- School of Dental Medicine, University of Pittsburgh, Pittsburgh, United States
The need to determine risk factors for complex diseases continues to drive efforts to identify etiological factors of common conditions. Molecular tools have created new opportunities to identify risk factors that may act interactively. The goal of this work was to explore potential interactions between oral microbial species and common genetic variants. Ninety-two 6- to 19-year-old individuals recruited through the University of Pittsburgh Dental Registry and DNA Repository project that had oral microbiome and genotyping of 44 single nucleotide polymorphism (SNP) data available were studied. Over-representation of alleles between individuals with or without particular microorganisms was determined using chi-square or Fisher's exact tests. An alpha of 0.001, to account for multiple testing (0.05/44), was considered statistically significant. Associations were found between Candida albicans and enamelin rs3796704 (p = 0.0006), and Staphylococcus epidermidis and tuftelin rs3828054 (p = 0.001). Microbiota and their metabolites might predispose to oral disease when interacting with the host genetic variation and future studies should address their causal roles in oral disease.
Introduction
Single nucleotide polymorphisms (SNPs) have been associated with microbial commensal species, and these associations may mean an interplay between overlapping pathophysiological processes. Several SNPs are associated with the gut microbiome (1–6), and among these initially identified associations, some were associated with outcomes such as food intake, hypertension, atopy, chronic obstructive pulmonary disease, body mass index, and lipids (7). Combined analyses of the oral microbiota and genetic variation of the host have been done in humans (8), indicating that genetic variation is an explanation for a stable or recurrent oral microbiome in each individual. These initial analyses unveiled associations between rs1196764 in the locus of the adaptor protein, phosphotyrosine interacting with pleckstrin homology domain and leucine zipper 2 (APPL2) and Prevotella jejuni, Oribacterium uSGB 3339, and Solobacterium uSGB 315 recovered from the tongue dorsum. The analysis also showed that carrying the variant allele of APPL2 rs1196764 and one of the three bacterial species made an individual less likely to have dental calculus.
An opportunity exists to identify disease associations by determining underlying associations between multiple risk factors. Previous work has suggested a number of associations between common genetic variation in humans and dental caries (9, 10), as well as that certain microorganisms are associated with the disease (11). Therefore, we tested for associations between oral microorganisms and genetic variation of a well-characterized cohort of 6- to 19-year-old individuals to unveil unknown associations between oral microbiota and host SNPs.
Material and methods
Data from 92 6- to 19-year-old individuals (12) (Supplemental Material) that had their whole saliva samples characterized using the Ibis Universal Biosensor, which combines polymerase chain reaction (PCR) and mass spectroscopy (MS), to detect species composition in a sample. Dental caries experience data available (DMFT/DMFS scores) referred to caries experience only in the permanent dentition. In brief, individuals were asked to expectorate (spit) 1 ml of unstimulated saliva in a plastic vial. Participants went at least 60 min without eating before sample collection. Saliva samples were immediately placed in a container with ice and then stored at −80°C. Subjects were recruited at the Department of Pediatric Dentistry of the University of Pittsburgh School of Dental Medicine between May and October 2012 as part of the Dental Registry and DNA Repository (DRDR) project. This study was approved by the University of Pittsburgh Institutional Review Board (approval number 0606091). All parents of the participating children provided written informed consent for their participation in the study after they provided written assent for their participation. For DNA extraction, the saliva was placed into a sterile microcentrifuge tube containing ATL lysis buffer (Qiagen, Germantown, MD, cat# 19076) and proteinase K (Qiagen, cat# 19131). Samples were incubated at 56 °C until lysis. One-hundred microliters of a mixture containing 50 μl each of 0.1 mm and 0.7 mm Zirconia beads (Biospec cat# 11079101z, 11079107zx, respectively) were added to the samples which were then homogenized for 10 min at 25 Hz using a Qiagen TissueLyser. Nucleic acid from the lysed sample was then extracted using the Qiagen DNeasy tissue kit (Qiagen cat# 69506). For microbiota analysis, 10 μl of each sample was loaded per well onto the BAC detection PCR plate (Abbott Molecular, cat# PN 05N13-01). The BAC detection plate is a 96-well plate containing 16 primers that survey all bacterial organisms by using the omnipresent loci (e.g., 16S rRNA sequences), while some are targeted to specific pathogens of interest (e.g., the Staphylococcus-specific tufB gene). The plate also includes primers for the detection of Candida species and some antibiotic resistance markers (e.g., mecA, vanA, vanB, and KPC). An internal calibrant of a synthetic nucleic acid template is also included in each assay, controlling for false negatives (e.g., from PCR inhibitors) and enabling a semi-quantitative analysis of the amount of template DNA present. PCR amplification was carried out, and the products were desalted in a 96-well plate format and sequentially electrosprayed into a mass spectrometer. The spectral signals were processed to determine the masses of each of the PCR products present with sufficient accuracy that the base composition of each amplicon could be unambiguously deduced. Using combined base compositions from multiple PCRs, the identities of the pathogens and a semi-quantitative determination of their relative concentrations in the starting sample were established by using a proprietary algorithm to interface with the Ibis database of known organisms. To display the distribution of species per patient, we generated heat map graphics using the “image()” and “grid()” functions in the “graphics” package part of R base distribution (version 3.2.2) (R Foundation for Statistical Computing, Vienna, Austria) (12).
