also successfully producing progeny that were rearing in the newly available habitat. The most pronounced physical effects of dam removal on aquatic habitat occurred in the years during and immediately following dam removal (e.g., East et al., 2015; Magirl et al., 2015; Randle et al., 2015; Morley et al., 2020), but these effects had largely dissipated by the time of our post-dam removal surveys (Peters et al., 2017; East et al., 2018; Ritchie et al., 2018). Such transient biophysical responses to dam removal have been noted elsewhere for small dam removals (Magilligan et al., 2021) but results from the Elwha showed a similar pattern with much larger dams and sediment loads. While additional studies are required to assess changes to population productivity and life-history diversity of the Elwha fish community, the restoration of connectivity is well underway (Liermann et al., 2017; Quinn et al., 2017; Lincoln et al., 2018; Brenkman et al., 2019).
Timeline of Fish Passage Upstream of the Former Dams
All nine runs of migratory fish species passed the Elwha Dam and were documented upstream within 31 months of complete dam removal (Figure 2). This response was predicted by Ward et al. (2008), but there are few examples in the literature of such a diverse migratory fish assemblage moving upstream following a dam removal (Wippelhauser, 2021). We hypothesized that the majority of these species would ascend past the former Glines Canyon Dam, but this did not occur until 60 months after dam removal. Adult Chum Salmon had not yet migrated to the Upper Elwha (UE) (Figure 2), although there were trace detections from eDNA surveys in January 2016 (Duda et al., 2021). The differences between the two dams in the timing and extent of upstream movements may be attributed to multiple factors. First, the large boulders that entered the channel in Glines Canyon downstream of the dam site created a temporary migration barrier in winter 2014 that delayed upstream migrations until full passage was restored in 2016 (Ertle et al., 2019). Second, the timing and magnitude of direct dam removal effects (e.g., sedimentation, channel restructuring) were different downstream of each dam, which can influence the outcomes or timeline of fish recovery following dam removal (Quinones et al., 2015). For example, removal began at the same time for both dams, but Elwha Dam was removed within the first 6 months of the 3-year project. Because 76% of the accumulated reservoir sediment was stored behind Glines Canyon Dam (Randle et al., 2015) and was not released until fall 2013, the conditions downstream of Elwha Dam were more favorable during the first year after removal of Elwha Dam compared to the first year after the removal of Glines Canyon Dam.
After fish passage was restored, salmonids extended their distribution upstream at different rates and to different extents. Because the dams were in place for nearly a century, limited empirical information exists on the historical spatial distribution of each salmon species (Ward et al., 2008). Based on life history characteristics of each species and the patterns of distribution in other watersheds in the Pacific Northwest, we hypothesized that Chinook Salmon, Steelhead, and Coho Salmon would ascend farther upstream than Pink Salmon, Chum Salmon and Sockeye Salmon. Predictions from intrinsic potential modeling suggested that upstream habitat conditions were more favorable for Chinook Salmon, Coho Salmon, and Steelhead than for Pink Salmon and Chum Salmon which typically spawn closer to saltwater (Pess et al., 2008). Our surveys showed that after dam removal, Summer Steelhead densities were highest in UE whereas adult Chinook Salmon densities were highest in ME and LE downstream of Grand Canyon. This is the longest in the series of canyons the river passes through and is most likely to inhibit some species from moving higher in the watershed due to potential seasonal waterfalls or other flow-related velocity barriers. Although we did detect a small number of Chinook Salmon upstream of this canyon during snorkeling surveys, it appears that they have not yet entered the high elevation reaches of the river in large numbers. This result also was consistent with a 4-year eDNA study (2014–2017), in which detection probabilities for Chinook Salmon and other anadromous species were higher in downstream reaches than in reaches upstream of Grand Canyon (Duda et al., 2021). We did observe low numbers of Pink Salmon in both even and odd years during our surveys, but they were uncommon and mostly seen in LE (downstream of the two former dams). Given the low population sizes of Pink Salmon prior to dam removal (Pess et al., 2008), this may have limited their opportunities to migrate past the former Glines Canyon Dam. Sockeye Salmon also were detected infrequently in our snorkeling surveys after dam removal, but eDNA detections occurred throughout the river. Subsequent genetic testing of tissues determined that these fish were strays from other systems on Vancouver Island and mainland British Columbia, Canada (Quinn et al., 2021). An expectation prior to dam removal (Ward et al., 2008) was that a Sockeye Salmon run in the Elwha would resume in Lake Sutherland at the headwaters of Indian Creek (a tributary between the former dams), but very few smolts and no adults have been documented. Because of their run timing later in the year than our surveys, our data cannot address the spatial distribution of adult Coho Salmon, Chum Salmon, and Winter Steelhead.
Spatial Patterns of Salmonid Density Before and After Dam Removal
Total abundance and reach-scale densities of salmonid species increased following dam removal, as both anadromous and potamodromous/resident fish species were able to migrate upstream and downstream in the free-flowing Elwha River. As anadromous species moved farther upstream into areas that were formerly unoccupied, locally high densities of Bull Trout and trout also shifted upstream. The total number of Bull Trout observed increased by 241% over the time period during which we conducted our snorkeling surveys. High density reaches near the former reservoirs that were “hotspots” for Bull Trout in 2007 and 2008 shifted upstream by 40 km (Brenkman et al., 2012). In contrast to areas upstream, Bull Trout densities declined downstream of Elwha Dam following dam removal. We hypothesize that the shift of higher density reaches into upstream areas (reaches 2–9) could be explained by multiple factors. First, Bull Trout prefer colder water associated with higher elevation and once connectivity was restored may have moved to access this better habitat. Although our surveys occurred prior to the spawning season, staging for spawning migrations to colder upstream areas could have influenced the patterns that we detected. Given seasonal opportunities to exploit estuarine food resources (Quinn et al., 2017) and a preference for colder waters for spawning, fluvial Bull Trout in a reconnected Elwha River display significant seasonal movement patterns both upstream and downstream (Brenkman et al., 2019). Another potential explanation for the higher Bull Trout densities upstream after dam removal was a fish rescue effort in June 2011. Bull Trout were removed from each reservoir and surrounding reaches prior to dam removal. Using angling, beach seining, and electrofishing, fish were captured and held until they could be translocated (Hayes and Banish, 2017) via helicopter to upstream areas in reaches 4 (Tipperary Camp, rkm 50) and 7 (Stoney Creek, rkm 43.5). Radiotelemetry data showed that the majority of fish with transmitters remained in upstream areas (NPS, unpublished data). The translocation of 82 fish into upstream areas 8 years prior to our first survey after removal may have contributed to the observed shift in high-density reaches, with progeny from transplanted fish contributing to larger population sizes upstream. Another potential explanation for the upstream shift in high-density reaches is related to Bull Trout that were present in the reservoirs but not counted because the areas were not surveyed due to their large size, depth, and poor visibility (<2 m).
