
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Dent. Med. , 09 August 2021
Sec. Pediatric Dentistry
Volume 2 - 2021 | https://doi.org/10.3389/fdmed.2021.703549
This article is part of the Research Topic Omics Research for Pediatric Dentistry in Health and Disease View all 4 articles
 Caio Luiz Bitencourt Reis1
Caio Luiz Bitencourt Reis1 Mariane Carolina Faria Barbosa2
Mariane Carolina Faria Barbosa2 Daniela Coelho de Lima3
Daniela Coelho de Lima3 Isabela Ribeiro Madalena1,4,5
Isabela Ribeiro Madalena1,4,5 Flares Baratto-Filho4
Flares Baratto-Filho4 Peter Proff6
Peter Proff6 Daniela Silva Barroso de Oliveira3
Daniela Silva Barroso de Oliveira3 Eva Paddenberg6
Eva Paddenberg6 Erika Calvano Küchler6
Erika Calvano Küchler6 Christian Kirschneck6*
Christian Kirschneck6*Parathyroid hormone (PTH) is essential for calcium and phosphate homeostasis in odontogenesis-related cells. Therefore, the present study aimed to investigate the association between single nucleotide polymorphisms in the gene encoding PTH, and dental caries in Brazilian children. Three hundred and fifty-three children (170 boys and 183 girls, age ranging from 8 to 11 years old) were included in this study. The International System for Detection and Assessment of Carious Lesions (ICDAS) was used for diagnosis of dental caries. Visible biofilm was also evaluated during the clinical examination. Genomic DNA was extracted from saliva for real-time PCR to evaluate the single nucleotide polymorphisms rs6256, rs307247 and rs694 in PTH gene. Dental caries was classified in ICDAS0 vs. ICDAS1−6 or ICDAS1−2 vs. ICDAS3−6. Chi-square test, binary logistic regression adjusted by biofilm and haplotype analyses were performed (p < 0.05). Biofilm was associated with dental caries (p < 0.05). There were no associations between dental caries and rs6256, rs307247, rs694 in none of the analyses performed (p > 0.05). In conclusion, the present study supports that the single nucleotide polymorphisms rs6256, rs307247, and rs694 in the PTH-encoding gene are not associated with dental caries in Brazilian children.
Parathyroid hormone (PTH) is essential for many biological processes, mostly for regulating extracellular fluid calcium and phosphate homeostasis (1). PTH is the product of chief cells of parathyroid glands in response to low calcium serum levels (2) and initiates a mechanism of bone resorption to stimulate calcium release (3). Besides that, PTH is involved in a complex interaction network in proliferation of mesenchymal stem cells and enhances the differentiation of osteo- and odontogenesis-related cells (4). Odontogenesis is a highly coordinated process of tooth formation and morphogenesis. This process relies upon protein-cell interaction, in which odontoblasts and ameloblasts are the cells responsible for dentin and enamel synthesis (5). PTH play a direct role in odontoblast and ameloblast (6, 7). Interestingly, during tooth formation, any imbalance in this network of protein-cell interaction may impacts dental tissues (8). This fact may lead to an enamel and dentin defects, contributing to an increased susceptibility risk and progression of dental caries (8, 9).
Dental caries is a multifactorial disease characterized by the demineralization of tooth minerals. It is one of the most common chronic diseases during childhood (10). The link between dental caries and host genetic background has been constantly studied due to the possibility that some genes may interfere in the function of amelogenesis and dentinogenesis related proteins and predicted the susceptibility or progression to dental caries (11). However, the association between dental caries and variants in PTH decoder gene was little investigated in children (12, 13).
Single nucleotide polymorphisms (SNPs) are a type of sequence variation within genomes that may modify the function and the quantity of protein expression. In fact, previous studies demonstrated that SNPs in PTH gene were associated with modifications of PTH serum levels (14–17). These modifications in PTH serum levels may both influence tooth formation and the remineralization and demineralization equilibrium of the tooth in occlusion (2, 18). Thus, it is possible that SNPs in PTH are involved in the risk of dental caries. Therefore, the present study aimed to investigate the association between SNPs rs6256, rs307247, rs694 in the gene encoding PTH, and dental caries in Brazilian children.