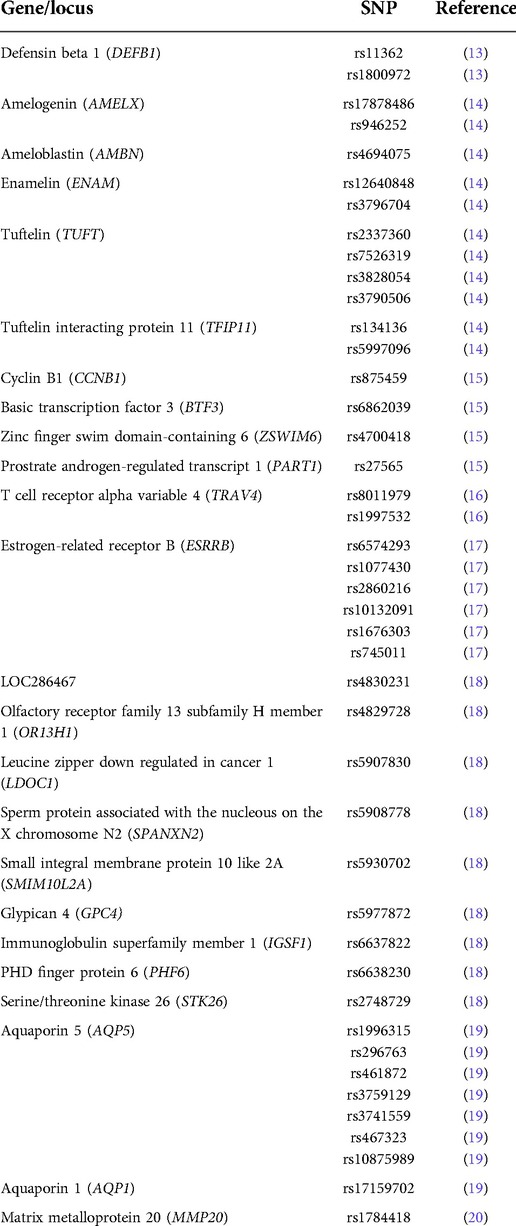
Further, genomic DNA was obtained from these same saliva samples and genotyping data for 43 single nucleotide polymorphisms (SNPs) were generated. These SNPs (Table 1) were selected based on previous data suggesting them to be marking genes that were associated with dental caries (13–20). TaqMan chemistry and endpoint analysis were used to determine genotypes (21). When a particular species was frequent enough in the group of 92 6- to 19-year-old individuals (present in at least 18 individuals), chi-square or Fisher's exact tests were used to test for overrepresentation of alleles in individuals carrying particular species, with an alpha of 0.001 (0.05/43). This cutoff of 20% aimed to avoid multiple comparisons of very small frequencies.