The largest increase in salmonid density after dam removal was for trout, with the total number increasing by 253% over the survey period (Figure 3). While we could not differentiate between Coastal Cutthroat Trout and Rainbow Trout during snorkeling surveys, previous research suggests that the vast majority of these fish are Rainbow Trout (Brenkman and Connolly, 2008; Brenkman et al., 2008). Of the 22 reaches, only those downstream of the former Elwha Dam had lower trout densities after dam removal. It is unclear why densities of trout increased following dam removal. These taxa are not as dependent on connectivity to complete their life-cycle as their anadromous counterparts. Previous genetic studies of Steelhead and resident Rainbow Trout found that Rainbow Trout populations were genetically segregated between ME and UE populations and that these differences likely existed prior to dam construction (Winans et al., 2017; Fraik et al., 2021). With the increase in the more fecund Summer Steelhead in the upper Elwha and the potential for interactions between resident and anadromous forms of O. mykiss (Brenkman et al., 2008), admixture of Steelhead and Rainbow Trout could have contributed to increased fitness and population sizes of resident fish (Ohms et al., 2014). Furthermore, as described for Bull Trout, we did not account for adfluvial Rainbow Trout in the reservoirs during our surveys. The additional ∼8 km of novel riverine habitat in the former reservoir reaches after dam removal most likely contributed to the increased productivity of trout.
The increased counts of adult Chinook Salmon are likely representative of an increase in overall escapement. While these observations were collected during “snapshot” riverscape surveys in 2018 and 2019 that limit our ability to assess continuous trends in abundance, contemporaneous sonar estimates taken in LE confirmed the second and third largest returns on record in 2019 and 2018, respectively (Denton et al., 2020). Estimates from previous years suggest that most of these returning adults (e.g., 96 percent in 2017) were hatchery-origin fish (Weinheimer et al., 2018). This increase in population size was likely due to ocean conditions, rather than improved river conditions or increasing freshwater capacity. Few adults were observed upstream of the Grand Canyon of the Elwha, the longest canyon (5.5 km) in the river. Grand Canyon was one of three canyons in the Elwha River with potential seasonal velocity barriers due to several cascades and low waterfalls at both the upstream and downstream ends of the canyon (Brenkman et al., 2008). This section of river may currently be limiting passage of Chinook salmon due to short sections with excessive gradient and velocities. Additionally, the Chinook Salmon hatchery located 5.6 km from the mouth of the river, coupled with the dominance of hatchery-origin fish in the post-dam removal runs may also have influenced the magnitude and spatial extent of migrations into the upper portions of the watershed. The propensity of Chinook Salmon and other hatchery-influenced stocks to favor the lower portion of the watershed (Dittman et al., 2010), or that natural origin fish are more likely to be found in upstream reaches are two hypotheses that might explain why the increase in spatial extent following dam removal has not yet translated into larger proportions of anadromous salmonids occurring in upstream waters.
Summer Steelhead were not observed during snorkeling surveys before dam removal, but they were relatively abundant after dam removal. A significant number of fish migrated up to 60 km into the upper reaches of the Elwha River. Their presence and remarkable increase in abundance are likely due to numerous factors, including the contribution from resident Rainbow Trout in the middle and upper river (Brenkman et al., 2008; Kendall et al., 2015; Fraik et al., 2021). Resident Rainbow Trout produced an estimated 350-700 emigrating fish that were fully capable of saltwater migration in the early 1990s (Hiss and Wunderlich, 1994). This level of emigration occurred during a low flow year which likely increased mortality of fish passing over Glines Canyon Dam (Hiss and Wunderlich, 1994). Thus, the potential emigration from upstream Rainbow Trout may have been much greater after dam removal. This emigration may have contributed to the apparent rapid recovery of Summer Steelhead in the Elwha and is supported by the observation of emigrating smolts after dam removal with genetic ancestry from populations upstream of the former dams (Fraik et al., 2021). In addition, Summer Steelhead appear to be favoring habitats within the boundaries of Olympic National Park. Furthermore, a commercial and recreational fishing moratorium in place since 2011 eliminated any potential catch-and-release or harvest impacts to Summer Steelhead, thereby removing this stressor on population recovery. With the rapid recovery and access to high-quality habitat, recovery of Summer Steelhead is likely to continue given prudent harvest management is maintained in the future.
Juveniles of Coho Salmon, Chinook Salmon, and trout were observed upstream of both former dams, but juvenile trout were much more common throughout the river. Although low numbers of adult Coho Salmon were observed upstream of the former dams, primarily due to survey timing, the observation of juvenile Coho Salmon within the former Lake Mills reach suggests that adult Coho Salmon may be spawning upstream of both dams. The presence of juvenile Coho Salmon also shows that successful spawning of natural-origin Coho Salmon may have continued following a program to protect returning adults from dam removal effects by relocating hatchery adults (Liermann et al., 2017). These juvenile Coho Salmon observations support the recommendation that the relocation of adult hatchery Coho Salmon is no longer necessary (McHenry et al., 2020a).
Juvenile Chinook Salmon were observed farther upstream in 2019 than in 2018 (Figure 5), supporting the hypothesis that Chinook Salmon may be expanding their distribution in the UE. The frequency of juvenile Chinook Salmon in the upper basin in 2019 may have been influenced by relatively high escapement of adults during the previous year (i.e., the third greatest escapement on record; Denton et al., 2020). This group of adults produced more than 500,000 Chinook migrants during the spring of 2019, which is the most abundant smolt production since dam removal (McHenry et al., 2020b). This was followed in the next year by over 1,000,000 smolts (McHenry et al., 2021). Greater numbers of Chinook Salmon adults may expand their distribution upstream in the future if these juveniles are able to return as adults and adapt to conditions in the upper river (UE). Future monitoring will be necessary to confirm whether Chinook Salmon are expanding their distribution and spawning in the upper river.