This study was designed and reported according to the Strengthening the Reporting of Genetic Association study (STREGA) statement checklist. After the consent by the Local Human Ethics committee (protocol: 78568217.7.0000.5142), this cross-sectional study recruited biologically unrelated schoolchildren of both genders, aged between 8 and 11 years, enrolled in schools in the city of Alfenas, Brazil. Parents/legal guardians agreed to participate in written informed consent and an assent document was developed for children. All standard biosecurity and institutional safety procedures have been adhered in this study. Children with unhealthy systemic conditions, diseases, endocrinal problems or hormonal treatment, and children with syndromes were excluded. This sample was previously described in Reis et al. (19).
A sample size calculation was performed [power = 80%; alpha = 95%; effect size (Cohen's w) = 0.20; Degrees of freedom: 3] and predicted a minimum of 273 children. The sample and data collection were performed in 2018 by one single examiner trained and calibrated (Kappa intra-examiner = 0.87). Information about health habits was obtained through the anamnesis answered by parents or caregivers.
Dental caries was accessed by ICDAS (International Caries Detection and Assessment System) according to Shoaib et al. (20). ICDAS was categorized as a dichotomic variable in two different cut-offs: Healthy (ICDAS0) vs. Caries (ICDAS1−6); or non-cavitated lesion (ICDAS1−2) vs. cavitated caries lesion (ICDAS3−6), as previously described (21).
Visible biofilm was also evaluated using the index described by Silness and Löe (22). The scores vary from 0 (absence) to 3 (abundant on the surface of the tooth).
For the genotyping analysis DNA samples from each child were used. Briefly, genomic DNA was isolated from buccal/oral epithelial cells in saliva samples and extracted from saliva following a pre-established protocol (23). The saliva tube was centrifuged and supernatant was discarded. Extraction solution (Tris- HCl 10 mmol/L, pH 7.8; EDTA 5 mmol/L; SDS 0.5%, 1 mL) and proteinase K (100 ng/mL) was added to the tube. Afterwards, for removal of non-digested proteins, ammonium acetate was added and centrifugation performed. The DNA was precipitated with isopropanol and washed with 70% ethanol. The tubes were allowed to air-dry on an absorbent paper and DNA resuspended in 100 μL of TE buffer (10 mmol/L Tris (pH 7.8) and 1 mmol/L EDTA). The concentration and purity of DNA was determined by spectrophotometry using NanoDrop 1000 (Thermo Scientific, Wilmington, DE, EUA).
Genotyping was performed by Real-time polymerase chain reactions (PCR) using TaqMan probes (Applied Biosystems®, 7500 Real-Time PCR System, Thermo Fisher Scientific). The allelic discrimination real-time PCR reactions were performed in a total volume of 5 μL (5 ng DNA/reaction in H2O, 2.5 μL TaqMan PCR master mix, 0.125 μL SNP assay; Applied Biosystems). The thermal cycling was carried out by starting with a hold cycle of 95°C for 10 min, followed by 40 amplification cycles of 92°C for 15 s and 60°C for 1 min. The SNPs selected in this study were previously associated with alterations in PTH serum level (14–16). Characteristics of the SNPs in the PTH gene are summarized in Table 1.
For statistical analyses, SPSS software version 25.0 (SPSS Inc. Chicago, IL, USA) was used. Chi-square test was applied for evaluating Hardy-Weinberg equilibrium genotypic distribution. Logistic regression adjusted by biofilm was also performed, and alleles and haplotypes were analyzed by PLINK software. A p < 0.05 was considered statistically significant.
Three hundred and fifty-three children were included in this study, 170 boys (48.2%) and 183 girls (51.8%). The mean age of the sample was 8.88 years (SD = 0.88), 8.82 years for girls (SD = 0.81), and 8.94 years (SD = 0.96) for boys. Biofilm was significantly more abundant in the ICDAS1−6 and ICDAS3−6 groups (p < 0.05). Detailed information is reported in Table 2.
All studied SNPs were within the Hardy-Weinberg equilibrium (p > 0.05). The amplification rate for rs6256 was 97.7% (n = 345), 94.3% for rs307247 (n = 333), and 98.0% for rs694 (n = 346).