Results
The 92 6- to 19-year-old individuals studied had a mean age of 12.74 years (ages ranged from 6 to 19 years), and 44 individuals were females. Most of them (N = 63) were white, and among the remaining individuals, 21 were Black and 8 were Asian. Twenty individuals were caries-free, and among the remaining individuals that had caries experience, DMFT scores (decayed, missing due to caries, filled teeth) ranged from 1 to 17 and DMFS scores (decayed, missing due to caries, filled surfaces) ranged from 1 to 41 (Supplemental Material). Sixty-seven microbial species were identified in the samples from the 92 6- to 19-year-old individuals studied (Table 2).
Five microbes (Candida albicans, Saccharomyces cerevisiae/paradoxus, Staphylococcus epidermidis, Stomatococcus mucilaginosus, and Streptococcus pneumoniae) that were at least frequent in 20% of the sample were used to create groups for testing for overrepresentation of alleles of the SNPs listed in Table 1, which are located in loci that were previously associated with dental caries (13–20). All genotypes were in Hardy-Weinberg equilibrium. Having Candida albicans was associated with ENAM rs3796704 (p = 0.0006). Half of the individuals colonized with Candida albicans carried the variant allele of ENAM rs3796704, whereas less than 10% of individuals without Candida albicans carried the allele. Being positive for Staphylococcus epidermidis was associated with TUFT rs3828054 (p = 0.001). No individuals colonized by Staphylococcus epidermidis carried the TUFT rs3828054 variant allele. No other comparison showed differences that were statistically significantly different (all data are available as Supplemental Materials). Microorganisms historically associated with caries lesions such as Streptococcus mutans and Lactobacilli were found in just a few individuals and did not provide a chance for testing for associations.
Discussion
Dental caries can be defined as having a complex mode of inheritance, which includes the influence of more than one gene with small effects and environmental factors. Putative associations between dental caries with certain microbes or with genomic variation have been reported. These results did not translate to new preventive strategies, in part due to the fact that we do not understand how these different contributing factors may interact. Here we report novel associations between microbial species found in the mouth and common genomic variants in the population that have been previously associated with dental caries. These kinds of analyses aim to provide new insight into how the host interacts with microorganisms and how these may impact health and disease.
The success of genome-wide association studies (GWAS) in determining associations between SNPs and disease has motivated the proposal of testing microorganism whole genomes as well. Initial attempts have identified associations between ranges of bacteria, viruses, and protozoa and traits under strong selection such as drug resistance. The challenge of identifying variants under moderate selection still exists, and solutions are needed for instances of increased population stratification in microorganisms due to selection and complex recombination patterns (22). To minimize the chance for spurious associations, we tested SNPs that were previously associated with dental caries, a meaningful oral health microbial-mediated outcome.
Candida albicans is suggested to play a role in the development of dental caries (23), and understanding this would provide useful new insights. We suggested that variation in enamel conformation, depending on genetic variation, may increase the risk of developing dental caries lesions (24). The association between ENAM rs3796704 and the presence of Candida albicans suggests that variation in enamel conformation may increase dental caries susceptibility in the presence of Candida albicans colonization. These possible interactions should be modeled in future studies.
The role of Staphylococcus epidermidis in dental caries is unclear, and it is possible that it exists in the presence of certain genetic variations. The meaning of the many species that can be found in the mouth after molecular technology became available is currently object of study. Interestingly, Streptococcus mutans, which is classically associated with initiation of dental caries lesions, was only detected in one individual out of 92, suggesting that the individuals studied here did not have newly forming dental caries lesions. We showed earlier (12) that when compared, the results between PCR-MS and 16S rRNA sequencing differ significantly. The samples we tested by PCR-MS displayed a range in the number of genera detected from 1 to 4, including both bacteria and fungi. Both PCR-MS and 16s rRNA sequencing detected a prevalence of Streptococci, Neisseria, and Rothia in the samples. Sequencing of the 16S rRNA gene suggests that Prevotella and Veillonella were not detected by PCR-MS; while staphylococci and fungi were not detected by 16S rRNA sequencing. Using 16S rRNA sequencing therefore should provide the opportunity for identification of additional associations.