Juvenile Trout were ubiquitous throughout the Elwha River, but their distribution shifted between 2018 and 2019. The percentage of channel units with juvenile trout decreased in ME from 2018 to 2019, but there was a slight increase in their frequency in UE. It is unclear what caused this decrease in the river section between the former dams. Potential causes of the decrease in juvenile trout occurrence in ME are not apparent based on our limited data for this life-stage. However, the increase in UE may be related to the presence of Summer Steelhead, which due to their larger size are more fecund than Rainbow Trout and therefore more likely to produce more progeny, resulting in local increases in juvenile trout occupancy.
Caveats
Riverscape surveys of salmonid distribution and density provided a comprehensive perspective on spatial patterns before and after dam removal. However, there are caveats associated with using these data for inferring the effects of dam removal on fish populations. We designed our surveys to coincide with low summer flows to ensure the best visibility while snorkeling and the safety of personnel working in a remote wilderness river. Limiting the surveys to summer, however, diminished our ability to assess spatial distribution for some species which enter the river to spawn in the spring, fall, and winter (e.g., Chum Salmon, Winter Steelhead). Thus, our surveys were focused on a subset of the migratory species in the Elwha River. Other approaches are needed to examine densities, spatial extent, and other important facets of fish ecology for these species.
Our fish surveys were high resolution and covered the majority of the migratory fish zone, but they are single, unreplicated ‘snapshots’ of dynamic processes. Several factors may contribute to bias, imprecision, and uncertainty in our results. The first factor is natural, interannual variability in population size caused by variation in processes that affect different parts of the salmon life cycle, such as smolt-to-adult survival, marine survival, and hydrologic variability (e.g., Kruse, 1998; Cross et al., 2009; Ward et al., 2015). The average discharge of the Elwha River was higher before dam removal than after (Table 1), but during low flows, this likely would have had a greater effect on observer error than on population size. Additionally, the potential for interannual variation in run timing and the short duration of our survey (typically 1 week) may contribute to apparent differences among years. Nevertheless, population estimates for Chinook Salmon based on redd counts (before dam removal) and sonar (after dam removal) showed that abundances after dam removal were significantly higher than before dam removal, similar to results from our snorkeling surveys.
The second source of uncertainty is observation error, the main components of which are inter-observer variability (where observers have different efficiencies in detecting, identifying, and counting fish underwater) and detectability (the degree to which fish are present but not visible to the diver). Snorkelers in our surveys were highly trained and had multiple years of experience, with many having conducted multiple riverscape surveys on the Olympic Peninsula within the past decade. Previous studies examining the precision of snorkeling counts on other rivers in the Olympic Peninsula showed that coefficients of variation ranged from 0.10 to 0.24 (Brenkman and Connolly, 2008) and across multiple species did not exceed 0.25. Given the objectives of our study, in particular our examination of fish counts over tens of kilometers, we determined that this level of variation was acceptable, as has been shown in comparisons of snorkeling surveys to other sampling methods (Thurow and Peterson, 2006; Plichard et al., 2016).
The spatially intensive riverscape approach that we employed mitigates, to a degree, our lack of formal error estimates for fish count data. Other common approaches used in fish ecology rely on probabilistic sampling by measuring a subset of “representative” or randomly selected habitats in river reaches to infer the statistical significance of changes in abundance (see review by Radinger et al., 2018). Spatially continuous sampling of reaches using a riverscape approach (sensu Fausch et al., 2002) allowed us to collect high-resolution data over a relatively large extent (i.e., the entire river, except for hazardous canyon sections). To determine the effectiveness of fish passage following dam removal and changes in spatial extent and heterogeneity (i.e., “hot spots”) of anadromous and potadromous fish, we found it necessary to apply a riverscape approach specifically designed for detecting changes in spatial patterns as opposed to average conditions (Torgersen et al., in press). Additionally, for the anadromous species that we examined in this study, the density and distribution patterns upstream of the dams were measured against known absence (i.e., zero counts) because fish passage was completely blocked. As such, we were certain that there were no anadromous fish (specifically Coho, Chinook, and Steelhead) in areas upstream of the dams. Thus, any changes in fish distribution and abundance upstream, even when estimates of bias and precision are lacking, provide a valuable measure of changes in anadromous fish populations. Coupling riverscape surveys with complementary approaches that provide robust estimates of population size (e.g., sonar), productivity (e.g., smolt trapping), and more detailed estimates of fish movement (e.g., telemetry) will provide a more comprehensive picture of the riverine ecosystem and the effects of fish restoration in the Elwha River.
Finally, our summary of first fish observations upstream of each dam provides a general timeline for understanding the temporal context of fish movement upstream after dam removal. We assembled this timeline from many different ongoing studies and researchers to provide documentation of fish passage past each dam. The timeline is based largely on one-time observations of species that may have been a single fish passing upstream of the dam. Thus, it does not address the rate of population expansion upstream of each dam, nor the overall temporal pattern of fish passage. Yet, it does provide an important historical record of how initial fish passage differed among species and between the dams.
Conclusion
The goal of the Elwha River dam removal project was to restore connectivity in the river ecosystem for the benefit of native anadromous fish. This is the largest dam removal to date and it cost over 340 million USD to purchase and remove the dams while mitigating the effects of dam removal. Connectivity was restored, allowing the return of anadromous and migratory fish populations to a watershed that previously had lost 90% of its anadromous fish habitat due to unpassable dams. It also restored approximately 8 km of river previously inundated by reservoirs. Given the ecosystem response to dam removal thus far, tracking the future trajectory of fish populations will be necessary to understand both short-term and long-term dam removal responses and how they may translate into long-term recovery (Bellmore et al., 2019; Magilligan et al., 2021).