Allele distribution and haplotype analysis are shown in Table 3. There was no association between alleles/haplotypes and dental caries (p > 0.05).
Dental caries was not associated with any of the SNPs in both comparisons (ICDAS0 vs. ICDAS1−6 and ICDAS0−2 vs. ICDAS1−6) in genotype distribution (Table 4).
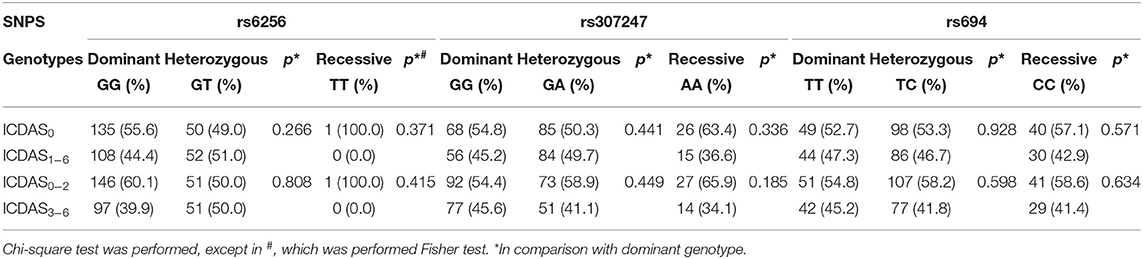
Table 4. Genotype distribution between Healthy vs. Dental caries (ICDAS0 vs. ICDAS1−6) and Healthy and non-cavitated caries lesion vs. Cavitated caries lesion (ICDAS0−2 vs. ICDAS3−6).
Multiple logistic regression adjusted by biofilm was also performed, but the SNPs were not associated with dental caries (Table 5).
ICDAS was also evaluated as a continuous variable, however, no association was also founded in this analysis (data not shown).
To the best of our knowledge, this is the first study to evaluate the association between SNPs in the PTH gene and dental caries in Brazilian children. PTH influences tooth formation, since the odontogenesis-related cells differentiate until dentin and enamel synthesis (4, 6, 7). There is evidence that odontoblasts, cementoblasts and ameloblasts, specific cells of tooth formation, express the PTH receptor 1 (PTHR1) and are sensible to PTH serum levels (7, 24). Although the effects of PTH on cementoblasts and ameloblasts are not fully understood (24), in vitro studies (7) and studies evaluating PTH-knockout mice (6) indicate that PTH modulates the proliferative and apoptotic responses of odontoblasts, suggesting that modifications in PTH serum levels during odontogenesis can affect dentine and enamel quality and increase the risk and progression of dental caries (6, 7). However, more studies should be developed to clarify the function of PTH in ameloblasts and odontoblasts and its impact on dental caries susceptibility and progression.
Although PTH serum levels have been investigated in a variety of conditions (14–17), the relationship between PTH serum levels and dental caries is not clarified yet. While Gyll et al. (25) did not observe differences in PTH serum levels between caries and caries-free children, on the other hand, Schroth et al. (26) and Deane et al. (27) showed an increase in PTH levels in children with Severe Early Childhood Caries, apart from observing a decrease of calcium and 1,25-dihydroxy vitamin D levels in these children. The authors suggest that changes in PTH levels can unbalance the levels of inflammatory mediators and un-regulate the host's response to dental caries. In fact, by a process not fully understood, the serum levels of some pro-inflammatory proteins, like interleukin-6 (IL-6) and IL-1β, are greater in patients diagnosed with primary or secondary hyperparathyroidism, a condition characterized by hypersecretion of PTH (17, 28, 29). Interestingly, IL-6 and IL-1β were already previously associated with dental caries (30–32). Future studies should investigate salivary and serum levels of these inflammatory proteins and PTH to clarify the relationship between PTH and the process of dental caries.