Larger sample sizes will allow for these analyses to be conducted taking into consideration age, sex, ethnicity, and dental caries severity. These data were available but were not used since initial comparisons were already with small numbers. Sex, age, and ethnicity variations were not accounted for in the comparisons presented here, and undetected population substructures can always unveil spurious associations. Another limitation is that the quantification of microorganisms is relevant data for interpreting associations with diseases and we utilized only representation of species in this report. Future approaches should include measures of quantification of microorganisms. Finally, some SNPs had lower amplification ratios, which also impacted some of the comparisons. In summary, associations between genomic variants that impact dental caries risk may be explained by individual microbial colonization, and genomic and microbiome host data should be considered when testing for associations between dental caries and other risk factors.
Data availability statement
The authors acknowledge that the data presented in this study must be deposited and made publicly available in an acceptable repository, prior to publication. Frontiers cannot accept a manuscript that does not adhere to our open data policies.
Ethics statement
The studies involving human participants were reviewed and approved by the University of Pittsburgh Institutional Review Board. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
ARV wrote the initial draft. AM critically revised and finalized the manuscript. Both authors contributed to design and resources. All authors contributed to the article and approved the submitted version.
Funding
The Dental Registry and DNA Repository project is supported by the University of Pittsburgh School of Dental Medicine.
Acknowledgments
The authors thank Dr. Studen-Pavlovich, Chair and Program Director of Pediatric Dentistry, for her support to the University of Pittsburgh School of Dental Medicine Dental Registry and DNA Repository project. The Dental Registry and DNA Repository project is also supported by the University of Pittsburgh School of Dental Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2022.875953/full#supplementary-material.
References
1. Davenport ER, Cusanovich DA, Michelini K, Barreiro LB, Ober C, Gilad Y. Genome-wide association studies of the human gut microbiota. PLoS One. (2015) 10(11):e0140301. doi: 10.1371/journal.pone.0140301
2. Bonder MJ, Kurilshikov A, Tigchelaar EF, Mujagic Z, Imhann F, Vila AV, et al. The effect of host genetics on the gut microbiome. Nat Genet. (2016) 48(11):1407–12. doi: 10.1038/ng.3663/
3. Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. (2016) 19(5):731–43. doi: 10.1016/j.chom.2016.04.017
4. Turpin W, Espin-Garcia O, Xu W, Silverberg MS, Kevans D, Smith MI, et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet. (2016) 48(11):1413–7. doi: 10.1038/ng.3693.27694960
5. Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, et al. Genome-wie association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. (2016) 48(11):1396–406. doi: 10.1038/ng.3695
6. Scepanovic P, Hodel F, Mondot S, Partula V, Byrd A, Hammer C, et al. Milieu intérieur consortium. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome. (2019) 7(1):130. doi: 10.1186/s40168-019-0747-x
7. Groot HE, van de Vegte YJ, Verweij N, Lipsic E, Karper JC, van der Harst P. Human genetic determinants of the gut microbiome and their associations with health and disease: a phenome-wide association study. Sci Rep. (2020) 10(1):14771. doi: 10.1038/s41598-020-70724-5
8. Liu X, Tong X, Zhu J, Tian L, Jie Z, Zou Y, et al. Metagenome-genome-wide association studies reveal human genetic impact on the oral microbiome. Cell Discov. (2021) 7(1):117. doi: 10.1038/s41421-021-00356-0
9. Vieira AR, Modesto A, Marazita ML. Caries: review of human genetics research. Caries Res. (2014) 48(5):491–506. doi: 10.1159/000358333