We showed that in only 5 years, dam removal profoundly influenced fish populations in the Elwha River. After dam removal, we counted 2–4 times as many Bull Trout, trout, and Chinook Salmon and hundreds of Summer Steelhead which were previously very rare in the river. All nine migratory fish runs passed the former Elwha Dam within 31 months, and 8 of 9 ascended through Glines Canyon within 60 months. Native anadromous salmonids were documented up to 40 km upstream of the upper dam and in approximately 8 km of novel river reaches contained in the footprint of the former reservoirs. Juvenile Coho Salmon and Chinook Salmon were also seen upstream of each dam, demonstrating that spawning adults and their progeny are utilizing upstream habitats. The reconnection of the Elwha River will have far-reaching implications for genetic diversity, life history diversity, and habitat use of these species, which has started to be documented for Steelhead (Fraik et al., 2021), Pacific Lamprey (Hess et al., 2021), Coho Salmon (Liermann et al., 2017), and Bull Trout (Quinn et al., 2017; Lincoln et al., 2018; Brenkman et al., 2019). Although our study was not designed to estimate population sizes, the observed densities increased and there was an upstream shift in the locations of higher density reaches for both trout and Bull Trout. Salmonid species expanded their upstream distributions to different degrees and Chinook Salmon have yet to occupy upstream reaches at densities observed downstream of the former Glines Canyon Dam. However, our surveys occurred before a full generation of Chinook Salmon had occurred after dam removal. More studies are needed to determine the mechanisms that may limit or promote spawning and rearing upstream of the former dams by the progeny of these early generations of post-dam removal salmonids. In the face of possible changing flow and temperature regimes due to climate change, such information will be particularly important for fish populations in the upper reaches of the watershed.
Prior to dam removal, there were uncertainties about the magnitude and duration of dam removal impacts and how this might influence the recovery of anadromous fish (McHenry and Pess, 2008; Ward et al., 2008). Our observations suggest that these short-term disturbances from dam removal (e.g., sedimentation, geomorphic responses) did not have long-term negative impacts on most salmonid populations documented in our study. In the planning stages of dam removal, natural resource managers aimed to recover self-sustaining anadromous salmonid populations in the Elwha River within 5-10 generations after dam removal. Large gains have already been made by the first generation of some species since the completion of dam removal, but the final outcome for the Elwha River ecosystem and its fish populations will continue to emerge over the coming decades.
Data Availability Statement
The datasets presented in this study can be found online at Torgersen et al. (2021).
Author Contributions
CT, JD, SB, GP, and RP conceptualized the study. SB, JD, CT, HC, and AG coordinated the field planning. All the authors participated in data collection, edited and approved the submitted version. JD, CT, RP, KS, EW, and SB conducted the data analysis and figure generation, and wrote the manuscript.
Funding
Funding was provided by the National Park Service, U.S. Fish and Wildlife Service, Trout Unlimited, and the U.S. Geological Survey Ecosystems Mission Area.
Author Disclaimer
This article has been peer reviewed and approved for publication consistent with USGS Fundamental Science Practices (https://pubs.usgs.gov/circ/1367/). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This project would not have been possible without the following volunteers who contributed as snorkelers and assisted with data collection: S. Anderson, T. Bennett, M. Beirne, N. Chambers, J. Deady, K. Denton, J. Dunham, M. Elofson, A. Fraik, J. Ganzhorn, C. Glenney, M. Groce, J Hagen, M. Hanks, T. Hoem-Neher, R. Hoffman, J. Johnson, T. Jurasin, L. Kelly, K. Kirkby, B. Krier, D. Lanz, T. Leavy, R. Masonis, J. Michel, A. Miller, L. Moulton, S. Neil, C. O’Connell, L. Ogg, C. Rice, I. Smith, J. Starr, S. Sampson, A. Simmons, J. Stapleton, L. Ward, J. Winkowski, and M. Zimmerman. We thank L. Baysinger, S. Baysinger, H. Brill, B. Lasswell, and B. Jones for horse packing support. We are grateful for comments by Joe Anderson and the two reviewers that helped us improve quality of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.765488/full#supplementary-material
References
Allen, M. B., Engle, R. O., Zendt, J. S., Shrier, F. C., Wilson, J. T., and Connolly, P. J. (2016). Salmon and steelhead in the White Salmon River after the removal of Condit Dam–Planning efforts and recolonization results. Fisheries 41, 190–203. doi: 10.1080/03632415.2016.1150839
American Rivers (2019). Data from: american rivers dam removal database. Figshare Dig. Rep. 2019:5234068636. doi: 10.6084/m9.figshare.5234068636
Battle, L., Chang, H. Y., Tzeng, C. S., and Lin, H. J. (2016). The impact of dam removal and climate change on the abundance of the Formosan landlocked salmon. Ecol. Modell. 2016:5. doi: 10.1016/j.ecolmodel.2016.08.005
Bednarek, A. T. (2001). Undamming rivers: A review of the ecological impacts of dam removal. Environ. Manage. 27, 803–814. doi: 10.1007/s002670010189
Bellmore, J. R., Pess, G. R., Duda, J. J., O’Connor, J. E., East, A. E., Foley, M. M., et al. (2019). Conceptualizing ecological responses to dam removal: If you remove it, what’s to come? Bioscience 69, 12–14. doi: 10.1093/biosci/biy152
Bellmore, R. J., Duda, J. J., Craig, L. S., Greene, S. L., Torgersen, C. E., Collins, M. J., et al. (2017). Status and trends of dam removal research in the United States. Wiley Interdiscip. Rev. Water 4:e164. doi: 10.1002/wat2.1164
Bisson, P. A., Montgomery, D. R., and Buffington, J. M. (2017). “Valley segments, stream reaches, and channel units,” in Methods in Stream Ecology: Ecosystem Structure, eds F. R. Hauer and G. Lamberti (San Diego, CA: Elsevier Science and Technology). doi: 10.1007/s00267-003-0157-4
Branco, P., Segurado, P., Santos, J. M., and Ferreira, M. T. (2014). Prioritizing barrier removal to improve functional connectivity of rivers. J. Appl. Ecol. 51, 1197–1206. doi: 10.1111/1365-2664.12317
Brenkman, S. J., and Connolly, P. J. (2008). Protocol for Monitoring Fish Assemblages in Pacific Northwest National Parks. Reston, VA: U.S. Geological Survey Techniques and Methods.