Calcium and 1,25-dihydroxy vitamin D homeostasis are essential for the formation and maintenance of teeth (33). In fact, low levels of calcium were already associated with dental caries in children (26, 27, 34, 35), as well as low levels of 1,25-dihydroxy vitamin D (36). Low calcium levels are detected by extracellular calcium receptors in the parathyroid glands, which increase PTH gene transcription and secretion. Besides calcium, phosphate and 1,25-dihydroxy vitamin D were also identified to regulate PTH gene transcription. In bones, PTH stimulates receptor activator for nuclear factor kappa-B ligand (RANKL) expression in osteoblasts, which initiates the differentiation process of immature to mature osteoclasts. This way, calcium is released into the bloodstream by osteoclastic dissolution of organic materials like hydroxyapatite (37, 38). Thus, PTH may indirectly interfere in dental caries susceptibility through calcium and vitamin D regulation.
Our hypothesis was that SNPs in the PTH gene that can alter PTH serum levels would affect tooth-forming tissues and, subsequently, later modify the host's immunoinflammatory response and the calcium regulation (14–17). We speculated that SNPs in the PTH gene may influence the quality/quantity and maintenance of tooth tissues, therefore increasing the susceptibility and risk of progression to dental caries. rs6256, rs307247, and rs694 SNPs were previously associated with a decrease of PTH serum levels (14–17). Besides that, Küchler et al. (39) associated the rs6256, rs307247, and rs694 SNPs in PTH with mandibular retrognathism in a Brazilian population. In this study, dental caries was not associated with these SNPs. A possible limitation of this study was that inflammatory mediators, calcium, vitamin D, and PTH levels were not measured during the formation of the child's dental tissue or during clinical examination.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Human Ethics committe of Federal University of Alfenas (protocol: 78568217.7.0000.5142). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
CK, EK, DO, FB-F, and DL conceptualize the study. EK, DO, and DL designed and organize the sample recruitment. MB performed the sample collection and the DNA extraction. CR, IM, and EP performed the genotyping analysis and analyzed the data. CR, MB, EK, and CK wrote the manuscript. EK, PP, CK, and FB-F funding support. All authors contributed to the article and approved the submitted version.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, and Alexander-von-Humboldt-Foundation (Küchler/Kirschneck accepted in July 4th, 2019). National Council for Scientific and Technological Development (CNPq) financed individual scholarship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES), Alexander-von-Humboldt-Foundation, and National Council for Scientific and Technological Development (CNPq).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2021.703549/full#supplementary-material
1. Mitra P, Maity B, Pal DK, Das M. Polymorphisms of PTH (Parathyroid hormone) gene and risk of kidney stone disease: a case-control study from West Bengal, India. Urology. (2018) 121:79–85. doi: 10.1016/j.urology.2018.06.033
2. Aggarwal P, Zavras A. Parathyroid hormone and its effects on dental tissues. Oral Dis. (2012) 18:48–54. doi: 10.1111/j.1601-0825.2011.01850.x
3. Tokunaga K, Seto H, Ohba H, Mihara C, Hama H, Horibe M, et al. Topical and intermittent application of parathyroid hormone recovers alveolar bone loss in rat experimental periodontitis. J Periodont Res. (2011) 46:655–62. doi: 10.1111/j.1600-0765.2011.01386.x
4. Ge X, Li Z, Jing S, Wang Y, Li N, Lu J, et al. Parathyroid hormone enhances the osteo/odontogenic differentiation of dental pulp stem cells via ERK and P38 MAPK pathways. J Cell Physiol. (2020) 235:1209–21. doi: 10.1002/jcp.29034
5. Cobourne MT. The genetic control of early odontogenesis. Br J Orthod. (1999) 26:21–8. doi: 10.1093/ortho/26.1.21
6. Sun W, Sun W, Liu J, Zhou X, Xiao Y, Karaplis A, et al. Alterations in phosphorus, calcium and PTHrP contribute to defects in dental and dental alveolar bone formation in calcium-sensing receptor-deficient mice. Development. (2010) 137:985–92. doi: 10.1242/dev.045898
7. Guimaraes GN, Rodrigues TL, de Souza AP, Line SR, Marques MR. Parathyroid hormone (1-34) modulates odontoblast proliferation and apoptosis via PKA and PKC-dependent pathways. Calcif Tissue Int. (2014) 95:275–81. doi: 10.1007/s00223-014-9892-1
8. Conrads G, About I. Pathophysiology of dental caries. Monogr Oral Sci. (2018) 27:1–10. doi: 10.1159/000487826
9. Kirthiga M, Murugan M, Saikia A, Kirubakaran R. Risk factors for early childhood caries: a systematic review and meta-analysis of case control and cohort studies. Pediatr Dent. (2019) 41:95–112.
10. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. (2015) 94:650–8. doi: 10.1177/0022034515573272
11. Opal S, Garg S, Jain J, Walia I. Genetic factors affecting dental caries risk. Aust Dent J. (2015) 60:2–11. doi: 10.1111/adj.12262
12. Shaffer JR, Wang X, Feingold E, Lee M, Begum F, Weeks, et al. Genome-wide association scan for childhood caries implicates novel genes. J Dental Res. (2011) 90:1457–62. doi: 10.1177/0022034511422910
13. Haworth S, Shungin D, Van Der Tas JT, Vucic S, Medina-Gomez C, Yakimov V, et al. Consortium-based genome-wide meta-analysis for childhood dental caries traits. Hum Mol Genet. (2018) 27:3113–27. doi: 10.1093/hmg/ddy237
14. Kanzawa M, Sugimoto T, Kobayashi T, Kobayashi A, Chihara K. Parathyroid hormone gene polymorphisms in primary hyperparathyroidism. Clin Endocrinol. (1999) 50:583–8. doi: 10.1046/j.1365-2265.1999.00685.x
15. Tenne M, McGuigan F, Jansson L, Gerdhem P, Obrant KJ, Luthman H, et al. Genetic variation in the PTH pathway and bone phenotypes in elderly women: evaluation of PTH, PTHLH, PTHR1 and PTHR2 genes. Bone. (2008) 42:719–27. doi: 10.1016/j.bone.2007.12.005
16. Ertl DA, Stary S, Streubel B, Raimann A, Haeusler G. A novel homozygous mutation in the parathyroid hormone gene (PTH) in a girl with isolated hypoparathyroidism. Bone. (2012) 51:629–32. doi: 10.1016/j.bone.2012.06.009
17. Venkatesan S, Chakkarai K, Arulvijayavani S, Senthilkumar GP, Manikandan R, Kalyaperumal M. Association between vitamin D, parathyroid hormone and inflammatory markers in urolithiasis patients. J Renal Inj Prev. (2017) 6:240–3. doi: 10.15171/jrip.2017.45
18. Neel EA, Aljabo A, Strange A, Ibrahim S, Coathup M, Young AM, et al. Demineralization-remineralization dynamics in teeth and bone. Int J Nanomed. (2016) 11:4743–63. doi: 10.2147/IJN.S107624
19. Reis CLB, Barbosa MCF, de Lima DC, Brancher JA, Lopes CMCF, Baratto-Filho F, et al. Risk factors for developmental defects of enamel in children from southeastern Brazil. Community Dent Health. (2021) doi: 10.1922/CDH_00242Reis04. [Epub ahead of print].
20. Shoaib L, Deery C, Ricketts DNJ, Nugent ZJ. Validity and reproducibility of ICDAS II in primary teeth. Caries Res. (2009) 43:442–8. doi: 10.1159/000258551
21. Barbosa MCF, Lima DC, Reis CLB, Reis ALM, Rigo D Jr, Segato RAB, et al. Vitamin D receptor FokI and BglI genetic polymorphisms, dental caries, and gingivitis. Int J Paediatr Dent. (2020) 30:642–9. doi: 10.1111/ipd.12631
22. Silness J, Loe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. (1964) 22:121–35. doi: 10.3109/00016356408993968
23. Küchler EC, Tannure PN, Falagan-Lotsch P, Lopes TS, Granjeiro JM, Amorim LM. Buccal cells DNA extraction to obtain high quality human genomic DNA suitable for polymorphism genotyping by PCR-RFLP and Real-Time PCR. J Appl Oral Sci. (2012) 20:467–71. doi: 10.1590/S1678-77572012000400013
24. Kato A, Suzuki M, Karasawa Y, Sugimoto T, Doi K. PTHrP and PTH/PTHrP receptor 1 expression in odontogenic cells of normal and HHM model rat incisors. Toxicol Pathol. (2005) 33:456–564. doi: 10.1080/01926230590959604
25. Gyll J, Ridell K, Öhlund I, Åkeson PK, Johansson I, Holgerson PL. Vitamin D status and dental caries in healthy Swedish children. Nutr J. (2018) 17:1–10. doi: 10.1186/s12937-018-0318-1