10. Nibali L, Di Iorio A, Tu YK, Vieira AR. Host genetics role in the pathogenesis of periodontal disease and caries. J Clin Periodontol. (2017) 44(Suppl 18):S52–78. doi: 10.1111/jcpe.12639
11. Pitts NB, Tweman S, Fisher J, Marsh PD. Understanding dental caries as a non-communicable disease. Br Dent J. (2021) 231(12):749–53. doi: 10.1038/s41415-021-3775-4
12. Vieira AR, Hiller NL, Powell E, Kim LH-J, Spirk T, Modesto A, et al. Profiling microorganisms in whole saliva of children with and without dental caries. Clin Exp Dent Res. (2019) 5(4):438–46. doi: 10.1002/cre2.206
13. Ozturk A, Famili P, Vieira AR. The antimicrobial peptide DEFB1 is associated with caries. J Dent Res. (2010) 89(6):631–6. doi: 10.1177/0022034510364491
14. Shimizu T, Ho B, Deeley K, Briseño-Ruiz J, Faraco IM Jr, Schupack BI, et al. Enamel formation genes influence enamel microhardness before and after cariogenic challenge. PLoS One. (2012) 7(9):e45022. doi: 10.1371/journal.pone.0045022
15. Shimizu T, Deeley K, Briseño-Ruiz J, Faraco IM Jr, Poletta FA, Brancher JA, et al. Fine-mapping of 5q12.1-13.3 unveils new genetic contributors to caries. Caries Res. (2013) 47(4):273–83. doi: 10.1159/000346278
16. Briseño-Ruiz J, Shimizu T, Deeley K, Dizak PM, Ruff TD, Faraco IM Jr, et al. Role of TRAV locus in low caries experience. Hum Genet. (2013) 132(9):1015–25. doi: 10.1007/s00439-013-1313-4
17. Weber ML, Hsin HY, Kalay E, BroŽková DS, Shimizu T, Bayram M, et al. Role of estrogen related receptor beta (ESRRB) in DFN35B hearing impairment and dental decay. BMC Med Genet. (2014) 15:81. doi: 10.1186/1471-2350-15-81
18. Küchler EC, Feng P, Deeley K, Fitzgerald CA, Meyer C, Gorbunov A, et al. Fine mapping of locus Xq25.1-27.2 for a low caries experience phenotype. Arch Oral Biol. (2014) 59(5):479–86. doi: 10.1016/j.archoralbio.2014.02.009
19. Anjomshoaa I, Briseño-Ruiz J, Deeley K, Poletta FA, Mereb JC, Leite AL, et al. Aquaporin 5 interacts with fluoride and possibly protects against caries. PLoS One. (2015) 10(12):e0143068. doi: 10.1371/journal.pone.0143068
20. Filho AV, Calixto MS, Deeley K, Santos N, Rosenblatt A, Vieira AR. MMP20 Rs1784418 protects certain populations against caries. Caries Res. (2017) 51(1):46–51. doi: 10.1159/000452345
21. Ranade K, Chang MS, Ting CT, Pei D, Hsiao CF, Olivier M, et al. High-throughput genotyping with single nucleotide polymorphisms. Genome Res. (2001) 11(7):1262–8. doi: 10.1101/gr.157801
22. Power RA, Parkhill J, Oliveira T. Microbial genome-wide association studies: lessons from human GWAS. Nat Rev Genet. (2017) 18(1):41–50. doi: 10.1038/nrg.2016.132
23. Cui Y, Wang Y, Zhang Y, Pang L, Zhou Y, Lin H, et al. Oral mycobiome differences in various spatial niches with and without severe early childhood caries. Front Pediatr. (2021) 9:748656. doi: 10.3389/fped.2021.748656
Keywords: single nucleotide (nt) polymorphism (SNP), oral microbiology, dental caries, genetics, linkage disequiblibrium
Citation: Vieira AR and Modesto A (2022) Oral microbiome dental caries associated genotypes analysis of 6- to 19-year-old individuals shows novel associations. Front. Dent. Med 3:875953. doi: 10.3389/fdmed.2022.875953
Received: 14 February 2022; Accepted: 7 September 2022;
Published: 23 September 2022.
Edited by:
Sreekanth Kumar Mallineni, Faculty of Dentistry at Al Zulfi, Majmaah University, Saudi ArabiaReviewed by:
Daniela Silva Barroso De Oliveira, Federal University of Alfenas, BrazilAhmad Faisal Ismail, Kulliyyah of Dentistry, International Islamic University Malaysia, Malaysia
Copyright: © 2022 Vieira and Modesto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandre Rezende Vieira, YXJ2MTFAcGl0dC5lZHU=
Specialty Section: This article was submitted to Pediatric Dentistry, a section of the journal Frontiers in Dental Medicine
 Alexandre R. Vieira
Alexandre R. Vieira Adriana Modesto
Adriana Modesto