Brenkman, S. J., Duda, J. J., Torgersen, C. E., Welty, E., Pess, G. R., Peters, R., et al. (2012). A riverscape perspective of Pacific salmonids and aquatic habitats prior to large-scale dam removal in the Elwha River. Washington, USA. Fish. Manag. Ecol. 19, 36–53. doi: 10.1111/j.1365-2400.2011.00815.x
Brenkman, S. J., Pess, G. R., Torgersen, C. E., Kloehn, K. K., Duda, J. J., and Corbett, S. C. (2008). Predicting recolonization patterns and interactions between potamodromous and anadromous salmonids in response to dam removal in the Elwha River, Washington. Northwest Sci. 82, (Suppl. 1), 91–106. doi: 10.3955/0029-344X-82.S.I.91
Brenkman, S. J., Peters, R. J., Tabor, R. A., Geffre, J. J., and Sutton, K. T. (2019). Rapid recolonization and life history responses of bull trout following dam removal in Washington’s Elwha River. North Am. J. Fish. Manag. 39, 560–573. doi: 10.1002/nafm.10291
Bunn, S. E., and Arthington, A. H. (2002). Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manage. 30, 492–507. doi: 10.1007/s00267-002-2737-0
Burdick, S. M., and Hightower, J. E. (2006). Distribution of spawning activity by anadromous fishes in an Atlantic slope drainage after removal of a low-head dam. Trans. Am. Fish. Soc. 135, 1290–1300. doi: 10.1577/t05-190.1
Catalano, M. J., Bozek, M. A., and Pellett, T. D. (2007). Effects of dam removal on fish assemblage structure and spatial distributions in the Baraboo River, Wisconsin. North Am. J. Fish. Manag. 27, 519–530. doi: 10.1577/M06-001.1
Cross, A. D., Beauchamp, D. A., Moss, J. A., and Myers, K. W. (2009). Interannual variability in early marine growth, size-selective mortality, and marine survival for Prince William Sound pink salmon. Mar. Coast. Fish. 2009, 57–70. doi: 10.1577/C08-005.1
Denton, K., McHenry, M., Moses, R., Ward, E., Stefankiv, O., Wells, W., et al. (2020). 2020 Elwha River Chinook escapement estimate based on DIDSON/ARIS multi-beam SONAR data. Lower Elwha Klallam Tribe. Port Angeles, WA. Lower Elwha Klallam Natural Resources Department
Dittman, A. H., May, D., Larsen, D. A., Moser, M. L., Johnston, M., and Fast, D. (2010). Homing and spawning site selection by supplemented hatchery- and natural-origin Yakima River spring Chinook salmon. Trans. Am. Fish. Soc. 139, 1014–1028. doi: 10.1577/T09-159.1
Draut, A. E., Logan, J. B., and Mastin, M. C. (2011). Channel evolution on the dammed Elwha River, Washington, USA. Geomorphology 127, 71–87. doi: 10.1016/j.geomorph.2010.12.008
Duda, J. J., and Bellmore, J. R. (in press). “Dam Removal and River Restoration,” in Encyclopedia of Inland Waters, eds K. Tockner and T. Mehner (Oxford, UK: Elsevier Ltd). doi: 10.1016/B978-0-12-819166-8.00101-8
Duda, J. J., Brenkman, S. J., Crain, P. Woodward, Jenkins, K., and Haggerty, P. (2018). “Pacific Salmonids,” in Natural Resource Condition Assessment: Olympic National Park, eds R. McCaffery and K. Jenkins (Port Angeles, WA: Natural Resource Report).
Duda, J. J., Freilich, J. E., and Schreiner, E. G. (2008). Baseline studies in the elwha river ecosystem prior to dam removal: introduction to the special issue. Northwest Sci. 82(Suppl. 1), 1–12. doi: 10.3955/0029-344X-82.S.I.1
Duda, J. J., Hoy, M. S., Chase, D. M., Pess, G. R., Brenkman, S. J., McHenry, M. M., et al. (2021). Environmental DNA is an effective tool to track recolonizing migratory fish following large-scale dam removal. Environ. DNA. 3, 121–141. doi: 10.1002/edn3.134
Duda, J. J., Warrick, J. A., and Magirl, C. S. (2011). “Coastal and lower Elwha River, Washington, prior to dam removal–History, status, and defining characteristics,” in Coastal Habitats of the Elwha River, Washington–Biological and Physical Patterns and Processes Prior to Dam Removal, eds J. J. Duda, J. A. Warrick, and C. S. Magirl (Port Angeles, WA: Geological Survey Scientific Investigations Report), doi: 10.3133/sir201151201
East, A. E., Logan, J. B., Mastin, M. C., Ritchie, A. C., Bountry, J. A., Magirl, C. S., et al. (2018). Geomorphic evolution of a gravel-bed river under sediment-starved versus sediment-rich conditions: river response to the world’s largest dam removal. J. Geophys. Res. Earth Surf. 123, 3338–3369. doi: 10.1029/2018jf004703
East, A. E., Pess, G. R., Bountry, J. A., Magirl, C. S., Ritchie, A. C., Logan, J. B., et al. (2015). Large-scale dam removal on the Elwha River, Washington, USA: River channel and floodplain geomorphic change. Geomorphology 246, 687–708. doi: 10.1016/j.geomorph.2015.04.027
Ertle, C. W., Roth, J. M., Judson, J. S., and Vankirk, G. H. (2019). Rock demolition and hazardous debris removal for ecosystem restoration on the Elwha River. Vicksburg, MS: ERDC/GSL TR, 19–21.
Fausch, K. D., Torgersen, C. E., Baxter, C. V., and Li, H. W. (2002). Landscapes to riverscapes : bridging the gap between research and conservation of stream fishes. Bioscience 52, 483–498.
Foley, M. M., Bellmore, J. R., O’Connor, J. E., Duda, J. J., East, A. E., Grant, G. E., et al. (2017a). Dam removal: Listening in. Water Resour. Res. 53, 5229–5246. doi: 10.1002/2017WR020457
Foley, M. M., Magilligan, F. J., Torgersen, C. E., Major, J. J., Anderson, C. W., Connolly, P. J., et al. (2017b). Landscape context and the biophysical response of rivers to dam removal in the United States. PLoS One 7:e0180107. doi: 10.1371/journal.pone.0180107
Foley, M. M., Warrick, J. A., Ritchie, A., Stevens, A. W., Shafroth, P. B., Duda, J. J., et al. (2017c). Coastal habitat and biological community response to dam removal on the Elwha River. Ecol. Monogr. 87, 552–577. doi: 10.1002/ecm.1268
Fraik, A. K., McMillan, J. R., Liermann, M., Bennett, T., McHenry, M. L., McKinney, G. J., et al. (2021). The impacts of dam construction and removal on the genetics of recovering steelhead (Oncorhynchus mykiss) populations across the Elwha River watershed. Genes 12:89. doi: 10.3390/genes12010089
Gelfenbaum, G., Stevens, A. W., Miller, I., Warrick, J. A., Ogston, A. S., and Eidam, E. (2015). Large-scale dam removal on the Elwha River, Washington, USA: Coastal geomorphic change. Geomorphology 246, 649–668. doi: 10.1016/j.geomorph.2015.01.002
Grill, G., Lehner, B., Lumsdon, A. E., MacDonald, G. K., Zarfl, C., and Reidy Liermann, C. (2015). An index-based framework for assessing patterns and trends in river fragmentation and flow regulation by global dams at multiple scales. Environ. Res. Lett. 10:15001. doi: 10.1088/1748-9326/10/1/015001
Grill, G., Lehner, B., Thieme, M., Geenen, B., Tickner, D., Antonelli, F., et al. (2019). Mapping the world’s free-flowing rivers. Nature 569, 215–221. doi: 10.1038/s41586-019-1111-9
Hayes, M. F., and Banish, N. P. (2017). Translocation and reintroduction of native fishes: a review of bull trout Salvelinus confluentus with applications for future reintroductions. Endanger. Species Res. 34, 191–209. doi: 10.3354/esr00849
Hess, J. E., Paradis, R. L., Moser, M. L., Weitkamp, L. A., Delomas, T. A., and Narum, S. R. (2021). Robust recolonization of Pacific lamprey following dam removals. Trans. Am. Fish. Soc. 150, 56–74. doi: 10.1002/tafs.10273
Hiss, J. M., and Wunderlich, R. C. (1994). Salmonid availability and migration in the middle Elwha River system. Olympia, WA: U.S. Fish and Wildlife Service.