26. Schroth RJ, Levi JA, Sellers EA, Friel J, Kliewer E, Moffatt ME. Vitamin D status of children with severe early childhood caries: a case-control study. BMC Pediatr. (2013) 13:174. doi: 10.1186/1471-2431-13-174
27. Deane S, Schroth RJ, Sharma A, Rodd C. Combined deficiencies of 25-hydroxyvitamin D and anemia in preschool children with severe early childhood caries: a case–control study. Paediatr Child Health. (2018) 23:e40–5. doi: 10.1093/pch/pxx150
28. Emam AA, Mousa SG, Ahmed KY, Al-Azab AA. Inflammatory biomarkers in patients with asymptomatic primary hyperparathyroidism. Med Princ Pract. (2012) 21:249–53. doi: 10.1159/000334588
29. Cheng SP, Liu CL, Liu TP, Hsu YC, Lee JJ. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm. (2014) 2014:709024. doi: 10.1155/2014/709024
30. Horst OV, Horst JA, Samudrala R, Dale BA. Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol. (2011) 12:1–13. doi: 10.1186/1471-2172-12-9
31. Gornowicz A, Bielawska A, Bielawski K, Grabwska SZ, Wojcicka A, Zalewska M, et al. Pro-inflammatory cytokines in saliva of adolescents with dental caries disease. Ann Agric Environ Med. (2012) 19:711–6.
32. Reis CLB, Barbosa MCF, Machado BMDSM, Baratto SSP, de Lima DC, Paza AO, et al. Genetic polymorphisms in interleukin-6 and interleukin-1-beta were associated with dental caries and gingivitis. Acta Odontol Scand. (2021) 79:96–102. doi: 10.1080/00016357.2020.1788722
33. Almoudi MM, Hussein AS, Hassan MIA, Schroth RJ. Dental caries and vitamin D status in children in Asia. Pediatr Int. (2019) 61:327–38. doi: 10.1111/ped.13801
34. Bener A, Al Darwish MS, Hoffmann GF. Vitamin D deficiency and risk of dental caries among young children: a public health problem. Indian J Oral Sci. (2013) 4:75–82. doi: 10.4103/0976-6944.119937
35. Pandey P, Reddy NV, Rao VAP, Saxena A, Chaudhary CP. Estimation of salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries severity, age and gender. Contemp Clin Dent. (2015) 6:S65–71. doi: 10.4103/0976-237X.152943
36. Navarro CLA, Grgic O, Trajanoska K, van der Tas JT, Rivadeneira F, et al. Associations between prenatal, perinatal, and early childhood vitamin D status and risk of dental caries at 6 years. J Nutr. (2021) 151:nxab075. doi: 10.1093/jn/nxab075
37. Khundmiri SJ, Murray RD, Lederer E. PTH and vitamin D. Compr Physiol. (2016) 6:561–601. doi: 10.1002/cphy.c140071
38. Khan M, Jose A, Sharma S. Physiology, Parathyroid Hormone. (2021). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK499940/ (accessed April 29, 2021).
Keywords: dental caries, parathyroid hormone, polymorphism, single nucleotid polymorphism, children
Citation: Reis CLB, Barbosa MCF, de Lima DC, Madalena IR, Baratto-Filho F, Proff P, de Oliveira DSB, Paddenberg E, Küchler EC and Kirschneck C (2021) Study of Dental Caries and PTH Gene. Front. Dent. Med. 2:703549. doi: 10.3389/fdmed.2021.703549
Received: 30 April 2021; Accepted: 12 July 2021;
Published: 09 August 2021.
Edited by:
Adriana Modesto Vieira, University of Pittsburgh, United StatesReviewed by:
Sivakumar Nuvvula, Narayana Dental College and Hospital, IndiaCopyright © 2021 Reis, Barbosa, de Lima, Madalena, Baratto-Filho, Proff, de Oliveira, Paddenberg, Küchler and Kirschneck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Kirschneck, Y2hyaXN0aWFuLmtpcnNjaG5lY2tAa2xpbmlrLnVuaS1yZWdlbnNidXJnLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.