Hitt, N. P., Eyler, S., and Wofford, J. E. B. (2012). Dam removal increases American Rel abundance in distant headwater streams. Trans. Am. Fish. Soc. 141, 1171–1179. doi: 10.1080/00028487.2012.675918
Hogg, R. S., Coghlan, S. M., Zydlewski, J., and Gardner, C. (2015). Fish community response to a small-stream dam removal in a Maine coastal river tributary. Trans. Am. Fish. Soc. 144, 467–479. doi: 10.1080/00028487.2015.1007164
Jones, P. E., Tummers, J. S., Galib, S. M., Woodford, D. J., Hume, J. B., Silva, L. G. M., et al. (2021). The use of barriers to limit the spread of aquatic invasive animal species: A global review. Front. Ecol. Evol. 2021:611631. doi: 10.3389/fevo.2021.611631
Kemp, P. S., and O’Hanley, J. R. (2010). Procedures for evaluating and prioritising the removal of fish passage barriers: A synthesis. Fish. Manag. Ecol. 17, 297–322. doi: 10.1111/j.1365-2400.2010.00751.x
Kendall, N. W., McMillan, J. R., Sloat, M. R., Buehrens, T. W., Quinn, T. P., Pess, G. R., et al. (2015). Anadromy and residency in steelhead and rainbow trout (Oncorhynchus mykiss): a review of the processes and patterns. Can. J. Fish. Aquat. Sci. 72, 319–342. doi: 10.1139/cjfas-2014-0192
Kornis, M., Weidel, B., Powers, S., Diebel, M., Cline, T., Justin, M., et al. (2014). Fish community dynamics following dam removal in a fragmented agricultural stream. Aquat. Sci. 77, 465–480. doi: 10.1007/s00027-014-0391-2
Kruse, G. H. (1998). Salmon run failures in 1997-1998: A link to anomalous ocean conditions? Alaska Fish. Res. Bull. 5, 55–63.
Lehner, B., Liermann, C. R., Revenga, C., Vörömsmarty, C., Fekete, B., Crouzet, P., et al. (2011). High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 9:494–502. doi: 10.1890/100125
Lejon, A. G. C., Renofalt, B. M., and Nilsson, C. (2009). Conflicts associated with dam removal in Sweden. Ecol. Soc. 14:19. doi: 10.5751/ES-02931-140204
Liermann, M., Pess, G., McHenry, M., McMillan, J., Elofson, M., Bennett, T., et al. (2017). Relocation and recolonization of coho salmon in two tributaries to the Elwha River: Implications for management and monitoring. Trans. Am. Fish. Soc. 146, 955–966. doi: 10.1080/00028487.2017.1317664
Lincoln, A. E., Shaffer, J. A., and Quinn, T. P. (2018). Opportunistic use of estuarine habitat by juvenile bull trout, Salvelinus confluentus, from the Elwha River before, during, and after dam removal. Env. Biol. Fish. 101, 1559–1569. doi: 10.1007/s10641-018-0800-9
Magilligan, F. J., Graber, B. H., Nislow, K. H., Chipman, J. W., Sneddon, C. S., and Fox, C. A. (2016). River restoration by dam removal: Enhancing connectivity at watershed scales. Elementa 4:e000108. doi: 10.12952/journal.elementa.000108
Magilligan, F. J., Nislow, K. H., Dietrich, J. T., Doyle, H., and Kynard, B. (2021). Transient versus sustained biophysical responses to dam removal. Geomorphology 389:e107836. doi: 10.1016/j.geomorph.2021.107836
Magirl, C. S., Hilldale, R. C., Curran, C. A., Duda, J. J., Straub, T. D., Domanski, M., et al. (2015). Large-scale dam removal on the Elwha River, Washington, USA: Fluvial sediment load. Geomorphology 246, 669–686. doi: 10.1016/j.geomorph.2014.12.032
McHenry, M., Elofson, M., Liermann, M., Bennett, T., Corbett, S., and Pess, G. (2020b). “2019 Elwha River smolt enumeration project report”. Report submitted to Olympic National park by the Lower Elwha Klallam Tribe. Port Angeles, WA.
McHenry, M., Elofson, M., Liermann, M., Bennett, T., and Pess, G. (2021). “2020 Elwha River smolt enumeration project report”. Report submitted to Olympic National park by the Lower Elwha Klallam Tribe. Port Angeles, WA.
McHenry, M., McMillan, J., Moses, R., and Pess, G. (2020a). “Coho salmon relocation and red surveys in the Elwha River 2011-2019: Summary report”. Report submitted to Olympic National park by the Lower Elwha Klallam Tribe. Port Angeles, WA.
McHenry, M. L., and Pess, G. R. (2008). An overview of monitoring options for assessing the response of salmonids and their aquatic ecosystems in the Elwha River following dam removal. Northwest Sci. 82, (Suppl. 1), 29–47. doi: 10.3955/0029-344X-82.S.I.29
McLaughlin, R. L., Hallett, A., Pratt, T. C., and O’Connor, L. M. and McDonald, G. D. (2007). Research to guide use of barriers, traps, and fishways to control sea lamprey. J. Great Lakes Res. 33, 7–19. doi: 10.3394/0380-1330
McMillan, J. R., Liermann, M. C., Starr, J., Pess, G. R., and Augerot, X. (2013). Using a stream network census of fish and habitat to assess models of juvenile salmonid distribution. Trans. Am. Fish. Soc. 142, 942–956. doi: 10.1080/00028487.2013.790846
Morley, S. A., Foley, M. M., Duda, J. J., Beirne, M. M., Paradis, R. L., Johnson, R. C., et al. (2020). Shifting food web structure during dam removal—Disturbance and recovery during a major restoration action. PLoS One 15:e0239198. doi: 10.1371/journal.pone.0239198
Muha, T. P., Rodriguez-Barreto, D., O’Rorke, R., Garcia de Leaniz, C., and Consuegra, S. (2021). Using eDNA metabarcoding to monitor changes in fish community composition after barrier removal. Front. Ecol. Evol. 2021:629217. doi: 10.3389/fevo.2021.629217
O’Connor, J. E., Duda, J. J., and Grant, G. E. (2015). 1000 dams down and counting. Science 348, 496–497. doi: 10.1126/science.aaa9204
Ohms, H. A., Sloat, M. R., Reeves, G. H., Jordan, C. E., and Dunham, J. B. (2014). Influence of sex, migration distance, and latitude on life history expression in steelhead and rainbow trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 71, 70–80. doi: 10.1139/cjfas-2013-0274
O’Neal, J. S. (2007). “Snorkel surveys,” in Salmonid Field Protocols Handbook: Techniques for Assessing Status and Trends in Salmon and Trout Populations, eds D. H. Johnson, B. M. Shrier, J. S. O’Neal, J. A. Knutzen, X. Augerot, T. A. O’Neil, et al. (Bethesda, MD: American Fisheries Society).
Palmer, M., and Ruhi, A. (2019). Linkages between flow regime, biota, and ecosystem processes: Implications for river restoration. Science 365:eaww2087. doi: 10.1126/science.aaw2087
Pecl, G. T., Araújo, M. B., Bell, J. D., Blanchard, J., Bonebrake, T. C., Chen, I. C., et al. (2017). Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355:eaai9214. doi: 10.1126/science.aai9214
Pess, G. R., McHenry, M. L., Beechie, T. J., and Davies, J. (2008). Biological impacts of the Elwha River dams and potential salmonid responses to dam removal. Northwest Sci. 82, (Suppl. 1), 72–90. doi: 10.3955/0029-344X-82.S.I.72
Pess, G. R., Quinn, T. P., Gephard, S. R., and Saunders, R. (2014). Re-colonization of Atlantic and Pacific rivers by anadromous fishes: linkages between life history and the benefits of barrier removal. Rev. Fish Biol. Fish. 24, 881–900. doi: 10.1007/s11160-013-9339-1
Peters, R. J., Duda, J. J., Pess, G. R., Zimmerman, M., Crain, P., Hughes, Z., et al. (2014). Guidelines for monitoring and adaptively managing Chinook salmon (Oncorhynchus tshawytscha) and steelhead (O. mykiss) restoration in the Elwha River. Lacey, WA: U.S. Fish and Wildlife Service, Washington Field Office.
Peters, R. J., Liermann, M., McHenry, M. L., Bakke, P., and Pess, G. R. (2017). Changes in streambed composition in salmonid spawning habitat of the Elwha River during dam removal. J. Am. Water Resour. Assoc. 53, 871–885. doi: 10.1111/1752-1688.12536
Petts, G. E. (1984). Impounded Rivers: Perspectives for Ecological Management. New York, NY: Wiley, doi: 10.1016/0006-3207(85)90110-7
Plichard, L., Capra, H., Mons, R., Pella, H., and Lamouroux, N. (2016). Comparing electrofishing and snorkeling for characterizing fish assemblages over space and time. Can. J. Fish. Aquat. Sci. 74, 75–86. doi: 10.1139/cjfas-2015-0578
Poff, N. L., Allan, J. D., Bain, M. B., Karr, J. R., Prestegaard, K. L., Richter, B. D., et al. (1997). The natural flow regime. Bioscience 47, 769–784. doi: 10.2307/1313099
Poff, N. L. R. (2018). Beyond the natural flow regime? Broadening the hydro-ecological foundation to meet environmental flows challenges in a non-stationary world. Freshwater. Biol. 63, 1011–1021. doi: 10.1111/fwb.13038
Poff, N. L. R., and Schmidt, J. C. (2016). How dams can go with the flow. Science 353, 1099–1100. doi: 10.1126/science.aah4926
Pohl, M. (2004). Channel bed mobility downstream from the Elwha dams. Washington. Prof. Geogr. 56, 422–431. doi: 10.1111/j.0033-0124.2004.05603010.x
Pohl, M. M. (2002). Bringing down our dams: Trends in American dam removal rationales. J. Am. Water Resour. Assoc. 38, 1511–1519. doi: 10.1111/j.1752-1688.2002.tb04361.x
Quinn, T. P., Bond, M. H., Brenkman, S. J., Paradis, R., and Peters, R. J. (2017). Re-awakening dormant life history variation: stable isotopes indicate anadromy in bull trout following dam removal on the Elwha River. Washington. Environ. Biol. Fishes. 100, 1659–1671. doi: 10.1007/s10641-017-0676-0
Quinn, T. P., Pess, G. P., Sutherland, B. J., Brenkman, S. J., and Withler, R. E. (2021). Resumption of anadromy or straying? Origins of sockeye salmon in the Elwha River. Trans. Am. Fish. Soc. 150, 452–464. doi: 10.1002/tafs.10294
Quinones, R. M., Grantham, T. E., Harvey, B. N., Kiernan, J. D., and Klasson. (2015). Dam removal and anadromous salmonid (Oncorhynchus spp.) conservation in California. Rev. Fish. Biol. Fisheries 25, 195–215. doi: 10.1007/s11160-014-9359-5
R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna: R Core Team.
Radinger, J., Britton, J. R., Carlson, S. M., Magurran, A. E., and Alcaraz-Hernandez, J. D. (2018). Effective monitoring of freshwater fish. Fish Fish. 20, 729–747.
Randle, T. J., Bountry, J. A., Ritchie, A., and Wille, K. (2015). Large-scale dam removal on the Elwha River, Washington, USA: Erosion of reservoir sediment. Geomorphology 246, 709–728. doi: 10.1016/j.geomorph.2014.12.045
Ritchie, A. C., Warrick, J. A., East, A. E., Magirl, C. S., Stevens, A. W., Bountry, J. A., et al. (2018). Morphodynamic evolution following sediment release from the world’s largest dam removal. Sci. Rep. 8:13279. doi: 10.1038/s41598-018-30817-8
Roy, S. G., Uchida, E., de Souza, S. P., Blachly, B., Fox, E., Gardner, K., et al. (2018). A multiscale approach to balance trade-offs among dam infrastructure, river restoration, and cost. Proc. Natl. Acad. Sci. USA. 115, 12069–12074. doi: 10.1073/pnas.1807437115
Su, G., Logez, M., Xu, J., Tao, S., Villeger, S., and Brosse, S. (2021). Human impacts on global freshwater fish biodiversity. Science 371, 835–838. doi: 10.1126/science.abd3369
Thurow, R. F., and Peterson, J. T. (2006). Utility and validation of day and night snorkel counts for estimating bull trout abundance in first- to third-order streams. N. Am. J. Fish. Manage. 26, 217–232. doi: 10.1577/M05-054.1
Torgersen, C. E., Duda, J. J., Brenkman, S. J., Peters, R. J., and Sutton, K. T. (2021). Riverscape snorkeling surveys of salmonid distribution and abundance before (2007, 2008) and after (2018, 2019) dam removal on the Elwha River. Washington: U.S. Geological Survey data release, doi: 10.5066/P9MFJXK1
Torgersen, C. E., Le Pichon, C., Fullerton, A. H., Dugdale, S. J., Duda, J. J., Giovannini, F., et al. (in press). Riverscape approaches in practice: Perspectives and applications. Biol. Rev. doi: 10.1111/brv.12810
USGS. (2021). Data inventory page for site 12045500—Elwha River at McDonald BR near. Port Angeles, WA. Available online at: https://waterdata.usgs.gov/nwis/inventory/?site_no=12045500. (accessed March 16, 2021).
Ward, E. J., Anderson, J. H., Beechie, T. J., and Pess, G. R. (2015). Increasing hydrologic variability threatens depleted anadromous fish populations. Glob. Change Biol. 21, 2500–2509. doi: 10.1111/gcb.12847
Ward, L., Crain, P., Freymond, B., McHenry, M., Morrill, D., and Pess, G. R. (2008). Elwha River fish restoration plan—developed pursuant to the Elwha River Ecosystem and Fisheries Restoration Act, Public Law 102-495. NOAA Tech. Memo. NMFS-NWFSC-90. Seattle, WA: U.S. Department of Commerce.
Warrick, J. A., Bountry, J. A., East, A. E., Magirl, C. S., Randle, T. J., Gelfenbaum, G., et al. (2015). Large-scale dam removal on the Elwha River, Washington, USA: Source-to-sink sediment budget and synthesis. Geomorphology 246, 729–750. doi: 10.1016/j.geomorph.2015.01.010
Weinheimer, J., Anderson, J., Cooper, R., Williams, S., McHenry, M., Crain, P., et al. (2018). Age structure and Hatchery Fraction of Elwha River Chinook Salmon: 2017 Carcass Survey Report. Olympia, WA: Washington Department of Fish and Wildlife, Fish Science Division Report FPA 18-05.
Welty, E. Z., Torgersen, C. E., Brenkman, S. J., Duda, J. J., and Armstrong, J. B. (2015). Multiscale analysis of river networks using the R package linbin. North Am. J. Fish. Manag. 35, 802–809. doi: 10.1080/02755947.2015.1044764
Whitelaw, E., and MacMullan, E. (2002). A framework for estimating the costs and benefits of dam removal: sound cost–benefit analyses of removing dams account for subsidies and externalities, for both the short and long run, and place the estimated costs and benefits in the appropriate economic context. BioScience 52, 724–730. doi: 10.1641/0006-3568(2002)052[0724:AFFETC]2.0.CO;2
Winans, G. A., Baker, J., McHenry, M., Ward, L., and Myers, J. (2017). Genetic characterization of Oncorhynchus mykiss prior to dam removal with implications for recolonization of the Elwha River Watershed, Washington. Trans. Am Fish. Soc. 146, 160–172. doi: 10.1080/00028487.2016.1249293
Winter, B. D., and Crain, P. (2008). Making the case for ecosystem restoration by dam removal in the Elwha River, Washington. Northwest Sci. 82, (Suppl. 1), 13–28. doi: 10.3955/0029-344X-82.S.I.13
Wippelhauser, G. (2021). Recovery of diadromous fishes: A Kennebec River case study. Trans. Am. Fish. Soc. 150, 277–290. doi: 10.1002/tafs.10292
Keywords: salmonids, spatially continuous survey, fish passage, restoration, connectivity, riverscape
Citation: Duda JJ, Torgersen CE, Brenkman SJ, Peters RJ, Sutton KT, Connor HA, Kennedy P, Corbett SC, Welty EZ, Geffre A, Geffre J, Crain P, Shreffler D, McMillan JR, McHenry M and Pess GR (2021) Reconnecting the Elwha River: Spatial Patterns of Fish Response to Dam Removal. Front. Ecol. Evol. 9:765488. doi: 10.3389/fevo.2021.765488
Received: 27 August 2021; Accepted: 21 October 2021;
Published: 09 December 2021.
Edited by:
Barbara Belletti, UMR 5600 Environnement, Ville, Societe (EVS), FranceReviewed by:
Christian Zimmerman, U.S. Geological Survey, United StatesKyle Martens, Washington State Department of Natural Resources, United States
At least a portion of this work is authored by Jeffrey J. Duda, Christian E. Torgersen, Samuel J. Brenkman, Roger J. Peters, Heidi A. Connor, Phil Kennedy, Stephen C. Corbett, Anna Geffre, Josh Geffre, Patrick Crain, and George R. Pess on behalf of the U.S. Government and as regards Dr. Duda, Dr. Torgersen, Dr. Brenkman, Dr. Peters, Dr. Connor, Dr. Kennedy, Dr. Corbett, Dr. Geffre, Dr. Geffre, Dr. Crain, Dr. Pess, and the U.S. Government, is not subject to copyright protection in the United States. Foreign and other copyrights may apply. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey J. Duda, jduda@usgs.gov
 Jeffrey J. Duda
Jeffrey J. Duda